Exploring the role of scaffolds in problem-based learning (PBL) in an undergraduate chemistry laboratory
Sujatha
Varadarajan
 * and
Savita
Ladage
* and
Savita
Ladage
 *
*
Homi Bhabha Centre for Science Education, Tata Institute of Fundamental Research, Mumbai, India. E-mail: sujsvarada@gmail.com; sujatha@hbcse.tifr.res.in
First published on 22nd October 2021
Abstract
The need for shifting the expository laboratory instruction style to inquiry-based approaches is widely acknowledged. Problem-based learning (PBL), one of the inquiry-based approaches, advocates students’ self-directed learning. The literature indicates that scaffolding students’ independent learning is necessary for a PBL environment. In our study, we provided scaffolds such as a precursor task, reading material, and structured group discussion to facilitate the planning of a PBL task on indigo dyeing wastewater treatment. In this paper, we describe how these three scaffolds integrated within the PBL style contributed to students’ learning as per the stages of Kolb's Experiential Learning Cycle (ELC). We analyzed the level of the abstract conceptualization stage of ELC through students’ experimental designs (EDs) of the PBL task. The results of the qualitative analysis of the scaffolded and un-scaffolded groups suggest that scaffolded students’ experimental design scores are higher. The qualitative results indicate that the structured group discussion influences the EDs planned by students more than the precursor task and the reading material. All three scaffolds help in the reflective learning and planning of executable EDs for a PBL task. The study indicates that students transitioning into the inquiry laboratory benefit from the inclusion of scaffolding.
Introduction
Chemistry education research (CER) on instruction in undergraduate chemistry laboratories has raised issues such as inadequate exposure to higher-order thinking skills, lack of meaningful investigation, and neglect of process skills, needing attention in curricula (Hodson, 1992; Reid and Shah, 2007; Zoller and Pushkin, 2007; Bennet et al. 2009). To address these issues, researchers have suggested various alternative instructional styles, for instance, open inquiry, guided inquiry (POGIL), discovery learning, and problem-based learning (PBL) (Domin, 1999; Daubenmire and Bunce, 2008; Bennet et al., 2009; Abraham, 2011). Each of these styles incorporates different levels of inquiry as described by Buck et al. (2008) and has both advantages and disadvantages. For example, the POGIL approach helps students arrive at concepts through the exploration of data inductively and then apply them deductively to a new situation. However, POGIL requires a good deal of teacher training, and researchers have demonstrated that long-term pedagogical support is required for the sustainability of the impact of teacher development groups (Stains et al., 2015). Open inquiry complies with authentic scientific practice; however, its pedagogical viability for large-scale implementation is not clear as the instructor cannot guarantee that all the needed chemicals and equipment will be available.Problem-based learning (PBL), a deductive and inquiry-based approach, emphasizes both processes and content as the learning objectives (Boud and Feletti, 2013). In this approach, students are given a real-life and contextual problem to solve, which may motivate them in their self-directed learning and fill the knowledge gap through collaborative knowledge building (Savery and Duffy, 1995; Hmelo-Silver and Barrows, 2008). Good PBL modules provide opportunities to understand problem scenarios holistically, i.e., with their linkages to the relevant interconnected components within and beyond the problem scope. Such exposure may help learners develop a Systems Thinking Approach and encourage formulating a more meaningful solution to global problems (Nagarajan and Overton, 2019). Thus, PBL is often perceived by students as more useful (George-Williams et al., 2020) and has been successfully implemented across different areas of chemistry (Ram, 1999; McDonnell et al. 2007; Flynn and Biggs, 2012; Hicks and Bevsek, 2012; Costantino and Barlocco, 2019).
Nevertheless, Kirschner et al. (2006) raised concerns regarding the effectiveness of minimally guided instructions like those in inquiry/problem-based learning, etc., arguing that these do not take into consideration the working memory overload. Additionally, researchers also suggest that students face difficulty when there is no direct instruction for the laboratory task (Seery et al., 2019). Thus, for students coming from a conventional instructional style, it is important to provide adequate and appropriate support/scaffolds to help them succeed in a complex investigatory task based on PBL or inquiry learning.
Researchers view scaffolds as tools that support learning. Scaffolds help students to articulate and reflect while completing a PBL task (Hmelo-Silver et al., 2007). Scaffolds can be soft scaffolds that include peer/teacher interactions and hard scaffolds which include artifacts/worksheets (Choo et al., 2011). These scaffolds should be distributed across the task so that students can best utilize them (Puntambekar and Hübscher, 2005). Hmelo-Silver et al. (2007) described in detail the scaffolding strategies to support disciplinary thinking, and to provide expert guidance. They suggested that structured tasks, tools, and artifacts such as whiteboards and storyboards can be used to reduce cognitive load. Though Van der Stuyf (2002) claimed that developing scaffolds to meet the needs of individual students is difficult, Quintana et al. (2004) contended that the scaffolding strategies lead to sense-making, process management, articulation, and reflection. Thus, the use of an effectively scaffolded pedagogy can help students accomplish success in cognitively complex tasks such as PBL.
Theory
Several learning theories support PBL, e.g., constructivism, humanist theory, and information processing theory (Savery and Duffy, 1995). We chose Kolb's Experiential Learning Cycle (ELC) as the theoretical framework for our work. Agustian and Seery (2017) contended that many theoretical frameworks for pre-lab activities including Kolb's ELC have some uniformity in terms of cognitive activation required for the laboratory work. We think Kolb's ELC suggests a way to sequence scaffolds for meaningful learning.According to Kolb's learning theory, the learner proceeds through four stages for effective learning (Kolb, 1984). These stages are Concrete Experience (CE), Reflective Observation (RO), Abstract Conceptualization (AC), and Active Experimentation (AE). The CE stage includes the active engagement of students with a concept. The RO stage helps students review the experience and may include giving and receiving feedback from their peers. The AC stage helps students interpret events and generate models of what is experienced. Finally, the AE stage involves students putting their learning into practice (Konak et al., 2014). All these stages constitute the experiential learning cycle (ELC). Kolb's theory finds its intellectual origin in the learning models of Dewey, Piaget, and Lewin, giving an integrative perspective on experience, perception, cognition, and behavior (Miettinen, 2000).
In the context of the PBL chemistry laboratory, we think the first three stages of Kolb's theory (CE, RO, and AC stages) can be experienced by students during pre-lab work to generate conceptual and procedural understanding followed by the laboratory work that can be aligned with the fourth stage (AE).
