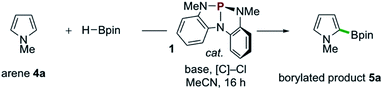Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Organophosphorus-catalyzed relay oxidation of H-Bpin: electrophilic C–H borylation of heteroarenes†
Jeffrey M.
Lipshultz
 a,
Yue
Fu
b,
Peng
Liu
a,
Yue
Fu
b,
Peng
Liu
 *b and
Alexander T.
Radosevich
*b and
Alexander T.
Radosevich
 *a
*a
aDepartment of Chemistry, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139, USA. E-mail: radosevich@mit.edu
bDepartment of Chemistry, University of Pittsburgh, 219 Parkman Avenue, Pittsburgh, PA 15260, USA. E-mail: pengliu@pitt.edu
First published on 19th November 2020
Abstract
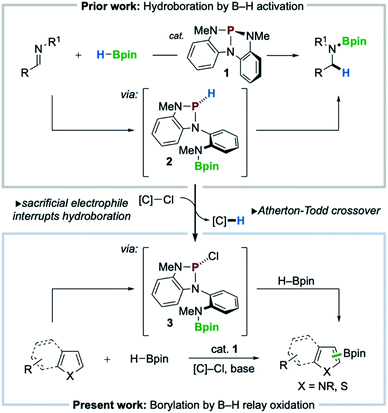
A nontrigonal phosphorus triamide (1, P{N[o-NMe-C6H4]2}) is shown to catalyze C–H borylation of electron-rich heteroarenes with pinacolborane (HBpin) in the presence of a mild chloroalkane reagent. C–H borylation proceeds for a range of electron-rich heterocycles including pyrroles, indoles, and thiophenes of varied substitution. Mechanistic studies implicate an initial P–N cooperative activation of HBpin by 1 to give P-hydrido diazaphospholene 2, which is diverted by Atherton–Todd oxidation with chloroalkane to generate P-chloro diazaphospholene 3. DFT calculations suggest subsequent oxidation of pinacolborane by 3 generates chloropinacolborane (ClBpin) as a transient electrophilic borylating species, consistent with observed substituent effects and regiochemical outcomes. These results illustrate the targeted diversion of established reaction pathways in organophosphorus catalysis to enable a new mode of main group-catalyzed C–H borylation.
Introduction
Recent innovations in synthetic organophosphorus chemistry are fueling new opportunities for catalysis.1 As a complement to well-known nucleophilic (Lewis basic) reactivity,2 new structural design principles are emerging that now enable organophosphorus catalysis to comprise Lewis acidic,3 dehydrative,4 redox O-atom transfer,5 and reductive6 activation modes for catalysis. Within this vein, nontrigonal phosphorus triamide 1 (ref. 7) was reported to catalyze the activation and transfer of H–Bpin to imines in a ligand cooperative8 fashion via the intermediacy of P-hydrido diazaphospholene 2 (Scheme 1, top).9 The pronounced hydricity of related P-hydrido diazaphospholenes—studied extensively by Gudat10— has been advanced by Kinjo,11 Speed,12 Cramer,13 and Melen14 within the context of hydroboration catalysis to effect either 1,2- or 1,4-addition of H–Bpin to π-electrophiles (e.g. imines, carbonyls, and pyridines).With a view toward enabling new reactivity, we considered that catalytic hydroboration by 1 might be interrupted by the inclusion of an exogenous sacrificial electrophile to scavenge the activated hydride of 2, achieving catalytic oxidative crossover to P-chloro diazaphospholene 3. Doing so would present the possibility that the established reductive manifold, i.e. hydroboration with H–Bpin, of diazaphospholene catalysis might be diverted via sequential oxidative transfers, or relay oxidation, to access electrophilic “pinB+” synthons15 for delivery to suitable nucleophilic substrates for C–H borylation (Scheme 1, bottom). Herein, we realize this vision and achieve a C–H borylation16 of electron-rich heteroarenes with H–Bpin as the boron donor under the catalytic action of phosphorus triamide 1 and a mild chloroalkane oxidant, establishing a new organophosphorus catalyzed platform for C–H borylation.
