 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Constructing nitrided interfaces for stabilizing Li metal electrodes in liquid electrolytes
Zhijie
Wang†
 a,
Yanyan
Wang†
a,
Chao
Wu
a,
Wei Kong
Pang
a,
Yanyan
Wang†
a,
Chao
Wu
a,
Wei Kong
Pang
 a,
Jianfeng
Mao
a,
Jianfeng
Mao
 ab and
Zaiping
Guo
ab and
Zaiping
Guo
 *ab
*ab
aInstitute for Superconducting & Electronic Materials, Australian Institute for Innovative Materials, University of Wollongong, NSW 2522, Australia
bSchool of Chemical Engineering and Advanced Materials, The University of Adelaide, Adelaide, South Australia 5005, Australia. E-mail: zaiping.guo@adelaide.edu.au
First published on 1st June 2021
Abstract
Traditional Li ion batteries based on intercalation-type anodes have been approaching their theoretical limitations in energy density. Replacing the traditional anode with metallic Li has been regarded as the ultimate strategy to develop next-generation high-energy-density Li batteries. Unfortunately, the practical application of Li metal batteries has been hindered by Li dendrite growth, unstable Li/electrolyte interfaces, and Li pulverization during battery cycling. Interfacial modification can effectively solve these challenges and nitrided interfaces stand out among other functional layers because of their impressive effects on regulating Li+ flux distribution, facilitating Li+ diffusion through the solid-electrolyte interphase, and passivating the active surface of Li metal electrodes. Although various designs for nitrided interfaces have been put forward in the last few years, there is no paper that specialized in reviewing these advances and discussing prospects. In consideration of this, we make a systematic summary and give our comments based on our understanding. In addition, a comprehensive perspective on the future development of nitrided interfaces and rational Li/electrolyte interface design for Li metal electrodes is included.
1. Introduction
Rechargeable lithium (Li) ion batteries (LIBs) have been shaping many aspects of our modern life.1–4 Nevertheless, the traditional graphite-based LIBs have nearly reached their theoretical limit in energy density (∼250 W h kg−1), which hinders the development of portable electrical devices and electric vehicles.5–8 Li metal has the lowest electrochemical potential (−3.04 V vs. the standard hydrogen electrode (SHE)) among the alkali metals and a much higher theoretical specific capacity of 3860 mA h g−1 (which is 10 times that of graphite) (Fig. 1a).4,9–15 When paired with high-voltage cathode materials, Li metal batteries (LMBs) are able to provide a 5 V-class output voltage and a 500 W h kg−1-class energy density (Fig. 1a).16–19 Therefore, reviving LMBs is an effective strategy to break the performance limitation of LIBs.20–23 The main challenge is that all liquid electrolytes are thermodynamically unstable at 0 V vs. Li/Li+, because the lowest unoccupied molecular orbital (LUMO) of the electrolyte is lower than the Fermi level of Li metal (Fig. 1b).3,24 Thus, the electrolyte accepts electrons from Li metal and reductively decomposes on the surface of the Li electrode to form a solid-electrolyte interphase (SEI).16,25–28 The inner layer of the SEI (close to Li metal) consists of inorganic components such as lithium oxide (Li2O), lithium fluoride (LiF), and lithium carbonate (Li2CO3), while the outer layer of the SEI (close to the electrolyte) mainly consists of organic components such as polyolefins and semicarbonates (Fig. 1c).25,29,30 The SEI layer is electrically non-conductive but ionically conductive, so that it can block the electron transport at the Li/electrolyte interface and stop the further decomposition of the electrolyte while Li+ diffuses through the layer.16,26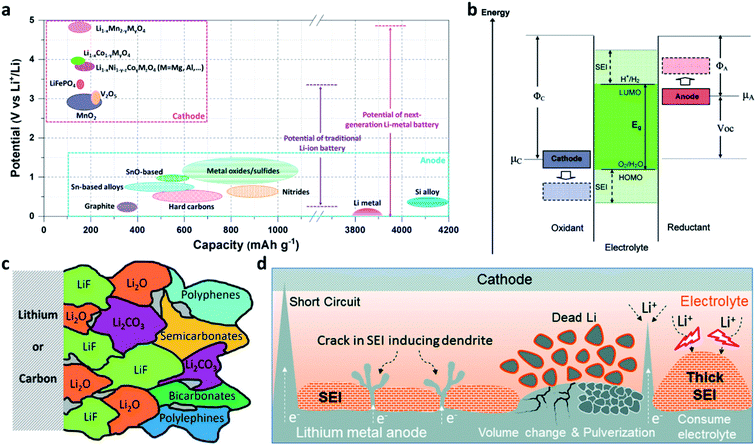 | ||
| Fig. 1 Opportunities and challenges of Li metal electrodes. (a) Voltage versus capacity for anode and cathode materials of Li-ion batteries and Li–metal batteries; (b) schematic of the open-circuit energy diagram of the electrolyte window (Eg) and the chemical potentials μA and μC of the anode and cathode; μA > LUMO and/or μC < HOMO, where the HOMO is the highest occupied molecular orbital.3 Reproduced with permission. Copyright 2008, American Chemical Society. (c) Illustration of the SEI formed on Li or graphite.30 Reproduced with permission. Copyright 2021, John Wiley and Sons. (d) Schematic illustration of the formation of Li dendrites, damage to the native SEI, and the formation of porous electrochemically non-active Li.38 Reproduced with permission. Copyright 2020, Elsevier. | ||
Unlike graphite which stores Li+ in its lattice with acceptable volumetric changes (∼12%), the Li metal anode accommodates Li+ at the Li/electrolyte interface, leading to unlimited volumetric changes during Li plating/stripping processes.11,12,31,32 Unfortunately, the native SEI formed on Li is brittle, so it fails to tolerate the stress caused by the volumetric changes of Li metal electrodes.25,33–35 In addition, Li+ is preferentially deposited on the protuberant tips with stronger electrical fields on the substrate, leading to the formation and growth of dendritic Li.26,36 An Li dendrite has a high Young's modulus of ∼5 GPa,37 so it can easily pierce the SEI. Once the SEI is damaged, the newly exposed Li would immediately react with the electrolyte to form a new SEI.16 Meanwhile, the cracked SEI layer may also expose defects and in turn accelerate the deposition of Li on the defects and form new Li dendrites. Furthermore, Li stripping from the roots of the dendrite would break the electrical contact and produce porous “dead” Li.38 With battery cycling and continuous SEI build-up, the above problems lead to electrolyte depletion, loss of electrochemically active Li, Li electrode pulverization, and battery performance decay (Fig. 1d).38,39 Even more troubling, the Li dendrites could lead to internal short-circuits or even safety hazards in working batteries (Fig. 1d).26,38,40–42
Interfacial engineering is critical to stabilize Li metal electrodes.16,20,43 Constructing nitrided interfaces on the surface of Li electrodes or current collectors has been proved to be effective to suppress Li dendrite formation and growth, as well as protecting Li from electrolyte erosion. The nitrided interfaces can regulate Li+ flux distribution near the Li electrodes or current collectors, facilitate Li+ diffusion through the SEI, and passivate the reductive surface of Li, thus improving the electrochemical performance of LMBs. In this paper, we review our current fundamental understanding and recent advances in developing nitrided interphases for stabilizing Li metal electrodes. The strategies for constructing nitrided interphases, including building artificial SEI layers, electrolyte engineering, substrate modification, and separator functionalization, have been comprehensively summarized and discussed. In addition, our perspective on the future development of nitrided interfaces and rational Li/electrolyte interface design for Li metal electrode is included.
2. Advantages of nitrided interfaces
2.1. Functions of nitrided interfaces
2.2. Comparison of nitride interfaces with other strategies
Besides nitride interfaces, metal oxides (such as MgO),55 phosphates (such as Li3PO4),56,57 some lithium halides (such as LiCl and LiI),56,58,59 and lithium chalcogenides (such as Li2S and Li2Se)60,61 have also been introduced as modification layers on Li metal. Generally, their precursors are hardly soluble in non-aqueous electrolytes, and as a consequence, these layers are usually fabricated by ex situ methods, serving as artificial SEI layers. These modification layers do play a positive role in protecting the Li metal, but they may be damaged by the interfacial stress during Li plating/stripping processes and therefore lose their functions. In contrast, in the case of nitride interfaces, some of their precursors (such as nitrates, amides, N-containing ionic liquids, and nitrocellulose) are soluble in certain non-aqueous solvents, which enables continuous repair of nitride interfaces during battery cycling when these precursors are introduced into the electrolyte as solvents or additives.Fluorinated interfaces, which feature LiF-rich SEI layers, are widely acclaimed for their outstanding effects on Li metal protection, which is based on their high Young's modulus and the high interfacial energy of LiF.49,62 Although fluorinated interfaces are excellent for inhibiting side reactions between Li metal and the electrolyte, nitride interfaces still show advantages over them in some aspects, especially in bulk ionic conductivity. The transport of Li ions in LiF is much more difficult than in Li3N or LiNxOy, obviously limiting the grain growth of deposited Li during the plating process. According to the morphologies, deposited Li with a nitrided interface has a larger grain size but smaller microstructural tortuosity compared with Li with a fluorinated interface, contributing to higher reversibility of the active Li during battery cycling.
3. Constructing a nitrided artificial SEI on Li metal electrodes
3.1. Methods to construct a nitrided SEI on Li metal electrodes
Since the formation of the SEI is a key factor in controlling the surface properties of Li, one of the effective approaches to stabilize the Li metal electrode is to construct functional artificial SEI layers on its surfaces.28,35,43,63,64 According to the preparation mechanism, the strategies to develop a nitrided artificial SEI can be divided into physical methods and chemical methods.![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) in the polymer chains with both Li+ and Li metal, the P(BMA-AN-St) cladding provided channels for regulating the Li+ (Fig. 2b),66 so that a dendrite-free surface and improved electrochemical performance of Li metal electrodes were realized, even with deep cycling. Paik et al. modified copper nitride nanowires (Cu3N NWs) on Li foil through one-step roll pressing. The Cu3N NWs could be conformally printed onto the Li metal and form a Li3N@Cu NW layer on the Li electrode (Fig. 2c).67 Yu et al. synthesized a polar polymer network (PPN) layer and coated it on a Li metal electrode during battery assembly.68 The C
O) in the polymer chains with both Li+ and Li metal, the P(BMA-AN-St) cladding provided channels for regulating the Li+ (Fig. 2b),66 so that a dendrite-free surface and improved electrochemical performance of Li metal electrodes were realized, even with deep cycling. Paik et al. modified copper nitride nanowires (Cu3N NWs) on Li foil through one-step roll pressing. The Cu3N NWs could be conformally printed onto the Li metal and form a Li3N@Cu NW layer on the Li electrode (Fig. 2c).67 Yu et al. synthesized a polar polymer network (PPN) layer and coated it on a Li metal electrode during battery assembly.68 The C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N groups of polyacrylonitrile polymer chains in the PPN could reduce the high reactivity of the C
N groups of polyacrylonitrile polymer chains in the PPN could reduce the high reactivity of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups of carbonate solvents and promote the decomposition of salt anions (PF6− and bis(trifluoromethane)sulfonimide (TFSI−)), forming a stable SEI (Fig. 2d). In addition to these artificial SEI layers, other different inorganic artificial SEIs were also prepared via physical methods on Li metal electrodes. For instance, a Li3N layer can be coated on the Li metal electrode via pressing and rubbing Li3N powder to suppress Li dendrite formation.69 Yang et al. coated a layer of acid-treated graphitic (g)-C3N4 on Li, and its N-containing groups were able to rearrange the concentration of Li+ and enhance the transfer of Li+.70
O groups of carbonate solvents and promote the decomposition of salt anions (PF6− and bis(trifluoromethane)sulfonimide (TFSI−)), forming a stable SEI (Fig. 2d). In addition to these artificial SEI layers, other different inorganic artificial SEIs were also prepared via physical methods on Li metal electrodes. For instance, a Li3N layer can be coated on the Li metal electrode via pressing and rubbing Li3N powder to suppress Li dendrite formation.69 Yang et al. coated a layer of acid-treated graphitic (g)-C3N4 on Li, and its N-containing groups were able to rearrange the concentration of Li+ and enhance the transfer of Li+.70
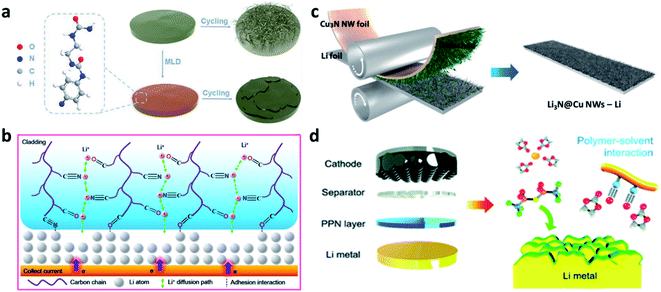 | ||
| Fig. 2 Constructing artificial SEI layers on Li metal electrodes via physical methods. (a) Schematic illustration of “polyurea” deposited on Li to guide Li uniform deposition;65 reproduced with permission. Copyright 2019, John Wiley and Sons. (b) Illustration of P(BMA-AN-St) cladding regulating Li+ flux;66 reproduced with permission. Copyright 2019, American Chemical Society. (c) Fabrication of a Cu3N layer on Li foil via physical rolling and printing method;67 reproduced with permission. Copyright 2020, John Wiley and Sons. (d) Schematic illustration of coating the PPN layer on Li metal in the battery assembly process.68 Reproduced with permission. Copyright 2019, Royal Society of Chemistry. | ||
It should be pointed out that the thickness of the nitrided artificial SEI developed via physical methods is normally more than a few micrometres (as summarized in Table 1), which would certainly impose a sacrifice on the overall volumetric energy density of Li metal electrodes. In addition, the physical methods could not well control the homogeneity of the artificial SEI on Li metal electrodes, and the adhesion between the SEI and the Li metal would not be strong enough, which may lead to the exfoliation of the artificial SEI during battery cycling. Besides, the organic artificial SEI layers modified by physical methods have poor ionic conductivity, so they normally lead to high electrochemical polarization for Li metal electrodes.
