Open Access Article
Open Access ArticleA chemical circular communication network at the nanoscale†
Beatriz
de Luis
 ab,
Ángela
Morellá-Aucejo
ab,
Antoni
Llopis-Lorente
ab,
Ángela
Morellá-Aucejo
ab,
Antoni
Llopis-Lorente
 ab,
Tania M.
Godoy-Reyes
ab,
Reynaldo
Villalonga
e,
Elena
Aznar
ab,
Tania M.
Godoy-Reyes
ab,
Reynaldo
Villalonga
e,
Elena
Aznar
 ab,
Félix
Sancenón
ab and
Ramón
Martínez-Máñez
ab,
Félix
Sancenón
ab and
Ramón
Martínez-Máñez
 *abcd
*abcd
aInstituto Interuniversitario de Investigación de Reconocimiento Molecular y Desarrollo Tecnológico (IDM), Universitat Politècnica de València, Universitat de València, Camino de Vera s/n, 46022 Valencia, Spain. E-mail: rmaez@qim.upv.es
bCIBER de Bioingeniería, Biomateriales y Nanomedicina (CIBER-BBN), Spain
cUnidad Mixta UPV-CIPF de Investigación en Mecanismos de Enfermedades y Nanomedicina, Universitat Politècnica de València, Centro de Investigación Príncipe Felipe, Valencia, Spain
dUnidad Mixta de Investigación en Nanomedicina y Sensores, Universitat Politècnica de València, Instituto de Investigación Sanitaria La Fe, Valencia, Spain
eNanosensors & Nanomachines Group, Department of Analytical Chemistry, Faculty of Chemistry, Complutense University of Madrid, Madrid, Spain
First published on 9th December 2020
Abstract
In nature, coordinated communication between different entities enables a group to accomplish sophisticated functionalities that go beyond those carried out by individual agents. The possibility of programming and developing coordinated communication networks at the nanoscale—based on the exchange of chemical messengers—may open new approaches in biomedical and communication areas. Here, a stimulus-responsive circular model of communication between three nanodevices based on enzyme-functionalized Janus Au–mesoporous silica capped nanoparticles is presented. The output in the community of nanoparticles is only observed after a hierarchically programmed flow of chemical information between the members.
Introduction
Chemical communication is based on the exchange of molecular messengers between different entities. In nature, living cells and organisms rely on chemical communication processes for sustaining vital biological functions.1–4 For instance, organelles exchange messengers that allow cellular metabolism; neurons communicate by exchanging neurotransmitters; and physiological functions are regulated by hormone molecules segregated by distant cells. At a larger scale, insects, bacteria and pluricellular organisms communicate with peers by means of pheromones. Communication networks enable a group to share information and act together towards the achievement of a common goal.5,6 Considering this aim, coordinated communication displays an essential role as it is necessary to organize the collective behaviour in a defined order to assure efficiency and productivity.7,8 In fact, nature life is based on communication processes developed in coordinated communities at the molecular scale involving the use of chemical messengers. As examples of coordinated groups, termite populations and fish shoals have chemically coordinated alarm systems in which a member detects an environment disturbance and secretes alarm molecules. Other members sense this message and expel chemical secretions, subsequently spreading alarm through the community and resulting in the articulation of a collective response (e.g., release of toxic/repellent chemicals or colony recruitment).9–11Transferring communication capabilities to human-made nanoscale systems has attracted significant attention in recent years due to potential applications in areas such as biomedicine or ICT (Information and Communication Technologies).12–16 Compared to traditional telecommunication technologies, chemical communication offers interesting features such as the reduced size of molecular transceivers and receivers, minimal power consumption and the ability to operate in biological and physiological environments. Several micro- and nanocarriers capable of interacting with living systems by means of sending or receiving chemical messengers have been developed.17–23 Linear communication between particles or feedback between two particles has also been reported.24,25 However, the field is still in its infancy and more complex communication communities should be demonstrated with the future aim to integrate coordinated multicomponent communities of nanodevices with advanced capabilities.26–29 Strategies of cooperation and coordination between different nanoparticles may enable sophisticated functionalities that go beyond those carried out by individual agents. However, regardless of the aforementioned advancements made in the last years, the definition of technologies to support practical and useful applications of communication at nanoscale, while essential to motivate further growth of this field in the research community, is still very limited and still scarcely explored.
