Pre-service chemistry teachers’ use of pedagogical transformation competence to develop topic-specific pedagogical content knowledge for planning to teach acid–base equilibrium
Sevgi
Aydin-Gunbatar
 *a and
Fatma Nur
Akin
*a and
Fatma Nur
Akin
 b
b
aVan Yuzuncu Yil University, Faculty of Education, Department of Mathematics and Science Education, 65100, Van, Turkey. E-mail: sevgi.aydin45@hotmail.com
bThe Scientific and Technological Research Council of Turkey, Department of Science and Society, 06680, Ankara, Turkey
First published on 4th November 2021
Abstract
In this study based on mixed-methods design, we aimed to examine to what extent the participants underwent pedagogical transformation and developed topic-specific pedagogical content knowledge (PCK) for planning to teach acid–base equilibrium. Training for the acquisition of pedagogical transformation competence to develop topic-specific PCK was provided for 12 weeks during the teaching methods course. Through this intervention, the participants were taught pedagogical transformation competence in different chemistry topics and were supposed to employ it in another topic that was not covered through the intervention. To fulfill this aim, the use of explicit discussions of the transformation of content knowledge as a teaching strategies and explicit topic-specific PCK use were provided throughout the intervention. A total of 13 junior pre-service chemistry teachers participated in the intervention. The content knowledge test, Content Representation (CoRe), semi-structured interviews, focus group interview, and reflection papers were the data sources. A rubric created by the researchers was used to analyse the pre- and post-CoRe data to determine to what extent the participants could develop topic-specific PCK for planning to teach the acid–base equilibrium topic, which was not discussed through the intervention. The results showed that 11 of the 13 participants could achieve transfer of what they had learnt and experienced during the intervention to plan for a new topic, and this was identified as an enrichment of at least one topic-specific PCK component. This study presented an uneven development of topic-specific PCK components as a product of the transformation. The findings revealed that the participants showed more progress in transferring content knowledge to students’ difficulties and alternative conceptions, and instructional strategies than in the assessment component of topic-specific PCK. This study provided valuable information about pre-service teachers’ use of the pedagogical transformation competence acquired during the intervention to develop topic-specific PCK for planning to teach acid–base equilibrium and the development of the knowledge of assessment. Implications for both the enrichment of science/chemistry teacher education and future research were presented.
Introduction
Recently, teacher educators have revealed a growing emphasis on the development of the knowledge base of pre-service teachers. In this respect, pedagogical content knowledge (PCK) has emerged as a significant form of knowledge that pre-service teachers need to develop through teacher training (Brown et al., 2013; Mavhunga, 2016; Sæleset and Friedrichsen, 2021). PCK was first introduced by Shulman (1986) as a central element in the knowledge base for teaching and for the specific practical knowledge teachers need to help learners comprehend specific content. It is usually pointed out that PCK is used to adapt content knowledge for pedagogical purposes via a process known as ‘transformation’ that ends with PCK (Shulman, 1987). Accordingly, Mavhunga (2016) put forward that “learning to transform content knowledge is a central element of the teaching of PCK to pre-service teachers” (p. 1081). Mavhunga (2016) suggested using the term ‘pedagogical transformation competence’ and stated that competence is just one side of the PCK coin. The construct is regarded as the generic competence to be gained. When the pedagogical transformation competence is implemented to make a particular topic more comprehensible for learners, the product will be PCK for a specific topic (Mavhunga, 2016), which is the most specific level of the general taxonomy of PCK proposed by Veal and MaKinster (1999). The topic-specific nature of PCK makes PCK development difficult and time-consuming (Rollnick and Mavhunga, 2015). To address this problem, Mavhunga (2016) stated that pre-service teachers could learn how to transform content knowledge into PCK for teaching a specific topic (gases) and could apply that competence in other topics (redox reactions), which is the motivation behind this current study. With these in mind, the primary purpose of this study was to examine to what extent the participants learned pedagogical transformation and developed topic-specific PCK for planning to teach acid–base equilibrium.The development of solid and quality PCK has positive influence on students’ learning, which lies at the heart of teacher education and professional development (Kind and Chan, 2019). Even though PCK development is a lifelong process, pre-service teacher education programs are a significant starting point in the teaching career (Friedrichsen et al., 2009; Brown et al., 2013; Ekiz-Kiran et al., 2021). The teacher education literature provides important points that should be taken into account regarding how to support pre-service teachers’ topic-specific PCK development through the program (e.g., Hume and Berry, 2011; Brown et al., 2013; Barnett and Friedrichsen, 2015). First, in most teacher education programs, pre-service teachers initially take content knowledge courses and then take teaching methods courses. It is assumed that pre-service teachers who are taking teaching methods courses have the necessary content knowledge; however, the related literature revealed that this is generally not the case (van Driel et al., 2002; Friedrichsen et al., 2009; Aydin et al., 2013). Therefore, it is suggested that explicit discussions of the transformation of content knowledge be addressed as a basis for the improvement of pre-service teachers’ PCK (Mavhunga and Rollnick, 2013; Mavhunga, 2016). Moreover, the explicit use of PCK components was suggested as a useful strategies (Aydin et al., 2013; Mavhunga, 2016).
In light of the aforementioned studies, in this study, the authors designed an intervention by integrating the strategies suggested in the literature (e.g., explicit topic-specific PCK use, explicit discussions of the transformation of content knowledge) in the context of the chemistry teaching methods course. By doing so, the authors aimed to teach pedagogical transformation competence that would result in giving topic-specific PCK to the participants. Then, to determine to what extent the participants managed to transfer what they learnt through the intervention into planning a lesson for teaching a topic that was not focused on in the intervention. This study has the potential to provide useful insights into how pedagogical transformation competence “would offer teacher educators a clearer insight into the kind of knowledge and the corresponding skills that ought to constitute the content of an initial teacher development program, with the fundamental aim of developing PCK as a goal” (Mavhunga, 2016, p. 1082).
Literature background
Theoretical framework for PCK and topic-specific PCK
Shulman (1986) argued that PCK “goes beyond the knowledge of subject matter per se to the dimension of subject matter for teaching” (p. 9) and described PCK as a kind of teachers’ special practical knowledge that differentiates a chemistry teacher from a chemist. Since the introduction of PCK, many scholars have studied PCK and developed different PCK models (e.g., Grossman, 1990; Magnusson et al., 1999; Carlson and Daehler, 2019). For example, Magnusson et al. (1999) developed a model of PCK for science teaching that is one of the most often cited models. Their PCK model was built on the idea that “how particular subject matter topics, problems, and issues can be organised, represented, and adapted to the diverse interests and abilities of learners and then presented for instruction” (Magnusson et al., 1999, p. 96). Their PCK model includes five components, namely: (a) science teaching orientations – teachers’ knowledge and beliefs about the goals and purposes of teaching science at a specific grade level; (b) knowledge of instructional strategies – teachers’ knowledge about science-specific strategies (e.g., argumentation) and topic-specific strategies (e.g., simulations); (c) knowledge of curriculum – teachers’ knowledge of goals and objectives concerning science and specific topics within that domain; (d) knowledge of learners – teachers’ knowledge of requirements for learning and areas of students’ difficulties, including alternative conceptions in specific science topics; and (e) knowledge of assessment – teachers’ knowledge of aspects of science learning to assess (e.g., science process skills) and knowledge of methods of assessment (e.g., test). In this model, PCK is conceptualised at the topic level and so the components are topic-specific PCK components. Accordingly, effective teachers should develop all components of PCK to teach a specific topic. In this study, Magnusson et al.'s PCK model (1999) has been used as the theoretical framework. Our PCK conceptualization mirrors Magnusson and her colleagues’ definition that focuses on the topic-specific nature of the PCK construct and has five components, one of which is knowledge of assessment.A recent PCK model: the Consensus Model of PCK
Recently, the Consensus Model of PCK, which focuses on teacher professional knowledge and skills, was developed (Gess-Newsome, 2015). In this model, knowledge from teacher professional knowledge bases informs, and is informed by, topic-specific professional knowledge. Four years after the introduction of the model, Carlson and Daehler (2019) presented the Refined Consensus Model (RCM) of PCK. The RCM model was built on the previous frameworks (e.g., Magnusson et al.'s model and the Consensus Model) and highlights many aspects of PCK. The centre of the RCM of PCK is around the practice of science teaching (Carlson and Daehler, 2019). This model describes the specialized professional knowledge that a teacher uses to engage in pedagogical reasoning during planning, teaching, and reflecting on a lesson. The RCM has a concentric representation and has enacted PCK (ePCK), personal PCK (pPCK), and collective PCK (cPCK), from the inner to the outer circle. A teacher uses ePCK while engaging in the practice of teaching, be it planning instruction, implementing that plan, or reflecting on instruction and student outcomes. In this model, enactment applies not only to the knowledge of and reasoning behind the act of teaching when cooperating directly with learners (reflection in action) but also to the acts of planning to teach and reflecting on instruction and student outcomes (reflection on action).A teacher's pPCK is the accumulative and dynamic PCK and skills of an individual teacher that reflect the teacher's own teaching and learning practices, with the influences of others, such as teaching colleagues, educational researchers, and other content specialists in the form of coursework, professional exchanges, social media, journal articles and professional learning experiences (Carlson and Daehler, 2019). As phases of the larger realm of pPCK are obtained and used, it becomes ePCK, and so ePCK is a subset of pPCK (Carlson and Daehler, 2019). cPCK is described by Carlson and Daehler as “a specialised knowledge base for science teaching that has been articulated and is shared among a group of professionals” (2019, p. 88). Accordingly, cPCK is consistently developing through professional interaction and shared by interventions (e.g., teacher training and workshops) (Coetzee et al., 2020). Fig. 1 shows the simplified illustration of the multidimensional nature of PCK in the RCM of PCK indicating how topic-specific PCK (and each of the other ‘grain sizes’) is embedded in the three realms of PCK, where the grain sizes refer to the discipline-specific, topic-specific, and concept-specific levels of PCK (Mavhunga, 2019). The idea of topic-specific PCK (e.g., Magnusson et al.'s model or the Consensus Model) is not new, but it is more obvious and given more clarity in the RCM of PCK for science teaching compared with the other grain sizes of PCK (Mavhunga, 2019).
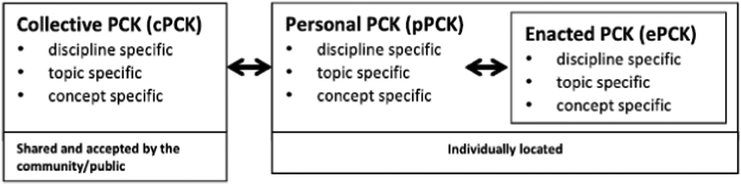 | ||
| Fig. 1 A simplified illustration of the multidimensional nature of PCK in the RCM of PCK (Mavhunga, 2019, p. 131). | ||
The relationships between pedagogical transformation competence and topic-specific PCK
Shulman (1987) stated that an important part of what skilful teachers do is to transform content into a more accessible form for students. This form of transformation is “the essence of the act of pedagogical reasoning, of teaching as thinking, and of planning – whether explicitly or implicitly – the performance of teaching” (p. 16). Shulman (1987) described pedagogical reasoning and action as the challenge of taking what the teacher understands and making it ready for effective instruction. His model of pedagogical reasoning and action has a cyclic nature and involves the six actions of a teacher, namely, comprehension, transformation, instruction, evaluation, reflection, and new comprehensions. The starting point is the act of comprehending facts, principles, purposes, subject matter structures, and ideas within and outside the discipline. According to Shulman (1987), “to teach is first to understand” (p. 14).Then, the understood ideas should be transformed using the following processes: (1) preparation of the given text materials, (2) representation of the ideas in the form of new analogies, metaphors, etc., (3) selections of instructional teaching methods and models, and (4) adaptation of these representations to the general characteristics of the learners. The process continues with instruction, evaluation and reflection, and new comprehension by the teacher. Pedagogical transformation refers to “the instructional principle in which scientific ideas are simplified and reconstructed into what can be readily accessible to and understood by students without distorting the essential features of the ideas” (Oh and Oh, 2011, p. 1124). Pedagogical transformation, a step within the pedagogical reasoning process, is considered to be the core of the act of pedagogical reasoning of teaching (Shulman, 1987).