However, there is evidence to suggest that many students use an algorithmic, ineffective approach to work with laboratory manuals (Hofstein and Lunetta, 2004; Reid and Shah, 2007; Bennet et al., 2009). In general, such an approach places less emphasis on the first three stages of ELC (Mahmoud and Nagy, 2009). We think the introduction of scaffolds sequenced according to the stages of ELC might contribute to formulating a successful experimental design (ED). A well-crafted ED is necessary and may lead students to an effective and concrete laboratory experience.
The PBL task and scaffolds
The PBL module used in this study was related to the analysis and treatment of wastewater generated from indigo dyeing of yarns in a chemistry laboratory. This PBL module may sensitize students to a contextual problem of environmental pollution caused by the processing of their lifestyle clothing (denim).In this PBL task, we provided two scaffolds for the first stage of ELC. The first one was a precursor task to plan and draw a flowchart for investigating the suitability of various vinegar samples for making pickles. This scaffold was planned to give students a concrete experience of developing an experimental design through active engagement in small-group discussion.
In addition, we also provided reading material containing facts, information, and chemical concepts related to indigo synthesis, dyeing, and textile wastewater treatment as the second scaffold to help students construct prior knowledge.
To scaffold the second stage, students were provided an opportunity to engage in dialectic conversation (reflective observation) across all the groups expressing their ideas. Table 1 summarizes the scaffolds provided to students for helping them in the development of their experimental design.
| Scaffold | Pedagogical purpose | Stage in Kolb's cycle |
|---|---|---|
| Precursor-task | Help students in logical sequencing of experimental processes | Stage 1: concrete experience (CE), developing a simple experimental design |
| Reading material | Provide pre-requisite knowledge for the PBL task | Stage 1: concrete experience (CE), accessing and acquiring prior knowledge |
| Group-discussion | Engage students in reflective thinking | Stage 2: reflective observation (RO), leading to Stage-3, i.e., abstract conceptualization (AC) |
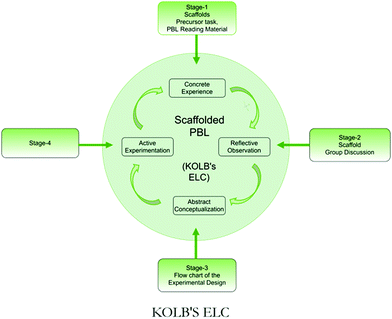
This paper presents a qualitative assessment of the intermediate achievement level of the abstract conceptualization stage indicated by the experimental design of the PBL task. The evaluation of the fourth stage, namely, the active experimentation stage involving laboratory work and report writing, is planned as a separate study. Fig. 1 presents the sequencing of the scaffolds as per Kolb's ELC.
Thus, our research questions are:
RQ 1: Do scaffolds (precursor task, reading material, group discussion) help students improve the quality of their experimental design (ED) of a PBL task?
RQ 2: How do scaffolds help students move through the planning stages of Kolb's Experiential Learning Cycle leading to meaningful learning?
Methods
The Institute and Authentic settings
The indigo module was implemented in two settings. The first one was the authors’ Educational Research Institute where students were invited for two inquiry lab sessions through announcements made on the Institute website. The Institute is dedicated to conducting various kinds of educational research and workshops. The second one was the Authentic setting of the students’ home colleges where undergraduate classes are held regularly. The second location was chosen to understand the transferability of the approach used in this study. Two workshops were conducted in each setting. The workshops in the Authentic setting were conducted in two colleges that are affiliated with the same University and are located 5 km apart. These colleges were selected based on the locational convenience of the researcher.Participants
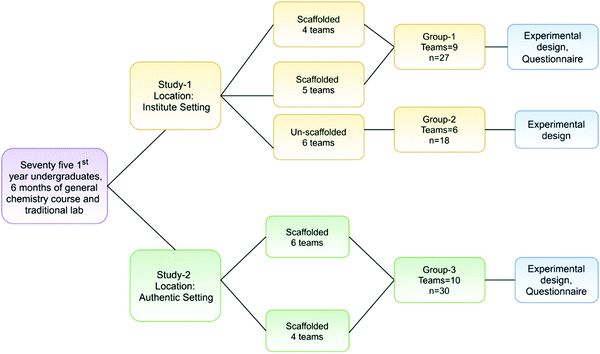
A total of 75 first-year undergraduates from both settings participated in our study. The participants had completed one semester of the first-year general chemistry course and had 6 month experience with the traditional undergraduate chemistry laboratory.In the Institute setting, 40 and 50 students came to participate in two inquiry lab sessions. For our workshops, we randomly selected 12 and 15 participants, respectively, for the scaffolded group. Of the remaining students of the second session, we invited voluntary participation for the un-scaffolded group. The students were informed about the scaffolded and un-scaffolded research for the comparative study of their experimental designs, following which, 18 students volunteered for the study. A random assignment would have been an appropriate way for the selection of the un-scaffolded group; however, participation for the un-scaffolded group was made voluntary due to the possibility of their designs being infeasible leading to partial engagement with the task. Thus, the total number of participants was 27 students for the scaffolded group and 18 students for the un-scaffolded group in the Institute setting. The students in the Institute setting were from different colleges across India and from diverse cultural, social, and language backgrounds.
In the Authentic setting, a scaffolded group of a cohort of 12 and 18 students participated in the two workshops respectively. We did not have an un-scaffolded group in the Authentic setting as the college authorities required that all participating students be given the same learning opportunity.
The number of students varied from 12 to 18 in each workshop to match it with the student to instructor ratio in the region where this study was carried out. All four workshops (Institute and Authentic) were conducted over the course of one calendar year. Informed written consent was obtained from all the participants from both the settings and were grouped into teams (n = 3) for collaborative learning as prescribed in a PBL approach.
The participants in the Institute and Authentic settings were equivalent in aspects such as age and background chemistry exposure; however, they were statistically different (Mann Whitney U = 75.5, p < 0.001, effect r = 0.68) in their 12th-grade prior academic achievement scores. The students in the Authentic setting had a lower mean score of 70.4 as compared to the 89.5 mean score of the Institute setting. Fig. 2 presents the research design.
Procedure and implementation
The module was validated for content and suitability for undergraduate students by four undergraduate chemistry teachers who also performed laboratory trials of the module. The input given by the teachers was incorporated into the module before carrying out trials with students.The indigo PBL task was divided into three parts – pre-lab work, lab work, and post-lab work. During the pre-lab work (first 2 days, 8 hours) students (a) devised an experimental plan for the precursor (vinegar) task, (b) went through the content of the reading material, (c) formulated an experimental design (ED) for analysis and treatment of indigo dyeing wastewater, and (d) constructed a flow chart of the process, and finally, engaged in group discussion. The facilitator of this task had 20 plus years of teaching secondary and tertiary levels of students. Her role was to define the task, carry out the group discussion, and encourage peer interaction in case of any clarification needed by students.