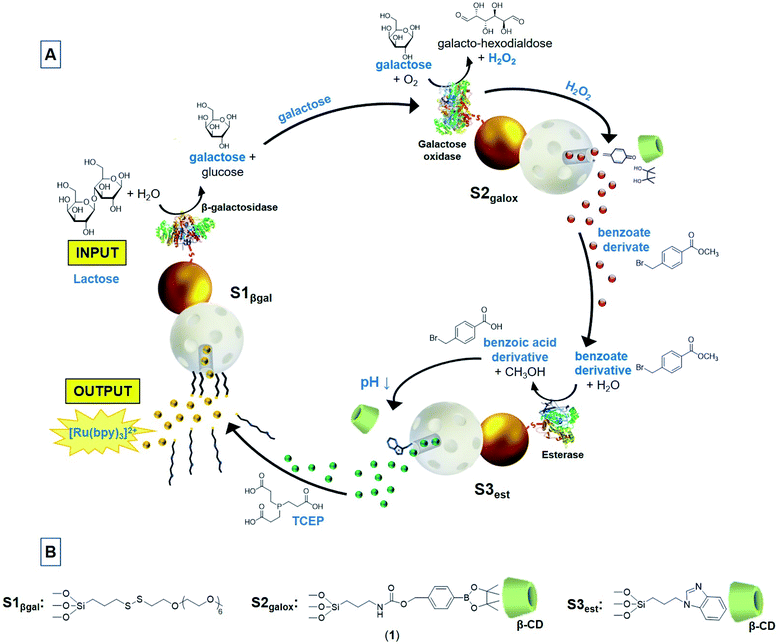
In this scenario, we report herein a circular model of a multicomponent communication network of nanoparticles based on the exchange of chemical messages. Today it is well recognized the ubiquity of cyclic organization in biology as it provides invaluable resources for controlling and orchestrating biological operations, giving rise to complex temporal dynamics in nature.30 For instance, dynamical feedback is observed in biological reaction networks where metabolic processes regulate their activity or resupply the initial substrate in a cyclic manner. Our communication system consists of three enzyme-functionalized Janus Au–mesoporous silica nanoparticles (Au–MSNPs) which display a double receiver-sender behaviour,31,32 as depicted in Fig. 1A. The mesoporous face of nanodevice 1 (S1βgal) is loaded with the fluorescent dye [Ru(bpy)3]Cl2 and capped with disulfide-containing oligo(ethylene glycol) chains (PEG) acting as gatekeepers, whereas the enzyme β-galactosidase (βgal) is attached to the gold face. In nanoparticle 2 (S2galox), the enzyme galactose oxidase (galox) is immobilized on the Au face, while the mesoporous silica is loaded with methyl 4-(bromomethyl)benzoate and the mesopores capped with a H2O2-sensitive self-immolative arylboronate derivative (1) which forms a host–guest complex with β-cyclodextrin (Fig. 1B).33 Finally, the nanodevice 3 (S3est) is functionalized with the enzyme esterase on the Au face, loaded with the reductive species tris(2-carboxyethyl)phosphine hydrochloride (TCEP) in the mesoporous face and capped with a pH responsive supramolecular nanovalve consisting of an inclusion complex between a benzimidazole moiety and β-cyclodextrin.34
As illustrated in Fig. 1A, this circular communication is triggered in the presence of lactose (input). Lactose is transformed into galactose by the enzyme β-galactosidase attached to the gold face of S1βgal. Galactose acts as a chemical messenger that is transmitted and sensed by galactose oxidase enzyme on S2galox resulting in the formation of galacto-hexodialdose and H2O2. Hydrogen peroxide triggers the self-immolative cleavage33 of the gatekeeper on S2galox allowing the delivery of a benzoate derivative (methyl 4-(bromomethyl)benzoate) as a second chemical messenger that is detected by the enzyme esterase on S3est and transformed into the corresponding benzoic acid derivative. Formation of benzoic acid induces a local drop of the pH causing the protonation of benzimidazole moieties,35 dethreading of the nanovalve and the delivery of the reductive agent (TCEP) from S3est. TCEP acts as a third chemical messenger that closes the loop by communicating S3est with S1βgal, as it produces the reductive cleavage of the disulfide bonds of the oligo(ethylene glycol) chains anchored to the mesoporous face of S1βgal. Eventually, the subsequent release of the dye [Ru(bpy)3]Cl2 from S1βgal is produced as the output collective response of the communication network.