By supporting Shulman's view, Geddis and Wood (1997) used this term for the pedagogical transformation of content knowledge and stated that the value of focusing on the transformation of content knowledge lies in that “…it directs attention simultaneously to subject matter, learners and educational purposes, and to the interactions among these different kinds of teacher knowledge in the pedagogical encounter” (p. 612). Some researchers have endorsed the opinion that the pedagogical transformation of content knowledge is the essence of teachers’ PCK (e.g., Magnusson et al., 1999, Mavhunga 2016). For instance, Magnusson et al. (1999) described PCK as the transformation of several types of knowledge for teaching, including content knowledge, and identified different kinds of knowledge which we have listed earlier as topic-specific PCK components. Likewise, Geddis and Wood (1997) stated that the product of the transformation is the knowledge of learners (difficulties and alternative conceptions), knowledge of representations and strategies, and knowledge of curriculum (saliency and materials), which are topic-specific PCK components.
The act of pedagogical transformation was viewed as competence and termed pedagogical transformation competence by Mavhunga (2016). It refers to “the understanding of the relevant kinds of knowledge, the components of TSPCK [topic-specific PCK], and the ability to create ‘interactions’ that need to be drawn into a pedagogical encounter” (Mavhunga, 2016, p. 1083). Pedagogical transformation competence is to be learnt and applied to a particular content knowledge to result in PCK for the topic in question. In light of the literature (Shulman, 1987; Geddis and Wood, 1997; Magnusson et al., 1999; Mavhunga, 2016), we regard competence as the key to being a successful teacher who comprehends the content and makes the content ready for learners’ comprehension. In this transformation, teachers need to (i) learn the parts of the specific content that are important for learners to understand, (ii) learn and anticipate these parts of the content that will be difficult for learners to comprehend, (iii) select strategies and representations to help learners to understand these parts, (iv) prepare the content for learners using these strategies, and (v) check to what extent learners understand the content. The competence can also be applied to planning or teaching other topics that have common attributes making learners confused (e.g., including reactions occurring at the particulate level, providing different observations for the macro and sub-micro levels). The process would need to be repeated to improve topic-specific PCK for different topics (Mavhunga, 2016).
Research on the relationship between pedagogical transformation competence and topic-specific PCK
Considering the history of PCK, only a few studies have been conducted to examine the link between pedagogical transformation competence and topic-specific PCK (e.g., Mavhunga and Rollnick, 2013; Mavhunga, 2016; Mavhunga et al., 2016). For example, based on the work of Shulman (1987) and Geddis and Wood (1997), Mavhunga and her colleagues focused on the construct and conducted studies to shed light on the idea of how pedagogical transformation competence is used to develop topic-specific PCK. Mavhunga and Rollnick (2013) examined to what extent the explicit discussion of the transformation of concepts in chemical equilibrium through five topic-specific PCK components impacts the development of PCK within the topic. Using a mixed-methods design, the researchers conducted an intervention for six weeks targeting the understanding of the transformation of chemical equilibrium concepts through the components identified earlier (learners’ prior knowledge, curricular saliency, what is difficult to understand, representations, and conceptual teaching strategies). The findings showed that the quality of topic-specific PCK developed significantly through such explicit discussions of the transformation of concepts in chemical equilibrium. The findings indicated a mutual interaction between the pedagogical transformation of concepts and PCK. Based on this finding, Mavhunga (2016) conducted a study to examine how the use of pedagogical transformation competence develops pre-service teachers’ topic-specific PCK in chemical equilibrium. Using a mixed-methods design, the author conducted an intervention for six weeks in a chemistry methodology class with 36 third-year pre-service teachers. The intervention consisted of explicit explanations of the idea of the transformation of content knowledge as a step in the process of pedagogical reasoning. The same five topic-specific PCK components identified earlier were discussed one at a time using the topic of the particulate nature of matter. The results showed that the participants used their learnt competence to transform content knowledge in the intervention's topic effectively, thereby developing their topic-specific PCK in that topic. The participants also effectively applied the competence to significantly improve their topic-specific PCK in the topic of transfer (chemical equilibrium). Similarly, another study investigated the extent to which the competence learnt in the intervention with the electric circuit is transferred for the improvement of topic-specific PCK in a new chemistry or physics topic (Mavhunga et al., 2016). This study was conducted in a physics methodology class with 10 pre-service teachers. The findings confirmed those of Mavhunga (2016) and revealed that the participants constantly showed higher performance in the topic-specific PCK components in the topic of choice, which they linked to transfer. In addition, some components of topic-specific PCK (e.g., representation) seemed to be more accessible than others (e.g., learners’ prior knowledge).Significance of the study
Mavhunga criticized “the common understanding that PCK observed in one topic is not transferable to another topic” (2016, p. 1094). Then, she asked a thought-provoking question, “if this is the case, what then could be transferred?” Given the importance of learning to transform content knowledge, Mavhunga (2016) opened a fruitful door to exploring how pedagogical transformation competence is used to develop topic-specific PCK. However, since this subject has been given little attention, there is a need for research that examines the transfer of pedagogical transformation competence within and across topics of the different disciplines (Mavhunga, 2016). Very few studies have looked at the transfer of pedagogical transformation competence by providing training on one topic (e.g., particulate nature of matter) or examined the transfer level in another topic (e.g., chemical equilibrium in Mavhunga, 2016).Based on Mavhunga's call (2016) to explore the transfer of pedagogical transformation competence further, we aimed to examine to what extent pedagogical transformation competence learnt through the intervention (the details will be provided in the Methodology section) including different chemistry topics is transferred to topic-specific PCK for planning to teach acid–base equilibrium. With explicit discussions on the transformation of different chemistry topics through a long intervention (when compared to intervention including one topic and one topic-specific PCK component each week), we argue that in this design the participants would have more chance to learn more transferable points across topics. The importance of this design lies in suggesting the existence of a generic pedagogical competence used to develop PCK from topic to topic. Moreover, focusing on all PCK components every week would also be useful for learning not only topic-specific PCK components but also the interplay among the components because the components are knowledge from which the transformation emerges (Mavhunga, 2016).
In addition to explicitly focusing on transformation content knowledge through different topics, the second significance of the study is the inclusion of the assessment component of topic-specific PCK. In the previous studies conducted to investigate pedagogical transformation competence, the assessment component was stated as hidden and not taken into consideration (Park and Oliver, 2008; Mavhunga, et al., 2016). Mavhunga and Rollnick (2013) used Geddis and Wood's (1997) components (e.g., curricular saliency and learners’ prior knowledge). However, Magnusson et al. (1999) defined ‘knowledge of assessment in science’ as a topic-specific PCK component. The model and components proposed by Magnusson et al. (1999) used in this study are the ones frequently cited by scholars (Brown et al., 2013; Barnett and Friedrichsen, 2015; Chan and Yung, 2018; Chan et al., 2019; Coetzee et al., 2020) and used as topic-specific PCK components (e.g., Aydin et al., 2013; Uzuntiryaki-Kondakci et al., 2017; Akın and Uzuntiryaki-Kondakci, 2018; Chan and Yung, 2018; Ekiz-Kiran et al., 2021). Therefore, the authors specifically aimed to focus on the assessment component in this study, which is supposed to contribute to the previous studies about the use of pedagogical transformation competence to develop pre-service teachers’ topic-specific PCK and provide evidence for the development of topic-specific assessment knowledge (e.g., Mavhunga, 2016; Mavhunga et al., 2016).
The research questions that guided the present study were:
1. To what extent did pre-service chemistry teachers undergo pedagogical transformation and develop topic-specific PCK for planning to teach acid–base equilibrium?
2. To what extent did pre-service chemistry teachers develop the knowledge of assessment, a topic-specific PCK component, for planning to teach acid–base equilibrium through pedagogical transformation?
Methodology
The study
This study used a mixed-methods design (Teddlie and Tashakkori, 2003). With more emphasis on qualitative data, both qualitative and quantitative data were collected simultaneously. Hence, the specific design of the study was qualitatively-driven qualitative and quantitative simultaneous design (Morse, 2003). In this study, the contribution of a special intervention designed in light of the useful practices to participants’ pedagogical transformation competence was explored, which is hoped to result in the development of topic-specific PCK.Participants and context
Thirteen junior chemistry pre-service teachers (eight female and five male) were enrolled in the chemistry teaching methods course. The participants were enrolled in a four-year program. Each year has two semesters (each semester is 12 weeks). Before taking the teaching methods course, all participants took content courses (e.g., Analytical Chemistry), pedagogy courses (e.g., Curriculum & Instruction), and two chemistry-specific PCK courses, namely, Material Development and Instruction, and Technology Use in Chemistry Teaching. Before the study began, the participants were informed about its nature. An informed consent form was signed by all participants. We also informed participants that pre- and post-data sources were not part of the course assessment and grading. They were all voluntary participants in the study. Moreover, necessary information about the research was given to the Head of the Department and the Dean of the Faculty of Education. Necessary permission from the IRB was also gained from Van-Yüzüncü Yil University, Ethical Board of Social Science and Human Subjects (approval number 85157263-604.01.02-E.89352). Pseudonyms were used in this study to preserve the confidentiality of the participants.The intervention
Training for the acquisition of pedagogical transformation competence to develop topic-specific PCK was provided for 12 weeks during the teaching methods course offered as four class hours each week (one class hour is 50 minutes). The details of the intervention are presented in Table 1. The intervention was designed considering Shulman's (1987) and Mavhunga's (2016) suggestions for the transformation of content knowledge (Table 1). Through the intervention, explicit emphasis was put on the transformation of content knowledge. Moreover, the topic-specific PCK components “were introduced as knowledge from which transformation of content knowledge emerges” (Mavhunga, 2016, p. 1084). To provide a clear picture of the intervention, how each topic-specific PCK component was discussed is explained below with examples from the intervention.| Chemistry topics covered | Topic-specific PCK components | |||
|---|---|---|---|---|
| Instructional strategies | Learners | Curriculum | Assessment | |
| a The intervention details were introduced to the participants in the first week. The CoRe and PCK introductions were held in the second week. The intervention began in the third week. b In this week of the intervention, the Arrhenius acid–base description was focused on. Acid–base equilibrium is a different topic that covers the Brønsted–Lowry acid–base description and weak acid–base equilibrium and their K constant calculations. | ||||
| Particulate nature of matter | Conceptual change | Alternative conceptions and difficulties related to the particulate nature of matter | Objectives, big ideas about the particulate nature of matter | Concept map and assessment of learners’ understanding of the sub-microscopic level |
| Chemical reactions | 5E | Alternative conceptions and difficulties related to chemical reactions | Objectives, big ideas about chemical reactions | Assessment of learners’ understanding of chemical reactions at the sub-microscopic level |
| Gases | Argumentation | Alternative conceptions and difficulties related to gases | Objectives, big ideas about gases | Concept cartoons for diagnosing alternative conceptions in gases |
| Solutions | Relating, experiencing, applying, cooperating, transferring (REACT) strategies & role-playing | Alternative conceptions and difficulties related to solutions | Objectives, big ideas about solutions | How to use and how to write questions that include daily-life events in solutions |
| Reaction rate | Inquiry | Alternative conceptions and difficulties related to the reaction rate and examining the patterns of students’ reasoning in chemistry (Talanquer, 2002) | Objectives, big ideas about the reaction rate | How to use and how to form graphs to assess learners’ understanding of the rate of reaction |
| Mid-term project: the CoRe project (participants prepared a CoRe on any topic except for the acid–base equilibrium topic individually) | ||||
| Chemical equilibrium | Analogies and games | Alternative conceptions and difficulties related to chemical equilibrium | Objectives, big ideas about chemical equilibrium | How to use and how to form a diagnostic tree and structured grid to diagnose learners’ alternative conceptions in chemical equilibrium |
| Acid–base: Arrhenius acids/bases & indicatorsb | Predict, observe, explain (POE) | Alternative conceptions and difficulties related to acids/bases | Objectives, big ideas about acids/bases | Forming open-ended questions to assess learners’ conceptual understanding of acids/bases and forming a rubric to assess learners’ performance in acid/base titration |
| Electrochemistry | Integrated science, technology, engineering and mathematics (STEM) approach | Alternative conceptions and difficulties related to electrochemistry | Objectives, big ideas about electrochemistry | Forming open-ended questions for the sub-microscopic level |
| Final project: the CoRe project (participants prepared a CoRe on any topic) | ||||
In the first week, the first meeting was held. Additionally, the aim of the intervention and the details were introduced to the participants. To make the participants familiar with the CoRe and PCK, in the second week, the researchers introduced a CoRe (i.e., the CoRe prepared by a chemistry education expert was provided to the participants) and a PCK construct (i.e., what PCK is and the components of PCK with explicit examples for teaching chemistry topics). The CoRe questions were focused on by examining the answers given in the prepared CoRe (i.e., the CoRe introduced was on the particulate nature of matter) (please see the Data Collection, The CoRe part). After the introduction of them, the intervention began in the third week of the semester.
