Open Access Article
Open Access ArticleFluorine-induced aggregate-interlocking for color-tunable organic afterglow with a simultaneously improved efficiency and lifetime†
Hui
Li‡
a,
Huanhuan
Li‡
a,
Jie
Gu
a,
Fei
He
a,
Hao
Peng
a,
Ye
Tao
*a,
Dan
Tian
b,
Qingqing
Yang
a,
Ping
Li
a,
Chao
Zheng
*a,
Wei
Huang
 ac and
Runfeng
Chen
ac and
Runfeng
Chen
 *a
*a
aKey Laboratory for Organic Electronics and Information Displays & Jiangsu Key Laboratory for Biosensors, Institute of Advanced Materials (IAM), Nanjing University of Posts & Telecommunications, 9 Wenyuan Road, Nanjing 210023, China. E-mail: iamytao@njupt.edu.cn; iamczheng@njupt.edu.cn; iamrfchen@njupt.edu.cn
bCollege of Materials Science and Engineering, Nanjing Forestry University, Nanjing 210037, China
cFrontiers Science Center for Flexible Electronics, Xi'an Institute of Flexible Electronics (IFE) and Xi'an Institute of Biomedical Materials & Engineering, Northwestern Polytechnical University (NPU), 127 West Youyi Road, Xi'an 710072, China
First published on 12th January 2021
Abstract
Designing organic afterglow materials with a high efficiency and long lifetime is highly attractive but challenging because of the inherent competition between the luminescence efficiency and lifetime. Here, we propose a simple yet efficient strategy, namely fluorine-induced aggregate-interlocking (FIAI), to realize both an enhanced efficiency and elongated lifetime of afterglow materials by stimulating the synergistic effects of the introduced fluorine atoms to efficiently promote intersystem crossing (ISC) and intermolecular non-covalent interactions for facilitating both the generation of triplet excitons and suppression of non-radiative decays. Thus, the fluorine-incorporated afterglow molecules exhibit greatly enhanced ISC with a rate constant up to 5.84 × 107 s−1 and suppressed non-radiative decay down to 0.89 s−1, resulting in efficient organic afterglow with a simultaneously improved efficiency up to 10.5% and a lifetime of 1.09 s. Moreover, accompanied by the efficient phosphorescence emission especially at cryogenic temperature, color-tunable afterglow was also observed at different temperatures. Therefore, tri-mode multiplexing encryption devices by combining lifetime, temperature and color, and visual temperature sensing were successfully established. The FIAI strategy by addressing fundamental issues of afterglow emission paves the way to develop high-performance organic afterglow materials, opening up a broad prospect of aggregated and excited state tuning of organic solids for emission lifetime-resolved applications.
Introduction
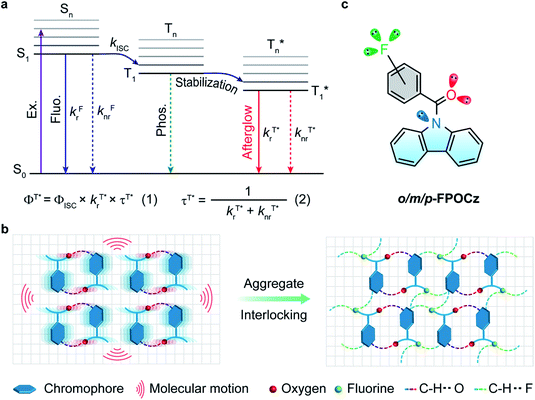
Long afterglow phosphorescent materials, which exhibit persistent luminescence after switching off the irradiation source,1,2 have recently received exponentially increasing attention, not only because of the significant scientific advance in understanding the rich excited state properties of optoelectronic materials but also owing to the substantial promising applications ranging from emergency display,3,4 information encryption5,6 and storage7 to bio-imaging and therapy.8–10 Compared to their inorganic afterglow counterparts,11,12 metal-free organic afterglow materials have many advantages including easy synthesis conditions, low production costs, large molecular versatility, excellent substrate compatibilities, and many others.2,13,14 However, because of their weak spin–orbit coupling (SOC) with inefficient intersystem crossing (ISC) and severe molecular motion and vibration with rapid non-radiative decays, the design and construction of high-performance organic afterglow materials with a high quantum yield and long lifetime are still very challenging even though they have been achieved in recent years.Principally, to achieve organic afterglow with a high phosphorescence quantum yield (ΦT*), it is essential to establish the rapid radiation decay rate 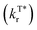 , slow non-radiation decay rate
, slow non-radiation decay rate 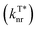 and high ISC efficiency (ΦISC) for the triplet excited state emission (Fig. 1a, eqn (1) and (2)); nevertheless, fast
and high ISC efficiency (ΦISC) for the triplet excited state emission (Fig. 1a, eqn (1) and (2)); nevertheless, fast 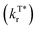 implies a short afterglow lifetime (τT*), resulting in an inherent interference between ΦT* and τT*. Up to now, most strategies including heavy atom,15 aromatic carbonyl,16 and heteroatom incorporation,17 and host–guest,18,19 crystallization20,21 and H-aggregation8,22 engineering have failed to achieve simultaneously improved ΦT* and τT*. Only limited examples through supramolecular self-assembly,23 free volume modulation24 and resonance-activated spin-flipping25 have shown both improved quantum yields and prolonged lifetimes by breaking through the intrinsic bottlenecks of organic afterglow, despite their design and synthetic complications.