Results and discussion
Preparation and characterization of the nanodevices
Janus Au–MSNPs were prepared using gold nanoparticles (of ca. 20 nm obtained by reduction of HAuCl4·3H2O with sodium citrate) and MSNPs (of ca. 100 nm MCM-41-type nanoparticles obtained by an alkaline hydrolysis reaction), as adapted from a previous study.31MSNPs were partially imbedded at the interface of a Pickering emulsion, formed by paraffin wax (oily phase) and water–ethanol (aqueous phase). The un-masked MSNPs surface was decorated with reactive thiol groups, by reaction with (3-mercaptopropyl)trimethoxysilane, on which citrate-capped Au nanoparticles were subsequently attached. After removing the paraffin with chloroform, the starting Janus Au–MSNPs (S0) were obtained. S1 was prepared loading S0 with the [Ru(bpy)3]Cl2·6H2O dye and protecting the Au face with 3-mercaptopropionic acid. The resulting solid was reacted with (3-mercaptopropyl)trimethoxysilane, with 2,2′-dipyridyl disulfide and then with O-(2-mercaptoethyl)-O′-methyl-hexa(ethylene glycol) to give the final capped Janus nanoparticles S1. S1βgal was obtained from S1 by anchoring β-galactosidase on the carboxylate-modified Au face by means of well-known crosslinking EDC/NHS chemistry resulting in the coupling between the free amino groups of lysine residues from the enzyme and the activated Au carboxyl groups.36S2 was prepared loading S0 with methyl 4-(bromomethyl)benzoate and adding 3-mercaptopropionic acid to functionalize the gold face. The mesoporous face was reacted with the self-immolative molecule (1) (Fig. 1B) and the system was capped by the formation of an inclusion complex between (1) and β-cyclodextrin (formation constant = 31.1 ± 0.7 M−1).37 For the preparation of S2galox, galactose oxidase was anchored on the Au face of S2 following a similar procedure as previously described for S1βgal. S3 was prepared by reacting the mesoporous face of S0 with (3-iodopropyl)trimethoxysilane and then with benzimidazole. The resulting solid was treated with 3-mercaptopropionic acid, loaded with tris(2-carboxyethyl)phosphine and capped with β-cyclodextrin as it threads onto benzimidazole forming an inclusion complex (formation constant = 104 ± 8 M−1).34S3est was obtained anchoring the esterase enzyme on the Au face of S3 following the above described EDC/NHS chemistry methodology. As control solids, S2blank and S3blank were prepared following the same procedure described for S2galox and S3est respectively but in this case the mesoporous container was not loaded.The nanoparticles were characterized using standard techniques (Fig. 2, for more details see ESI†). Powder X-ray diffraction patterns at low (1.5 < 2θ < 7) and at high angles (35 < 2θ < 80) of different prepared nanoparticles showed in all cases low-angle reflections of mesoporous silica and high-angle cubic gold characteristic diffraction peaks (see also Fig. S3†). UV/vis of the synthesized Au nanoparticles showed a single surface plasmon absorption band at 524 nm, characteristic of spherically shaped nanospheres with approximately 20 nm diameter that was redshifted to 533 nm in S0 (Fig. S4†). The presence of the mesoporous structure as well as Au nanoparticles was confirmed by transmission electron microscopy (TEM) (Fig. 2B, additional images in Fig. S2†), obtaining similar Au–MSNPs ratios as previously reported.38 N2 adsorption–desorption isotherms of the calcined MSNPs and Janus Au–MS nanoparticles S0 showed an adsorption step at intermediate P/P0 value 0.3 (Fig. S5†), which is characteristic for mesoporous solids with empty pores. Application of the BET model resulted in a value for the total specific surface of 1079 m2 g−1 for calcined MSNPs and 802 m2 g−1 for S0. Pore sizes and total pore volumes were calculated with the BJH model (Table S1†). The hydrodynamic size and zeta potential of different solids were measured by dynamic light scattering (DLS) (Fig. 2C and Table S2†). S0 showed a hydrodynamic diameter of 115 ± 4 nm that increased to 133 ± 18 nm, 143 ± 15 nm and 148 ± 18 nm for S1βgal, S2galox and S3est