In the third week, we focused on the particulate nature of matter topic. In that week, we started by focusing on the basic concepts (e.g., particles, particles’ movement, particles’ organisation in the solid, liquid, and gas phases) of the topic. The first part is related to Shulman's (1987) comprehension, which states that to teach, teachers first need to learn the content. Moreover, the sequence of the concepts was focused on. For example, it was discussed that the particulate nature of matter should be emphasized before empty space between particles. This part is a starting point for the transformation of content knowledge to make it more understandable for learners.
Later, the points that may make the topic difficult for learners to understand (transformation of content knowledge) were discussed. For example, since the particles cannot be seen with the naked eye, learners have difficulty in understanding and visualising the particulate nature of matter and the space between the particles. This part was the explicit discussion of the knowledge of learners component of topic-specific PCK with specific attention to content knowledge. In other words, it is the transformation of content knowledge into a form more accessible to learners. Regarding the learners component, in addition to those discussions, Talanquer (2002) was used to examine how learners think about chemical reactions and processes, and how to reason the changes occurring (Table 1). Talanquer (2002) gave examples of the patterns of reasoning that learners have such as “most properties or changes in a system depend on a single independent variable; students tend to focus their attention on a variable whose change is most evident” (p. 48). With those specific points revealed by Talanquer (2002), we, as a group, brainstormed the single variable that learners may focus on in the topics discussed each week (e.g., “the polarity of a molecule depends only on the polarity of its bonds”) (Talanquer, 2002, p. 48).
After determining the points making the topic difficult for learners, we moved to discuss how to address those points (interplay between the knowledge of learners and instructional strategies components of topic-specific PCK). This part is also related to the selection of instructional teaching methods and models in Shulman's (1987) transformation of content knowledge process. Then, the instructor (the first author) started with an alternative conception, that is, “Matter has a continuous structure and does not have any space”, and assumed that some of the learners in our imaginary high school class had that alternative conception. To address it, she implemented a conceptual change strategies (as if she were a teacher and the participants were the high school students in an imaginary high school class). Due to the lack of an internship component in the teaching methods course, the instructor carried out a mock lesson with the participants as learners.
During the mock lesson, the participants formed small groups (three or four pre-service teachers in each group) and experienced the conceptual change strategies step-by-step (cognitive conflict, intelligibility, plausibility, and fruitfulness) (Posner et al., 1982). In the cognitive conflict step, the instructor mixed 25 mL ethyl alcohol and 25 mL water in a beaker as a demonstration. Then, she asked the learners to guess the final volume of the mixture. After taking the learners’ guesses, she mixed the liquids and read the volume, which was less than 50 mL. She asked the learners to think about the reason for that volume difference, which is related to the space between the particles and intermolecular forces. Later, the instructor used another activity in which she gave syringes to the groups. Then, she told them to fill the syringes with air. After that, she asked them to push the piston of the syringe and read the volume that they could not push any further. Her aim was to create a conflict in the learners’ minds about the alternative conception that could not explain the observations made in both activities. The other steps of conceptual change were also implemented using different activities, and videos and discussions on the observations were made.
After the mock lesson, the conceptual change strategies was introduced with the help of presentation software. During this stage, the instructor linked the theoretical information about the strategies to what they did during the previous implementation phase regarding each step of the conceptual change strategies (instructional strategies component of topic-specific PCK). The introduction of the instructional strategies was specifically linked to chemistry teaching and learning, which differentiates this part from general pedagogical knowledge. The specific reason behind the use of the instructional strategies (e.g., the use of the Conceptual Change strategies to address the learners’ “matter has a continuous structure and does not have any space” alternative conception) was also explicitly stated each week by the instructor. This is associated with the adaptation of these representations to the general characteristics of the learners of Shulman's (1987) transformation of content knowledge process. In this way, how the transformation of content knowledge emerged from the instructional strategies component was emphasized explicitly.
Later, as a group, we examined the Turkish high school chemistry curriculum for the concepts emphasized that week (curriculum component of topic-specific PCK) and determined the big ideas that should be focused on. For example, for the particulate nature of matter, the participants focused on the Nature of Matter topic in the 9th grade. The big ideas stated in the curriculum document were highlighted (e.g., the matter is composed of the particles; there is empty space between the particles). In this part of the intervention, we also explicitly emphasized the link between the chemistry topics. A concept learnt in a topic (polarity of molecules) is necessary to understand a concept (dissolution) that would be learnt in a later topic. For example, in the third week, the instructor emphasized that the ‘matter is composed of particles’ concept would be necessary for learning such concepts as dissolution and diffusion. The emphasis shows that the teaching of transformation of content knowledge was highlighted whenever possible through the intervention. In this case, the network that exists among the chemistry concepts was pointed out in line with the curriculum component of topic-specific PCK.
Finally, the instructor introduced an assessment technique appropriate to both the strategies and the topic covered in that week (assessment component of topic-specific PCK). In the particulate nature of matter topic, the instructor created a concept map with the help of the participants at the end of the lesson and stated explicitly that the topic included many concepts that learners might find it difficult to link to each other. Therefore, to determine to what extent the learners could link the concepts, the use of a concept map as a strategies was implemented as an example of transforming content knowledge into the assessment component of topic-specific PCK. Additionally, the instructor shared some open-ended questions using sub-microscopic level figures of atoms, molecules, and ions (the explicit emphasis on the interplay between the instructional strategies and assessment components of topic-specific PCK). The use of those questions was linked to the learners’ difficulty in comprehending the particulate nature of matter, which was focused on at the beginning of the lesson. The instructor explicitly stated the necessity of using the sub-microscopic level representations to assess learners’ visualisation of the chemical events.
Through the intervention, the instructor used explicit topic-specific PCK language to get the participants accustomed to using each component in the transformation process. Each week, she explicitly used the names of the components for the topic focused on. For example, she started the group discussion on the points that make learning the particulate nature of matter difficult for learners (knowledge of learners component of PCK for teaching the particulate nature of matter topic). To conclude, the intervention design was inspired by Shulman (1987) and Mavhunga (2016) and paid explicit attention to the transformation of content knowledge to make it more readily accessible for learners.
Finally, regarding the use of different levels of representations, through the intervention, the instructor paid specific attention to use representations at different levels. Additionally, hybrid representations including at least two levels together were utilized to focus on the reactions. By the use of hybrid representations, the instructor aimed to support the pre-service teachers’ understanding of the reactions at all levels rather than only focusing on the symbolic or macroscopic level. As Table 1 shows, the representations were not only linked to the instructional strategies but also the learners’ difficulty and assessment components of the PCK. For instance, in the 11th week, the instructor focused on ‘writing open-ended questions for the sub-microscopic level’ for assessing the learners’ understanding of electrochemical reactions. Likewise, in the week that the Solutions topic was addressed, during the introduction of the role-playing strategies, the participants experienced dissolution of NaCl in water through role-playing, which emphasizes the sub-microscopic level.
Data sources
The details of the data sources are summarized in Table 2. The literature concerning the pedagogical transformation process and topic-specific PCK informed us about the data that should be collected. As mentioned earlier, the literature highlighted the role of content knowledge and reasoning the content knowledge to make it more accessible for learners (Shulman, 1987). Moreover, as a consequence of this focus on teaching the transformation of content knowledge, Geddis and Wood (1997) proposed different kinds of knowledge which are topic-specific PCK components. To conclude, content knowledge development should be assessed throughout the study (with a content knowledge test), as should topic-specific PCK development (with a lesson plan including all components of topic-specific PCK, which could be the CoRe), and the details showing how the intervention helped participants reason and transfer that reasoning into planning a new topic (interviews and reflection papers).| Data sources | Time | The purpose | |
|---|---|---|---|
| Data collected before the training | Pre-content knowledge test | In the first week | To assess participants’ content knowledge at the beginning |
| Pre-CoRe | In the second week after the introduction of a CoRe prepared by an expert | To assess participants’ topic-specific PCK level for planning to teach acid–base equilibrium at the beginning | |
| Pre-interviews | In the second week, after pre-CoRe administration | To get details about the pre-CoRe | |
| During training | Reflection papers | At the end of the sixth week | To learn participants’ views about how the intervention influenced their topic-specific PCK development |
| Data collected after the training | Post-content knowledge test | At the end of the intervention | To assess participants’ content knowledge level at the end of the intervention |
| Post-CoRe | At the end of the intervention | To assess the topic-specific PCK level for planning to teach acid–base equilibrium at the end of the intervention | |
| Post-interviews | After the last week, after post-CoRe administration | To get details about the post-CoRe | |
| Focus group interview | At the end of the intervention | To learn participants’ views about how the intervention influenced their topic-specific PCK development |
The interviews and reflection papers were prepared and applied in Turkish because the institution in which the research was conducted uses Turkish as the medium of instruction. The quotes taken from the interviews and/or reflection papers were translated from Turkish into English by the authors who are bilingual chemistry education experts with a PhD from an English-medium university and lived in the United States for a year. Moreover, they have been working on chemistry education and chemistry teacher education for about 15 years. In this translation process, the issues raised by Taber (2018) were considered. The CoRe was originally developed in English by Loughran et al. (2004) and applied in Turkish to the participants. The validity checks of the translated version of the CoRe were done in previous studies (e.g., Aydin et al., 2013; Aydin et al., 2015).
The test included 10 multiple-choice items from general chemistry topics covered in the intervention. For the validity, we prepared a specification table. Then, the opinions of two experts with a PhD in chemistry education were taken. After revisions, the final form of the test was prepared. As for reliability, the small sample size did not let us calculate the reliability score for the test. This is one of the limitations of the study. It was administered both at the beginning and at the end of the intervention. Both the pre-test and post-test took around 50 minutes to administer. An example item from the content knowledge test is included in Appendix A.
