Open Access Article
Open Access ArticleTandem [4 + 2]/[2 + 2] cycloaddition of o-carboryne with enynes: facile construction of carborane-fused tricyclics†
Jie
Zhang
and
Zuowei
Xie
 *
*
Department of Chemistry and State Key Laboratory of Synthetic Chemistry, The Chinese University of Hong Kong, Shatin, N. T., Hong Kong, China. E-mail: zxie@cuhk.edu.hk
First published on 22nd February 2021
Abstract
o-Carboryne (1,2-dehydro-o-carborane) is a very useful synthon for the synthesis of a variety of carborane-functionalized molecules. With 1-Li-2-OTf-o-C2B10H10 as the precursor, o-carboryne undergoes an efficient [4 + 2] cycloaddition with various conjugated enynes, followed by a subsequent [2 + 2] cycloaddition at room temperature, generating a series of carborane-fused tricyclo[6.4.0.02,7]dodeca-2,12-dienes in moderate to high isolated yields. This reaction is compatible with many functional groups and has a broad substrate scope. A reactive carborane-fused 1,2-cyclohexadiene intermediate is involved, which is supported by experimental results and DFT calculations. This protocol offers a convenient strategy for the construction of complex carborane-functionalized tricyclics.
Introduction
Strained cyclic organic molecules, such as arynes, cyclic alkynes and cyclic allenes, have intrigued chemists for more than a century with their unusual structures and high chemical reactivity (Scheme 1a).1 The considerable ring strain (30–50 kilocalories per mole)2,3 that characterizes these transient intermediates imparts high reactivity in many reactions, including cycloadditions and nucleophilic trappings, often generating structurally complex products.4 Cyclic allenes are a relatively less studied class of highly strained intermediate as compared with benzynes and cyclic alkynes. The generation and reactivity of 6-membered cyclic allenes such as the parent 1,2-cyclohexadiene have attracted much research interest in recent years.5 Strained six-membered-ring allenes are also found as common intermediates in the [4 + 2] cycloaddition of conjugated enynes with unsaturated molecules, including alkenes and alkynes.6,7 Aryne, a very reactive archetypal two-electron component in [4 + 2] cycloadditions, can react with conjugated enynes to generate isoaromatic cyclic allenes, which undergo isoaromatization to afford the polycyclic aromatics (Scheme 1b).8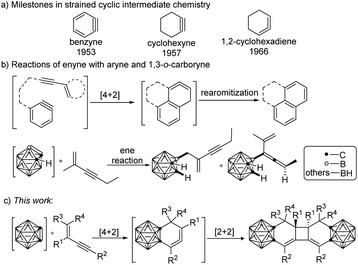 | ||
| Scheme 1 Reactions involving the enynes, aryne and carboryne: (a) strained cyclic intermediates. (b) Reactions of enyne with aryne and 1,3-o-carboryne. (c) This work. | ||
On the other hand, carborane (o-C2B10H12), a three-dimensional relative of benzene, is a molecular boron–carbon cluster.9 Owing to their unique properties, functionalized carboranes are now finding a broad range of applications encompassing organic synthesis, drug design, polymers, cancer therapy, catalysis, metal–organic frameworks, electronic devices, and more.9–11 Similarly, 1,2-dehydro-o-carborane (o-carboryne) can be viewed as a three-dimensional relative of benzyne, which has been widely employed as a useful synthon for generating a wide range of functional carboranes over the past two decades.12 It can undergo cycloadditions,13–15 the ene reaction16 and the C–H bond insertion reaction,17 with a variety of organic molecules to afford a large class of functionalized carboranes. Cycloadditions involving an o-carboryne intermediate have been developed to enable the synthesis of various carbocyclic carborane derivatives.12 In our recently reported work, the ene reaction was observed between 1,3-dehydro-o-carboryne and a conjugated enyne due probably to the polarized “C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B” multiple bond (Scheme 1b).18 Surprisingly, 1,2-dehydro-o-carboryne (o-carboryne) reacted with conjugated enynes in an unprecedented tandem [4 + 2]/[2 + 2] cycloaddition manner, generating a new class of rigid carborane-fused tricyclo[6.4.0.02,7]dodeca-2,12-dienes (Scheme 1c). Herein, we reported a general method for the construction of such carborane-fused tricyclics.