respectively, whereas Z potential of S0 was −28.4 ± 0.5 and −31.3 ± 0.9, −26.3 ± 0.4 and −30.4 ± 0.7 for S1βgal, S2galox and S3est. From elemental analysis and delivery studies the amounts of different components on the nanoparticles were calculated (Table S3†). The presence of β-galactosidase, galactose oxidase and esterase enzymes on S1βgal, S2galox and S3est was confirmed by enzyme activity assays (Fig. S6–S8, see ESI† for details) revealing activities of 0.001 U, 0.124 U and 0.165 U per mg of solid, respectively. TEM-EDX mapping of the final nanodevices S1βgal, S2galox and S3est confirmed the presence of the expected atoms in the solids. Images showed that gold surfaces were rich in sulfur atoms, strongly suggesting the preferential localization of the enzymes in the Au face (Fig. 2D, S9–S11†) as they were immobilized by means of 3-mercaptopropionic acid. Moreover, the remarkable presence of sulfur atoms in the whole scaffold S1βgal is attributed to the disulfide bonds of the gatekeeper PEG in the mesoporous face. In turn, the slight signal of sulfur atoms in S2galox and S3est is attributed to the (3-mercaptopropyl)trimethoxysilane employed to attach the gold nanoparticles to the silica container during the scaffold synthesis. Boron atoms and nitrogen atoms in S2galox are ascribed to the boronic esters and carbamate groups of the gatekeepers in S2galox, whereas the abundance of nitrogen atoms in S3est is due to the presence of benzimidazole moieties.
Release studies
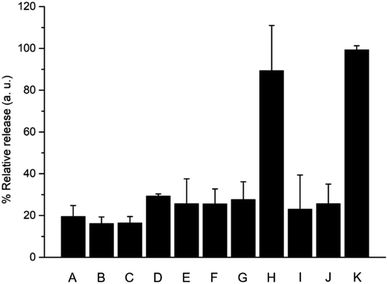
When building cooperative networks of nanoparticles, it is crucial to understand each component's function and rule out potential side interactions and/or incompatibilities between the communities of nanodevices, resulting for instance in “unintended cross-talk” between nanodevices. In order to discard such effects, we performed additional delivery studies in the same conditions previously described using different combinations with “uncomplete” nanoparticles (i.e., lacking either immobilized enzyme or cargo). As shown in Fig. 6, all combinations tested — S1/S2galox/S3est (lacking the enzyme on nanoparticle 1, bar B), S1βgal/S2/S3est (lacking the enzyme on nanoparticle 2, bar C), S1βgal/S2galox/S3 (lacking the enzyme on nanoparticle 3, bar D), S1βgal/S2blank/S3est (lacking the cargo in nanoparticle 2, bar E), and S1βgal/S2galox/S3blank (lacking the cargo in nanoparticle 3, bar F) — showed much lower delivery in the presence of lactose compared to the complete community of nanoparticles (S1βgal/S2galox/S3est, bar H). When one nanoparticle was not complete, the communication channel was disrupted and information was lost. In addition, we also studied the effect of having the enzymes in the solution instead of anchored to the nanoparticles. In a typical experiment the communities of nanoparticles S1/S2/S3 (all lacking the enzyme) were brought in aqueous solution and enzymes at equivalent catalytic concentrations than in S1βgal/S2galox/S3est were added to the mixture. As observed in Fig. 6 (bar G), the output signal (i.e. [Ru(bpy)3]Cl2 release) of the combination S1/S2/S3/free enzymes was significantly lower compared to the response of the complete community which is ascribed to the dilution of the (free) enzyme-generated species in the solution. Moreover, the combinations S1βgal/S2/S3est/free galactose oxidase (bar I) and S1βgal/S2galox/S3/free esterase (bar J) at equivalent catalytic concentrations also showed a low delivery. These data demonstrate the importance of having the enzymes anchored to nanoparticles, which allows the generation of chemical microenvironments around the nanoparticles enabling the effective intracommunication between the enzyme on the Au face and silica gatekeepers as previously reported.25,31 Furthermore, the mixture S1/S2galox/S3est/free β-galactosidase (bar H) displayed a result comparable to the complete network; as β-galactosidase products act over a different nanoparticle (nanoparticle 2) the enzyme may or may not be attached to the nanoparticle 1 without disrupting the communication loop.