All the teams had access to the Internet for planning during the prelab work. The teams in the un-scaffolded group were not given any scaffold, whereas the teams in the scaffolded group were additionally provided scaffolds as mentioned below.
• After going through the reading material, the students discussed and finalized the ED with their team members. The facilitator did not intervene in their discussion; however, she did make a judgment for herself of the clarity and completeness of the initial ED by each of the teams.
• Each team presented its initial design to the remaining teams. The first presentation was given by the team with the least comprehensive ED and the last one was given by the team which had the most comprehensive ED, i.e., the presentation of the ED was planned by the facilitator in the order of increasing comprehensiveness. The objective was to ensure equitable discussion of each experimental design irrespective of how comprehensive it was and to engage all students regardless of how complete or incomplete their EDs were in the group discussion. The order of increasing comprehensiveness was also to help students realize how their plans could be improved both incrementally and overall.
• To initiate discussion across the teams, the facilitator asked a few questions and then encouraged questioning by students. Later, when students were comfortable, they took over the discussion.
• The facilitator prevented digression and bridged the gaps in discussion with guiding questions. She also ensured that students did not discourage others while identifying flaws in a design or raising questions about a group's plan.
At the end of the prelab, we expected students to choose parameters for analysis (e.g., pH, color, COD) and to suggest a method to treat the wastewater with the help of the scaffolds provided to them. Students also had the freedom to devise a design based on their prior experience or knowledge.
We provided another 8 hours spread over two days for the lab work and four hours for post-lab work.
Data: Students’ EDs and flow charts of the precursor (vinegar) task and the indigo task were evaluated for the quality of the experimental designs. We also collected the teams’ EDs after giving two and three scaffolds to the scaffolded group in the Institute setting. A questionnaire was administered to capture the students’ perceptions of the module in general. The questionnaire included a five-point Likert scale question to investigate the students’ perceptions of the usefulness of scaffolds for the indigo task.
Additionally, students were asked to highlight the following information in the reading material.
(a) Information that discusses theory/concepts related to the task.
(b) Important information that is needed for planning the laboratory work.
(c) Useful background information related to the task.
The annotated reading material was used as data to understand whether students selected the required information needed for the design of the indigo task. Audio transcripts of group discussions were used as the additional data.
The data from the scaffolded teams from the two workshops within the Institute setting were combined. Likewise, the data from the two workshops within the Authentic setting were combined since variables such as the age group, chemistry background (theory as well as traditional nature of lab experience), facilitator, implementation, and intervention protocols were held constant.
Data analysis
The rubric for the indigo task was reviewed by two experts who had five and thirty years of teaching experience, including mentoring inquiry projects, and assessing the laboratory work of undergraduate students. We invited two undergraduate college teachers with fourteen and twenty-five years of teaching experience, respectively, to evaluate the students’ EDs of the precursor task, the indigo task, and the student-annotated reading material. As part of the reliability exercise and use of the rubric, one of the students’ EDs was jointly evaluated by reviewers and the researcher. We determined the inter-evaluator consistency for the ED scores by the two evaluators and was found to be high (Cronbach's alpha = 0.93).
The results of the student perception questionnaire were used to test the convergence of our findings of the scaffolds.
Though students completed the given PBL task and submitted the lab investigation report, in the present paper, we analyzed data only from the prelab work.
Results
The following section presents the results of the comparison of experimental design (ED) scores of the indigo task for (a) the scaffolded vs. un-scaffolded groups in the Institute setting, (b) two scaffolds vs. three scaffolds in the Institute setting, and (c) the Institute setting vs. the Authentic setting (in the students’ colleges). Additionally, we present the analysis of students’ responses to individual scaffolds in the Authentic setting and the analysis of students’ responses to the questionnaire.Institute setting
The comparison of the ED scores of the un-scaffolded and scaffolded groups in the Institute setting indicates improvement of the mean scores after giving the scaffolds. The mean score was found to be 6 and 17 for the un-scaffolded and scaffolded groups out of a maximum score of 28. Further, the EDs of the un-scaffolded and scaffolded groups differed in terms of providing a feasible design with regard to the availability of time, chemicals, and equipment in undergraduate labs. The other difference was in the sequencing of the steps of the task. We describe the EDs in the following section.Experimental designs of the teams from the un-scaffolded group
The teams in the un-scaffolded group provided only a treatment method based on the information perhaps obtained through browsing the Internet. No team in the un-scaffolded group included sequencing of wastewater analysis pre- and post-treatment. Three of the teams provided an infeasible design and the EDs of the remaining three teams were feasible but did not meet the module objectives. Most of the ideas were interesting but no ED could be executed in the lab. Table 2 gives an example of feasible and infeasible EDs by the un-scaffolded teams. The ED by Team A2 (Table 2) was feasible because it could be executed even though the module objectives were not met by this design. The phytoremediation suggested by Team A4 (Table 2) was infeasible because it would require that the students grow and test the suitability of the plant to treat the given wastewater.| Description | Exemplar | |
|---|---|---|
| Feasible design | “This problem could potentially be solved by satisfying the COD by oxidizing the substrates before their disposal. A favourable green oxidizing agent would be hydrogen peroxide, which is itself reduced to water. The sulphate salt, already being at the apex of the latimer diagram, is unaffected by the addition of hydrogen peroxide, but the sulfite and thiosulfate ions face oxidation, giving sulfate ions.” | |
| Team A-2 | ||
| Score = 6/28 | ||
| Infeasible design | Process | Reason |
| Team A-4 | “Wastewater | Indigo wastewater with a larger number of debris, pebbles, mesh-like structure, and many organic and inorganic substances with indigo pigment. |
| Score = 8/28 | Series of filters to remove larger particles | Larger pebbles, insoluble materials, and mesh-like structures are essentially removed. |
| Phytoremediation to reduce BOD and COD, some pigments, minerals/metal ions | Many inorganic (most of) ions and organic compounds which are essential for minerals nutrition of plants are taken up by the roots (essentially water is also consumed which meets the water requirement of the plant) plant used: Canna indica. | |
| Clean coloured water to a separate unit | Indigo dye which is now the leftover constituent is taken up by the hairs (wig). | |
| Dye water to use to dye hair which retains a large amount of colour and uses up extra indigo dye in the wastewater | # Artificial synthetic wig production (commercial process) maybe by soaking or providing mechanical stress to the hair on leftover indigo water.” | |
The EDs by the un-scaffolded group indicate that providing only the problem statement is closer to open inquiry requiring more time for a concrete understanding of experimental designs. Further, the difficulties mentioned by students of the un-scaffolded group in a written response to a question in the questionnaire are – (a) difficulty in understanding the online information, (b) insufficient accessible information to plan, (c) time-consuming and (d) difficulty in accessing the restricted articles. We infer that comprehending the online content for application in a specific situation and designing the task within the stipulated time are cognitively demanding. Thus, students need a scaffold in the form of reading material to make the fact and information readily available.
