Effects of a context-based approach with prediction–observation–explanation on conceptual understanding of the states of matter, heat and temperature†
Fethiye
Karsli Baydere

Giresun University, Faculty of Education, Department of Science Education, Giresun, Turkey
First published on 18th March 2021
Abstract
The main purpose of this study was to investigate the effect of the Context-Based Approach (CBA) enriched by Prediction–Observation–Explanation (POE) on 5th grade students’ conceptual understanding of the States of Matter, Heat and Temperature. In this study, quantitative and qualitative data collection methods were employed. The quantitative element used a quasi-experimental design involving a non-equivalent pretest–posttest control group. This research was performed with a total number of 38 (N control group = 18, N experimental group = 20) 5th grade students (aged 10 to 11 years) in a school in a village located in the East Blacksea region in the 2016–2017 academic year. In the study, a two-tier concept questionnaire entitled ‘the States of Matter, Heat and Temperature (SMHTQ)’ and a semi-structured interview were used as data collection tools. In the experimental group, the topics were taught using the CBA enriched with the POE strategy while in the control group the topics were taught using the 5E teaching model (Engage–Explore–Explain–Elaborate–Evaluate) of the constructivist approach. The results indicated that the CBA enriched with the POE strategy was more effective in improving students’ conceptual understanding and reducing students’ alternative concepts than the 5E teaching model on the States of Matter, Heat and Temperature concepts. The results of the study provided helpful information for science teachers and researchers in science/chemistry education since the teaching materials used enriched the learning environment. Similar studies can be applied to different and wider sample groups and concepts.
Introduction
This study focuses on conceptual understanding of 5th grade students regarding the States of Matter, Heat and Temperature which are inclusive of a big portion in chemistry. Students in different grade levels, such as primary school, high school and college/university, may have alternative understanding about these concepts, which may significantly differ from those widely accepted by the scientific community (Gilbert and Swift, 1985). Such alternative understanding will be called ‘alternative concepts’ in this study. In the process of laying the first foundations of abstract topics in science/chemistry teaching, it is of great importance for students to move on to higher learning processes without any alternative concepts. Therefore, an innovative teaching approach is highly important in the early stages of the science learning process in order to draw students' attention and enable them to establish a relationship between science concepts and daily life. A context-based approach (CBA) adopting students' learning by associating the concepts of science with the contexts from daily life and providing permanent learning for them has been very successful in overcoming students’ alternative concepts (Bulte et al., 2006; Gilbert, 2006; Pilot and Bulte, 2006). In the meantime, it is important to use teaching strategies that will help students to expand their understanding in science concepts, encourage student discussions in the learning process, and eliminate alternative concepts. It has been reported that each stage of the Prediction–Observation–Explanation (POE) strategy provides a rich discussion/learning environment for students, and addresses alternative concepts (Kearney and Treagust, 2001). In this study, a rich learning environment that is composed of a combination of CBA and POE strategy (CBA enriched by POE) has been designed and investigated to improve the conceptual understanding of 5th grade students on the States of Matter, Heat and Temperature.The context-based approach (CBA)
The CBA is one of the most widely used approaches allowing learning to take place with real and relevant context by finding the answers to questions, such as (i) How will this information be useful for me? and (ii) Why do I need to know this information? With the CBA, students are provided with scientific content in a learning environment, in which they understand why they need to learn the new knowledge (e.g.Bulte et al., 2006; Gilbert, 2006; Pilot and Bulte, 2006; Gilbert et al., 2011; Stolk et al., 2011). The CBA also facilitates learning by helping learners to associate abstract topics or concepts with daily life experiences (e.g.Campbell et al., 2000; Gilbert et al., 2011), and develops scientific understanding for the learners (e.g.Bennett et al., 2005; Karslı and Kara-Patan, 2016; Karsli and Yigit, 2017). The reason that the CBA has been applied in this study is to enable students to perceive events occurring in their daily lives with their science classes and thus to develop a scientific understanding.The prediction–observation–explanation (POE) strategy
Students’ learning process in the POE strategy begins with prediction of a situation or an event provided to them. Students thereafter indicate reasons for their predictions, called the prediction stage. After this stage, they observe the event or the situation, reaching out to the observation stage. In the final step, discrepancies between the predictions and the observations are presented and explained in the explanation stage (White and Gunstone, 1992; Liew and Treagust, 1998; Kearney and Treagust, 2001; Coştu et al., 2012). The POE strategy may effectively be used in teaching and students may able to view the topic from a different perspective. This approach does not only provide information but also increases students’ curiosity and stimulates their active involvement in the learning process (White and Gunstone, 1992; Liew and Treagust, 1998; Kearney et al., 2001). Researchers have already suggested that the POE is helpful to promote student discussions in the learning process and increase their conceptual science topic understanding and reduce their alternative concepts (White and Gunstone, 1992; Liew and Treagust, 1998; Kearney and Treagust, 2001; Kearney et al., 2001; Kearney, 2004; Tokur et al., 2014; Bilen et al., 2016). The POE may embed scientific ideas in everyday contexts, and the contextual features may influence how a problem is interpreted. Moreover, it is reported that every stage of the POE strategy provides a rich discussion/learning environment for students (Kearney and Treagust, 2001). Hence, given the advantages of both the CBA and POE strategies, the current study intended to combine both strategies within an enriched learning environment (the CBA enriched with the POE) to increase students’ conceptual understanding. Although many studies applied CBA in chemistry education (King et al., 2008; Cam and Geban 2013; Demircioğlu et al., 2013; Cigdemoglu and Geban, 2015; Karslı and Yigit, 2016, 2017), none of them employed the CBA enriched with the POE strategy.Meaningful learning in social constructivism occurs when students are engaged in social activities/events, such as peer-to-peer and peer-to-teacher discussions, interactions and collaborations (Derry, 1999; Kearney, 2004). From a social constructivist perspective, the collaborative use of CBA with the POE strategy may offer students some opportunities, such as articulating, justifying, debating and reflecting on their own and peers’ scientific views, and negotiating novel and shared implications (Kearney, 2004). Therefore, the current work adopts this view while designing the perspective of the study. It is also believed that the combined pedagogical features (i.e., content knowledge, CBA and POE) may improve the 5th grade students’ learning capacities in practicum.
Pedagogical approaches to the concepts of states of matter, heat and temperature
Conceptual change studies have employed varied pedagogical approaches regarding the concepts of the States of Matter, Heat and Temperature. These include the problem based learning approach (Ayyildiz and Tarhan, 2018); the common knowledge constructing model (Bakırcı and Ensari, 2018); experimenting with physical and virtual manipulatives (Zacharia et al., 2008); the conceptual change oriented approach (Ma-Naim et al., 2002; Jabot and Kautz, 2003; Baser and Geban, 2007); cognitive conflict based instruction (Stavy and Berkovitz, 1980; Baser, 2006; Madu and Orji, 2015); misconception-guided instruction, based on conceptual change theory (Luera et al., 2005); an integrated sensory model (Clark and Jorde, 2004); the inquiry based teaching model coupled with concept substitution strategies (Harrison et al., 1999) and a constructivist teaching approach (Thomaz et al., 1995). As seen in the relevant literature, the CBA enriched with POE strategy is not well-implemented. Most approaches have also focused on diverse samples: children (Stavy and Berkovitz, 1980), seventh grade students (Baser and Geban, 2007), eighth-grade students (Clark and Jorde, 2004), high school students (Harrison et al., 1999; Madu and Orji, 2015; Ayyildiz and Tarhan, 2018; Bakırcı and Ensari, 2018), undergraduate students (Zacharia et al., 2008), preservice teachers (Jabot and Kautz, 2003; Luera et al., 2005; Baser, 2006) and teachers (Thomaz et al., 1995; Ma-Naim et al., 2002). In summary, these studies regarding the concepts of the States of Matter, Heat and Temperature have generally involved either high school or undergraduate students while a very limited number of them have studied 5th grade students.The most recent version of the Turkish science curricula emphasizes student engagement, creative thinking, innovative thinking, constructivist learning theory, inquiry-based learning and varied teaching methods/strategies to stimulate students’ interests in science (MNE, 2018). All Turkish secondary schools have to follow the same science curriculum for compulsory courses offered by the Ministry of National Education. In this regard, all students take compulsory science courses in lower and upper secondary schools since the Turkish science curriculum follows a top-down model in curriculum development. The States of Matter concept is introduced to 3rd, 4th, 5th and 8th grade students first, and the Heat and Temperature concepts to the 5th and 8th grades in their science courses. These concepts are highly essential in the Turkish science curriculum at all grade levels because they are relevant to other courses and supportive in explaining several everyday phenomena. Moreover, 14% of the 5th grade science curriculum in Turkey is allocated to the States of Matter, Heat and Temperature. The researcher has therefore hypothesized that the CBA enriched with POE would result in better conceptual understanding of these topics at the 5th grade level.
Aim of this study
The main goal of this study is to investigate the influence of the CBA enriched with POE on 5th grade students’ conceptual understanding of the States of Matter, Heat and Temperature. Therefore, the study seeks responses to the following research questions:1. Does CBA enriched with the POE strategy create a significant statistical difference in the students' conceptual understanding of the States of Matter, Heat and Temperature?
2. What kind of difference, if any, does the CBA enriched with the POE strategy make to the students’ conceptual understanding of The States of Matter, Heat and Temperature?
Methodology
Experimental design of the study
In educational research, it is almost impossible to conduct a full-experimental research design due to difficulties in the student selection process with full efficiency or formation of student groups or classes (Gravetter and Wallnau, 1996; Shadish et al., 2002) because such difficulties could not only hinder the school schedules, but also result in negative outcomes in terms of research instruction. Therefore, a quasi-experimental research design is mostly used to examine outcomes of an experimental study in educational research (Çepni, 2014).This study has planned to investigate the effects of independent variables (e.g., the CBA enriched with the POE and 5E teaching model of the constructivist approach) on the dependent variables (e.g., 5th grade students’ conceptual understanding). In the experimental group, the topics have been taught by using the CBA enriched with the POE strategy while the topics have been introduced using the 5E teaching model of the constructivist approach in the control group. The quantitative and qualitative data collection methods have also been employed in this study. The quantitative part of the study has a quasi-experimental design involving a non-equivalent pretest-posttest control group while the qualitative data involve semi-structured interviews to support quantitative data and explore the participants’ personal thoughts, experiences and perspective with their own expressions. In this context, the quantitative data in the study have been supported by qualitative data.
The participants
The research was performed with a total number of 38 (N control group = 18, N experimental group = 20) 5th grade students in a school located in the East Black Sea region. Convenience sampling was used because it enabled the researcher to invite participants that it was easy to access (Merriam, 2009). The school where the practices were carried out was selected according to the adequacy of education-assistive elements, such as the status of the laboratory, materials, projector, etc. The teacher applying the teaching materials in the research took a course titled “Context-based Learning Approach” during her graduate education. Since she is a teacher with knowledge on the subject of the CBA and POE strategies, students were specifically selected as participants from her classroom. The research subject was taught by the same teacher in both the experimental and control groups. Some ethical measures (what the features of the sampling are, how the experimental and control groups are determined, how the practices are carried out, etc.) were taken into consideration as stated in the studies involving human participants (Taber, 2014). The study was conducted with two separate 5th grade classes (5A and 5B) in the school. Student names and classroom labels (written each on a piece of paper in two separate boxes) were randomly picked by an independent authority who was not involved in the study in order to establish 5A and 5B classes with their selected students. While the first picked piece of paper was assigned as the control group (N = 18), the other one was assigned as the experimental group (N = 20). Required permissions were taken from the school administration prior to the initiation of the practices. The author reassured the students that they were not obliged to participate and were able to opt out any time from the study. They also would not get extra points for their participation or lose any points if they opt out of their participation. Students who refused to participate were not included in the study.The students in these classes were taught similar subjects until the 5th grade level. They were from similar social environments and had similar socio-economic statuses. The students had superficially learned the subjects of the States of Matter, Heat and Temperature for the first time at 3rd grade level. In this study, a two-tier concept questionnaire, entitled ‘The States of Matter, Heat and Temperature’, was initially applied to the students as a pre-test. It was found that the students’ results regarding this pre-test did not show any significant difference (p > 0.05) as shown in Table 2. Therefore, it was interpreted that the students had similar backgrounds and experiences.
Data collection tools
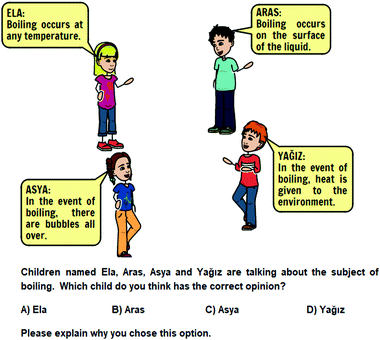
In the research, a two-tier questionnaire, titled as “The States of Matter, Heat and Temperature Concept Questionnaire (SMHTQ),” was used as a data collection tool. Two-tier questionnaires are frequently used for reaching in-depth information about students’ alternative concepts and comprehension levels. While developing the SMHTQ for this study, the following steps were taken into consideration: (1) identifying what concepts would be included; (2) examining alternative concepts from the relevant literature, and the unstructured student interviews, in which open-ended questions, such as ‘What is heat?’, ‘Explain what happens when solids are heated?’, and ‘What are the differences between evaporation and boiling?’, aiming to reveal current alternative concepts, were asked to the students in order to identify alternative concepts in the subject area under investigation, and (3) developing two-tier multiple-choice items for the questionnaire (Chandrasegaran et al., 2007). The first stage of the two-tier questionnaire simply had one question asking the students to make a guess based on a particular situation, and one correct answer with three incorrect answers were included. The second stage, stating “Please explain why you chose this option,” asked the students to write down a reasoning for the answer they picked from the multiple-choice items, for which an empty space was provided right below. The reason why an open-ended question was preferred in the second stage was to reach more in-depth information about the students’ cognitive understandings and determine whether the students have any alternative concepts other than the ones previously determined (Voska and Heikkinen, 2000). The two-tier SMHTQ, containing 14 items with the first stage being multiple-choice and the second being open-ended, was prepared as described. The items of the questionnaire were presented to a chemistry teacher, a science instructor and a science teacher for the evaluation of their appropriateness for the 5th grade level and coverage of all the concepts within the subjects in the curriculum and the benchmarks. Moreover, they evaluated the questionnaire for its item readability and for any scientific mistakes/misleading in the questions and answer choices. The latest version of the questionnaire took its final form after the experts’ recommendations and minor corrections. The concept cartoons (an example shown in Fig. 1) in the two-tier SMHTQ were prepared using the StoryboardThat software. Fig. 1 shows a sample question from the two-tier SMHTQ.In this research, a semi-structured interview protocol was used as a secondary data collection tool. The questions of this protocol consisted of 14 open-ended questions in total, which were on the subjects of states of matter (melting (1), freezing (1), vaporization (1), boiling (1), condensation (1), sublimation (1), and deposition (1)), points of change of state (2), and heat-temperature (6). The appearance and content validity of the questions of the protocol were established as a result of the opinions of two science instructors. In order for the determination of the intelligibility of the questions and the duration of the interview, a pilot study was carried out with one student outside of the sampling. Following the expert opinions and the pilot study, the interview took its final version. Examples from the questions of the semi-structured interview are presented below:
What do you think of when you hear ‘melting’? Could you please give examples of melting from everyday life? When matter is melting, does it absorb or give off heat? What would you say about the temperature of matter before and after melting?
Could you please explain by drawing the transitions in the matters’ changes of state? How does absorption or the giving off of heat take place during these transitions? Could you please indicate on the drawing?
What do you think ‘heat’ is? Can it be measured? If so, what is it measured with and what is its unit?
What do you think ‘temperature’ is? Can it be measured? If so, what is it measured with and what is its unit?
Think of a mother bear and her cub, how big do you think the amounts of their temperatures are? Which bear has the higher body temperature? Why?
The interviews were conducted by the teacher carrying out the instruction practice with 15 students in total, 7 students from the control group and 9 students from the experimental group. Since the confidentiality of the identities of the students in the research was crucial, the control group students were labelled as C1–C7, and the experimental group students were labelled as E1–E9. Prior to the interviews, the teacher was informed with details about how the interviews would be conducted, and a pilot study was carried out with one student. The interviewed students were randomly chosen. The interviews were conducted by the students’ classroom teacher with a total of 15 students and each interview lasted 15 to 20 min. In order to prevent data loss at the time of the interview, the interviews were recorded with the consent of all teachers and students. The recorded data were first transcribed, followed by the elimination of unrelated text to the interview questions from the transcription.
Data analysis
In the analysis of the two-tier SMHTQ, scoring was made on the basis of categories (for the first stage of the questionnaire: Correct Answer, Incorrect Answer, and Unanswered; for the second stage of the questionnaire: Correct Explanation, Partially Correct Explanation, Explanation with Alternative Concepts or Incorrect Explanation, and Unanswered). The categories used in both stages of the two-tier SMHTQ, their abbreviations and their quantitative values are provided in Table 1. Similar categories have been used in several studies (Karslı and Kara-Patan, 2016; Çalık and Cobern, 2017). The scores of the students in the pretest and the posttest were calculated according to the categories given in Table 1. The answers that students replied to the second stages of the test were categorized by both the author and the teacher of the course according to the codings given in Table 1. Scoring was made for the questions in the second stage of the test according to the coding agreed upon jointly. The percentage of agreement between encodings made by independent researchers was calculated and it was found to be 85%.| The categories used in the first stage of the two-tier SMHTQ | The categories used in the second stage of the two-tier SMHTQ | Abbreviation | Total Score |
|---|---|---|---|
| Correct Answer | Correct Explanation | CA-CE | 10 |
| Correct Answer | Partially Correct Explanation | CA-PCE | 9 |
| Incorrect Answer | Correct Explanation | IA-CE | 8 |
| Unanswered | Correct Explanation | U-CE | 7 |
| Incorrect Answer | Partially Correct Explanation | IA-PCE | 6 |
| Correct Answer | Explanation with Alternative Concepts/Incorrect Explanation | CA-EAC | 5 |
| Correct Answer | Unanswered/Repeated/Irrelevant | CA-U | 4 |
| Incorrect Answer | Explanation with Alternative Concepts/Incorrect Explanation | IA-EAC | 3 |
| Unanswered | Explanation with Alternative Concepts/Incorrect Explanation | U-EAC | 2 |
| Incorrect Answer | Unanswered/Irrelevant | IA-U | 1 |
| Unanswered | Unanswered/Irrelevant | U-U | 0 |
Non-parametric statistical techniques were applied using SPSS.16 Package Software since the two-tier SMHTQ did not correspond to the hypotheses of the parametric tests as the data acquired were ordinal (Garcia et al., 2009). Mann–Whitney U test was used in the comparison of the scores between the experimental and control groups, while Wilcoxon Signed-Rank Test was used for the comparisons within the groups themselves (Karslı and Kara-Patan, 2016). In addition, the influence quantity values were also calculated. According to Cohen (1988), the influence quantity (r) has a low effect if it is between 0 and 0.3; a medium effect if it is around 0.5 and a great effect if its value is 0.8 and above.
The interviews were subjected to content analysis by the author. In this analysis, codes and themes were created from the students’ responses to the questions. The responses under these codes were then classified under three categories of understanding: correct understanding, partially correct understanding and understanding with alternative concepts (Yıldırım and Şimşek, 2013). As an example, these categories were explained by taking students responses into consideration to the question of “Think of a mother bear and her cub, how big do you think the amounts of their temperatures are? Which bear has the higher body temperature? Why?” For the Correct Understanding (CU) category, the students were expected to provide a scientifically correct answer to the question for their responses to fall in this category. For instance, responses such as “The mother bear's body heat is higher because she is bigger than her cub, and she has a thicker fat layer and skin” classified under the CC category. For the Partially Correct Understanding (PCU) category, the responses were scientifically correct answers but had some deficiencies. For instance, student responses such as “The mother bear's body heat is higher because her fat layer is more developed and she has more skin” were categorized under the PCU category. For the Understanding with Alternative Concepts (UAC) category, the responses were incorrect and contradicted with the scientific facts. For instance, responses such as “The bear cub's body heat is higher because it is a newborn and it is small” were put into the understanding with alternative concepts category. In other words, it was used to categorize incorrectly explained expressions.
In the process of the creation of these categories, an independent researcher outside of this study was asked to identify which categories the student responses would fall into. This way, the categories that were consistent among the researchers were created and the consistency was determined as of 90%.
Practice stage and teaching material
This study examined the effects of the CBA enriched with the POE strategy on 5th grade students' conceptual understandings of The States of Matter, Heat and Temperature. Therefore, in the experimental group, classes were taught using the CBA enriched with the POE, while in the control group, the same concepts were taught according to the 5E teaching model of the constructivist approach. The constructivist learning approach is based on the principle that students construct their own meaning through pre-existing knowledge, activities and social interaction. In other words, new learning builds on pre-existing knowledge (Karslı-Baydere et al., 2020). The 5E model is based on a constructivist view of learning (Bybee and Landes, 1990). This model consists of 5 stages. These are: Engage–Explore–Explain–Elaborate–Evaluate. In the first stage of the model, the learning task is initiated. Various questions were asked to the students in this stage to inquire about their prior knowledge on the subject in the control group. In the exploration phase, students actively explore their environment or do experiments. At this stage, students in the control group conducted experiments on the subject. The explanation stage of the teaching model provides students with opportunities to verbalize their conceptual understanding. During the explanation stage, students were given opportunities to explain their own explanations and to ask questions about the subjects they discovered. The purpose of the elaborate stage is to help develop deeper and broader understandings of concepts (Duran and Duran 2004). At this stage, examples of the application areas of the subject in daily life are given to the students in the control group. At the evaluate stage of the teaching model in the control group, student progress towards achieving educational goals was evaluated.In CBA, a context is chosen from everyday life in order for the students to be able to visualize it better in their minds and attract their attention to the subject. Context must be chosen among the circumstances that the students are familiar with and be appropriate to their age level. It must neither distract students from the relevant subject nor be difficult or confusing to comprehend (De Jong, 2008). Therefore, ‘the lives of polar bears and grizzly bears’ was chosen as the context for the presentation of the subjects on the States of Matter, Heat and Temperature. Within the scope of the chosen context, the lives of polar bears and grizzly bears were made into a story and turned into worksheets in accordance to the POE strategy. The worksheets used during the teaching of the class had been presented to one chemistry teacher, three science instructors and two science teachers for evaluation, and they were given their final version after the suggested revisions from the experts.
Some examples presented according to the CBA within POE strategy are provided below.
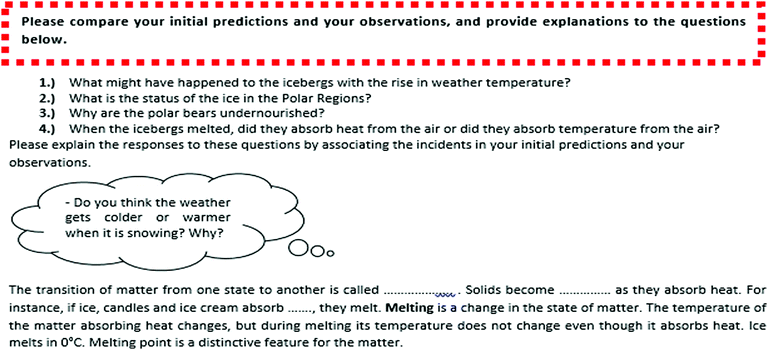
1. Prediction: Owing to the nature of the prediction stage of the POE, at this stage of the practice, the lives and skins etc. of polar bears and grizzly bears were presented to the students with various questions in order for the students to associate them with the subject on the States of Matter, Heat and Temperature. An example of the prediction stage of the worksheet used in the teaching of the melting subject is shown in Fig. 2.
2. Observation: In the observation stage, students were told to carry out experiments under the guidance of the teacher on the situation they had made predictions about and they were asked to write down their observations in the relevant spaces on the worksheet. An example of the observation stage of the worksheet used in the teaching of the melting subject is shown in Fig. 3.
3. Explanation: In the explanation stage, the students were guided to explain the contradictory incidents between their initial predictions and their observations. An example of the explanation stage of the worksheet used in the teaching of the melting subject is shown in Fig. 4.
The worksheets were applied as a pilot study with 14 fifth grade students enrolled in the science course in the 2015–2016 academic years in a public elementary school in Turkey. The pilot study was carried out in a different sample group for the main study at the same school. The teacher was involved in both the pilot and the main studies. All official permissions for the pilot study were obtained from the school administration and the classroom teacher. Minor revisions were made after the pilot application on the worksheets. Firstly, the two-tier SMHTQ was given to the students as a pretest prior to the teaching practice. The students answered the two-tier SMHTQ for approximately 25 min. The entire teaching process took place with the students in groups of 3 or 4 in the science laboratory of the school. The teaching practice was completed in 8 h within 2 weeks (8 × 40 min). To avoid disconnection between practices, a brief summary of the subjects covered in the previous class was reviewed at the beginning of each class, and each class was evaluated. After the instruction, the two-tier SMHTQ was given to the students for the second time as a post-test. One week after the post-test, interviews on concepts were conducted with the students.
Findings
Findings acquired from the two-tier SMHTQ
Below are the findings acquired as a result of the comparison of the scores of the experimental and control groups in the two-tier SMHTQ pre-test and the post-test.As shown in Table 2, a Mann–Whitney U test did not demonstrate a significant difference between the pre-test scores of the experimental and control groups (U = 175.000 p = 0.146 (p > 0.05), z = −0.146, r = −0.02). In the results of the post-test, however, a significant difference was observed in favor of the experimental group (U = 112.000 p = 0.047 (p < 0.05), z = −1.990, r = −0.32). When the influence quantity (r) findings in the comparison of the post-tests of the experimental and control groups were analyzed, there was a small influence quantity (r = 0.32) in favor of the experimental group.
| Tests | Group | N | Mean rank (X) | Sum of ranks | U | Z | p | r |
|---|---|---|---|---|---|---|---|---|
| N = sample size; U = calculated U value; Z: calculated Z value; p = significance; r (influence quantity); r = Z/√N | ||||||||
| Pre-test | Control | 18 | 19.22 | 346.000 | 175.000 | −0.146 | 0.884 | 0.02 |
| Experimental | 20 | 19.75 | 395.000 | |||||
| Post-test | Control | 18 | 15.22 | 283.000 | 112.000 | −1.990 | 0.047 | 0.32 |
| Experimental | 20 | 22.90 | 458.000 | |||||
According to Table 3, there was a significant difference in favor of the post-test in the scores of both the experimental and control groups in the two-tier SMHTQ pre-test and the post-test (p < 0.05, z = −3.413; −3.921, r = −0.80; 0.87). Moreover, when the influence quantity (r) of the scores of the students of the experimental and control groups in the pre-test and post-test were analyzed, there was a high influence quantity in favor of the post-test.
| Group | Tests | N | Mean rank | Sum of ranks | Z | p | r | |
|---|---|---|---|---|---|---|---|---|
| p = significance; r (influence quantity); r = Z/√N. | ||||||||
| Control | Pre-test–post test | Negative ranks | 1 | 2.00 | 2.00 | −3.413 | 0.001 | 0.80 |
| Positive ranks | 15 | 8.93 | 134.00 | |||||
| Ties | 2 | |||||||
| Experimental | Pre-test–post test | Negative ranks | 0 | 0.00 | 0.00 | −3.921 | 0.000 | 0.87 |
| Positive ranks | 20 | 10.50 | 210.00 | |||||
| Ties | 0 | |||||||
The change in the alternative concepts revealed in the two-tier SMHTQ pre-test and post-test of the students in the experimental and control groups are presented in Table 4.
| Category | The students’ alternative concepts | Control group (f) | Experimental group (f) | ||||
|---|---|---|---|---|---|---|---|
| PrT | PoT | CC | PrT | PoT | CC | ||
| PrT: pre-test; PoT: post-test, CC: conceptual change; +: positive conceptual change; −: negative conceptual change. | |||||||
| Melting | Ice cannot melt at 0 °C | 3 | — | +3 | 4 | 2 | +2 |
| Melting point is not distinctive for the matter | 1 | — | +1 | — | — | — | |
| The matter gives off heat when it transitions into the liquid state from the solid state | 1 | — | +1 | 1 | 1 | 0 | |
| Freezing | The freezing point of water is 4 °C | 1 | — | +1 | — | — | — |
| When turning into water, ice absorbs heat and leaves it stabilized | 1 | — | +1 | — | — | — | |
| Freezing point is not a distinctive feature | 1 | — | +1 | — | — | — | |
| Boiling | Heat is given off during boiling | 5 | 2 | +3 | 6 | — | +6 |
| Boiling point is not distinctive for the matter | 1 | — | +1 | 2 | — | +2 | |
| Boiling point is the hottest form of the matter | 3 | — | +3 | 3 | — | +3 | |
| Boiling starts from the bottom and reaches to the top | 1 | — | +1 | — | — | — | |
| Boiling point is where the heat remains stable | 1 | 1 | 0 | — | — | — | |
| When the matter is boiling, the heat does not remain stable | — | — | — | 1 | 1 | 0 | |
| Boiling takes place on the surface of the liquid | 1 | 1 | 0 | 2 | — | +2 | |
| Boiling can occur at any temperature | 1 | 2 | −1 | 1 | 1 | 0 | |
| Boiling and vaporization are the same thing | — | — | — | 1 | — | +1 | |
| Vaporization | In snowy weather, clothes dry by absorbing heat from the snow | 2 | 2 | 0 | 3 | — | +3 |
| Clothes give off heat while drying | 2 | 1 | +1 | 3 | — | +3 | |
| Clothes do not always need to absorb heat to dry | 2 | 1 | +1 | 1 | — | +1 | |
| The clouding on a window when we blow on it is an example of vaporization | 1 | 1 | 0 | 1 | — | +1 | |
| Clothes do not dry without air | 1 | — | +1 | — | — | — | |
| The droplets on a bottle taken out of a fridge occur as a result of vaporization | 2 | 2 | 0 | 3 | — | +3 | |
| Condensation | Water condenses and rises to the air and returns as rain | 1 | — | +1 | 1 | — | +1 |
| Condensation point cannot be 100 °C | 1 | 1 | 0 | 6 | 2 | +4 | |
| There is no such thing as a condensation point | 1 | — | +1 | 1 | — | +1 | |
| The matter cannot transition from the gas state to the liquid state | — | — | — | 1 | — | +1 | |
| The matter absorbs heat during the transition from the gas state to the liquid state | — | — | — | 2 | — | +2 | |
| Deposition | Deposition occurs when water turns into ice | 1 | — | +1 | — | — | — |
| The matter absorbs heat during deposition | — | — | — | 1 | — | +1 | |
| Sublimation | The matter gives off heat during sublimation | 1 | — | +1 | 2 | 1 | +1 |
| Heat-temperature | Heat and temperature are measured by thermometer | 7 | 2 | +5 | 6 | 3 | +3 |
| The unit for heat is °C | 1 | — | +1 | 1 | — | +1 | |
| The matter loses volume when heated | 1 | — | +1 | 2 | — | +2 | |
| The matter does not change size whether heated or not | — | — | — | 2 | 1 | +1 | |
| The temperature of the matter absorbing heat does not change on a phase change | — | 2 | −2 | 1 | — | +1 | |
| Total | 45 | 18 | +27 | 58 | 12 | +46 | |
As shown in Table 4, student responses were also analyzed in order to determine specific alternative concepts about the States of Matter, Heat and Temperature based on pre-, and post-tests. Table 4 summarizes the frequency of alternative concepts in pre-test, and post-test of the two-tier SMHTQ. Table 4 shows that the students’ conceptual understanding about the States of Matter, Heat and Temperature was improved after the teaching intervention because the frequency of the alternative concepts on the pre-test were more than the post-test. For example, the ‘heat is given off during boiling’ concept was observed in the pre-test, but it was not observed in the post-test. In another example, there were two students in the control group and three students in the experimental group in the pre-test with the alternative concept suggesting that ‘in snowy weather, clothes dry by absorbing heat from the snow’. However, there were only two students in the control group and none in the experimental group in the post-test. This shows that a positive conceptual change took place regarding this alternative concept after the teaching intervention (0; +3). In the total number of alternative concepts, the number of students with the alternative concept in the control group was 45 in the pre-test and 18 in the post-test while in the experimental group there were 58 students in the pre-test and 12 in the post-test. In addition, the alternative concepts identified in the students were focused on the ‘Boiling’, ‘Vaporization’ and ‘Heat-Temperature’ concepts in both the two-tier SMHTQ pre-test and post-test.
Table 5 shows the distribution of student answers in the semi-structured interview on the concepts being studied with the students about the subjects on the States of Matter, Heat and Temperature with regard to categories as correct understanding (CU), partially correct understanding (PCU), and understanding with alternative concepts (UAC). Table 5 also shows examples of responses that correspond to the codes and understanding categories.
| Theme | Code | UC | Quotes from the student responses | f | |
|---|---|---|---|---|---|
| Control | Experimental | ||||
| UC: Understanding Categories; CU: Correct Understanding, PCU: Partially Correct Understanding; UAC: Understanding with Alternative Concepts. 7 students from the control group and 9 students from the experimental group participated in the interview. | |||||
| Changes of state | Melting | CU | “In melting, the matter transitions from the solid state to the liquid state.” | 5 | 9 |
| UAC | “The matter gives off heat while melting.” | 1 | 0 | ||
| “The temperature of the matter decreases after melting” | 1 | 1 | |||
| “The temperature of the matter remains the same after melting” | |||||
| Freezing | CU | “Freezing is when liquid matter turns into solid” | 3 | 9 | |
| UAC | “If we put ice cream in the fridge after it melts, it freezes. In this incident, the ice cream transitions from the liquid state to the gas state.” | 1 | 0 | ||
| Vaporization | CU | “Vaporization is matter transitioning from liquid to gas.” | 5 | 8 | |
| UAC | “Droplets outside of a teapot is vaporizing” | 1 | 0 | ||
| “Matter gives off heat while vaporizing” | 1 | 0 | |||
| Boiling | CU | “The matter absorbs heat while boiling” | 5 | 9 | |
| PCU | “When you heat the water and it bubbles up, it is boiling” | 4 | 4 | ||
| UAC | “The matter gives off heat while boiling” | 1 | 0 | ||
| “I think boiling and vaporization are the same thing.” | 1 | 0 | |||
| “For example, when we put water in the pot it boils after a while, and then it vaporizes.” | 1 | 0 | |||
| “Vaporization happens after boiling” | 1 | 0 | |||
| “Boiling occurs when the matter is really hot” | 1 | 0 | |||
| Condensation | CU | “The matter transitions from gas to liquid while condensation” | 5 | 9 | |
| PCU | “It is the forming of droplets outside a teapot when there is water boiling inside” | 1 | 0 | ||
| UAC | “The matter absorbs heat during condensation” | 2 | 1 | ||
| “ The matter transitions from liquid to gas while condensation” | 1 | 0 | |||
| Sublimation | CU | “The matter transitioning from solid to gas” | 2 | 7 | |
| Deposition | CU | “Deposition is when the matter transitions from the gas state directly into the solid state.” | 3 | 9 | |
| PCU | “The air turning solid like ice particles on the window of the car on cold days” | 1 | 1 | ||
| State change points | CU | “Points of change of state are where the temperature remains stable” | 2 | 5 | |
| PCU | “Points of change of state are the melting point and the freezing point”. | 3 | 0 | ||
| UAC | “The highest temperature of matter is the boiling point” | 1 | 0 | ||
| “Heat is measured by a thermometer” | 5 | 1 | |||
| “Heat is something hot” | 1 | 0 | |||
| “Heat is measured by degrees” | 1 | 0 | |||
| “Heat is a temperature” | 1 | 0 | |||
| “The unit for heat is centigrade Celsius” | 5 | 1 | |||
| “The heat of the cub is higher, because it is a newborn and is small.” | 2 | 0 | |||
| “I think the heat of the cub is higher than the mother bear. Because the cub is a newborn. Since the cub is warmer, there is a heat transfer from the cub to the mother.” | |||||
| “The energy flow from the mother bear to her cub does not continue forever. Because the energy finishes” | 1 | 0 | |||
| “The energy flow from the mother bear to her cub does not continue forever. It goes on until their energies are even” | 2 | 0 | |||
| “I remember the temperature as Joule” | 1 | 0 | |||
When student responses in the semi-structured interview were analysed (Table 5), they were divided into codes such as ‘Melting’, ‘Freezing’, ‘Vaporization’, ‘Boiling’, ‘Condensation’, ‘Sublimation’, ‘Deposition’, ‘Points of Change of State’, ‘Heat’, and ‘Temperature’. The results show that the students had various alternative concepts besides the CU and PCU regarding the States of Matter, Heat and Temperature. After the teaching intervention, a majority of the 5th grade students provided responses under the CU and PCU categories in the semi-structured interview, and the students under these categories were generally in the experimental group. At the same time, the students with alternative concepts were generally the ones in the control group as seen in the code of ‘Melting’ (e.g. C1, C6), ‘Freezing’ (e.g. C1), ‘Vaporization’ (e.g. C1, C5), ‘Boiling’ (e.g. C1, C4, C5 and C6), and ‘Condensation’ (e.g. C2, C4, C6). Additionally, the alternative concepts identified in the students in the semi-structured interview were generally in the ‘Boiling’ and ‘State change points’ codes.
Discussion
The main purpose of this study was to investigate the effects of the CBA enriched with POE on 5th grade students' conceptual understanding of the States of Matter, Heat and Temperature concepts. With this purpose, the first research question was “Does CBA enriched with the POE strategy create a significant statistical difference in the students' conceptual understanding of the States of Matter, Heat and Temperature?”, which was addressed by the quantitative data, and the second was “What kind of difference, if any, does the CBA enriched with the POE strategy lead to the students’ conceptual understanding of The States of Matter, Heat and Temperature?” addressed by the qualitative data in this study.The statistical results showed that there was no significant difference among the students’ pre-test scores (Table 2). In other words, the students in the experimental and control group have similar conceptual understanding of the States of Matter, Heat and Temperature before the teaching intervention, which may be the result of teaching the same curriculum to students (MNE, 2018). However, comparisons of the Mann–Whitney U test results showed that there were significant differences in favor of the post-test in the experimental group (Table 2). In other words, the CBA enriched with the POE strategy was more effective in improving conceptual understanding of students than the 5E teaching model of the constructivist approach on the States of Matter, Heat and Temperature after the teaching intervention. This may be the result of the CBA positively affecting conceptual understanding (Campbell et al., 2000; Bennett et al., 2005; Gilbert et al., 2011; Karslı and Kara-Patan, 2016; Karslı and Yigit, 2017). Another reason may be the use of the POE strategy in the study. Indeed, it is known that the POE is useful to promote students’ conceptual understanding of science topics and student discussions in the learning process, and reduce their alternative concepts (White and Gunstone, 1992; Liew and Treagust, 1998; Kearney and Treagust, 2001; Kearney et al., 2001; Kearney, 2004; Tokur et al., 2014; Bilen et al., 2016; Yaman et al., 2019). This may also be due to the use of the POE strategy in conjunction with the CBA. As emphasized in many studies, it is necessary to bring various pedagogical approaches together and provide students an enriched learning environment (Karsli and Çalık, 2012; Karslı-Baydere et al., 2020). As shown in Table 3, there was a significant difference in favor of the post-test in the scores of both the experimental and control groups. In other words, both the 5E teaching model of the constructivist approach and the CBA enriched with the POE strategy have led to an increase in students' conceptual understanding. As a matter of fact, the students also made experiments in the control group, and in the elaborate phase they tried to establish a relationship between the science concepts and daily life. There are also studies in the literature that only apply the 5E teaching model and have positive results (e.g.Karslı-Baydere et al., 2020). However, it seems that, based on this work, the CBA with POE was more effective than the 5E teaching model in increasing students' conceptual understanding. The design of the teaching and learning materials to align with the CBA with POE approach is likely to be the reason why this increased conceptual understanding has been observed. Moreover, the CBA enriched with the POE strategy may provide a rich discussion/conversation environment for the 5th grade students (Kearney and Treagust, 2001; Yaman et al., 2019). This may also stem from the effect of the daily life examples and materials based on the CBA used during the intervention (Campbell et al., 2000; Gilbert et al., 2011; Cigdemoglu and Geban, 2015; Karslı and Yigit, 2016, 2017). As shown in Table 4, the conceptual change in the positive direction was higher in the experimental group. We can also see this from the change in the total number of alternative concepts in Table 4. This is further supported by the data in Table 5. For example, when the answers were divided into the comprehensive categories, the answers in the UA and PUA categories consisted of the students in the experimental group, whereas the students in the UAC category consisted of the students in the control group in Table 5. In other words, the intervention to the experimental group was more effective in eliminating students' alternative concepts. This result is also consistent with findings of other studies in terms of supporting the idea that the CBA causes a decrease in students' alternative concepts (Bennett et al., 2005; Karslı and Kara-Patan, 2016; Karslı and Yigit, 2017; Karslı-Baydere and Aydın, 2019). This may be the result of providing students experience with a familiar and remarkable context (Belt et al., 2005). In addition, the students had the opportunity to observe the prediction questions presented to them and to discuss the results in the POE stages. This may be the result of the POE strategy adopting student and activity-based activities rather than teacher-centered activities (Kearney et al., 2001; Yaman et al., 2019). Additionally, POE has an important role in finding students' prior knowledge and thought processes. This feature helps educators to identify students' alternative concepts about topics and find sustainable solutions to eliminate students' alternative concepts (Kibirige et al., 2014).
In this work, the effect of the teaching and learning materials using the CBA with POE approach on the long term memory of students in relation to the concepts studied was not examined. Future studies may be conducted to investigate the effects of combined pedagogical features (i.e., content knowledge, CBA and POE) on the retention of students’ foundational understanding on the States of Matter, Heat and Temperature. The results of the study provide helpful information for science teachers and researchers in science/chemistry education through the teaching materials used to enrich the learning environment. Similar studies can be applied to different and wider sample groups and their effects can be examined. Moreover, the current study recommends that the CBA enriched with the POE strategy can be tested on other topics throughout a long-term teaching intervention. Teaching materials whose effectiveness has been studied could potentially be shared through a database under a creative commons license to enable the implementation for a larger audience of researchers and teachers.
Conclusions
In this study, it is concluded that the use of these important features of the CBA and the POE strategies together outweighs the 5E teaching model of the constructivist approach in overcoming students’ existing alternative concepts.The research findings reported that the conceptual change in the positive direction is higher in the experimental group.
Although the 5E teaching model of the constructivist approach did not particularly focus on the common alternative concepts of “the States of Matter, Heat and Temperature”, the alternative concepts of 5th grade students were somewhat reduced and showed improvements although it was not as much as the experimental group. This may be due to the teacher because she might have intuitively addressed these alternative concepts during the question/answer sessions. Phrased differently, the teacher who taught the subject to both the experimental and control groups may have transferred her awareness of common alternative conceptions to the existing instruction (control group). This may be seen as an uncontrolled variable (limitation) of the current study.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
I would like to thank science teacher Nurgül Çakmak in the pilot and actual work, Dr Çiğdem Şahin Çakir from Giresun University, Dr Fatma Yaman from Bozok University, Turkey and chemistry teacher Çiğdem Yildirim who assisted with checking interview questions, the SMHTQ and evaluating the CBA with POE worksheets for their kind help. Also I would like to thank Dr M. Mustafa Çetin from Kadir Has University, Turkey for his kind help in language polishing.References
- Ayyildiz Y. and Tarhan L., (2018), Problem-based learning in teaching chemistry: Enthalpy changes in systems, Res. Sci. Technol. Educ., 36(1), 35–54.
- Bakırcı H. and Ensari Ö., (2018), The effect of common knowledge construction model on academic achievement and conceptual understandings of high school students on heat and temperature, Educ. Sci., 43(196).
- Baser M., (2006), Fostering conceptual change by cognitive conflict based instruction on students’ understanding of heat and temperature concepts, Eurasia J. Math., Sci. Technol. Educ., 2(2), 96–114.
- Baser M. and Geban Ö., (2007), Effectiveness of conceptual change instruction on understanding of heat and temperature concepts, Res. Sci. Technol. Educ., 25(1), 115–133.
- Belt S. T., Leisvik M. J., Hyde A. J. and Overton T. L., (2005), Using a context-based approach to undergraduate chemistry teaching – a case study for introductory physical chemistry, Chem. Educ. Res. Pract., 6(3), 166–179.
- Bennett J., Gräsel C., Parchmann I. and Waddington D., (2005), Context-based and conventional approaches to teaching chemistry: Comparing teachers’ views, Int. J. Sci. Educ., 27(13), 1521–1547.
- Bilen K., Özel M. and Köse S., (2016), Using action research based on the predict-observe-explain strategy for teaching enzymes, Turkish J. Educ., 5(2), 72–81.
- Bulte A. M., Westbroek H. B., de Jong O. and Pilot A., (2006), A research approach to designing chemistry education using authentic practices as contexts, Int. J. Sci. Educ., 28(9), 1063–1086.
- Bybee R. and Landes N. M., (1990), Science for life and living: An elementary school science program from Biological Sciences Improvement Study (BSCS), Am. Biol. Teach., 52(2), 92–98.
- Çalık M. and Cobern W. W., (2017), A cross-cultural study of CKCM efficacy in an undergraduate chemistry classroom, Chem. Educ. Res. Pract., 18, 691–730.
- Cam A. and Geban Ö., (2013), Effectiveness of case-based learning instruction on students' understanding of solubility equilibrium concepts, Hacettepe Üniversitesi Eğitim Fakültesi Dergisi, 44(44), 97–108.
- Campbell B., Lubben F. and Dlamini Z., (2000), Learning science through contexts: Helping pupils make sense of everyday situations, Int. J. Sci. Educ., 22, 239–252.
- Çepni S., (2014), Introduction to research and project work, 6th edn, Trabzon: Celepler Printing.
- Chandrasegaran A. L., Treagust D. F. and Mocerino M., (2007), The development of a two-tier multiple-choice diagnostic instrument for evaluating secondary school students’ ability to describe and explain chemical reactions using multiple levels of representation, Chem. Educ. Res. Pract., 8(3), 293–307.
- Cigdemoglu C. and Geban O., (2015), Improving students’ chemical literacy levels on thermochemical and thermodynamics concepts through a context-based approach, Chem. Educ. Res. Pract., 16(2), 302–317.
- Clark D. and Jorde D., (2004), Helping students revise disruptive experientially supported ideas about thermodynamics: Computer visualizations and tactile models, J. Res. Sci. Teach., 30, 1–23.
- Cohen J., (1988), Statistical power analysis for the behavioral sciences, New Jersey: Lawrence Erlbaum Associates, Publishers.
- Coştu B., Ayas A. and Niaz M., (2012), Investigating the effectiveness of a POE-based teaching activity on students’ understanding of condensation, Instruct. Sci., 40(1), 47–67.
- De Jong O., (2008), Context-based chemical education: How to improve it?, Chem. Educ. Int., 8(1), 1–7.
- Demircioğlu H., Dinç M. and Çalık M., (2013), The effect of storylines embedded within context-based learning approach on grade 6 students’ understanding of ‘physcial and chemcal change’ concepts, J. Baltic Sci. Educ., 12(5), 682–691.
- Derry S. J., (1999), A fish called peer learning: Searching for common themes, Cogn. Perspect. Peer Learn., 9(1), 197–211.
- Duran L. B. and Duran E., (2004), The 5E instructional model: A learning cycle approach for inquiry-based science teaching, Sci. Educ. Rev., 3(2), 49–58.
- Garcia S., Molina D., Lozano M. and Herrera F., (2009), A study on the use of non-parametric tests for analyzing the evolutionary algorithms’ behaviour: A case study on the CEC’ 2005 special session on real parameter optimization, J. Heuristics, 15(6), 617–644.
- Gilbert J. K., (2006), On the nature of ‘‘Context’’ in chemical education, Int. J. Sci. Educ., 28 (9), 957–976.
- Gilbert J. K. and Swift D. J., (1985), Towards a Lakatosian analysis of the Piagetian and alternative conceptions research programs, Sci. Educ., 69(5), 681–696.
- Gilbert J. K., Bulte A. M. W. and Pilot A., (2011), Concept development and transfer in context-based science education, Int. J. Sci. Educ., 33(6), 817–837.
- Gravetter F. J. and Wallnau L. B., (1996), Statistics for the behavioralsciences, Minneapolis, MN: West.
- Harrison A. G., Grayson D. J. and Treagust D. F., (1999), Investigation a grade 11 student's evolving conceptions of heat and temperature. J. Res. Sci. Teach., 36, 55–87.
- Jabot M. and Kautz C. K., (2003), A model for preparing preservice physics teachers using inquiry-based methods, J. Teach. Educ. Online, 1, 25–32.
- Karsli F. and Çalık M., (2012), Can freshman science student teachers' alternative conceptions of ‘electrochemical cells’ be fully diminished? Asian J. Chem., 24(2), 485.
- Karslı F. and Kara-Patan K., (2016), Effects of the context-based approach on students’ conceptual understanding: “The Umbra, the Solar Eclipse and the Lunar eclipse”, J. Baltic Sci. Educ., 15(2), 246–260.
- Karsli F. and Yigit M., (2016), 12 th grade students' views about an alkanes worksheet based on the REACT strategy, Necatibey Faculty Educ. Electron. J. Sci. Math. Educ., 10(1), 472–499.
- Karsli F. and Yigit M., (2017), Effectiveness of the REACT strategy on 12th grade students’ understanding of the alkenes concept, Res. Sci. Technol. Educ., 35(3), 274–291 DOI:10.1080/02635143.2017.1295369.
- Karslı-Baydere F. and Aydın E., (2019), “The Eye” topic through the explanation assisted REACT strategy of the context-based approach, Gazi Univ. J. Gazi Educ. Faculty (GUJGEF), 39(2), 755–791.
- Karslı-Baydere F. K., Ayas A. and Çalik M., (2020), Effects of a 5Es learning model on the conceptual understanding and science process skills of pre-service science teachers: The case of gases and gas laws, J. Serb. Chem. Soc., 85(4), 559–573.
- Kearney D. M., (2004), Classroom use of multimedia supported predict-observe-explain tasks in a social constructivist learning environment, Res. Sci. Educ., 34(4), 427–453.
- Kearney M. and Treagust D. F., (2001), Constructivism as a referent in the design and development of a computer program using interactive digital video to enhance learning in physics, Australasian J. Educ. Technol., 17(1).
- Kearney M., Treagust D., Yeo S. and Zadnik M. G., (2001), Student and teacher perceptions of the use of multimedia supported predict-observe-explain tasks to probe understanding, Res. Sci. Educ., 31(4), 589–615.
- Kibirige I., Osodo J. and Tlala K. M., (2014), The effect of predict–observe–explain strategy on learners’ misconceptions about dissolved salts, Mediterranean J. Soc. Sci., 5(4), 300.
- King D., A. Bellocchi and S. M. Ritchie, (2008), “Making connections: Learning and teaching chemistry in context.” Res. Sci. Educ.38, 365–384.
- Liew C. W. and Treagust D F., (1998), The effectiveness of predict–observe–explain tasks in diagnosing students’ understanding of science and in identifying their levels of achievement. Paper presented at the Annual Meeting of the American Educational Research Association, April 13–17, San Diago.
- Luera G. R., Otto C. A. and Zitzewitz P. W., (2005), A conceptual change approach to teaching energy and thermodynamics to pre-service elementary teachers, J. Phys. Teach. Educ. Online, 2(4), 3–8.
- Madu B. C. and Orji E., (2015), Effects of cognitive conflict instructional strategy on students’ conceptual change in temperature and heat, Sage Open, 5(3), 2158244015594662.
- Ma-Naim C., Bar V. and Zinn B., (2002), Integrating microscopic macroscopic and energetic descriptions for a Conceptual Change in Thermodynamics. Paper presented in the third European Symposium on Conceptual Change, June 26-28. 2002, Turku, Finland.
- Merriam S. B., (2009), Qualitative research: A guide to design and implementation, San Francisco, CA: Jossey-Bass.
- MNE, (2018), Science course curriculum, MEB Talim ve Terbiye Kurulu Başkanlığı, Ankara, Retrieved from http://mufredat.meb.gov.tr/Dosyalar/201812312311937-FEN%20B%C4%B0L%C4%B0MLER%C4%B0%20%C3%96%C4%9ERET%C4%B0M%20PROGRAMI2018.pdf.
- Pilot A. and Bulte A. M. W., (2006), Why do you ‘‘need to know’’? Context-based education, Int. J. Sci. Educ., 28 (9), 953–956.
- Shadish W. R., Cook T. D. and Campbell D. T., (2002), Experimental and quasi-experimental designs for generalized causal inference, Boston, MA: Houghton Mifflin Company.
- Stavy R. and Berkovitz B., (1980), Cognitive conflict as a basis for teaching quantitative aspects of the concept of temperature, Sci. Educ., 64, 679–692.
- Stolk M. J., De Jong O., Bulte A. M. and Pilot A., (2011), Exploring a framework for professional development in curriculum innovation: Empowering teachers for designing context-based chemistry education, Res. Sci. Educ., 41(3), 369–388.
- Taber K. S., (2014), Ethical considerations of chemistry education research involving ‘human subjects’, Chem. Educ. Res. Pract., 15(2), 109–113.
- Thomaz M. F., Malaquias I. M., Valente M. C. and Antunes M. J., (1995), An attempt to overcome alternative conceptions related to heat and temperature, Phys. Educ., 30, 19–26.
- Tokur F., Duruk Ü. and Akgün A., (2014), Investigation of the effect of POE activities on remedying preservice science teachers’ misconceptions in the context of growing and developing in flowery plants unit, Route Educ. Soc. Sci. J., 1(1), 65–80.
- Voska K. W. and Heikkinen H. W., (2000), Identification and analysis of student conceptions used to solve chemical equilibrium problems, J. Res. Sci. Teach., 37(2), 160–176.
- White R. and Gunstone R., (1992), Prediction-observation-explanation. Probing understanding, 1st edn, London and New York, pp. 44–64.
- Yaman F., Ayas A. and Çalık M., (2019), Facilitating grade 11 students' conceptual understanding of fundamental acid-base models, Turkish J. Educ., 8(1), 16–32 DOI:10.19128/turje.449100.
- Yıldırım A. and Şimşek H., (2013), Qualitative research methods in social sciences, 9th edn, Ankara: Seckin Publishing.
- Zacharia Z. C., Olympiou G. and Papaevripidou M., (2008), Effects of experimenting with physical and virtual manipulatives on students' conceptual understanding in heat and temperature, J. Res. Sci. Teach., 45(9), 1021–1035.
Footnote |
| † An earlier version of this study was presented at the ESERA 2017, Dublin, Ireland. |
| This journal is © The Royal Society of Chemistry 2021 |