The behaviour of this circular model of communication between three nanodevices can be expressed in a Boolean logic table of 6 elements:39,40 (i.e. the triggering species (lactose), enzyme β-galactosidase, enzyme galactose oxidase, cargo of nanoparticle 2 (methyl 4-(bromomethyl)benzoate), enzyme esterase and cargo of nanoparticle 3 (TCEP)). Among the 64 possible entries, only the combination of fully-equipped nanodevices S1βgal/S2galox/S3est leads to a circular communication and efficient [Ru(bpy)3]Cl2 release (Tables S4 and S5 in ESI†).
Altogether, the results described in this section illustrate how the analysis of process/communication efficiencies can help understand the complex behaviour of communication systems, identify critical steps, and facilitate the design of optimized networks. In addition, chemical communication networks could be affected by spatial effects such as the relative distance between nanodevices/communication sites. Molecular messengers could be diluted in the medium before reaching its target,41 directional information flow over distances in molecular communication circuits would require a fine control over the location of interacting nanodevices and messenger diffusion kinetics should be considered. In this study, we have conducted the experiments under stirring of solutions containing nanodevices; thus, diminishing the effect of particle location. Notwithstanding, other works about chemical communication within communities of immobilized abiotic elements using microfluidic technologies have observed the importance of controlling geometrical patterning, chemical input concentrations and flow rate. Although so-far scarce, further studies in this direction will be important for advancing towards the design of chemical communication networks of nanodevices with spatiotemporal control over the information transmission.42,43
Conclusions
In summary, we present herein a circular model of communication between nanodevices based on enzyme-functionalized Janus Au–mesoporous silica capped nanoparticles. In the community of nanoparticles, the release of a cargo from a first nanoparticle (output) after the addition of a stimulus (i.e. lactose) is only observed after the circular communication of the nanoparticles in the sequence S1βgal–S2galox–S3est–S1βgal as a result of the exchange of chemical messengers between the components of the group. The relatively slow communication (maximum delivery after 16 h) is ascribed to the number of chemical processes that have to occur to communicate, including enzymatic activation of different molecules, cargo diffusion from the pores to the solution and chemical messenger diffusion from one nanoparticle to another. Moreover a calculation of the communication efficiency, process efficiency and information loss for the different steps of the communication path allowed us to better understand the communication network and determine the rate limiting processes. Several are the options to enhance communication; one of them is the use of nanoparticles equipped with nanomotors and several studies in this direction are being carried by us currently.44,45 Overall, our network involves three enzymatic processes and the exchange of three chemical messengers (galactose, an ester derivative and the reducing agent TCEP). Only the complete community of nanoparticles is capable of producing the desired output phenomenon (dye release), while incomplete communities do not succeed in transmitting the information. In spite of being similar to a cascade-like system, its potential scope is beyond a typical metabolomic pathway because it is not only limited to the chained enzyme substrates and products. For instance, the possibility of encapsulating a cargo enables communication between enzymes that otherwise could not naturally interact. Moreover, nanoparticles can be directed, concentrated in certain places, etc., allowing designing more advance communication systems, when compared with cascade-like system using chained enzymes in diluted solutions. Circular communication could be of relevance for designing networks enabling initiation of the communication and output from the same nanodevice after sequentially-programmed steps of information transmission with other nanodevices, thus potentially allowing feedback – this is an aspect that would not be possible with a linear pathway since signal detection and final response would occur on different sites. Although the presented design is a proof of concept which leads to a dye release as final output instead of the usual dynamical feedback observed in biological reaction cycles, it illustrates the potential of using abiotic nanodevices to design multistep signalling pathways. Our results demonstrate how artificial nanodevices can be connected by means of molecular communication, yielding systems that show a collective synergic behaviour. Although modelling of the present system was beyond of our scope, advances in the modelling of molecular communications could help in the design and understanding of chemical networks at the micro/nanoscale. A search of different modes of communication within groups of nanoparticles (such as the circular mode of communication reported here) is a key step to further develop more realistic nano-communities to perform specific and complex tasks at the nanoscale. Moreover, the possibility of combining different nanoparticles and communicating them with living organisms46–49 would allow to develop swarms of nanodevices able to interact with their neighbours and local environment leading to advanced systems with new cooperative functionalities. This would cause a deep impact in the way we understand the interaction between artificial nanodevices and nanodevices with living systems. We believe that the idea of developing multicomponent nanoscale cooperative communities capable of communicating and performing coordinated may open new directions in the near future in areas such as biomedicine and ICT.50–55Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors wish to thank the Spanish Government (projects RTI2018-100910-B-C41 (MCUI/AEI/FEDER, UE), CTQ2017-87954-P), the Generalitat Valenciana (PROMETEO 2018/024), the Comunidad de Madrid (IND2017/BMD-7642) and CIBER-BBN (NANOCOMMUNITY project) for support.References
- D. Malak and O. B. Akan, Nano Commun. Netw., 2012, 3, 19 CrossRef.
