Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Long-lived triplet charge-separated state in naphthalenediimide based donor–acceptor systems†
Alexander
Aster
a,
Christopher
Rumble
 a,
Anna-Bea
Bornhof
b,
Hsin-Hua
Huang
a,
Anna-Bea
Bornhof
b,
Hsin-Hua
Huang
 c,
Naomi
Sakai
b,
Tomáš
Šolomek
c,
Naomi
Sakai
b,
Tomáš
Šolomek
 c,
Stefan
Matile
c,
Stefan
Matile
 b and
Eric
Vauthey
b and
Eric
Vauthey
 *a
*a
aDepartment of Physical Chemistry, University of Geneva, CH-1211 Geneva, Switzerland. E-mail: eric.vauthey@unige.ch
bDepartment of Organic Chemistry, University of Geneva, CH-1211 Geneva, Switzerland
cDepartment of Chemistry, University of Basel, St. Johanns-Ring 19, Basel 4056, Switzerland
First published on 26th February 2021
Abstract
1,4,5,8-Naphthalenediimides (NDIs) are widely used motifs to design multichromophoric architectures due to their ease of functionalisation, their high oxidative power and the stability of their radical anion. The NDI building block can be incorporated in supramolecular systems by either core or imide functionalization. We report on the charge-transfer dynamics of a series of electron donor–acceptor dyads consisting of a NDI chromophore with one or two donors linked at the axial, imide position. Photo-population of the core-centred π–π* state is followed by ultrafast electron transfer from the electron donor to the NDI. Due to a solvent dependent singlet–triplet equilibrium inherent to the NDI core, both singlet and triplet charge-separated states are populated. We demonstrate that long-lived charge separation in the triplet state can be achieved by controlling the mutual orientation of the donor–acceptor sub-units. By extending this study to a supramolecular NDI-based cage, we also show that the triplet charge-separation yield can be increased by tuning the environment.
1 Introduction
Due to their high stability, good solubility and ease of functionalization, 1,4,5,8-naphthalenediimides (NDIs) are among the most used molecular building blocks in supramolecular chemistry.1–7 Their high oxidative power and the good stability of their radical anions make NDIs popular electron accepting units in multichromophoric systems.8–26 In principle, they can also act as chromophores due to their strong π–π* transition around 375 nm. In contrast to their bigger brother, the perylenediimide,27 unsubstituted NDIs have a low fluorescence quantum yield and a very short S1 state lifetime arising from a fast intersystem crossing (ISC).28–31 In agreement with the El Sayed's rule,32 the ISC is facilitated by a low lying Tn state of n–π* character, which enables a change of the spin angular momentum thanks to a concomitant change of orbital angular momentum.28,33 We have recently shown that, directly after photexcitation to the S1(π–π*) state, an equilibrium between this state and the almost isoenergetic Tn(n–π*) state is established before the population is funnelled down to the T1(π–π*) state via internal conversion (IC).34The photophysics of the electron deficient NDI can change drastically upon chemical functionalization with electron donors (Ds), depending on the site of modification. Core substitution makes the whole colour palette accessible due to the presence of a charge transfer (CT) state below the NDI-centred π–π* state.35–37 Since this CT state is located below the Tn(n–π*) state, ISC is no longer allowed and the fluorescence lifetime increases from the ps to the ns regime.35–37
On the other hand, imide functionalization of NDIs with Ds has only a negligible impact on the absorption spectrum and reduction potential due to a weaker coupling to the NDI core.2,4,28,33 The high transition energy in the near UV, paired with the high oxidative power already in the ground state, renders the S1(π–π*) state of NDI a very strong electron acceptor (A) for photoinduced electron transfer (PET) reactions. As a consequence, PET is energetically feasible with relatively weak electron donors such as benzene, which is often used to axially link the NDI core to other chromophores or catalysts in multichromophoric systems.21,38–40
Even though imide functionalized NDIs are extensively used in model systems for artificial photosynthesis,8,10,11,14,18–21 and other supramolecular chemistry,41–46 their complex excited-state dynamics and the mechanistic cascade following PET are not fully understood.33 The main reason for this is that, in most cases, the NDIs are intended to serve as electron accepting units only. However, as they can absorb the high-energy side of the solar spectrum, they can also act as chromophores and, thus, their PET dynamics need to be better understood.
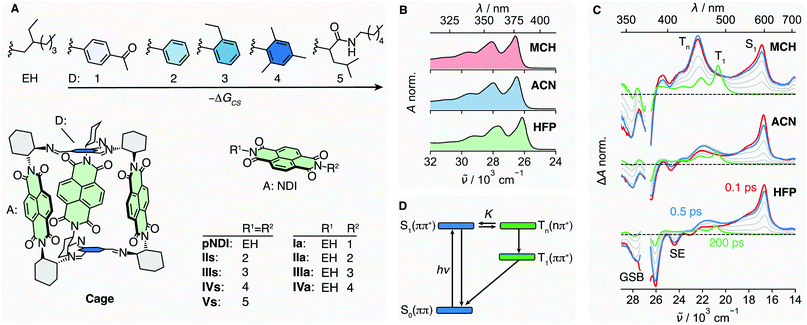
Herein we report on our investigation of axially-linked NDIs, aiming at a comprehensive picture of their excited-state dynamics. For this, we prepared a series of donor–acceptor (DA) dyads in which a NDI core is symmetrically (s) or asymmetrically (a) linked to 5 different Ds (Fig. 1A). The oxidation potential of the phenyl-based Ds decreases from 1 to 4, allowing –ΔGCS, the driving force for charge separation (CS), to be varied from <0.35 to about 0.85 eV,47 while keeping the coupling to the NDI core nearly constant. This series is compared with the reference NDI (pNDI), which is decorated with two innocent ethyl-hexyl (EH) linkers as well as to Vs with two non-aromatic and weakly coupled Ds 5. By applying transient-absorption spectroscopy in the UV-Vis and mid-infrared (IR), we will show that the ISC to the triplet manifold depends on the solvent and allows for the population of a long-lived triplet charge-separated state (3CSS) from the triplet Tn(n–π*) state. Based on these results, we reinterpret the excited-state dynamics of a recently studied multichromophoric Cage,45 and show how the yield of the long-lived 3CSS can be enhanced by supramolecular chemistry.
2 Results and discussion
2.1 Solvent-dependent intersystem crossing of pNDI
We address the solvent dependence of the ISC dynamics of pNDI, which first needs to be clarified for understanding the more complex dyads. As expected for the π–π* transition of a symmetric chromophore, the stationary electronic absorption spectra of pNDI are nearly identical in solvents of different polarity and only a small red shift is observed in the strong hydrogen-bonding hexafluoro-2-propanol (HFP) (Fig. 1B and S1A†), pointing to stronger H-bond interactions in the excited than in the ground state.48 However, the transient absorption (TA) spectra, which report on excited-state processes occurring after excitation to the S1(π–π*) state at 375 nm, differ significantly (Fig. 1C). In acetonitrile (ACN), the excited-state absorption (ESA) band around 600 nm, the ground-state bleach (GSB), and the stimulated emission (SE) observed in the early spectra are indicative of the photo-population of the bright S1(π–π*) state. The latter decays partially within the first few hundreds of fs, whereas a band around 450 nm rises, which can be attributed to a Tn(n–π*) state.34 Both S1 and Tn spectral features then decay simultaneously, and the T1(π–π*) band appears with its characteristic vibronic progression.49 These dynamics can be explained by a S1-Tn equilibrium established faster than the IC from the Tn to the T1 state (Fig. 1D).34 The Tn to S1 band-intensity ratio at equilibrium, i.e. after about 2 ps, decreases from 1.4 to 0.7 and 0.4 upon going from methylcyclohexane (MCH) to ACN and HFP, respectively (Fig. S9–S11†). Given that these ultrafast transitions occur on a similar timescale as those of vibrational/solvent relaxation, a true equilibrium constant cannot be defined.50 Therefore, K is only used here on a qualitative basis.A similar dependence on the solvent polarity and hydrogen-bonding affinity was found with naphthalenemonoimides and attributed to the different electronic character of the two states involved in the equilibrium.28,51 Solvent polarity as well as hydrogen-bonding interactions destabilise the Tn(n–π*) state relative to the S1(π–π*) state, allowing for a control of the equilibrium constant, K.52–54 Therefore, the equilibrium is shifted toward the S1(π–π*) state when going from MCH to ACN and HFP and K decreases accordingly. This trend is corroborated by measurements in the intermediate polarity solvents dibutylether (NBE) and tetrahydrofuran (THF) (Fig. S1B†). The solvent dependence of K additionally manifests as a slowing down of the concurrent decay of the S1 and Tn bands from MCH to ACN and HFP (Fig. S9–S11†). This can be explained using the pre-equilibrium approximation, 1/τobs ∝ KkTIC, where τobs is the decay time of the S1 and Tn bands after equilibration and kTIC is the rate constant of the Tn → T1 IC.
Even though the instantaneous population of the Tn(n–π*) state is relatively low in polar and protic solvents, the faster Tn → T1 IC compared to the S1 → S0 IC funnels a substantial fraction of the excited-state population toward the T1 state (Fig. 1D). Triplet quantum yields for the population of the T1 state of 0.95, 0.7 and 0.5 in MCH, ACN and HFP, respectively, were estimated from the partial decrease of the GSB (ESI Section S4†). However, these values should be considered with some caution as the T1 state also absorbs in the GSB region (Fig. S2–S4†). Despite this, these triplet yields smaller than 1, together with the presence of a hot-ground state feature on the red side of the GSB in the TA spectra (Fig. S9–S10†), suggest the existence of an ultrafast deactivation channel to the ground state competing with ISC. Such an ultrafast non-radiative decay of a π–π* state was recently reported for a disulfide core-substituted NDI.55
In addition to the effect of solvent polarity on K, it is also important to note that the high oxidative power of the excited pNDI, Ered(pNDIS1) = −3.7 V vs. SCE,36 enables PET from usually inert solvents such as toluene, dimethylsulfoxide or dimethylformamide. If these solvents are used in organic synthesis, excitation to the S1(π–π*) state should therefore be prevented to avoid the formation of radicals, possibly leading to unwanted side products.
2.2 Charge separation in I–IV
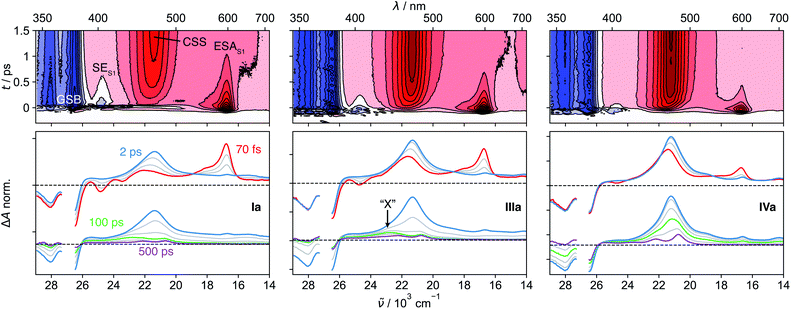
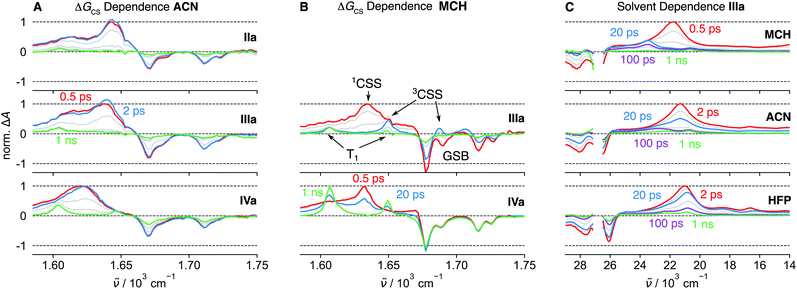
The π–π* absorption band of the NDI core remains almost unchanged upon covalent addition of Ds at the imide position. However, the bandwidth increases significantly with –ΔGCS and the number of Ds (Fig. S5†). This band broadening was recently shown to originate from the ultrafast decay of the NDI-centred π–π* excited state upon CS.47The spectral features observed with pNDI, namely the ESA and the SE bands of the S1 state, are also present in the TA spectra measured directly after excitation of the dyads at 375 nm (Fig. 2). These bands, however, decay on the sub-ps timescale together with the rise of the NDI radical anion band at 470 nm,56 indicative of the population of the 1CSS upon charge separation. Although the 1CSS is the lowest singlet excited state of the dyads, S1 will be still used further on to designate the NDI-centred singlet π–π* state. The ultrafast CS dynamics of these dyads, measured by UV-Vis TA in ACN, were already discussed earlier.47 In brief, CS takes place with an average time constant ranging from 30 to 200 fs, depending on ΔGCS and the number of Ds. CS accelerates with increasing driving force and is approximately twice as fast in the symmetric than the asymmetric dyads. Finally, the CS dynamics in MCH, measured with IVs only, was found to be the same as in ACN. For dyads I–III, an additional TA band “X” appears upon CS. This will be discussed in detail in Section 2.4.
TA measurements in the mid-infrared (IR) region reveal that the charge distribution in the 1CSS also depends on ΔGCS. We monitored the symmetric and antisymmetric stretches of the imide carbonyls, which are sensitive to subtle changes of the charge distribution57–59 that are otherwise not visible in the broad transient electronic absorption bands. As illustrated in Fig. 3A, the splitting of the two IR bands of the 1CSS located between 1600 and 1650 cm−1 decreases with increasing driving force and vanishes for IVa. This effect is mainly due to the frequency downshift of the most intense band that can be attributed to an increased electronic density on the carbonyl groups. Consequently, these results suggest an increase of the extent of charge separation in the 1CSS with the driving force.60
2.3 Charge recombination to the T1 state in IV
The UV-Vis TA spectra measured with IVa and IVs show that the decay of the 1CSS band is accompanied by the appearance of the T1 band, pointing to triplet charge recombination (CR) of the 1CSS, i.e. charge recombination to the triplet manifold (Fig. 2). This usually spin-forbidden process is enabled here due to the spin–orbit charge-transfer intersystem crossing (SOCT-ISC) mechanism. If the frontier molecular orbitals, localised mainly on the D and A subunits, have nearly perpendicular orientation, the change of orbital angular momentum upon CR allows for a change of spin angular momentum.61–65 The branching ratio of the singlet CR, i.e. recombination to S0, and triplet CR can be estimated from the IR-TA spectra, where the ESA bands do not overlap with the GSB (Fig. 3A and B). In the apolar MCH, the GSB remains constant during the decay of the 1CSS, indicating quantitative triplet CR via SOCT-ISC, which, combined with the sub-ps CS, gives a quantitative triplet yield. On the other hand, the GSB in ACN decreases to about 50% of its initial value during the decay of the 1CSS, pointing to equally probable triplet and singlet CR. In addition, CR is four times as fast in MCH (∼30 ps) compared to ACN (∼120 ps), suggesting that the stabilisation of the 1CSS by polar solvation has a significant impact on the SOCT-ISC dynamics (Fig. S22–S27†). This agrees with CR in the Marcus normal region,66 which is expected to slow down with decreasing 1CSS–T1 gap.Contrary to CS, the CR dynamics are identical for the symmetric and asymmetric dyads in both ACN and MCH (Fig. S6†). This indicates that CS leads to symmetry breaking even in low-polarity solvents, rendering the probability for CR identical in the symmetric and asymmetric species.
2.4 Origin of the band “X” in I–III
In contrast to the UV-Vis TA spectra measured with IVa and IVs, where only the S1, 1CSS and T1 bands are visible, those measured with the dyads I–III exhibit an additional band, “X”, around 430 nm that is clearly visible after about 100 ps (Fig. 2). This feature was already observed in a previous study, but its origin could not be established.33 The knowledge acquired above with the solvent dependence of the S1–Tn equilibrium of pNDI can be used to interpret this band “X”. It is the most intense in MCH, is weaker in ACN, and is not visible in HFP (Fig. 3C). The S1(π–π*)–Tn(n–π*) equilibrium constant K shows a similar solvent dependence, i.e., decreases from MCH to ACN and HFP. Until now, we have only considered the population of 1CSS from the S1(π–π*) state. However, given the fast S1–Tn equilibrium, the population of the triplet charge-separated state (3CSS) from the Tn(n–π*) state, which is thermodynamically favourable, should also be considered. Based on the similar solvent dependence of band “X” and equilibrium constant K, we propose that this band originates from the 3CSS, itself populated upon CS from the Tn state, in competition with IC to the T1 state.According to this hypothesis, the 3CSS yield should depend on K, the dynamics of equilibration and the CS rate constants from the S1 and Tn states. In MCH, the equilibrium is shifted towards the Tn state, leading to a significant population of the 3CSS. By contrast, the equilibrium in HFP is strongly shifted towards the S1 state, whose population undergo CS to the 1CSS.
The population of the Tn state prior to CS can be clearly distinguished by comparing the UV-Vis TA spectra of the dyads with those of pNDI (Fig. S8†). For I, II and III, a shoulder that resembles the Tn band observed with pNDI is apparent at early times on the high-energy side of the anion band. This feature is absent with IV, the only dyad for which no 3CSS band is observed even in the apolar MCH. The absence of 3CSS with IV can be accounted for by the faster CS from the S1 state, which outcompetes the equilibration and a significant population of the Tn state.
Due to a smaller signal-to-noise ratio in the mid-IR region, spectral features that can be attributed to the 3CSS are only visible in MCH, as illustrated by the spectrum at 20 ps in Fig. 3B. The carbonyl stretching bands of the 3CSS are shifted to higher frequencies compared to T1, with one band overlapping with the 1690 cm−1 GSB and the other with the T1 band at 1650 cm−1. The 1CSS of I–III can either recombine to the S0 or the T1 state via the SOCT-ISC mechanism, as already discussed for IV. The recombination of the 3CSS to the S0 takes place via the same mechanism. It is visible in the mid-IR (Fig. 3B), where the 3CSS features and the GSB decay in parallel, while the amplitude of the most intense T1 band remains constant. Rather counter-intuitively, the opening of the triplet CS channel to the 3CSS thereby results in a smaller T1 yield. This is evident by comparing how the GSB decreases for IIIa but not for IVa (Fig. 3B).
2.5 Aromatic vs. non-aromatic electron donor
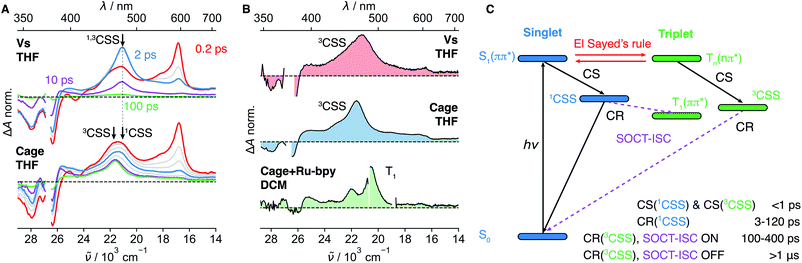
To strengthen our assignment of band “X” to the 3CSS, we turned to dyad Vs in THF, where the non-aromatic D does not ensure a perpendicular arrangement of the frontier molecular orbitals. Similarly to the dyads with phenyl donors, the 1CSS band appears on the sub-ps timescale upon excitation of Vs to the S1 state (Fig. 4A and S28†). The 1CSS band does however decay significantly faster (∼5 ps) than with the other dyads and no T1 band is visible. As expected, SOCT-ISC is not operative and the 1CSS can only undergo singlet CR to the S0 state. Since CR occurs on the sub-10 ps timescale, hot ground-state features can be observed on the low-energy side of the GSB.67 As ground-state recovery upon CR is faster than vibrational relaxation, the instantaneous population of the vibrationally hot S0 state is large enough to be visible in the transient absorption spectrum. The faster CR compared to I–IV can be attributed to the non-aromatic character of the donor.68,69Fig. 4A, B, S28 and S29† reveal that, after the sub-10 ps decay of the 1CSS band, a small residual signal, which does not decay on the ns timescale and is spectrally similar to the 1CSS, persists. The relative amplitude of this signal is slightly higher in the medium-polar THF than the highly-polar ACN and, thus, follows the same solvent dependence as the S1–Tn equilibrium constant K (Fig. S28–S29†). Triplet sensitization measurements reveal that this residual signal is spectrally different from the T1 absorption band of Vs (Fig. 4B). By analogy to the results obtained with I–III, the residual signal is attributed to the 3CSS. However, since the SOCT-ISC channel is not operative and CR of the 3CSS to the ground state is spin forbidden, the lifetime of the 3CSS is extended to a few μs at least (Fig. S31†). The slow decay of the 3CSS is biphasic, which could be due to the occurrence of a bimolecular process such as triplet–triplet annihilation or an interaction with some impurity. This decay was however not investigated in further detail, being outside the main scope of this study. The 3CSS yield could be expected to be larger in the non-polar MCH than in THF. Unfortunately, as Vs is not soluble in MCH, this could not be verified.
TA spectra recorded on the μs timescale do not exhibit the T1 band (Fig. S31†). This can be explained by a 3CSS located below the T1 state or by a small 3CSS–T1 gap, that results in a very slow CR to T1.
2.6 Increasing the 3CSS yield by supramolecular chemistry
Now, we want to illustrate with an example the importance of the above results for achieving long-lived charge separation. PET in a covalent organic cage (Cage, Fig. 1A) consisting of three NDI cores linked via an aromatic imine bridge was described recently.45 Upon excitation of a NDI sub-unit, an electron is transferred from the aromatic imine bridge to the NDI core. After CR, a residual spectral feature persisting on the ns timescale was attributed to the T1 state. This interpretation was based on the spectral and kinetic similarity with the T1 state of pNDI (Fig. 4B). Without the above results, we would have reached at the same conclusion.We remeasured this cage upon 375 nm excitation in THF and could reproduce the TA spectra reported in ref. 45 (Fig. 4B). We furthermore carried out triplet sensitization measurements, as for Vs, which reveal that the long-lived spectral feature and the T1 spectrum of the cage are clearly different (Fig. 4B and S7†). Based on our results obtained with the dyads, we can re-interpret this long-lived spectral feature and assign it to the 3CSS. At early times, the CSS band is broader than that measured with Vs and consists of two partially overlapping bands. The low-frequency band matches that measured with Vs, decays within the first 10 ps and can thus be attributed to the 1CSS. The high-frequency band persists and is consequently assigned to the 3CSS, which, as for Vs, cannot recombine to the S0 state since this process is spin forbidden. The 3CSS yield is considerably higher for Cage than for Vs even though the same solvent was used. In view of the solvent dependence of the 3CSS yield found above, this suggests that the supramolecular architecture of Cage leads to a decrease of the local polarity and, thus, to a shift of the S1–Tn equilibrium towards the Tn state.45 Indeed, only three solvent molecules can occupy the restricted inner space of the Cage. As dipolar solvation is a long range interaction, the solvent field around the NDI cores is expected to strongly differ from that in bulk solvent. Additionally, the coupling between the three NDI sub-units can be expected to have a different effect on the two states in equilibrium. However, electrochemical measurements pointed to a weak interchromophoric coupling in Cage.45
Additional spectral features are visible on the μs timescale (Fig. S31†). They could arise from an intermolecular reaction of the 3CSS, and could be related to the sample degradation observed after prolonged laser irradiation. Although the decay of the 3CSS was not investigated in detail, these results indicate that it is slow enough to enable intermolecular reactions. This observation is highly relevant to photoredox catalysis with organic supramolecular cages that has emerged only very recently.70,71 The presence of the radical anion and the radical cation in the 3CSS state suggests that both reduction and oxidation reactions, respectively, could be achieved directly or photochemically, using a near IR light source around 800 nm that matches the lowest-energy absorption band of the NDI radical anion.72,73
2.7 Spectral differences between 1CSS and 3CSS
The positions of the 1CSS and 3CSS bands are nearly identical for Vs, slightly shifted for Cage and about 2000 cm−1 apart for the phenyl donor series. In principle, the electronic absorption spectra of 1CSS and 3CSS should be identical in the limit of zero electronic coupling between the D and A moieties. However, if the coupling is not negligible, the exchange interaction lifts the degeneracy of these two states and renders them spectrally distinct. An inert alkyl group separates the D and NDI sub-units in Vs and leads to a weak coupling. This is experimentally observable as a slow CS and a negligible spectral difference between 1CSS and 3CSS. By contrast, the coupling is larger for the phenyl Ds, which are directly linked to the imide nitrogen. This results in a stabilisation of the 3CSS relative to the 1CSS and in a blue shift of the 3CSS band. The exchange interaction, which is responsible for the 1CSS–3CSS energy splitting, also affects the electronic distribution that can be probed by vibrational spectroscopy (Fig. 3B).3 Conclusion
A comprehensive picture of the photophysics of axially modified NDIs could be drawn by systematically varying the N-substituted donors as well as the environment. Two channels, which efficiently connect the singlet to the triplet manifolds, were revealed and lead to rich photophysics (Fig. 4C). The efficiency of the first channel is due to the conservation of the overall angular momentum upon S1(π–π*) to Tn(n–π*) transition, in agreement with the El-Sayed's rule, and to the energetic proximity of these two states, which is intrinsic to the NDI core. We have herein shown that an equilibrium is reached on the sub-ps timescale and the associated constant K strongly depends on the polarity and hydrogen-donating ability of the solvent. The relative population of the triplet state and K are the highest in apolar solvents, decrease in polar solvent and are the lowest in strong hydrogen-bond donating solvents. Upon linking a D at the axial imide position, PET occurs from both equilibrated singlet and triplet states. The relative 1CSS and 3CSS populations depends on K and on the respective CS rate constants. However, these processes are too fast and the spectra of the different species involved overlap too much to allow determining a rate constant for each of these CS pathways. The second singlet-triplet channel becomes active after population of the two CSS. It depends on the spatial arrangement of the NDI core and the Ds. With phenyl-based donors, the frontier molecular orbitals on the NDI core and D are arranged at near 90°, enabling CR of the 1CSS and 3CSS to the T1 and S0 states, respectively. Without such near-perpendicular dihedral angle between the NDI core and D planes, the SOCT-ISC channel is shut down and the lifetime of the 3CSS is significantly increased. The yield of the long-lived 3CSS can be controlled by the polarity of the solvent and is the highest in non-polar solvents. In addition, we have shown that the 3CSS yield can be enhanced by incorporating the NDI unit in a multichromophoric structure. These remarkable excited-state properties of NDIs could be further exploited by integrating these chromophores in molecular architectures developed for applications based on long-lived photoinduced charge separation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the Fonds National Suisse de la Recherche Scientifique (Project Nr. 200020-184607, 200020-175486, PZ00P2-174175) as well as the University of Geneva for financial support. All experimental data can be downloaded from https://doi.org/10.26037/yareta:ijrkot57u5bkblnplfua2uec5m.References
- X. Zhan, A. Facchetti, S. Barlow, T. J. Marks, M. A. Ratner, M. R. Wasielewski and S. R. Marder, Adv. Mater., 2011, 23, 268–284 CrossRef CAS.
- S.-L. Suraru and F. Würthner, Angew. Chem., Int. Ed., 2014, 53, 7428–7448 CrossRef CAS.
- L. Rocard, A. Berezin, F. De Leo and D. Bonifazi, Angew. Chem., Int. Ed., 2015, 54, 15739–15743 CrossRef CAS.
- M. Al Kobaisi, S. V. Bhosale, K. Latham, A. M. Raynor and S. V. Bhosale, Chem. Rev., 2016, 116, 11685–11796 CrossRef.
- S. Kumar, J. Shukla, Y. Kumar and P. Mukhopadhyay, Org. Chem. Front., 2018, 5, 2254–2276 RSC.
- S. Maniam, H. F. Higginbotham, T. D. M. Bell and S. J. Langford, Chem.–Eur. J., 2019, 25, 7044–7057 CrossRef CAS.
- S. K. Keshri, A. Takai, T. Ishizuka, T. Kojima and M. Takeuchi, Angew. Chem., Int. Ed., 2020, 59, 5254–5258 CrossRef CAS.
- M. P. Debreczeny, W. A. Svec and M. R. Wasielewski, Science, 1996, 274, 584–587 CrossRef CAS.
- S. E. Miller, A. S. Lukas, E. Marsh, P. Bushard and M. R. Wasielewski, J. Am. Chem. Soc., 2000, 122, 7802–7810 CrossRef CAS.
- M. S. Cubberley and B. L. Iverson, J. Am. Chem. Soc., 2001, 123, 7560–7563 CrossRef CAS.
- M. Borgström, N. Shaikh, O. Johansson, M. F. Anderlund, S. Styring, B. Åkermark, A. Magnuson and L. Hammarström, J. Am. Chem. Soc., 2005, 127, 17504–17515 CrossRef.
- K. P. Ghiggino, J. A. Hutchison, S. J. Langford, M. J. Latter, M. A. P. Lee, P. R. Lowenstern, C. Scholes, M. Takezaki and B. E. Wilman, Adv. Funct. Mater., 2007, 17, 805–813 CrossRef CAS.
- I. V. Sazanovich, M. A. H. Alamiry, J. Best, R. D. Bennett, O. V. Bouganov, E. S. Davies, V. P. Grivin, A. J. H. M. Meijer, V. F. Plyusnin, K. L. Ronayne, A. H. Shelton, S. A. Tikhomirov, M. Towrie and J. A. Weinstein, Inorg. Chem., 2008, 47, 10432–10445 CrossRef CAS.
- F. Chaignon, F. Buchet, E. Blart, M. Falkenström, L. Hammarström and F. Odobel, New J. Chem., 2009, 33, 408–416 RSC.
- C. Liao, J. E. Yarnell, K. D. Glusac and K. S. Schanze, J. Phys. Chem. B, 2010, 114, 14763–14771 CrossRef CAS.
- Y. Pellegrin and F. Odobel, Coord. Chem. Rev., 2011, 255, 2578–2593 CrossRef CAS.
- J. M. Keller, K. D. Glusac, E. O. Danilov, S. McIlroy, P. Sreearuothai, A. R. Cook, H. Jiang, J. R. Miller and K. S. Schanze, J. Am. Chem. Soc., 2011, 133, 11289–11298 CrossRef CAS.
- E. Iengo, G. Dan Pantoş, J. K. M. Sanders, M. Orlandi, C. Chiorboli, S. Fracasso and F. Scandola, Chem. Sci., 2011, 2, 676–685 RSC.
- V. L. Gunderson, A. L. Smeigh, C. H. Kim, D. T. Co and M. R. Wasielewski, J. Am. Chem. Soc., 2012, 134, 4363–4372 CrossRef CAS.
- S. F. Völker, A. Schmiedel, M. Holzapfel, C. Böhm and C. Lambert, Phys. Chem. Chem. Phys., 2013, 15, 19831–19844 RSC.
- M. Natali, M. Ravaglia, F. Scandola, J. Boixel, Y. Pellegrin, E. Blart and F. Odobel, J. Phys. Chem. C, 2013, 117, 19334–19345 CrossRef CAS.
- O. Yushchenko, R. V. Hangarge, S. Mosquera-Vazquez, S. V. Bhosale and E. Vauthey, J. Phys. Chem. B, 2015, 119, 7308–7320 CrossRef CAS.
- Z. Ji and Y. Wu, J. Phys. Chem. C, 2013, 117, 18315–18324 CrossRef CAS.
- M. Skaisgirski, X. Guo and O. S. Wenger, Inorg. Chem., 2017, 56, 2432–2439 CrossRef CAS.
- J. Jiang, A. Alsam, S. Wang, S. M. Aly, Z. Pan, O. F. Mohammed and K. S. Schanze, J. Phys. Chem. A, 2017, 121, 4891–4901 CrossRef CAS.
- A. L. Jones, M. K. Gish, C. J. Zeman, J. M. Papanikolas and K. S. Schanze, J. Phys. Chem. A, 2017, 121, 9579–9588 CrossRef CAS.
- F. Würthner, Chem. Commun., 2004, 1564–1579 RSC.
- V. Wintgens, P. Valat, J. Kossanyi, L. Biczok, A. Demeter and T. Bérces, J. Chem. Soc., Faraday Trans., 1994, 90, 411–421 RSC.
- T. C. Barros, S. Brochsztain, V. G. Toscano, P. Berci Filho and M. J. Politi, J. Photochem. Photobiol., A, 1997, 111, 97–104 CrossRef CAS.
- S. Alp, S. Erten, C. Karapire, B. Koz, A. O. Doroshenko and S. Icli, J. Photochem. Photobiol., A, 2000, 135, 103–110 CrossRef CAS.
- M. S. Refat, I. Grabchev, J. M. Chovelon and G. Ivanova, Spectrochim. Acta, Part A, 2006, 64, 435–441 CrossRef CAS.
- S. K. Lower and M. A. El-Sayed, Chem. Rev., 1966, 66, 199–241 CrossRef CAS.
- P. Ganesan, J. Baggerman, H. Zhang, E. J. R. Sudhölter and H. Zuilhof, J. Phys. Chem. A, 2007, 111, 6151–6156 CrossRef CAS.
- O. Yushchenko, G. Licari, S. Mosquera-Vazquez, N. Sakai, S. Matile and E. Vauthey, J. Phys. Chem. Lett., 2015, 6, 2096–2100 CrossRef CAS.
- F. Würthner, A. Shahadat, C. Thalacker and T. Debaerdemaeker, Chem.–Eur. J., 2002, 8, 4742–4750 CrossRef.
- N. Sakai, J. Mareda, E. Vauthey and S. Matile, Chem. Commun., 2010, 46, 4225–4237 RSC.
- S. V. Bhosale, S. V. Bhosale and S. K. Bhargava, Org. Biomol. Chem., 2012, 10, 6455–6468 RSC.
- W.-S. Han, B. S. Veldkamp, S. M. Dyar, S. W. Eaton and M. R. Wasielewski, Tetrahedron, 2017, 73, 4925–4935 CrossRef CAS.
- B. Küçüköz, B. Adinarayana, A. Osuka and B. Albinsson, Phys. Chem. Chem. Phys., 2019, 21, 16477–16485 RSC.
- A. L. Jones, J. Jiang and K. S. Schanze, J. Am. Chem. Soc., 2020, 142, 12658–12668 CrossRef CAS.
- M. B. Avinash and T. Govindaraju, Adv. Funct. Mater., 2011, 21, 3875–3882 CrossRef CAS.
- A.-J. Avestro, D. M. Gardner, N. A. Vermeulen, E. A. Wilson, S. T. Schneebeli, A. C. Whalley, M. E. Belowich, R. Carmieli, M. R. Wasielewski and J. F. Stoddart, Angew. Chem., Int. Ed., 2014, 53, 4442–4449 CrossRef CAS.
- A. Das and S. Ghosh, Chem. Commun., 2016, 52, 6860–6872 RSC.
- K. S. Lee and J. R. Parquette, Chem. Commun., 2015, 51, 15653–15656 RSC.
- T. Šolomek, N. E. Powers-Riggs, Y.-L. Wu, R. M. Young, M. D. Krzyaniak, N. E. Horwitz and M. R. Wasielewski, J. Am. Chem. Soc., 2017, 139, 3348–3351 CrossRef.
- Q.-H. Ling, J.-L. Zhu, Y. Qin and L. Xu, Mater. Chem. Front., 2020, 4, 3176–3189 RSC.
- A. Aster, A.-B. Bornhof, N. Sakai, S. Matile and E. Vauthey, J. Phys. Chem. Lett., 2021, 12, 1052–1057 CrossRef CAS.
- P. Suppan, J. Photochem. Photobiol., A, 1990, 50, 293–330 CrossRef CAS.
- S. Green and M. A. Fox, J. Phys. Chem., 1995, 99, 14752–14757 CrossRef CAS.
- A. Rosspeintner, B. Lang and E. Vauthey, Annu. Rev. Phys. Chem., 2013, 64, 247–271 CrossRef CAS.
- P. Nemes, A. Demeter, L. Biczok, V. Wintgens, P. Valat and J. Kossanyi, J. Photochem. Photobiol., A, 1998, 113, 225–231 CrossRef CAS.
- G. Porter and P. Suppan, Pure Appl. Chem., 1964, 9, 499–505 CAS.
- P. Suppan and N. Ghoneim, Solvatochromism, The Royal Society of Chemistry, Cambridge, 1997 Search PubMed.
- C. Reichardt and T. Welton, Solvents and Solvent Effects in Organic Chemistry, John Wiley & Sons, 2011 Search PubMed.
- I. Shybeka, A. Aster, Y. Cheng, N. Sakai, A. Frontera, E. Vauthey and S. Matile, Chem.–Eur. J., 2020, 26, 14059–14063 CrossRef CAS.
- D. Gosztola, M. P. Niemczik, W. Svec, A. S. Lukas and M. R. Wasielewski, J. Phys. Chem. A, 2000, 104, 6545–6551 CrossRef CAS.
- E. T. J. Nibbering, H. Fidder and E. Pines, Annu. Rev. Phys. Chem., 2005, 56, 337–367 CrossRef CAS.
- S. G. Boxer, J. Phys. Chem. B, 2009, 113, 2972–2983 CrossRef CAS.
- M. Koch, R. Letrun and E. Vauthey, J. Am. Chem. Soc., 2014, 136, 4066–4074 CrossRef CAS.
- I. R. Gould and S. Farid, J. Phys. Chem. B, 2007, 111, 6782–6787 CrossRef CAS.
- T. Okada, I. Karaki, E. Matsuzawa, N. Mataga, Y. Sakata and S. Misumi, J. Phys. Chem., 1981, 85, 3957–3960 CrossRef CAS.
- M. R. Wasielewski, D. G. Johnson, W. A. Svec, K. M. Kersey and D. W. Minsek, J. Am. Chem. Soc., 1988, 110, 7219–7221 CrossRef CAS.
- Z. E. X. Dance, S. M. Mickley, T. M. Wilson, A. B. Ricks, A. M. Scott, M. A. Ratner and M. R. Wasielewski, J. Phys. Chem. A, 2008, 112, 4194–4201 CrossRef CAS.
- R. Letrun, B. Lang, O. Yushchenko, R. Wilcken, D. Svechkarev, D. Kolodieznyi, E. Riedle and E. Vauthey, Phys. Chem. Chem. Phys., 2018, 20, 30219–30230 RSC.
- D. Sasikumar, A. T. John, J. Sunny and M. Hariharan, Chem. Soc. Rev., 2020, 49, 6122–6140 RSC.
- R. A. Marcus and N. Sutin, Biochim. Biophys. Acta, 1985, 811, 265–322 CrossRef CAS.
- S. A. Kovalenko, R. Schanz, H. Hennig and N. Ernsting, J. Chem. Phys., 2001, 115, 3256–3274 CrossRef CAS.
- D. Burget, P. Jacques, E. Vauthey, P. Suppan and E. Haselbach, J. Chem. Soc., Faraday Trans., 1994, 90, 2481–2487 RSC.
- E. Vauthey, J. Phys. Chem. A, 2000, 104, 1804–1810 CrossRef CAS.
- X. Feng, P. Liao, J. Jiang, J. Shi, Z. Ke and J. Zhang, ChemPhotoChem, 2019, 3, 1014–1019 CrossRef CAS.
- C. Liu, K. Liu, C. Wang, H. Liu, H. Wang, H. Su, X. Li, B. Chen and J. Jiang, Nat. Commun., 2020, 11, 1047 CrossRef CAS.
- N. T. L. Porte, J. F. Martinez, S. Hedström, B. Rudshteyn, B. T. Phelan, C. M. Mauck, R. M. Young, V. S. Batista and M. R. Wasielewski, Chem. Sci., 2017, 8, 3821–3831 RSC.
- J. F. Martinez, N. T. La Porte and M. R. Wasielewski, J. Phys. Chem. C, 2018, 122, 2608–2617 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, solvent dependent photophysics of pNDI, estimation of the triplet quantum yields of pNDI, stationary electronic absorption spectra of I–V, electron-transfer dynamics of I–V in ACN, triplet sensitisation experiments, additional transient absorption spectra, global analysis of the transient absorption data. See DOI: 10.1039/d1sc00285f |
| This journal is © The Royal Society of Chemistry 2021 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 follows the same solvent dependence as the S1(π–π*)–T
000 cm−1 follows the same solvent dependence as the S1(π–π*)–T