Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Intermediates involved in serotonin oxidation catalyzed by Cu bound Aβ peptides†
Arnab Kumar
Nath
,
Arnab
Ghatak
,
Abhishek
Dey
 * and
Somdatta Ghosh
Dey
* and
Somdatta Ghosh
Dey
 *
*
Indian Association for the Cultivation of Science, 2A & 2B, Raja S. C. Mullick Road, Jadavpur, Kolkata 700032, India. E-mail: icsgd@iacs.res.in
First published on 22nd December 2020
Abstract
The degradation of neurotransmitters is a hallmark feature of Alzheimer's disease (AD). Copper bound Aβ peptides, invoked to be involved in the pathology of AD, are found to catalyze the oxidation of serotonin (5-HT) by H2O2. A combination of EPR and resonance Raman spectroscopy reveals the formation of a Cu(II)–OOH species and a dimeric, EPR silent, Cu2O2 bis-μ-oxo species under the reaction conditions. The Cu(II)–OOH species, which can be selectively formed in the presence of excess H2O2, is the reactive intermediate responsible for 5-HT oxidation. H2O2 produced by the reaction of O2 with reduced Cu(I)–Aβ species can also oxidize 5-HT. Both these pathways are physiologically relevant and may be involved in the observed decay of neurotransmitters as observed in AD patients.
Introduction
Alzheimer's disease (AD) is a terminal neurodegenerative disease which is characterized by the deposition of insoluble plaques in the hippocampus of an AD affected brain.1,2 It is clinically characterized by progressive dementia.3 The exact cause for this disease is still unclear. Over the past two decades extensive research has focused on determining the actual cause of this disease, resulting in important hypotheses relating to the pathology of AD. Among them, one of the more accepted hypothesis is the “amyloid hypothesis”.4–6 The amyloid β-peptide is the main constituent of this hypothesis and is found in high proportions in the plaques that are diagnostic of AD.5,6 Moreover transition metals like Cu(II) and Zn(II) and cofactors like heme are found in the plaques of an AD brain.7–9 Apart from the ability of these bivalent metals to aggregate the monomeric Aβ peptides, redox active metals like Cu and Fe are proposed to cause oxidative stress resulting in damage to the neuronal cell membrane making it “leaky”.7,9 The oxidative damage is proposed to be an upstream event which eventually leads to the formation of insoluble plaques which is a hallmark of the disease.7,10 The oxidative stress can be due to hydrogen peroxide (H2O2), which, apart from its natural availability, can also be produced by the reaction of the reduced redox active metals bound Aβ peptides with O2.9,11,12The recent investigation of Cu bound Aβ peptides and their site-directed mutants using a combination of spectroscopic techniques and theoretical calculations has revealed a Cu active site coordinated to histidine residues and exchangeable ligands.13–18 The Cu–Aβ complexes exhibit peroxidase activity in the presence of H2O2.12 Presently several naturally occurring copper containing metalloenzymes are known which can oxidize/hydroxylate organic compounds in the presence of H2O2. Enzymes like amine oxidase participate in the breakdown of amines to produce an aldehyde and ammonia.19–21 Alternatively, copper enzymes like dopamine β-hydroxylase (DβM) are involved in the synthesis of a small-molecule neurotransmitter by catalyzing the hydroxylation of dopamine to convert it to norepinephrine.19,22–24 Note that the Cu active sites of these above-mentioned proteins also have histidine and water derived exchangeable ligands in their active sites similar to that of Cu–Aβ. Now oxidative degradation of neurotransmitters like serotonin generating neurotoxins like tryptamine-4,5-dione is a hallmark of AD, which can lead to impaired neuronal signaling.25–33 This raises the possibility of Cu bound Aβ reacting with H2O2 and catalyzing the degradation of neurotransmitters (Scheme 1). Previously Ming et al. have kinetically shown the catalytic hydroxylation/oxidation of substrates like dopamine, catechol derivatives, phenol and serotonin and proposed a side-on μ-peroxo dicopper(II) intermediate as the active species, though no spectroscopic proof was provided.34–37 Since the oxidation of neurotransmitters by Cu–Aβ in the presence of H2O2 is a likely physiological process occurring in the human brain, the identification and characterization of the reactive intermediates involved in this chemical oxidation process are crucial for the understanding of this disease.