- N. Deisig, F. Dupuy, S. Anton and M. Renou, Insects, 2014, 5, 399 CrossRef PubMed.
- M. E. Taga and B. L. Bassler, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 14549 CrossRef CAS PubMed.
- J. T. Hancock in Cell Signalling, Oxford University Press, Oxford, UK, 4th edn, 2016 Search PubMed.
- D. J. Levi in Group Dynamics for Teams, SAGE Publications, Thousand Oaks, CA, USA, 5th edn, 2016 Search PubMed.
- T. E. Harris and J. C. Sherblom in Small Group and Team Communication, Pearson, London, UK, 5th edn, 2010 Search PubMed.
- J. H. Miller and S. Moser, Complexity, 2004, 9, 31 CrossRef.
- R. E. Rice, E. J. Zackrison and D. R. Seibold in The International Encyclopedia of Organizational Communication, John Wiley & Sons, NJ, USA, 1st edn, 2017 Search PubMed.
- P. F. Cristaldo, V. Jandák, K. Kutalová, V. B. Rodrigues, M. Brothánek, O. Jirícek, O. DeSouza and J. Sobotník, Biol. Open, 2015, 4, 1649 CrossRef CAS PubMed.
- J. S. M. Chia, E. S. Wall, C. L. Wee, T. A. J. Rowland, R.-K. Cheng, K. Cheow, K. Guillemin and S. Jesuthasan, Nat. Commun., 2019, 10, 3831 CrossRef PubMed.
- T. Eisner, I. Kriston and D. J. Aneshansley, Behav. Ecol. Sociobiol., 1976, 1, 83 CrossRef.
- I. F. Akyildiz, F. Brunetti and C. Blázquez, Comput. Netw., 2008, 52, 2260 CrossRef.
- J. L. Marzo, J. M. Jornet and M. Pierobon, Curr. Drug Targets, 2019, 20, 800 CrossRef CAS PubMed.
- I. F. Akyildiz, M. Pierobon, S. Balasubramaniam and Y. Koucheryavy, IEEE Commun. Mag., 2015, 53, 32 Search PubMed.
- M. E. Roth, O. Green, S. Gnaim and D. Shabat, Chem. Rev., 2016, 116, 1309 CrossRef CAS PubMed.
- X. Sun, D. Shabat, S. T. Phillips and E. V. Anslyn, J. Phys. Org. Chem., 2018, 31, e3827 CrossRef PubMed.
- S. Campuzano, B. Esteban-Fernandez de Ávila, P. Yáñez-Sedeño, J. M. Pingarrón and J. Wang, Chem. Sci., 2017, 8, 6750 RSC.
- E. Aznar, M. Oroval, L. Pascual, J. R. Murguía, R. Martínez-Máñez and F. Sancenón, Chem. Rev., 2016, 116, 561 CrossRef CAS PubMed.
- C.-A. Cheng, T. Deng, F.-C. Lin, Y. Cai and J. I. Zink, Theranostics, 2019, 9, 3341 CrossRef CAS PubMed.