Experimental designs by the teams in the scaffolded group
The teams in the scaffolded group could sequence the task to analyze the wastewater prior to and after the chosen treatment (Table 3). This sequencing is important to understand the efficacy of the proposed treatment.| Description | Exemplar |
|---|---|
| Scaffolded team | (1) “10 ml of sample: Filter the sample, pH, color of the sample |
| Team C-2 | • 10 ml of sample: COD (filtration for obtaining COD). (Process-2) |
| Score = 19/28 | • COD: |
| ∘ 100 ml of K2Cr2O7, 0.693 g in 100 ml | |
| ∘ 250 ml of 0.25 M FAS (Ferrous Ammonium Sulphate), 2.45 g in 25 | |
| ml of DW (Distilled Water), 5 ml Conc. H 2 SO 4 | |
| • Conduct a blank titration of K2Cr2O7and FAS | |
| • 10 ml of sample + 5 ml of K2Cr2O7+ 15 ml of Ag2SO4, H2SO4 | |
| ∘ Add to the flask and reflux | |
| ∘ Add ferroin indicator and titrate with FAS | |
| • Calculate the COD | |
| (2) 20 ml of the sample treated with activated charcoal | |
| 10 ml (COD) | |
| 10 ml: Filter, pH, color | |
| (3) Repeat process (2) with chemical coagulation | |
| (4) COD value in (2) and (3) subtract from the original COD value and obtain the percentage of the organic matter that has been treated.” |
The ED in the exemplar describes all the steps, the quantities and concentrations of the chemicals, and the optimization process too. However, some of the components included in the rubric for assessment, such as the tools for measurement, are missing.
Further, we carried out the analysis within the scaffolded group to know whether the reading material is sufficient and/or the structured group discussion has added value. The following section presents this comparison.
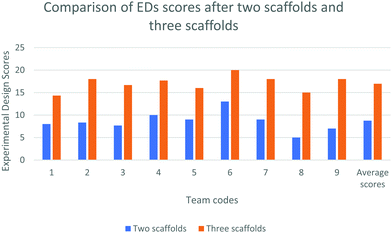
EDs with two vs. three scaffolds
We collected the EDs from the teams of the scaffolded group in the Institute setting twice, once after giving two scaffolds (precursor task and reading material) and then again after giving three scaffolds (precursor task, reading material, and group discussion).Analysis of EDs suggests that seven teams out of nine included the quantities of various chemicals and optimizations of the treatment process (one of the module objectives) in their EDs only after the third scaffold. Table 4 presents the design of a team after giving two and three scaffolds respectively.
| Description | Exemplar |
|---|---|
| Two scaffolds | “Step 1: To the wastewater sample containing indigo white (whose COD is known), we add small amount of (estimated) of cellulose. |
| Team-B1 | Step 2: We allow the cellulose bonded to indigo white to settle as precipitate. |
| Score = 8/28 | Step 3: We then carry out separation of cellulose wastewater by filtration. |
| Step 4: We then measure the COD of the waste water. | |
| Step 5: Depending on the change in COD, further cellulose is added and/or reused in an estimated quantity. | |
| Step 6: The process is repeated in this fashion till significant reduction in COD is observed.” | |
| Three scaffolds | “COD of the sample is measured by the standard procedure. To 10 ml of the sample arbitrarily small amount of cellulose is added. The sample is stirred and then allowed to stay. Cellulose settles, followed by which filtration is carried out. COD of the filtrate is measured. Decrease in the COD is noted and depending on the change, the process is repeated: |
| Team-B1 | (a) with different amount of cellulose at the same temperature. |
| Score = 17/28 | (b) with the same amount of cellulose at different temperature. |
| If COD increases, the process is repeated at higher temperature.” | |
The ED scores of Team-B1 indicate that group discussion as the third scaffold contributed to the improved experimental design by the students in the Institute setting.
The average scores of the teams after giving two scaffolds and three scaffolds were found to be 9 and 17. Fig. 3 presents improvement in the scores of each team after two and three scaffolds.
We further compared the results obtained in the Institute setting with the Authentic setting of students’ colleges. The results are presented in the following section.
Experimental designs by students in the Institute setting vs. the Authentic setting
We gave all three scaffolds to students in the Authentic setting since the results from the Institute setting indicated that students arrived at an executable experimental design at the end of the three scaffolds.We analyzed the EDs by the teams in the two settings for the presence of three of the important components of the ED. These components are (a) the feasibility of the design for execution in undergraduate laboratories, (b) the analysis of wastewater pre- and post-treatment, and (c) the optimization of the treatment method or treatment conditions such as pH, temperature, and quantities. We counted the number of teams in the Authentic and Institute settings that included these components in their EDs. Table 5 presents the results of this analysis.
| Components included by teams in the experimental design | No. of teams | No. of teams |
|---|---|---|
| Authentic setting | Institute setting | |
| Total = 10 teams | Total = 9 teams | |
| Feasibility of the design for execution in an undergraduate lab | 10 (100%) | 9 (100%) |
| Wastewater analysis pre- and post-treatment | 8 (80%) | 9 (100%) |
| Optimization of the treatment process/conditions | 6 (60%) | 7 (78%) |
The numbers of teams that included these components in their EDs were almost identical in the two settings. All the teams gave a feasible plan in both settings, whereas optimization was missed by some teams in both settings. The audio transcript of the group discussion indicated that the teams were pondering whether they had to consider the most optimal method of treatment or the optimum conditions within a method such as pH and temperature. Possibly, understanding the term optimization was unclear for some students.
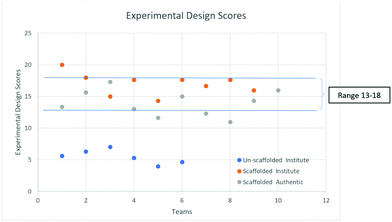
Further, to understand the trend in ED scores in the two settings, we looked for the range of scores where the maximum number of teams lies. Fig. 3 gives this comparison.
Fig. 4 suggests that most of the teams had ED scores in the range of 13–18 in both settings (Institute and Authentic). The mean scores of the Institute and Authentic settings were found to be 17 and 14 respectively. However, no team scored a full 28 because no team included the tools of measurement in their EDs.
Thus, we infer that the qualitative and quantitative trends in the experimental design of the scaffolded PBL are similar in both settings. We anticipated a larger difference due to the significantly different prior academic achievement scores. It is surprising but encouraging that the participants in the Authentic setting scored similarly to the Institute participants. This can be attributed to the fact that in both the settings the prior knowledge required for the task was built through the reading material and the EDs of the complex task were formulated with support from peers through structured group discussion.
Students’ perceptions about the usefulness of the scaffolds
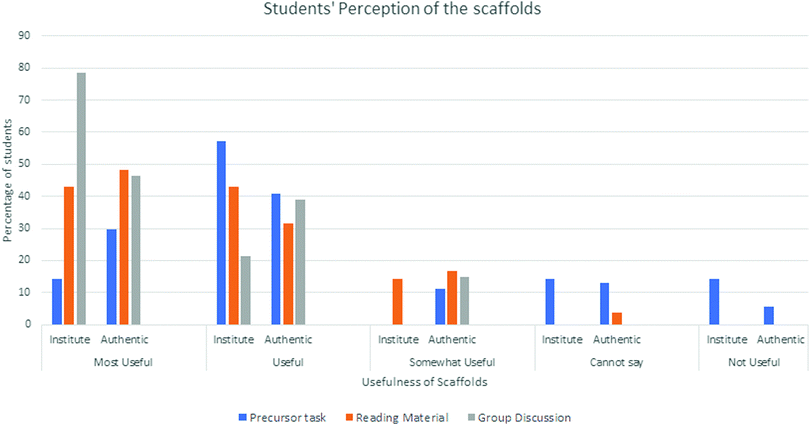
We gave a five-point Likert scale questionnaire to individual students in both settings. Fig. 5 presents the analysis of these data.The results suggest that most of the students in both settings considered all three scaffolds either useful or most useful. A small number of students were not as enthusiastic about the precursor (vinegar) task. In our future studies, we need to explore whether all students can extrapolate the learning outcomes of the precursor task to the main PBL (indigo) task.
We further explored how each one of the scaffolds helped the teams in the Authentic setting in their planning of EDs and whether the purpose of the scaffolds was met. The next section presents the results of this analysis.
Authentic setting
| Score range | Teams in the given score range | Exemplar experimental designs from students’ quotes in the given range of scores |
|---|---|---|
| Max. score = 6 | Max teams = 10 | |
| Low (1 to 2 | 0 | -----NA---- |
| Medium (3 to 4) | 2 | “Identification of concentration of acetic acid in various types of vinegars. |
| By titrating above mentioned types of vinegars against NaOH and phenolphthalein as indicator. It is a neutralization reaction. | ||
| Different concentrations of single type of vinegar can be tested for the same kind of pickle to now exact concentration of vinegar, best suitable as a preservative for that pickle, same with other two types of vinegar.” | ||
| Team code D3 | ||
| High (5 to 6) | 7 teams | “Wash all the required apparatus, rinse the burettes. |
| NaOH being a secondary standard substance, its normality should be calculated before using it as a titrant for the further experiment. Thus, we standardize NaOH by titrating against HCl (0.01 M) using phenolphthalein as an indicator, endpoint being pink to colourless. | ||
| After standardization of NaOH and calculating its normality and strength, we can use it as a titrant to test the conc. of acetic acid of each sample procured from the market. | ||
| Now wash the apparatus, put NaOH (standardized) in burette-1 and put apple cider vinegar in burette-2. Calculate the normality of the solution and calculate its strength and concentration v/V. | ||
| Repeat the procedure for other samples and compare the values to the required concentrations given for vinegar which is 5–6%. | ||
| The 5%, 6% v/V is converted to Normality which is 0.083–0.1 N | ||
| Phenolphthalein is used as an indicator and the endpoint is colourless to pink.” | ||
| Team code D8 |
Such performance is most likely due to the familiar and easy content of the task. Since all teams completed the given precursor task, we infer that the precursor task provided students with a concrete experience of planning an experimental design of a simple PBL task and helping them to move through the first stage of Kolb's ELC as was hypothesized.
Six of the 10 teams in the Authentic setting submitted the highlighted reading material which we used for assessment. Table 7 presents the number of teams that selected specific content from the reading material.
| Type of selected content | Number of teams that selected the contents, max. = 6 teams |
|---|---|
| The concepts/theory | 5 |
| Two parameters of analysis | 4 |
| Two treatment methods | 4 |
| Effluent discharge limits | 2 |
| Category | Code | Description |
|---|---|---|
| Students’ statements | Explanations/reasoning | Reason for why the student is suggesting/narrating something |
| Suggestions/personal views | Opinions/statements that do not refer to any resource or are not supported by a reason | |
| Acknowledge flaws/doubts | To accept or suggest some mistake/problem/inadequacy or express uncertainty in their ideas | |
| Agreement/disagreement | To agree or disagree with the other students’ viewpoints | |
| Processes/procedures/concepts | To describe plans related to the wastewater treatment | |
| Comparison | To judge which process, procedure, materials, chemicals, etc., may be better for selection | |
| Students’ questions | Seeking explanations/elaborations | To know the other students’ reasoning, response to these questions would need extra information than what is previously stated |
| Seeking clarifications | To understand better the other students’ ideas | |
| Can be answered by | ||
| • repeating what was said | ||
| • agreeing (yes) | ||
| • without adding extra information | ||
Table 7 indicates that most of the teams who supplied their highlighted reading material could identify relevant concepts, but not all could select the expected number of parameters or treatment. To understand students’ viewpoints about the content of the reading material, we gave a 3-point Likert scale questionnaire (easy, moderately difficult, and challenging). The following section describes this analysis.
Students’ perceptions about the reading material
The analysis of questionnaire data of 30 students indicated that 56% of them found the content of reading material easy to comprehend. Responding to another item in the questionnaire on the planning of the task based on the reading material, a large number of students (60%) found it moderately difficult and 23% found it challenging. This suggests, though many students found the contents of the reading material easy to comprehend, synthesis of the content to develop an experimental design for the given task was difficult for the students. The content in the reading material was new to students and thus might have added to the cognitive load, or possibly the content was cognitively less engaging.Group discussion as the third scaffold
The audio transcript indicated various components of reflective thinking during the group discussion which are coded as given in Table 8.The following section provides the analysis of the audio transcript.
Reflective learning and collaborative knowledge building through structured group discussion
The discussion started with each team describing its plans. Three teams out of ten included only the plans, while the others offered reasons for their choices. For example,Student-D1:
“The third thing is about the color and we have to reduce the color, there are six methods given in the manual. For each method, the drawback is given in that itself. We are choosing activated carbon adsorption though it is expensive but is a comparatively very tricky manner to cover the color and remove color by 90%. And about 90% of the color is removed.
The sixth method is also good like ultrafiltration. It is a physical procedure. We will be going with a chemical procedure.”
Such a description of the initial plans was the basis for further discussion leading students to ask clarificatory questions. For example,
Student-D4:
“So, you are dividing the given sample, right? into different parts and each of them is treated in different ways and then you are comparing them and suggesting which one is better?”
The clarificatory questions might have helped students compare implicitly or explicitly their plans with the ones stated by other students. However, the explanations offered by some students were not always agreeable to other students and that led to further questioning and reasoning. For example,
Student-D6:
“It is actually a great and easy method if you can actually confirm” (whether sawdust would work for vat dyes).
This process of negotiation continued till a consensus was reached between the presenting students’ ideas and those of the others. This series of agreement, disagreement, and explanations improved the coherence and quality of the experimental design. For example,
Student-D9:
“After that, you add 4 g per litre of i.e., 4 g activated carbon per litre of the effluent you need to treat, and leave them for 2.5 hours at room temperature… at 60 °C but at 2.5 hours in the room temperature, in the long run, it won’t make a difference and after 2.5 hours, the coarse particles of activated carbon, we can actually remove, the activated carbon can be filtered. We have the filtrate. Now the original wastewater you run a spectrophotometer on that at some frequency of close to 650 nm and …once again you can note down the spectrophotometer and you can compare the two to find the effectiveness of the two.”
Questioning peers or disagreement with peers not only led to sense-making as students articulated their ideas/thinking, but also helped students see the flaws in the plans/ideas. For example,
Student-D5:
“She has not specified the amount she is taking.”
As the discussion progressed, students tended to evaluate theirs as well as the others’ plans. For example,
Student-D7:
“I like that they separated each step on the flow chart… it makes it clear, there are multiple procedures even though, each one is indicated in great detail, so I like that.”
Seeking explanations or clarifications by students suggests that they were perhaps engaging in reflective thinking. For example,
Student-D1:
“My question is, why are you using two methods coagulation and activated carbon?”
Student-D8:
“Everything as in, what all will it adsorb; we have to make sure that every value lies under the tally, (discharge limits) right?”
Thus, these students’ quotes indicate that the structured group discussion might have provided an opportunity for reflective thinking which led students to a better understanding of their choice of the wastewater treatment processes.
The logic that went into designing the EDs was very different for each team and these varied perspectives became visible to many teams through the structured group discussion. Apart from getting clarity and the rationale for the steps that had to be included in the EDs, students compared the flow chart of the precursor (vinegar) task with the indigo ED flowchart during the group discussion. This led some students to identify the gaps in their EDs such as the quantities of chemicals.
Since the designing of the experiment happened at the theoretical level, students got a chance to have their ideas validated by their peers, while questions from peers probed the presenting students’ thinking. For example,
“SS-1: So basically, you will be doing all of them?
SS-2: Not all of them.
SS-3: You have to do all of them. Because, each of them comes at a different purpose. Chlorination is for disinfecting or killing bacteria, activated carbon is for adsorption and coagulation is mostly for the dyes, these two will result in colourless water. This can be done as a last process because this will be disinfecting, that will kill off the bacteria, each of them serves a different purpose, you want to make sure.
SS-1: This is just for clarification, you are basically going to do all of them one by one, how strong the effects are and then decide which order to move.
SS-2: What is the effect on the economics of the situation?
SS-3: That is why we are first testing on the samples.”
Thus, such reflective thinking might have contributed towards an improved score of the EDs after the group discussion.
Further, we analyzed the frequency of the codes in the group discussion.
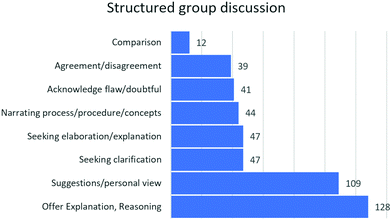
Fig. 6 indicates the frequency of the codes in the transcript.
Fig. 6 indicates that students’ explanations and reasoning are the most frequent code in structured group discussions. The substantial number of students’ questions (Fig. 6) seeking an explanation as well as seeking a clarification might have contributed to the students’ reflection on their initial experimental design while presenting the ED. This might have led to improved EDs.
Each aspect of the group discussion mentioned in Fig. 6 gives affordance to reflect on the adequacy/gaps of students’ plans. Students need an opportunity to reflect on the connection between the content of the reading material and their experimental design. The structured group discussion provided the opportunity for the same.
Thus, we infer that the structured group discussion supported reflective thinking when students justified their stance or revisited their ideas and improved the quality of the experimental design.
Discussion
The PBL environment offers challenges and students require adequate scaffolding to accomplish the task. We provided scaffolds to facilitate the self-directed learning of the indigo PBL task. Our work examined whether the scaffolds were helpful to students in the planning of their experimental design and in moving through the first three stages of Kolb's ELC.Our study, to answer the first research question, suggests that scaffolds make a qualitative difference in the experimental design of a complex PBL laboratory task. Our study corroborates the previous research (Hmelo-Silver et al., 2007) that students need support in the PBL approach. The scaffolds provided an opportunity for students to experience the first three stages of Kolb's ELC, i.e., the Concrete Experience stage (through the precursor task and reading material), the Reflective Observation stage (through the structured group discussion) that led to the Abstract Conceptualization stage (indicated by the experimental design), which answers our second research question.
On one hand, the scaffolds were planned to align with the stages of Kolb's ELC; on the other hand, they also followed some of the prelab strategies suggested by Agustian and Seery (2017); for example, a simple precursor task given before the main PBL task aligns with a simple to complex task strategy; the representation of the procedure in the form of a flow chart is aligned with the whole task strategy of the Agustian/Seery model.
Agustian and Seery (2017) also recommended that students need to be prepared for lab work. Since prior knowledge is an important determinant for activation of new information (Bruck and Towns, 2009) the precursor task and reading material were designed to introduce various aspects of planning a lab task and the contents needed for developing prior knowledge respectively. Further, the precursor task helped students to identify quantities and concentrations of chemicals as one of the missing components in their experimental design while comparing the flowchart of the precursor task with that of the indigo task.
It is difficult for students to effectively select the scattered information available online for planning the task as was indicated by the EDs of the un-scaffolded group in our study. This view is supported by another study that suggests that students need chemistry-specific searches to help them understand the nature of the problem and the required planning (Shultz and Zemke, 2019). Thus, there is a need to provide the reading material.
We expected that the targeted reading material which we provided would help students to focus on the information required for the task and thus prepare students for the laboratory task. Based on our results we infer that presenting compiled information in the form of reading material may not be sufficient for students to plan executable experimental designs aligned with all the module objectives. One possible way to enhance the impact of reading material is to include reflective questions at the end of each section of the reading material. It may help students to be better engaged cognitively with the reading material.
The third scaffold, the structured group discussion, is important as it provides an opportunity for the metacognitive engagement required for the reconstruction and reorganization of students’ initial experimental designs. This finding is supported by a study by Lu Bridges and Hmelo-Silver (2014) that group discussion gives affordance for making students’ thinking visible leading to correction, modification, and knowledge-building through collaboration.
Thus, our study indicates that the use of all three scaffolds, i.e., the precursor task, reading material, and group discussion, results in a quantifiable and quality difference in the experimental design of a PBL task such as the indigo task.
Limitations
In our study, we considered a sample size of 4 to 6 teams of students for each workshop and did not explore how the sample size affected the group discussion. Further, the absence of an un-scaffolded group in the Authentic setting did not allow us to draw a comparison between the un-scaffolded and scaffolded groups in the Authentic setting. The individual students’ contributions to the group discussion were not explored too. We consider these as our limitations.Though the study was carried out in the two settings with a difference in the prior academic achievement levels of students, the transferability of the study to a different setting may be a limitation.
Future direction
The most interesting work to explore in the future is related to the influence of the scaffolds on the fourth stage of ELC, i.e., the active experimentation stage. The evaluation of the experimental design in our study also suggests that the students need an additional scaffold or possibly a couple of more exposure to PBL for planning an experimental design devoid of shortcomings. This and the effect of the precursor task on the main PBL task can be explored in future work. Further work could be carried out to understand the role of the facilitator and the learning outcome achieved by individual students vs a team during the structured group discussion. Possible ways to help students bridge the gap between learning the content of the reading material and using it for problem-solving need further exploration that can be done in future work.Implications for research and practice
By using Kolb's theory, we were able to investigate students’ engagement with the first three stages of this theory. We also investigated how a combination of scaffolds sequenced according to the stages of Kolb's ELC facilitated students to accomplish the given task in a complex learning environment such as the PBL laboratory. Further, students’ responses to the targeted scaffolds helped us understand the engagement level by students at each stage of Kolb's cycle. Thus, by using multiple data sources, that is, the ED scores, students’ perception questionnaire, and group discussion, we could gather evidence for the effectiveness of the scaffolds for student-groups that differed in their prior academic scores.Our work presents a pedagogical approach for the transition from a traditional laboratory to a PBL environment with the help of multiple scaffolds. Our study suggests that PBL chemistry laboratory curriculum development should consider the use of a combination of scaffolds with special emphasis on the structured group discussion. Such development also needs to take into consideration the local instructional needs/contextual effects for scaffolding a simple or complex PBL task. The complexity of the task, we suggest, can be judged based on the number of variables, steps involved in the investigation, new concepts involved, and minimum time required for completion of the task.
Conclusion
Scaffolds helped students to devise a feasible experimental design and provided students with opportunities to engage in reflective learning. As per Kolb's Experiential Learning Cycle (ELC), students undergo four stages for meaningful learning. This article presents a study that explored the first three stages with the use of a scaffolded PBL approach for the planning of the experimental design. The scaffolds were designed to contribute to different aspects of the ELC: for example, the precursor task, reading material and group discussion helped with the first three stages (Fig. 1). Our study suggests that the scaffolds may provide a way for students to move through the first three stages in the ELC sequence for effective planning of the given PBL task. Thus, the scaffolds appear to be useful and to have helped students plan the experimental design which is an important antecedent to the PBL laboratory investigation.Conflicts of interest
The authors have no conflict of interest to disclose.Appendix 1: Rubric for assessing the experimental design of the vinegar precursor task
| Description | Points, if all three components | Points, if two components | Point, if one component | Remarks |
|---|---|---|---|---|
| Logical steps | 3 | 2 | 1 | * Indicates conversion of molarity to % strength |
| • Titrations | ** Three vinegar samples | |||
| • Molar calculations* | ||||
| • Trials with three different samples** | ||||
| Procedure | 3 | 2 | 1 | |
| • Clear | ||||
| • Correct | ||||
| • Glassware mentioned | ||||
Appendix 2: Rubric for evaluation of the experimental design by students for the indigo PBL task
| Descriptor | 0 point | 1 point | 1 point | 1 point | 1 point | Total/4 |
|---|---|---|---|---|---|---|
| Parameters chosen for analysis | None | pH | COD | Suspended solids | Colour | |
| Treatment chosen | None | For pH | For the COD | For suspended solids | For colour | |
| Executable plan (feasibility in an undergraduate lab) | Not executable | Available time (3 lab sessions) | Available equipment | Available chemicals/substances | Available glassware | |
| Quantities/concentrations | Not mentioned | Quantity of wastewater | Quantities for the chosen treatment method | COD (quantities of chemicals) | COD (concentrations of chemicals) | |
| Optimization | None considered | Amount of AC/saw dust/coagulant | Treatment conditions (pH/temperature) | Time for equilibration of AC/saw dust/or coagulant | Minimally two trials | |
| Flow-chart | Not presented | Pre-treatment analysis | Treatment after initial analysis | Post-treatment analysis | Clarity of representation | |
| Tools for measurement | None indicated | pH meter/pH paper | Glassware and indicator for the COD | Weighing balance | Colorimeter/visual comparison |
Acknowledgements
We thank the students who participated in the study and the undergraduate teachers who helped us complete the study. We also thank the reviewers for their valuable suggestions and comments. We express our immense gratitude to Prof. Diane Bunce for her constant encouragement and comprehensive feedback. We acknowledge the support of the Department of Atomic Energy, Govt. of India, under Project Identification No. RTI4001.Note: We invite email @ sujsvarada@gmail.com for instructional materials used in implementation of the indigo PBL module.
References
- Abraham M. R., (2011), What can be learned from laboratory activities? Revisiting 32 years of research, J. Chem. Educ., 88, 1020–1025.
- Agustian H. Y. and Seery M. K., (2017), Reasserting the role of pre-laboratory activities in chemistry education: A proposed framework for their design, Chem. Educ. Res. Pract., 18, 518–532.
- Bennet S. W., Seery M. and Sovegjarto-Wigbers D., (2009), Practical work in higher-level chemistry education, in Eilks I. and Byers B. (ed.), Innovative Methods of Teaching and Learning Chemistry in Higher Education, London: Royal Society of Chemistry, pp. 85–102.
- Boud D. and Feletti G., (2013), The challenge of problem-based learning, London: Kogan Page Limited, ch. 1.
- Bruck L. B. and Towns M. H., (2009), Preparing students to benefit from inquiry-based activities in the chemistry laboratory: Guidelines and suggestions. J. Chem. Educ., 86(7), 820–822.
- Buck L. B., Bretz S. L. and Towns M. T., (2008), Characterizing the level of inquiry in the undergraduate laboratory, J. Col. Sci. Teach., 52–58.
- Choo S. S. Y., Rotgans J. I., Yew E. H. J. and Schmidt H. G., (2011), Effect of worksheet scaffolds on student learning in problem-based learning, Adv. Health Sci. Educ., 16, 517–528.
- Costantino L. and Barlocco D., (2019), Teaching an undergraduate organic chemistry laboratory course with a tailored problem-based learning approach, J. Chem. Educ., retrieved from DOI:10.1021/acs.jchemed.8b01027.
- Daubenmire P. L. and Bunce D. M., (2008), What do students experience during POGIL instruction? in Moog R. S. and Spencer J. N. (ed.), Process Oriented Guided Inquiry Learning (POGIL), ACS Symposium Series, pp. 87–99.
- DeVellis R. F., (2005), Inter-rater reliability, in Kempf-Leonard K. (ed.), Encyclopedia of Social Measurement, San Diego: Elsevier, pp. 317–322.
- Domin D. S., (1999), A review of laboratory instructional styles, J. Chem. Educ., 76 (4), 543–547.
- Donnell C. M., O’Connor C. and Seery M. K., (2007), Developing practical chemistry skills by means of student-driven problem-based learning mini-projects, Chem. Educ. Res. Pract., 8(2), 130–139.
- Flynn A. B. and Bigg R., (2012), The development and implementation of a problem-based learning format in fourth-year undergraduate synthetic organic and medicinal chemistry laboratory course, J. Chem. Educ., 89, 52–57.
- George-Williams S. R., Ziebell A. L., Thompson C. D., Overton T., (2020), Inquiry-, problem-, context- and industry-based laboratories: An investigation into the impact of large-scale, longitudinal redevelopment on student perceptions of teaching laboratories, Int. J. Sci. Educ., 42(3), 451–468.
- Hicks R. W. and Bevsek H. M., (2012), Utilizing problem-based learning in qualitative analysis lab experiments, J. Chem. Educ., 89, 254–257.
- Hmelo-Silver C. E. and Barrows H. S., (2008), Facilitating collaborative knowledge building, Cogn. Instruct., 26(1), 48–94.
- Hmelo-Silver C. E., Duncan R. G. and Chinn C. A., (2007), Scaffolding and achievement in problem-based and inquiry learning: A response to Kirschner, Sweller, and Clark (2006), Educ. Psychol., 42(2), 99–107.
- Hodson D., (1992), Assessment of practical work, Sci. Educ., 1, 115–144.
- Hofstein A. and Lunetta V. N., (2004), The laboratory in science education: Foundations for the twenty-first century, Sci. Educ., 88(1), 28–54.
- Jonsson A. and Svingby G., (2007), The use of scoring rubrics: Reliability, validity and educational consequences, Educ. Res. Rev., 2 130–144.
- Kirschner P., Sweller J. and Clark R. E., (2006), Why minimal guidance during instruction does not work: An analysis of the failure of constructivist, discovery, problem-based, experiential, and inquiry-based teaching, Educ. Psychol., 41(2), 75–86.
- Kolb D. A., (1984), Experiential learning: Experience as the source of learning and development, Englewood Cliffs, NJ: Prentice-Hall, p. 21.
- Konak A., Clark T. and Nasereddin M. (2014), Using Kolb's Experiential Learning Cycle to improve student learning in virtual computer laboratories. Comput. Educ. 72. p11–22.
- Lu J., Bridges S. and Hmelo-Silver C. E., (2014), Problem-based learning, in R. K. Sawyer (ed.), The Cambridge Handbook of the Learning Science, 2nd edn, Cambridge: Cambridge University Press, pp. 298–318.
- Mahmoud A. and Nagy Z., (2009), Applying Kolb's experiential learning cycle for laboratory education, J. Eng. Educ., 98, 283–294.
- Miettinen R., (2000), The concept of experiential learning and John Dewey's theory of reflective thought and action, Int. J. Lifelong Educ., 19(1), 54–72.
- Nagarajan S. and Overton T., (2019), Promoting systems thinking using project- and problem-based learning, J. Chem. Educ., 96(12), 2901–2909.
- Puntambekar S. and Hübscher R., (2005), Tools for scaffolding students in a complex learning environment: What we gained and what have we missed? Educ. Psychol., 40(1), 1–12.
- Quintana C., Reiser B. J., Davis E. A., Krajcik J., Fretz E., Duncan R. G., Kyza E., Edelson D. and Soloway E., (2004), A scaffolding design framework for software to support science inquiry, J. Learn. Sci, 13(3), 337–386.
- Ram P., (1999), Problem-based learning in undergraduate instruction: A sophomore chemistry laboratory, J. Chem. Educ., 76(8), 1122–1126.
- Reid N. and Shah S., (2007), The role of laboratory work in university chemistry, Chem. Educ. Res. Pract., 8(2), 172–185.
- Savery J. R. and Duffy T. M., (1995), Problem-based learning: An instructional model and its constructivist framework, Educ. Tech., 35(5), 31–38.
- Seery M. K., Jones A. B., Kew W. and Mein T., (2019), Unfinished recipes: Structuring upper-division laboratory work to scaffold experimental design skills, J. Chem. Educ., 96(1), 53–59.
- Shultz G. V. and Zemke J. M., (2019), “I wanna just google it and find the answer”: Student information searching in a problem-based inorganic chemistry laboratory experiment, J. Chem. Educ., 96(4), 618–628.
- Stains M., Pilarz M. and Chakraverty D., (2015), Short and long-term impacts of the Cottrell scholars collaborative new faculty workshop, J. Chem. Educ., 92(9), 1466–1476.
- Van der Stuyf R. R., (2002). Scaffolding as a teaching strategy. Adolescent learning and development, 52(3), 5–18.
- Zoller U. and Pushkin D., (2007), Matching higher-order cognitive skills (hots) promotion goals with problem-based laboratory practice in a freshman organic chemistry course, Chem. Educ. Res. Pract., 8(2), 153–171.
| This journal is © The Royal Society of Chemistry 2022 |