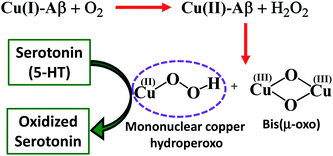
In this manuscript, we experimentally determine the reaction intermediates involved in serotonin oxidation as well as in the peroxidase activity pathway of Cu(II) bound Aβ peptides in the presence of H2O2. Spectroscopic evidence reveals that two intermediates, Cu2O2-bis-μ-oxo and Cu(II)–OOH, are formed in the reaction of Cu–Aβ and H2O2.
Results and discussion
Neurotransmitter serotonin (5-HT) oxidation by Cu–Aβ with H2O2
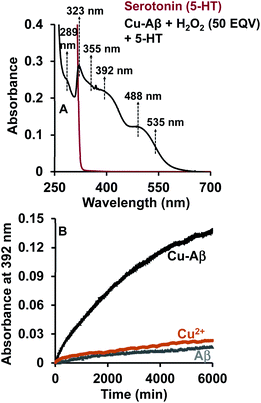
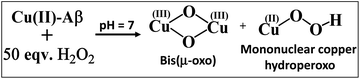
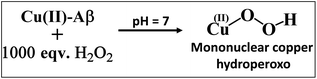
The reaction of the neurotransmitter serotonin (5-HT) with Cu–Aβ in the presence of H2O2 produces absorption peaks at 289, 323, 355, 392, 488 and 535 nm (Fig. 1A and S1†). These peaks are indicative of serotonin oxidation. Note that, as Cu–Aβ (1–16) and Cu–Aβ (1–40) produce similar serotonin oxidized products, all the other experiments have been performed with Cu–Aβ (1–16). The oxidized products of serotonin are further characterized using HPLC as previously reported (Fig. S2 and Scheme S1†).38,39 The products are found to be tryptamine-4,5-dione (T-4,5-D), 5-hydroxy-3-ethylamino-2-oxindole (5-HEO) and 3,3′-bis(2-aminoethyl)-5-hydroxy-[3,7′-bi-1H-indole]-2,4′,5′(3H)-trione, which is the aerially oxidized dimer of T-4,5-D and 5-HEO. The pseudo 1st order rate for this reaction is found to be (4 ± 0.2) × 10−4 s−1 (Fig. 1B and S3†). Note that the slow kinetics of this oxidation matches the slow onset and progression of this disease. Control experiments without Cu–Aβ, i.e. H2O2 or Cu(II) (Fenton reaction conditions) alone, show barely any oxidation of 5-HT (Fig. 1B).The oxidation of 5-HT by H2O2 catalyzed by Cu(II)–Aβ can proceed via several reactive intermediates similar to those which have been observed in different Cu enzymatic active sites and their synthetic analogues19,40–43 (Scheme 2). Hence the reaction of Cu–Aβ with H2O2 is monitored with the aim of trapping and characterizing the active oxidant responsible for oxidizing substrates like serotonin and other neurotransmitters.