- A. Llopis-Lorente, B. Lozano-Torres, A. Bernardos, R. Martínez-Máñez and F. Sancenón, J. Mater. Chem. B, 2017, 5, 3069 RSC.
- M. Karimi, A. Ghasemi, P. Sahandi Zangabad, R. Rahighi, S. Masoud Moosavi Basri, H. Mirshekari, M. Amiri, Z. Shafaei Pishabad, A. Aslani, M. Bozorgomid, D. Ghosh, A. Beyzavi, A. Vaseghi, A. R. Aref, L. Haghani, S. Bahramia and M. R. Hamblin, Chem. Soc. Rev., 2016, 45, 1457 RSC.
- A. Llopis-Lorente, P. Díez, A. Sánchez, M. D. Marcos, F. Sancenón, P. Martínez-Ruiz, R. Villalonga and R. Martínez-Máñez, Nano Today, 2018, 18, 8 CrossRef CAS.
- H. Wang and M. Pumera, Chem. Soc. Rev., 2020, 49, 3211 RSC.
- C. Giménez, E. Climent, E. Aznar, R. Martínez-Máñez, F. Sancenón, M. D. Marcos, P. Amorós and K. Rurack, Angew. Chem., Int. Ed., 2014, 53, 12629 ( Angew. Chem. , 2014 , 126 , 12838 ) Search PubMed.
- A. Llopis-Lorente, P. Díez, A. Sánchez, M. D. Marcos, F. Sancenón, P. Martínez-Ruiz, R. Villalonga and R. Martínez-Máñez, Nat. Commun., 2017, 8, 15511 CrossRef CAS PubMed.
- C. Chen, X. Chang, H. Teymourian, D. E. Ramírez-Herrera, B. Esteban-Fernández de Ávila, X. Lu, J. Li, S. He, C. Fang, Y. Liang, F. Mou, J. Guan and J. Wang, Angew. Chem., Int. Ed., 2018, 57, 241 ( Angew. Chem. , 2018 , 130 , 247 ) CrossRef CAS PubMed.
- Y. Qiao, M. Li, D. Qiu and S. Mann, Angew. Chem., Int. Ed., 2019, 58, 17758 ( Angew. Chem. , 2019 , 131 , 17922 ) CrossRef CAS PubMed.
- T. Farrugia, A. W. Perriman, K. P. Sharma and S. Mann, Chem. Commun., 2017, 53, 2094 RSC.
- W. Wang, W. Duan, S. Ahmed, A. Sen and T. Mallouk, Acc. Chem. Res., 2015, 48, 1938 CrossRef CAS PubMed.
- W. Bechtel and A. Abrahamsen in Philosophy of Complex Systems, Elsevier, Amsterdam, Netherlands, 1st edn, 2011, Part 2, Ch. 1 Search PubMed.
- R. Villalonga, P. Díez, A. Sánchez, E. Aznar, R. Martínez-Máñez and J. M. Pingarrón, Chem.–Eur. J., 2013, 19, 7889 CrossRef CAS PubMed.
- A. Llopis-Lorente, B. de Luis, A. García-Fernández, P. Díez, A. Sánchez, M. D. Marcos, R. Villalonga, R. Martínez-Máñez and F. Sancenón, J. Mater. Chem. B, 2017, 5, 6734 RSC.
- T. M. Godoy-Reyes, A. Llopis-Lorente, A. García-Fernández, P. Gaviña, A. M. Costero, R. Villalonga, F. Sancenón and R. Martínez-Máñez, Org. Chem. Front., 2019, 6, 1058 RSC.
- F. O. Yousef, M. B. Zughul and A. A. Badwan, J. Inclusion Phenom. Macrocyclic Chem., 2007, 57, 519 CrossRef CAS.
- G. Jerez, G. Kaufman, M. Prystai, S. Schenkeveld and K. K. Donkor, J. Sep. Sci., 2009, 32, 1087 CrossRef CAS PubMed.
- S. S. Wong and L.-J. C. Wong, Enzyme Microb. Technol., 1992, 14, 866 CrossRef CAS PubMed.