| Artificial SEI | Thickness | Fabrication method | Electrolyte | Current density (mA cm−2) | Capacity (mA h cm−2) | Lifespan (h) | Polarization (mV) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Polar polymer network | N/A | Physical pressing in battery assembly | LiTFSI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EC = 1 EC = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
10 | 1 | 200 | ∼300 | 68 |
| Polyurea | ∼4 nm | Atomic layer deposition | 1 M LiPF6 in EC/DEC/DMC | 1 | 2 | 400 | ∼170 | 65 |
| P(BMA-AN-St) | ∼4 μm | Drop coating | 1 M LiPF6 in EC/DEC/DMC | 0.5 | 1 | 800 | ∼200 | 66 |
| Acid-treated g-C3N4 | ∼5 μm | Physical pressing | 1 M LiTFSI in DOL/DME with 2 wt% LiNO3 | 1 | 1 | 400 | ∼240 | 70 |
| Li3N | N/A | Pressing and rubbing | 1 M LiPF6 in EC/DEC | 1 | 2 | 360 | ∼240 | 69 |
| Cu3N nanowires | ∼3 μm | Roll-printing | 1.3 M LiPF6 in EC/DEC with 5% FEC | 3 | 1 | 250 | ∼240 | 67 |
| AgNO3 | N/A | Drop coating | 1 M LiTFSI in DOL/DME with 1 wt% LiNO3 | 5 | 0.5 | 50 | ∼400 | 83 |
| PEO–UPy | 70 nm | Drop coating | 1 M LiTFSI in DOL/DME with 2 wt% LiNO3 | 5 | 10 | 1000 | 300 | 80 |
| CTF + LiI | ∼20 μm | Drop coating | 1 M LiPF6 in EC/DEC | 10 | 1 | 500 | 500 | 77 |
| Li3N | N/A | N2 flow treatment | 1 M LiPF6 in EC/DMC | N/A | N/A | N/A | N/A | 71 |
| Li3N | 8.25 μm | N2 flow treatment | 1 M LiPF6 in EC/DMC | N/A | N/A | N/A | N/A | 72 |
| Pinhole-free Li3N | 50–400 nm | N2 based reaction | 1 M LiTFSI in DOL/DME with 1 wt% LiNO3 | N/A | N/A | N/A | N/A | 74 |
| Li3N | ∼8 μm | Plasma activation under N2 | 1 M LiPF6 in EC/DMC | 0.5 | 1 | 500 | ∼250 | 73 |
| LiPON | 250 nm | N2 plasma-assisted deposition | 1 M LiTFSI in DOL/DME with 1 wt% LiNO3 | 3 | 1 | 600 | ∼160 | 75 |
| N-organic@Li3N | 950 nm | C3N4 based surface reaction | 1 M LiTFSI in DOL/DME with 1 wt% LiNO3 | 1 | 2 | 1100 | ∼80 | 78 |
| PECA–Li3N/LiNxOy | ∼4 μm | In situ polymerization of ECA with a LiNO3 additive | 1 M LiPF6 and EC/DMC | 1 | 1 | 200 | ∼160 | 79 |
| [LiNBH]n | 140–160 nm | Two-step dehydrogenation reaction | 1 M LiTFSI in DOL/DME with 1 wt% LiNO3 | 3 | 1 | 700 | 204 | 82 |
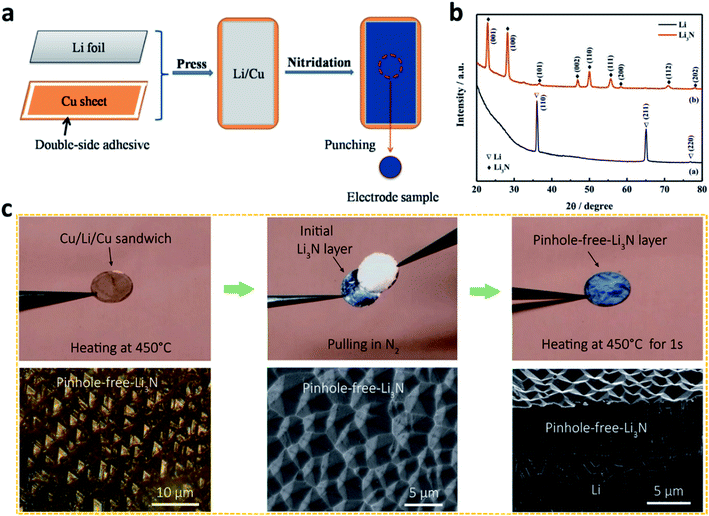 | ||
| Fig. 3 Constructing artificial SEI layers on Li metal electrodes via chemical methods. (a) Preparation of a Li3N film on an Li surface by utilizing the reaction between N2 gas and Li metal; (b) characterization of the Li3N film by X-ray diffraction (XRD);72 reproduced with permission. Copyright 2015, Elsevier. (c) Preparation of a pinhole-free Li3N layer to protect the Li metal electrode, and optical and scanning electron microscope (SEM) images of the pinhole-free Li3N layer.74 Reproduced with permission. Copyright 2018, American Chemical Society. | ||
Despite these achievements, the effect of Li3N is limited to some extent by its small grain size (<160 nm), which leads to weak interconnections between the Li3N particles. To solve this challenge, Cui et al. heated Li metal in a N2 atmosphere at a high temperature to develop a pinhole-free Li3N layer on the Li metal surface (Fig. 3c).74 The dense, large, and strongly interconnected grains of Li3N in the film reduced the defects in the artificial SEI and effectively improved the stability of the Li metal electrode during battery cycling.
Apart from forming Li3N, Xie et al. also dropped AgNO3/tetrahydrofuran (THF) solution on the Li surface, and the AgNO3 particles would further react with Li to form LiNO3, which is useful for regulating Li+ plating behaviour and suppressing Li dendrite growth. A lithium phosphorus oxynitride layer on a Li metal anode with high ionic conductivity and chemical stability was developed via a nitrogen plasma-assisted deposition method to suppress the corrosion from the electrolyte and promote uniform Li plating/stripping.75
As summarized in Table 1, the nitrided artificial SEI layers prepared via chemical methods are generally inorganic, and most of them are thinner as well as having higher ionic conductivity, so they could reduce the polarization of Li metal electrodes. These inorganic artificial SEI layers are normally brittle, however, so the integrity of the SEI would be damaged by the interfacial stress changes caused by Li plating/stripping processes, which would shorten the lifespan of Li metal electrodes.
3.2. Building nitrided organic–inorganic composite artificial SEIs
Building nitrided organic–inorganic composite interfaces is a good idea that takes advantage of the merits of both individual components and overcome their disadvantages. In this regard, Cui et al. developed a reactive interface constructed from Cu3N nanoparticles joined together by styrene butadiene rubber (SBR) as an artificial SEI for Li metal electrodes.76 The inorganic Cu3N has high ionic conductivity, and the organic SBR has high mechanical strength and high flexibility (Fig. 4a). The Cu3N further reacted with Li and a composite artificial SEI composed of Li3N/SBR/Cu was formed on the surface of the Li metal electrode. The Li3N particles provided ionically conductive paths, while the SBR confined the Li3N particles and buffered the volume changes of the Li anode. Zheng et al. coated a covalent triazine framework (CTF)-LiI hybrid artificial SEI on Li by the doctor blade method (Fig. 4b).77 The N in CTF could bind with Li+ from the electrolyte to form Li–N bonds and thus facilitate uniform Li deposition. The uniformly distributed LiI particles could help to improve the mechanical stress to suppress Li dendrite growth. Yu et al. reported a composite artificial SEI consisting of an N-containing organic phase (N-organic) and an inorganic Li3N phase by utilizing the hyperthermal reduction of Li and g-C3N4 (Fig. 4c).78 The obtained N-organic phase could link with the Li3N phase and form a conformal and compact coating on Li. The authors believed that the C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and N–(C)3 groups realized the homogeneous distribution of Li+ and provided nucleation sites for Li deposition, while the Li3N reduced the resistance to Li+ transfer across the Li/electrolyte interfaces. Besides, a dual-layer artificial SEI was constructed via in situ polymerization of ethyl α-cyanoacrylate (ECA) monomers on the Li metal surface, in which LiNO3 was introduced with the ECA monomers as an additive (Fig. 4d).79 The CN− groups in ECA and the LiNO3 additive reacted with Li to form a nitrided inorganic interface on Li during battery cycling. Poly(ethyl α-cyanoacrylate) (PECA) was used to cover the outer surface to accommodate the volume changes and buffer the interfacial stress during the Li plating/stripping processes. Xiong et al. modified a self-healing supramolecular copolymer, which consisted of pendant poly(ethylene oxide) (PEO) segments and ureido-pyrimidinone (UPy) quadruple-hydrogen-bonding moieties, on a Li metal electrode via a drop coating method.80 During the following drying process, the amide and heterocyclic amine groups in PEO–UPy polymer reacted with Li metal and formed a stable artificial SEI (LiPEO–UPy) layer on the Li metal electrode. The developed LiPEO–UPy layer could protect the electrolyte from side reactions and homogenize the fast Li+ flux to the surface of the Li metal. Lee et al. developed hybrid polyion complex micelles and coated them on Li foil, in which ionized LiNO3 combined with block copolymer micelles, polystyrene-block-poly(2-vinyl pyridine) (S2VP), via electrostatic interaction (Fig. 4e).81 It was believed that the S2VP polymer could isolate the active Li from carbonates so as to reduce the side reaction between them, and meanwhile, the introduced LiNO3 could further dissolve into the electrolyte during battery cycling. As a result, a composite N-rich SEI with a multilayered structure could be formed on the Li electrode (Fig. 4f). With the designed protective layer, Li metal full cells with a high voltage cathode (LiNi0.8Co0.1Mn0.1O2) delivered superior performance, even under harsh test conditions (thin Li anode, high areal-capacity of 4.0 mA h cm−2, and high current density of 4.0 mA cm−2).
C and N–(C)3 groups realized the homogeneous distribution of Li+ and provided nucleation sites for Li deposition, while the Li3N reduced the resistance to Li+ transfer across the Li/electrolyte interfaces. Besides, a dual-layer artificial SEI was constructed via in situ polymerization of ethyl α-cyanoacrylate (ECA) monomers on the Li metal surface, in which LiNO3 was introduced with the ECA monomers as an additive (Fig. 4d).79 The CN− groups in ECA and the LiNO3 additive reacted with Li to form a nitrided inorganic interface on Li during battery cycling. Poly(ethyl α-cyanoacrylate) (PECA) was used to cover the outer surface to accommodate the volume changes and buffer the interfacial stress during the Li plating/stripping processes. Xiong et al. modified a self-healing supramolecular copolymer, which consisted of pendant poly(ethylene oxide) (PEO) segments and ureido-pyrimidinone (UPy) quadruple-hydrogen-bonding moieties, on a Li metal electrode via a drop coating method.80 During the following drying process, the amide and heterocyclic amine groups in PEO–UPy polymer reacted with Li metal and formed a stable artificial SEI (LiPEO–UPy) layer on the Li metal electrode. The developed LiPEO–UPy layer could protect the electrolyte from side reactions and homogenize the fast Li+ flux to the surface of the Li metal. Lee et al. developed hybrid polyion complex micelles and coated them on Li foil, in which ionized LiNO3 combined with block copolymer micelles, polystyrene-block-poly(2-vinyl pyridine) (S2VP), via electrostatic interaction (Fig. 4e).81 It was believed that the S2VP polymer could isolate the active Li from carbonates so as to reduce the side reaction between them, and meanwhile, the introduced LiNO3 could further dissolve into the electrolyte during battery cycling. As a result, a composite N-rich SEI with a multilayered structure could be formed on the Li electrode (Fig. 4f). With the designed protective layer, Li metal full cells with a high voltage cathode (LiNi0.8Co0.1Mn0.1O2) delivered superior performance, even under harsh test conditions (thin Li anode, high areal-capacity of 4.0 mA h cm−2, and high current density of 4.0 mA cm−2).
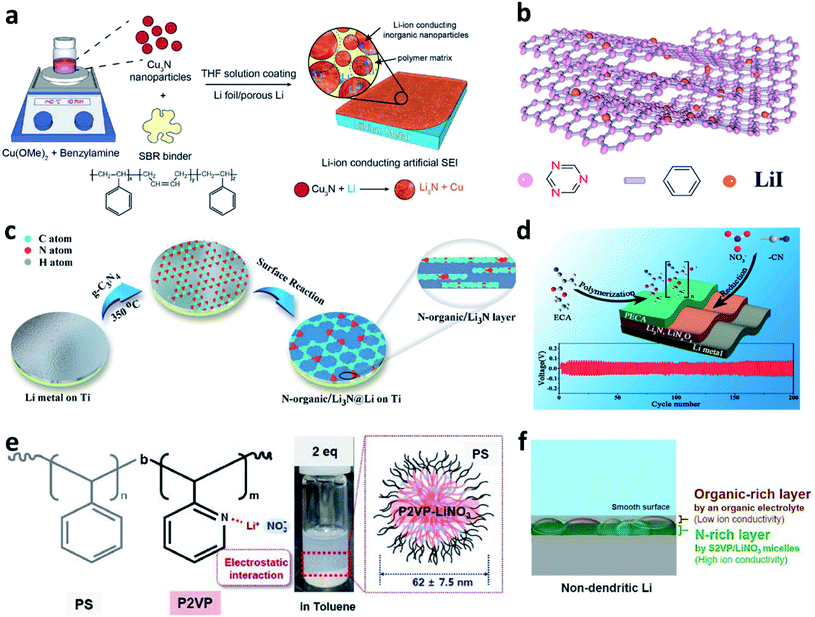 | ||
| Fig. 4 Constructing nitrided organic–inorganic composite interfaces for Li metal electrodes. (a) Preparation of a Cu3N-SBR interface on a Li metal electrode;76 reproduced with permission. Copyright 2017, John Wiley and Sons. (b) Structural illustration of the CTF–LiI composite layer;77 reproduced with permission. Copyright 2020, John Wiley and Sons. (c) Preparation of an N-organic/Li3N composite layer on a Li metal electrode;78 reproduced with permission. Copyright 2020, John Wiley and Sons. (d) Schematic illustration of the preparation of a PECA–Li3N/LiNxOy dual protection layer for a Li metal electrode and the subsequent electrochemical performance.79 Reproduced with permission. Copyright 2017, American Chemical Society. (e) Chemical structure, optical image, and schematic illustration of the S2VP/LiNO3 micelles; (f) schematic illustration of the multilayered structure formed on the surface of S2VP/LiNO3–Li.81 Reproduced with permission. Copyright 2021, Elsevier. | ||
Despite the advantages of organic/inorganic composite artificial SEIs, it is challenging to control the homogeneous distribution of organic and inorganic phases. To overcome this, our group synthesized a multi-functional [LiNBH]n layer as an artificial SEI for Li metal anodes by utilizing a two-step dehydrogenation reaction between Li and ammonia borane (Fig. 5a–c), which features the properties of both organic and inorganic SEIs.82 The obtained ASEI is composed of [LiNBH]n chains, which are cross-linked and self-reinforced by their intermolecular Li–N ionic bonds, and thus give rise to a flexible nature (Fig. 5d). Because of the higher charge density of N in the polar [LiNBH]n chain, Li+ from the electrolyte will be absorbed by the N to form additional Li–N ionic bonds, which helps to regulate the homogeneous distribution of the Li+ flux on Li electrodes (Fig. 5e). In addition, the [LiNBH]n layer is electrically isolated but has high ionic conductivity, thus facilitating Li+ diffusion and deposition beneath the artificial SEI layer. Therefore, with the protection of the [LiNBH]n layer, Li dendrite growth has been successfully suppressed and a denser and flatter surface was achieved after Li plating/stripping cycles (Fig. 5f and g).
