Open Access Article
Open Access ArticleA pyrone remodeling strategy to access diverse heterocycles: application to the synthesis of fascaplysin natural products†
Vignesh
Palani
 ,
Melecio A.
Perea
,
Melecio A.
Perea
 ,
Kristen E.
Gardner
,
Kristen E.
Gardner
 and
Richmond
Sarpong
and
Richmond
Sarpong
 *
*
Department of Chemistry, University of California, Berkeley, California 94720, USA. E-mail: rsarpong@berkeley.edu
First published on 27th November 2020
Abstract
The synthesis of diverse N-fused heterocycles, including the pyrido[1,2-a]indole scaffold, using an efficient pyrone remodeling strategy is described. The pyrido[1,2-a]indole core was demonstrated to be a versatile scaffold that can be site-selectively functionalized. The utility of this novel annulation strategy was showcased in a concise formal synthesis of three fascaplysin congeners.
Introduction
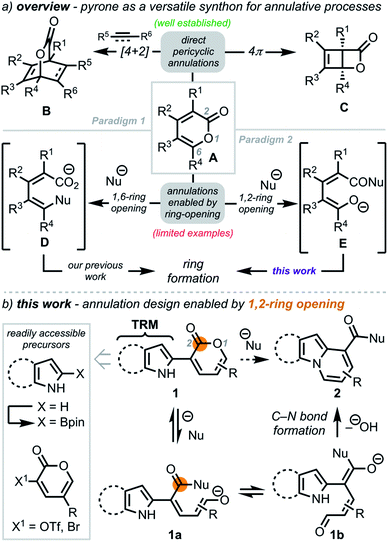
The use of annulation reactions to construct complex structures remains a powerful strategy in chemical synthesis.1 For almost a century, 2-pyrones (A, Scheme 1a) have served as valuable heterocycles for annulations due to their versatile reactivity, which can be broadly categorized into two main paradigms: (1) pericyclic annulative processes and (2) regioselective opening via nucleophilic addition to unveil reactive intermediates poised for subsequent annulation. With respect to the first paradigm, pericyclic reactions, such as [4+2]-cycloadditions2 and 4π electrocyclizations,3 have been well documented to provide rapid access to bicycles such as B and C, which have been exploited in myriad ways.4,5 In contrast, there have been limited examples within the second paradigm. While nucleophilic 1,6-ring opening of 2-pyrones has proven to be a particularly effective strategy for orchestrating novel cyclization events via reactive intermediate D6 (our previous work6a,b), leveraging the dienolate functionality (E) accessible through 1,2-ring opening in annulation reactions remains underexplored.7We envisioned a strategy to N-fused bicycles in which a tethered reactive moiety (TRM) on 2-pyrone would engage an in situ generated dienolate (such as 1b) in an annulation reaction (Scheme 1b). The precursor N-heterocycle–pyrone adducts (e.g., 1) were anticipated to arise modularly by coupling N-heterocycle boronate esters and pyrones (e.g., 3-OTf pyrone)8via Suzuki coupling. The C2-borylated N-heterocycles were expected to arise directly from the precursor heterocycles by leveraging existing methods (e.g., C–H functionalization),9 thus enhancing the practicality of this approach. We hypothesized that opening 1 with a suitable nucleophile would first unveil dienolate 1a, which upon equilibration to 1b, would set the stage for annulation via direct capture of the aldehyde group by the TRM to provide N-fused heterocycle 2. Notably, varying the TRM would provide a general platform for diverse heterocycle synthesis.
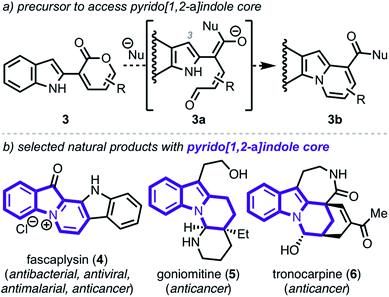
To demonstrate the viability of this strategy, we initially focused on converting indole–pyrone adduct 3 to the pyrido[1,2-a]indole scaffold (3b, Scheme 2a)—a key structural motif present in a number of biologically active natural products including fascaplysin (4, Scheme 2b),10 goniomitine (5),11 and tronocarpine (6).12 While there exists numerous methods to access this biologically relevant scaffold,13–17 many of these tactics rely on reaction precursors with highly specific substitution patterns and, therefore, are unfortunately not general or modular. Specifically, we recognized that while heterocyclic–dienolate adducts (such as C3-substituted intermediate 3a) have proven to be effective precursors for benzannulation processes, strategies to install dienol/dienolate functionality at C2 of 1H-indoles lacking C3-substitution have remained elusive due to regioselectivity challenges.13b,18,19 Overall, we envisioned that our approach to coupling pyrone—a masked dienolate—to the C2-position of 1H-indole would provide a unique opportunity to address this longstanding regioselectivity challenge.