- A. Kasprzak, K. M. Borys, S. Molchanov and A. Adamczyk-Wozniak, Carbohydr. Polym., 2018, 198, 294 CrossRef CAS PubMed.
- A. Llopis-Lorente, B. de Luis, A. García-Fernández, S. Jiménez-Falcao, M. Orzáez, F. Sancenón, R. Villalonga and R. Martínez-Máñez, ACS Appl. Mater. Interfaces, 2018, 10, 26494 CrossRef CAS PubMed.
- S. Erbas-Cakmak, S. Kolemen, A. C. Sedgwick, T. Gunnlaugsson, T. D. James, J. Yoon and E. U. Akkaya, Chem. Soc. Rev., 2018, 47, 2228 RSC.
- Biomolecular Information Processing: From Logic Systems to Smart Sensors and Actuators, ed. E. Katz, John Wiley & Sons, 1st edn, NJ, USA, 2012 Search PubMed.
- G. Gines, A. S. Zadorin, J.-C. Galas, T. Fujii, A. Estevez-Torres and Y. Rondelez, Nat. Nanotechnol., 2017, 12, 351 CrossRef CAS PubMed.
- L. Tian, M. Li, J. Liu, A. J. Patil, B. W. Drinkwater and S. Mann, ACS Cent. Sci., 2018, 4, 1551 CrossRef CAS PubMed.
- J. Liu, L. Tian, Y. Qiao, S. Zhou, A. J. Patil, K. Wang, M. Li and S. Mann, Angew. Chem., Int. Ed., 2020, 59, 6853 CrossRef CAS PubMed.
- A. Llopis-Lorente, A. García-Fernández, E. Lucena-Sánchez, P. Díez, F. Sancenón, R. Villalonga, D. A. Wilson and R. Martínez-Máñez, Chem. Commun., 2019, 55, 13164 RSC.
- A. Llopis-Lorente, A. García-Fernández, N. Murillo-Cremaes, A. C. Hortelão, T. Patiño, R. Villalonga, F. Sancenón, R. Martínez-Mañez and S. Sánchez, ACS Nano, 2019, 13, 12171 CrossRef CAS PubMed.
- R. Lentini, N. Y. Martin, M. Forlin, L. Belmonte, J. Fontana, M. Cornella, L. Martini, S. Tamburini, W. E. Bentley, O. Jousson and S. S. Mansy, ACS Cent. Sci., 2017, 3, 117 CrossRef CAS PubMed.
- B. de Luis, A. Llopis-Lorente, P. Rincón, J. Gadea, F. Sancenón, E. Aznar, R. Villalonga, J. R. Murguía and R. Martínez-Máñez, Angew. Chem. Int. Ed, 2019, 58, 14986 ( Angew. Chem. , 2019 , 131 , 15128 ) CrossRef CAS PubMed.
- C. G. Hebert, A. Gupta, R. Fernandes, C. Y. Tsao, J. J. Valdes and W. E. Bentley, ACS Nano, 2010, 4, 6923 CrossRef CAS PubMed.
- M. Schwarz-Schilling, L. Aufinger, A. Mückl and F. C. Simmel, Integr. Biol., 2016, 8, 564 CrossRef CAS PubMed.
- S. Hauert and S. N. Bhatia, Trends Biotechnol., 2014, 32, 448 CrossRef CAS PubMed.
- N. Agoulmine, K. Kim, S. Kim, T. Rim, J.-S. Lee and M. Meyyappan, IEEE Wirel. Commun., 2012, 19, 42 Search PubMed.
- N. Farsad, Mob. Comput. Commun. Rev., 2018, 22, 5 Search PubMed.
- T. Nakano, M. J. Moore, F. Wei, A. V. Vasilakos and J. Shuai, IEEE Trans. Nanobioscience, 2012, 11, 135 Search PubMed.
- A. García-Fernández, E. Aznar, R. Martínez-Máñez and F. Sancenón, Small, 2020, 16, 1902242 CrossRef PubMed.
- O. B. Akan, H. Ramezani, T. Khan, N. A. Abbasi and M. Kuscu, Proc. IEEE, 2017, 105, 306 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Chemicals, general methods, characterization, procedures and additional figures. See DOI: 10.1039/d0sc04743k |
| This journal is © The Royal Society of Chemistry 2021 |