implies a short afterglow lifetime (τT*), resulting in an inherent interference between ΦT* and τT*. Up to now, most strategies including heavy atom,15 aromatic carbonyl,16 and heteroatom incorporation,17 and host–guest,18,19 crystallization20,21 and H-aggregation8,22 engineering have failed to achieve simultaneously improved ΦT* and τT*. Only limited examples through supramolecular self-assembly,23 free volume modulation24 and resonance-activated spin-flipping25 have shown both improved quantum yields and prolonged lifetimes by breaking through the intrinsic bottlenecks of organic afterglow, despite their design and synthetic complications.
Non-covalent interactions that can form an interlocked molecular aggregate have been proved to be effective in suppressing nonradiative decay.26 Fluorine with a small size and high electronegativity has been known as an influential moiety to construct unique intra- and intermolecular non-covalent interactions in the fields of optoelectronic materials27 for achieving a dense molecular aggregation network in the solid state,28 especially in preparing donor and acceptor molecules for organic photovoltaics.29,30 Inspired by the intensive fluorine-induced non-covalent interactions, we envision that the incorporation of fluorine atoms into molecular crystals would greatly inhibit non-radiative transition. Particularly, the motion of an aggregated dimer in a conventional molecular crystal would induce the strong consumption of triplet excitons under ambient conditions (Fig. 1b, left), leading to relatively poor afterglow properties. After the introduction of fluorine atoms, the adjacent dimers can be effectively interlocked through efficient non-covalent interactions (Fig. 1b, right), leading to the efficient stabilization of triplet excitons for reinforced afterglow performance; meanwhile, the multiple lone-pair electrons of the fluorine atom could potentially favor n–π* transition to facilitate the ISC process for the population of triplet excitons. With these extraordinary characteristics of the fluorine atom in mind, we propose a new strategy of fluorine-induced aggregate-interlocking (FIAI) to design and prepare three afterglow isomers of o/m/p-FPOCz at different fluorine substitution positions to simultaneously improve the lifetime and promote the ISC of organic afterglow (Fig. 1c). Indeed, significantly boosted afterglow performance with enhanced ISC rates (kminISCs) up to 5.84 × 107 s−1 and suppressed 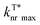 down to 0.89 s−1 for excellent afterglow emission with both an enlarged ultralong lifetime up to 1.09 s and an improved afterglow yield of 10.5% was achieved in the resultant afterglow molecules under ambient conditions, breaking the inherent conflict between the afterglow lifetime and efficiency. More interestingly, these afterglow isomers designed by the FIAI strategy also demonstrate both phosphorescence emission from T1 and afterglow emission from
down to 0.89 s−1 for excellent afterglow emission with both an enlarged ultralong lifetime up to 1.09 s and an improved afterglow yield of 10.5% was achieved in the resultant afterglow molecules under ambient conditions, breaking the inherent conflict between the afterglow lifetime and efficiency. More interestingly, these afterglow isomers designed by the FIAI strategy also demonstrate both phosphorescence emission from T1 and afterglow emission from 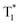 at cryogenic temperature, leading to color-tunable afterglow emission at different temperatures. On account of these distinguished afterglow properties, a tri-mode (lifetime, temperature and color) multiplexing encryption device and temperature sensing chart were realized on flexible substrates for the first time. These findings illustrate a fundamental advancement of purely organic afterglow molecules with the aid of the FIAI tactic in simultaneously elongating the emission lifetime and enhancing the afterglow efficiency, shedding light on the rational molecule design of high-performance organic afterglow materials for advanced lifetime-resolved applications.
at cryogenic temperature, leading to color-tunable afterglow emission at different temperatures. On account of these distinguished afterglow properties, a tri-mode (lifetime, temperature and color) multiplexing encryption device and temperature sensing chart were realized on flexible substrates for the first time. These findings illustrate a fundamental advancement of purely organic afterglow molecules with the aid of the FIAI tactic in simultaneously elongating the emission lifetime and enhancing the afterglow efficiency, shedding light on the rational molecule design of high-performance organic afterglow materials for advanced lifetime-resolved applications.