B” multiple bond (Scheme 1b).18 Surprisingly, 1,2-dehydro-o-carboryne (o-carboryne) reacted with conjugated enynes in an unprecedented tandem [4 + 2]/[2 + 2] cycloaddition manner, generating a new class of rigid carborane-fused tricyclo[6.4.0.02,7]dodeca-2,12-dienes (Scheme 1c). Herein, we reported a general method for the construction of such carborane-fused tricyclics.
Results and discussion
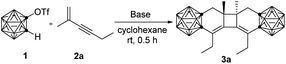
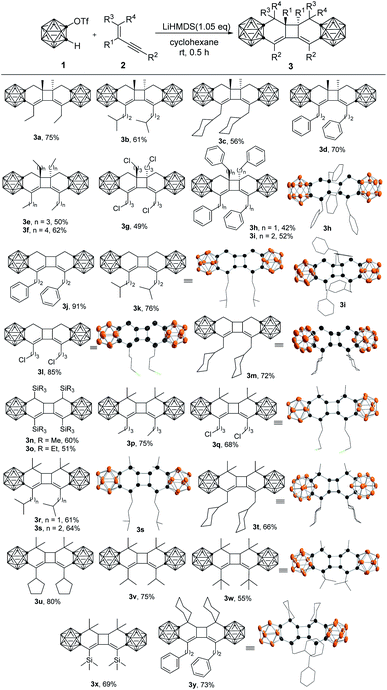
In our initial study, the reaction of o-carboryne, generated in situ by treatment of 1-OTf-o-C2B10H11 (1) with LiHMDS (lithium bis(trimethylsilyl)amide), with 1.2 equiv. of 2-methyl-1-hexen-3-yne (2a) in cyclohexane at room temperature afforded carborane-fused tricyclo[6.4.0.02,7]dodeca-2,12-diene 3a in 84% GC yield (Table 1, entry 1). Several common bases, such as organic lithium reagents and Grignard reagents, were screened (Table 1, entries 2–5), and the results suggested that LiHMDS was the best choice (Table 1, entry 1).With the optimal reaction conditions in hand, the scope of this tandem [4 + 2]/[2 + 2] cycloaddition of o-carboryne with a series of conjugated enynes was examined and the desired carborane-fused tricyclic compounds were obtained in moderate to high yields (Table 2). Various substituents, including linear, branched and cyclic alkyl groups, silyl groups, and distal chloro and phenyl groups, were compatible with this reaction. It was noted that the reaction of the ornamented conjugated enynes directly with the phenyl group led to very complicated results due to the side reactions of the styrene14i,l or phenylacetylene15b moiety with o-carboryne. It was found that the steric hindrance of the substituents may play a role in the reaction, especially for the ones (R1) attached to the internal alkenyl carbon atom (3a–3ivs.3j–3m). For instance, the reaction of 2j or 2l proceeded smoothly to generate cycloadducts 3j and 3l in >85% yields, whereas 3d, 3g and 3i were isolated in <70% yields. When the two terminal alkenyl C–H groups were replaced by methyl groups, the yield of the desired products decreased slightly (3qvs.3l, 3svs.3k, 3tvs.3m, and 3yvs.3j). Moreover, the substituents (R2) at the terminal alkynyl carbon atom showed no obvious effect on the yields of 3.