Regarding the content knowledge necessary for preparing the CoRe on teaching the acid–base equilibrium topic, the participants learnt the topic in high school and the analytical chemistry course that they took one year ago. During the intervention, the participants did not receive any other intervention and/or training for either topic-specific PCK development or acid–base equilibrium. The topic is covered by the 11th-grade Chemistry Curriculum, which means that the participants will teach it after graduating from the teacher education program. Given the importance of content knowledge for topic-specific PCK development, we photocopied the acid–base equilibrium part of the Turkish 11th-grade chemistry textbook and gave it to all participants during pre- and post-CoRe preparations.
In this study, we utilized a modified version of the CoRe that was prepared and validated in our previous work (e.g., Aydin et al., 2013). As a research group, we made several modifications to the original one to make the prompts more understandable for pre-service teachers (Table 3). This form of the CoRe is more consistent with the topic-specific PCK components of Magnusson et al.'s (1999) model. Likewise, other scholars, for instance, Mavhunga (2016), modified the CoRe in light of Geddis and Wood (1997) and used the revised version that highlights the components of Geddis and Wood's (1997) topic-specific PCK components (teachers’ knowledge of learners’ prior knowledge, curricular saliency, difficult points of the content taught, representations, and instructional strategies). As stated earlier, the assessment component has been viewed as ‘hidden,’ so we paid specific attention to developing the assessment component through the intervention. In this way, we aimed to contribute to the available body of knowledge (e.g., Mavhunga 2016; Mavhunga et al., 2016).
| Modified CoRe prompts | Topic-specific PCK components |
|---|---|
| Q2. Why is it important for learners to know this big idea/s? | Knowledge of curriculum |
| Q3. What difficulties do learners have when learning the big idea? | Knowledge of learners (difficulty & prior knowledge including alternative conceptions) |
| Q4. What alternative conceptions do learners typically have about each big idea? | |
| Q5. Which teaching strategies and what specific activities might be useful for helping learners develop an understanding of the big idea? | Knowledge of instructional strategies |
| Q6. How would you assess learners’ understanding/alternative conceptions about the big idea? | Knowledge of assessment |
After the introduction to the intervention, in the second week, a CoRe (prepared by a chemistry education expert with a PhD in the field) was introduced to the participants. In the introduction, what the big ideas might be and how to set big ideas were discussed. The CoRe, which was readily available and introduced, was about teaching the particulate nature of matter topic. The CoRe introduction was made using a smart board and took about two class hours. All the questions asked in the CoRe were analysed by examining the answers given in the prepared CoRe. After the CoRe introduction, the participants were asked to prepare a CoRe on acid–base equilibrium. The participants determined the big ideas in both the pre- and post-data administration of the CoRe. The high school chemistry curriculum objectives for the topic were photocopied and given to the participants. Then, at the end of the intervention, they were once again asked to prepare another CoRe on acid–base equilibrium. Their preparation took about 60–70 minutes. The participants were free to use chemistry textbooks and other resources during both pre- and post-CoRe preparation.
We studied acid–base equilibrium because (i) acids and bases and their properties are important in chemistry curricula all around the world; and (ii) learners have many alternative conceptions of and difficulties with acid–base strength (Drechsler and van Driel, 2008). Moreover, the nature of the topic makes it possible to use different activities in class and to focus on three levels of representation in chemistry.
Before the focus group interview, the participants were informed about the purpose of the focus group interview and were asked to take part in it. Nine volunteer participants talked about how learning pedagogical transformation competence resulted in their topic-specific PCK development, and how the intervention taught them that transformation. The focus group interview took about 35 minutes. All interviews (individual interviews and the focus group) were recorded and transcribed verbatim.
To conclude, in this study, the transformation of content knowledge was focused on and presented to the pre-service teachers via the intervention, which is supposed to result in a product, namely, topic-specific PCK. The end product was assessed using the CoRes, semi-structured and focus group interviews, and reflection papers. The content knowledge test was used to examine to what extent the participants’ content knowledge, which is important for topic-specific PCK development, had developed.
Data coding
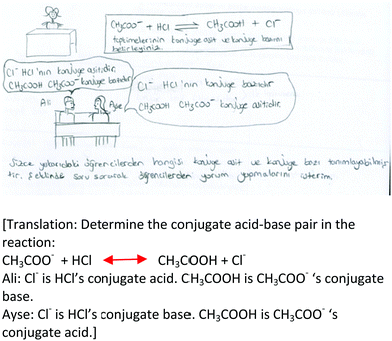
For the content knowledge test, a key was prepared and used to code the data. A score of 1 point was given to each correct answer and 0 was given to each incorrect answer. Then, the results for pre- and post-administration were calculated for each participant and run through the SPSS 22.0 package program. We ran the Wilcoxon signed-rank test analysis, which is the nonparametric test equivalent of the dependent t-test (Pallant, 2007). Due to the low number of participants, the assumptions of the parametric tests were not met. Therefore, we had to run a non-parametric test.For the CoRe analysis, we first examined the literature for a suitable rubric for the study. We found several rubrics, but they had some points that did not apply to our data and the aim. For instance, while the useful rubric developed by Mavhunga and Rollnick (2012) paid considerable attention to the three levels of representation in chemistry (e.g., symbolic levels), it did not apply to our data. The rubric prepared by Mavhunga (2016) did not include the assessment component of topic-specific PCK. Given that the assessment component was stated as ‘hidden’ (Park and Oliver, 2008; Mavhunga, et al., 2016), as mentioned earlier, we specifically aimed to focus on the assessment component in this study, which is supposed to contribute to the available findings regarding how pre-service teachers learn pedagogical transformation competence (e.g., Mavhunga, 2016; Mavhunga et al., 2016). Therefore, we decided to create a new rubric that had several properties in common with the existing ones (e.g., similar levels of performance to those of Park et al., 2011) while being designed for the revised CoRe prompts (see Appendix B). When developing the rubric, we paid attention to the description of each level for each CoRe prompt. Each cell described what the participants could do related to that prompt. Moreover, the cell descriptions were checked to see whether they could categorize the participants by examining the existing CoRe data. The performance expectations of the rubric (e.g., what difficulties do learners have while learning the big idea?) are the topic-specific PCK components that are explicitly focused on with the intervention. To conclude, the rubric created is suitable for coding the CoRe data. The rubric was utilized to determine the depth of the participants’ responses to each topic-specific PCK component for planning a lesson for teaching acid–base equilibrium, and to compare and contrast pre- and post-CoRes as evidence of the pedagogical transformation process learnt through the intervention. For example, regarding the assessment component, in their pre-Core some participants provided either no assessment idea at all or inappropriate assessment ideas for the big ideas set; therefore, based on the rubric we coded this CoRe prompt as limited (0). In their post-CoRe, some participants planned to assess the imaginary learners’ understanding of at least one big idea successfully by using only one classic assessment technique (e.g., the use of multiple-choice items). Accordingly, based on the rubric we coded this CoRe prompt as basic (1). If the participants planned to assess the imaginary learners’ understanding of at least two big ideas by using alternative assessment techniques (e.g., concept cartoon), we coded this prompt as developing (2). Additionally, if the participants planned to assess the imaginary learners’ understanding of at least two big ideas by using alternative assessment techniques (e.g., concept cartoon) and planned to assess different variables such as alternative conceptions in addition to learners’ understanding of chemistry concepts, we coded this prompt as exemplary (3). To illustrate, in her pre-CoRe, Nicole planned to assess learners’ understandings of acid–base strength using an open-ended question (“describe the ionization of strong acids and bases and give examples”). She attempted to assess one big idea by using one classic assessment technique and got the score basic (1). In the post-CoRe, in addition to learners’ understanding of acid–base strength, she planned to assess learners’ alternative conceptions related to acid–base strength using a concept cartoon. She also assessed two big ideas that she wrote. She planned to assess the learners’ alternative conceptions in addition to their understanding of chemistry concepts by using alternative assessment techniques and got the score exemplary (3).
After this stage, the authors coded three participants’ pre-CoRes independently and got together to compare the independent coders’ coding. We determined the number of agreements and disagreements to calculate the interrater reliability, which was calculated as 0.83 (Miles and Huberman, 1994). After resolving the inconsistencies, we coded the other three CoRes and got together again. In the second round, we had very high reliability, at 0.96. Then, the authors coded the remainder of the data independently. The raw data were entered into Excel, and then tables were formed to summarize the results.
Deductive analysis was used to analyse the reflection papers and interviews, which are the secondary sources used to validate the results received from the CoRes (Marshall and Rossman, 2006). To be specific, when analysing the reflection papers and the focus group interview transcripts, the focus was on how the intervention supported participants’ topic-specific PCK development using pedagogical transformation competence.
Results
In this part of the study, to be able to understand to what extent pre-service chemistry teachers underwent pedagogical transformation and developed topic-specific PCK for planning to teach acid–base equilibrium, first, the contribution of the intervention to the participants’ content knowledge, and then the comparison of the pre- and post-CoRe scores of the participants are provided. Later, the changes for each topic-specific PCK component, showing the extent of engagement with the content knowledge (reflection of the transformation of content knowledge competency), are presented through the analysis of each of the CoRe's prompts (those corresponding to topic-specific PCK components, see Table 3) with the support of semi-structured and focus group interviews, and reflection papers.Comparison of the participants’ content knowledge test scores
The Wilcoxon signed-rank test was run to compare and construct the content test results. The results revealed that the difference between the pre- and post-test scores was statistically significant (z = −3.086, p < 0.05). The effect size was r = 0.605. It is a large effect size according to Cohen's (1988) criteria. Apart from Zane, who received the same score in both the pre-test and post-test, all participants increased their scores in the post-test.Comparison of the participants’ pre- and post-CoRe scores
Table 4 shows to what extent the participants’ topic-specific PCK for planning to teach acid–base equilibrium changed through the intervention by presenting the pre-CoRe and post-CoRe scores (in parentheses) for each question, and the median scores for each question (in the last row of the table).| Topic-specific PCK components | ||||||
|---|---|---|---|---|---|---|
| Curriculum | Learners | Instructional strategies | Assessment | |||
| Participants | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 |
| a The scores correspond to a category of topic-specific PCK. (0) is limited, (1) is basic, (2) is developing, and (3) corresponds to the exemplary category. The numbers outside the parentheses are pre-test scores, while the ones are in the parentheses are post-test scores. | ||||||
| Nicole | 2(3)a | 2(3) | 1(3) | 2(3) | 1(3) | 1(3) |
| Edward | 1(1) | 1(3) | 0(1) | 0(1) | 0(1) | 0(1) |
| Elsa | 1(3) | 2(3) | 3(3) | 0(1) | 0(1) | 1(2) |
| Helen | 1(1) | 0(0) | 0(0) | 0(1) | 1(1) | 1(1) |
| Carla | 1(1) | 1(1) | 1(0) | 1(0) | 0(1) | 0(1) |
| Sandy | 1(1) | 0(2) | 1(1) | 0(0) | 0(1) | 1(2) |
| Nancy | 1(3) | 0(3) | 0(1) | 0(1) | 0(3) | 0(3) |
| Calvin | 1(1) | 0(2) | 1(1) | 1(3) | 0(1) | 0(1) |
| Tony | 1(1) | 2(2) | 1(2) | 0(1) | 0(2) | 1(1) |
| Zoe | 3(1) | 1(1) | 0(0) | 0(3) | 0(1) | 0(0) |
| Zane | 1(1) | 2(3) | 0(3) | 0(2) | 0(1) | 0(1) |
| Henry | 1(3) | 2(3) | 0(3) | 2(2) | 0(0) | 1(1) |
| Beyonce | 1(1) | 1(2) | 1(2) | 1(1) | 0(0) | 1(1) |
| Median scores | 1(1) | 1(2) | 1(1) | 0(1) | 0(1) | 1(1) |
The results showed that 11 of the 13 participants improved at least one topic-specific PCK component through the intervention (at the individual level) (Table 4). Only Beyonce and Helen remained almost the same. Helen showed development in Q4 (alternative conceptions of learners), whereas Beyonce showed improvement in Q2 and Q3, the importance of the big idea and the learners’ difficulties in learning the big idea, respectively. If the results are examined for each topic-specific PCK component, we see improvements in the curriculum (Q2), learners (learners’ alternative conceptions) (Q4), and instructional strategies (Q5) scores. Although the median scores of the curriculum (Q1, setting big ideas), learners (Q3, learners’ difficulties) and assessment (Q6) components did not change from pre- to post-CoRe administration, at a personal level, four, six, and eight participants improved their scores for the curriculum (Q1), learners’ difficulty (Q3), and assessment components (Q6), respectively (Table 4). Finally, there are some participants whose scores did not change for topic-specific PCK components. In the following part, evidence of pedagogical transformation competence resulting in development in each component will be presented in detail.