Results and discussion
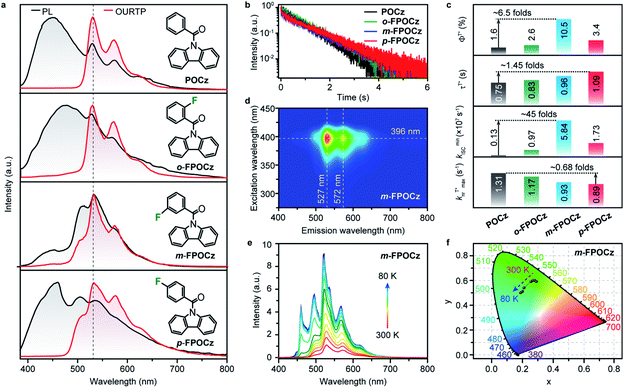
The fluorine-incorporated isomers of o/m/p-FPOCz designed by the FIAI strategy were facilely synthesized by a one-step C–N coupling31 with high yields up to 85% and completely characterized using nuclear magnetic resonance (NMR) spectra, high resolution mass spectra (HRMS), and high-performance liquid chromatogram (HPLC) spectra as well as single crystal and thermal property analyses (Scheme S1 and Fig. S1–S14†). The recently reported afterglow molecule of (9H-carbazol-9-yl)(phenyl) methanone (POCz) without fluorine substitution was also prepared for comparative investigation (Scheme S1†).32The photophysical properties of o/m/p-FPOCz in dilute tetrahydrofuran solution are almost identical to those of fluorine-free POCz, showing absorption peaks at ∼279, 305 and 316 nm and steady-state photoluminescence (SSPL) bands at ∼338 and 354 nm with nanosecond lifetimes (Fig. S15 and S16†) at room temperature. The low temperature SSPL spectra of o/m/p-FPOCz and POCz exhibit not only fluorescence bands at ∼338 and 354 nm but also obvious phosphorescence emission bands peaking at ∼415 and 445 nm with lifetimes up to several seconds because of the suppressed nonradiative transition processes at 77 K (Fig. S17–S19†); notably, the phosphorescence intensities of o/m/p-FPOCz are even stronger than those of their corresponding fluorescence bands, while the fluorescence intensity of POCz is higher than phosphorescence intensity (Fig. S17†). The large difference between the fluorescence spectra of o/m/p-FPOCz and POCz at 77 K in solution should be due to the combined results of the fluorine promoted ISC for the enhanced population of triplet excitons and low-temperature suppressed non-radiative transitions for achieving enhanced phosphorescence emission of o/m/p-FPOCz. Strikingly, as shown in Fig. 2a, the crystals of the four molecules show intense blue short-lived fluorescence bands with nanosecond lifetimes (Fig. S20†) and efficient yellow afterglow emission with an ultralong lifetime up to several seconds at room temperature (Fig. 2b and c). o/m/p-FPOCz reveal a shorter fluorescence lifetime than POCz (Fig. S20†), confirming again the promoted ISC process induced by fluorine incorporation. These afterglow molecules demonstrate quite similar afterglow spectra profiles, showing unchanged main afterglow emission peaks at ∼527 nm when tuning the excitation light from 300 to 400 nm (Fig. 2d and S21–S24†), the irradiation excitation power from 0.11 to 1.33 mW cm−2 (Fig. S25†) and the irradiation time from 0.01 to 10 s, respectively (Fig. S26†). The τT*and ΦT* of POCz are 0.75 s and 1.6%. These data are slightly different from the previous results,31,32 which may be due to the different aggregation states of the crystals. Importantly, the afterglow performance of the o/m/p-FPOCz crystals is much better than that of POCz; τT*/ΦT* increases from 0.75 s/1.6% for POCz to 0.83 s/2.6%, 0.98 s/10.5% and 1.09 s/3.4% for o-FPOCz, m-FPOCz and p-FPOCz, respectively (Fig. 2c and Table S1†), clearly illustrating simultaneous increments in both afterglow lifetime and efficiency.
To quantify the enhancement effects of the FIAI strategy on afterglow performance, the rate constant analyses of kISC, 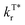 and
and 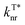 of o/m/p-FPOCz and POCz were performed. As shown in Fig. 2c, the kminISC of o-FPOCz, m-FPOCz and p-FPOCz crystals reached 9.70 × 106, 5.84 × 107 and 1.73 × 107 s−1 respectively, which are about 7-, 54- and 13-folds larger than that of POCz (1.32 × 106 s−1), confirming experimentally the enhanced ISC for increasing the afterglow efficiency; meanwhile, the
of o/m/p-FPOCz and POCz were performed. As shown in Fig. 2c, the kminISC of o-FPOCz, m-FPOCz and p-FPOCz crystals reached 9.70 × 106, 5.84 × 107 and 1.73 × 107 s−1 respectively, which are about 7-, 54- and 13-folds larger than that of POCz (1.32 × 106 s−1), confirming experimentally the enhanced ISC for increasing the afterglow efficiency; meanwhile, the 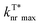 , which plays a key role in manipulating the phosphorescence lifetime, of o-FPOCz, m-FPOCz and p-FPOCz crystals also decreases to 1.17, 0.93 and 0.89 s−1, which are 0.89-, 0.71- and 0.68-folds that of POCz (1.31 s−1), respectively; and o/m/p-FPOCz crystals also exhibit higher
, which plays a key role in manipulating the phosphorescence lifetime, of o-FPOCz, m-FPOCz and p-FPOCz crystals also decreases to 1.17, 0.93 and 0.89 s−1, which are 0.89-, 0.71- and 0.68-folds that of POCz (1.31 s−1), respectively; and o/m/p-FPOCz crystals also exhibit higher 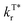 than POCz (Table S1†). The enhanced kISC, and
than POCz (Table S1†). The enhanced kISC, and 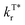 , and decreased
, and decreased 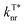 of o/m/p-FPOCz should be responsible for the simultaneously elongated lifetime and high efficiency,33 verifying the significant role of fluorine-substitution in boosting afterglow performance.
of o/m/p-FPOCz should be responsible for the simultaneously elongated lifetime and high efficiency,33 verifying the significant role of fluorine-substitution in boosting afterglow performance.
We further explore the influence of temperature and atmosphere on the unique afterglow emission. On decreasing from 300 to 80 K, the intensity of the afterglow emission of POCz and o/m/p-FPOCz at 527 nm from 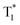 increases; interestingly, in comparison to POCz, a new emission band centered at about 460 nm appears in o/m/p-FPOCz crystals and the corresponding intensities also increase when the temperature decreases; and because of different increases in phosphorescent intensity at 527 nm and 460 nm, color-tunable afterglow emissions at different temperatures in o/m/p-FPOCz crystals were achieved (Fig. 2e and f and S27–S30†). To figure out the origin of the new emission band, the phosphorescence spectra of m-FPOCz, as a model molecule, at 80 K were recorded. As shown in Fig. S31,†m-FPOCz reveals a main emission band around 460 nm in both pure and doped films, suggesting that the emission band at ∼460 nm in o/m/p-FPOCz crystals should be attributed to the radiative decay from T1 of the amorphous state. The crystals of o/m/p-FPOCz show almost identical afterglow spectra and lifetime decay profiles in air, argon (Ar) and oxygen (O2). In contrast, the afterglow emission and lifetime of POCz were slightly enhanced in Ar and decreased in O2 compared to those in an air atmosphere (Fig. S32–S36†).
increases; interestingly, in comparison to POCz, a new emission band centered at about 460 nm appears in o/m/p-FPOCz crystals and the corresponding intensities also increase when the temperature decreases; and because of different increases in phosphorescent intensity at 527 nm and 460 nm, color-tunable afterglow emissions at different temperatures in o/m/p-FPOCz crystals were achieved (Fig. 2e and f and S27–S30†). To figure out the origin of the new emission band, the phosphorescence spectra of m-FPOCz, as a model molecule, at 80 K were recorded. As shown in Fig. S31,†m-FPOCz reveals a main emission band around 460 nm in both pure and doped films, suggesting that the emission band at ∼460 nm in o/m/p-FPOCz crystals should be attributed to the radiative decay from T1 of the amorphous state. The crystals of o/m/p-FPOCz show almost identical afterglow spectra and lifetime decay profiles in air, argon (Ar) and oxygen (O2). In contrast, the afterglow emission and lifetime of POCz were slightly enhanced in Ar and decreased in O2 compared to those in an air atmosphere (Fig. S32–S36†).
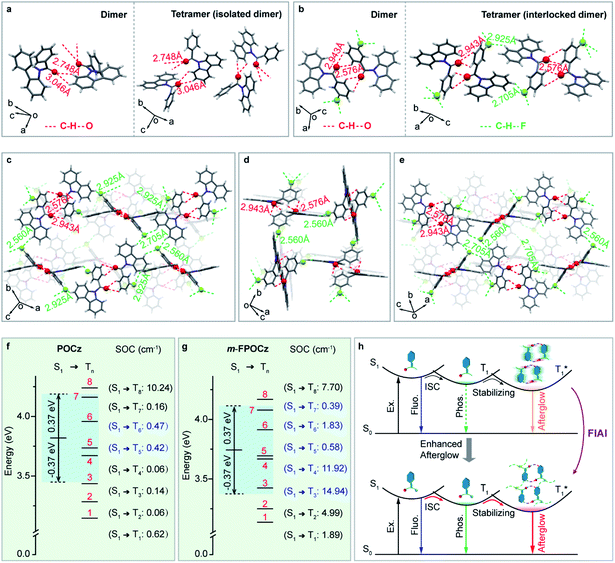
To exactly understand the improved afterglow performance of fluorine-substituted afterglow molecules, we further analyzed the molecular packing modes and intermolecular interactions in single crystals. As shown in Fig. 3a (left), obvious intermolecular interactions of C–H⋯O with distances of 3.046 and 2.748 Å were observed in the dimer of the POCz crystal. Nevertheless, because there are no intermolecular interactions between adjacent dimers (Fig. 3a, right and S37†), the facile molecular motion and vibration induce severe non-radiative decays (Fig. 1b, left), thereby leading to relatively poor afterglow performance of POCz. In contrast, besides various C–H⋯O interactions in the dimers of the o/m/p-FPOCz crystals (Fig. 3b, left and S38a and S39a†), there are also abundant intermolecular interactions of C–H⋯F in the neighboring dimers for further suppressed non-radiative decays (Fig. 3c–e, S38b, c and S39b, c†). The enhanced intermolecular interactions of o/m/p-FPOCz compared to POCz can be experimentally verified by more stable afterglow emission and lifetimes in different atmospheres of Ar, air and O2 (Fig. S32–S36†) and theoretically revealed by independent gradient model (IGM) analysis showing a larger IGM isosurface with stronger interactions between neighboring molecules (Fig. S40†). Meanwhile, powder XRD measurements were also performed to further verify the enhanced intermolecular interactions in the o/m/p-FPOCz crystals. An obvious difference could be visually observed in their XRD spectra (Fig. S41†), showing much more sharp peaks ranging from 20 to 50° in the curve of o/m/p-FPOCz, which indicate their enhanced interactions and rigidities compared to POCz. Additionally, multiple H-aggregations that are very crucial in achieving efficient afterglow emission were found in the o/m/p-FPOCz single crystals (Table S3†). These strong intermolecular interactions can effectively facilitate the formation of steric-interlocked molecular aggregates along different directions in single crystals to greatly inhibit non-radiative decays, leading to high afterglow performance.
To theoretically investigate the promoted ISC through fluorine incorporation, time dependent density functional theory (TD-DFT) and Dalton calculations were carried out.34,35 From the simulated excited state energy levels, there are multiple triplet states in o/m/p-FPOCz molecules with the singlet and triplet splitting energy (ΔEST) lower than 0.37 eV for achieving possible ISC channels according to the energy gap law.36 Moreover, the feasible ISC process can be further confirmed by large SOC values according to the EI-sayed rule.37 Indeed, the SOC values of o/m/p-FPOCz are greatly larger than that of POCz (Fig. 3f and g and S42†). On the basis of small ΔEST (<0.37 eV) and large SOC values (>0.3 cm−1), the number of efficient ISC channels predicted in POCz, o-FPOCz, m-FPOCz and p-FPOCz is 2, 3, 5 and 4, clearly indicating the enhanced ISC process in fluorine-substituted isomers;38 moreover, the larger SOC values and stronger intermolecular interactions in m-FPOCz crystals should be the main reason for their higher afterglow efficiency. On account of both experimental and theoretical investigations, the facilitated organic afterglow of the fluorine-incorporated molecules could be ascribed to suppressed non-radiative transition and promoted ISC achieved by the FIAI strategy (Fig. 3h).