Results and discussion
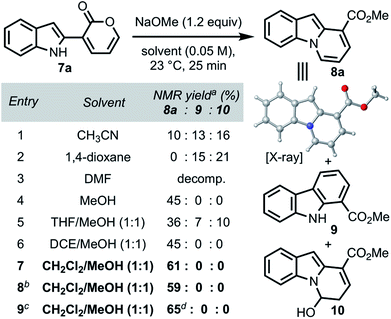
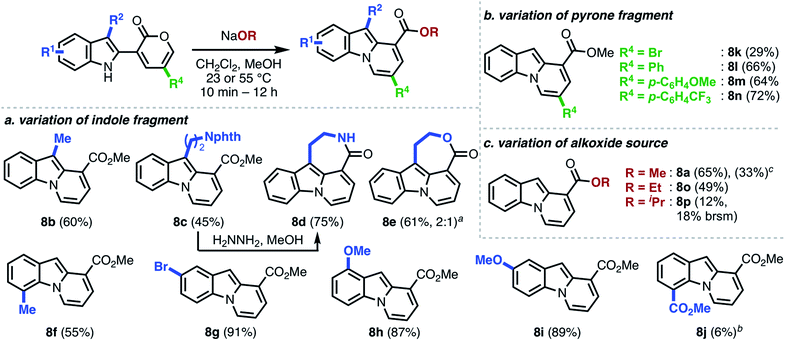
We commenced our investigations with indole–pyrone 7a (Table 1) and sodium methoxide as the nucleophile. Initially, we observed the formation of the desired pyrido[1,2-a]indole (8a) along with carbazole 9 and hemiaminal 10 as side products (entry 1). Changing the solvent from acetonitrile to 1,4-dioxane enhanced the formation of 9, which was generally more pronounced in relatively non-polar solvents.20 However, the use of polar solvents such as dimethylformamide resulted in complete decomposition of 7a (entry 3). The formation of hemiaminal 10 corroborates the proposed reaction mechanism illustrated in Scheme 1b and led us to investigate the use of polar protic solvents, such as methanol, to favor the conversion of 10 to 8a. We found, at this stage, that conducting the annulation in methanol furnished 8a in 45% yield (entry 4). Further investigation using co-solvents (entries 5–7) led to the identification of a dichloromethane/methanol solvent mixture as optimal, furnishing 8a in 61% yield (entry 7),21 presumably due to the increased solubility of 7a. Gratifyingly, the yield remained unaffected when the annulation was conducted both under open-flask conditions (entry 8) and on 1.3 g scale (entry 9). The structure of 8a was unambiguously confirmed by single-crystal X-ray analysis.With optimized conditions in hand, we investigated the scope of this operationally simple pyrido[1,2-a]indole synthesis (Scheme 3). Indole–pyrone substrates with varied substitution patterns were readily synthesized through Suzuki coupling of indole boronate esters9 with either 3-bromo-8a or 3-triflyloxy-2-pyrones.8b Indole substitution at both C3 and C7 had minimal influence on the ring-opening/annulation process, and the corresponding pyrido[1,2-a]indoles were isolated in comparable yields (8b–f, Scheme 3a). Interestingly, tetracyclic scaffolds such as lactam 8d and lactone 8e were accessed from indole–pyrones derived from tryptamine and tryptophol, respectively. Notably, 8d represents the core framework of tronocarpine (6). Next, we sought to investigate the tolerance of the overall transformation toward alterations of the electronics of the indole moiety. We observed that the presence of an electron-donating group, irrespective of the position, furnished the corresponding pyrido[1,2-a]indoles in high yields (8g–8i), whereas the product bearing an electron-withdrawing substituent (8j) was isolated in poor yield.22
As shown in Scheme 3b, the established reaction conditions were also applicable to the efficient preparation of pyrido[1,2-a]indoles 8k–n bearing various substituents on the pyrone moiety. Unlike the electronic influence exerted by the substituents on the indole, C5-substitution on the pyrone moiety had little to no effect on the final reaction outcome with the sole exception being product 8k, which was isolated in diminished yield. Additionally, we investigated the effect of other alkoxide nucleophiles (Scheme 3c). With increasing basicity and sterics of the alkoxide, more forcing conditions were generally required, and the yield of the final products (8a, 8o–p) were also diminished.22
To further demonstrate the generality and versatility of our strategy, we next explored the synthesis of structurally diverse heterocyclic systems by subjecting various N-heterocyclic–pyrone adducts to the established reaction conditions (Scheme 4).23 Gratifyingly, upon coupling various TRMs, such as pyrrole, 7-aza-indole, pyrazole, and aniline moieties, to the C3 position of 2-pyrones, heterocycles such as indolizine 11, pyrido[3,2-b]indolizine 12, 3-aza-indolizine 13, and 1-naphthylamine 14 were isolated in moderate to high yields.