Results and discussion
The success of the proposed catalytic platform is predicated upon the compatibility and interplay of both reducing (HBpin) and oxidizing (sacrificial electrophile) reagents with the organophosphorus catalyst 1. Inspired by an observed Atherton–Todd-like17 chlorination of P-hydrido diazaphospholene 2,9 we considered the possibility that even a weak electrophilic reagent such as chloroform might efficiently serve to capture the P–H hydride of 2 and thus divert H–Bpin activation toward electrophilic borylation via relay oxidation. As an initial probe of this hypothesis, the reaction of N-Me-pyrrole (4a) with HBpin (1 equiv.) and NEt3 under the action of catalytic phosphorus triamide 1 (10 mol%) in chloroform at 80 °C was attempted. In the event, C–H borylation of 4a was indeed observed, giving C2-functionalized product 5a in 8% yield (Table 1, entry 1). Use of CHCl3 in reagent quantities in MeCN proved equally effective (entry 2), and increasing the reaction temperature to 100 °C resulted in improved 29% yield (entry 3). Although cationic borenium reagents are known to produce a mixture of borylation regioisomers,15b the C3-functionalized isomer was not detected under these catalytic conditions. When bromoform was used in place of chloroform over a range of temperatures, no borylated product was observed (entry 4), and the use of exogenous bromide with CHCl3 saw no improvement in yield (28% yield, entry 5). An evaluation of organic and inorganic bases determined that Hünig's base was optimal, providing 35% yield (entry 6), while optimization of reagent and catalyst loading resulted in further improvement to 50% yield (entry 7). Replacement of CHCl3 with a higher-boiling chloroalkane (α,α-dichlorotoluene,18 entries 9 and 10) improved the efficiency to 60% yield. Use of catecholborane in place of pinacolborane resulted in no borylation, as rapid decomposition of catalyst 1 was observed (entry 11). Control experiments establish that catalyst 1, base, and chloroalkane are each required for C–B bond formation (entries 12–14). Thus, phosphorus triamide 1 is indeed responsible for catalyzing this C–H borylation reaction, establishing new precedent for organophosphorus catalysis of this valuable transformation as a complement to established transition metal-8b,19,29 and organoboron-catalyzed20 methods.| Entry | 1 (mol%) | HBpin (equiv.) | Base (equiv.) | [C]–Cl (equiv.) | Temp (°C) | 5a (%) |
|---|---|---|---|---|---|---|
| a Reactions conducted on 0.125 mmol scale, 0.25 M in MeCN. b 1H NMR yields compared to internal standard. c CHCl3 used as solvent in place of MeCN. d Reactions conducted at 25, 50, and 80 °C also yielded 0% 5a. e Et4NBr (0.1 equiv.) additive. f HBcat (2 equiv.) used in place of HBpin. HBpin = pinacolborane. HBcat = catecholborane. | ||||||
| 1 | 10 | 1 | NEt3 (1) | CHCl3c | 80 | 8 |
| 2 | 10 | 1 | NEt3 (1) | CHCl3 (2) | 80 | 8 |
| 3 | 10 | 1 | NEt3 (1) | CHCl3 (2) | 100 | 29 |
| 4 | 10 | 1 | NEt3 (1) | CHBr3 (2) | 100d | 0 |
| 5 | 10 | 1 | NEt3 (1) | CHCl3 (2)e | 100 | 28 |
| 6 | 10 | 1 | EtNiPr2 (1) | CHCl3 (2) | 100 | 35 |
| 8 | 20 | 2 | EtNiPr2 (2) | CHCl3 (2) | 100 | 50 |
| 9 | 20 | 2 | EtNiPr2 (2) | PhCHCl2 (2) | 100 | 60 |
| 10 | 20 | 2 | EtNiPr2 (2) | PhCHCl2 (1) | 100 | 60 |
| 11 | 20 | 0f | EtNiPr2 (2) | PhCHCl2 (1) | 100 | 0 |
| 12 | 0 | 2 | EtNiPr2 (2) | PhCHCl2 (1) | 100 | 0 |
| 13 | 20 | 2 | 0 | PhCHCl2 (1) | 100 | 0 |
| 14 | 20 | 2 | EtNiPr2 (2) | None | 100 | 0 |
Examples of the C–H borylation of electron-rich heteroarenes illustrating the scope and limitations of this phosphacatalytic method are shown in Scheme 2. While the borylation of N-Me-pyrrole (4a) provided exclusively C2-borylated product 5a in 60% yield, borylation is efficiently diverted to the C3 position by blocking both the C2- and C5-positions with methyl groups as in 5b (67% yield at 80 °C). When the same conditions were applied to N-Me-indole, similarly efficient C3-borylation was achieved, delivering product 5c as the sole regioisomer in 73% yield. Substitution on the 5-membered ring, as in 1,2-dimethylindole, led to increased reactivity at the sterically encumbered C3-position even at lower temperature (5d, 86% yield, 60 °C), indicating an overwhelming electronic bias relative to steric effects. Substitution at all positions of the benzenoid ring could be similarly tolerated, as methyl substitution at the C4-position had no effect on the efficiency with respect to unsubstituted indole substrate, yielding 5e in 74% yield. Substitution at each of the C5-, C6-, and