Evidence of pedagogical transformation competence resulting in the development of topic-specific PCK for planning to teach acid–base equilibrium
Regarding the development observed in the participants’ determination of the big ideas, throughout the intervention, the instructor paid specific attention to the big ideas of the topic that they focused on each week (explicit discussion of the transformation of content knowledge). The importance of formulating big ideas in planning and organising the lesson plan while paying attention to the sequence of big ideas was the first and most important part of the explicit discussion of the transformation of content knowledge every week. For example, in the week when chemical equilibrium was focused on, the meaning of the equilibrium concept in chemical reactions, the equilibrium constant, and later the Le Chatelier principle were sequentially discussed. In addition, the importance of the sequence among those chunks of content knowledge was explicitly highlighted, which is more than just content knowledge discussion. The instructor stated that their organisation plays a vital role in planning and teaching. These highlights are starting points for transforming content knowledge for teaching (pedagogical transformation process), which is supposed to teach pre-service teachers how to formulate big ideas for successful planning and teaching. Provided with this type of transformation experience through the intervention each week, the participants were supposed to learn how to comprehend the content, and later how to transform it for teaching. As Shulman (1987) stated, the transformation process starts with comprehension and continues with transformation. In the current study, the participants most probably learnt that for successful planning, they first need to focus on the big ideas of the topic and their organisation. Rather than engaging with the content knowledge superficially as in the pre-CoRe (e.g., formulating big ideas such as the ‘acidity and basicity of solutions’ because the topic includes acids and bases), the participants were able to engage deeply with the content knowledge of the acid–base equilibrium topic and set the related big ideas in the post-CoRe.
Moreover, the big ideas stressed in the objectives of the chemistry curriculum were also examined each week. The data received from the reflection papers verified this contribution and revealed that before the intervention the participants had no idea about what to teach or how to decide what constituted a big idea. All participants stated that they started to learn how to use the national chemistry curriculum regarding big ideas, the objectives to be focused on, and the limitations necessary to teach a topic for a specific grade (they stated the spiral nature of the curriculum). For example, Calvin stated, “I did not know much about big ideas, the curriculum, or objectives. Now, when I teach a topic, I first look at the topics and concepts in the curriculum, and then I decide what to teach and how.” This is the ability to determine the important parts of the content taught and sequence them in a meaningful order. The participants had the opportunity to observe and experience how to determine big ideas using the high school chemistry curriculum, then transfer this awareness and competence to a new topic that was not focused on during the intervention.
Q2. Why is it important for learners to know this big idea/s? In the pre-CoRe, Edward, Sandy, Nancy, Calvin, and Beyonce gave reasons unrelated to the big ideas or stated generic benefits of education. In the post-CoRe, however, they provided at least one topic-specific reason for why the big ideas set are important for learners. For example, Beyonce wrote generic reasons in the pre-CoRe: “To create a basis for learners’ learning and to be able to comment when faced with these concepts in educational life or daily life.” In her post-CoRe, she provided a topic-specific reason, namely, “learning the concepts of acid–base strength helps learners to understand and conjugate acid–base pairs easily.” In addition, Nicole, Elsa, Zane, and Henry stated more than one topic-specific reason for the big ideas in their post-CoRe. For example, Henry focused on conceptual scaffolding with reference to the next topic in his post-CoRe by writing “to be able to write the Ka and Kb equations.” Besides that, he emphasized the importance of learning acid–base strength in daily life in his post-CoRe: “to be able to evaluate substances as weak or strong when encountering them in daily life.”
Regarding the improvement observed, each week, the intervention included a specific discussion on why learning A (where A represents the big idea/s of the week) is important for learners and what happens if the learners do not learn A? For instance, in the seventh week of the intervention when the focus was on the reaction rate and what the rate concept means in chemistry (we differentiated the definition of the rate concepts in physics and chemistry), we discussed why learning the reaction rate is important and how learners use the reaction rate in the following topics. In that discussion, the instructor mentioned that chemical equilibrium is based on the rates of forward and backward reactions. In addition, the importance of learning the concepts focused on each week was also reasoned regarding its link to daily life. For example, while discussing why learning the rate of a reaction is important for our lives, as a group we came up with the idea that learning the rate of reactions is important because people need to make some reactions that we encounter slower (food spoilage) or faster to make our lives better. By doing so (focusing on the links between concepts and their use in our lives), the researchers planned to make the pre-service teachers think about the links between chemistry topics and how they apply to daily-life. By asking those questions, the researchers aimed to alert the participants to focus on the relationships between the chemistry topics. This is different from focusing only on the content knowledge that is supposed to be taught. The pedagogical transformation of content knowledge in this case means that a concept learnt in a previous topic (e.g., the rate of reaction concept) is essential for learning a concept that will be taught later (e.g., learning the chemical equilibrium concept). This transformation is also related to the knowledge of learners component (learners’ prior knowledge necessary to learn the new topic).
By emphasizing the links between the chemistry topics each week, the intervention allowed the participants to learn the links between the chemistry concepts. Moreover, they learnt the reasoning behind the importance of the links for successful teaching. As a result, they were able to transfer that competency for reasoning why acid–base equilibrium is important for learners to learn. In the reflection papers, Nicole stated that with the help of those discussions, she started to think about the big ideas and tried to understand the importance of the big idea for learning later concepts. The specific discussion of big ideas helped the pre-service teachers understand that chemistry topics are related. Moreover, they also came up with the idea that learning a big idea in one topic will help in learning the other one.
Regarding the contribution made by the intervention, in the focus group interview Tony stated that as a result of the explicit discussion of the transformation of content knowledge at the beginning of each week, they came to realize the points that make the topic difficult to learn. In this discussion, the instructor and the participants examined the nature of each topic each week. For instance, in the week when the focus was on chemical equilibrium, the entire group discussion centred on why the equilibrium topic is difficult to learn. Since two reactions are occurring at the same time, learners generally have difficulties in imagining the two reactions are happening simultaneously in the same container. We concluded that the difficulty is related to the learners’ familiarity with one-sided reactions. Likewise, acid–base equilibrium is a special type of chemical equilibrium. The participants learnt that the chemical equilibrium topic is different from other topics due to reversible reactions. When they planned a lesson in the post-CoRe, they could transfer those points discussed in previous weeks into their lesson plan for acid–base equilibrium. At this time, they focused on a special equilibrium description for weak acids or bases. Hence, when there are two reactions, they tend to think of these reactions taking place in different containers. Likewise, Nicole thought that focusing on the difficulties that learners may face each week helped the pre-service teachers understand the points that make topics difficult to learn (e.g., the inclusion of so many abstract concepts and the occurrence of reactions at the sub-microscopic level). For instance, almost every week, the instructor emphasized three levels of representations (macroscopic, symbolic, and sub-microscopic) and the link between the levels. Moreover, the instructor used multiple representations to help the participants link those levels in their minds whenever possible. In this way, the participants learnt that chemistry topics should be taught at different levels using three types of representations, something that is difficult for learners to comprehend. When the big idea includes a reaction that should be imagined at the sub-microscopic level, they learned that sub-microscopic level representations and animations are key for the difficulties encountered (this point will be discussed under the knowledge of instructional strategies component). In this case, the transfer included the need to imagine the reactions occurring at the sub-microscopic level, the difficulty in linking the three types of representations, and reactions occurring simultaneously. To conclude, by taking part in those discussions each week, the pre-service teachers began to reason about and to identify the points that make chemistry topics different from other topics and difficult for learners to comprehend. Finally, they started to anticipate possible difficulties that learners may experience in learning acid–base strength. This is an indicator of the pedagogical transformation of content knowledge.
Q4. What alternative conceptions do learners typically have about each big idea? In the pre-CoRe, eight participants could write any alternative conceptions for the big ideas they stated or they provided unrelated alternative conceptions to the big ideas stated. After the intervention, they could state at least one alternative conception related to one big idea that they focused on. For example, as alternative conceptions, Zoe stated: (i) “The strength of bases depends on the number of OH− that the base has” (revealed by Demircioğlu Ayas and Demircioğlu, 2005), (ii) “If the pH increases, the acidity increases” (revealed by Sheppard, 2006), (iii) “A concentrated base is always stronger than a dilute one” (revealed by Özmen et al., 2009), and (iv) “The pH values of strong acids are higher than those of weak acids.” In addition, Nicole wrote that “with the increase in the number of H+ that an acid has, the acid strength increases” and “if pH increases, acidity increases.”
When the possible sources of development were examined, all participants stated in their reflection papers that they learnt during the intervention what alternative conceptions learners might have. For example, Elsa wrote:
This course taught me where learners might have an alternative conception and how to address this. Each week, we first talked about the topic's concepts, and then we focused on the alternative conceptions that learners might have. As a result, we learnt the alternative conceptions and learnt that we should pay attention to them while teaching.
Similarly, other participants emphasized the importance of focusing on alternative conceptions each week during the focus group interview. For example, Nicole said:
When we learn about the alternative conceptions of each topic every week, it becomes permanent, and we can associate it with other topics. The instructor addressed alternative conceptions for each topic after the concepts were focused on.
As the participants stated, paying specific attention to learners’ alternative conceptions alerted the pre-service teachers to the existence of alternative conceptions and made them think about other possible alternative conceptions that learners might develop. In the discussions, the instructor paid specific attention to the points revealed by Talanquer (2002). Through the intervention, we examined how learners think about chemical reactions and processes, and how they reason about the changes occurring. For instance, Talanquer stated the patterns of reasoning that learners have. For instance, “most properties or changes in a system depend on a single independent variable. Students tend to focus their attention on the variable whose change is most evident” (p. 48). Those patterns listed may help the participants realize how learners misconstrue chemistry concepts or develop alternative conceptions. Therefore, although we did not discuss the alternative conceptions of acid–base equilibrium through the intervention, most of the participants (n = 9) could reason about them. For instance, Zoe and Nicole came to realize that learners tend to focus on one specific point about a phenomenon such as the number of the H+ that an acid has, which is an example of the patterns stated by Talanquer (2002). In other words, the transformation observed here is that the participants learnt that learners tend to focus only on a single factor in chemical reactions or processes and use that single factor to explain everything or all properties. Later, they used that knowledge to determine which factor may be most important for learners to pay attention to when determining the strength of an acid. Zoe and Nicole most probably reasoned that learners would focus only on the number of H atoms that an acid has. This is why both Zoe and Nicole stated that learners might focus on the number of the H of an acid to determine the strength of the acid or the strength of its conjugate base.
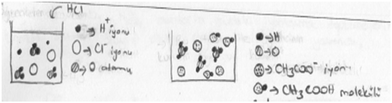 | ||
| Fig. 2 Nancy's sub-microscopic level drawings for helping learners visualise their ionization in aqueous solutions. | ||
Nancy wrote “using those representations, I help learners visualise how acids ionize in aqueous solution.” She stressed that in the first container there was no HCl in the solution but there were acetic acid molecules in the second container. Tony's, Nicole's, and Nancy's strategies choices to use sub-microscopic level representations were in parallel with the emphasis made throughout the intervention. The instructor consistently highlighted that teaching most of the chemistry topics (e.g., dissolution, diffusion, ionization, collision theory, chemical equilibrium, electrolysis) is difficult due to their abstract nature (apart from some changes that can be seen at the macroscopic level such as colour change, reactions that occur at the particulate level cannot be observed with the naked eye). Therefore, to address that difficulty, the instructor stated that to teach chemistry with reactions or events that need to be imagined at the particulate level, strategies and representations should be used to help learners visualise them (i.e., an interplay between the PCK components through which the knowledge of learners component informs the knowledge of instructional strategies component). This is an example of explicit content knowledge engagement for making it easily understandable for learners. Moreover, the instructor also used representations at three levels throughout the intervention. This emphasis on content knowledge transformation to make the topic more accessible to learners was highlighted in different weeks (e.g., the week when solutions, gases, and reaction rate topics were focused on). In this way, the participants came to realize that chemical events should be examined at the sub-microscopic level, but this is not available when the instruction is only at the macro and symbolic levels. As the post-CoRes showed, the participants appeared to learn the reasoning for the content regarding the reactions that should be presented at the sub-microscopic level. They could then transfer that competency to determine the reactions’ acid–base equilibrium requiring sub-microscopic representations (e.g., weak and strong acid solutions). Based on the examples given, although those points were discussed for different chemistry topics in the intervention, the participants could transfer that learnt competence to address learners’ difficulties in visualisation at the particulate level in the acid–base equilibrium topic.
Other evidence of participants’ transfer was seen in Elsa's post-CoRe. Elsa interplayed her knowledge about learners’ alternative conception (she wrote “learners may think that the conjugate base of a strong acid is also strong” in the alternative conception part of the post-CoRe) to her knowledge of instructional strategies. Based on the alternative conception that Elsa stated, she planned to implement the 5E learning cycle in the post CoRe. Elsa started the lesson by asking, “How can we determine whether an acid/base is strong or weak?” After taking learners’ ideas, she stated that she moved to an exploration step that included an activity. In the activity, learners were supposed to observe that when pH strips were immersed in acid solutions with the same concentration this resulted in different pH readings. The differences were determined by comparing the colours of the strips and the colours’ meanings provided on the pH strip package. She made learners realize that acids have different properties even if they have the same concentration. In the explanation step, Elsa explained what acid/base strength means by focusing on the ionization percentage of an acid/base and then moved on to the strength of conjugate acid–base pairs. In this step, she related acid–base strength with Ka and Kb and had learners recall what K represents (equilibrium constant). Later, she focused on acetic acid's equilibrium. She wrote the equation at the symbolic level and wrote the Ka equation for the equilibrium. She then asked the learners to determine the conjugate pair of acetic acid in light of the description provided earlier. She continued with the acetate ion's reaction with water. Then, she asked learners to write the Kb of acetate ions. Elsa asked, “what happens if Ka is multiplied by Kb?” They came to realize that they obtained Kwater, which is a constant. Through these steps, she helped learners notice that if an acid is strong (the Ka of the acid is high), its conjugate base is weak (the Kb of the base pair must be small).
To be more specific about what Elsa could transfer in the previous part, first of all, she could engage with the content knowledge and manage to determine the point that learners could misconstrue (the conjugate base of a strong acid is also strong). At this point, the explicit discussion of the transformation of content knowledge made each week most probably taught them to reason why topics are difficult to learn and why learners have alternative conceptions, etc. Although it was not focused on in the intervention, Elsa could transfer what she learnt and anticipate that learners would most probably tend to generalize that if an acid is strong so is its conjugate base. Second, after reasoning the content knowledge and determining the specific alternative conception, she could decide to implement the 5E strategies. In the intervention, the 5E strategies (knowledge of instructional strategies component) was implemented to replace the ‘chemical reactions are non-reversible whereas physical events are reversible’ idea with the ‘through chemical reactions the existing bonds are broken, and new ones are made’ big idea (knowledge of learners component). The instructor explicitly explained that she preferred to use the 5E strategies, which is an example of the transformation of content knowledge, to address the alternative conception (chemical reactions are non-reversible, whereas physical events are reversible) and help learners understand the big idea (through chemical reactions the existing bonds are broken, and new ones are made). As Shulman (1987) stated, the instructor modelled the participants on how to engage with the content knowledge and select suitable instructional teaching methods. As a result, Elsa transformed what she experienced and learnt into her post-CoRe to make the topic more understandable for learners. Finally, Elsa's planning is also good evidence of the usefulness of the intervention for interplay among the topic-specific PCK components (teachers’ knowledge of learners’ alternative conceptions and instructional strategies). The interplay among the components was continually stressed throughout the intervention as can be seen in the explanation provided for using 5E for the chemical reactions mentioned above.
In the reflection papers, all participants stated that they enhanced their knowledge of instructional strategies for teaching chemistry topics through the intervention with explicit emphasis on how content knowledge informs the selection of the instructional strategies. They revealed that discussing the reasons for the instructional strategies selection, observing the instructors’ implementation of teaching methods, experiencing the methods for different topics each week as if they were high school students, and practicing the method by doing assignments were beneficial to their learning of how to implement teaching methods to address learners’ alternative conceptions or difficulties in learning chemistry topics. For example, Tony stated in his reflection paper:
Discussion of the content knowledge each week was very useful in understanding the use of the teaching method. After all, if we know the method but do not know the content knowledge, we cannot fully understand the reason for choosing that method. Without content knowledge, it is like knowing how to drive while not knowing the traffic regulations. If you do not know the traffic regulations, you would have trouble.
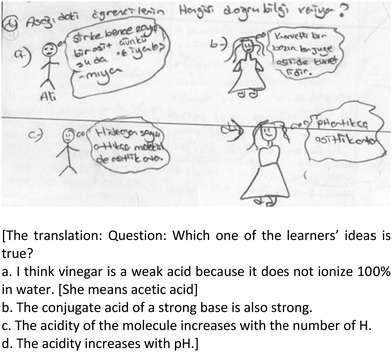 | ||
| Fig. 3 The concept cartoon that Nicole drew in the post-CoRe to determine learners’ alternative conceptions. | ||
Rather than just picking a concept (i.e., ionization of acids) from the textbook and writing a question for the assessment part as in the pre-CoRe, Nicole's use of a concept cartoon in the post-CoRe revealed that she could focus on the content knowledge taught and the points making learners confused (conjugate acid–base pair strength), and could then transfer what she learnt previously into her planning (using a concept cartoon to diagnose learners’ alternative conceptions in chemistry topics). It is also an interplay between the knowledge of learners and assessment components of PCK. The knowledge of learners component informed the assessment component in this example. Related to Nicole's transformation and use of a concept cartoon, in the fifth week of the intervention (the gases topic was focused on), the use of a concept cartoon for diagnosing learners’ alternative conceptions in the gases topic was introduced to the participants. With specific examples of learners’ possible alternative conceptions in gases (e.g., gases have no mass), the participants had a chance to engage with gases content knowledge, alternative conceptions that learners may have in gases, and how to diagnose alternative conceptions in the topic. That transformation seemed to help Nicole. To be specific, she reasoned about the possible alternative conceptions of learners and how to identify them in the assessment part. Also, learning the use of a concept cartoon for diagnosing alternative conceptions in a chemistry topic was useful for her transformation of acid–base strength content knowledge into a possible alternative conception and its diagnosis for another topic. In that week, the interplay between the content knowledge, knowledge of learners (alternative conception), and knowledge of assessment components of topic-specific PCK was also highlighted just as in all weeks in the intervention. Nicole's transformation presented above reminded us of Geddis and Wood's (1997) point, which says transformation “directs attention simultaneously to subject matter, learners, and educational purposes, and to the interactions among these different kinds of teacher knowledge in the pedagogical encounter” (p. 612). Similarly, in Elsa's post-CoRe, the interplay between the content knowledge of acid–base equilibrium, knowledge of learners, instructional strategies, and assessment components of topic-specific PCK was identified. Elsa also planned to assess whether learners have difficulty in determining conjugate acid–base pairs in the post-CoRe (Fig. 4).
Discussion and conclusion
In this study, we explored to what extent pre-service chemistry teachers underwent pedagogical transformation and developed topic-specific PCK for planning to teach acid–base equilibrium (which was not considered at all during the intervention). The intervention with explicit emphasis on the transformation of content knowledge is supposed to produce topic-specific PCK. Moreover, the intervention was planned in a different way that provided more chance for the participants to reason and utilize the content knowledge of different topics for planning to teach acid–base equilibrium. That difference is valuable because, for a complete understanding of the content knowledge of chemistry and the nature of the chemistry knowledge, we believe it is necessary to cover different topics and reason them. Additionally, it has the potential to offer pre-service teachers the opportunity to put themselves into learners’ shoes throughout the semester for different topics. The results of the study supported Mavhunga's (2016) and Mavhunga et al.'s (2016) findings that learning pedagogical transformation competence is possible and results in topic-specific PCK. Moreover, this study specifically focused on the knowledge of assessment component that was not addressed by previous studies. As the results showed, to most extent the participants developed assessment knowledge for assessing learners’ conceptions of acid–base strength, conjugate acid–base pairs, and Ka–Kb relations.The intervention with the explicit discussion of the transformation of content knowledge helped the participants to engage with the content knowledge and transform it into a more accessible form for learners, culminating in topic-specific PCK. Based on Mavhunga's (2016) great question asking whether PCK is topic-specific and cannot be transferred to another topic, we asked, “What can then be transferred in the context of learning and acquiring PCK?” (p. 1081). This study aimed to inform the literature to what extent the pre-service teachers could transfer the pedagogical transformation of content knowledge learnt through the intervention to plan a lesson for teaching acid–base equilibrium if different chemistry topics are focused on each week rather than a single topic. This topic (including strong and weak acids/bases, equilibrium for weak acids with Ka and Kb calculations, and conjugate acid–base pairs) was not focused on during the intervention (see Table 1). The results showed that 11 of the 13 participants could achieve transfer of what they had learnt and experienced during the intervention to plan for a new topic, and this was identified as an enrichment of at least one topic-specific PCK component. Although two participants had difficulties in that transfer and could not show development after the intervention, it still seems promising to see the development of the 11 pre-service teachers reported in the study for teacher education. Finally, acid–base equilibrium is a specialized type of equilibrium applied to acid–base ideas. Therefore, the transformation that we claimed may also stem from content integration rather than some generalized competence that was evidenced. This alternative claim may also explain at least some part of the growth observed from the intervention.
In light of the results, first, through the intervention, it seems that the pre-service teachers’ comprehension of the content knowledge of chemistry improved, as the content knowledge test scores revealed. This part is related to Shulman's (1987) comprehension of the content knowledge step in the transformation process. The explicit discussion on the concepts of the topic each week, and the network and the sequence of the concepts through the intervention seem to enrich the participants’ content knowledge. Moreover, after the comprehension, as the results revealed, the participants began to notice that content knowledge informs teachers in planning and implementing a lesson. The results give the impression that the participants could also learn to engage with the content knowledge and anticipate the points that make it difficult for students to learn (e.g., including reactions at the particulate level or including two reactions occurring simultaneously in the same container). In other words, they started to transform the content knowledge into an easily comprehensible form for learners (Shulman, 1987). Furthermore, the evidence provided in the Results section shows that the participants learnt how to prepare the topic, how to decide what representations and instructional strategies to use, and how to adapt those representations to the general characteristics of the learners, all of which are later steps in Shulman's transformation process (1987). As in Nicole's, Tony's, and Nancy's cases, they focused on the content knowledge of the acid–base strength topic, and anticipated learners in visualising the ionization of acids/bases at the particulate level. Based on those points, they decided to use representations to show HCl and CH3COOH solutions, and role modelling to represent those solutions at the particulate level. Regarding this development, Shulman (1987) argued that it is related to engaging with the content knowledge and selecting the appropriate instructional teaching methods. In addition, those examples also showed that the participants learnt the interplay between content knowledge and the components of topic-specific PCK, and how to transfer that competency into planning a new topic. Similar transformations were also reported for a sequence of big ideas, Elsa's preference for the use of 5E for addressing an alternative conception, and the network among the chemistry concepts. Regarding those transformations, Geddis and Wood (1997) stated that “representations and teaching strategies are not just examples of pedagogical content knowledge,…, but that they are also (as in this case) the end products of the knowledge transformation itself” (p. 624). In short, we argue that experiencing explicit discussions of the transformation of content knowledge many times with different topics (reasoning about teaching as Mavhunga 2016 stated) helped the participants learn pedagogical transformation competence. Through that lens, we argue that the participants found that the content knowledge (raw material) should be reasoned, which can be done in light of the guidance provided by the topic-specific PCK components. This competence development was promoted by explicit focus on topic-specific PCK components when we engaged with the content knowledge to transform it for learners.
Regarding the use of the pedagogical transformation of content knowledge for developing topic-specific PCK for teachers, this study presented an uneven development of topic-specific PCK components as a product of the transformation. The findings revealed that the participants showed more progress in transferring content knowledge when they learnt about students’ difficulties and alternative conceptions, and instructional strategies than in the assessment component. Other studies have reported the varying accessibility levels of topic-specific PCK components (e.g., Henze et al., 2008; Mavhunga, 2016; Mavhunga et al., 2016). Mavhunga (2016) reported that pre-service teachers’ development in the learners and curriculum components was greater than those in the other components. Regarding the curriculum component, Mavhunga et al. (2016) revealed that the use of classification maps for establishing big ideas focused on the CoRe is valuable for pre-service teachers. Regarding the learners component, Mavhunga et al. (2016) stated that through the intervention the participants found that research papers published on learners’ alternative conceptions and the effectiveness of different teaching methods could inform pre-service teachers about the points that learners may have difficulty with and how teaching methods enrich learners’ comprehension. In other words, the instructor's implementation of teaching methods and the use of the literature were useful strategies for the development of the knowledge of learners component. The explicit discussions on the topic-specific PCK components and the instructor's explicit engagement with the content knowledge for developing the components are important aspects that require attention. Another possible reason is the accessibility of the learners component in that the participants’ personal experience in learning chemistry since secondary school may inform them about the learners’ difficulties. Regarding the assessment component, teachers and interns have a limited repertoire of assessment strategies (Friedrichsen et al., 2009). Accordingly, the assessment component particularly needs more time to develop (Henze et al., 2008) than other topic-specific PCK components. These may give reasons for the difficulties in this area and be obstacles to planning the acid–base equilibrium topic effectively. Nevertheless, it seems that the time and effort needed for the development of topic-specific PCK components through the use of pedagogical transformation competence is different.
In the present study, the context, the courses taken previously, and the lack of teaching experience are the same for all participants. Additionally, their educational backgrounds were more or less similar. However, some participants were more successful in their use of pedagogical transformation competence for topic-specific PCK development than others, which makes us think that learning pedagogical transformation competence and using it to plan a new topic is person-specific as well. In other words, as the PCK construct has a person-specific nature (Hashweh, 2005), pedagogical transformation competence is also person-specific. A possible explanation for this situation may be that pedagogical transformation competence includes reasoning the content knowledge, and individuals may reason about the content at different levels. In addition to that difference, based on the comparison of pre- and post-content knowledge tests, it is possible to argue that the level of content knowledge that pre-service teachers have may make a difference in pedagogical transformation competence use. Given the fact that content knowledge is the raw material of the transformation process (Shulman, 1986), the participants’ difference in content knowledge seems to be a valid claim. Correspondingly, van Driel et al. (2002) revealed that teachers with rich content knowledge were more successful in determining learners’ difficulties in learning the topic in question. Also, Kind (2009) reported that teachers with deep content knowledge could implement different instructional strategies. Likewise, in our research, the participants with a good score in the post-content knowledge test (e.g., Nancy, Elsa, and Nicole) could transform it into components (e.g., selection of useful instructional and assessment strategies).
Implications
This study provides valuable information about pre-service teachers’ use of pedagogical transformation competence acquired during the intervention to develop topic-specific PCK for planning to teach the acid–base equilibrium topic and the development of the knowledge of assessment. This study has valuable implications, for both the enrichment of science/chemistry teacher education and future research. First, for science/chemistry teacher education, the findings of this study imply the need to focus on pre-service teachers’ acquisition of pedagogical transformation competence to develop topic-specific PCK. If pre-service teachers are supported in their understanding of pedagogical transformation competence during pre-service teacher education programs, this will positively contribute to their professional development. In the teaching methods courses, pre-service teachers should explicitly be taught about reasoning the content knowledge, the aspects of the topics that make it difficult for learners to conceptualise, how to make those points more understandable for learners by focusing on the reasons behind instructional strategies selection, and how to assess those parts in line with the nature of the topic.In order to develop assessment knowledge, in teacher education courses (e.g., practicum, measurement and evaluation, teaching methods courses), the assessment component with explicit examples for teaching science/chemistry topics should be discussed. Moreover, the assessment component should be emphasized by asking questions about how teachers can use the knowledge obtained from assessment to design their instruction. Additionally, in these courses, pre-service teachers should be offered explicit opportunities to reflect on their own assessment purposes, challenges, and consequences. This reflection can facilitate pre-service teachers to align their assessment purposes with their assessment consequences. Hence, after reflection they could be able to consider how they could utilize the assessment results to tailor their instruction and improve their students’ understanding of science/chemistry concepts. Furthermore, teaching experience may have the potential to develop pre-service teachers’ assessment knowledge by giving a chance to pre-service teachers to integrate assessment into their planning and teaching.
In future studies, researchers should focus on analysing pedagogical transformation competence with other suggested practices. For instance, in their exploratory study, Hume and Berry (2011) revealed that examining and exploring the CoRes prepared by experienced teachers is very useful for pre-service teachers’ pedagogical transformation competence and topic-specific PCK development. Finally, in future research, pedagogical transformation competence should be examined for enactive PCK (ePCK), which is presented in a classroom. In this study, we focused on participants’ pedagogical transformation competence at the planning level. Furthermore, future research that examines a group of pre-service teachers’ pedagogical transformation competence rather than personal ones would also be informative regarding the understanding of the pedagogical transformation competence construct and its nature.
Limitations
One of the limitations of this study was the lack of teaching practice held in the classroom context. However, this limitation was due to the nature of the intervention offered through the teaching methods course in Turkey (the teachings method course does not have a practice teaching component) (see Aydin-Gunbatar and Demirdogen, 2017, for the details).Another limitation was related to the small number of participants in the study. Although our aim was not to generalize our results, it would be informative to apply the design with a larger group of pre-service teachers as this would result in quantitative results as in Mavhunga (2016). In addition to that, due to the small number of the participants, the reliability coefficient for the content knowledge test was not calculated.
Conflicts of interest
There are no conflicts to declare.Appendix A
An example item from the content knowledge test.Q6. Related to acid strength, which one of the following will be correct?
I. Acetic acid, a weak acid, does not dissociate into its ions completely.
II. Among HI, HBr, HCl and HF acids, HI has the highest acid strength, whereas HF has the lowest acid strength.
III. HNO3, a strong acid, dissociates into its ions completely.
A. Only I B. Only II C. I and II.
D. I and III E. I, II, and III.
Appendix B
The rubric used to code pre- and post-CoRes prepared by the participants.| Performance expectation | Limited | Basic | Developing | Exemplary |
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Q1. What would you consider a big idea/ideas for the topic? | Provides unrelated big ideas to the objectives | • Provides at least two big ideas | • Provides at least two big ideas related to the objectives | • Provides at least two big ideas related to the objectives |
| • Provides a big idea unrelated to the objectives or no logical sequence | • Provides a logical sequence but no clarity | • Provides a logical sequence | ||
| • Clarity (provides big ideas in a clear way) | ||||
| Q2. Why is it important for learners to know this big idea/s? | No and unrelated reasons to the big ideas | Reasons given are generic benefits of education | Include at least one topic-specific reason (scientific literacy, daily life, conceptual scaffolding with reference to the next topic/unit, etc.) with examples | • Include more than one topic-specific reason (scientific literacy, daily life, conceptual scaffolding with reference to the next topic/unit, etc.) with examples |
| Q3. What difficulties do learners have while learning the big idea? | • No information about what makes topic difficult for learners | • Identifies specific concepts but provides broad generic reasons such as ‘abstract’ | • Identifies specific concepts leading to learners’ difficulties | • Identifies specific concepts leading to learners’ difficulties with reasons |
| • Provides unrelated points that make the topic difficult to the big idea | • Only one point making the topic difficult for only one big idea | • Identifies one point for each big idea | • Identifies two or more points making the topic difficult for each big idea | |
| Q4. What alternative conceptions do learners typically have about each big idea? | • No alternative conception | • One is related but the other is unrelated | • Identifies one alternative conception for each big idea | • Identifies two or more alternative conceptions for each big idea |
| • Provides unrelated alternative conceptions to the big idea | • Alternative conception/s for only one big idea | • Alternative conception is related to the big idea | • Alternative conceptions are related to the big idea | |
| Q5. Which teaching strategies and what specific activities might be useful for helping learners develop an understanding of the idea? | • No activity/representation | Use of representations/activities (analogies, demos, etc.) without explanatory notes to make the links with the aspects of the concept being explained | Use of representations/activities with explanatory notes linking to the aspect(s) of the concept being explained | • Use of representations/activities at least one big idea with explanatory notes linked to the aspect(s) of the concept being explained |
| • Provide unrelated activity/representation for the big ideas | • Use of the above representations/activities taking alternative conceptions/difficulty into consideration | |||
| Or | ||||
| • Use of representations/activities for all big ideas with explanatory notes linking to the aspect(s) of the concept being explained | ||||
| Q6. In what ways would you assess learners’ understanding/alternative conceptions about the big idea? | • No attempt to assess big ideas | • Attempt to assess at least one big idea successfully | • Assess students’ understanding for two big ideas | • Assess students’ understanding for two big ideas |
| • Provide inappropriate assessment for the big ideas | • Include some unrelated assessment | • Assess two big ideas using alternative assessment techniques | • Assess two big ideas using alternative assessment techniques | |
| Or | ||||
| • Use only one classic assessment technique | • At least one big idea + with an alternative conception for one big idea | • Assess different variables in addition to understanding/knowledge (e.g., science process skills, alternative conceptions, graphic reading, nature of science, particulate level drawings, etc.) |
References
- Akın F. N. and Uzuntiryaki-Kondakci E., (2018), The nature of the interplay among components of pedagogical content knowledge in reaction rate and chemical equilibrium topics of novice and experienced chemistry teachers, Chem. Educ. Res. Pract., 19(1), 80–105 10.1039/C7RP00165G.
- Apedoe X. S., Reynolds B., Ellefson M. R. and Schunn C. D., (2008), Bringing engineering design into high school science classrooms: the heating/cooling unit, J. Sci. Educ. Technol., 17(5), 454–465 DOI:10.1007/s10956-008-9114-6.
- Aydin-Gunbatar S. and Demirdogen B., (2017), Chemistry teaching method course for secondary science teacher training: an example from Turkey, in A. J. Sickel and S. B. Witzig (ed.), Designing and Teaching the Secondary Science Methods Course: An International Perspective, Sense International Publishers, pp. 129–148.
- Aydin S., Demirdogen B., Tarkin A., Kutucu S., Ekiz B., Akin F. N., Tuysuz M. and Uzuntiryaki E., (2013), Providing a set of research-based practices to support preservice teachers’ long-term professional development as learners of science teaching, Sci. Educ., 97(6), 903–935 DOI:10.1002/sce.21080.
- Aydin S., Demirdogen B., Akin F. N., Uzuntiryaki-Kondakci E. and Tarkin A., (2015), The nature and development of interaction among components of pedagogical content knowledge in practicum, Teach. Teach. Educ., 46, 37–50 DOI:10.1016/j.tate.2014.10.008.
- Aydin-Gunbatar S., Tarkin-Celikkiran A., Kutucu E. S. and Ekiz-Kiran B., (2018), The influence of a design-based elective STEM course on pre-service chemistry teachers’ content knowledge, STEM conceptions, and engineering views, Chem. Educ. Res. Pract., 19, 954–972 10.1039/c8rp00128f.
- Barnett E. and Friedrichsen P. J., (2015), Educative mentoring: How a mentor supported a preservice biology teacher's pedagogical content knowledge development, J. Sci. Teach. Educ., 26(7), 647–668 DOI:10.1007/s10972-015-9442-3.
- Brown P., Friedrichsen P. and Abell S., (2013), The development of prospective secondary biology teachers PCK. J. Sci. Teach. Educ., 24(1), 133–155 DOI:10.1007/s10972-012-9312-1.
- Carlson J. and Daehler K. R., (2019), The refined consensus model of pedagogical content knowledge in science education, in A. Hume, R. Cooper and A. Borowski (ed.), Repositioning pedagogical content knowledge in teachers’ knowledge for teaching science, Springer, pp. 77–92.
- Chan K. K. H. and Yung B. H. W., (2018), Developing pedagogical content knowledge for teaching a new topic: More than teaching experience and subject matter knowledge, Res. Sci. Educ., 48(2), 233–265.
- Chan K. K. H., Rollnick M. and Gess-Newsome J., (2019), A Grand Rubric for Measuring Science Teachers’ Pedagogical Content Knowledge, Repositioning Pedagogical Content Knowledge in Teachers’ Knowledge for Teaching Science, Springer, pp. 251–269.
- Coetzee C., Rollnick M. and Gaigher E., (2020), Teaching electromagnetism for the first time: A case study of pre-service science teachers’ enacted pedagogical content knowledge, Res. Sci. Educ., 1–22 DOI:10.1007/s11165-020-09948-4.
- Cohen J., (1988), Statistical power analysis for the behavioral sciences, 2nd edn, Hillsdale, NJ: Lawrence Erlbaum Associates.
- Demircioğlu G., Ayas A. and Demircioğlu H., (2005), Conceptual change achieved through a new teaching program on acids and bases, Chem. Educ. Res. Pract., 6(1), 36–51 10.1039/B4RP90003K.
- Drechsler M. and Van Driel J., (2008), Experienced teachers’ pedagogical content knowledge of teaching acid–base chemistry, Res. Sci. Educ., 38(5), 611–631 DOI:10.1007/s11165-007-9066-5.
- Ekiz-Kiran B., Boz Y. and Oztay E. S., (2021), Development of pre-service teachers’ pedagogical content knowledge through a PCK-based school experience course, Chem. Educ. Res. Pract., 22(2), 415–430 10.1039/D0RP00225A.
- Friedrichsen P. J., Abell K. A., Pareja E. M., Brown P. L., Lankford D. M. and Volkmann M. J., (2009), Does teaching experience matter? Examining biology teachers’ prior knowledge for teaching in an alternative certification program, J. Res. Sci. Teach., 46(4), 357–383 DOI:10.1002/tea.20283.
- Geddis A. N., and Wood E., (1997), Transforming subject matter and managing dilemmas: A case study in teacher education, Teach. Teach. Educ., 13, 611–626.
- Gess-Newsome J., (2015), A model of teacher professional knowledge and skill including PCK, in A. Berry, P. Friedrichsen and J. Loughran (ed.), Re-examining pedagogical content knowledge in science education, Routledge, pp. 28–42.
- Grossman P. L., (1990), The making of a teacher: Teacher knowledge and teacher education, New York: Teachers College Press.
- Hashweh M. Z., (2005), Teacher pedagogical constructions: A reconfiguration of pedagogical content knowledge, Teach. Teach.: Theory and Pract., 11, 273–292 DOI:10.1080/13450600500105502.
- Henze I., van Driel J. H. and Verloop N., (2008), Development of experienced science teachers’ pedagogical content knowledge of models of the solar system and the universe, Int. J. Sci. Educ., 30(10), 1321–1342 DOI:10.1080/09500690802187017.
- Hume A. and Berry A., (2011), Constructing CoRes—a strategies for building PCK in pre-service science teacher education, Res. Sci. Educ., 41(3), 341–355 DOI:10.1007/s11165-010-9168-3.
- Kind V., (2009), Pedagogical content knowledge in science education: perspectives and potential for progress, Stud. Sci. Educ., 45(2), 169–204 DOI:10.1080/03057260903142285.
- Kind V. and Chan K. K., (2019), Resolving the amalgam: connecting pedagogical content knowledge, content knowledge and pedagogical knowledge, Int. J. Sci. Educ., 41(7), 964–978 DOI:10.1080/09500693.2019.1584931.
- Loughran J., Mulhall P. and Berry A., (2004), In search of pedagogical content knowledge in science: Developing ways of articulating and documenting professional practice, J. Res. Sci. Teach., 41, 370–391 DOI:10.1002/tea.20007.
- Magnusson S., Krajcik J. and Borko H., (1999), Nature, sources and development of pedagogical content knowledge for science teaching, in J. Gess-Newsome and N.G. Lederman (ed.), Examining pedagogical content knowledge: The construct and its implications for science education, Kluwer Academic Publishers, pp. 95–132.
- Marshall C. and Rossman G. B., (2006), Designing qualitative research, 4th edn, Thousand Oaks, CA: Sage.
- Mavhunga E., (2016), Transfer of the pedagogical transformation competence across chemistry topics, Chem. Educ. Res. Pract., 17(4), 1081–1097.
- Mavhunga E., (2019), Exposing pathways for developing teacher pedagogical content knowledge at the topic level in science, in A. Hume, R. Cooper and A. Borowski (ed.), Repositioning pedagogical content knowledge in teachers’ knowledge for teaching science, Springer, pp. 129–148.
- Mavhunga M. E. and Rollnick M., (2012), Development and piloting a tool for measuring topic-specific PCK in chemical equilibrium, in C. Bruguière, A. Tiberghien and P. Clément (ed.), Proceedings of the ESERA 2011 Conference: Science learning and Citizenship, Lyon, France: European Science Education Research Association.
- Mavhunga E. and Rollnick M., (2013), Improving PCK of chemical equilibrium in pre-service teachers, Afr. J. Res. Math., Sci. Technol. Educ., 17(1–2), 113–125 DOI:10.1080/10288457.2013.828406.
- Mavhunga E., Ibrahim B., Qhobela M. and Rollnick M., (2016), Student teachers’ competence to transfer strategies for developing PCK for electric circuits to another physical sciences topic, Afr. J. Res. Math., Sci. Technol. Educ., 20(3), 299–313.
- McConnell T. J., Parker J. M. and Eberhardt J., (2013), Assessing teachers* science content knowledge: A strategy for assessing depth of understanding. J. Sci. Teacher Educ., 24(4), 717–743.
- Miles M. B. and Huberman A. M., (1994), Qualitative data analysis: An expanded sourcebook, 2nd edn, Thousand Oaks, CA: Sage.
- Morse J. M., (2003), Principles of mixed methods and multimethod research design, in A. Tashakkori and C. Teddlie (ed.), Handbook of mixed methods in social and behavioral research, Sage Publications, pp. 189–208.
- Nilsson P. and Loughran J., (2012), Exploring the development of pre-service science elementary teachers’ pedagogical content knowledge, J. Sci. Teach. Educ., 23(7), 699–721 DOI:10.1007/s10972-011-9239-y.
- Oh P. S. and Oh S. J., (2011), What teachers of science need to know about models: An overview. Int. J. Sci. Educ., 33(8), 1109–1130 DOI:10.1080/09500693.2010.502191.
- Özmen H., Demircioğlu G. and Coll R. K., (2009), A comparative study of the effects of a concept mapping enhanced laboratory experience on Turkish high school students' understanding of acid–base chemistry, Int. J. Sci. Math. Educ., 7, 1–24.
- Pallant J., (2007), SPSS survival manual: a step by step guide to data analysis using SPSS for Windows, 15th edn, Buckingham: Open University Press.
- Park S. and Oliver J. S., (2008), Revisiting the conceptualisation of pedagogical content knowledge (PCK): PCK as a conceptual tool to understand teachers as professionals, Res. Sci. Educ., 38(3), 261–284 DOI:10.1007/s11165-007-9049-6.
- Park S., Jang J. Y., Chen Y. C. and Jung J., (2011), Is pedagogical content knowledge (PCK) necessary for reformed science teaching?: Evidence from an empirical study, Res. Sci. Educ., 41(2), 245–260 DOI:10.1007/s11165-009-9163-8.
- Posner G. J., Strike K. A., Hewson P. W. and Gertzog W. A., (1982), Accommodation of a scientific conception: Toward a theory of conceptual change, Sci. Educ., 66(2), 211–227.
- Rollnick M. and Mavhunga E., (2015), The PCK Summit and its effect on work in South Africa, in A. Berry, P. Friedrichsen and J. Loughran (ed.), Re-examining pedagogical content knowledge in science education, Routledge, pp. 135–146.
- Sæleset J. and Friedrichsen P., (2021), Pre-service science teachers’ pedagogical content knowledge integration of students’ understanding in science and instructional strategies, Eurasia J. Math., Sci. Technol. Educ., 17(5) DOI:10.29333/ejmste/10859.
- Sheppard K., (2006), High school students’ understanding of titrations and related acid–base phenomena, Chem. Educ. Res. Pract., 7(1), 32–45.
- Shulman L. S., (1986), Those who understand: Knowledge growth in teaching, Educ. Res., 15(2), 4–14.
- Shulman L. S., (1987), Knowledge and training: Foundations of the new reform, Harvard Educ. Rev., 57(1), 1–22.
- Taber K. S., (2018), Lost and found in translation: guidelines for reporting research data in an ‘other’ language, Chem. Educ. Res. Pract., 19(3), 646–652 10.1039/C8RP90006J.
- Talanquer V., (2002), Minimizing misconceptions, Sci. Teach., 69(8), 46–49.
- Teddlie C. and Tashakkori A., (2003), Major issues and controversies in the use of mixed methods in the social and behavioral sciences, in A. Tashakkori and C. Teddlie (ed.), Handbook of mixed methods in social and behavioral research, Sage Publications, pp. 3–50.
- Uzuntiryaki-Kondakci E., Demirdöğen B., Akın F. N., Tarkin A. and Aydın-Günbatar S., (2017), Exploring the complexity of teaching: the interaction between teacher self-regulation and pedagogical content knowledge, Chem. Educ. Res. Pract., 18(1), 250–270 10.1039/C6RP00223D.
- Van Driel J., De Jong O. and Verloop N., (2002), The development of preservice chemistry teachers’ pedagogical content knowledge, Sci. Educ., 86, 572–590 DOI:10.1002/sce.10010.
- Veal W. R. and MaKinster J. G., (1999), Pedagogical content knowledge taxonomies, Electron. J. Sci. Educ., 3(4).
| This journal is © The Royal Society of Chemistry 2022 |