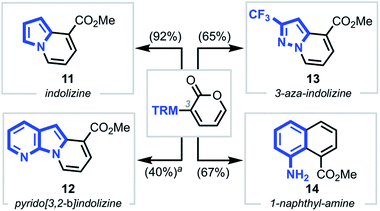 | ||
| Scheme 4 Access to other novel heterocyclic cores. Conditions: NaOMe, CH2Cl2/MeOH, 23 or 55 °C, 10 min. aYield over two steps starting from SEM-protected 7-azaindole–pyrone substrate. | ||
Each of the pyrone–heterocycle substrates described to this point contain a free N–H group, thus enabling cyclization directly from nitrogen to form a new N–C bond, with the sole exception being 1-naphthylamine 14.24 On the basis of the latter result and our initial hypothesis (Scheme 1b), we envisioned that employing N-protected substrates would direct the cyclization to the reactive carbon center, thus facilitating C–C bond formation25 and carbazole synthesis (Scheme 5). Interestingly, we found the annulation to be tolerant of various indole N-substituents, providing carbazoles 15a–c and 9 in high yields. Notably, unlike the pyrido[1,2-a]indole scope, the nature of the substituents—both on the indole and pyrone moieties—had little influence on the final reaction outcome, delivering the corresponding carbazoles (15d–g) in good yields.26
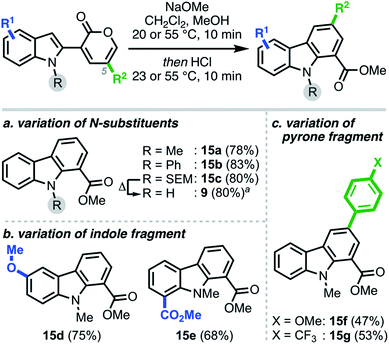 | ||
| Scheme 5 Scope of modular carbazole synthesis. aSEM cleavage can also proceed in the same pot upon prolonged heating to furnish the free N–H carbazole 9. | ||
We next sought to explore the subsequent reactivity of the C7-ester functionalized pyrido[1,2-a]indole products (Scheme 6). Friedel–Crafts acylation,27 copper-catalyzed carbenoid C–H insertion,28 Lewis acid-mediated epoxide opening/attendant lactonization,29 and chlorination30 all proceeded to provide the corresponding C10-functionalized pyrido[1,2-a]indoles 16–19. The structure of 18 and 19 were unambiguously confirmed by single-crystal X-ray analysis. Hydrogenation proceeded smoothly to furnish tetrahydro pyrido[1,2-a]indole 20. Treating 8a under Hartwig borylation conditions9,20 yielded boronate ester 21, resulting from borylation at the C7 position. Photo-mediated Heck coupling20,31 of 8a with iodobenzene gave biaryl compound 22, thus providing a platform to functionalize the C6 position as well, albeit at low conversion.32
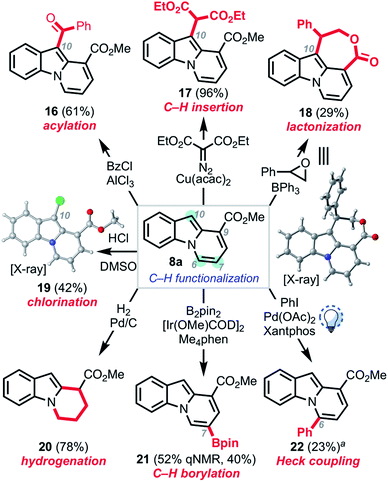 | ||
| Scheme 6 Derivatizations of pyrido[1,2-a]indoles. aSignificant portion of 8a (75%) remained unreacted. | ||
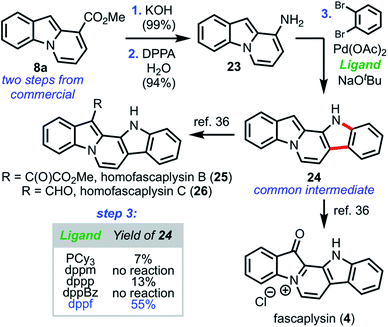
With the generality of this strategy successfully established, we next turned our attention toward applying our pyrone remodeling strategy to access the fascaplysin family of natural products. As illustrated in Scheme 7, we began by hydrolyzing ester 8a to afford the intermediate carboxylic acid, which smoothly underwent Curtius rearrangement33 to furnish amine 23 in high yield.