C7-positions with a methoxy group boosted efficiency, delivering C3-borylated product in excellent yield (5f–5h, 83–87% yields). While N-H and N-silyl indoles did not deliver synthetically useful yield of borylation products, N-Bn-indole could be borylated in good efficiency (5i, 45% yield, >99% yield based on recovered starting material). Also, tricyclic indole alkaloid lilolidine could be borylated in near-quantitative efficiency to deliver 5j (98% yield). Other electron-rich heterocycles are subject to borylation, as demonstrated by the formation of 5k from 2-OMe-thiophene. However, π-rich benzenes are unreactive, as exemplified by no formation of 5l from N,N-dimethylaniline.21
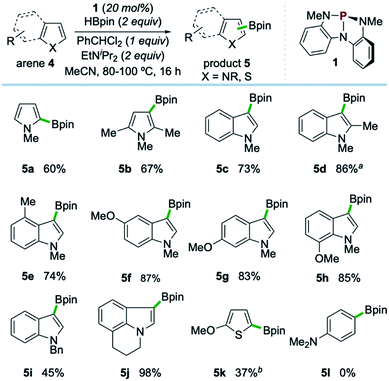 | ||
| Scheme 2 Synthetic scope of organophosphorus-catalyzed C–H borylation of electron-rich heterocycles. All yields isolated from 0.5 mmol scale reactions conducted for 16 hours. See ESI† for full synthetic details. aReaction conducted at 60 °C. bReaction conducted with 2 equivalents of 2-methoxythiophene (4k), yield based on HBpin as limiting reagent. See ESI.† | ||
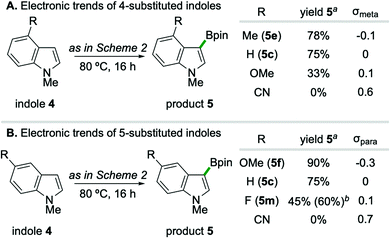
A systematic variation of substituents on the benzenoid ring of indole substrates revealed a reactivity pattern which is gated by heteroarene nucleophilicity (Scheme 3),22 indicative of a borylation event proceeding via an electrophilic aromatic substitution reaction (SEAr) pathway.23 In the case of C4- substituted indoles (Scheme 3A), the formation of borylation products 5 correlates with Hammett substituent constant σmeta; specifically, inclusion of a 4-methoxy substituent (σmeta = 0.1) leads to lower yield (33%) than the parent 5-unsubstituted substrate (75%). Relatedly, C5-substitution of indoles trend with substituent constant σpara (Scheme 3B), such that 5-methoxy substitution (σpara = −0.3) gives higher yield (90%) than the parent 4-unsubstituted substrate.24 While strongly electron-withdrawing substituents such as CN completely suppressed borylation independent of C4/C5 position on the indole, weakly withdrawing 5-F substitution on 1-methylindole provided synthetically useful levels of efficiency upon conducting the reaction at 100 °C for 24 hours (5m, 60% yield).
In situ NMR analysis of a representative catalytic reaction is consistent with the evolution of compounds 1, 2, and 3 as envisioned (Scheme 4). Specifically, 31P{1H} NMR spectral monitoring of the catalytic borylation of 1,2-dimethylindole (4d) with HBpin and CHCl3 by catalyst 1 shows initial conversion of 1 (δ 159.7 ppm) to P-hydrido diazaphospholene 2 (δ 85.4 ppm) by reaction with HBpin. Compound 2 is further converted to P-chloro diazaphospholene 3 (δ 147.0 ppm) within ca. 2 h, ultimately reaching a steady state ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (ca. 4
2 (ca. 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) that persists for the duration of the borylation reaction (96% yield of 5d after 16 h). Complementary monitoring in the 1H and 11B NMR channels indicates a delay in formation of borylation product 5d until a significant concentration of 3 is accrued (2% yield of 5d at 2 h). Evidently, P-chloro diazaphospholene 3 is necessary for product formation; indeed, when 3 is employed directly as precatalyst under otherwise identical conditions, formation of product 5d is observed without an induction period. Moreover, 31P NMR spectra confirm the formation of 2 under these catalytic reaction conditions with precatalyst 3, converging on a 4
1) that persists for the duration of the borylation reaction (96% yield of 5d after 16 h). Complementary monitoring in the 1H and 11B NMR channels indicates a delay in formation of borylation product 5d until a significant concentration of 3 is accrued (2% yield of 5d at 2 h). Evidently, P-chloro diazaphospholene 3 is necessary for product formation; indeed, when 3 is employed directly as precatalyst under otherwise identical conditions, formation of product 5d is observed without an induction period. Moreover, 31P NMR spectra confirm the formation of 2 under these catalytic reaction conditions with precatalyst 3, converging on a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 steady state ratio of 3
1 steady state ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 as was observed by reaction with precatalyst 1. Taken together, these spectroscopic results are consistent with sequential activation of HBpin by 1 and of CHCl3 by 2, followed by turnover-limiting reaction of 3, presumably to effect C–H borylation of 4d.