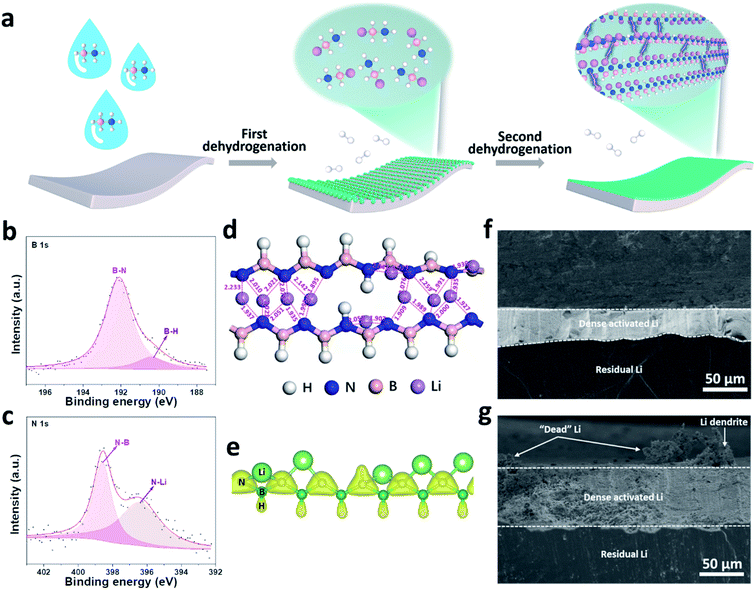 | ||
| Fig. 5 Building a [LiNBH]n artificial SEI for a Li metal electrode. (a) Schematic illustration of the fabrication process for the [LiNBH]n layer; surface XPS spectra of B 1s (b) and N 1s (c) for the [LiNBH]n coated Li; (d) relaxed atomic configurations of two adjacent [LiNBH]n chains with bond lengths between Li and N atoms (the unit of the values is Å), which reveals that the Li–N distances are comparable to the corresponding bond lengths in the crystalline phases of Li3N; (e) charge density distribution in the chain of [LiNBH]n with an isosurface of 0.15 e Å−3, which confirms that the N is negatively charged; cross-sectional SEM images of (f) bare Li and (g) Li@[LiNBH]n after 20 cycles.82 Reproduced with permission. Copyright 2020, John Wiley and Sons. | ||
In a short summary, inorganic nitrided artificial SEI layers have high ionic conductivity and relatively low thickness, but they suffer from low integrity and mechanical flexibility. Organic nitrided artificial SEI layers (normally prepared via physical methods) can regulate the Li+ flux distribution and buffer the volume changes of Li metal electrodes, while their poor ionic conductivity and high thickness usually lead to high electrochemical polarization. Building nitrided inorganic–organic composite artificial SEI layers is useful to take advantage of the individual components. Most of the composite artificial SEI layers delivered enhanced lifespan compared with pure organic or pure inorganic artificial SEIs. Unfortunately, it is still difficult to control the homogeneous distribution of organic and inorganic components in the SEI. In addition, the thickness of artificial SEI layers varies from a few nanometres to tens of micrometres. To avoid the sacrifice of the volumetric energy density, the thickness of the artificial SEI should be lower than one micrometre, especially considering that the anodes in practical LMBs are thin foils with a thickness of <50 μm. Another big challenge to artificial SEI layers is their stability during battery cycling. With continuous Li plating/stripping cycles, the structure and the integrity of artificial SEI layers would be destroyed by the interfacial mechanical strength, particularly during long-term cycling. Therefore, improving the stability, reducing the thickness, and increasing the flexibility are critical to boost the practical applications of nitrided artificial SEI layers in LMBs.
4. Electrolyte engineering
The deposition behaviour of Li+ is strongly related to the physical and chemical properties of the electrolyte.16,45,48 Electrolyte engineering, including introducing functional additives, using new solvents, regulating the Li+ solvation structure, etc., is useful to stabilize Li metal electrodes. The most efficient method to evaluate the effects of these electrolytes is to test Li plating/stripping reversibility in Li–Cu cells.4.1. LiNO3 additive
LiNO3 is a practical and economical additive to improve the electrochemical performance of LMBs. It was first used in an ether-based electrolyte to suppress the shuttle effect of polysulphides in Li–S batteries by Aurbach et al.87 They studied the surface of the Li anode cycled in the LiNO3-containing ether electrolyte and proposed that the LiNO3 additive was decomposed on Li to form LiNxOy and LiOR. Wen et al. further proved that LiNO3 is able to improve the coulombic efficiency (CE) of the Li plating/stripping processes in Li–Cu cells (Fig. 6a), as well as suppressing Li dendrite growth.84 A smoother surface of the Li metal anode was obtained after adding 0.4 M LiNO3 into the ether-based electrolyte (Fig. 6b and c). By using the more accurate and sensitive X-ray photoelectron spectroscopy (XPS) depth profile method, Xiong et al. revealed that LiNO3 in the ether-based electrolyte is reduced on the Li metal surface and forms a complex product consisting of Li3N, LiNxOy, and RCH2NO2 (Fig. 6d).85 Brezesinski et al. also demonstrated that LiNO3 can form a protective layer on the Li metal anode and suppress gas evolution in the Li–S battery in conjunction with a diglyme-based electrolyte. In particular, the amount of flammable CH4 and H2 is dramatically decreased, and either very little or no H2 is generated during discharge (Fig. 6e).86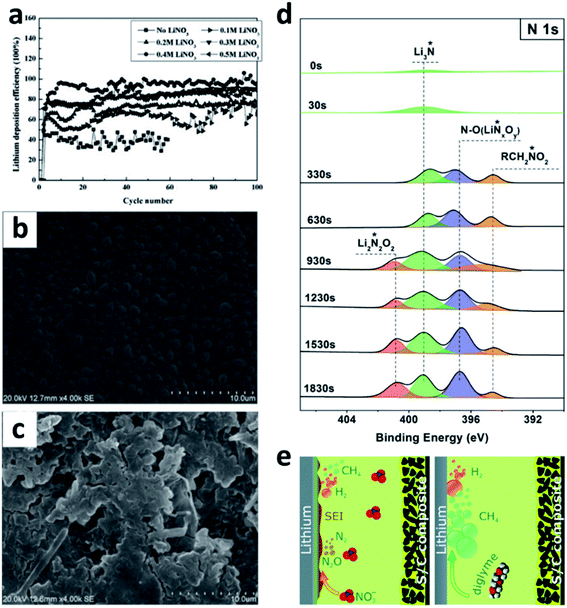 | ||
| Fig. 6 The use of a LiNO3 additive in ether-based electrolytes. (a) The effects of the LiNO3 additive towards improving the CE of Li–Cu cells in the ether electrolyte; comparison of SEM images of the Li electrode cycled in the ether electrolyte with LiNO3 (b) and without LiNO3 (c);84 reproduced with permission. Copyright 2011, Elsevier. (d) Surface characterization by XPS of the Li electrode cycled in the ether-based electrolyte with the LiNO3 additive;85 reproduced with permission. Copyright 2014, Elsevier. (e) Schematic illustration of the passivation of the Li surface and the suppression of gas evolution in the ether electrolyte by the LiNO3 additive.86 Reproduced with permission. Copyright 2016, Royal Society of Chemistry. | ||
Even though LiNO3 has achieved a big success in ether-based electrolytes and it also has good solubility in ether-based electrolytes (up to ∼5 wt%), its application in high-voltage carbonate-based electrolytes is limited due to its ultralow solubility in carbonate solvents (lower than 10−5 g mL−1).88,89 To boost the application of the LiNO3 additive in carbonate-based electrolytes, various solubilizers have been utilized to promote its dissolution in carbonate solvents. It was initially reported that 2% vinylene carbonate (VC) can promote the dissolution of 0.1 M LiNO3 in an ethylene carbonate/dimethyl carbonate (EC/DMC)-based electrolyte and effectively improve the reversibility of Li plating/stripping processes in Li–Cu cells (Fig. 7a).90 By analysing the surface of the cycled Li metal electrode with XPS, the existence of Li3N in the SEI was confirmed (Fig. 7b). Huang et al. further used a trace amount of CuF2 to promote the dissolution of 1 wt% LiNO3 in an EC/diethyl carbonate (DEC)-based electrolyte, and proved that LiNO3 was reduced on Li and formed a nitrided SEI (Fig. 7c).91
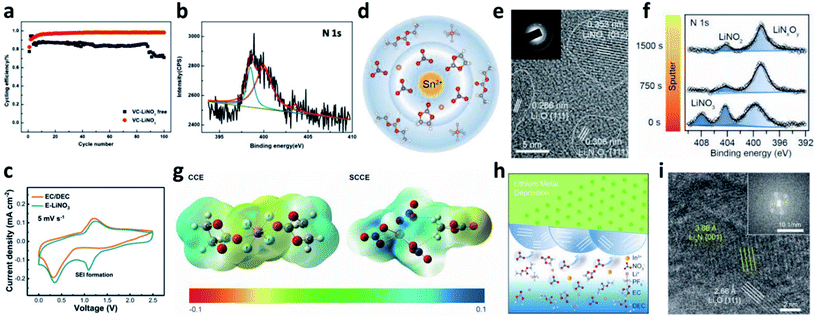 | ||
| Fig. 7 The use of the LiNO3 additive in carbonate-based electrolytes. (a) CE of a Li–Cu cell in a carbonate electrolyte with and without VC–LiNO3 as the additive and (b) N 1s XPS spectrum of the cycled Li;90 reproduced with permission. Copyright 2015, Elsevier. (c) Cyclic voltammetry (CV) curves of Li–Cu cells with and without CuF2–LiNO3 co-additives, where an additional peak belonging to LiNO3 decomposition at ∼1.1 V can be observed;91 reproduced with permission. Copyright 2018, John Wiley and Sons. (d) Structural illustration of the Sn2+ solvated sheath; (e) high resolution TEM (HRTEM) image of the SEI formed in the carbonate electrolyte with Sn(OTf)2–LiNO3 additives, with the corresponding selected area electron diffraction pattern in the inset; (f) N 1s XPS depth profiles for the SEI formed in the electrolyte with Sn(OTf)2–LiNO3 additives;35 reproduced with permission. Copyright 2020, John Wiley and Sons. (g) Electrostatic potential (ESP) images for the solvated EC and DEC molecules in the electrolyte with and without In(OTf)3–LiNO3 as an additive; (h) schematic illustration of the formation of an inorganic wavy SEI; (i) cryo-TEM image of the inorganic wavy SEI showing the presence of Li3N.92 Reproduced with permission. Copyright 2020, John Wiley and Sons. | ||
Increasing the concentration of LiNO3 in carbonate-based electrolytes could improve the electrochemical performance of LMBs. In this regard, Lu et al. used 0.5 wt% Sn(OTf)2, where OTf is trifluoromethanesulfonate, as a solubilizer to increase the solubility of LiNO3 in carbonate electrolytes to as high as 5 wt%.35 Tin(II), which is a Lewis acid, can effectively coordinate NO3− and promote complete dissociation between ion pairs without decomposing the solvent molecules (Fig. 7d). By using high-resolution transmission electron microscopy (TEM, Fig. 7e) and XPS depth profiling (Fig. 7f), they confirmed that N-containing species, such as Li3N and LiNxOy, were formed in the SEI of the Li metal electrode. With the benefits of the nitrided SEI, the CE in Li–Cu cells was improved to 98.14% at a high capacity of 3 mA h cm−2 over 150 cycles, and the cycling performance of Li‖NCM811 full cells delivered superior electrochemical performance under practical conditions. Similarly, they also used In(OTf)3 to dissolve 3 wt% LiNO3 in a carbonate-based electrolyte and achieved a high CE of >98% in Li–Cu cells at a high plating capacity of 4 mA h cm−2.92 They demonstrated that, because of the presence of In3+, the reactivity of the EC molecule was reduced, and the NO3− anions were more likely to undergo a site-selective reaction at the inner Helmholtz plane and form an N and O-rich inorganic wavy SEI (Fig. 7g and h), which was experimentally proved by cryo-TEM results (Fig. 7i).
The use of extra solubilizers has improved the solubility of LiNO3 in a carbonate-based electrolyte, but they also increase the cost of the electrolyte. In addition, the solubilizers could be reduced on Li, so that they may destabilize the SEI. Sulfones (such as dimethyl sulfoxide (DMSO), sulfolane, etc.) have high solvability towards LiNO3, so they can replace the extra solubilizers and be used as solvents in the electrolyte to dissolve LiNO3. In this aspect, Wang et al. used DMSO solvent to dissolve LiNO3 and prepared a 4 M LiNO3/DMSO solution as an additive.93 They added 5 wt% of this additive into a carbonate-based electrolyte and achieved an ultrahigh CE of 99.55% in Li–Cu cells. It was indicated that distinct NO3− anions were involved in the Li+ solvation sheath, and a small number of DMSO molecules were also found in the Li+ solvation sheath (Fig. 8a and b). The NO3− in the solvation sheath could be reduced on the Li surface and formed a nitrided inorganic-rich SEI, which was more stable than the SEI formed in the LiNO3-free electrolyte (Fig. 8c and d), while the DMSO molecules could not be decomposed. Therefore, denser and more compact plated Li was obtained on the Cu substrate (Fig. 8e and f). Wang et al. also used pure sulfolane as the solvent in their electrolyte for LMBs, which contained 3.25 M lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) as a salt and 0.1 M LiNO3 as an additive.94 By using molecular dynamics (MD) simulations, they pointed out that the NO3− anions in the Li+ solvation sheath could promote the coordination of TFSI− anions with Li+ (Fig. 8e). During battery cycling, these anions in the Li+ solvation sheath would be reduced and formed an inorganic SEI. It should be emphasized that LiNO3 is strongly oxidizing, so it will increase the safety risk of the battery after being added into the electrolyte, although most of the reported work failed to mention this safety issue. To address this problem, Guo et al. used triethyl phosphate as a solvent to dissolve 1 M LiNO3 into the electrolyte as well as an extinguishant to eliminate fire risk.95 The developed electrolyte not only generated a nitrided SEI that could suppress Li dendrite growth (Fig. 8f), but also improved the safety of the resultant LMBs. The CE for Li plating/stripping processes only reached ∼97%, however, which was not high enough for practical LMBs.
 | ||
| Fig. 8 The use of the LiNO3 additive in other ester-based electrolytes. (a) MD simulation of a carbonate electrolyte with LiNO3/DMSO as the additive; (b) structure of the Li+ solvated sheath; schematic illustration of the SEI and Li deposition in the electrolyte without the LiNO3/DMSO additive (c) and with the LiNO3/DMSO additive (d); SEM images and corresponding optical images of deposited Li from the electrolyte without the LiNO3/DMSO additive (e) and with the LiNO3/DMSO additive (f);93 reproduced with permission. Copyright 2020, John Wiley and Sons. (g) MD simulations and the Li+ solvation sheath of a sulfolane-based electrolyte with and without LiNO3;94 reproduced with permission. Copyright 2020, John Wiley and Sons. (h) Schematic illustration of building a nitrided interface on a Li metal electrode by adding LiNO3 into a triethyl phosphate-based electrolyte.95 Reproduced with permission. Copyright 2019, John Wiley and Sons. | ||
In short, the use of LiNO3 as an additive has effectively optimized the SEI and improved the Li plating/stripping reversibility. The application of LiNO3 in high-voltage and more practical carbonate-based electrolytes for LMBs is limited, however, due to its low solubility. Different solubilizers were used to increase the solubility of LiNO3 in carbonate-based electrolytes, although these solubilizers increase the cost of the electrolyte and their decomposition on Li would destabilize the SEI. LiNO3 has high solubility in organic phosphate esters, sulfones, and amides, and they can be used as solvents or liquid solubilizers to dissolve LiNO3 in the electrolyte. The thermodynamic stability of these solvents is poorer than that of carbonate solvents, however, which increases the undesirable side reactions between the electrolyte and the Li metal electrode. Furthermore, for the development of safe and practical LMBs, the fire risk caused by the oxidizing properties of LiNO3 should be carefully considered.
4.2. Other N-containing additives
Apart from LiNO3, some other N-containing additives can also be used to build a nitrided SEI on Li metal electrodes. In this regard, Sun et al. reported that Mg(NO3)2 can be dissolved in a carbonate-based electrolyte as an additive.96 They suggested that Mg(NO3)2 can be dissolved directly as Mg2+ and NO3− ions in the electrolyte even at a concentration of 0.1 M, which was quite different from the situation for LiNO3 (Fig. 9a). The NO3− in the electrolyte could also form a LiNxOy-based SEI and improve the performance of both Li–Cu cells and Li–metal full cells. Wu et al. used metal–organic frameworks (MOF-808) as nanocapsules to load LiNO3, and used the MOF-808/LiNO3 composite as an additive for LMBs (Fig. 9b).97 The MOF-808 has an internal diameter of 18.4 Å and a pore window of 14 Å, which can efficiently encapsulate and diffuse LiNO3. During battery cycling, the LiNO3 will be released to react with Li and form a nitrided-rich SEI. Xie et al. introduced nitrofullerene (nitro-C60) as a bifunctional electrolyte additive to smooth the Li surface.98 The nitro-C60 in the electrolyte was designed to gather on the protuberances of the Li metal electrode and decompose to NO2− and insoluble C60. After that, NO2− further reacted with Li metal and formed a compact and stable Li3N/LiNxOy protective layer. The C60 was anchored on the uneven grooves of the Li surface and resulted in a homogeneous distribution of Li+ (Fig. 9c). Similarly, a paradigmatic N-rich polyether, nitrocellulose (NC), was used as an electrolyte additive to stabilize the Li metal electrode (Fig. 9d).99 The NC additive has low LUMO energy so that it reacts with Li to form an endogenous symbiotic Li3N/cellulose double SEI. However, the Li plating/stripping CE only reached ∼92%, even though the base electrolyte used ethers as the solvents. | ||
| Fig. 9 The use of other electrolyte additives for constructing nitrided interfaces on Li metal electrodes. (a) The use of Mg(NO3)2 as an additive in a carbonate electrolyte;96 reproduced with permission. Copyright 2020, John Wiley and Sons. (b) Schematic illustration of the use of MOF-808/LiNO3 as an electrolyte additive for LMBs;97 reproduced with permission. Copyright 2020, Springer Nature. (c) Schematic illustration of nitro-C60 as a bifunctional electrolyte additive for LMBs;98 reproduced with permission. Copyright 2019, American Chemical Society. (d) Illustration of the endogenous symbiotic Li3N/cellulose double SEI using nitrocellulose.99 Reproduced with permission. Copyright 2021, John Wiley and Sons. | ||
The use of these N-containing additives also introduces extra cations and organic components into the electrolyte. Their influence on the SEI composition and the performance of LMBs has not been clearly revealed, however. In addition, as shown in Table 2, the CE for Li plating/stripping in most of these electrolytes is lower than 98%, suggesting that they are not promising for practical applications at the current stage. Also, the stability of these N-containing additives has not been studied.
| Electrolyte | N-Containing precursor | N-Containing SEI components | Current density (mA cm−2) | Capacity (mA h cm−2) | Lifespan (cycles) | Coulombic efficiency (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 0.38 M LiTFSI + 0.31 M LiNO3 + 0.23 M Li2S6 in DOL | LiNO3 | Li3N and LiNxOy | N/A | N/A | N/A | N/A | 87 |
| 0.5 M LiCF3SO3 + 0.4 M LiNO3 | LiNO3 | Li3N | N/A | N/A | 100 | 90 | 84 |
| 0.1 M LiNO3 + 0.1 M Li2S6 in DOL/DME | LiNO3 | Li3N and LiNxOy | N/A | N/A | N/A | N/A | 85 |
| 0.5 M LiNO3 in DOL/DME | LiNO3 | Li3N, LiNxOy and Li2N2O2 | N/A | N/A | N/A | N/A | 110 |
| 1 M LiTFSI in DOL/DME + 0.18 M Li2S8 + 5 wt% LiNO3 | LiNO3 | N–S groups | 2 | 1 | 400 | 99.1 | 111 |
| 2.3 M LiFSI in DME + 20 mM CuF2 and 20 mM LiNO3 | LiNO3 | Li3N and LiNxOy | 1 | 1 | 500 | 99.5 | 112 |
| 1 M LiPF6 in EC/DMC with 2 v% VC + 0.1 M LiNO3 | LiNO3 | Li3N | N/A | N/A | 100 | ∼98 | 90 |
| 1 M LiPF6 in EC/DEC with 0.2 wt% CuF2 and 1 wt% LiNO3 | LiNO3 | Li3N | 0.5 | 0.5 | 20 | 98.1 | 91 |
| 1 M LiPF6 in FEC/DMC/DME with 1.1 wt% LiNO3 | LiNO3 | Li3N and LiNxOy | N/A | N/A | N/A | N/A | 103 |
| 1 M LiTFSI in TEP/VC + 5% LiNO3 | LiNO3 | Li3N and N–S groups | N/A | N/A | N/A | 97.3 | 95 |
| 1 M LiDFOB in TEP/EC with 0.2 M LiNO3 | LiNO3 | Li3N | N/A | N/A | 100 | ∼95 | 113 |
| 1 M LiPF6 in FEC/EMC with 1 wt% TPFPB and 3 wt% LiNO3 | LiNO3 | Li3N | 1 | 1 | 300 | 98.5 | 105 |
| 1 M LiPF6 in FEC/EMC with 0.5 wt% Sn(OTf)2 and 5 wt% LiNO3 | LiNO3 | LiNxOy | 1 | 3 | 150 | 98.14 | 35 |
| 1 M LiPF6 EC/DEC with 10 mM In(OTf)3 and 0.5 M LiNO3 | LiNO3 | Li3N and LiNxOy | 1 | 4 | 100 | 98.2 | 92 |
| 1 M LiFSI in FEC/GBL with 0.3 M LiNO3 | LiNO3 | Li3N and N–S groups | 0.5 | 1 | 200 | 98.8 | 89 |
| 3.25 M LiTFSI in SL with 0.1 M LiNO3 | LiNO3 | Li3N and N–S groups | 0.5 | 1 | 100 | 98.5 | 93 |
| 0.8 M LiPF6 FEC/DMC with a 5 wt% additive of 4 M LiNO3/DMSO | LiNO3 | LiNxOy | 1 | 1 | 100 | 99.42 | 94 |
| 1 M LiPF6 EC/DEC with a 50 mg mL−1 LiNO3–MOF composite | LiNO3-MOF | Li3N and LiNxOy | 0.5 | 0.5 | 20 | 98.8 | 97 |
| 0.8 M LiTFSI + 0.2 M LiDFOB + 0.05 M LiPF6 with 0.1 M Mg(NO3)2 in EMC/FEC | Mg(NO3)2 | LiNxOy and N–S groups | 2 | 2 | 100 | ∼94 | 96 |
| 1 M LiPF6 EC/DEC with a 5 M nitro-C60 derivative | Nitro-C60 | Li3N and LiNxOy | 0.1 | 0.5 | 150 | ∼92 | 98 |
| 1 M LiTFSI in DOL/DME with 1 wt% LiNO3 and ∼10 wt% TiN | Mainly TiN | TiN and N–H groups | 1 | 1 | 270 | 97.19 | 114 |
| 1 M LiTFSI in DOL/DME with 2% nitrocellulose | Nitrocellulose | Li3N and LiNxOy | 1 | 1 | 150 | 92 | 99 |
| 1 M LiTFSI in DOL/DME with 1 wt% LiNO3 with 0.5 mg mL−1 AlN | AlN | N/A | 2 | 1 | 170 | 94.68 | 115 |
| 2 M LiTFSI in Py13TFSI/DOL/DME | Py13TFSI | Li3N and N+(Py13) | 1 | 3 | 50 | 99.1 | 100 |
| 1 M LiTFSI in DOL/DME with 1 M Pyr1(4) FSI | Pyr1(4) FSI | Li3N and N+(Pyr1(4)) | 1 | 1 | 50 | 97.7 | 102 |
| 1 M LiTNFSI in DOL/PI13FASI | LiTNFSI and PI13FASI | Li3N and N+(PI13) | 0.5 | N/A | 300 | 98.7 | 101 |
| 1 M LiTFSI in FDMA/FEC | FDMA | Li3N | 0.5 | 1 | 100 | ∼99.3 | 107 |
4.3. N-Containing ionic liquids
The decomposition of normal organic solvents on Li metal electrodes leads to the formation of organic components such as ROCOOLi or the inorganic component Li2CO3 in the SEI, both of which have ultralow Li+ ionic conductivity and limit the kinetics of Li plating. N-Containing ionic liquids can be used to optimize the SEI composition and generate more effective species for conducting Li+. Due to the high viscosity of ionic liquids, however, they are normally used in mixed solvents. For example, Guo et al. developed an electrolyte with a mixed solvent consisting of N-propyl-N-methylpyrrolidinium bis(trifluoromethanesulfonyl)amide (Py13TFSI) and normal ether solvents 1,3-dioxolane/1,2-dimethoxyethane (DOL/DME) for LMBs.100 The Py13TFSI was reduced to form N+(Py13) and N−(TFSI) species in the SEI, which was able to passivate the active surface of the Li electrode (Fig. 10a). In addition, more ionically conductive Li3N was generated on the Li surface during battery cycling. Peng et al. developed an electrolyte that used a mixture of N-propyl-N-methylpiperidinium bis(fluorosulfonyl)imide (PI13FSI) and DOL as the solvent and Li[(CF3SO2)(n-C4F9SO2)N] (LiTNFSI) as the salt.101 The ionic liquid and the salt decomposed on the surface of the Li metal anode to form an Li3N-containing SEI that was highly ionically conductive and flexible (Fig. 10b), and a CE of 98.2% was achieved in Li–Cu cells, even at a high current density of 10 mA cm−2. Choi et al. reported the use of 1-dodecyl-1-methylpyrrolidinium (Pyr1(12)+) bis(fluorosulfonyl)imide (FSI−) in ordinary electrolyte solutions.102 The Pyr1(12)+ cation with a long aliphatic chain mitigated dendrite growth via the synergistic effects of electrostatic shielding and lithiophobicity, and the FSI− anion induced the generation of a rigid nitrided SEI (Fig. 10c).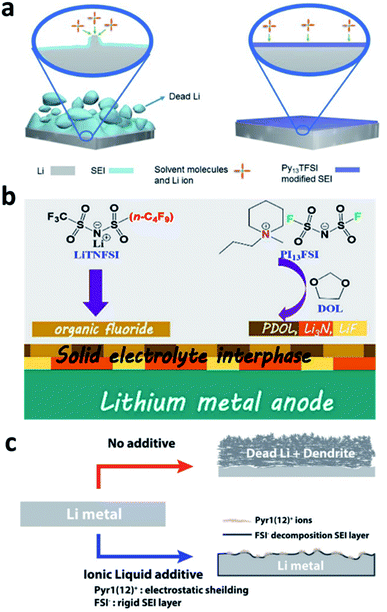 | ||
| Fig. 10 The use of an ionic liquid in the electrolyte for forming nitrided interfaces on Li metal electrodes. (a) Schematic illustration of the use of the Py13TFSI ionic liquid in an ether electrolyte for stabilizing the Li metal electrode;100 reproduced with permission. Copyright 2017, John Wiley and Sons. (b) Illustration of the co-use of the PI13FSI ionic liquid and LiTNFSI to construct a nitrided composite SEI on the Li metal electrode;101 reproduced with permission. Copyright 2018, American Chemical Society. (c) Illustration of the application of Pyr1(12)FSI to regulate the uniform deposition of Li+ and reduce dead Li.102 Reproduced with permission. Copyright 2018, John Wiley and Sons. | ||
Even the ether solvents reduce the viscosity of N-containing ionic liquid-based electrolytes, and they also limit the voltage window of the electrolyte. In addition, the high volatility and flammability of ether solvents diminish the safety advantages of ionic liquids in the electrolyte. The high price of ionic liquids is another problem that should be addressed before large-scale applications.