Taking inspiration from methodology developed by Ackermann and co-workers,34 a palladium-catalyzed amination/C–H arylation domino coupling35 was employed to couple 23 and 1,2-dibromobenzene to furnish the pentacyclic core of the fascaplysin natural products (24), which possessed analytical data (1H and 13C NMR, HRMS, melting point, IR) in full agreement with those previously reported. The synthesis of 24 constitutes formal syntheses of fascaplysin (1) and homofascaplysins B and C (25 and 26), which can all be accessed independently in a single step from 24.36
Conclusions
In summary, we have developed a general, novel pyrone remodeling strategy, which capitalizes on the 1,2-ring opening of 2-pyrones, to construct diverse heterocyclic scaffolds. This transformation, which was initially validated through pyrido[1,2-a]indole synthesis, features a diverse substrate scope, with varied substitution patterns on both the indole and pyrone moieties. The scope was additionally extended to access carbazole cores and other N-fused heterocycles, thus, showcasing the generality of this strategy. The unusual reactivity of the pyrido[1,2-a]indole core was explored in several synthetic transformations, which enabled selective functionalization of three distinct carbon positions. Finally, the utility of this strategy was further demonstrated in a concise formal synthesis of three fascaplysin congeners. Studies to further expand the non-intuitive potential of 2-pyrone and its derivatives in the total synthesis of complex natural products are the focus of our current efforts.Conflicts of interest
There are no conflicts to declare.Acknowledgements
V. P. acknowledges TRDRP for a predoctoral fellowship. M. A. P. and K. E. G. thank the NSF for graduate research fellowships (DGE 1752814). Financial support for this research was provided to R. S. by the National Science Foundation (CHE-18566228). We thank Dr Hasan Celik and UC Berkeley's NMR facility in the College of Chemistry (CoC-NMR) for spectroscopic assistance. Instruments in CoC-NMR are supported in part by NIH S10OD024998. We are also grateful to Dr Nicholas Settineri (UC Berkeley) for single-crystal X-ray diffraction studies, and Dr Miao Zhang (UC Berkeley) for support with the acquisition of HRMS and IR data.Notes and references
- (a) L. Wu, B. Yu and E.-Q. Li, Recent advances in organocatalyst-mediated benzannulation reactions, Adv. Synth. Catal., 2020, 362, 4010 CrossRef CAS; (b) J. Li, Y. Ye and Y. Zhang, Cycloaddition/annulation strategies for the construction of multisubstituted pyrrolidines and their applications in natural product synthesis, Org. Chem. Front., 2018, 5, 864 RSC.
- For selected examples of [4+2]-cycloadditions with 2-pyrones, see: (a) C. J. F. Cole, L. Fuentes and S. A. Snyder, Asymmetric pyrone Diels–Alder reactions enabled by dienamine catalysis, Chem. Sci., 2020, 11, 2175 RSC; (b) X.-W. Liang, Y. Zhao, X.-G. Si, M.-M. Xu, J.-H. Tan, Z.-M. Zhang, C.-G. Zheng, C. Zheng and Q. Cai, Enantioselective synthesis of arene cis-dihydrodiols from 2-pyrones, Angew. Chem., Int. Ed., 2019, 58, 14562 CrossRef CAS; (c) Y. Wang, H. Li, Y.-Q. Wang, Y. Liu, B. M. Foxman and L. Deng, Asymmetric Diels–Alder reactions of 2-pyrones with a bifunctional organic catalyst, J. Am. Chem. Soc., 2007, 129, 6364 CrossRef CAS.