Compounds 3 were fully characterized by 1H, 13C, and 11B NMR spectroscopy as well as by HRMS. The 11B{1H} NMR spectra exhibited a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 pattern for 3a and 3d, a 6
2 pattern for 3a and 3d, a 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 pattern for 3c, 3e–j, 3n, and 3o, and a 4
14 pattern for 3c, 3e–j, 3n, and 3o, and a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 pattern for 3p–u and 3y, spanning the range δ = −2 to −14 ppm. The molecular structures of 3h–3i, 3k–3m, 3q, 3s–3t, 3w and 3y were further confirmed by single-crystal X-ray analyses.19
16 pattern for 3p–u and 3y, spanning the range δ = −2 to −14 ppm. The molecular structures of 3h–3i, 3k–3m, 3q, 3s–3t, 3w and 3y were further confirmed by single-crystal X-ray analyses.19
To gain some insight into the reaction mechanism, several control experiments were conducted (Scheme 2). Under standard reaction conditions, o-carboryne was treated with a mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) of 2j and 2m, yielding three products 3j, 3jm, and 3m in a molar ratio of around 1
1 molar ratio) of 2j and 2m, yielding three products 3j, 3jm, and 3m in a molar ratio of around 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The isolation of the crossover product 3jm suggested that this is a stepwise process and the [2 + 2] cycloaddition step is an intermolecular reaction (Scheme 2a). On the other hand, 1,4-bis(triisopropylsilyl)-1-buten-3-yne 4, providing two highly sterically demanding silyl groups at the terminals of 1-buten-3-yne, reacted smoothly with o-carboryne to afford 5 in 30% yield (Scheme 2b). In this reaction, the in situ generated carborane-fused 1,2-cyclohexadiene intermediate was trapped preferentially by less hindered o-carboryne, which can be ascribed to the fact that the resultant sterically demanding cyclic allene intermediate prevents its dimerization.
1. The isolation of the crossover product 3jm suggested that this is a stepwise process and the [2 + 2] cycloaddition step is an intermolecular reaction (Scheme 2a). On the other hand, 1,4-bis(triisopropylsilyl)-1-buten-3-yne 4, providing two highly sterically demanding silyl groups at the terminals of 1-buten-3-yne, reacted smoothly with o-carboryne to afford 5 in 30% yield (Scheme 2b). In this reaction, the in situ generated carborane-fused 1,2-cyclohexadiene intermediate was trapped preferentially by less hindered o-carboryne, which can be ascribed to the fact that the resultant sterically demanding cyclic allene intermediate prevents its dimerization.
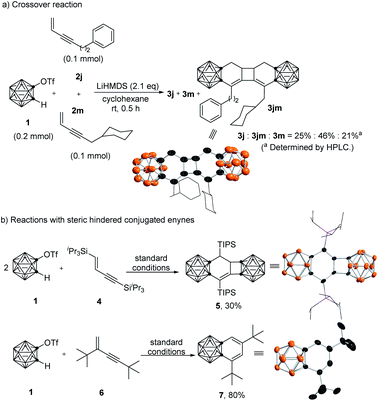 | ||
| Scheme 2 Control experiments: (a) crossover reaction. (b) Reactions with sterically hindered enynes. | ||
Furthermore, the treatment of 2,4-bis(tert-butyl)-1-buten-3-yne 6 with o-carboryne gave benzo-o-carborane 7 in 80% yield, which might result from the 1,3-H-migration of the extremely sterically demanding carborane-fused 1,3-di(tert-butyl)-1,2-cyclohexadiene intermediate (Scheme 2b). These results further supported that this reaction proceeded via a carborane-fused 1,2-cyclohexadiene intermediate. The molecular structures of 3jm, 5 and 7 were further confirmed by single-crystal X-ray analyses.19
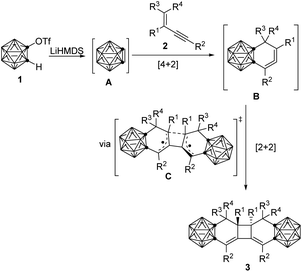
Based on the above experimental results, a plausible reaction mechanism is proposed in Scheme 3. At first, in situ generated o-carboryne A by reaction of 1 with LiHMDS, reacts with conjugated enyne 2 to form highly reactive carborane-fused 1,2-cyclohexadiene Bvia [4 + 2] cycloaddition.20 Two molecules of cyclic allenes B undergo a stepwise [2 + 2] cycloaddition via a singlet bis-allyl diradical C intermediate,21 affording the desired carborane-fused tricyclo[6.4.0.02,7]dodeca-2,12-diene 3. This proposed mechanism is supported by DFT calculations (see ESI† for details).