2 as was observed by reaction with precatalyst 1. Taken together, these spectroscopic results are consistent with sequential activation of HBpin by 1 and of CHCl3 by 2, followed by turnover-limiting reaction of 3, presumably to effect C–H borylation of 4d.
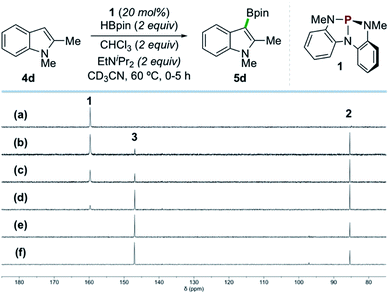 | ||
| Scheme 4 Time-stacked in situ31P NMR spectra of the borylation of 4d by HBpin as in Scheme 2 (CD3CN, 202 MHz, 60 °C) at the following time points: (a) 0 min, (b) 15 min, (c) 30 min, (d) 1 h, (e) 3 h, and (f) 5 h, showing 1 (δ 159.7 ppm), 2 (δ 85.4 ppm), and 3 (δ 147.0 ppm). | ||
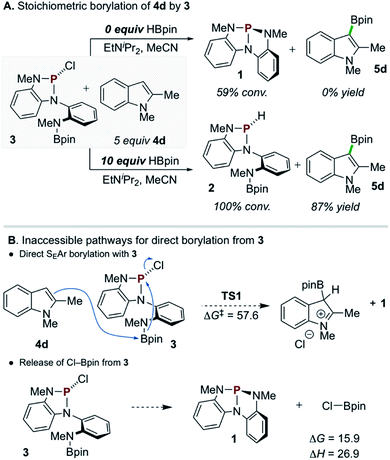
A single turnover experiment reacting 3 with 4d under conditions that lack exogenous chloroalkane reagent but otherwise approximate the catalytic reaction (i.e. containing HBpin and EtNiPr2) resulted in formation of borylated product 5d in 87% yield with respect to 3 (Scheme 5A, bottom). In this reaction, P-chloro diazaphospholene 3 is converted cleanly into P-hydrido diazaphospholene 2 with no observable intermediates along the reaction pathway (see ESI† for in situ1H and 31P NMR reaction profile). However, an analogous stoichiometric reaction of 3 with 4d omitting the additional HBpin did not lead to C–H borylation (Scheme 5A, top); moreover, 31P NMR spectra demonstrate the conversion of P-chloro diazaphospholene 3 to the nontrigonal phosphorus triamide 1 in this experiment. Evidently, P-chloro diazaphospholene 3 is, in itself, necessary but insufficient to effect the C–H borylation of 4d under these single turnover conditions. Although a downstream borylating species arising from the interaction of 3 and HBpin might be presumed, the simple mixture of 3 and HBpin at 60 °C does not yield any spectroscopic changes. Regrettably, then, the identity of the active borylating species cannot be unambiguously assigned at this time via experimental means.
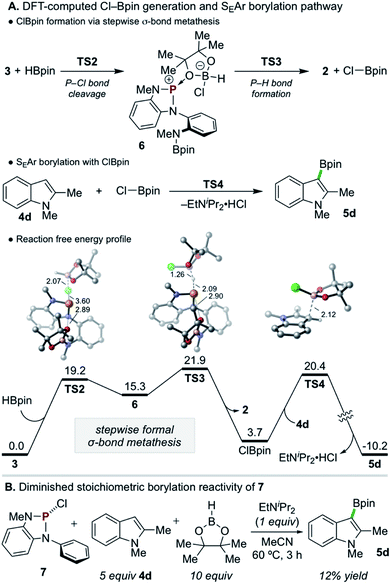
Density functional theory (DFT) calculations were performed to investigate the reactivity of 3 as the active borylating species or precursor. Consistent with the aforementioned experimental results, direct SEAr borylation of 4d with the pendant Bpin moiety of 3 (TS1, ΔG‡ = 57.6 kcal mol−1) requires an insurmountably high activation energy (Scheme 5B, top).25 Further, unimolecular decomposition of 3 to generate 1 and electrophilic Cl–Bpin was found to be substantially uphill (Scheme 5B, bottom, ΔG = 15.9 kcal mol−1),26 indicating that, while 1 is observed as a decay product of 3 in the presence of 4d and EtNiPr2 (see Scheme 5A, top), it is likely not generating Cl–Bpin in this process due to the energetic penalty.
Given the evident requirement of H–Bpin in addition to 3 to achieve C–H borylation, DFT calculations were performed on higher-order reaction pathways (see ESI† for full details). These calculations suggest that highly electrophilic chloropinacolborane (Cl–Bpin)27 can be generated via a stepwise, formal σ-bond metathesis between H–Bpin and the P–Cl bond of 3 with kinetically accessible barriers of ΔG‡ = 19.2 and 21.9 kcal mol−1 for P–Cl cleavage (TS2) and P–H formation (TS3), respectively, via the intermediacy of 6 (Scheme 6A). Although the conversion of H–Bpin to Cl–Bpin is endergonic by 3.7 kcal mol−1, the subsequent borylation of 4d with Cl–Bpin proceeds with a low relative barrier of 16.7 kcal mol−1 (TS4) and is highly exergonic upon deprotonation and rearomatization.
Although such an endergonic exchange reaction between HBpin and 3 was not observed experimentally (via in situ NMR of the full reaction mixture or isolated reaction of the two species), insights can be drawn from analysis of the stepwise metathesis pathway. In the course of the reaction of 3 and HBpin to form 2, the distance between an O-atom of the Bpin moiety of 3 and the electropositive P-atom shortens in the rate-limiting TS (d(P–O) = 2.95 Å and 2.90 Å in 3 and TS3, respectively), indicating the formation of Cl–Bpin is possibly promoted by a stabilizing P–O interaction in the transition state. To probe this hypothesis, diazaphospholene 7, which lacks the pendant N(Me)Bpin moiety, was synthesized and exposed to the single turnover conditions (Scheme 6B). Consistent with the delineated hypothesis, product 5d was formed in substantially diminished yield of 12%.28 Thus, this unexpected Lewis base-stabilization effect provides a potential guiding principle for future development.
In accordance with the preceding experimental and computational results, the mechanism in Scheme 7 is proposed. First, during the induction period phosphorus triamide 1 activates HBpin to generate P-hydrido diazaphospholene 2. Then, catalytic relay oxidation occurs in which 2 reacts with chloroalkane to generate P-chloro diazaphospholene 3, followed by stepwise metathesis with H–Bpin to provide Cl–Bpin and regenerate 2. In effect, the facile oxidation of the P–H bond of 2 enables the downstream oxidation of the B–H bond of H–Bpin. Subsequently, the catalytically generated, substoichiometric electrophile Cl–Bpin can undergo SEAr borylation with substrate to provide borylated product. Notably, Cl–Bpin is known to be extremely unstable and difficult to prepare,27 and its reactivity in SEAr borylation has therefore not been previously reported. Thus, a catalytic platform for the in situ substoichiometric generation of Cl–Bpin from H–Bpin is a novel approach to borylative chemistry via relay oxidation, as proposed in Scheme 1.
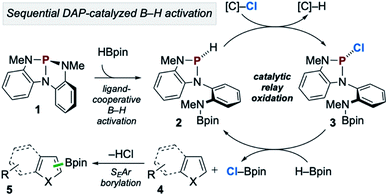 | ||
| Scheme 7 Proposed catalytic reaction pathway for C–H borylation of heteroarenes via organophosphorus-catalyzed relay oxidation. | ||
Conclusions
To summarize, nontrigonal phosphorus triamide 1 represents the first organophosphorus catalyst to enable C–H borylation of electron-rich heteroarenes. In this transformation, a novel mode of catalysis is realized by targeted diversion of an established hydroboration pathway of 2 with a sacrificial chloroalkane electrophile via Atherton–Todd oxidation, diverting the reactivity towards electrophilic borylation from 3. Computational studies support the in situ generation of the highly electrophilic Cl–Bpin, which serves as a fleeting intermediate for SEAr borylation, avoiding the difficulties inherent to working with stoichiometrically-generated Cl–Bpin. This novel phosphacatalytic system is poised for further study of the impact of catalyst structure on this mode of catalysis in borylative transformations.Conflicts of interest
There are no conflicts to declare.Acknowledgements
ATR acknowledges support from the NSF (CHE-1900060) and MIT. PL acknowledges support from the NSF (CHE-1654122). J. M. L. thanks the Camille and Henry Dreyfus Foundation for a postdoctoral fellowship in Environmental Chemistry. DFT calculations were performed at the Center for Research Computing at the University of Pittsburgh, the TACC Frontera supercomputer, and the Extreme Science and Engineering Discovery Environment (XSEDE) supported by the National Science Foundation grant number ACI-1548562. The authors acknowledge J. Connor Gilhula and Hye Won Moon for assistance in acquiring HRMS data.Notes and references
- H. Guo, Y. C. Fan, Z. Sun, Y. Wu and O. Kwon, Chem. Rev., 2018, 118, 10049–10293 CrossRef CAS.
- E. Vedejs and S. E. Denmark, Lewis Base Catalysis in Organic Synthesis, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2016 Search PubMed.
- (a) J. M. Bayne and D. W. Stephan, Chem. Soc. Rev., 2016, 45, 765–774 RSC; (b) C. B. Caputo, L. J. Hounjet, R. Dobrovetsky and D. W. Stephan, Science, 2013, 341, 1374–1377 CrossRef CAS; (c) J. C. Gilhula and A. T. Radosevich, Chem. Sci., 2019, 10, 7177–7182 RSC.
- (a) H. A. Van Kalkeren, S. H. A. M. Leenders, C. R. A. Hommersom, F. P. J. T. Rutjes and F. L. Van Delft, Chem.–Eur. J., 2011, 17, 11290–11295 CrossRef CAS; (b) R. H. Beddoe, K. G. Andrews, V. Magné, J. D. Cuthbertson, J. Saska, A. L. Shannon-Little, S. E. Shanahan, H. F. Sneddon and R. M. Denton, Science, 2019, 914, 910–914 CrossRef; (c) M. Lecomte, J. M. Lipshultz, S.-H. Kim-Lee, G. Li and A. T. Radosevich, J. Am. Chem. Soc., 2019, 141, 12507–12512 CrossRef CAS.
- (a) W. Zhao, P. K. Yan and A. T. Radosevich, J. Am. Chem. Soc., 2015, 137, 616–619 CrossRef CAS; (b) T. V. Nykaza, T. S. Harrison, A. Ghosh, R. A. Putnik and A. T. Radosevich, J. Am. Chem. Soc., 2017, 139, 6839–6842 CrossRef CAS; (c) T. V. Nykaza, A. Ramirez, T. S. Harrison, M. R. Luzung and A. T. Radosevich, J. Am. Chem. Soc., 2018, 140, 3103–3113 CrossRef CAS; (d) A. Ghosh, M. Lecomte, S. H. Kim-Lee and A. T. Radosevich, Angew. Chem., Int. Ed., 2019, 58, 2864–2869 CrossRef CAS.
- (a) N. L. Dunn, M. Ha and A. T. Radosevich, J. Am. Chem. Soc., 2012, 134, 11330–11333 CrossRef CAS; (b) K. D. Reichl, N. L. Dunn, N. J. Fastuca and A. T. Radosevich, J. Am. Chem. Soc., 2015, 137, 5292–5295 CrossRef CAS.
- (a) W. Zhao, S. M. McCarthy, T. Y. Lai, H. P. Yennawar and A. T. Radosevich, J. Am. Chem. Soc., 2014, 136, 17634–17644 CrossRef CAS; (b) K. Lee, A. V. Blake, A. Tanushi, S. M. McCarthy, D. Kim, S. M. Loria, C. M. Donahue, K. D. Spielvogel, J. M. Keith, S. R. Daly and A. T. Radosevich, Angew. Chem., Int. Ed., 2019, 58, 6993–6998 CrossRef CAS.
- For ligand-cooperativity of p-block compounds, see: (a) L. Greb, F. Ebner, Y. Ginzburg and L. M. Sigmund, Eur. J. Inorg. Chem., 2020, 3030–3047 CAS . For organometallic ligand-cooperative activation of HBpin for C–H borylation, see: ; (b) T. Stahl, K. Müther, Y. Ohki, K. Tatsumi and M. Oestreich, J. Am. Chem. Soc., 2013, 135, 10978–10981 CrossRef CAS . For other examples of organometallic ligand-cooperative activation of HBpin, see: ; (c) A. Anby, B. Butschke, Y. Ben-David, L. J. W. Shimon, G. Leitus, M. Feller and D. Milstein, Organometallics, 2014, 33, 3716–3726 CrossRef; (d) L. T. Scharf, J. Weismann, K.-S. Feichtner, F. Lindl and V. H. Gessner, Chem.–Eur. J., 2018, 24, 3439–3443 CrossRef CAS; (e) M. Pang, C. Wu, X. Zhuang, F. Zhang, M. Su, Q. Tong, C.-H. Tung and W. Wang, Organometallics, 2018, 37, 1462–1467 CrossRef CAS.
- Y. C. Lin, E. Hatzakis, S. M. McCarthy, K. D. Reichl, T. Y. Lai, H. P. Yennawar and A. T. Radosevich, J. Am. Chem. Soc., 2017, 139, 6008–6016 CrossRef CAS.
- (a) D. Gudat, A. Haghverdi and M. Nieger, Phosphorus, Sulfur Silicon Relat. Elem., 2001, 168–169, 203–208 CrossRef CAS; (b) D. Gudat, Acc. Chem. Res., 2010, 43, 1307–1316 CrossRef CAS; (c) D. Gudat, in Encycl. Inorg. Bioinorg. Chem., Wiley, 2018, pp. 1–23 Search PubMed; (d) D. Gudat, A. Haghverdi and M. Nieger, Angew. Chem., Int. Ed., 2000, 39, 3084–3086 CrossRef CAS; (e) D. Gudat, A. Haghverdi, H. Hupfer and M. Nieger, Chem.–Eur. J., 2000, 6, 3414–3425 CrossRef CAS; (f) D. Gudat, A. Haghverdi and W. Hoffbauer, Magn. Reson. Chem., 2002, 40, 589–594 CrossRef CAS; (g) S. Burck, D. Gudat, M. Nieger and W. W. Du Mont, J. Am. Chem. Soc., 2006, 128, 3946–3955 CrossRef CAS; (h) S. Burck, D. Förster and D. Gudat, Chem. Commun., 2006, 2810–2812 RSC.
- (a) C. C. Chong, H. Hirao and R. Kinjo, Angew. Chem., Int. Ed., 2014, 53, 3342–3346 CrossRef CAS; (b) C. C. Chong, H. Hirao and R. Kinjo, Angew. Chem., Int. Ed., 2015, 54, 190–194 CrossRef CAS; (c) C. C. Chong and R. Kinjo, ACS Catal., 2015, 5, 3238–3259 CrossRef CAS; (d) C. C. Chong and R. Kinjo, Angew. Chem., Int. Ed., 2015, 54, 12116–12120 CrossRef CAS; (e) C. C. Chong, B. Rao and R. Kinjo, ACS Catal., 2017, 7, 5814–5819 CrossRef CAS; (f) B. Rao, C. C. Chong and R. Kinjo, J. Am. Chem. Soc., 2018, 140, 652–656 CrossRef CAS.
- (a) M. R. Adams, C. H. Tien, B. S. N. Huchenski, M. J. Ferguson and A. W. H. Speed, Angew. Chem., Int. Ed., 2017, 56, 6268–6271 CrossRef CAS; (b) C. H. Tien, M. R. Adams, M. J. Ferguson, E. R. Johnson and A. W. H. Speed, Org. Lett., 2017, 19, 5565–5568 CrossRef CAS; (c) M. R. Adams, C. H. Tien, R. McDonald and A. W. H. Speed, Angew. Chem., Int. Ed., 2017, 56, 16660–16663 CrossRef CAS; (d) T. Hynes, E. N. Welsh, R. McDonald, M. J. Ferguson and A. W. H. Speed, Organometallics, 2018, 37, 841–844 CrossRef CAS; (e) T. Lundrigan, E. N. Welsh, T. Hynes, C.-H. Tien, M. R. Adams, K. R. Roy, K. N. Robertson and A. W. H. Speed, J. Am. Chem. Soc., 2019, 141, 14083–14088 CrossRef CAS.
- (a) S. Miaskiewicz, J. H. Reed, P. A. Donets, C. C. Oliveira and N. Cramer, Angew. Chem., Int. Ed., 2018, 57, 4039–4042 CrossRef CAS; (b) J. H. Reed, P. A. Donets, S. Miaskiewicz and N. Cramer, Angew. Chem., Int. Ed., 2019, 58, 8893–8897 CrossRef CAS.
- D. M. C. Ould, T. T. P. Tran, J. M. Rawson and R. L. Melen, Dalton Trans., 2019, 48, 16922–16935 RSC.
- (a) A. Del Grosso, M. D. Helm, S. A. Solomon, D. Caras-Quintero and M. J. Ingleson, Chem. Commun., 2011, 47, 12459–12461 RSC; (b) A. Del Grosso, P. J. Singleton, C. A. Muryn and M. J. Ingleson, Angew. Chem., Int. Ed., 2011, 50, 2102–2106 CrossRef CAS; (c) V. Bagutski, A. Del Grosso, J. A. Carrillo, I. A. Cade, M. D. Helm, J. R. Lawson, P. J. Singleton, S. A. Solomon, T. Marcelli and M. J. Ingleson, J. Am. Chem. Soc., 2013, 135, 474–487 CrossRef CAS; (d) A. Del Grosso, J. Ayuso Carrillo and M. J. Ingleson, Chem. Commun., 2015, 51, 2878–2881 RSC; (e) T. S. De Vries, A. Prokofjevs and E. Vedejs, Chem. Rev., 2012, 112, 4246–4282 CrossRef CAS; (f) M. Ingleson, Synlett, 2012, 23, 1411–1415 CrossRef CAS.
- (a) L. Xu, G. Wang, S. Zhang, H. Wang, L. Wang, L. Liu, J. Jiao and P. Li, Tetrahedron, 2017, 73, 7123–7157 CrossRef CAS; (b) Y. Li and X. F. Wu, Angew. Chem., Int. Ed., 2020, 59, 1770–1774 CrossRef CAS.
- (a) F. R. Atherton and A. R. Todd, J. Chem. Soc., 1947, 674 RSC; (b) F. R. Atherton, H. T. Openshaw and A. R. Todd, J. Chem. Soc., 1945, 660 RSC; (c) S. S. Le Corre, M. Berchel, H. Couthon-Gourvès, J. P. Haelters and P. A. Jaffrès, Beilstein J. Org. Chem., 2014, 10, 1166–1191 CrossRef.
- L. Longwitz, S. Jopp and T. Werner, J. Org. Chem., 2019, 84, 7863–7870 CrossRef CAS.
- (a) I. A. I. Mkhalid, J. H. Barnard, T. B. Marder, J. M. Murphy and J. F. Hartwig, Chem. Rev., 2010, 110, 890–931 CrossRef CAS; (b) J. F. Hartwig, Chem. Soc. Rev., 2011, 40, 1992–2002 RSC; (c) J.-Y. Cho, M. K. Tse, D. Holmes, R. E. Maleczka Jr and M. R. Smith III, Science, 2002, 295, 305–308 CrossRef CAS.
- (a) K. Chen, L. Wang, G. Meng and P. Li, Synthesis, 2017, 49, 4719–4730 CrossRef CAS; (b) M.-A. Légaré, M.-A. Courtemanche, É. Rochette and F.-G. Fontaine, Science, 2015, 349, 513–516 CrossRef; (c) J. Légaré Lavergne, A. Jayaraman, L. C. Misal Castro, É. Rochette and F. G. Fontaine, J. Am. Chem. Soc., 2017, 139, 14714–14723 CrossRef; (d) Q. Yin, H. F. T. Klare and M. Oestreich, Angew. Chem., Int. Ed., 2017, 56, 3712–3717 CrossRef CAS; (e) F. Kitani, R. Takita, T. Imahori and M. Uchiyama, Heterocycles, 2017, 95, 158–166 CrossRef CAS; (f) J. S. McGough, J. Cid and M. J. Ingleson, Chem.–Eur. J., 2017, 23, 8180–8184 CrossRef CAS; (g) Y. L. Liu, G. Kehr, C. G. Daniliuc and G. Erker, Chem.–Eur. J., 2017, 23, 12141–12144 CrossRef CAS; (h) S. Zhang, Y. Han, J. He and Y. Zhang, J. Org. Chem., 2018, 83, 1377–1386 CrossRef CAS.
- Reactions conducted with anisole, 1,3-dimethoxybenzene, and mesitylene also provided 0% yield of product.
- S. Lakhdar, M. Westermaier, F. Terrier, R. Goumont, T. Boubaker, A. R. Ofial and H. Mayr, J. Org. Chem., 2006, 71, 9088–9095 CrossRef CAS.
- S. Bähr and M. Oestreich, Pure Appl. Chem., 2018, 90, 723–731 Search PubMed.
- C. Hansch, A. Leo and R. W. Taft, Chem. Rev., 1991, 91, 165–195 CrossRef CAS.
- When 3 and 4d were reacted under single-turnover conditions employing HB(pin-d6) in place of HBpin, 89% yield was obtained with in a ratio of 14
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 86 5d:5d-d6 (see ESI† page S22).
86 5d:5d-d6 (see ESI† page S22). - 3 is thermally stable in solution at elevated temperature.
- The synthesis of Cl–Bpin has been reported: (a) G. E. Herberich and A. Fischer, Organometallics, 1996, 15, 58–67 CrossRef CAS; (b) H. F. Bettinger, M. Filthaus, H. Bornemann and I. M. Oppel, Angew. Chem., Int. Ed., 2008, 47, 4744–4747 CrossRef CAS; (c) Y. Cui, W. Li, T. Sato, Y. Yamashita and S. Kobayashi, Adv. Synth. Catal., 2013, 355, 1193–1205 CrossRef CAS; (d) S. J. Geier, C. M. Vogels, N. R. Mellonie, E. N. Daley, A. Decken, S. Doherty and S. A. Westcott, Chem.–Eur. J., 2017, 23, 14485–14499 CrossRef CAS . Its instability has been noted as an impediment to its use for C–H borylation, see ref. 15b, and other C–B bond-forming reactions, see: ; (e) D. J. Faizi, A. Issaian, A. J. Davis and S. A. Blum, J. Am. Chem. Soc., 2016, 138, 2126–2129 CrossRef CAS; (f) D. J. Faizi, A. J. Davis, F. B. Meany and S. A. Blum, Angew. Chem., Int. Ed., 2016, 55, 14286–14290 CrossRef CAS.
- The related reaction pathway with 7 was calculated to proceed with ΔG‡ = 22.3 kcal mol−1. See ESI Fig. S24 and Table S1.†.
- Y.-M. Tan, X.-N. Guo, Z. Wu, A. Friedrich, S.A. Westcott, H. Braunschweig, U. Radius and T. B. Marder, J. Am. Chem. Soc., 2020, 142, 13136–13144 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0sc05620k |
| This journal is © The Royal Society of Chemistry 2021 |