- For selected examples of 4π electrocyclization of 2-pyrones, see: (a) O. L. Chapman, C. L. McIntosh and J. Pacansky, Photochemical transformations. XLVIII. Cyclobutadiene, J. Am. Chem. Soc., 1973, 95, 614 CrossRef CAS; (b) R. G. S. Pong and J. S. Shirk, Photochemistry of alpha-pyrone in solid argon, J. Am. Chem. Soc., 1973, 95, 248 CrossRef CAS; (c) W. H. Pirkle and L. H. McKendry, Photochemical reactions of 2-pyrone and thermal reactions of the 2-pyrone photoproducts, J. Am. Chem. Soc., 1969, 91, 1179 CrossRef CAS; (d) E. J. Corey and J. Streith, Internal photoaddition reactions of 2-pyrone and N-methyl-2-pyridone: a new synthetic approach to cyclobutadiene, J. Am. Chem. Soc., 1964, 86, 950 CrossRef CAS.
- Q. Cai, The [4+2]-cycloaddition of 2-pyrone in total synthesis, Chin. J. Chem., 2019, 37, 946 CrossRef CAS.
- S. C. Coote, 4-π-photocyclization: scope and synthetic applications, Eur. J. Org. Chem., 2020, 1405 CrossRef CAS.
- For selected examples of annulation strategies involving 1,6-ring opening of 2-pyrones, see: (a) V. Palani, C. L. Hugelshofer and R. Sarpong, A unified strategy for the enantiospecific total synthesis of delavatine A and formal synthesis of incarviatone A, J. Am. Chem. Soc., 2019, 141, 14421 CrossRef CAS; (b) V. Palani, C. L. Hugelshofer, I. Kevlishvili, P. Liu and R. Sarpong, A short synthesis of delavatine A unveils new insights into site-selective cross-coupling of 3,5-dibromo-2-pyrone, J. Am. Chem. Soc., 2019, 141, 2652 CrossRef CAS; (c) W. Disadee, A. Lekky and S. Ruchirawat, Metal-free, one-pot cascade annulation of 2-pyrones in water for the synthesis of peptidomimetics, J. Org. Chem., 2020, 85, 1802 CrossRef CAS; (d) H. K. Maurya, P. G. Vasudev and A. Gupta, A regioselective synthesis of 2,6-diarylpyridines, RSC Adv., 2013, 3, 12955 RSC; (e) B. I. Usachev, S. A. Usachev, G.-V. Röschenthaler and V. Y. Sosnovskikh, A simple and convenient synthesis of 3-[5-(trifluoromethyl)-1,2,3-triazol-4-yl]cinnamic acids from 4-aryl-6-(trifluoromethyl)-2H-pyran-2-ones and sodium azide, Tetrahedron Lett., 2011, 52, 6723 CrossRef CAS; (f) A. Goel, D. Verma, M. Dixit, R. Raghunandan and P. R. Maulik, Acetyltrimethylsilane: a novel reagent for the transformation of 2H-pyran-2-ones to unsymmetrical biaryls, J. Org. Chem., 2006, 71, 804 CrossRef CAS.
- For known annulation strategies involving 1,2-ring opening of 2-pyrones, see: (a) S. A. Usachev, B. I. Usachev and V. Y. Sosnovskikh, Synthesis of 6-hydroxy-5,6-dihydro-2-pyrones and pyridones by reaction of 4-aryl-6-trifluoromethyl-2-pyrones with water, hydrazine, and hydroxylamine, Chem. Heterocycl. Compd., 2017, 53, 1294 CrossRef CAS; (b) C. A. Hansen and J. W. Frost, Deoxygenation of polyhydroxybenzenes: an alternate strategy for the benzene-free synthesis of aromatic chemicals, J. Am. Chem. Soc., 2002, 124, 5926 CrossRef CAS; (c) C. Tanyeli and O. Tarhan, Annulation reactions of 4-methoxy-2-pyrone with various active methyl compounds, Synth. Commun., 1989, 19, 2749 CrossRef CAS.