Conclusions
Using 1-OTf-o-C2B10H10 (1) as an o-carboryne precursor, an unprecedented tandem [4 + 2]/[2 + 2] cycloaddition reaction of o-carboryne with conjugated enynes was developed with a broad substrate scope, affording a series of carborane-fused tricyclo[6.4.0.02,7]dodeca-2,12-dienes in moderate to high yields. In this reaction, a reactive carborane-fused 1,2-cyclohexadiene intermediate was formed, followed by a stepwise [2 + 2] cycloaddition via a diallyl diradical to give the final product. This protocol provided a feasible strategy for the synthesis of complex carborane-fused tricyclic compounds in a single process, which is otherwise inaccessible by other means.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by grants from the Research Grants Council of HKSAR (Project No. 14306519) and NSFC/RGC Joint Research Scheme (Project No. N_CUHK402/18), as well as by the CUHK Impact Postdoctoral Fellowship Scheme (IPDFS to Z.J.).Notes and references
- H. H. Wenk, M. Winkler and W. Sander, Angew. Chem., Int. Ed., 2003, 42, 502–528 CrossRef CAS PubMed.
- J. F. Liebman and A. A. Greenberg, Chem. Rev., 1976, 76, 311–365 CrossRef CAS.
- R. O. Angus, M. W. Schmidt Jr and R. P. Johnson, J. Am. Chem. Soc., 1985, 107, 532–537 CrossRef CAS.
- H. Pellissier and M. Santelli, Tetrahedron, 2003, 59, 701–730 CrossRef CAS.
- (a) I. Quintana, D. Peña, D. Pérez and E. Guitián, Eur. J. Org. Chem., 2009, 2009, 5519–5524 CrossRef; (b) J. S. Barber, E. D. Styduhar, H. V. Pham, T. C. McMahon, K. N. Houk and N. K. Garg, J. Am. Chem. Soc., 2016, 138, 2512–2515 CrossRef CAS; (c) V. A. Lofstrand and F. G. West, Chem.−Eur. J., 2016, 22, 10763–10767 CrossRef CAS PubMed; (d) J. S. Barber, M. M. Yamano, M. Ramirez, E. R. Darzi, R. R. Knapp, F. Liu, K. N. Houk and N. K. Garg, Nat. Chem., 2018, 10, 953–960 CrossRef CAS; (e) V. A. Lofstrand, K. C. McIntosh, Y. A. Almehmadi and F. G. West, Org. Lett., 2019, 21, 6231–6234 CrossRef CAS PubMed; (f) M. Yamano, R. Knapp, A. Ngamnithiporn, M. Ramirez, K. Houk, B. Stoltz and N. K. Garg, Angew. Chem., Int. Ed., 2019, 58, 5653–5657 CrossRef CAS PubMed; (g) Y. A. Almehmadi and F. G. West, Org. Lett., 2020, 22, 6091–6095 CrossRef CAS PubMed; (h) M. M. Yamano, A. V. Kelleghan, Q. Shao, M. Giroud, B. J. Simmons, B. Li, S. Chen, K. N. Houk and N. K. Garg, Nature, 2020, 586, 242–247 CrossRef CAS PubMed.
- (a) P. Wessig and G. Muller, Chem. Rev., 2008, 108, 2051–2063 CrossRef CAS PubMed; (b) V. Gevorgyan and O. V. Zatolochnaya, [4 + 2] Benzannulation of enynes with alkynes, in Transition-Metal-Mediated Aromatic Ring Construction, ed. K. Tanaka, 2013, pp. 355–377 Search PubMed.
- (a) R. L. Danheiser, R. G. Brisbois, J. J. Kowalczyk and R. F. Miller, J. Am. Chem. Soc., 1990, 112, 3093–3100 CrossRef CAS; (b) J. R. Dunetz and R. L. Danheiser, J. Am. Chem. Soc., 2005, 127, 5776–5777 CrossRef CAS PubMed; (c) S. Saito, M. M. Salter, V. Gevorgyan, N. Tsuboya, K. Tando and Y. Yamamoto, J. Am. Chem. Soc., 1996, 118, 3970–3971 CrossRef CAS; (d) V. Gevorgyan, A. Takeda and Y. Yamamoto, J. Am. Chem. Soc., 1997, 119, 11313–11314 CrossRef CAS; (e) V. Gevorgyan, A. Takeda, M. Homma, N. Sadayori, U. Radhakrishnan and Y. Yamamoto, J. Am. Chem. Soc., 1999, 121, 6391–6402 CrossRef CAS; (f) M. Rubina, M. Conley and V. Gevorgyan, J. Am. Chem. Soc., 2006, 128, 5818–5827 CrossRef CAS PubMed; (g) Y. Nakao, Y. Hirata, S. Ishihara, S. Oda, T. Yukawa, E. Shirakawa and T. Hiyama, J. Am. Chem. Soc., 2004, 126, 15650–15651 CrossRef CAS PubMed; (h) F. Punner and G. Hilt, Chem. Commun., 2012, 48, 3617–3619 RSC.
- (a) M. E. Hayes, H. Shinokubo and R. L. Danheiser, Org. Lett., 2005, 7, 3917–3920 CrossRef CAS PubMed; (b) S. Yoshida, K. Shimizu, K. Uchida, Y. Hazama, K. Igawa, K. Tomooka and T. Hosoya, Chem.–Eur. J., 2017, 23, 15332–15335 CrossRef CAS PubMed.
- (a) R. N. Grimes, Carboranes, Academic Press, Amsterdam, 3rd edn, 2016 Search PubMed; (b) N. S. Hosmane, Boron Science: New Technologies and Applications, CRC Press, Boca Raton, FL, 2011 Search PubMed; (c) Z. Xie and G. X. Jin, Carborane Themed Issue, Dalton Trans., 2014, 43, 4911–5232 RSC; (d) V. I. Bregadze and Z. Xie, Boron Chemistry Themed Issue, Eur. J. Inorg. Chem., 2017, 4344–4692 Search PubMed.
- For reviews, see: (a) R. Núñez, M. Tarrés, A. Ferrer-Ugalde, F. F de Biani and F. Teixidor, Chem. Rev., 2016, 116, 14307–14378 CrossRef PubMed; (b) S. Mukherjee and P. Thilagar, Chem. Commun., 2016, 52, 1070–1093 RSC; (c) X. Li, H. Yan and Q. Zhao, Chem.−Eur. J., 2016, 22, 1888–1898 CrossRef CAS PubMed; (d) Z. Xie, Acc. Chem. Res., 2003, 36, 66–77 CrossRef PubMed; (e) W.-B. Yu, P.-F. Cui, W.-X. Gao and G.-X. Jin, Coord. Chem. Rev., 2017, 350, 300–319 CrossRef CAS; (f) Z. Qiu, S. Ren and Z. Xie, Acc. Chem. Res., 2011, 44, 299–309 CrossRef CAS PubMed; (g) S. P. Fisher, A. W. Tomich, S. O. Lovera, J. F. Kleinsasser, J. Guo, M. J. Asay, H. M. Nelson and V. Lavallo, Chem. Rev., 2019, 119, 8262–8290 CrossRef CAS PubMed; (h) J. Zhang and Z. Xie, Acc. Chem. Res., 2014, 47, 1623–1633 CrossRef CAS PubMed; (i) M. F. Hawthorne, Angew. Chem., Int. Ed. Engl., 1993, 32, 950–984 CrossRef; (j) M. F. Hawthorne and A. Maderna, Chem. Rev., 1999, 99, 3421–3434 CrossRef CAS PubMed; (k) A. F. Armstrong and J. F. Valliant, Dalton Trans., 2007, 4240–4251 RSC; (l) F. Issa, M. Kassiou and L. M. Rendina, Chem. Rev., 2011, 111, 5701–5722 CrossRef CAS PubMed; (m) M. Scholz and E. Hey-Hawkins, Chem. Rev., 2011, 111, 7035–7062 CrossRef CAS PubMed; (n) R. Núñez, I. Romero, F. Teixidor and C. Viñas, Chem. Soc. Rev., 2016, 45, 5147–5173 RSC; (o) Y. Quan and Z. Xie, Chem. Soc. Rev., 2019, 48, 3660–3673 RSC; (p) Y. K. Au and Z. Xie, Bull. Chem. Soc. Jpn., 2021 DOI:10.1246/bcsj.20200366R; (q) N. Grimes, Dalton Trans., 2015, 44, 5939–5956 RSC; (r) P. Stockmann, M. Gozzi, R. Kuhnert, M. B. Sárosi and E. Hey-Hawkins, Chem. Soc. Rev., 2019, 48, 3497–3512 RSC.
- For selected examples, see: (a) H. Jude, H. Disteldorf, S. Fischer, T. Wedge, A. M. Hawkridge, A. M. Arif, M. F. Hawthorne, D. C. Muddiman and P. J. Stang, J. Am. Chem. Soc., 2005, 127, 12131–12139 CrossRef CAS PubMed; (b) B. H. Northrop, H.-B. Yang and P. J. Stang, Chem. Commun., 2008, 5896–5908 RSC; (c) J. M. Ludlow III, M. Tominaga, Y. Chujo, A. Schultz, X. Lu, T. Xie, K. Guo, C. N. Moorefield, C. Wesdemiotis and G. R. Newkome, Dalton Trans., 2014, 43, 9604–9611 RSC; (d) D. Jung, L. M. A. Saleh, Z. J. Berkson, M. F. El-Kady, J. Y. Hwang, N. Mohamed, A. I. Wixtrom, E. Titarenko, Y. Shao, K. McCarthy, J. Guo, I. B. Martini, S. Kraemer, E. C. Wegener, P. Saint-Cricq, B. Ruehle, R. R. Langeslay, M. Delferro, J. L. Brosmer, C. H. Hendon, M. Gallagher-Jones, J. Rodriguez, K. W. Chapman, J. T. Miller, X. Duan, R. B. Kaner, J. I. Zink, B. F. Chmelka and A. M. Spokoyny, Nat. Mater., 2018, 17, 341–348 CrossRef CAS PubMed; (e) J. Guo, D. Liu, J. Zhang, J. Zhang, Q. Miao and Z. Xie, Chem. Commun., 2015, 51, 12004–12007 RSC; (f) B. P. Dash, R. Satapathy, E. R. Gaillard, J. A. Maguire and N. S. Hosmane, J. Am. Chem. Soc., 2010, 132, 6578–6587 CrossRef CAS PubMed; (g) M. Koshino, T. Tanaka, N. Solin, K. Suenaga, H. Isobe and E. Nakamura, Science, 2007, 316, 853 CrossRef CAS PubMed; (h) D. Brusselle, P. Bauduin, L. Girard, A. Zaulet, C. Viñas, F. Teixidor, I. Ly and O. Diat, Angew. Chem., Int. Ed., 2013, 52, 12114–12118 CrossRef CAS PubMed; (i) O. K. Farha, A. M. Spokoyny, K. L. Mulfort, M. F. Hawthorne, C. A. Mirkin and J. T. Hupp, J. Am. Chem. Soc., 2007, 129, 12680–12681 CrossRef CAS PubMed; (j) J. J. Schwartz, A. M. Mendoza, N. Wattanatorn, Y. Zhao, V. T. Nguyen, A. M. Spokoyny, C. A. Mirkin, T. Baše and P. S. Weiss, J. Am. Chem. Soc., 2016, 138, 5957–5967 CrossRef CAS; (k) S. G. McArthur, L. Geng, J. Guo and V. Lavallo, Inorg. Chem. Front., 2015, 2, 1101–1104 RSC; (l) D. J. Clingerman, W. Morris, J. E. Mondloch, R. D. Kennedy, A. A. Sarjeant, C. Stern, J. T. Hupp, O. K. Farha and C. A. Mirkin, Chem. Commun., 2015, 51, 6521–6523 RSC; (m) A. M. Spokoyny, C. W. Machan, D. J. Clingerman, M. S. Rosen, M. J. Wiester, R. D. Kennedy, C. L. Stern, A. A. Sarjeant and C. A. Mirkin, Nat. Chem., 2011, 3, 590–596 CrossRef CAS PubMed; (n) C. A. Lugo, C. E. Moore, A. L. Rheingold and V. Lavallo, Inorg. Chem., 2015, 54, 2094–2096 CrossRef CAS; (o) L. M. A. Saleh, R. M. Dziedzic, S. I. Khan and A. M. Spokoyny, Chem.–Eur. J., 2016, 22, 8466–8470 CrossRef CAS PubMed; (p) X. Wei, M.-J. Zhu, Z. Cheng, M. Lee, H. Yan, C. Lu and J.-J. Xu, Angew. Chem., Int. Ed., 2019, 58, 3162–3166 CrossRef CAS PubMed.
- For reviews on carboryne chemistry, see: (a) Z. Qiu and Z. Xie, Dalton Trans., 2014, 43, 4925–4934 RSC; (b) D. Zhao and Z. Xie, Coord. Chem. Rev., 2016, 314, 14–33 CrossRef CAS.
- Example for [5 + 2]/[3 + 2] cycloaddition of o-carboryne: D. Zhao, J. Zhang and Z. Xie, J. Am. Chem. Soc., 2015, 137, 13938–13942 CrossRef CAS PubMed.
- Select examples for [4 + 2] cycloaddition of o-carboryne: (a) H. L. Gingrich, T. Ghosh, Q. Huang and M. Jones Jr, J. Am. Chem. Soc., 1990, 112, 4082–4083 CrossRef CAS; (b) J. Jeon, T. Kitamura, B.-W. Yoo, S. O. Kang and J. Ko, Chem. Commun., 2001, 2110–2111 RSC; (c) T. Ghosh, H. L. Gingrich, C. K. Kam, E. C. M. Mobraaten and M. Jones Jr, J. Am. Chem. Soc., 1991, 113, 1313–1318 CrossRef CAS; (d) L. Barnett-Thamattoor, G.-X. Zheng, D. M. Ho, M. Jones Jr and J. E. Jackson, Inorg. Chem., 1996, 35, 7311–7315 CrossRef CAS PubMed; (e) J. H. Atkins, D. M. Ho and M. Jones Jr, Tetrahedron Lett., 1996, 37, 7217–7220 CrossRef CAS; (f) S. R. Wang and Z. Xie, Organometallics, 2012, 31, 3316–3323 CrossRef CAS; (g) J. U. Kahlert, A. Rawal, J. M. Hook, L. M. Rendina and M. Choucair, Chem. Commun., 2014, 50, 11332–11334 RSC; (h) J. Zhang, Z. Qiu, P.-F. Xu and Z. Xie, ChemPlusChem, 2014, 79, 1044–1052 CrossRef CAS; (i) S. R. Wang and Z. Xie, Tetrahedron, 2012, 68, 5269–5278 CrossRef CAS; (j) J. Zhang, Z. Qiu and Z. Xie, Organometallics, 2017, 36, 3806–3811 CrossRef CAS; (k) D. Zhao, J. Zhang and Z. Xie, Angew. Chem., Int. Ed., 2014, 53, 8488–8491 CrossRef CAS PubMed; (l) J. Zhang and Z. Xie, Chin. J. Chem., 2018, 36, 1041–1046 CrossRef CAS.
- Select examples for [2 + 2] cycloaddition of o-carboryne: (a) T. Lee, J. Jeon, K. H. Song, I. Jung, C. Baik, K.-M. Park, S. S. Lee, S. O. Kang and J. Ko, Dalton Trans., 2004, 933–937 RSC; (b) R. J. Cunningham, N. Bian and M. Jones Jr, Inorg. Chem., 1994, 33, 4811–4812 CrossRef CAS; (c) D. M. Ho, R. J. Cunningham, J. A. Brewer, N. Bian and M. Jones Jr, Inorg. Chem., 1995, 34, 5274–5278 CrossRef CAS; (d) S. R. Wang, Z. Qiu and Z. Xie, J. Am. Chem. Soc., 2010, 132, 9988–9989 CrossRef CAS PubMed; (e) D. Zhao, J. Am. Chem. Soc., 2015, 137, 9423–9428 CrossRef CAS PubMed; (f) J. Zhang and Z. Xie, Organometallics, 2020, 39, 4214–4220 CrossRef CAS.
- Select examples for ene reactions of o-carboryne: (a) Q. Huang, H. L. Gingrich and M. Jones Jr, Inorg. Chem., 1991, 30, 3254–3257 CrossRef CAS; (b) H. L. Gingrich, Q. Huang, A. L. Morales and M. Jones Jr, J. Org. Chem., 1992, 57, 3803–3806 CrossRef CAS; (c) D. Zhao, J. Zhang and Z. Xie, Chem.–Eur. J., 2015, 21, 10334–10337 CrossRef CAS PubMed.
- Select examples for C−H insertion reactions of o-carboryne: (a) S. R. Wang, Z. Qiu and Z. Xie, J. Am. Chem. Soc., 2011, 133, 5760–5763 CrossRef CAS PubMed; (b) D. Zhao, J. Zhang and Z. Xie, Angew. Chem., Int. Ed., 2014, 53, 12902–12906 CrossRef CAS PubMed; (c) S. R. Wang and Z. Xie, Organometallics, 2012, 31, 4544–4550 CrossRef CAS; (d) R. Cheng, J. Zhang, J. Zhang, Z. Qiu and Z. Xie, Angew. Chem., Int. Ed., 2016, 55, 1751–1754 CrossRef CAS.
- D. Zhao, J. Zhang and Z. Xie, Chem.−Eur. J., 2015, 21, 10334–10337 CrossRef CAS PubMed.
- CCDC 2052100 (3h), CCDC 2052101 (3i), CCDC 2052102 (3k), CCDC 2052103 (3l), CCDC 2052104 (3m), CCDC 2052105 (3q), CCDC 2052106 (3s), CCDC 2052107 (3t), CCDC 2052108 (3w), CCDC 2052109 (3y), CCDC 2052110 (3jm), CCDC 2052111 (5) and CCDC 2052112 (7) contain the supplementary crystallographic data for this paper.
- (a) A. Ajaz, A. Z. Bradley, R. C. Burrell, W. H. H. Li, K. J. Daoust, L. B. Bovee, K. J. DiRico and R. P. Johnson, J. Org. Chem., 2011, 76, 9320–9328 CrossRef CAS PubMed; (b) V. P. Ananikov, J. Phys. Org. Chem., 2001, 14, 109–121 CrossRef CAS; (c) V. P. Ananikov and E. G. Gordeev, Chem. Sci., 2011, 2, 2332–2341 RSC.
- (a) R. P. Johnson, Chem. Rev., 1989, 89, 1111–1124 CrossRef CAS; (b) S. L. Skraba and R. P. Johnson, J. Org. Chem., 2012, 77, 11096–11100 CrossRef CAS PubMed; (c) T. L. Jacobs, J. R. McClenon and O. J. Muscio Jr, J. Am. Chem. Soc., 1969, 91, 6038–6041 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC [2052100–2052112]. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0sc07047e |
| This journal is © The Royal Society of Chemistry 2021 |