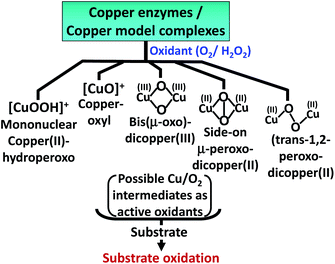 | ||
| Scheme 2 Possible reactive Cu/O2 intermediates for substrate oxidation by different copper enzymes and copper model complexes in the presence of oxidants (O2 or H2O2).19,40–42 | ||
Characterization of Cu/O2 intermediates formed in the reaction of Cu–Aβ with H2O2
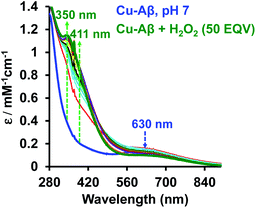
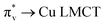 . CT bands in these regions hint at the plausible formation of either a side-on μ-peroxo-dicopper(II) complex or a bis(μ-oxo)dicopper(III) complex as depicted in Scheme 2.41,42,44–46 Previously Ming and coworkers proposed the formation of a side-on μ-peroxo dicopper(II) intermediate.34,36
. CT bands in these regions hint at the plausible formation of either a side-on μ-peroxo-dicopper(II) complex or a bis(μ-oxo)dicopper(III) complex as depicted in Scheme 2.41,42,44–46 Previously Ming and coworkers proposed the formation of a side-on μ-peroxo dicopper(II) intermediate.34,36
| Complex | A ll | g ll |
|---|---|---|
| Cu–Aβ | 173 | 2.234 |
| Cu–Aβ + 50 eq. H2O2 | 166 | 2.206 |
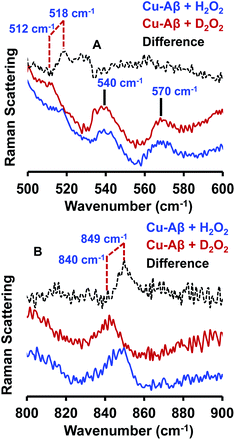 | ||
| Fig. 4 Resonance Raman spectra of (A) Cu–Aβ + 50 eq. H2O2, blue and Cu–Aβ + 50 eq. D2O2 in, red; difference spectrum of H2O2 data from D2O2 data, dashed black; lower energy region; (B) Cu–Aβ + 50 eq. H2O2, blue and Cu–Aβ + 50 eq. D2O2 in, red; difference spectrum of H2O2 data from D2O2 data, dashed black; higher energy region. Data were obtained with an excitation wavelength of 415.4 nm (10 mW at the sample) at 77 K (full spectra in Fig. S5†). | ||
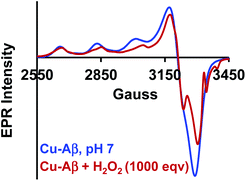 | ||
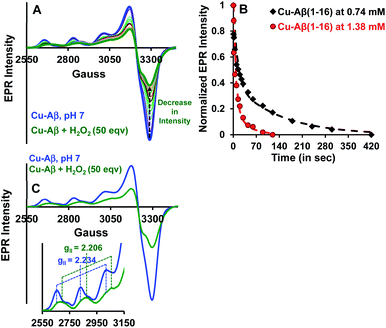
| Fig. 5 EPR spectra of Cu–Aβ, blue; for the reaction of Cu–Aβ with 1000 eq. H2O2, red; in 100 mM HEPES buffer at pH 7, at 77 K. | ||
Finally, reduced Cu–Aβ can react with O2, where the Cu gets oxidized and H2O2 is generated via disproportionation of the O2− produced.11 The H2O2 generated from dissolved oxygen in blood can trigger the oxidation of serotonin. This is exactly the same case as indicated by the gradual appearance of the bands corresponding to the oxidized products of 5-HT when incubated with a solution of Cu–Aβ reduced with ascorbic acid in aerated buffer solutions (Fig. 6, blue), albeit the extent of oxidized HT produced is much less under these stoichiometric conditions. Ming et al. have previously observed a similar result as Cu–Aβ significantly accelerated the aerobic oxidation of the neurotransmitters.34Scheme 5 demonstrates the possible routes for the oxidation of serotonin.
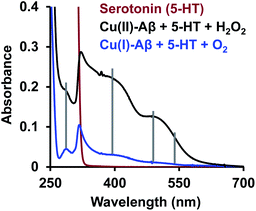 | ||
| Fig. 6 Absorption spectra of serotonin (5-HT), red; oxidation of 5-HT by H2O2 catalyzed by Cu(II)–Aβ, black and by O2 catalyzed by Cu(I)–Aβ, blue; in 100 mM HEPES buffer at pH 7. | ||
Conclusion
Cu–Aβ oxidizes the neurotransmitter serotonin (5-HT) in the presence of H2O2. The combined absorption, EPR and resonance Raman data indicate that Cu(II)–Aβ reacts with H2O2 to produce bis(μ-oxo)dicopper and mononuclear copper hydroperoxo intermediates. This is the first experimental characterization of the active oxidants originating from Cu bound Aβ that can oxidize neurotransmitters like serotonin (5-HT) and generate neurotoxins like tryptamine-4,5-dione, which are also observed in an AD brain. Cu(II)–Aβ can be reduced by physiologically relevant reductants like ascorbic acid and this reduced Cu center can generate H2O2 by reacting with dissolved oxygen, which can then oxidize 5-HT catalyzed by the Cu(II)–Aβ produced. Both these pathways are accessible under physiological conditions and may account for the abnormal neurotransmission, a key pathological feature of AD.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank DST, SERB India for financial support (grants EMR/2014/000392 and EMR/2015/0008063). A. K. N. and A. G. are funded by the University Grants Commission Fellowship. The authors thank CSS and IACS for providing HPLC facility.Notes and references
- T. Lührs, C. Ritter, M. Adrian, D. Riek-Loher, B. Bohrmann, H. Döbeli, D. Schubert and R. Riek, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 17342 CrossRef.
- J. J. Balbach, A. T. Petkova, N. A. Oyler, O. N. Antzutkin, D. J. Gordon, S. C. Meredith and R. Tycko, Biophys. J., 2002, 83, 1205–1216 CrossRef CAS.
- C. Schmidt, M. Wolff, M. Weitz, T. Bartlau, C. Korth and I. Zerr, Arch. Neurol., 2011, 68, 1124–1130 CrossRef.
- J. Hardy and D. J. Selkoe, Science, 2002, 297, 353 CrossRef CAS.
- F. Kametani and M. Hasegawa, Front. Neurosci., 2018, 12, 25 CrossRef.
- L. M. F. Gomes, J. C. Bataglioli and T. Storr, Coord. Chem. Rev., 2020, 412, 213255 CrossRef CAS.
- A. Rauk, Chem. Soc. Rev., 2009, 38, 2698–2715 RSC.
- S. Bagheri, R. Squitti, T. Haertlé, M. Siotto and A. A. Saboury, Front. Aging Neurosci., 2018, 9, 446 CrossRef.
- C. Garza, Y. Posadas, L. Quintanar, M. Gonsebatt and R. Franco, Antioxid. Redox Signaling, 2018, 28, 1669–1703 CrossRef.
- C. Cheignon, M. Tomas, D. Bonnefont-Rousselot, P. Faller, C. Hureau and F. Collin, Redox Biol., 2018, 14, 450–464 CrossRef CAS.
- D. Pramanik and S. G. Dey, J. Am. Chem. Soc., 2011, 133, 81–87 CrossRef CAS.
- D. Pramanik, C. Ghosh and S. G. Dey, J. Am. Chem. Soc., 2011, 133, 15545–15552 CrossRef CAS.
- C. Ghosh and S. G. Dey, Inorg. Chem., 2013, 52, 1318–1327 CrossRef CAS.
- I. Pal, M. Roy and S. G. Dey, JBIC, J. Biol. Inorg. Chem., 2019, 24, 1245–1259 CrossRef CAS.
- S. C. Drew, C. J. Noble, C. L. Masters, G. R. Hanson and K. J. Barnham, J. Am. Chem. Soc., 2009, 131, 1195–1207 CrossRef CAS.
- P. Dorlet, S. Gambarelli, P. Faller and C. Hureau, Angew. Chem., Int. Ed., 2009, 48, 9273–9276 CrossRef CAS.
- B.-k. Shin and S. Saxena, Biochemistry, 2008, 47, 9117–9123 CrossRef CAS.
- S. Furlan, C. Hureau, P. Faller and G. La Penna, J. Phys. Chem. B, 2012, 116, 11899–11910 CrossRef CAS.
- E. I. Solomon, D. E. Heppner, E. M. Johnston, J. W. Ginsbach, J. Cirera, M. Qayyum, M. T. Kieber-Emmons, C. H. Kjaergaard, R. G. Hadt and L. Tian, Chem. Rev., 2014, 114, 3659–3853 CrossRef CAS.
- R. H. Holm, P. Kennepohl and E. I. Solomon, Chem. Rev., 1996, 96, 2239–2314 CrossRef CAS.
- J. P. Klinman, Chem. Rev., 1996, 96, 2541–2562 CrossRef CAS.
- A. Beliaev, H. Ferreira, D. A. Learmonth and P. Soares-da-Silva, Curr. Enzyme Inhib., 2009, 5, 27–43 CrossRef.
- J. P. Evans, K. Ahn and J. P. Klinman, J. Biol. Chem., 2003, 278, 49691–49698 CrossRef CAS.
- J. P. Klinman, J. Biol. Chem., 2006, 281, 3013–3016 CrossRef CAS.
- R. Kandimalla and P. H. Reddy, J. Alzheimer's Dis., 2017, 57, 1049–1069 CAS.
- D. M. Bowen, AGE, 1988, 11, 104–109 CrossRef.
- S. A. Lyness, C. Zarow and H. C. Chui, Neurobiol. Aging, 2003, 24, 1–23 CrossRef CAS.
- S. G. Snowden, A. A. Ebshiana, A. Hye, O. Pletnikova, R. O'Brien, A. Yang, J. Troncoso, C. Legido-Quigley and M. Thambisetty, J. Alzheimer's Dis., 2019, 72, 35–43 CAS.
- H. Förstl and P. Fischer, Eur. Arch. Psychiatr. Clin. Neurosci., 1994, 244, 252–260 CrossRef.
- S. Kaur, G. DasGupta and S. Singh, Neurophysiology, 2019, 51, 293–309 CrossRef CAS.
- M. Z. Wrona and G. Dryhurst, Chem. Res. Toxicol., 1998, 11, 639–650 Search PubMed.
- L. Volicer and P. B. Crino, Neurobiol. Aging, 1990, 11, 567–571 CrossRef CAS.
- P. B. Crino, B. A. Vogt, J.-C. Chen and L. Volicer, Brain Res., 1989, 504, 247–257 CrossRef CAS.
- G. F. Z. da Silva and L.-J. Ming, Angew. Chem., Int. Ed., 2007, 46, 3337–3341 CrossRef CAS.
- G. F. Z. da Silva and L.-J. Ming, Angew. Chem., Int. Ed., 2005, 44, 5501–5504 CrossRef CAS.
- G. F. da Silva, V. Lykourinou, A. Angerhofer and L. J. Ming, Biochim. Biophys. Acta, 2009, 1792, 49–55 CrossRef CAS.
- G. F. da Silva, W. M. Tay and L. J. Ming, J. Biol. Chem., 2005, 280, 16601–16609 CrossRef CAS.
- M. Z. Wrona, Z. Yang, M. McAdams, S. O'Connor-Coates and G. Dryhurst, J. Neurochem., 1995, 64, 1390–1400 CrossRef CAS.
- V. F. Ximenes, G. J. Maghzal, R. Turner, Y. Kato, C. C. Winterbourn and A. J. Kettle, Biochem. J., 2009, 425, 285–293 CrossRef.
- C. E. Elwell, N. L. Gagnon, B. D. Neisen, D. Dhar, A. D. Spaeth, G. M. Yee and W. B. Tolman, Chem. Rev., 2017, 117, 2059–2107 CrossRef CAS.
- L. M. Mirica, X. Ottenwaelder and T. D. P. Stack, Chem. Rev., 2004, 104, 1013–1046 CrossRef CAS.
- E. I. Solomon, P. Chen, M. Metz, S.-K. Lee and A. E. Palmer, Angew. Chem., Int. Ed., 2001, 40, 4570–4590 CrossRef CAS.
- F. Nastri, M. Chino, O. Maglio, A. Bhagi-Damodaran, Y. Lu and A. Lombardi, Chem. Soc. Rev., 2016, 45, 5020–5054 RSC.
- J. A. Halfen, S. Mahapatra, E. C. Wilkinson, S. Kaderli, V. G. Young, L. Que, A. D. Zuberbühler and W. B. Tolman, Science, 1996, 271, 1397 CrossRef CAS.
- M. J. Henson, M. A. Vance, C. X. Zhang, H.-C. Liang, K. D. Karlin and E. I. Solomon, J. Am. Chem. Soc., 2003, 125, 5186–5192 CrossRef CAS.
- S. Mahapatra, J. A. Halfen, E. C. Wilkinson, G. Pan, C. J. Cramer, L. Que Jr and W. B. Tolman, J. Am. Chem. Soc., 1995, 117, 8865–8866 CrossRef CAS.
- E. I. Solomon, J. W. Ginsbach, D. E. Heppner, M. T. Kieber-Emmons, C. H. Kjaergaard, P. J. Smeets, L. Tian and J. S. Woertink, Faraday Discuss., 2011, 148, 11–108 RSC.
- D. J. E. Spencer, A. M. Reynolds, P. L. Holland, B. A. Jazdzewski, C. Duboc-Toia, L. Le Pape, S. Yokota, Y. Tachi, S. Itoh and W. B. Tolman, Inorg. Chem., 2002, 41, 6307–6321 CrossRef CAS.
- P. L. Holland, C. J. Cramer, E. C. Wilkinson, S. Mahapatra, K. R. Rodgers, S. Itoh, M. Taki, S. Fukuzumi, L. Que and W. B. Tolman, J. Am. Chem. Soc., 2000, 122, 792–802 CrossRef CAS.
- N. Lehnert, K. Fujisawa and E. I. Solomon, Inorg. Chem., 2003, 42, 469–481 CrossRef CAS.
- Y. J. Choi, K.-B. Cho, M. Kubo, T. Ogura, K. D. Karlin, J. Cho and W. Nam, Dalton Trans., 2011, 40, 2234–2241 RSC.
- P. Chen, K. Fujisawa and E. I. Solomon, J. Am. Chem. Soc., 2000, 122, 10177–10193 CrossRef CAS.
- B. Kim, D. Jeong, T. Ohta and J. Cho, Commun. Chem., 2019, 2, 81 CrossRef.
- P. J. Mak, W. Thammawichai, D. Wiedenhoeft and J. R. Kincaid, J. Am. Chem. Soc., 2015, 137, 349–361 CrossRef CAS.
- N. Lehnert, F. Neese, R. Y. N. Ho, L. Que and E. I. Solomon, J. Am. Chem. Soc., 2002, 124, 10810–10822 CrossRef CAS.
- R. Y. N. Ho, G. Roelfes, B. L. Feringa and L. Que, J. Am. Chem. Soc., 1999, 121, 264–265 CrossRef CAS.
- A. Ghatak, S. Bhunia and A. Dey, ACS Catal., 2020, 10, 13136–13148 CrossRef CAS.
- C. Citek, S. Herres-Pawlis and T. D. P. Stack, Acc. Chem. Res., 2015, 48, 2424–2433 CrossRef CAS.
- M. T. Kieber-Emmons, J. W. Ginsbach, P. K. Wick, H. R. Lucas, M. E. Helton, B. Lucchese, M. Suzuki, A. D. Zuberbühler, K. D. Karlin and E. I. Solomon, Angew. Chem., Int. Ed., 2014, 53, 4935–4939 CrossRef CAS.
- P. Chen and E. I. Solomon, J. Am. Chem. Soc., 2004, 126, 4991–5000 CrossRef CAS.
- A. Crespo, M. A. Martí, A. E. Roitberg, L. M. Amzel and D. A. Estrin, J. Am. Chem. Soc., 2006, 128, 12817–12828 CrossRef CAS.
- P. Chen and E. I. Solomon, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 13105–13110 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0sc06258h |
| This journal is © The Royal Society of Chemistry 2021 |