- For synthesis of 3,5-dibromo-2-pyrone and 3-triflyloxy-2-pyrone for cross-coupling, see: (a) H.-K. Cho and C.-G. Cho, Preparation of 3,5-dibromo-2-pyrone from coumalic acid, Org. Synth., 2015, 92, 148 CrossRef; (b) F. Frébault, M. T. Oliveira, E. Wöstefeld and N. Maulide, A concise access to 3-substituted 2-pyrones, J. Org. Chem., 2010, 75, 7962 CrossRef.
- The C–H borylation chemistry developed by Hartwig and co-workers can be employed to synthesize the N-heterocycle boronate ester precursors. For selected literature examples, see: (a) M. A. Larsen and J. F. Hartwig, Iridium-catalyzed C–H borylation of heteroarenes: scope, regioselectivity, application to late-stage functionalization, and mechanism, J. Am. Chem. Soc., 2014, 136, 4287 CrossRef CAS; (b) T. Ishiyama, Y. Nobuta, J. F. Hartwig and N. Miyaura, Room temperature borylation of arenes and heteroarenes using stoichiometric amounts of pinacolborane catalyzed by iridium complexes in an inert solvent, Chem. Commun., 2003, 2924 RSC.
- (a) S. B. Bharate, S. Manda, N. Mupparapu, N. Battini and R. A. Vishwakarma, Chemistry and biology of fascaplysin, a potent marine-derived CDK-4 inhibitor, Mini-Rev. Med. Chem., 2012, 12, 650 CrossRef CAS; (b) N. L. Segraves, S. J. Robinson, D. Garcia, S. A. Said, X. Fu, F. J. Schmitz, H. Pietraszkiewicz, F. A. Valeriote and P. Crews, Comparison of fascaplysin and related alkaloids: a study of structures, cytotoxicities, and sources, J. Nat. Prod., 2004, 67, 783 CrossRef CAS; (c) N. L. Segraves, S. Lopez, T. A. Johnson, S. A. Said, X. Fu, F. J. Schmitz, H. Pietraszkiewicz, F. A. Valeriote and P. Crews, Structures and cytotoxicities of fascaplysin and related alkaloids from two marine phyla—Fascaplysinopsis sponges and Didemnum tunicates, Tetrahedron Lett., 2003, 44, 3471 CrossRef CAS.
- (a) H.-Y. Bin, K. Wang, D. Yang, X.-H. Yang, J.-H. Xie and Q.-L. Zhou, Scalable enantioselective total synthesis of (−)-goniomitine, Angew. Chem., Int. Ed., 2019, 58, 1174 CrossRef CAS; (b) S. Zhou and Y. Jia, Total synthesis of (−)-goniomitine, Org. Lett., 2014, 16, 3416 CrossRef CAS; (c) F. De Simone, J. Gertsch and J. Waser, Catalytic selective cyclizations of aminocyclopropanes: formal synthesis of aspidospermidine and total synthesis of goniomitine, Angew. Chem., Int. Ed., 2010, 49, 5767 CrossRef CAS; (d) L. Randriambola, J.-C. Quirion, C. Kan-Fan and H.-P. Husson, Structure of goniomitine, a new type of indole alkaloid, Tetrahedron Lett., 1987, 28, 2123 CrossRef CAS.
- (a) D.-X. Tan, J. Zhou, C.-Y. Liu and F.-S. Han, Enantioselective total synthesis and absolute configuration assignment of (+)-tronocarpine enabled by an asymmetric Michael/aldol reaction, Angew. Chem., Int. Ed., 2020, 59, 3834 CrossRef CAS; (b) T.-S. Kam, K.-M. Sim and T.-M. Lim, Tronocarpine, a novel pentacyclic indole incorporating a seven-membered lactam moiety, Tetrahedron Lett., 2000, 41, 2733 CrossRef CAS.
- For selected examples involving annulation strategy to access the pyrido[1,2-a]indole core, see: (a) P. Chuentragool, Z. Li, K. Randle, F. Mahchi, I. Ochir, S. Assaf and V. Gevorgyan, General synthesis of pyrido[1,2-a]indoles via Pd-catalyzed cyclization of o-picolylbromoarenes, J. Organomet. Chem., 2018, 867, 273 CrossRef CAS; (b) S. G. Dawande, B. S. Lad, S. Prajapati and S. Katukojvala, Rhodium-catalyzed pyridannulation of indoles with diazoenals: a direct approach to pyrido[1,2-a]indoles, Org. Biomol. Chem., 2016, 14, 5569 RSC; (c) Y. Jung and I. Kim, Deformylative intramolecular hydroarylation: synthesis of benzo[e]pyrido[1,2-a]indoles, Org. Lett., 2015, 17, 4600 CrossRef CAS; (d) I. Karthikeyan and G. Sekar, Iron-catalyzed C–H bond functionalization for the exclusive synthesis of pyrido[1,2-a]indoles or triarylmethanols, Eur. J. Org. Chem., 2014, 8055 CrossRef CAS; (e) L.-L. Sun, Z.-Y. Liao, R.-Y. Tang, C.-L. Deng and X.-G. Zhang, Palladium and copper cocatalyzed tandem N–H/C–H bond functionalization: synthesis of CF3-containing indolo- and pyrrolo[2,1-a]isoquinolines, J. Org. Chem., 2012, 77, 2850 CrossRef CAS; (f) D. C. Rogness, N. A. Markina, J. P. Waldo and R. C. Larock, Synthesis of pyrido[1,2-a]indole malonates and amines through aryne annulation, J. Org. Chem., 2012, 77, 2743 CrossRef CAS.
- For examples involving aza-Nazarov type cyclization to access the pyrido[1,2-a]indole core, see: (a) I. Karthikeyan, D. Arunprasath and G. Sekar, An efficient synthesis of pyrido[1,2-a]indoles through aza-Nazarov type cyclization, Chem. Commun., 2015, 51, 1701 RSC; (b) R. R. Naredla, C. Zheng, S. O. N. Lill and D. A. Klumpp, Charge delocalization and enhanced acidity in tricationic superelectrophiles, J. Am. Chem. Soc., 2011, 133, 13169 CrossRef CAS.
- For benzyne-mediated rearrangement to access the pyrido[1,2-a]indole core, see: I. L. Nikonov, D. S. Kopchuk, I. S. Kovalev, G. V. Zyryanov, A. F. Khasanov, P. A. Slepukhin, V. L. Rusinov and O. N. Chupakhin, Benzyne-mediated rearrangement of 3-(2-pyridyl)-1,2,4-triazines into 10-(1H-1,2,3-triazol-1-yl)pyrido[1,2-a]indoles, Tetrahedron Lett., 2013, 54, 6427 CrossRef CAS.
- For cycloaddition strategy to access the pyrido[1,2-a]indole core, see: E. M. Beccalli, G. Broggini, C. L. Rosa, D. Passarella, T. Pilati, A. Terraneo and G. Zecchi, Access to pyrrolo- and pyrido[1,2-a]indole derivatives by intramolecular nitrone cycloadditions. Effect of steric factors on the regioselective product formation, J. Org. Chem., 2000, 65, 8924 CrossRef CAS.
- For multicomponent fragment coupling strategy to access the pyrido[1,2-a]indole core, see: (a) H. Zhu, J. Stöckigt, Y. Yu and H. Zou, “One-pot” multicomponent approach to indolizines and pyrido[1,2-a]indoles, Org. Lett., 2011, 13, 2792 CrossRef CAS; (b) T. Li, Z. Wang, M. Zhang, H.-J. Zhang and T.-B. Wen, Rh/Cu-catalyzed multiple C–H, C–C, and C–N bon cleavage: facile synthesis of pyrido[2,1-a]indoles from 1-(pyridin-2-yl)-1H-indoles and γ-substituted propargyl alcohols, Chem. Commun., 2015, 51, 6777 RSC.
- J.-Q. Wu, Z. Yang, S.-S. Zhang, C.-Y. Jiang, Q. Li, Z.-S. Huang and H. Wang, From indoles to carbazoles: tandem Cp*Rh(III)-catalyzed C–H activation/Brønsted acid-catalyzed cyclization reactions, ACS Catal., 2015, 5, 6453 CrossRef CAS.
- K. S. Rathore, M. Harode and S. Katukojvala, Regioselective π-extension of indoles with rhodium enalcarbenoids – synthesis of substituted carbazoles, Org. Biomol. Chem., 2014, 12, 8641 RSC.
- See the ESI† for detailed discussions.
- Alternatively, pyrido[1,2-a]indole core can also be accessed from the Boc protected indole–pyrone precursor albeit in poor yield. See the ESI† for detailed experimental results.
- Mechanistically, having an electron-withdrawing substituent on the indole moiety renders the free N–H of the precursor indole–pyrone more acidic, which upon exposure to sodium methoxide results in undesired deprotonation to yield the corresponding indole-1-ide, which is resistant toward the desired ring-opening/annulative process. For the same reason, increasing the basicity of the alkoxide source also has a negative effect on this overall transformation.
- In general, N-heterocyclic–pyrone adducts with enhanced N–H acidity were more resistant toward the desired ring-opening/annulative process as mentioned in ref. 24. For instance, both the 7-aza-indole and pyrazole substrate required more forcing conditions to effect the desired transformation.
- For the aniline substrate, the cyclization did not occur from the nitrogen center to provide the corresponding benzazepine core.
- For the carbazole formation, addition of HCl was crucial to effect the C-addition to the unveiled aldehyde group.
- As the precursors for carbazole synthesis lack a free N–H, the substituents on the indole fragment have little to no influence on the reaction outcome. This hypothesis supports the rationalization provided in ref. 22.
- O. Ottoni, A. V. F. Neder, A. K. B. Dias, R. P. A. Cruz and L. B. Aquino, Acylation of indole under Friedel–Crafts conditions – an improved method to obtain 3-acylindoles regioselectively, Org. Lett., 2001, 3, 1005 CAS.
- B. E. Maryanoff, Reaction of dimethyl diazomalonate and ethyl-2-diazoacetoacetate with N-methylpyrrole, J. Org. Chem., 1982, 47, 3000 CrossRef CAS.
- S. Sueki, Z. Wang and Y. Kuninobu, Manganese- and borane-mediated synthesis of isobenzofuranones from aromatic esters and oxiranes via C–H bond activation, Org. Lett., 2016, 18, 304 CrossRef CAS . However, as reported in this reference, we did not observe the anticipated lactone formation.
- W. W. Epstein and F. W. Sweat, Dimethyl sulfoxide oxidations, Chem. Rev., 1967, 67, 247 CrossRef CAS.
- (a) P. Chuentragool, D. Kurandina and V. Gevorgyan, Catalysis with Palladium Complexes Photoexcited by Visible Light, Angew. Chem., Int. Ed., 2019, 58, 11586 CrossRef CAS; (b) D. Kurnadina, M. Rivas, M. Radzhabov and V. Gevorgyan, Heck Reaction of Electronically Diverse Tertiary Alkyl Halides, Org. Lett., 2018, 20, 357 CrossRef; (c) M. Parasram, P. Chuentragool, D. Sarkar and V. Gevorgyan, Photoinduced Formation of Hybrid Aryl Pd-Radical Species Capable of 1,5-HAT: Selective Catalytic Oxidation of Silyl Ethers into Silyl Enol Ethers, J. Am. Chem. Soc., 2016, 138, 6340 CrossRef CAS.
- Failed attempts to functionalize C6 include Lewis acid-mediated conjugate addition, nucleophilic radical addition, C–H insertion reactions, and [4+2]-cycloadditions.
- A. K. Ghosh, A. Sarkar and M. Brindisi, The Curtius rearrangement: mechanistic insight and recent applications in natural product syntheses, Org. Biomol. Chem., 2018, 16, 2006 RSC.
- L. Ackermann and A. Althammer, Domino N–H/C–H bond activation: palladium-catalyzed synthesis of annulated heterocycles using dichloro(hetero)arenes, Angew. Chem., Int. Ed., 2007, 46, 1627 CrossRef CAS.
- After an extensive screening, a combination of Pd(OAc)2 and dppf in substoichiometric amounts have provided the best yields. See the ESI† for detailed optimization efforts.
- For synthesis of fascaplysin congeners, see: (a) H. Waldmann, L. Eberhardt, K. Wittstein and K. Kumar, Silver catalyzed cascade synthesis of alkaloid ring systems: concise total synthesis of fascaplysin, homofascaplysin C and analogues, Chem. Commun., 2010, 46, 4622 RSC; (b) G. W. Gribble and B. Pelcman, Total syntheses of the marine sponge pigments fascaplysin and homofascaplysin B and C, J. Org. Chem., 1992, 57, 3636 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2034052–2034054. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0sc06317g |
| This journal is © The Royal Society of Chemistry 2021 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: