Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Expanded porphyrins: functional photoacoustic imaging agents that operate in the NIR-II region†
Jingqin
Chen‡
a,
Adam C.
Sedgwick‡
 b,
Sajal
Sen‡
b,
Sajal
Sen‡
 b,
Yaguang
Ren
a,
Qinchao
Sun
a,
Calvin
Chau
b,
Yaguang
Ren
a,
Qinchao
Sun
a,
Calvin
Chau
 b,
Jonathan F.
Arambula
b,
Jonathan F.
Arambula
 b,
Tridib
Sarma
b,
Tridib
Sarma
 *b,
Liang
Song
*b,
Liang
Song
 a,
Jonathan L.
Sessler
a,
Jonathan L.
Sessler
 *b and
Chengbo
Liu
*b and
Chengbo
Liu
 *a
*a
aResearch Center for Biomedical Optics and Molecular Imaging, Shenzhen Institute of Advanced Technology, CAS Key Laboratory of Health Informatics, Chinese Academy of Sciences, Shenzhen 518055, China. E-mail: cb.liu@siat.ac.cn
bDepartment of Chemistry, University of Texas at Austin, 105 East 24th Street A5300, Austin, Texas 78712-1224, USA. E-mail: kumartridib@gmail.com; sessler@cm.utexas.edu
First published on 23rd June 2021
Abstract
Photoacoustic imaging (PAI) relies on the use of contrast agents with high molar absorptivity in the NIR-I/NIR-II region. Expanded porphyrins, synthetic analogues of natural tetrapyrrolic pigments (e.g. heme and chlorophyll), constitute as potentially attractive platforms due to their NIR-II absorptivity and their ability to respond to stimuli. Here, we evaluate two expanded porphyrins, naphthorosarin (1) and octaphyrin (4), as stimuli responsive PA contrast agents for functional PAI. Both undergo proton-coupled electron transfer to produce species that absorb well in the NIR-II region. Octaphyrin (4) was successfully encapsulated into 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-poly(ethylene glycol) (DSPE-PEG2000) nanoparticles to afford OctaNPs. In combination with PAI, OctaNPs allowed changes in the acidic environment of the stomach to be visualized and cancerous versus healthy tissues to be discriminated.
1. Introduction
Photoacoustic imaging (PAI) is an imaging modality that combines the high contrast and sensitivity of optical imaging with the tissue penetration depths of ultrasound (US).1 This “light in – sound out” approach relies on the light absorption of either an endogenous or exogenous chromophore, typically excited by a pulsed laser, to produce heat and generate acoustic pressure waves (thermoelastic expansion).2,3 These acoustic signals are then detected using ultrasound transducers and reconstructed to form the photoacoustic (PA) image. One of the most important preclinical and clinical applications of PAI is mapping blood oxygenation within tissue through the excitation of the endogenous chromophore, hemoglobin, (Hb).1,4–6 Heme is an iron porphyrin (cf.Fig. 1 for generic structure) that displays distinct absorption differences between its unbound and oxygen-bound forms, allowing imaging of both oxygenated and deoxygenated tissues.4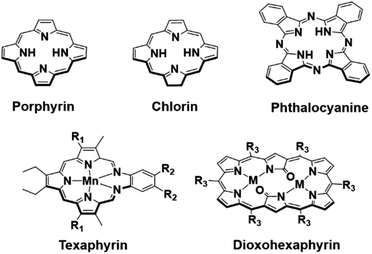 | ||
| Fig. 1 Top row: chemical structures of the porphyrin, chlorin and phthalocyanine scaffolds. Bottom row: chemical structures of manganese texaphyrin (R1 = –CH2CH2CH2OH, R2 = –(CH2CH2O)3CH3) and 26 π-electron-conjugated bis-metal (Zn and Cu) dioxohexaphyrin complexes (M = Cu or Zn, R3 = C6F5) reported by Furuta and collaborators.22 | ||
In recent years, a number of classic tetrapyrrolic pigments, including porphyrins, chlorins and phthalocyanines, have been demonstrated as PA-responsive systems for the detection of biologically important species (i.e. low pH, enzymes, and hydrogen peroxide (H2O2)).7–10 However, most tetrapyrrolic PA systems are limited to the NIR region (NIR-I: 700–950 nm).7,11 Absorption greater than 1000 nm, termed the second near-infrared region (NIR-II: 1000–1350 nm), allows for deeper tissue penetration and reduced light scattering.12–16 Current NIR-II PA contrast agents comprise of organic semiconducting conjugated polymers; very few are solely organic-based.17,18 In recent years, numerous groups have focused on developing new porphyrinoid systems.19,20 Many of these, particularly the so-called expanded porphyrins, show promise as NIR-I and NIR-II absorbers.20–23 Early on, our group reported a penta-aza Schiff base porphyrinoid known as texaphyrin that absorbs >700 nm and forms stable 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes with a large array of metal cations. Recently, a Mn(II) texaphyrin was shown to be effective as a PA contrast agent using NIR-I light (Fig. 1).24 To broaden the application of PAI, it is useful to develop systems that are capable of absorbing in the NIR-II region. In 2020, Furuta and collaborators reported 26 π-electron-conjugated bis-metal (Zn and Cu) dioxohexaphyrin complexes as potential NIR-II PA contrast agents (Fig. 1).25 Since then, Furuta and collaborators have reported several other expanded porphyrin platforms that produce a PA signal in the NIR-II and NIR-III region (NIR-III: 1550–1870 nm).15,26–28 However, no biological studies were carried out nor were these compounds shown responsive to biological stimuli. There thus remains an unmet need for functional PAI agents that function in the NIR-II region. Here, we report the use of two expanded porphyrins, naphthorosarin (1) and octaphyrin (4), as proton-coupled electron transfer (PCET)-activated PA imaging agents for functional PAI.29,30 As detailed below, octaphyrin (4) proved effective for the imaging of stomach pH and allowed the discrimination between cancerous tissue (HepG2) and healthy tissue in BALB/c nude mice model.
1 complexes with a large array of metal cations. Recently, a Mn(II) texaphyrin was shown to be effective as a PA contrast agent using NIR-I light (Fig. 1).24 To broaden the application of PAI, it is useful to develop systems that are capable of absorbing in the NIR-II region. In 2020, Furuta and collaborators reported 26 π-electron-conjugated bis-metal (Zn and Cu) dioxohexaphyrin complexes as potential NIR-II PA contrast agents (Fig. 1).25 Since then, Furuta and collaborators have reported several other expanded porphyrin platforms that produce a PA signal in the NIR-II and NIR-III region (NIR-III: 1550–1870 nm).15,26–28 However, no biological studies were carried out nor were these compounds shown responsive to biological stimuli. There thus remains an unmet need for functional PAI agents that function in the NIR-II region. Here, we report the use of two expanded porphyrins, naphthorosarin (1) and octaphyrin (4), as proton-coupled electron transfer (PCET)-activated PA imaging agents for functional PAI.29,30 As detailed below, octaphyrin (4) proved effective for the imaging of stomach pH and allowed the discrimination between cancerous tissue (HepG2) and healthy tissue in BALB/c nude mice model.
2. Results and discussion
The two porphyrinoids considered in this study are naphthorosarin 1 and octaphyrin 4 (Schemes 1 and 2).29,30 Unique to both systems is their propensity to undergo PCET, this process is currently being used to describe any reaction that involves both a proton transfer (PT) and electron transfer (ET).31–33 In the case of naphthorosarin 1 and octaphyrin 4, it is believed that both undergo stepwise proton transfer electron transfer (PTET) or concerted proton electron transfer (CPET) processes depending on the chosen acid and reductant.29,30 The addition of a proton source and an oxidizable anion, e.g., HCl, to 24 π-electron antiaromatic 1 results in a quasi-stable non-aromatic triprotonated monoradical dication 25 π-electron species (2) being readily formed.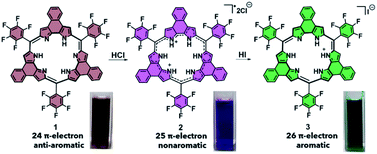 | ||
| Scheme 1 PCET-mediated reduction of naphthorosarin 1 using HCl and HI and photographs of each species in dichloromethane (DCM). | ||
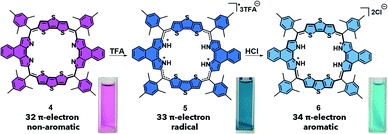 | ||
| Scheme 2 PCET-mediated reduction of octaphyrin 4 using TFA and HCl and the photographs of each species in DCM. | ||
This radical is characterized by an absorption wavelength at ∼900 nm. In the presence of stronger reductants, such as HI, conversion to the two-electron reduced 26 π-electron aromatic species (3), absorbing at ca. 1000 nm, occurs (Scheme 1). In contrast, the 32 π-electron non-aromatic octaphyrin 4 undergoes a concerted two-electron reduction in the presence of a proton source such as HCl. This yields the corresponding 34 π-electron aromatic form (6) with an absorption maximum at ca. 1200 nm (Scheme 2). In the presence of less redox active acid trifluoroacetic acid (TFA), a 33 π-electron radical 5 is formed, which can then be further reduced with the addition of reductants.30 Therefore, a critical feature to PCET is that it requires both a reductant and a proton source. To our knowledge this has not previously been explored in the context of photoacoustic imaging.
The importance of pH in human health is well appreciated. For instance, numerous studies have shown that changes in the upper gastrointestinal tract pH are implicated in pathological processes.34,35 A high pH has been observed in the stomach of gastric ulcer (pH = 3.4) and gastric cancer patients (pH = 6.6) compared to healthy subjects (pH = 2.9), whereas, a low pH has been seen in the stomach of esophageal ulcer (pH = 1.9) and duodenal ulcer patients (pH = 2.1).35 Thus, the ability to probe non-invasively the dynamics of stomach pH could prove useful in monitoring stomach health. Cancer, broadly speaking represents another area where monitoring pH via PAI could be beneficial in the context of diagnostic and therapeutic applications.8,36 It is well-known that the extracellular tumor microenvironment is slightly acidic, pH = 6.4–7.0.37 Most cancer environments are also highly reducing.37,38 We thus postulated that stomach and cancer imaging would provide useful testbeds for evaluating 1 and 4 as possible PCET-based PAI agents.
The pH responsiveness of 1 and 4 were evaluated in THF solution through the careful addition of 1 M aqueous HCl. Both 1 and 4 demonstrated a strong PA response when subject to 900 and 1200 nm pulsed laser excitation (15 mJ), respectively, as the apparent39 pH decreased (see Fig. S1–S3†). This was considered indicative of the formation of the non-aromatic triprotonated monoradical dication 25 π-electron species (2) and 34 π-electron aromatic (6), respectively. No 26 π-electron naphthorosarin species (3) was observed, presumably reflecting its less positive reduction potential ((1: +0.42 V and +0.04 V); (4: +0.56 V and +0.25 V)).25,26
A nanoparticle (NP) encapsulation strategy was employed to allow studies of 1 and 4 in biological milieus. Both 1 and 4 could be encapsulated using 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-poly(ethylene glycol) (DSPE-PEG2000) (compound: DSPE-PEG2000 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 (w/w)). Unfortunately, the absorption and PA features of NaphthNPs (from 1) proved unresponsive to pH changes (see ESI – Fig. S4 and S5†). In the case of the OctaNPs (from 4) the mean size (106 nm) and zeta potential (−55 mV) were considered suitable for biological testing (Fig. 2D). The ratio between 4 and DSPE-PEG2000 was found to be critical for the PCET-mediated conversion of 4 to 6 (see ESI – Fig. S6†). No changes in acidic media were observed for OctaNPs at ratios 1
750 (w/w)). Unfortunately, the absorption and PA features of NaphthNPs (from 1) proved unresponsive to pH changes (see ESI – Fig. S4 and S5†). In the case of the OctaNPs (from 4) the mean size (106 nm) and zeta potential (−55 mV) were considered suitable for biological testing (Fig. 2D). The ratio between 4 and DSPE-PEG2000 was found to be critical for the PCET-mediated conversion of 4 to 6 (see ESI – Fig. S6†). No changes in acidic media were observed for OctaNPs at ratios 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and 1
10 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (4:DSPE-PEG2000). On the other hand a ratio of 1
100 (4:DSPE-PEG2000). On the other hand a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
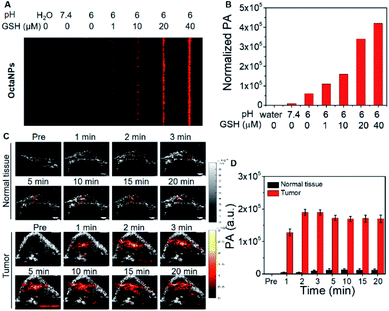
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 (4:DSPE-PEG2000) was found to provide an optimal formulation for the formation of 6 in acidic media. A similarly responsive formulation was not found in the case of the NaphthNPs. While studies are ongoing in an effort to determine an optimal nanoparticle strategy for 1, this failure is ascribed to the less positive reduction potential of this particular expanded porphyrin. No changes in color were observed for OctaNPs in aqueous solution over the course of 7 days. In contrast, 4 in THF was observed to gradually became darker in color, which we ascribe to partial degradation (Fig. 2G). On this basis, we suggest that the use of this nanoparticle strategy improves the solution phase stability of 4. Moreover, OctaNPs displayed a pH dependent increase in the long-wavelength absorption band expected for a PCET process wherein the components of the medium (e.g., chloride anions) serve as the reductant (Fig. 2E and F). The importance of reductants to this PCET-response was further confirmed using the less-redox active acid trifluoroacetic acid (TFA) to adjust the pH of the aqueous test solutions. At pH 5, a minimal increase in absorption at ∼1200 nm was observed for OctaNPs. In contrast, co-treatment with both TFA and the biological reductant glutathione (GSH, 40 μM) led to a significant increase in this absorption feature (Fig. S7†). We thus focused our attention on OctaNPs for the remainder of this study.
750 (4:DSPE-PEG2000) was found to provide an optimal formulation for the formation of 6 in acidic media. A similarly responsive formulation was not found in the case of the NaphthNPs. While studies are ongoing in an effort to determine an optimal nanoparticle strategy for 1, this failure is ascribed to the less positive reduction potential of this particular expanded porphyrin. No changes in color were observed for OctaNPs in aqueous solution over the course of 7 days. In contrast, 4 in THF was observed to gradually became darker in color, which we ascribe to partial degradation (Fig. 2G). On this basis, we suggest that the use of this nanoparticle strategy improves the solution phase stability of 4. Moreover, OctaNPs displayed a pH dependent increase in the long-wavelength absorption band expected for a PCET process wherein the components of the medium (e.g., chloride anions) serve as the reductant (Fig. 2E and F). The importance of reductants to this PCET-response was further confirmed using the less-redox active acid trifluoroacetic acid (TFA) to adjust the pH of the aqueous test solutions. At pH 5, a minimal increase in absorption at ∼1200 nm was observed for OctaNPs. In contrast, co-treatment with both TFA and the biological reductant glutathione (GSH, 40 μM) led to a significant increase in this absorption feature (Fig. S7†). We thus focused our attention on OctaNPs for the remainder of this study.
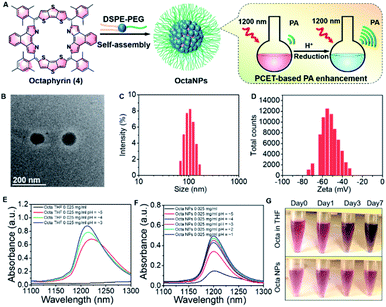 | ||
| Fig. 2 (A) Preparation of nanoparticles (OctaNPs) from octaphyrin 4. (B) TEM image of OctaNPs. (C and D) Size and zeta potential distribu-tion of OctaNPs. (E and F) Absorbance spectra of 4 in THF at varying apparent39 pH values and OctaNPs in aqueous solution at varying pH values. pH was adjusted through the careful addition of 1 M HCl. (G) Stability studies of octaphyrin 4 in THF and OctaNPs in neutral aqueous media (pH = 7.40) that involved monitoring over the course of 7 days at room temperature. | ||
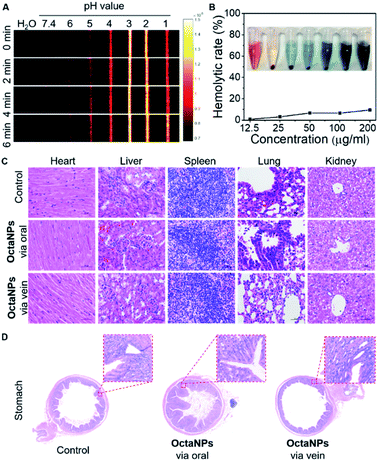
Next, the ability of OctaNPs to detect changes in pH was evaluated using PAI. The maximum PA intensity was seen at pH = 3 with the response being essentially immediate on the laboratory time scale. In contrast, a gradual turn-on response was observed over the 5–6 pH range that peaked after 6 min (Fig. 3). The lower PA signals observed at pH 1 and pH 2 are ascribed to partial degradation of 4. No adverse toxicities were seen for OctaNPs, as inferred from histological analyses and evaluation of hemolysis rates (Fig. 3B and C).
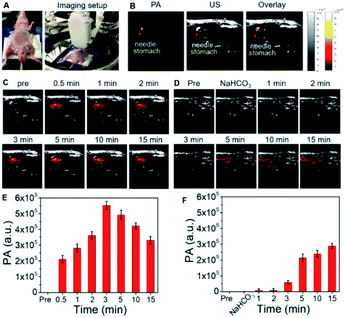
OctaNPs were then tested as PAI agents in vivo. As shown in Fig. 4, direct injection (intragastric) of OctaNPs (200 μL, 0.1 mg mL−1) into the stomach resulted in an immediate PA signal being observed (Fig. 4C and E). This is due to the presence of HCl within the stomach and the stomach having a known pH range of 1.5–3.5. These environment are thus conducive to PCET reduction of 4.34 To test whether OctaNPs could be used not just to visualize the stomach, but to monitor directly dynamic changes in the stomach pH, mice were pretreated through the injection of a saturated NaHCO3 solution (50 μL) to raise the pH (Fig. 4D and F). This was followed by the direct injection of OctaNPs (200 μL, 0.1 mg mL−1, neutral aqueous) into the stomach. No PA signal was initially observed under 1200 nm excitation (15 mJ), a finding ascribed to the bicarbonate-induced neutralization of the gastric acid. As time progressed the PA signal at 1200 nm increased reflecting the presumed secretion of gastric acid by the mice.
Efforts were then made to explore OctaNPs as PCET-triggered PA agents for cancer imaging. As noted above, the extracellular tumor microenvironment is slightly acidic (pH 6.4–7.0).37 As shown in Fig. 3A, OctaNPs produce a minimal PA “turn on” response at 1200 nm at pH 6. However, we considered it likely that the reducing nature of tumor tissues38,40 would facilitate PCET and afford a significant PA response at 1200 nm. To test this hypothesis, increasing concentrations of the endogenous reducing agent GSH were added to a pH 6 solution containing OctaNPs. This resulted in a marked increase in the PA signal intensity (Fig. 5A and B). In contrast, no changes in absorptivity were observed when GSH was added to a neutral pH 7.4 solution of OctaNPs (see ESI – Fig. S8†). OctaNPs were then injected into the right flank of BALB/c nude mice as well as into a HepG2 mice tumor model. A statistically significant PA signal at 1200 nm was observed for the tumor region into which the OctaNPs (200 μL, 0.1 mg mL−1) had been injected. In contrast, minimal PA signals were observed for healthy tissues. Overall, 2 minutes post-injection, a 42 (±7)-fold difference in PA signal intensity between cancerous and healthy tissue was observed (Fig. 5C and D).
3. Conclusion
The results presented here lend support to the suggestion that expanded porphyrins could prove useful as PAI agents. Systems such as 4, that are known to undergo PCET, provide for environmental responsive PA imaging, while allowing access to the NIR-II spectral region. Encapsulation of 4 in DSPE-PEG afforded biocompatible nanoparticles (OctaNPs) that were shown to be stable for over 7 days. OctaNPs enabled the visualization of acidic environments such as in the stomach, along with changes in the stomach pH. OctaNPs also proved effective at discriminating between cancerous and healthy tissues with a 42-fold difference in the PA intensity being observed. Overall, this work serves to highlight the role that expanded porphyrins may have to play in functional photoacoustic imaging in the NIR-II region.Ethical statement
All animal experiments and procedures were performed in compliance with the requirements of the National Act on the Use of Experimental Animals (People's Republic of China) and were approved by the Experimental Animal Ethical Committee of Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences. The accreditation number is SIAT-IRB-180205-YYS-CJQ-A0413.Data availability
All relevant data supporting the key findings of this study are available within the article and its ESI or from the corresponding author upon request.Author contributions
J. Chen and A. C. Sedgwick conceived the project and designed the experiments. J. Chen performed the nanoparticles preparation, characterization and biological experiment. A. C. Sedgwick and S. Sen synthesized compounds. A. C. Sedgwick and J. Chen wrote the paper and prepared the manuscript. Y. Ren conducted partial PA imaging experiments. J. L. Sessler and C. Liu contributed writing, funding acquisition and supervision. All authors provided input on the manuscript.Conflicts of interest
J. F. A. and J. L. S. currently serve, respectively, as Vice President and a non-executive Director for OncoTEX, Inc. that provided partial support for this work.Acknowledgements
The initial work in Austin was supported by the National Institutes of Health (grant CA68682 to J. L. S.), with subsequent funding being provided by the Robert A. Welch Foundation and OncoTEX, Inc. The work in Shenzhen was supported by grants from the National Natural Science Foundation of China 81801758 and 92059108; Chinese Academy of Sciences (2019352, YJKYYQ20190078, GJJSTD20210003); National Key R&D Program of China (2020YFA0908800); CAS Key Laboratory of Health Informatics (2011DP173015); Guangdong Provincial Key Laboratory of Biomedical Optical Imaging (2020B121201010); Science and Technology Innovation Fund of Shenzhen (JCYJ20190806150001764). All animal experiments and procedures were performed in compliance with the Animal Study Committee of Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences.Notes and references
- I. Steinberg, D. M. Huland, O. Vermesh, H. E. Frostig, W. S. Tummers and S. S. Gambhir, Photoacoustics, 2019, 14, 77–98 CrossRef PubMed.
- J. Weber, P. C. Beard and S. E. Bohndiek, Nat. Methods, 2016, 13, 639–650 CrossRef CAS PubMed.
- H. J. Knox and J. Chan, Acc. Chem. Res., 2018, 51, 2897–2905 CrossRef CAS PubMed.
- M. C. Li, Y. Q. Tang and J. J. Yao, Photoacoustics, 2018, 10, 65–73 CrossRef PubMed.
- J. Lv, S. Li, J. D. Zhang, F. Duan, Z. Y. Wu, R. H. Chen, M. M. Chen, S. S. Huang, H. S. Ma and L. M. Nie, Theranostics, 2020, 10, 816–828 CrossRef CAS PubMed.
- J. Yang, G. Zhang, Q. Q. Li, C. D. Liao, L. Huang, T. F. Ke, H. B. Jiang and D. Han, Quantitative Imaging in Medicine and Surgery, 2019, 9, 160–170 CrossRef PubMed.
- J. M. Merkes, L. M. Zhu, S. B. Bahukhandi, M. Rueping, F. Kiessling and S. Banala, Int. J. Mol. Sci., 2020, 21, 3082 CrossRef CAS PubMed.
- W. J. Liu, D. Zhang, L. L. Li, Z. Y. Qiao, J. C. Zhang, Y. X. Zhao, G. B. Qi, D. Wan, J. Pan and H. Wang, ACS Appl. Mater. Interfaces, 2016, 8, 22875–22883 CrossRef CAS PubMed.
- L. L. Li, Q. Zeng, W. J. Liu, X. F. Hu, Y. S. Li, J. Pan, D. Wan and H. Wang, ACS Appl. Mater. Interfaces, 2016, 8, 17936–17943 CrossRef CAS PubMed.
- Z. S. Yang, Y. H. Yao, A. C. Sedgwick, C. C. Li, Y. Xia, Y. Wang, L. Kang, H. M. Su, B. W. Wang, S. Gao, J. L. Sessler and J. L. Zhang, Chem. Sci., 2020, 11, 8204–8213 RSC.
- J. M. Merkes, M. Rueping, F. Kiessling and S. Banala, ACS Sens., 2019, 4, 2001–2008 CrossRef CAS PubMed.
- J. Cao, B. L. Zhu, K. F. Zheng, S. G. He, L. Meng, J. B. Song and H. H. Yang, Front. Bioeng. Biotechnol., 2020, 7, 487 CrossRef PubMed.
- K. Y. Duan and B. Liu, Adv. Mater., 2018, 30, e1802394 CrossRef PubMed.
- M. Y. Lucero, A. K. East, C. J. Reinhardt, A. C. Sedgwick, S. Su, M. C. Lee and J. Chan, J. Am. Chem. Soc., 2021, 143, 7196–7202 CrossRef CAS PubMed.
- E. Hemmer, A. Benayas, F. Legare and F. Vetrone, Nanoscale Horiz., 2016, 1, 168–184 RSC.
- M. Kamimura, T. Matsumoto, S. Suyari, M. Umezawa and K. Soga, J. Mater. Chem. B, 2017, 5, 1917–1925 RSC.
- E. D. Cosco, A. L. Spearman, S. Ramakrishnan, J. G. P. Lingg, M. Saccomano, M. Pengshung, B. A. Arus, K. C. Y. Wong, S. Glasl, V. Ntziachristos, M. Warmer, R. R. McLaughlin, O. T. Bruns and E. M. Sletten, Nat. Chem., 2020, 12, 1123–1130 CrossRef PubMed.
- D. Jung, S. Park, C. Lee and H. Kim, Polymers, 2019, 11, 1693 CrossRef CAS PubMed.
- J. L. Sessler, Z. Gross and H. Furuta, Chem. Rev., 2017, 117, 2201–2202 CrossRef CAS PubMed.
- W. Y. Cha, T. Kim, A. Ghosh, Z. Zhang, X. S. Ke, R. Ali, V. M. Lynch, J. Jung, W. Kim, S. Lee, S. Fukuzumi, J. S. Park, J. L. Sessler, T. K. Chandrashekar and D. Kim, Nat. Chem., 2017, 9, 1243–1248 CrossRef CAS PubMed.
- C. M. Davis, K. Ohkubo, I. T. Ho, Z. Zhang, M. Ishida, Y. Y. Fang, V. M. Lynch, K. M. Kadish, J. L. Sessler and S. Fukuzumi, Chem. Commun., 2015, 51, 6757–6760 RSC.
- V. G. Anand, S. K. Pushpan, S. Venkatraman, A. Dey, T. K. Chandrashekar, B. S. Joshi, R. Roy, W. J. Teng and K. R. Senge, J. Am. Chem. Soc., 2001, 123, 8620–8621 CrossRef CAS PubMed.
- J. Ajay, S. Shirisha, M. Ishida, K. Ito, S. Mori, H. Furuta and S. Gokulnath, Chem.–Eur. J., 2019, 25, 2859–2867 CrossRef CAS PubMed.
- Y. G. Ren, A. C. Sedgwick, J. Q. Chen, G. Thiabaud, C. V. Chau, J. S. An, J. F. Arambula, X. P. He, J. S. Kim, J. L. Sessler and C. B. Liu, J. Am. Chem. Soc., 2020, 142, 16156–16160 CrossRef CAS PubMed.
- K. Shimomura, H. Kai, Y. Nakamura, Y. Hong, S. Mori, K. Miki, K. Ohe, Y. Notsuka, Y. Yamaoka, M. Ishida, D. Kim and H. Furuta, J. Am. Chem. Soc., 2020, 142, 4429–4437 CrossRef CAS PubMed.
- Y. Wang, H. Kai, M. Ishida, S. Gokulnath, S. Mori, T. Murayama, A. Muranaka, M. Uchiyama, Y. Yasutake, S. Fukatsu, Y. Notsuka, Y. Yamaoka, M. Hanafusa, M. Yoshizawa, G. Kim, D. Kim and H. Furuta, J. Am. Chem. Soc., 2020, 142, 6807–6813 CrossRef CAS PubMed.
- Y. Wang, K. Ogasahara, D. Tomihama, R. Mysliborski, M. Ishida, Y. Hong, Y. Notsuka, Y. Yamaoka, T. Murayama, A. Muranaka, M. Uchiyama, S. Mori, Y. Yasutake, S. Fukatsu, D. Kim and H. Furuta, Angew. Chem., Int. Ed., 2020, 59, 16161–16166 CrossRef CAS PubMed.
- L. A. Sordillo, Y. Pu, S. Pratavieira, Y. Budansky and R. R. Alfano, J. Biomed. Opt., 2014, 19, 056004 CrossRef PubMed.
- M. Ishida, S. J. Kim, C. Preihs, K. Ohkubo, J. M. Lim, B. S. Lee, J. S. Park, V. M. Lynch, V. V. Roznyatovskiy, T. Sarma, P. K. Panda, C. H. Lee, S. Fukuzumi, D. Kim and J. L. Sessler, Nat. Chem., 2013, 5, 15–20 CrossRef CAS PubMed.
- T. Sarma, G. Kim, S. Sen, W. Y. Cha, Z. Duan, M. D. Moore, V. M. Lynch, Z. Zhang, D. Kim and J. L. Sessler, J. Am. Chem. Soc., 2018, 140, 12111–12119 CrossRef CAS PubMed.
- D. R. Weinberg, C. J. Gagliardi, J. F. Hull, C. F. Murphy, C. A. Kent, B. C. Westlake, A. Paul, D. H. Ess, D. G. McCafferty and T. J. Meyer, Chem. Rev., 2012, 112, 4016–4093 CrossRef CAS PubMed.
- J. W. Darcy, B. Koronkiewicz, G. A. Parada and J. M. Mayer, Acc. Chem. Res., 2018, 51, 2391–2399 CrossRef CAS PubMed.
- J. R. Mayfield, E. N. Grotemeyer and T. A. Jackson, Chem. Commun., 2020, 56, 9238–9255 RSC.
- P. Holzer, Am. J. Physiol., 2007, 292, G699–G705 CAS.
- P. J. Lu, P. I. Hsu, C. H. Chen, M. Hsiao, W. C. Chang, H. H. Tseng, K. H. Lin, S. K. Chuah and H. C. Chen, World J. Gastroenterol., 2010, 16, 5496–5501 CrossRef PubMed.
- S. Siriwibool, N. Kaekratoke, K. Chansaenpak, K. Siwawannapong, P. Panajapo, K. Sagarik, P. Noisa, R.-Y. Lai and A. Kamkaew, Sci. Rep., 2020, 10, 1283 CrossRef CAS PubMed.
- E. Boedtkjer and S. F. Pedersen, in Annual Review of Physiology, ed. M. T. Nelson and K. Walsh, 2020, vol. 82, pp. 103–126 Search PubMed.
- V. V. Khramtsov and R. J. Gillies, Antioxid. Redox Signaling, 2014, 21, 723–729 CrossRef CAS PubMed.
- Z. Yang, D. Zacherl and A. J. Russell, J. Am. Chem. Soc., 1993, 115, 12251–12257 CrossRef CAS.
- P. Kuppusamy, H. Q. Li, G. Ilangovan, A. J. Cardounel, J. L. Zweier, K. Yamada, M. C. Krishna and J. B. Mitchell, Cancer Res., 2002, 62, 307–312 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: General synthetic experimental details. See DOI: 10.1039/d1sc01591e |
| ‡ Equal contributions. |
| This journal is © The Royal Society of Chemistry 2021 |