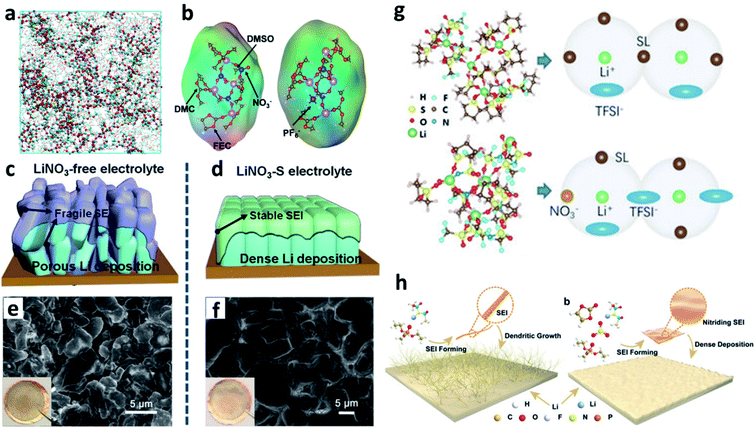
4.4. Constructing F-rich and N-rich composite interfaces
LiF has an ultra-high Young's modulus, so it can suppress Li dendrite growth.49 Although its bulk ionic conductivity is poor, it could form a compact structure and conduct Li+via grain boundaries because of the high grain boundary energy. Inorganic nitrides such as Li3N have much higher bulk ionic conductivity, but their particle size is larger than that of LiF, and the connections between the nitride grains are not as compact as that between LiF grains. The F-rich (LiF) and N-rich (Li3N and LiNxOy) composite interface is more effective for stabilizing the Li metal anode. The most common approach to introduce the LiF species on the surface of Li is using F-containing solvents. In this regard, Zhang et al. designed an electrolyte with a mixed carbonate ester containing fluoroethylene carbonate (FEC) as the solvent and LiNO3 as an additive.103 The FEC and LiNO3 in the electrolyte altered the solvation sheath of Li+ (Fig. 11a), and formed a uniform SEI with an abundance of LiF and LiNxOy on the Li metal anode. Wang et al. pointed out that the simultaneous use of LiNO3 and FEC in a carbonate-based electrolyte reduced the reactivity of the electrolyte and formed a more compact SEI, thus shortening the diffusion paths of Li+ through the SEI and improving the CE of the resultant LMBs (Fig. 11b).104 Lu et al. dissolved 3 wt% LiNO3 with the aid of 1 wt% tris(pentafluorophenyl)borane as a solubilizer in FEC-based carbonate solvents and produced an nitrided and fluorinated composite SEI.105 The co-existence of LiF and Li3N in the SEI on Li was verified with a cryo-TEM (Fig. 11c). Zhang et al. proved that under the protection of this F- and N-rich SEI, LMBs delivered much superior electrochemical performance and a prolonged lifespan (Fig. 11d).106 Li et al. designed an electrolyte with a mixed solvent consisting of fluoro-amide (2,2,2-trifluoro-N,N-dimethylacetamide (FDMA)) and FEC.107 The FDMA would react with Li via a three-step decomposition mechanism and finally form Li3N (Fig. 11e), while FEC would react with Li to generate LiF on the surface of the Li metal electrode. Benefiting from the composite SEI, the plated Li was much denser and the stripping of Li was much more homogeneous. Zeng et al. also formulated an electrolyte with a mixture of fluorine-rich carbonate and cyclophosphonitrile as the solvent, and formed a fluoride-nitride ion-conducting interphase to suppress Li dendrite growth.108 Optimizing the solvation structure of the electrolyte is another approach to generate a composite SEI. For instance, Zhang et al. proposed that the presence of NO3− in the electrolyte could alter the structure of the Li+ solvation sheath and promote the decomposition of the FSI− anion, so that an SEI containing LiF and LiNxOy was formed on the Li surface (Fig. 11f).109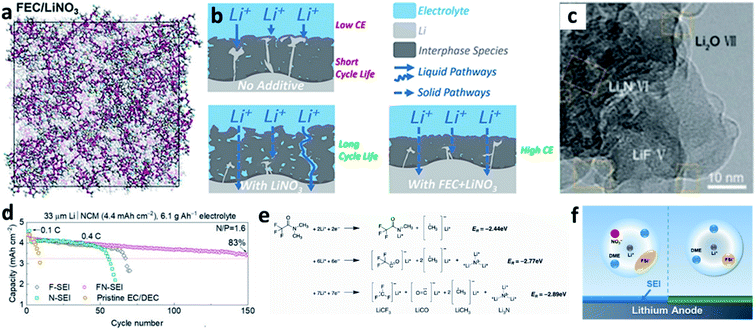 | ||
| Fig. 11 Constructing F-rich and N-rich composite interfaces for stabilizing Li metal electrodes. (a) MD results for a carbonate electrolyte with FEC as the solubilizer to improve the solvability of LiNO3;103 reproduced with permission. Copyright 2018, John Wiley and Sons. (b) Schematic illustration of the formation of a denser and more effective SEI on the Li metal electrode due to the synergistic effects of FEC and LiNO3 in the electrolyte;104 reproduced with permission. Copyright 2019, American Chemical Society. (c) Cryo-TEM image of the SEI formed in the electrolyte with tris(pentafluorophenyl)borane and LiNO3 as a dual additive;105 reproduced with permission. Copyright 2020, John Wiley and Sons. (d) Cycling performance of Li-NCM batteries with different types of SEIs;106 reproduced with permission. Copyright 2020, John Wiley and Sons. (e) Decomposition routes of the FDMA solvent to form Li3N;107 reproduced with permission. Copyright 2020, Springer Nature. (f) Structural illustration of the Li+ solvation structure of an ether electrolyte with or without NO3−.109 Reproduced with permission. Copyright 2019, American Chemical Society. | ||
As summarized in Table 2, the reported ether-based electrolytes normally deliver higher CE than ester-based electrolytes, because ether solvents are more stable against Li metal than ester solvents, although the low anodic decomposition voltage (<4 V) of ether-based electrolytes limits their application potential in high-voltage LMBs. In addition, although these reported electrolytes have improved the reversibility of the Li plating/stripping process, most of their CEs were still lower than 99.5%, which is not high enough for long-term and high-volumetric-energy-density LMBs. Furthermore, in most of the published results, the CE of the electrolytes was evaluated under a current density and an areal capacity lower than required for practical applications (higher than 3 mA cm−2 and 3 mA h cm−2, respectively).
To maintain reasonable viscosity and stability of the electrolyte, the amount of LiNO3 or other additives has been limited in the reported results. Nevertheless, these additives are continuously consumed/decomposed during battery cycling, and once the additives are depleted, the electrochemical performance of LMBs would decay rapidly. In contrast, the amounts of amides, fluoroamides, and N-containing ionic liquids are higher than that of additives when they are used as solvents in the electrolyte, and they can quickly repair the damaged SEI via reacting with newly exposed Li. Therefore, from the point of view of prolonging the lifespan of LMBs, the use of these N-containing solvents is more promising than the use of N-containing additives.
5. Substrate modification
The Li plating behaviours on the surfaces of metallic Li anodes and current collectors are quite different. The lithiophilicity of the substrate determines the over-potential for Li plating and the size of the nuclei at the initial stage of Li deposition.33,116–119 Nevertheless, most substrates, such as Cu, are lithiophobic.120–122 In addition, a practical current collector substrate is uneven with some protuberant tips on the surface, which induce a non-uniform distribution of the electric field and inhomogeneous charge distribution near the substrate, which eventually leads to the formation and growth of Li dendrites. Constructing nitrided interfaces on current collector substrates is important to improve their lithiophilicity and adjust the local electric field, thus regulating the uniform deposition of Li+.5.1. Cu substrate
Cu is the most popular substrate/current collector for negative electrodes in LMBs or anode-free batteries. Nitrided interfaces are able to guide Li+ homogeneous plating and improve the Li plating/stripping reversibility on a Cu substrate. Cui et al. synthesized an adaptive polymer with abundant N–H hydrogen bonding sites and applied it for Cu foil modification, where they achieved a much more uniform morphology of plated Li (Fig. 12a).123 Li et al. used a reactive sputtering method to develop a Cu3N layer on Cu foil (Fig. 12b).124 The Cu3N layer was believed to promote uniform surface electronic conductivity of the Cu foil, and it could further react with the deposited Li to form Li3N after the first plating process. The modified layer improved the CE in Li–Cu cells and the cycling stability of anode-free LiFePO4 (LFP)‖Cu cells.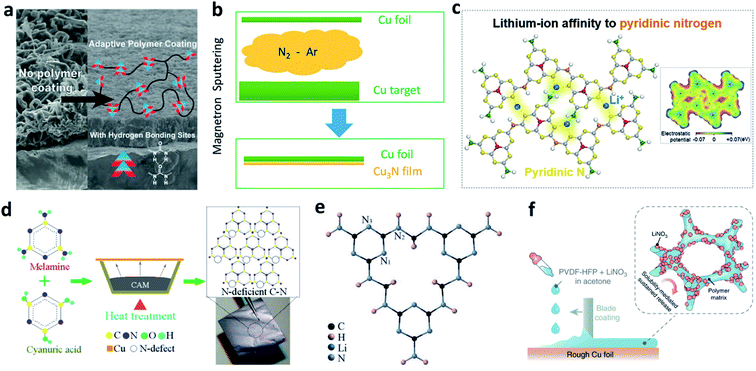 | ||
| Fig. 12 Modification of a Cu substrate with nitrided interfaces. (a) SEM images showing the effect of an adaptive polymer coated on Cu foil to suppress Li dendrite growth, and the structure of the adaptive polymer layer;123 reproduced with permission. Copyright 2016, American Chemical Society. (b) Schematic illustration of the preparation of a Cu3N layer on Cu foil by using the reaction of Cu and N2 gas;124 reproduced with permission. Copyright 2018, American Chemical Society. (c) Illustration of the pyridinic nitrogen in g-C3N4, which serves as a Li+ affinity centre;125 reproduced with permission. Copyright 2020, Elsevier. (d) Preparation of a defect-rich C–N polymer on Cu foil;126 reproduced with permission. Copyright 2020, American Chemical Society. (e) Structure of the poly-melamine-formaldehyde framework;127 reproduced with permission. Copyright 2018, John Wiley and Sons. (f) Schematic illustration of blade coating of a PVDF-HFP + LiNO3 layer on rough Cu foil.128 Reproduced with permission. Copyright 2018, Springer Nature. | ||
In addition to its function in protecting the Li metal electrode, as discussed above, g-C3N4 can also regulate Li+ deposition behaviour on Cu foil. Song et al. reported that the pyridinic nitrogen of g-C3N4 can serve as a Li+ affinity centre and help to improve the lithiophobicity of Cu foil (Fig. 12c).125 The g-C3N4 layer can also facilitate Li+ conduction at the SEI through a site-to-site hopping mechanism. In addition, defect engineering of a C–N polymer was proposed to construct an N-deficient ultrathin layer (27 nm) on Cu foil via reactive thermal evaporation (Fig. 12d).126 The lithiophilicity of the defective C–N layer triggered a space charge effect in the SEI and enhanced its charge-transfer capability, leading to a lower nucleation over-potential. In addition, a three-dimensional (3D) porous poly-melamine-formaldehyde (PMF) framework was developed to modify Cu foil and prepare a PMF/Li composite anode. The amine and triazine groups in the PMF can homogenize Li+ concentration near the Cu surface and regulate the uniform deposition of Li (Fig. 12e).127
Decorating LiNO3 on Cu foil is also effective for forming nitrided interfaces, but the main problem is that LiNO3 consists of inorganic particles so that it cannot closely adhere to the Cu foil. To solve this issue, with the aid of a polymeric matrix of poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP), Cui et al. coated a thin layer of LiNO3 on the surface of rough Cu.128 In this design, NO3− can be continuously released from the layer into the carbonate-based electrolyte during the Li plating process to maintain an appreciable local NO3− concentration at the anode surface (Fig. 12f). In addition, Xie et al. immersed commercially available Cu foam into LiNO3 aqueous solution to load LiNO3 particles into the pores and inner surface of the Cu foam.129 When operating in a carbonate-based electrolyte, the LiNO3 was reduced and formed an N-rich SEI on the outer and inner surfaces of the Cu foam. The authors believe that this facile method can be applied in large-scale production.
Apart from these advances, polyacrylonitrile (PAN) or PAN-based materials were used as interfacial functional layers to guide the uniform deposition of Li+ ions.130,131 AlN interlayers, which simultaneously possessed high Li affinity and an insulating nature, were also used as a surface stabilizer for Li metal anodes.132 Metal–organic framework (MOF) or MOF-derived materials could increase the affinity of Li+ to the Cu substrate, so they were also reported to suppress Li dendrite growth.121
5.2. Other substrates
3D nickel (Ni) foam can also be used as a substrate to accommodate Li. Similar to Cu, the lithiophobic and uneven surface of Ni leads to uniform deposition of Li. Compared with two-dimensional (2D) planar Cu, the 3D porous structure of Ni foam offers space to alleviate the volume changes of Li during plating/stripping processes, so it could accommodate high capacity Li plating. To improve the surface lithiophobicity of Ni, Nan et al. decorated cobalt nitride (Co3N) nanobrushes on Ni foam.133 The Co3N enabled a low over-potential for nucleation, leading to homogeneous plating of dendrite-free Li. Yang et al. used experimental results and theoretical simulations to prove that a micro-electric field can be formed by the tri-s-triazine units of g-C3N4 that were used to modify Ni foam, which induced numerous Li nuclei during the initial plating and guided the uniform growth of Li on the substrate (Fig. 13a and b).134 Sun et al. decorated NixN on the surface of Ni foam, which improved the specific surface area to reduce the local current density and promoted the uniform plating of Li by the formation of Li3N.135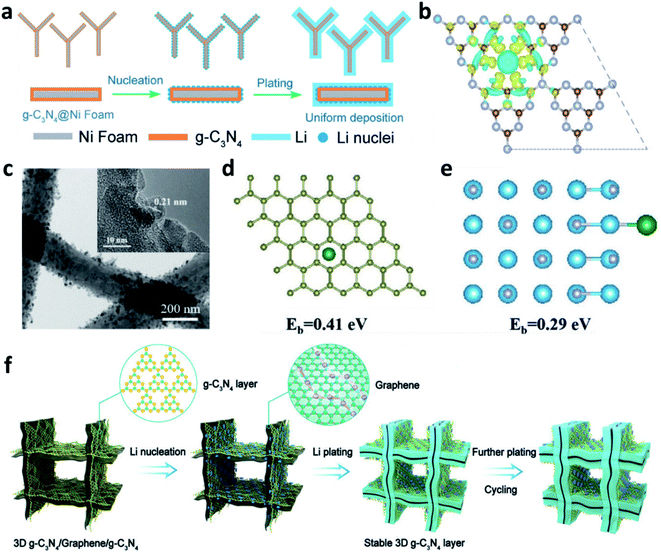 | ||
| Fig. 13 (a) Schematic illustration of the preparation of a g-C3N4 layer on Ni foam and its effects towards regulating Li+ uniform deposition; (b) charge distribution of the g-C3N4 layer revealing that N atoms have higher electron density and can absorb positively charged Li+;134 reproduced with permission. Copyright 2019, John Wiley and Sons. (c) TEM images of the TiN-decorated 3D carbon fibre; (d) optimized geometries for calculating the binding energy of a Li atom adsorbed on TiN, and (e) the corresponding charge density difference;136 reproduced with permission. Copyright 2019, John Wiley and Sons. (f) Schematic illustration of the Li deposition on the 3D g-C3N4/G/g-C3N4 electrode.137 Reproduced with permission. Copyright 2021, John Wiley and Sons. | ||
3D carbon is also a good scaffold to host Li. The modification achieved by nitrided materials can help to increase the affinity of Li+ to the carbon matrix and thus decrease the over-potential for Li plating. For example, Li et al. prepared TiN-decorated 3D carbon fibres as a scaffold for Li metal anodes (Fig. 13c).136 The TiN sheath on the carbon fibre could absorb Li+ and reduce the diffusion energy barrier, thus providing uniform nucleation sites for Li and suppressing the formation of Li dendrites (Fig. 13d and e). Gong et al. decorated g-C3N4 on 3D graphene to develop a g-C3N4/graphene/g-C3N4 architecture, which can be used as an electrode to accommodate the Li metal anode (Fig. 13f).137 The sandwiched structure can guide uniform Li plating/stripping in the van der Waals gap between the graphene and the g-C3N4. The g-C3N4 can be regarded as an artificial SEI to prevent Li deposition on its surface and prevent the direct contact of the electrolyte with the Li metal because of its isolating nature. Other polar nitrides, such as AlN and Mg3N2, were also reported to modify 3D carbon hosts to regulate the Li plating behaviour and suppress Li dendrite growth.115,138,139
In a brief summary, nitrided interface modification has improved the lithiophilicity and adjusted the local charge distribution at the surface of substrates/current collectors. As summarized in Table 3, with the functionalization of nitrided interfaces, the Li plating/stripping efficiency on Cu substrates or other substrates has been effectively enhanced to 96–99%. Nevertheless, for real LMBs, especially for anode-free LMBs (without excess Li), the CE should reach a level of 99.9%. This means that the effects of nitrided interfaces need to be further improved. Furthermore, most of the reported results were obtained in ether-based electrolytes, which can undoubtedly improve the CE. As discussed above, evaluating the electrochemical performance with a high-voltage ester-based electrolyte is more practically significant. Besides, most of the reported results did not mention anode-free battery testing, while one of the most important aims of modifying substrates/current collectors is to build anode-free batteries.
| Substrate | Modification | Electrolyte | Current density (mA cm−2) | Capacity (mA h cm−2) | Lifespan (cycles) | Coulombic efficiency (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 2D Cu | Adaptive polymer | 1 M LiTFSI in DOL/DME with 1 wt% LiNO3 | 1 | 1 | 180 | 97 | 123 |
| 2D Cu | Cu3N | 1 M LiPF6 in EC/DMC | 0.5 | 1 | 130 | ∼90 | 124 |
| 2D Cu | SBR + Cu3N | 1 M LiPF6 EC/DEC | 1 | 1 | 100 | 97.4 | 76 |
| 2D Cu | g-C3N4 | 1 M LiTFSI in DOL/DME | 1 | 1 | 350 | ∼99 | 125 |
| 2D Cu | g-C3N4 | 1 M LiTFSI in DOL/DME with 0.2 M LiNO3 | 3 | 1 | 450 | ∼96 | 126 |
| 2D Cu | Polyacrylonitrile | 1 M LiTFSI in DOL/DME with 2 wt% LiNO3 | 0.5 | 1 | 250 | 97.4 | 131 |
| 2D Cu | Aluminum nitride | 1 M LiPF6 in EC/DEC with 5 v% FEC | 0.5 | 1 | 125 | ∼97 | 132 |
| 2D Cu | Metal–organic framework | 1 M LiTFSI in DOL/DME with 1 wt% LiNO3 | 1 | 1 | 300 | 99.1 | 121 |
| 2D Cu | MOF comprising bipyridinic nitrogen linker | 1 M LiTFSI in DOL/DME with 2 wt% LiNO3 | 1 | 1 | 600 | ∼96 | 114 |
| 2D Cu | Carbon@PVDF@LiNO3 | 1 M LiPF6 in EC/DEC with 5 wt% VC | 1 | 1 | 200 | 97.9 | 140 |
| 2D Cu | Polyethylene terephthalate | 1 M LiTFSI in DOL/DME with 2 wt% LiNO3 | 1 | 1 | 100 | 98 | 120 |
| 2D Cu | Polyacrylonitrile/polyimide | 1 M LiTFSI in DOL/DME with 2 wt% LiNO3 | 2 | 2 | 130 | 97.3 | 130 |
| Rough Cu | PVDF-HFP + LiNO3 | 0.5 M LiPF6 EC/DEC | 1 | 1 | 200 | 98.1 | 128 |
| Ni foam | g-C3N4 | 1 M LiTFSI in DOL/DME with 1 wt% LiNO3 | 2 | 2 | 140 | 97 | 134 |
| 2D Cu | 3D porous polymelamine-formaldehyde | 1 M LiTFSI in DOL/DME with 2 wt% LiNO3 | 10 | 1 | 50 | 94.7 | 127 |
| Carbon nanofiber mat | TiN | 1 M LiTFSI in DOL/DME with 1 wt% LiNO3 | 2 | 1 | 250 | 97.5 | 136 |
| Ni foam | Co3N4 nanobrush | 1 M LiTFSI in DOL/DME with 2 wt% LiNO3 | 1 | 1 | 120 | 96.9 | 133 |
| 3D carbon paper | Mg3N2 | 1 M LiTFSI in DOL/DME with 1 wt% LiNO3 | 0.5 | 0.5 | 240 | 98.2 | 139 |
| Ni foam | NixN (x = 3, 4) | 1 M LiTFSI in DOL/DME with 1 wt% LiNO3 | 1 | 1 | 300 | 97 | 135 |
| Cu foam | LiNO3 | 1 M LiPF6 in EC/DEC with 10% FEC | 1 | 1 | 300 | 95.5 | 129 |
| 3D graphene | g-C3N4 | 1 M LiTFSI in DOL/DME with 1 wt% LiNO3 | 1 | 1 | 500 | 99.1 | 137 |
It is worth noting that 3D Cu, Ni foam and 3D carbon hosts are all electronically conductive, so Li+ from the electrolyte may accept electrons and be plated on their upper surfaces (separator side). Once this occurs, the effects of the 3D structure towards accommodating Li will be greatly weakened, while the volumetric energy density of the Li anode will be sacrificed. As modified nitrides normally have poor electronic conductivity, they could decrease the surface electronic conductivity of the host and thus force Li+ diffusion into the pores and lead to Li deposition on the inner surface of the 3D scaffolds.
6. Separator functionalization
Separators play a key role in all batteries. In LIBs and LMBs, the separator is a porous membrane placed between the positive electrode and negative electrode, which is permeable to ionic flow but prevents electric contact between the electrodes.144 Previous results proved that coating a polypropylene (PP)-based separator with BN nanosheets was useful for suppressing Li dendrite growth and prolonging the lifespan of LMBs.145 The separator can also be used to support N-containing materials to induce nitriding of the interface on the Li metal anode. Wang et al. creatively immersed a glass fibre separator in a LiNO3 solution to impregnate the separator with sub-micrometer-scale particles of LiNO3.88 During battery cycling, the LiNO3 was released into the carbonate-based electrolyte and reacted with Li to form a nitrided protective layer, which could suppress the formation of Li dendrites and “dead” Li. Li et al. proposed a similar concept for sustainably releasing NO3− in a carbonate electrolyte by intercalating superfluous LiNO3 particles between bi-layer polypropylene membranes (PP/LiNO3/PP).146 Wu et al. developed a composite separator coated with polyacrylamide-grafted graphene oxide molecular brushes (GO-g-PAM) (Fig. 14a and b).141 The polyacrylamide chains contained abundant N–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups and thus enabled a molecular-level homogeneous and fast Li+ flux on the surface of Li. Besides, a layer of g-C3N4 on commercially available PP separators was prepared (Fig. 14d and e), and the g-C3N4 on the PP film was grafted to the Li metal surface after cell assembly.142 It was proposed that the g-C3N4 can form transient Li–N bonds at the electrode/electrolyte interface to effectively stabilize the Li+ flux and thus enable smooth Li deposition at high current densities and capacities (Fig. 14f and g). Besides, Huang et al. modified a hybrid layer of silk fibroin and polyvinyl alcohol (SF–PVA) on a PP separator via a freeze drying method. The SF–PVA layer will auto-transferred from the PP separator to the Li surface (Fig. 14h).143 The N–H and C
O groups and thus enabled a molecular-level homogeneous and fast Li+ flux on the surface of Li. Besides, a layer of g-C3N4 on commercially available PP separators was prepared (Fig. 14d and e), and the g-C3N4 on the PP film was grafted to the Li metal surface after cell assembly.142 It was proposed that the g-C3N4 can form transient Li–N bonds at the electrode/electrolyte interface to effectively stabilize the Li+ flux and thus enable smooth Li deposition at high current densities and capacities (Fig. 14f and g). Besides, Huang et al. modified a hybrid layer of silk fibroin and polyvinyl alcohol (SF–PVA) on a PP separator via a freeze drying method. The SF–PVA layer will auto-transferred from the PP separator to the Li surface (Fig. 14h).143 The N–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups in SF are able to regulate the Li+ flux distribution, and the SF–PVA layer can form a Li3N rich SEI. Therefore, uniform Li nuclei deposition was achieved.
O groups in SF are able to regulate the Li+ flux distribution, and the SF–PVA layer can form a Li3N rich SEI. Therefore, uniform Li nuclei deposition was achieved.
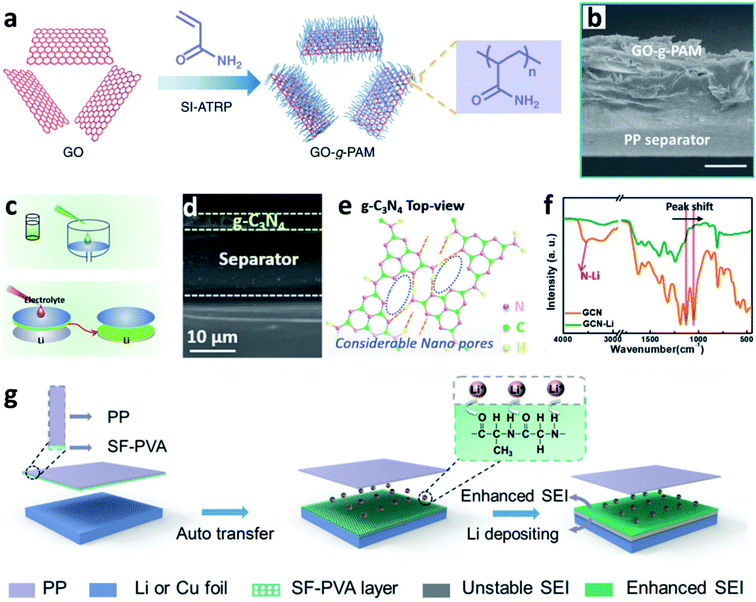 | ||
| Fig. 14 Separator functionalization for constructing nitrided interfaces on Li metal electrodes. (a) Schematic illustration of the synthesis of GO-g-PAM molecular brushes; (b) cross-sectional SEM images of the GO-g-PAM@PP separator; scale bar: 20 μm;141 reproduced with permission. Copyright 2019, Springer Nature. (c) Preparation of a g-C3N4 layer on the PP separator; (d) SEM image of the g-C3N4 layer supported on the PP separator; (e) Fourier transform infrared (FT-IR) spectra showing the formation of transient Li–N bonds in the g-C3N4 layer after it is immersed in the electrolyte; (f) schematic illustration of the process of Li+ adsorption and the formation of Li–N bonds in the g-C3N4 layer;142 reproduced with permission. Copyright 2019, John Wiley and Sons. (g) Schematic illustration of the modification of the SF–PVA layer on a Li metal electrode and its function in forming an enhanced SEI.143 Reproduced with permission. Copyright 2021, John Wiley and Sons. | ||
The separator functionalization strategies can remarkably improve the lifespan and the electrochemical performance of Li metal electrodes, even under high current density and high capacity conditions. These strategies are also convenient for large-scale production. Unfortunately, the introduction of N-containing materials increases the overall thickness of the separator, which will certainly sacrifice the volumetric energy density of LMBs.
7. Prospects and outlook
Nitrided interfaces have effectively stabilized Li metal electrodes and improved their electrochemical performance as well as lifespan of LMBs. Despite this success, many critical issues and challenges remain to be carefully considered in the future. They are summarized below.7.1. Investigating how nitrided interfaces affect the stripping process
The majority of research has only focused on the plating process, while the stripping process, deciding the utilization of deposited Li, was rarely noticed. There is no doubt that a uniform stripping step will lead to less pulverization and depletion of active Li during each cycle, which is a significant factor in promoting the lifespan and electrochemical performance of LMBs. The course of Li dissolution with and without nitrided interfaces during the stripping step deserves to be carefully studied.7.2. Understanding how nitrided interfaces affect the components and microstructure of the final SEI layer
The introduction of nitrogenous additives will definitely influence the solvent structure of the electrolyte, changing the final decomposition products, while for nitrided interfaces fabricated ex situ, their existence also alters the decomposition of the electrolyte. In short, the final SEI layer is composed of nitrogenous compounds and other components, and these products and their distributions have a direct correlation with the Li ion transport. If we only target nitrogenous compounds, we cannot achieve a comprehensive outlook with respect to the interfaces. A better knowledge of the interaction between nitrogenous compounds and other components will help to understand the differences in performance among the various nitrided interfaces.7.3. Developing high-voltage N-containing electrolytes
The application of LiNO3 and other N-containing additives is mainly confined to low-voltage electrolytes due to their low solubility in most high-voltage non-aqueous solvents. Increasing their solubility in high-voltage electrolytes is of great importance.7.4. Controlling the thickness of nitrided interfaces
The thickness of reported nitrided or nitride-based composite interfaces varies from a few nanometres to more than 20 μm. To avoid the sacrifice of the volumetric-energy-density of the Li metal battery, the thickness of the modification layers should be much thinner than that of the Li foil, especially considering that the Li foil used in the practical batteries is only 50 μm in thickness.7.5. Improving the lifespan of the nitrided artificial SEI
The reported nitrided artificial SEI layers do exhibit positive effects towards suppressing Li dendrite growth and passivating the active Li surface, but their stability is far from satisfactory. They may be destroyed by the interfacial stress resulting from the huge volume changes of the Li metal electrode, so the real effects of nitrided artificial SEI layers would be sacrificed. Developing flexible and robust nitrided artificial SEI layers with longer lifespans is critical to promoting their practical effects.7.6. Evaluating the effects of nitrided interfaces under practical conditions
According to the reported designs, most of the electrochemical performance was tested under mild conditions different from the real application situation. To make it more objective, the electrolyte and Li metal should be well quantified, while the cathode capacity should reach the commercial scale. In consideration of the mass energy density and the cost, the amount of electrolyte should be limited to less than 10 μL mA h−1 (lean electrolyte). Meanwhile, the areal capacity of the cathode should be higher than 3 mA h cm−2, and the thickness of the Li metal anode should be less than 50 μm (∼10 mA h cm−2), with the capacity ratio of the negative electrode (Li) to the positive electrode (n/p ratio) lower than 3. In addition, the current density and areal capacity in coulombic efficiency and symmetric cell tests should be increased to higher than 3 mA cm−2 and 3 mA h cm−2, respectively.Data availability
There is no origional experimental or computational data associated with this article, as it is a Perspective.Author contributions
Z. Wang and Y. Wang wrote the manuscript. Z. Guo supervised this project. All the authors discussed and polished the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support provided by the Australian Research Council (ARC) (LP160101629, LE120100104, DP200101862, DP210101486, and LE180100141) is gratefully acknowledged. Y. W. and Z. W. acknowledge the China Scholarship Council for scholarship support (No. 201808440447 and 201706340049). The authors thank Dr Tania Silver at UOW for critical reading and polishing of the manuscript.References
- M. Winter, B. Barnett and K. Xu, Chem. Rev., 2018, 118, 11433–11456 CrossRef CAS PubMed.
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359–367 CrossRef CAS PubMed.
- J. B. Goodenough and Y. Kim, Chem. Mater., 2010, 22, 587–603 CrossRef CAS.
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 652–657 CrossRef CAS PubMed.
- Q. Wang, H. Wang, J. Wu, M. Zhou, W. Liu and H. Zhou, Nano Energy, 2021, 80, 105516 CrossRef CAS.
- Y. Ding, Z. P. Cano, A. Yu, J. Lu and Z. Chen, Electrochem. Energy Rev., 2019, 2, 1–28 CrossRef CAS.
- J. Duan, X. Tang, H. Dai, Y. Yang, W. Wu, X. Wei and Y. Huang, Electrochem. Energy Rev., 2020, 3, 1–42 CrossRef CAS.
- X. Zeng, M. Li, D. Abd El-Hady, W. Alshitari, A. S. Al-Bogami, J. Lu and K. Amine, Adv. Energy Mater., 2019, 9, 1900161 CrossRef.
- B. Liu, J.-G. Zhang and W. Xu, Joule, 2018, 2, 833–845 CrossRef CAS.
- H. Ye, Y. Zhang, Y.-X. Yin, F.-F. Cao and Y.-G. Guo, ACS Cent. Sci., 2020, 6, 661–671 CrossRef CAS PubMed.
- W. Xu, J. Wang, F. Ding, X. Chen, E. Nasybulin, Y. Zhang and J.-G. Zhang, Energy Environ. Sci., 2014, 7, 513–537 RSC.
- X.-B. Cheng, R. Zhang, C.-Z. Zhao and Q. Zhang, Chem. Rev., 2017, 117, 10403–10473 CrossRef CAS PubMed.
- Y. Wang, Z. Wang, L. Zhao, Q. Fan, X. Zeng, S. Liu, W. K. Pang, Y.-B. He and Z. Guo, Adv. Mater., 2021, 33, 2008133 CrossRef CAS PubMed.
- Z.-J. Zheng, H. Ye and Z.-P. Guo, Adv. Sci., 2020, 7, 2002212 CrossRef CAS PubMed.
- C.-Y. Wang, Z.-J. Zheng, Y.-Q. Feng, H. Ye, F.-F. Cao and Z.-P. Guo, Nano Energy, 2020, 74, 104817 CrossRef CAS.
- L. Suo, W. Xue, M. Gobet, S. G. Greenbaum, C. Wang, Y. Chen, W. Yang, Y. Li and J. Li, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 1156 CrossRef CAS PubMed.
- B. Han, D. Xu, S.-S. Chi, D. He, Z. Zhang, L. Du, M. Gu, C. Wang, H. Meng, K. Xu, Z. Zheng and Y. Deng, Adv. Mater., 2020, 32, 2004793 CrossRef CAS PubMed.
- Y. Lu, X. Rong, Y.-S. Hu, L. Chen and H. Li, Energy Storage Mater., 2019, 23, 144–153 CrossRef.
- J. Liu, Z. Bao, Y. Cui, E. J. Dufek, J. B. Goodenough, P. Khalifah, Q. Li, B. Y. Liaw, P. Liu, A. Manthiram, Y. S. Meng, V. R. Subramanian, M. F. Toney, V. V. Viswanathan, M. S. Whittingham, J. Xiao, W. Xu, J. Yang, X.-Q. Yang and J.-G. Zhang, Nat. Energy, 2019, 4, 180–186 CrossRef CAS.
- D. Lin, Y. Liu and Y. Cui, Nat. Nanotechnol., 2017, 12, 194–206 CrossRef CAS PubMed.
- X. Shen, X.-Q. Zhang, F. Ding, J.-Q. Huang, R. Xu, X. Chen, C. Yan, F.-Y. Su, C.-M. Chen, X. Liu and Q. Zhang, Energy Mater. Adv., 2021, 2021, 1205324 Search PubMed.
- P. Albertus, S. Babinec, S. Litzelman and A. Newman, Nat. Energy, 2018, 3, 16–21 CrossRef CAS.
- M. D. Tikekar, S. Choudhury, Z. Tu and L. A. Archer, Nat. Energy, 2016, 1, 16114 CrossRef CAS.
- Y. Jie, X. Ren, R. Cao, W. Cai and S. Jiao, Adv. Funct. Mater., 2020, 30, 1910777 CrossRef CAS.
- E. Peled and S. Menkin, J. Electrochem. Soc., 2017, 164, A1703–A1719 CrossRef CAS.
- X.-B. Cheng, R. Zhang, C.-Z. Zhao, F. Wei, J.-G. Zhang and Q. Zhang, Adv. Sci., 2016, 3, 1500213 CrossRef PubMed.
- M. D. Tikekar, S. Choudhury, Z. Tu and L. A. Archer, Nat. Energy, 2016, 1, 16114 CrossRef CAS.
- C.-Z. Zhao, H. Duan, J.-Q. Huang, J. Zhang, Q. Zhang, Y.-G. Guo and L.-J. Wan, Sci. China: Chem., 2019, 62, 1286–1299 CrossRef CAS.
- E. Peled, D. Golodnitsky and G. Ardel, J. Electrochem. Soc., 1997, 144, L208–L210 CrossRef CAS.
- K. Qin, K. Holguin, M. Mohammadiroudbari, J. Huang, E. Y. S. Kim, R. Hall and C. Luo, Adv. Funct. Mater., 2021, 31, 2009694 CrossRef CAS.
- Y. Zhao, Y. Ye, F. Wu, Y. Li, L. Li and R. Chen, Adv. Mater., 2019, 31, 1806532 CrossRef PubMed.
- Z. Wang, H. Gao, Q. Zhang, Y. Liu, J. Chen and Z. Guo, Small, 2019, 15, 1803858 CrossRef PubMed.
- X.-R. Chen, B.-C. Zhao, C. Yan and Q. Zhang, Adv. Mater., 2021, 33, 2004128 CrossRef CAS PubMed.
- C. Fang, X. Wang and Y. S. Meng, Trends Chem., 2019, 1, 152–158 CrossRef CAS.
- W. Zhang, Q. Wu, J. Huang, L. Fan, Z. Shen, Y. He, Q. Feng, G. Zhu and Y. Lu, Adv. Mater., 2020, 32, 2001740 CrossRef CAS PubMed.
- F. Ding, W. Xu, G. L. Graff, J. Zhang, M. L. Sushko, X. Chen, Y. Shao, M. H. Engelhard, Z. Nie, J. Xiao, X. Liu, P. V. Sushko, J. Liu and J.-G. Zhang, J. Am. Chem. Soc., 2013, 135, 4450–4456 CrossRef CAS PubMed.
- J. Zheng, Q. Zhao, T. Tang, J. Yin, C. D. Quilty, G. D. Renderos, X. Liu, Y. Deng, L. Wang, D. C. Bock, C. Jaye, D. Zhang, E. S. Takeuchi, K. J. Takeuchi, A. C. Marschilok and L. A. Archer, Science, 2019, 366, 645 CrossRef CAS PubMed.
- R. Wang, W. Cui, F. Chu and F. Wu, J. Energy Chem., 2020, 48, 145–159 CrossRef.
- L. Chen, X. Fan, X. Ji, J. Chen, S. Hou and C. Wang, Joule, 2019, 3, 732–744 CrossRef CAS.
- Y. He, X. Ren, Y. Xu, M. H. Engelhard, X. Li, J. Xiao, J. Liu, J.-G. Zhang, W. Xu and C. Wang, Nat. Nanotechnol., 2019, 14, 1042–1047 CrossRef CAS PubMed.
- R. Zhang, N.-W. Li, X.-B. Cheng, Y.-X. Yin, Q. Zhang and Y.-G. Guo, Adv. Sci., 2017, 4, 1600445 CrossRef PubMed.
- F. Wu, Y.-X. Yuan, X.-B. Cheng, Y. Bai, Y. Li, C. Wu and Q. Zhang, Energy Storage Mater., 2018, 15, 148–170 CrossRef.
- R. Xu, X.-B. Cheng, C. Yan, X.-Q. Zhang, Y. Xiao, C.-Z. Zhao, J.-Q. Huang and Q. Zhang, Matter, 2019, 1, 317–344 CrossRef.
- I. Skarmoutsos, V. Ponnuchamy, V. Vetere and S. Mossa, J. Phys. Chem. C, 2015, 119, 4502–4515 CrossRef CAS.
- K. Xu, Chem. Rev., 2004, 104, 4303–4418 CrossRef CAS PubMed.
- M. Li, C. Wang, Z. Chen, K. Xu and J. Lu, Chem. Rev., 2020, 120, 6783–6819 CrossRef CAS PubMed.
- W. Cai, Y.-X. Yao, G.-L. Zhu, C. Yan, L.-L. Jiang, C. He, J.-Q. Huang and Q. Zhang, Chem. Soc. Rev., 2020, 49, 3806–3833 RSC.
- P. Zhai, L. Liu, X. Gu, T. Wang and Y. Gong, Adv. Energy Mater., 2020, 10, 2001257 CrossRef CAS.
- T. Li, X.-Q. Zhang, P. Shi and Q. Zhang, Joule, 2019, 3, 2647–2661 CrossRef CAS.
- R. A. Vilá, W. Huang and Y. Cui, Cell Rep. Phys. Sci., 2020, 1, 100188 CrossRef.
- R. Guo and B. M. Gallant, Chem. Mater., 2020, 32, 5525–5533 CrossRef CAS.
- W. Qi, L. Ben, H. Yu, W. Zhao, G. Zhao and X. Huang, Prog. Nat. Sci.: Mater. Int., 2020, 30, 321–327 CrossRef CAS.
- W. Li, G. Wu, C. M. Araújo, R. H. Scheicher, A. Blomqvist, R. Ahuja, Z. Xiong, Y. Feng and P. Chen, Energy Environ. Sci., 2010, 3, 1524–1530 RSC.
- U. v. Alpen, J. Solid State Chem., 1979, 29, 379–392 CrossRef.
- Y. Zhou, X. Zhang, Y. Ding, J. Bae, X. Guo, Y. Zhao and G. Yu, Adv. Mater., 2020, 32, 2003920 CrossRef CAS PubMed.
- S. Li, Q. Liu, X. Wang, Q. Wu, L. Fan, W. Zhang, Z. Shen, L. Wang, M. Ling and Y. Lu, ACS Mater. Lett., 2020, 2, 1–8 CrossRef.
- N.-W. Li, Y.-X. Yin, C.-P. Yang and Y.-G. Guo, Adv. Mater., 2016, 28, 1853–1858 CrossRef CAS PubMed.
- L. Lin, F. Liang, K. Zhang, H. Mao, J. Yang and Y. Qian, J. Mater. Chem. A, 2018, 6, 15859–15867 RSC.
- Y. Lin, Z. Wen, J. Liu, D. Wu, P. Zhang and J. Zhao, J. Energy Chem., 2021, 55, 129–135 CrossRef.
- H. Chen, A. Pei, D. Lin, J. Xie, A. Yang, J. Xu, K. Lin, J. Wang, H. Wang, F. Shi, D. Boyle and Y. Cui, Adv. Energy Mater., 2019, 9, 1900858 CrossRef.
- F. Liu, L. Wang, Z. Zhang, P. Shi, Y. Feng, Y. Yao, S. Ye, H. Wang, X. Wu and Y. Yu, Adv. Funct. Mater., 2020, 30, 2001607 CrossRef CAS.
- X. Fan, L. Chen, O. Borodin, X. Ji, J. Chen, S. Hou, T. Deng, J. Zheng, C. Yang, S.-C. Liou, K. Amine, K. Xu and C. Wang, Nat. Nanotechnol., 2018, 13, 715–722 CrossRef CAS PubMed.
- J. Yu, L. Zhao, Y. Huang, Y. Hu, L. Chen and Y.-B. He, Front. Mater., 2020, 7, 71 CrossRef.
- D. Kang, M. Xiao and J. P. Lemmon, Batteries Supercaps, 2020, 4, 445–455 CrossRef.
- Y. Sun, Y. Zhao, J. Wang, J. Liang, C. Wang, Q. Sun, X. Lin, K. R. Adair, J. Luo, D. Wang, R. Li, M. Cai, T.-K. Sham and X. Sun, Adv. Mater., 2019, 31, 1806541 CrossRef PubMed.
- X. Wang, Z. Pan, J. Zhuang, G. Li, X. Ding, M. Liu, Q. Zhang, Y. Liao, Y. Zhang and W. Li, ACS Appl. Mater. Interfaces, 2019, 11, 5159–5167 CrossRef CAS PubMed.
- D. Lee, S. Sun, J. Kwon, H. Park, M. Jang, E. Park, B. Son, Y. Jung, T. Song and U. Paik, Adv. Mater., 2020, 32, 1905573 CrossRef CAS PubMed.
- J. Bae, Y. Qian, Y. Li, X. Zhou, J. B. Goodenough and G. Yu, Energy Environ. Sci., 2019, 12, 3319–3327 RSC.
- K. Park and J. B. Goodenough, Adv. Energy Mater., 2017, 7, 1700732 CrossRef.
- X. Luan, C. Wang, C. Wang, X. Gu, J. Yang and Y. Qian, ACS Appl. Mater. Interfaces, 2020, 12, 11265–11272 CrossRef PubMed.
- M. Wu, Z. Wen, Y. Liu, X. Wang and L. Huang, J. Power Sources, 2011, 196, 8091–8097 CrossRef CAS.
- Y. J. Zhang, W. Wang, H. Tang, W. Q. Bai, X. Ge, X. L. Wang, C. D. Gu and J. P. Tu, J. Power Sources, 2015, 277, 304–311 CrossRef CAS.
- K. Chen, R. Pathak, A. Gurung, E. A. Adhamash, B. Bahrami, Q. He, H. Qiao, A. L. Smirnova, J. J. Wu, Q. Qiao and Y. Zhou, Energy Storage Mater., 2019, 18, 389–396 CrossRef.
- Y. Li, Y. Sun, A. Pei, K. Chen, A. Vailionis, Y. Li, G. Zheng, J. Sun and Y. Cui, ACS Cent. Sci., 2018, 4, 97–104 CrossRef CAS PubMed.
- W. Wang, X. Yue, J. Meng, J. Wang, X. Wang, H. Chen, D. Shi, J. Fu, Y. Zhou, J. Chen and Z. Fu, Energy Storage Mater., 2019, 18, 414–422 CrossRef.
- Y. Liu, D. Lin, P. Y. Yuen, K. Liu, J. Xie, R. H. Dauskardt and Y. Cui, Adv. Mater., 2017, 29, 1605531 CrossRef PubMed.
- Y. Zheng, S. Xia, F. Dong, H. Sun, Y. Pang, J. Yang, Y. Huang and S. Zheng, Adv. Funct. Mater., 2021, 31, 2006159 CrossRef CAS.
- S. Ye, L. Wang, F. Liu, P. Shi, H. Wang, X. Wu and Y. Yu, Adv. Energy Mater., 2020, 10, 2002647 CrossRef CAS.
- Z. Hu, S. Zhang, S. Dong, W. Li, H. Li, G. Cui and L. Chen, Chem. Mater., 2017, 29, 4682–4689 CrossRef CAS.
- G. Wang, C. Chen, Y. Chen, X. Kang, C. Yang, F. Wang, Y. Liu and X. Xiong, Angew. Chem., Int. Ed., 2020, 59, 2055–2060 CrossRef CAS PubMed.
- J.-I. Lee, S. Cho, T. T. Vu, S. Kim, S. Ryu, J. Moon and S. Park, Energy Storage Mater., 2021, 38, 509–519 CrossRef.
- Z. Wang, Y. Wang, Z. Zhang, X. Chen, W. Lie, Y.-B. He, Z. Zhou, G. Xia and Z. Guo, Adv. Funct. Mater., 2020, 30, 2002414 CrossRef CAS.
- J. Gu, C. Shen, Z. Fang, J. Yu, Y. Zheng, Z. Tian, L. Shao, X. Li and K. Xie, Front. Chem., 2019, 7, 572 CrossRef CAS PubMed.
- X. Liang, Z. Wen, Y. Liu, M. Wu, J. Jin, H. Zhang and X. Wu, J. Power Sources, 2011, 196, 9839–9843 CrossRef CAS.
- S. Xiong, K. Xie, Y. Diao and X. Hong, J. Power Sources, 2014, 246, 840–845 CrossRef CAS.
- A. Jozwiuk, B. B. Berkes, T. Weiß, H. Sommer, J. Janek and T. Brezesinski, Energy Environ. Sci., 2016, 9, 2603–2608 RSC.
- D. Aurbach, E. Pollak, R. Elazari, G. Salitra, C. S. Kelley and J. Affinito, J. Electrochem. Soc., 2009, 156, A694 CrossRef CAS.
- Q. Shi, Y. Zhong, M. Wu, H. Wang and H. Wang, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 5676 CrossRef CAS PubMed.
- Y. Jie, X. Liu, Z. Lei, S. Wang, Y. Chen, F. Huang, R. Cao, G. Zhang and S. Jiao, Angew. Chem., Int. Ed., 2020, 59, 3505–3510 CrossRef CAS PubMed.
- J. Guo, Z. Wen, M. Wu, J. Jin and Y. Liu, Electrochem. Commun., 2015, 51, 59–63 CrossRef CAS.
- C. Yan, Y.-X. Yao, X. Chen, X.-B. Cheng, X.-Q. Zhang, J.-Q. Huang and Q. Zhang, Angew. Chem., Int. Ed., 2018, 57, 14055–14059 CrossRef CAS PubMed.
- W. Zhang, Z. Shen, S. Li, L. Fan, X. Wang, F. Chen, X. Zang, T. Wu, F. Ma and Y. Lu, Adv. Funct. Mater., 2020, 30, 2003800 CrossRef CAS.
- S. Liu, X. Ji, N. Piao, J. Chen, N. Eidson, J. Xu, P. Wang, L. Chen, J. Zhang, T. Deng, S. Hou, T. Jin, H. Wan, J. Li, J. Tu and C. Wang, Angew. Chem., Int. Ed., 2021, 60, 3661–3671 CrossRef CAS PubMed.
- J. Fu, X. Ji, J. Chen, L. Chen, X. Fan, D. Mu and C. Wang, Angew. Chem., Int. Ed., 2020, 59, 22194–22201 CrossRef CAS PubMed.
- S.-J. Tan, J. Yue, X.-C. Hu, Z.-Z. Shen, W.-P. Wang, J.-Y. Li, T.-T. Zuo, H. Duan, Y. Xiao, Y.-X. Yin, R. Wen and Y.-G. Guo, Angew. Chem., Int. Ed., 2019, 58, 7802–7807 CrossRef CAS PubMed.
- S. H. Lee, J.-Y. Hwang, J. Ming, Z. Cao, H. A. Nguyen, H.-G. Jung, J. Kim and Y.-K. Sun, Adv. Energy Mater., 2020, 10, 2000567 CrossRef CAS.
- Q. Liu, Y. Xu, J. Wang, B. Zhao, Z. Li and H. B. Wu, Nano-Micro Lett., 2020, 12, 176 CrossRef CAS PubMed.
- Z. Jiang, Z. Zeng, C. Yang, Z. Han, W. Hu, J. Lu and J. Xie, Nano Lett., 2019, 19, 8780–8786 CrossRef CAS PubMed.
- Y. Luo, T. Li, H. Zhang, W. Liu, X. Zhang, J. Yan, H. Zhang and X. Li, Angew. Chem., Int. Ed., 2021, 60, 11718–11724 CrossRef CAS PubMed.
- N.-W. Li, Y.-X. Yin, J.-Y. Li, C.-H. Zhang and Y.-G. Guo, Adv. Sci., 2017, 4, 1600400 CrossRef PubMed.
- B. Tong, X. Chen, L. Chen, Z. Zhou and Z. Peng, ACS Appl. Energy Mater., 2018, 1, 4426–4431 CrossRef CAS.
- D.-J. Yoo, K. J. Kim and J. W. Choi, Adv. Energy Mater., 2018, 8, 1702744 CrossRef.
- X.-Q. Zhang, X. Chen, X.-B. Cheng, B.-Q. Li, X. Shen, C. Yan, J.-Q. Huang and Q. Zhang, Angew. Chem., Int. Ed., 2018, 57, 5301–5305 CrossRef CAS PubMed.
- Y. Zhang, Y. Zhong, S. Liang, B. Wang, X. Chen and H. Wang, ACS Mater. Lett., 2019, 1, 254–259 CrossRef CAS.
- S. Li, W. Zhang, Q. Wu, L. Fan, X. Wang, X. Wang, Z. Shen, Y. He and Y. Lu, Angew. Chem., Int. Ed., 2020, 59, 14935–14941 CrossRef CAS PubMed.
- X.-Q. Zhang, T. Li, B.-Q. Li, R. Zhang, P. Shi, C. Yan, J.-Q. Huang and Q. Zhang, Angew. Chem., Int. Ed., 2020, 59, 3252–3257 CrossRef CAS PubMed.
- Q. Wang, Z. Yao, C. Zhao, T. Verhallen, D. P. Tabor, M. Liu, F. Ooms, F. Kang, A. Aspuru-Guzik, Y.-S. Hu, M. Wagemaker and B. Li, Nat. Commun., 2020, 11, 4188 CrossRef PubMed.
- Y.-F. Liu, H.-R. Wang, J.-Y. Li, M.-J. Chen, H. Chen, B.-Y. Lu, Q. Ma, X.-W. Wu and X.-X. Zeng, Appl. Surf. Sci., 2021, 541, 148294 CrossRef CAS.
- X.-Q. Zhang, X. Chen, L.-P. Hou, B.-Q. Li, X.-B. Cheng, J.-Q. Huang and Q. Zhang, ACS Energy Lett., 2019, 4, 411–416 CrossRef CAS.
- S. Xiong, K. Xie, Y. Diao and X. Hong, Electrochim. Acta, 2012, 83, 78–86 CrossRef CAS.
- W. Li, H. Yao, K. Yan, G. Zheng, Z. Liang, Y.-M. Chiang and Y. Cui, Nat. Commun., 2015, 6, 7436 CrossRef CAS PubMed.
- C. Yan, H.-R. Li, X. Chen, X.-Q. Zhang, X.-B. Cheng, R. Xu, J.-Q. Huang and Q. Zhang, J. Am. Chem. Soc., 2019, 141, 9422–9429 CrossRef PubMed.
- Z. L. Brown, S. Heiskanen and B. L. Lucht, J. Electrochem. Soc., 2019, 166, A2523–A2527 CrossRef CAS.
- C. Gao, K. Sun, B. Hong, K. Zhang, Z. Zhang and Y. Lai, Int. J. Hydrogen Energy, 2020, 45, 28294–28302 CrossRef CAS.
- C. Gao, B. Hong, K. Sun, H. Fan, K. Zhang, Z. Zhang and Y. Lai, Energy Technol., 2020, 8, 1901463 CrossRef CAS.
- C. Wei, H. Fei, Y. An, Y. Tao, J. Feng and Y. Qian, J. Mater. Chem. A, 2019, 7, 18861–18870 RSC.
- W. Liu, P. Liu and D. Mitlin, Chem. Soc. Rev., 2020, 49, 7284–7300 RSC.
- K.-C. Pu, X. Zhang, X.-L. Qu, J.-J. Hu, H.-W. Li, M.-X. Gao, H.-G. Pan and Y.-F. Liu, Rare Met., 2020, 39, 616–635 CrossRef CAS.
- Y. Cheng, X. Ke, Y. Chen, X. Huang, Z. Shi and Z. Guo, Nano Energy, 2019, 63, 103854 CrossRef CAS.
- W. Zhang, H. L. Zhuang, L. Fan, L. Gao and Y. Lu, Sci. Adv., 2018, 4, eaar4410 CrossRef PubMed.
- T.-S. Wang, X. Liu, X. Zhao, P. He, C.-W. Nan and L.-Z. Fan, Adv. Funct. Mater., 2020, 30, 2000786 CrossRef CAS.
- X. Chen, X.-R. Chen, T.-Z. Hou, B.-Q. Li, X.-B. Cheng, R. Zhang and Q. Zhang, Sci. Adv., 2019, 5, eaau7728 CrossRef CAS PubMed.
- G. Zheng, C. Wang, A. Pei, J. Lopez, F. Shi, Z. Chen, A. D. Sendek, H.-W. Lee, Z. Lu, H. Schneider, M. M. Safont-Sempere, S. Chu, Z. Bao and Y. Cui, ACS Energy Lett., 2016, 1, 1247–1255 CrossRef CAS.
- Q. Li, H. Pan, W. Li, Y. Wang, J. Wang, J. Zheng, X. Yu, H. Li and L. Chen, ACS Energy Lett., 2018, 3, 2259–2266 CrossRef CAS.
- Y. Jeon, S. Kang, S. H. Joo, M. Cho, S. O. Park, N. Liu, S. K. Kwak, H.-W. Lee and H.-K. Song, Energy Storage Mater., 2020, 31, 505–514 CrossRef.
- Q. Yang, M. Cui, J. Hu, F. Chu, Y. Zheng, J. Liu and C. Li, ACS Nano, 2020, 14, 1866–1878 CrossRef CAS PubMed.
- L. Fan, H. L. Zhuang, W. Zhang, Y. Fu, Z. Liao and Y. Lu, Adv. Energy Mater., 2018, 8, 1703360 CrossRef.
- Y. Liu, D. Lin, Y. Li, G. Chen, A. Pei, O. Nix, Y. Li and Y. Cui, Nat. Commun., 2018, 9, 3656 CrossRef PubMed.
- Z. Jiang, L. Jin, Z. Zeng and J. Xie, Chem. Commun., 2020, 56, 9898–9900 RSC.
- F. Shen, K. Wang, Y. Yin, L. Shi, D. Zeng and X. Han, J. Mater. Chem. A, 2020, 8, 6183–6189 RSC.
- J. Lang, J. Song, L. Qi, Y. Luo, X. Luo and H. Wu, ACS Appl. Mater. Interfaces, 2017, 9, 10360–10365 CrossRef CAS PubMed.
- Y. Zhu, F. Meng, N. Sun, L. Huai, M. Wang, F. Ren, Z. Li, Z. Peng, F. Huang, H. Gu and D. Wang, ACS Appl. Mater. Interfaces, 2019, 11, 42261–42270 CrossRef CAS PubMed.
- M. Lei, J.-G. Wang, L. Ren, D. Nan, C. Shen, K. Xie and X. Liu, ACS Appl. Mater. Interfaces, 2019, 11, 30992–30998 CrossRef CAS PubMed.
- Z. Lu, Q. Liang, B. Wang, Y. Tao, Y. Zhao, W. Lv, D. Liu, C. Zhang, Z. Weng, J. Liang, H. Li and Q.-H. Yang, Adv. Energy Mater., 2019, 9, 1803186 CrossRef.
- J. Zhu, J. Chen, Y. Luo, S. Sun, L. Qin, H. Xu, P. Zhang, W. Zhang, W. Tian and Z. Sun, Energy Storage Mater., 2019, 23, 539–546 CrossRef.
- K. Lin, X. Qin, M. Liu, X. Xu, G. Liang, J. Wu, F. Kang, G. Chen and B. Li, Adv. Funct. Mater., 2019, 29, 1903229 CrossRef CAS.
- P. Zhai, T. Wang, H. Jiang, J. Wan, Y. Wei, L. Wang, W. Liu, Q. Chen, W. Yang, Y. Cui and Y. Gong, Adv. Mater., 2021, 33, 2006247 CrossRef CAS PubMed.
- Y. Xu, T. Li, L. Wang and Y. Kang, Adv. Mater., 2019, 31, 1901662 CrossRef PubMed.
- Q. Dong, B. Hong, H. Fan, H. Jiang, K. Zhang and Y. Lai, ACS Appl. Mater. Interfaces, 2020, 12, 627–636 CrossRef CAS PubMed.
- H. Liu, X. Yue, X. Xing, Q. Yan, J. Huang, V. Petrova, H. Zhou and P. Liu, Energy Storage Mater., 2019, 16, 505–511 CrossRef.
- C. Li, S. Liu, C. Shi, G. Liang, Z. Lu, R. Fu and D. Wu, Nat. Commun., 2019, 10, 1363 CrossRef PubMed.
- Y. Guo, P. Niu, Y. Liu, Y. Ouyang, D. Li, T. Zhai, H. Li and Y. Cui, Adv. Mater., 2019, 31, 1900342 CrossRef PubMed.
- X. Li, L. Yuan, D. Liu, M. Liao, J. Chen, K. Yuan, J. Xiang, Z. Li and Y. Huang, Adv. Funct. Mater., 2021, 31, 2100537 CrossRef.
- S. Santhanagopalan and Z. Zhang, in Encyclopedia of Sustainability Science and Technology, ed. R. A. Meyers, Springer New York, New York, NY, 2012, pp. 8715–8757, DOI:10.1007/978-1-4419-0851-3_505.
- W. Luo, L. Zhou, K. Fu, Z. Yang, J. Wan, M. Manno, Y. Yao, H. Zhu, B. Yang and L. Hu, Nano Lett., 2015, 15, 6149–6154 CrossRef CAS PubMed.
- Y. Liu, X. Qin, D. Zhou, H. Xia, S. Zhang, G. Chen, F. Kang and B. Li, Energy Storage Mater., 2020, 24, 229–236 CrossRef.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2021 |