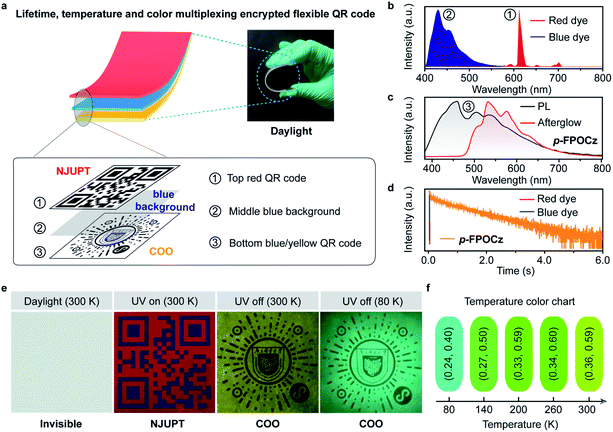
Given the extraordinary ultralong-lived and temperature-dependent color-tunable feature of the afterglow molecules designed via the FIAI strategy, we take one step further to demonstrate the potential applications of these promising afterglow materials in the fields of lifetime, temperature and color tri-mode multiplexing encryption quick response (QR) code as well as visual sensing of cryogenic temperature (Fig. 4 and S43†). The multilayer QR code comprises a top short-lived fluorescence red and blue QR layer (NJUPT) and a bottom ultralong lived yellow afterglow QR (COO) layer (Fig. 4a–d). As shown in Fig. 4e, the multilayer QR code is very difficult to observe in daylight. Under irradiation using a 365 nm UV-lamp, the top red and blue QR layer can be facilely captured and identified using a commercial mobile-phone displaying the information of “NJUPT” and the bottom QR layer showing blue emission under 365 nm irradiation is actually indiscernible because of the interference of the middle blue fluorescence background layer. After the ceasing of 365 nm irradiation light, the short-lived background fluorescence interference immediately vanishes; the bottom yellow afterglow QR code layer appears and can be seen showing the varied information of “COO” (Fig. 4e). And, on cooling from room temperature to 80 K, the emission color of the bottom afterglow QR code layer changes from yellow to blue-green, demonstrating its unique feature of lifetime, temperature and color-based tri-mode encryption, and visual multicolor display for quantitative temperature sensing (Fig. 4f).
Conclusions
In summary, we propose a new strategy to simultaneously enhance the efficiency and lifetime of organic afterglow materials by fluorine-induced molecular aggregate interlocking. The introduction of fluorine atoms can not only favor n–π* transition to promote the ISC process, but can also form strong non-covalent intermolecular interactions between adjacent molecules to suppress the molecular vibration and motion of dimers. Consequently, o/m/p-FPOCz demonstrate greatly elevated ISC rates up to 5.84 × 107 s−1 and remarkably suppressed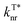 down to 0.89 s−1, thus resulting in significantly boosted afterglow emission with a lifetime up to 1.09 s and efficiency of 10.5% under ambient conditions. More impressively, with dual phosphorescence from amorphous T1 and aggregated
down to 0.89 s−1, thus resulting in significantly boosted afterglow emission with a lifetime up to 1.09 s and efficiency of 10.5% under ambient conditions. More impressively, with dual phosphorescence from amorphous T1 and aggregated 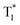 at cryogenic temperature, color-tunable afterglow emission at different temperatures was achieved. Thus, the tri-mode multiplexing encryption QR code and temperature sensing were successfully established based on the prominent high-efficiency, ultralong-lifetime and color-tunable nature of the organic afterglow molecules designed by the FIAI strategy. These results of simultaneously achieving the afterglow lifetime and efficiency via FIAI furnish a simple but efficient approach in developing high-performance organic afterglow molecules, providing an exciting and promising avenue to overcome the intrinsic conflict between the afterglow efficiency and lifetime for fascinating applications.
at cryogenic temperature, color-tunable afterglow emission at different temperatures was achieved. Thus, the tri-mode multiplexing encryption QR code and temperature sensing were successfully established based on the prominent high-efficiency, ultralong-lifetime and color-tunable nature of the organic afterglow molecules designed by the FIAI strategy. These results of simultaneously achieving the afterglow lifetime and efficiency via FIAI furnish a simple but efficient approach in developing high-performance organic afterglow molecules, providing an exciting and promising avenue to overcome the intrinsic conflict between the afterglow efficiency and lifetime for fascinating applications.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported in part by the National Natural Science Foundation of China (21704042, 62075102, 21604039, 61875090, 91833306, 22075149 and 21674049), the Six Talent Plan of Jiangsu Province (XCL-049), 1311 Talents Program of Nanjing University of Posts and Telecommunications (Dingshan), Natural Science Fund for Colleges and Universities in Jiangsu Province (Grant No. 17KJB150017), China Postdoctoral Science Foundation funded project (2018M642284), Nanjing University of Posts and Telecommunications Start-up Fund (NUPTSF) (NY219007 and NY217140), and Postgraduate Research & Practice Innovation Program of Jiangsu Province (46030CX18011).Notes and references
- Kenry, C. Chen and B. Liu, Nat. Commun., 2019, 10, 2111 CrossRef CAS.
- S. Xu, R. Chen, C. Zheng and W. Huang, Adv. Mater., 2016, 28, 9920 CrossRef CAS.
- L. Gu, H. Shi, L. Bian, M. Gu, K. Ling, X. Wang, H. Ma, S. Cai, W. Ning, L. Fu, H. Wang, S. Wang, Y. Gao, W. Yao, F. Huo, Y. Tao, Z. An, X. Liu and W. Huang, Nat. Photonics, 2019, 13, 406 CrossRef CAS.
- W. Li, Q. Huang, Z. Mao, J. Zhao, H. Wu, J. Chen, Z. Yang, Y. Li, Z. Yang, Y. Zhang, M. P. Aldred and Z. Chi, Angew. Chem., Int. Ed., 2020, 59, 3739 CrossRef CAS.
- H. Li, H. Li, W. Wang, Y. Tao, S. Wang, Q. Yang, Y. Jiang, C. Zheng, W. Huang and R. Chen, Angew. Chem., Int. Ed., 2020, 59, 4756 CrossRef CAS.
- Y. Su, S. Z. F. Phua, Y. Li, X. Zhou, D. Jana, G. Liu, W. Q. Lim, W. K. Ong, C. Yang and Y. Zhao, Sci. Adv., 2018, 4, eaas9732 CrossRef.
- M. Gmelch, H. Thomas, F. Fries and S. Reineke, Sci. Adv., 2019, 5, u7310 CrossRef.
- J. Jin, H. Jiang, Q. Yang, L. Tang, Y. Tao, Y. Li, R. Chen, C. Zheng, Q. Fan, K. Y. Zhang, Q. Zhao and W. Huang, Nat. Commun., 2020, 11 Search PubMed.
- J. Yang, X. Zhen, B. Wang, X. Gao, Z. Ren, J. Wang, Y. Xie, J. Li, Q. Peng, K. Pu and Z. Li, Nat. Commun., 2018, 9, 840 CrossRef.
- X. Chen, C. Xu, T. Wang, C. Zhou, J. Du, Z. Wang, H. Xu, T. Xie, G. Bi, J. Jiang, X. Zhang, J. N. Demas, C. O. Trindle, Y. Luo and G. Zhang, Angew. Chem., Int. Ed., 2016, 55, 9872 CrossRef CAS.
- R. Hu, Y. Zhang, Y. Zhao, X. Wang, G. Li and C. Wang, Chem. Eng. J., 2020, 392, 124807 CrossRef CAS.
- K. Van den Eeckhout, D. Poelman and P. Smet, Materials, 2013, 6, 2789 CrossRef CAS.
- L. Huang, B. Chen, X. Zhang, C. O. Trindle, F. Liao, Y. Wang, H. Miao, Y. Luo and G. Zhang, Angew. Chem., Int. Ed., 2018, 57, 16046 CrossRef CAS.
- S. Zheng, T. Zhu, Y. Wang, T. Yang and W. Z. Yuan, Angew. Chem., Int. Ed., 2020, 59, 10018 CrossRef CAS.
- P. Xue, P. Wang, P. Chen, B. Yao, P. Gong, J. Sun, Z. Zhang and R. Lu, Chem. Sci., 2017, 8, 6060 RSC.
- W. Zhao, Z. He, J. W. Y. Lam, Q. Peng, H. Ma, Z. Shuai, G. Bai, J. Hao and B. Z. Tang, Chem, 2016, 1, 592 CAS.
- H. Feng, J. Zeng, P. Yin, X. Wang, Q. Peng, Z. Zhao, J. W. Y. Lam and B. Z. Tang, Nat. Commun., 2020, 11, 2617 CrossRef CAS.
- Z. Lin, R. Kabe, K. Wang and C. Adachi, Nat. Commun., 2020, 11, 191 CrossRef CAS.
- X. Zhang, L. Du, W. Zhao, Z. Zhao, Y. Xiong, X. He, P. F. Gao, P. Alam, C. Wang, Z. Li, J. Leng, J. Liu, C. Zhou, J. W. Y. Lam, D. L. Phillips, G. Zhang and B. Z. Tang, Nat. Commun., 2019, 10, 5161 CrossRef.
- Y. Gong, L. Zhao, Q. Peng, D. Fan, W. Z. Yuan, Y. Zhang and B. Z. Tang, Chem. Sci., 2015, 6, 4438 RSC.
- W. Zhao, T. S. Cheung, N. Jiang, W. Huang, J. W. Y. Lam, X. Zhang, Z. He and B. Z. Tang, Nat. Commun., 2019, 10, 1595 CrossRef.
- Z. An, C. Zheng, Y. Tao, R. Chen, H. Shi, T. Chen, Z. Wang, H. Li, R. Deng, X. Liu and W. Huang, Nat. Mater., 2015, 14, 685 CrossRef CAS.
- L. Bian, H. Shi, X. Wang, K. Ling, H. Ma, M. Li, Z. Cheng, C. Ma, S. Cai, Q. Wu, N. Gan, X. Xu, Z. An and W. Huang, J. Am. Chem. Soc., 2018, 140, 10734 CrossRef CAS.
- Z. Mao, Z. Yang, Z. Fan, E. Ubba, W. Li, Y. Li, J. Zhao, Z. Yang, M. P. Aldred and Z. Chi, Chem. Sci., 2019, 10, 179 RSC.
- Y. Tao, R. Chen, H. Li, J. Yuan, Y. Wan, H. Jiang, C. Chen, Y. Si, C. Zheng, B. Yang, G. Xing and W. Huang, Adv. Mater., 2018, 30, 1803856 CrossRef.
- H. Ma, H. Yu, Q. Peng, Z. An, D. Wang and Z. Shuai, J. Phys. Chem. Lett., 2019, 10, 6948 CrossRef CAS.
- A. A. Berger, J. Völler, N. Budisa and B. Koksch, Acc. Chem. Res., 2017, 50, 2093 CrossRef CAS.
- K. Reichenbächer, H. I. Süss and J. Hulliger, Chem. Soc. Rev., 2005, 34, 22 RSC.
- Y. Li, J. Wang, Y. Liu, M. Qiu, S. Wen, X. Bao, N. Wang, M. Sun and R. Yang, ACS Appl. Mater. Interfaces, 2016, 8, 26152 CrossRef CAS.
- N. Bauer, Q. Zhang, J. J. Rech, S. Dai, Z. Peng, H. Ade, J. Wang, X. Zhan and W. You, Nano Res., 2019, 12, 2400 CrossRef CAS.
- Y. Xie, Y. Ge, Q. Peng, C. Li, Q. Li and Z. Li, Adv. Mater., 2017, 29, 1606829 CrossRef.
- S. Cai, H. Shi, J. Li, L. Gu, Y. Ni, Z. Cheng, S. Wang, W. Xiong, L. Li, Z. An and W. Huang, Adv. Mater., 2017, 29, 1701244 CrossRef.
- S. Hirata, Adv. Opt. Mater., 2017, 5, 1700116 CrossRef.
- J. Yuan, R. Chen, X. Tang, Y. Tao, S. Xu, L. Jin, C. Chen, X. Zhou, C. Zheng and W. Huang, Chem. Sci., 2019, 10, 5031 RSC.
- J. Yuan, S. Wang, Y. Ji, R. Chen, Q. Zhu, Y. Wang, C. Zheng, Y. Tao, Q. Fan and W. Huang, Mater. Horiz., 2019, 6, 1259 RSC.
- T. Chen, L. Zheng, J. Yuan, Z. An, R. Chen, Y. Tao, H. Li, X. Xie and W. Huang, Sci. Rep., 2015, 5, 10923 CrossRef.
- J. Zhao, W. Wu, J. Sun and S. Guo, Chem. Soc. Rev., 2013, 42, 5323 RSC.
- Y. Tao, K. Yuan, T. Chen, P. Xu, H. Li, R. Chen, C. Zheng, L. Zhang and W. Huang, Adv. Mater., 2014, 26, 7931 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 149367, 1975648, 1975612, and 1975625. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0sc06025a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |