University instructors’ knowledge for teaching organic chemistry mechanisms
Eleni K.
Zotos
 ,
Jordan J.
Tyo
and
Ginger V.
Shultz
,
Jordan J.
Tyo
and
Ginger V.
Shultz
 *
*
Department of Chemistry, University of Michigan, Ann Arbor, Michigan 48109, USA. E-mail: gshultz@umich.edu
First published on 26th April 2021
Abstract
Many recent studies document the difficulties that students experience when learning organic chemistry, often due to the complex visualization and reasoning skills required to successfully understand the ways molecules interact in specific environments. Many of these studies call on instructors to improve their teaching strategies to support students’ learning of organic chemistry mechanisms, but few have focused on instructors’ knowledge of organic chemistry and how they use their knowledge to teach this topic. To investigate university instructors’ knowledge for teaching organic chemistry mechanisms, we utilized a task-based think-aloud interview protocol where graduate teaching assistants (GTAs) and faculty instructors assessed authentic undergraduate student responses to three organic chemistry mechanism questions. We describe this knowledge for a substitution, an acid–base, and an addition reaction. For all mechanisms, we describe how GTA participants’ knowledge for teaching related to their content knowledge. This result revealed differences between GTA and faculty participants’ knowledge for teaching mechanisms that were specific to features of each mechanistic task. For example, in a substitution reaction question, all faculty participants recognized and explained issues with a student's drawing of a transition state and apparent understanding of partial bonds. These features of the student's drawing were not recognized by any GTA participants, who focused instead on the student's prior knowledge about ionic bonding. These findings qualitatively illuminate strengths and weaknesses in graduate students’ knowledge for teaching which can guide how they are supported as instructors.
Introduction
Organic chemistry courses are some of the most difficult introductory chemistry courses for undergraduate students. To understand how to support student learning in organic chemistry, many researchers have focused on the ways students work through organic chemistry content (Graulich, 2015). Studies focused on representational competence have found that novice learners struggle to make the necessary connections between representations and their underlying meaning—e.g., identifying a molecular structure based on its formula (Bodner and Domin, 2000; Kozma, 2003; Anderson and Bodner, 2008). This struggle often impacts students’ abilities to mechanistically reason through a chemical transformation because students need to more deeply interpret many symbolic representations to do so. One of the most ubiquitous representations used in a chemical transformation is the curved arrow formalism, which undergraduate and graduate students reportedly struggle to fully understand (Bhattacharyya and Bodner, 2005; Ferguson and Bodner, 2008; Grove et al., 2012), with the exception of some students enrolled in a patterns-of-mechanisms organic chemistry curriculum and flipped course format (Webber and Flynn, 2018; Galloway et al., 2019).While many studies have focused on undergraduate student and graduate student learning of organic chemistry, the same attention has not yet been paid to the instructors of these courses. At large research institutions, graduate students are hired as graduate teaching assistants (GTAs) to teach the lab and discussion classes that accompany large introductory chemistry courses. Through these teaching assistantships, GTAs often spend more instructional time with undergraduate students than professors (Bond-Robinson and Rodriques, 2006). At these types of institutions, it is often assumed that graduate students enter graduate school with sufficient content knowledge from their undergraduate studies and that content knowledge alone is sufficient for adequate teaching. However, it has been shown that some graduate students lack fundamental chemistry knowledge (Bhattacharyya and Bodner, 2005, 2014) and, while content knowledge is a prerequisite to the development of teaching knowledge, high levels of content knowledge do not guarantee high levels of teaching knowledge (Shulman, 1986; Grossman, 1990; Connor and Shultz, 2018; Lutter et al., 2019).
The goal of this study was to investigate GTAs’ knowledge for teaching organic chemistry mechanisms to (1) better understand how GTAs address the challenges and alternative conceptions that their students are known to face and (2) inform training designs to better support GTAs in their instructor role.
Background
Learning to reason mechanistically
Introductory organic chemistry courses are required for many different undergraduate majors, and in these courses, students learn many foundational chemistry concepts and problem-solving techniques. One of the most challenging tasks in the organic chemistry curriculum is using chemical knowledge to propose mechanistic transformations correctly. While a consensus definition of mechanistic reasoning has not yet been achieved, practitioners and researchers have worked toward a common definition (Bhattacharyya, 2013). For the context of this study, our definition is similar to Watts et al. (2020), drawn from Bhattacharyya (2013). Students with successful mechanistic reasoning skills are able to describe what changes in a reaction, how the changes happen, and why the changes happen. This skill involves interpreting symbolic representations of molecules to understand underlying behaviors (e.g., which molecules act as acids or bases, possible resonance structures, partial or formal charges), the role of the molecules included (e.g., reagents, solvents, catalysts), and how each of these entities interacts with the others to produce appropriate products. Reasoning through how entities interact involves considering the logical movement of atoms and electrons (represented by curved arrows) and the formation of intermediate structures and products. Note that this definition does not include a connection to the energetics of reactions, which is a requirement of causal mechanistic reasoning (Caspari et al., 2018). To master the ability to propose mechanistic transformations, students must first develop the necessary chemical knowledge (e.g., understanding valence shells, electronegativity, bond polarity, etc.). As they learn to draw on this knowledge, they must also learn to interpret representations used in organic chemistry (e.g., reaction arrows, resonance contributors, etc.) to create a mental representation and make meaning of the transformation of interest (Bhattacharyya, 2013). This is no small task but results in students strengthening their problem-solving skills (Bodner and Domin, 2000).Many researchers have focused on investigating how students undertake the pieces of this task (i.e., interpreting representations) and the task as a whole (i.e., completing a mechanism) (Graulich, 2015). Symbolic representations like elemental symbols, curved arrows to indicate electron flow, and plus and minus symbols to indicate charge are fundamental components of communicating chemistry. To understand how novices (undergraduate students) and experts (practicing chemists) differ in their understanding and use of chemical representations, we turn to research on representational competence (Kozma and Russell, 1997, 2005; Kozma, 2000). Kozma and Russell (2005) found that undergraduate students struggle to utilize multiple representations, so their understanding and communication of chemistry are hindered as they rely primarily on surface features of representations, findings that are consistent with organic chemistry-specific studies (Bhattacharyya and Bodner, 2005; Anderson and Bodner, 2008; Grove et al., 2012; Bode and Flynn, 2016; Weinrich and Talanquer, 2016). On the other hand, practicing chemists can utilize multiple representations, swiftly moving within and across them, and can communicate chemistry using symbols to represent underlying processes and entities (Kozma and Russell, 1997; Kozma et al., 2000). Developing representational competence is key for success in chemistry, as students’ problem-solving abilities are hindered if they cannot translate between different representations (e.g., drawing a molecule based on a structural formula) (Bodner and Domin, 2000). Furthermore, in a case study of seven undergraduate students enrolled in organic chemistry, Anderson and Bodner (2008) described one student who struggled to attribute useful meaning to chemical symbols. He was a successful chemistry student until it was necessary for him to consider the underlying meaning of the symbols used to represent mechanistic transformations, further pointing to the conclusion that representational competence is key for success in organic chemistry.
As Anderson and Bodner (2008) indicate, reasoning through a mechanism is incredibly difficult without understanding what chemical symbols represent. Research focused on undergraduate students’ ability to reason mechanistically through a chemical transformation overwhelmingly indicated that students could complete mechanisms and use the arrow-pushing formalism without understanding what the curved arrow symbols represent (Bhattacharyya and Bodner, 2005; Ferguson and Bodner, 2008; Bhattacharyya, 2014; Galloway et al., 2017). Students often rely on memorization; they categorize mechanism types in their minds and follow the patterns blindly. This tendency allows students to correctly reproduce mechanisms without understanding underlying causes (Kraft et al., 2010; Grove and Bretz, 2012). When students approached reactions unfamiliar to them, they sometimes proposed mechanistic steps that were productive but chemically inaccurate (Bhattacharyya and Bodner, 2005; Ferguson and Bodner, 2008; Caspari et al., 2018). When students considered underlying properties, they often focused on one feature (e.g., charges or resonance) to explain chemical behavior (Bhattacharyya, 2014; Galloway et al., 2017).
While many studies have demonstrated students’ mechanical and meaningless use of mechanistic arrows, there are few cases were students demonstrated an understanding of what mechanistic arrows represent (Webber and Flynn, 2018; Galloway et al., 2019; Watts et al., 2020). Students enrolled in a patterns-of-mechanisms organic chemistry curriculum with a flipped course format, where the curriculum specifically addresses the symbolism of mechanistic arrows, attributed meaning to mechanistic arrows when solving familiar and unfamiliar mechanisms (Webber and Flynn, 2018; Galloway et al., 2019). Results from these studies indicated that the modified curriculum in which students learn about the underlying meaning of mechanistic arrows positively impacts their understanding. In another study by Watts et al. (2020), students enrolled in a more traditional curriculum were asked to specifically describe steps of a mechanism in writing. Of these students, 85% explicitly described the movement of electrons in a given mechanism (Watts et al., 2020). Webber and Flynn (2018), Galloway et al., (2019), and Watts et al. (2020) all demonstrate that undergraduate students are capable of attributing meaning to mechanistic arrows but need specific instruction to do so.
In a study by Bode and Flynn (2016), 700 student responses to mechanism exam questions were analyzed to determine problem-solving strategies commonly used by successful and unsuccessful students. They identified six strategies that were common in successful students’ problem-solving strategies, but rare among unsuccessful students: identifying new bonds in the target molecule, identifying which atoms are added to the starting material, identifying key regiochemical relationships, mapping starting material atoms to the product, using a partial or complete retrosynthetic analysis, and drawing reaction mechanisms. The degree to which instructors influence students’ use of these strategies has not been studied. Bode and Flynn (2016) suggest that research on the instruction of organic chemistry mechanisms is needed to understand another dimension of students’ understanding of this topic.
Instructors’ knowledge for teaching
While researchers have not yet investigated GTAs’ knowledge for teaching organic chemistry mechanisms, others have investigated various facets of GTAs’ experiences. In a qualitative study focused on the teaching knowledge and identities of chemistry GTAs, GTAs reported very few opportunities to develop as instructors. They were often discouraged from doing so (Zotos et al., 2020). This discouragement, along with the pressure to conduct good research, caused GTAs to identify as tutors or lab managers, which inhibited self-investment in their teaching role. Although there is an assumption that graduate students have the content knowledge necessary to teach introductory chemistry courses, GTAs reported struggling with the content in this study (Zotos et al., 2020). Other studies have also documented the specific ways GTAs struggled with certain content (Bhattacharyya and Bodner, 2005; Kraft et al., 2010). Studies of GTAs’ pedagogical content knowledge (PCK) relevant to organic chemistry, specifically of thin layer chromatography (Hale et al., 2016) and 1H NMR spectroscopy (Connor and Shultz, 2018), demonstrated that GTAs had high levels of content knowledge. Yet, GTAs exhibited generally lower levels of PCK, further indicating that even with appropriate content knowledge, instructors need spaces to develop their PCK (Bond-Robinson, 2005).In a study comparing GTAs’ and high school teachers’ content knowledge and TS-PCK of high school organic chemistry content, GTAs demonstrated higher levels of content knowledge and lower (but not significantly lower) levels of TS-PCK than high school teachers (Rollnick et al., 2017). These results suggest that GTAs can develop TS-PCK even with their relatively limited teaching experience. Further, this study underscores the importance of studying the development of knowledge for teaching among different types of instructors and contexts. Given that many studies have demonstrated how students grapple with organic chemistry mechanisms (Graulich, 2015), that GTAs often spend more instructional time with undergraduate students than professors (Bond-Robinson and Rodriques, 2006), and that GTAs themselves struggle with organic chemistry mechanisms (Bhattacharyya and Bodner, 2005), we sought to characterize GTAs’ PCK of organic chemistry mechanisms.
Theoretical perspective
Since its conception by Shulman in 1986, PCK has gained traction in education research communities across the disciplines (Shulman, 1986, 1987). PCK refers to the special knowledge that teachers possess at the intersection of content knowledge and pedagogical knowledge. With strong PCK, teachers can transform content in a way that enhances student learning of the particular content. In the years following Shulman's introduction of PCK, many conceptions of PCK were published, with some of them being discipline-specific (Grossman, 1990; Magnusson et al., 1999; Park and Oliver, 2008; Mavhunga and Rollnick, 2013; Chan and Hume, 2019).The number of different frameworks has prompted science PCK summits, the most recent in the year 2016 (Hume et al., 2019). This summit resulted in the reformed consensus model of PCK, which detailed several realms of PCK and the interactions between them (Hume et al., 2019). Most central is “enacted PCK,” which refers to the actions teachers take to plan, teach, and reflect on their teaching. This domain directly interacts with “personal PCK,” the domain that includes teachers’ own pedagogical content knowledge. Teachers draw upon their personal PCK to inform their enacted PCK. Both personal and enacted PCK are situated within teachers’ individual contexts, implying that these two realms of PCK are influenced by the teachers’ experiences, the classroom environment in which they teach, and the students that make up their class. The third PCK realm is “collective PCK,” which includes PCK constructed by many science educators and researchers. It is defined by Hume et al. (2019) as “a specialized knowledge base for science teaching that has been articulated and is shared among a group of professionals, which is related to teaching that particular subject matter knowledge to particular students in a particular learning context” (Hume et al., 2019, p. 88). It is within this realm that Shulman's original conception of PCK belongs (Shulman, 1986, 1987). The study presented herein, in which we investigate the PCK of individual university instructors, is situated within the realm of personal PCK.
Because we are investigating PCK of a particular topic—organic chemistry mechanisms—we used the topic-specific PCK (TS-PCK) framework defined by Mavhunga and Rollnick (2013) to inform our data collection and analysis. This framework acknowledges that an instructor's PCK within a discipline (e.g., chemistry) can vary depending on the topic (e.g., stoichiometry versus reaction mechanisms). Studies have reported the topic-specific nature of PCK (Aydin et al., 2014), and this framework has been used to analyze chemistry instructors' PCK for many different topics like chemical equilibrium, thin-layer chromatography, and 1H NMR spectroscopy (Mavhunga and Rollnick, 2013; Hale et al., 2016; Rollnick, 2017; Connor and Shultz, 2018).
This framework for TS-PCK includes five components: knowledge of (1) learner's prior knowledge, (2) what makes a topic difficult to learn, (3) representations, (4) curricular saliency, and (5) conceptual teaching strategies (Mavhunga and Rollnick, 2013). Learner's prior knowledge refers to the knowledge students have or do not have that is relevant to learning a new concept, including alternative conceptions about relevant content. Knowledge of what makes a topic difficult includes the ability to identify specific concepts that are difficult for students to understand. Knowledge of representations refers to instructors’ knowledge for representing content, including examples, illustrations, demonstrations, etc. and the benefits and limitations of those representations. Curricular saliency refers to the ability to arrange various concepts within a curriculum and to understand which concepts are most central and should be covered in-depth and which concepts are more peripheral. Knowledge of conceptual teaching strategies includes instructors’ knowledge of instructional strategies to address particular alternative conceptions or areas of difficulty and teach important concepts (Mavhunga and Rollnick, 2013). Using this framework, we have defined the following research question to guide our study in investigating graduate students’ TS-PCK of organic chemistry mechanisms: What is the nature of chemistry graduate teaching assistants’ knowledge for teaching organic chemistry mechanisms?
Methods
Context
This project was conducted at a large, public, research-intensive university in the Midwestern United States. GTA participants were recruited from a first-semester organic chemistry course. This course consists of a one-hour lecture three days per week and an optional one-hour discussion section one day per week. Lectures are taught by professors, lecturers, or post-doctoral fellows and have hundreds of students, while discussions are taught by graduate students and have 20–30 students. An in-house textbook accompanies course lectures, which follows a fairly standard curriculum, as seen in other studies (Houseknecht, 2010). Assessment is based solely on scores from three midterm exams and one final exam. Students primarily use a workbook containing sample problems from previous years’ exams to study and become accustomed to the exam format. Most students in the course are first- or second-year students. Few students in the course are chemistry majors; the majority of students are pre-medicine.Graduate students are hired as teaching assistants for this course, which serves as a source of funding via a graduate teaching assistantship. Teaching assistants receive a two-day teacher training during summer orientation before obtaining their specific teaching assignments. Weekly staff meetings are held throughout each semester, during which the instructors and GTAs discuss the topics for the week, anticipate student questions, and discuss the logistics of the course. The instructors may provide guidelines and suggestions to support GTAs in their discussion sections, but the structure of each discussion section is ultimately determined by the GTA teaching that section.
Participants
We interviewed 17 graduate students with a range of teaching experience. In the fall of 2018, incoming graduate students were emailed to participate in this study. Because content knowledge is a prerequisite for PCK (Shulman, 1987; Mavhunga and Rollnick, 2013; Connor and Shultz, 2018), we specifically invited graduate students who intended to join organic chemistry research laboratories with the assumption that they are more likely to possess a foundation of organic chemistry content knowledge than their peers in other subdisciplines (e.g., inorganic chemistry). At this time, we interviewed seven graduate students to capture TS-PCK of novice university instructors. To capture TS-PCK from a more experienced population, we invited graduate students who had taught organic chemistry discussions to participate. All experienced GTAs had held a teaching assistantship position for at least two semesters, though most experienced GTA participants had been teaching for three or more semesters. Four graduate students agreed to participate. After a preliminary analysis of the first eleven interviews, we determined that we had not reached saturation. Three additional experienced graduate students were interviewed in April of 2019, and three additional incoming graduate students were interviewed in September of 2019. Saturation—no new themes appeared in preliminary analysis—was reached with this set of 17 interviews. Seven of ten incoming GTA participants and six of seven experienced GTA participants had prior teaching experience during their undergraduate studies.We also interviewed three faculty members who had taught the organic chemistry lecture course to capture an upper-bound of university instructors’ PCK of organic chemistry mechanisms. One faculty participant had taught the organic chemistry lecture course five times, and two faculty participants had taught the course over twenty times.
Interview protocol
The think-aloud interview protocol used in this study was developed to elicit our participants’ TS-PCK of organic chemistry mechanisms. As such, we initially chose three organic chemistry mechanism questions that covered a range of reaction types—substitution, acid–base, and addition. For each question, participants filled out a blank exam question themselves, compared their answer to an answer key (see Appendix 2), and then assessed an authentic undergraduate student response pulled from a past exam to the same question (Fig. 1, 3, and 5). Participants were asked to think-aloud as they completed each task to capture their thought processes (Ericsson and Simon, 1980). When participants were presented with the authentic undergraduate student response, they were asked to give their overall impression and to respond to specific questions:1. Was this student correct? Why or why not?
2. What makes this problem difficult?
3. What thinking on the part of the student may have led to this response?
4. How would you respond if one of your students showed this answer to you?
The questions in our interview protocol specifically probed participants’ knowledge of what makes the problem difficult (question 2), knowledge of learners’ prior knowledge (question 3), and knowledge of teaching strategies (question 4). Although knowledge of representations and curricular saliency are not specifically probed by our interview protocol, the components of TS-PCK are interconnected (Park and Chen, 2012); it is often impossible to isolate only one component and eliminate the potential to elicit others. So, while the interview protocol specifically elicits participants’ TS-PCK of what makes the topic difficult, learners’ prior knowledge, and teaching strategies, we were also able to elicit and identify the remaining components of TS-PCK in some interviews. Moreover, by assessing student responses, participants offered approximations of some of the cognitive work involved in their teaching. Through these approximations, we are better able to understand TS-PCK as a whole, rather than solely as its individual components (Howell et al., 2017). The authentic undergraduate student responses were chosen to demonstrate what we know students struggle with based on previous research focused on student learning of organic chemistry (Bhattacharyya, 2006; Kozma and Russell, 2005; Ferguson and Bodner, 2008; McClary and Talanquer, 2011a, 2011b; McClary and Bretz, 2012; Bhattacharyya, 2014). More detail regarding these student responses is included in the results section.
Pilot interviews were conducted with an incoming graduate student, a graduate student who taught organic chemistry discussion, and a postdoctoral fellow with a PhD in organic chemistry who taught organic chemistry lecture. The pilot interviews were conducted to ascertain that the chosen mechanism questions were representative of the content taught in introductory organic chemistry and that the student responses to the mechanism questions were representative of student responses in the course. After the pilot interviews, one question was removed because all pilot interview participants noted that its content was not commonly discussed in introductory organic chemistry. It was replaced with another question that was more representative of topics covered in the course (an acid–base question).
For each interview, audiovisual data was recorded, and all written work was collected at the end of the interview. The audio data were transcribed verbatim using a secure online service and served as our primary sources of data. Visual recordings were used as a supplementary source of data for when participants used vague language. For example, if a participant said, “this atom would be the most basic,” we would use the visual recordings to identify the atom to which they were referring. Participants’ written work was scanned, stored digitally, and was used in a similar manner as the visual recordings. IRB approval was obtained for this study, and all participants gave informed consent to participate. All data were anonymized, and participants were given pseudonyms.
Data analysis
Analysis of the audiovisual interview data was approached with a theoretical grounding in the TS-PCK framework (Mavhunga and Rollnick, 2013). All transcripts were qualitatively analyzed using the NVivo 11 Pro software for participants’ TS-PCK of organic chemistry mechanisms through provisional coding methods (Saldaña, 2016), where data were coded using predetermined codes. Namely, we coded for the components of TS-PCK described above, including learner's prior knowledge, what makes a topic difficult, curricular saliency, representations, and conceptual teaching strategies. Transcripts were coded at the paragraph-level, and one paragraph could receive multiple codes if warranted. The first author coded a subset of interviews and slightly adjusted the codebook. More specifically, we noted that most participants’ responses to the question, “How would you respond if one of your students showed this answer to you?” did not elicit conceptual teaching strategies that aligned the Mavhunga and Rollnick (2013) definition: knowledge of instructional strategies to address particular alternative conceptions or areas of difficulty and to teach important concepts. Many responses, for example, followed a knowledge-telling format in which the participant described the content they would tell the student to correct their answer. Rather than leaving these responses uncoded, we adjusted this code to be named “teaching strategies,” defined as any method of supporting the undergraduate student in correcting their response. Our codebook is included in Appendix 1.The first and second authors met to discuss the final codebook, and then independently coded 20% of the data. The first two authors then compared their codes, discussed any discrepancies, and used differences to refine the codebook. The first two authors then independently coded an additional subset of 20% of the data. Inter-rater reliability (IRR) was calculated with this subset of data to determine the degree to which the codebook could be consistently applied to our data set. IRR was determined using a modified Cohen's kappa, the Fuzzy kappa statistic, which allows for more than one code to be applied to a single unit of analysis (Kirilenko and Stepchenkova, 2016). Our interrater reliability value (0.84, Fuzzy kappa) indicated a consistent implementation of our coding scheme (McHugh, 2012). The first and second authors then coded each of the remaining interviews.
Coded excerpts underwent a second round of analysis during which the TS-PCK for each subset of participants (incoming GTAs, experienced GTAs, and faculty) was summarized for each reaction question. Common elements of TS-PCK were identified across all participants and within each subset of participants.
Limitations
There are several important limitations to discuss regarding our participant population and our interview protocol. First, all participants were from one university, limiting the transferability of our results to other contexts. Graduate students and professors volunteered to participate in this study, which introduces the possibility of self-selection bias. Furthermore, the desire to include authentic student responses to organic chemistry mechanism questions in our interview protocol limited the pool of organic mechanism questions to those used as exam questions at this particular university. All chosen questions were from the first-semester organic chemistry course. While instructors at this university are familiar with the question format, it may not reflect question formats used at other universities. The interview format—focused on exam questions—may have unintentionally elicited specific components of TS-PCK more so than others. More specifically, exams are meant to be challenging and to measure students’ knowledge. Thus, focusing on exams during interviews may have prompted participants to focus more on their knowledge of what makes a topic difficult and learner's prior knowledge than on representations, curricular saliency, or teaching strategies. Because all components of PCK are interconnected (Park and Chen, 2012), we were able to elicit all components of TS-PCK with the protocol described above. However, additional research focused on GTAs’ knowledge of representations, curricular saliency, and teaching strategies is needed to describe these components in a more complete manner. Additionally, asking participants to describe what made the problem difficult after showing the undergraduate student responses may have influenced participants to focus specifically on what challenged the particular student. Should this study be repeated, we recommend asking this question prior to showing the undergraduate response, and about the reaction type in general. Finally, the use of interviews provided insight into participants’ conceptions and knowledge for teaching but did not provide direct evidence for the practices that teachers use in their classroom (Chan and Hume, 2019). Further research through observations is needed to investigate the actions GTAs take as they teach organic chemistry discussion courses.Trustworthiness
While the generalizability of our results may be limited by our participant population and the specific organic chemistry mechanism questions used in the interview protocol, we strove to maintain the trustworthiness of our results through multiple facets (Lincoln and Guba, 1985). First, we have provided a detailed description of our participants, interview protocol, and analysis methods. Second, at the end of each interview with faculty members, we asked (1) if the mechanism questions presented were typical for their course and (2) if the undergraduate student responses were representative of how they would expect their students to respond. All faculty participants voiced that the substitution and addition reactions aligned well with the types of questions they ask on their written exams in their classes. The acid–base reaction, however, was not met with as much agreement—faculty noted that it was a fair question, but they did not like it since the reaction is unfavorable (this is further described in the results section). Faculty overwhelmingly agreed that the student responses for all three questions were representative of the most common mistakes made by their students.After all the interviews were conducted and all the data were analyzed, we presented our results describing GTAs’ TS-PCK to two faculty participants. We intended to evaluate the degree to which our results were consistent with their experiences as organic chemistry instructors who often teach alongside chemistry GTAs. This check was completed through a questionnaire using the Qualtrics software. For each mechanism question, the faculty members were presented with the undergraduate student response to the question. They were given space to record how they would expect an organic chemistry GTA to respond to this undergraduate student. They were presented with our results describing the organic chemistry content knowledge and TS-PCK for our GTA participants. They were then asked to comment on whether the results were surprising to them and if they would expect GTAs to say anything else to this student. The outcomes and perspectives gained from this process are reported in the following sections.
Results and discussion
Question 1: substitution reaction
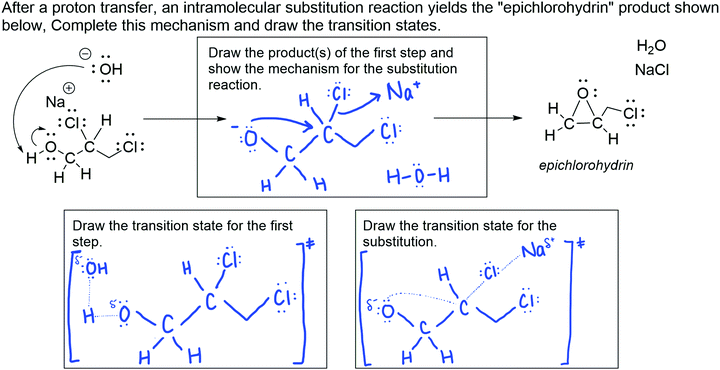
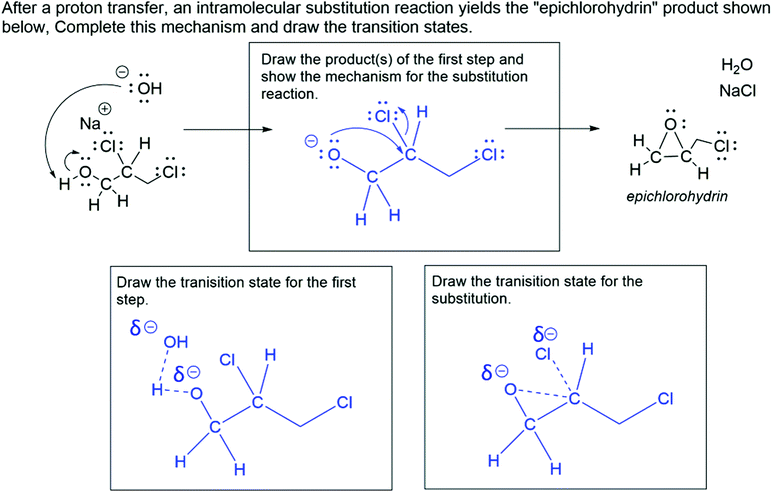
The substitution reaction question (see Fig. 1) prompted students to draw the product of a proton transfer reaction given the starting material and curved arrow mechanism of the proton transfer, then to draw the curved arrow mechanism for the substitution reaction that yields the given product. The exam question also prompted students to draw the transition state for each step. The answer key is provided in Appendix 2, Fig. 7. All participants except for one incoming GTA answered this question correctly. A few incoming GTA participants were unfamiliar with drawing transition states but were ultimately able to answer the question correctly. Both of the faculty members who reviewed our results were unsurprised that some GTAs struggled with drawing the transition states because, as one faculty member said, they “likely have to dust off the rust of old knowledge.”The undergraduate student response selected for the substitution reaction question (Fig. 1) represents students with a misunderstanding of the nature of ionic bonds or how to represent their formation. In the first box, the student correctly drew the product of the first proton transfer step. When drawing the curved arrow mechanism for the intramolecular substitution reaction, the student incorrectly drew a curved arrow from the carbon–chlorine bond of the intermediate to the sodium ion, indicating a covalent bond forming between sodium and chlorine. The student response also had errors in their transition states. In both transition states, the student represented the electrons that form bonds twice, both as a partial bond and as a lone pair. In the second transition state, the student showed a partial bond between the chlorine and sodium, a partial-positive charge on the sodium, and no charge on the chlorine. From inspecting this undergraduate student's response, it is possible that this student understands that sodium chloride is an ionic compound but holds an alternative conception about the curved arrow mechanism and what it represents, and/or that this student understands the curved arrow mechanism but does not recognize the ionic nature of the sodium chloride bond.
Alternatively, four GTA participants (three incoming GTAs and one experienced GTA) thought that the student knew that sodium chloride is ionic and tried to use curved arrows to indicate the formation of an ionic bond. For example, an incoming GTA stated, “In my perspective, they probably had that knowledge that these form an ionic bond, and they'll come together. So, this is then trying to show that this chlorine is about to go to that sodium.” This notion is consistent with previous studies of undergraduate students’ use and understanding of the curved arrow mechanism, which suggests that students draw curved arrows with little to no meaning associated with their use (Ferguson and Bodner, 2008), however, it is concerning that only four participants considered this, all of whom were GTAs.
Interestingly, all three faculty participants, but none of the GTAs, noticed a specific feature of this student's drawn transition states that indicated another alternative conception. In many instances, the student overcounted the electrons around a specific atom. Faculty participants noticed that when the student drew transition states, they sometimes drew too many electrons on atoms that had partial bonds. For example, in the transition state for the first step of this reaction, the student drew three sets of lone pair electrons around the hydroxide oxygen and drew the partial bond between that oxygen and the hydrogen on the other molecule. One faculty participant said:
It looks like they double counted the electrons. […] That would make me think that they don't quite understand what this partial bond is indicating. They're showing that it means a bond is forming or a bond is breaking, but they don't know what those dots are corresponding to, other than that there's going to be a bond there, not that those might be electrons, which is the way I usually teach it.
Our faculty participants noted that this indicates that the undergraduate student lacks an understanding of partial bonds and the electrons that form those bonds. They specifically interpreted this to mean that though the student knew that partial bonds indicate a bond breaking or forming, they did not understand how partial bonds relate to electrons.
One of the faculty members that reviewed our results specifically noted that they were surprised that GTAs did not notice this as well. Another stated that partial bonds are an extension of the curved arrow formalism; curved arrows show the movement of electrons to break or form bonds, and partial bonds drawn in a transition state demonstrate a snapshot of those bonds breaking or forming. As such, it was not surprising that GTAs did not notice this misrepresentation in the undergraduate student's work, given that graduate students have limited conceptions of the curved arrow mechanism as well (Bhattacharyya and Bodner, 2005). The limitation described here in GTAs’ ability to recognize potential gaps in students’ prior knowledge could be a result of the limitations in some of the GTAs’ content knowledge, though for others it is the result of limitations in PCK.
Only two participants (one incoming GTA and one experienced GTA) did not provide a teaching strategy for this question. One of them did not recognize the error in the undergraduate student response and thus did not provide a teaching strategy. Thirteen participants presented knowledge-telling teaching strategies (nine incoming GTAs and four experienced GTAs), in which they described the knowledge that they would tell the student to help them without asking the student questions or inquiring about their thinking. For example, when asked how they would respond to this student, an incoming GTA said, “I would say that your indication that the covalent bond is formed between chlorine and sodium is inaccurate… Otherwise, it's correct. You should just draw the chloride being a free ion, and sodium being a free ion.” Two experienced GTA participants discussed an activity they would have their students complete to guide them to the correct answer. For instance, one experienced GTA said they would point to the sodium ion and ask the student, “What do you know about this ion? What can you tell me about the sort of bonds that it likes to make or not thereof, and hopefully guide them to getting the correct answer themselves.” As demonstrated in Fig. 2, incoming GTAs primarily provided knowledge-telling teaching strategies, with just one incoming GTA not reporting a teaching strategy. Experienced GTAs were distributed across the three groups, with over half in the knowledge-telling group, two in the activity-based group, and one with no teaching strategy. All three faculty provided a teaching strategy involving an activity. One faculty pointed at the sodium chloride product and said,
I'd go right to there and have the student explore the business of an ionic compound and so, if there's a bond there, what is the nature of the bond? And then push back onto [the incorrect curved arrow] to say, what is that arrow telling me? Where is that pair of electrons in the structure of the ionic compound?
Studies have reported that developing knowledge of conceptual teaching strategies is the most difficult component of PCK as it requires knowledge from the other components of PCK (De Jong et al., 2005). Furthermore, studies have shown that PCK develops with experience (Lederman and Gess-Newsome, 1999; Davis and Krajcik, 2005; Hale et al., 2016). Our results echo these sentiments, as the majority of our GTA participants provided a knowledge-telling teaching strategy, and our participants with more experience show evidence of having sophisticated knowledge of teaching strategies.
Knowledge-telling teaching strategies alone are rarely effective at promoting students’ conceptual understanding of a topic (Bodner, 1986). The undergraduate student response to the substitution reaction question provides a valuable example of the importance of drawing out students’ knowledge through an activity-based teaching strategy. As noted above, the student's alternative conception leading to their response could be that sodium chloride is a covalent compound, that curved arrows are used to show the formation of ionic bonds, or some other alternative conception that was not discussed by our participants. If an instructor assumes this student thinks sodium chloride is a covalent compound and provides a knowledge-telling teaching strategy to address this and help the student answer the question correctly, the instructor could be misidentifying the gap in the student's knowledge. Using an activity to elicit the student's prior knowledge would allow the instructor to better support this student in learning this fundamental chemistry content. Ideally, instructors should support students through an activity-based teaching strategy, but here, it is only the case for five of our twenty participants—three of whom were faculty members. Given GTAs’ role in undergraduate education, this indicates the importance of placing focus on the development of GTAs’ teaching strategies during instructor training.
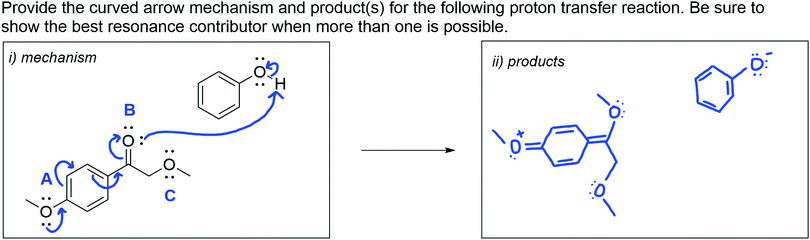
Question 2: acid–base reaction
The acid–base reaction question (see Fig. 3) provided starting materials and prompted students to draw the curved arrow mechanism and products for a proton transfer. The prompt also reminded students to show the best resonance contributor when more than one is possible. The answer key is provided in Appendix 2, Fig. 8.This problem was challenging for our participants. In general, the proton transfer reactions possible with the given starting materials are quite unfavorable, with the equilibrium lying to the left. Faculty noted that they did not like this question for that reason. Indeed, we urge readers not to use this task as an assessment in their own teaching contexts for the same reason. Nonetheless, including this question proved to be informative because participants needed to consider multiple effects on basicity to complete the mechanism, which is a major challenge for both undergraduate and graduate chemistry students (Bhattacharyya, 2006; McClary and Talanquer, 2011a, 2011b; McClary and Bretz, 2012; de Arellano and Towns, 2014). Furthermore, it is not uncommon for imperfectly written questions to arise in discussion sections, during which GTAs are expected to teach. For the acid–base question, three GTA participants chose the most likely oxygen atom (C) as the most basic atom. One of whom confidently stated:
I mean, [oxygen C is] the most basic one there out of all of those. So if you think about it, [oxygen A] is an sp 2 oxygen, [oxygen B] is sp 2 oxygen localizing pair, and [oxygen C is an] sp 3 oxygen that's not delocalized into anything, and so that's gonna have the highest basicity, so that's definitely gonna take the proton.
This GTA demonstrated how they thought through this mechanism by considering the hybridization of the three oxygen atoms. Very few GTA participants mentioned hybridization during the interview when responding to this question, which is consistent with previous studies on undergraduate students’ understanding of acid–base chemistry (McClary and Talanquer, 2011a, 2011b; McClary and Bretz, 2012). When reviewing the results for this part of the interview, one of the faculty members mentioned that this type of problem is difficult for undergraduate and graduate students because (1) acid–base chemistry is challenging in and of itself, and (2) there is a tendency to apply rules (e.g. resonance decreases pKa), which leads them to make quick assessments rather than using the tools provided (e.g., the pKa table).
Ten of our GTA participants (five incoming GTAs and five experienced GTAs) chose the carbonyl oxygen (B) as the most basic site and drew the products accordingly. Participants rationalized this response by drawing one of the resonance contributors of the base where a negative charge is present on the carbonyl oxygen (B). As one experienced GTA stated,
The most basic atom in this molecule is going to be one that might, at some point, carry a negative charge on it. So, by resonance, [oxygen B is] the only oxygen that can do that. […] Here, you usually think of these lone pairs as being pushed into the ring. So [oxygen A] has a net positive charge or delta positive at some point. So, it's also not very basic. And then kind of conversely, these electrons can be removed from the ring and delocalized to [oxygen B], so it has some negative charge on it. So, then it's the most basic.
This GTA described their reasoning for choosing the most basic atom in the molecule shown above, which was similar to the processes observed from students in other research studies where students relied on resonance and charges to identify acids and bases (Bhattacharyya, 2006; McClary and Talanquer, 2011a, 2011b; McClary and Bretz, 2012). Neither of the faculty members who reviewed our results were surprised that GTAs responded in this way and expected this response to be common among undergraduates as well.
The undergraduate student response chosen for this acid–base problem demonstrated similar reasoning as the ten GTA participants who chose oxygen B as the most basic site (Fig. 3). This response was representative of students who prioritize resonance when considering the acidity or basicity of molecules. The undergraduate student demonstrated their knowledge of resonance and used resonance as a rationalization for oxygen B being the most basic. They correctly chose the phenol proton as the most acidic proton and drew the corresponding products. Participants discussed different reasons why a student would respond this way, which demonstrated their TS-PCK for this problem.
I guess what the student here was thinking that if you take the electrons from the oxygen furthest away from the carbonyl, you could resonate it through the system, and you would be able to have a negatively charged oxygen that wants to be protonated and go, and then that nucleophilic [oxygen B] would be able to go and attack the hydrogen and remove it from the phenol.
As this and other participants note, the undergraduate student used their knowledge of resonance to complete the proton transfer between the two given molecules. While it is unclear whether the undergraduate student considered other effects (i.e., hybridization) when completing this mechanism, participants assumed that they did not. Using one parameter to reason through a mechanism has been seen in other research, and often leads to limited and incorrect problem solving in students (Bhattacharyya, 2014).
I think the difficulty in this question comes from remembering how to balance the difference between the effects of hybridization and localized versus delocalized electrons, and then kind of where thinking about partial charges could fit into all of that. […] They were more concerned with showing the partial negative charge on the oxygen, or at least that you could have a partial negative here to make it more basic, over considering hybridization of this oxygen here.
Again, participants identified one of the major difficulties of proposing mechanisms: considering many contributing variables (Kraft et al., 2010; Bhattacharyya, 2014).
In addition to balancing effects on basicity, seven GTA participants (four incoming GTAs and three experienced GTAs) and all three faculty participants noted that the question statement made this problem difficult. Namely, the part of the question that prompted students to consider resonance may have misled students. An incoming GTA stated, “Definitely I think what makes this response difficult is, ‘Show the best resonance contributor when more than one is possible.’” Participants recognized that this was misleading to students, as it encouraged considering resonance when completing a mechanism in which considering resonance leads to the incorrect products. One of the faculty members who reviewed our results noted that the problem statement likely had the strongest influence on the student's response, and “is more to blame than anything else.”
I would say look on your pK a table. And so, what you can see is, yes, for sure an oxygen, an sp 3 hybridized oxygen atom with lone pairs available definitely would be difficult to protonate. But it would be more difficult to protonate something that is a carbonyl oxygen, even an ester.
Two experienced graduate students also mentioned the same teaching strategy, but with less confidence:
I would definitely first review my own knowledge and make sure that I could explain it first. […] Once I really gathered a strong explanation, I would try to explain to them in terms of always using their pK a table, especially when they are given more than one option. Hopefully, they recognized that it probably could have been any of these lone pairs to reach out, and then if they weren't able to identify that, at least it would be an oxygen lone pair. Talk to them about how to read their pK a table about that.
In this quote from an experienced GTA—one of the eleven participants that initially responded incorrectly and chose the carbonyl oxygen (B) as the most basic oxygen—they explained that they would first review the content to make sure they understood it themselves before using the pKa table to explain the mechanism to a student. This experienced GTA demonstrated that content knowledge is a prerequisite for developing PCK, aligning with findings from prior studies (Shulman, 1987; Mavhunga and Rollnick, 2013; Hale et al., 2016; Connor and Shultz, 2018). This finding is emphasized even further in the cases where graduate students answered the question incorrectly and then did not provide a teaching strategy.
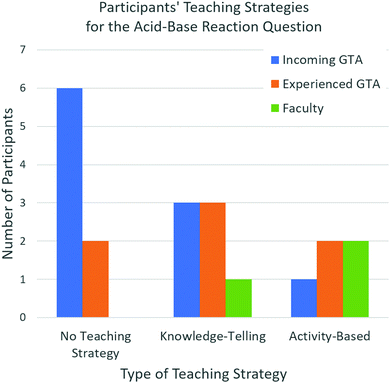
Through further inductive analysis, we identified that eight participants (six incoming GTAs and two experienced GTAs) did not provide a teaching strategy for the acid–base reaction question (Fig. 4). This number is likely much higher than for the substitution reaction question, where only two participants did not provide a teaching strategy, because this problem proved to be much more challenging. When asked how they would help this student, participants responded with, “hopefully, I’d be prepared and explain why that oxygen is not the one that attacks,” or “I honestly probably would have said that it's correct, unfortunately.” Six GTA participants (three incoming GTAs, three experienced GTAs) provided a knowledge-telling teaching strategy. For example, an experienced GTA stated, “I would have told them that the sp3 oxygen is probably more basic because it is less electronegative, so it's more likely to pull off that hydrogen.” Three GTA participants (one incoming GTA and two experienced GTAs) provided a teaching strategy that involved some sort of activity to help this student. One experienced GTA, for example, noted that they “would ask them to draw both resonance structures—or all the resonance structures for this, and […] based on that, give me some reasons as like, is there aromaticity effect?” Fig. 4 demonstrates the distribution of each teaching strategy type across our participant groups.
As demonstrated in Fig. 4, incoming graduate students tend toward the left portion of the graph, with over half of the incoming GTAs not providing a teaching strategy, one third providing a knowledge-telling teaching strategy, and one providing a teaching strategy involving an activity. Faculty members tend toward the right portion of the graph, with two providing a teaching strategy involving an activity, and one providing a knowledge-telling teaching strategy. Experienced GTAs are distributed over all three categories of the graph.
Question 3: addition reaction
The third exam question was focused on an acid-catalyzed addition reaction (see Fig. 5). Students were given the structures of the starting material, reagent, and product, and the catalyst was given as a molecular formula. Students were asked to provide the curved arrow mechanism for the transformation and were prompted to use appropriate acid/base choices, to show and use the best resonance contributor, and to draw a three-step mechanism. The answer key is provided in Appendix 2, Fig. 9.Overall, there was variety in the ways participants completed this mechanism. For example, in the first step of the mechanism in which a proton is added to the triple bond, four participants first showed the protonation of acetic acid at the carbonyl oxygen. They used this protonated acetic acid in the first step of the mechanism as the proton source. Two participants protonated acetic acid at the sp3 oxygen and used this protonated acetic acid in the first step of the mechanism as the proton source. Four participants used sulfuric acid in the first step of the mechanism, and two participants used a generic proton. Four participants used neutral acetic acid, and one participant did not respond at all.
The rest of the mechanism varied as well. The faculty members that reviewed our results were unsurprised by the variety in GTAs’ responses to this mechanism question. They noted that across undergraduate institutions, there is a variety in how the source of protons are taught, and in some cases, the proton source is unspecified. This sometimes occurs even at a single institution; when completing this mechanism, one faculty member gave an “Organic Chemistry I” answer and an “Organic Chemistry II” answer, where the structure of protonated acetic acid varied. Faculty participants noted that identifying how the catalyst is used is the most difficult part of this mechanism. Overall, more concepts need to be considered when completing this mechanism than in the previous two mechanisms.
In the undergraduate student response to this problem (Fig. 5), the student did not incorporate the sulfuric acid catalyst (pKa = −9) into their mechanism, but rather protonated the alkyne starting material with acetic acid (pKa = 4.8). Again, this is consistent with the findings of Kozma and Russell (2005) and others that have shown that students struggle to interpret different representations and often focus on surface-level features. In this particular problem, all molecules are represented in their structural form, except for the sulfuric acid catalyst. It follows that this student may not have recognized the role of sulfuric acid because surface-level features are not as evident in the molecular formula representation (Decocq and Bhattacharyya, 2019). It is also important to consider Bhattacharyya and Bodner's (2005) research in which students’ primary focus when completing a mechanism was to draw curved arrows that provided the correct product without always considering the feasibility of the chemical transformations. We see the same sort of method in Fig. 5, where the student drew curved arrows that lead to the product but did not consider the acidic conditions when drawing the curved arrows.
Additionally, five GTA participants (four incoming GTAs and one experienced GTA), along with two faculty participants explained that this student mapped the starting material to the product and tried to complete the mechanism in the most simple way to get to the product. For example, one experienced GTA stated,
I think this is the most direct mechanism you could think of drawing. It's just not accurate with the pH you're in. They see they know they have to protonate [the starting material]. They have an acid (acetic acid). […] So they never involve H 2 SO 4 ; maybe they thought it was just there to be confusing or something.
This GTA noted that while this was the most direct mechanism that could be drawn, it did not consider the conditions in which the mechanism occurs. Similarly, an incoming GTA explained,
He strangely drew an anion. We can see we are under an acid situation. There should not be an anion because any anion if it occurs, will be neutralized by the proton in the system. It is not allowed, and I don't know where he came up with this idea. Maybe he just cannot think of any way to lead to the product, so he just made up his own reagent. And yeah, this happens when you can't think of anything, you just make up your own.
This GTA assumed that the undergraduate student did not know how the product forms, so they made up their own path without making chemical sense. These sentiments reflect other studies in which students use the curved arrow formalism to “connect the dots” between the starting material and product without always making chemical sense (Bhattacharyya and Bodner, 2005).
I would take them back to thinking about how fundamentally why we have this happening so that they realize that this needs to be protonated and then they can, I think their understanding of at least their pushing… if they understood that and I gave them that protonated molecule and I would ask them to do this again and with that protonated species and see if they can get there.
One faculty member provided a knowledge-telling teaching strategy, and two provided an activity-based teaching strategy. One of the faculty members who provided an activity-based teaching strategy said,
I would probably start by reminding them about equilibria in acid–base reactions and have them try to draw it, like how much of the [deprotonated acetic acid] species would actually be present, to get them to take home the idea that you wouldn't have [deprotonated acetic acid] present in solution and you have lots of [acetic acid] but it's not the strongest acid, and you really need the strongest acid to do this. So, I'd probably review acid–base equilibria with them. Then I would ask them why they put the carbocation here. And if it was simply because of the product there, then I would remind them about resonance stabilization of cations. I'd probably simplify this example and give them a different one that was a little bit more straightforward to see if they understood what I just reviewed.
This faculty member described how they would review the content that is relevant to completing this type of mechanism question, then ask the student to complete a simplified version of this mechanism before jumping back into this mechanism question.
Participants did not demonstrate consistent knowledge of what makes this topic difficult, knowledge of representations, or curricular saliency for the addition reaction question. We suspect this is due to the specialized knowledge that is needed to understand this mechanism, and because PCK is tied to content knowledge, how an instructor conceptualizes this mechanism will influence their knowledge for teaching this mechanism. While there is often consistency in the ways mechanisms are taught in one class at one institution, there can, in fact, be different probable ways in which a mechanism proceeds and thus nuanced differences across institutions and even across class levels at a single institution. GTAs come from different undergraduate institutions, which, as we have described, can influence their content knowledge for specific topics and their TS-PCK as a result.
Conclusions and implications
Given the difficulty in proposing and explaining mechanistic transformations in organic chemistry experienced by both undergraduate and graduate students, and that little is known about university instructors’ knowledge for teaching this topic, we interviewed twenty university instructors of organic chemistry to gain insight into this knowledge. Participants thought aloud as they completed three mechanism questions chosen from introductory organic chemistry exams and assessed an authentic undergraduate student's response to each question. Through this interview, we elicited participants’ TS-PCK for teaching organic chemistry mechanisms.GTA participants’ knowledge of what makes the problem difficult for the substitution and acid–base reaction questions was often consistent with literature on student understanding of organic chemistry—this was a strength in GTAs’ TS-PCK. More specifically, GTAs noted that the representation of sodium chloride made the substitution question difficult (Kozma and Russell, 2005), and considering different effects on basicity to identify the most basic atom made the acid–base question difficult (Kraft et al., 2010; Bhattacharyya, 2014). It is possible that GTAs can identify the difficulties of organic mechanism questions because they currently do or recently have struggled with the same aspects themselves. Strengths in this component of PCK can be leveraged to help develop other components of PCK in GTA training—like teaching strategies to support struggling students (Charalambous et al., 2011; Connor and Shultz, 2018).
Prior studies have indicated that graduate students struggle with introductory chemistry content (Bhattacharyya and Bodner, 2005; Kraft et al., 2010; Zotos et al., 2020). In this study, we found that our GTA participants also exhibited weaknesses in their content knowledge of organic chemistry mechanisms, and we describe how this impacted their TS-PCK. In the substitution reaction question, the faculty participants noticed a limitation in the undergraduate student's understanding of the curved arrow formalism through an overcounting of electrons in the transition states. None of the GTAs noticed this mistake, and most GTAs assumed the undergraduate student completed the mechanism incorrectly because they thought sodium chloride was a covalent molecule. In an aforementioned study by Bhattacharyya and Bodner (2005), graduate students demonstrated a limited understanding of the curved arrow formalism. It is likely that our GTA participants also have a limited understanding of the curved arrow formalism. This weakness in content knowledge may have prevented our GTA participants from identifying the mistake in the student response (overcounting electrons in the transition states) that revealed a significant gap in the undergraduate student's knowledge. Identifying this mistake could in turn better influence GTAs’ teaching strategies.
Moreover, in the acid–base reaction question, many GTA participants made the same mistake as the undergraduate student. They relied on resonance to identify the basic site of a molecule when hybridization needed to be considered as well. Additionally, almost half of our participants did not provide a teaching strategy to support the undergraduate student. Many were unable to provide a teaching strategy to help the undergraduate student achieve the correct answer because they did not understand the correct answer themselves.
Finally, in the addition reaction, there was such a variety in the way participants completed the mechanism that we detected few patterns in their TS-PCK. GTAs come from different undergraduate institutions where there may be nuanced differences in how complex mechanisms are discussed, which can be responsible for the variety in responses that we saw from our GTA participants. Accordingly, TS-PCK was limited and inconsistent for this mechanism. Aside from knowledge of learner's prior knowledge, we could not identify any facets of our participants’ TS-PCK that was consistent across multiple participants.
To better understand these differences, research is needed to investigate how organic chemistry mechanisms are taught in various institutions and to identify how these methods of teaching are similar or different. Furthermore, it is important to investigate how practicing organic chemists reason through unknown mechanisms in their research. While we have many learning goals for students in organic chemistry courses, the goals should be informed by the ways mechanisms are used in practical situations.
The undergraduate student responses used in our interview protocol represented students with a limited understanding of the curved arrow mechanism and other symbols used in organic chemistry (Bhattacharyya and Bodner, 2005; Ferguson and Bodner, 2008; Caspari et al., 2018). The undergraduate students’ responses indicated that the student focused more on surface-level features than the underlying meaning of symbols (Kozma and Russell, 2005), which requires specific instruction to mediate (Kozma, 2003). Galloway et al. (2019) and Webber and Flynn (2018) demonstrated that in an organic chemistry course with a flipped course format and in which instructors precisely describe the use of the curved arrow mechanism, undergraduate students attributed meaning to the curved arrows when proposing both familiar and unfamiliar mechanisms. These findings indicate that when undergraduate students have opportunities to work through mechanisms with an instructor present to help guide them, they develop a conceptual understanding of the curved arrow mechanism.
The study presented here unveiled GTAs’ limited knowledge of teaching strategies. Many GTA participants offered knowledge-telling teaching strategies, and some did not offer a teaching strategy at all. GTAs who demonstrated high levels of content knowledge in another study by Rollnick et al. (2017) also offered limited teaching strategies by often just stating relevant content without considering students’ prior knowledge. GTAs’ deficiency in knowledge of teaching strategies is not entirely surprising since knowledge of conceptual teaching strategies is the most difficult component of PCK because it requires knowledge from the other components of PCK (De Jong et al., 2005). Without consistent responses, we cannot determine how GTAs might use their knowledge of learner's prior knowledge, representations, what makes the topic difficult, and curricular saliency to address students that these question responses represent—though GTAs are instructing students regularly in discussion and laboratory sections. Further research specifically investigating the knowledge of teaching strategies and the actual teaching strategies used in practice to address student difficulties with mechanisms is needed, as well as opportunities for GTAs to develop their knowledge of teaching strategies to best support students learning organic chemistry. One possible avenue to support GTAs’ development of their PCK is through structured observations of high-quality GTA instruction with subsequent discussions of the observed instruction (Ekiz-Kiran et al., 2021). Mavhunga and Rollnick (2013) and Rollnick (2017) demonstrated that content knowledge also improves as instructors engage with TS-PCK interventions.
While we found that GTAs hold both content knowledge and TS-PCK of organic chemistry mechanisms, the assumption that graduate students begin graduate school with sufficient content knowledge to teach introductory organic chemistry courses should be carefully considered when designing training. GTA training should connect to the knowledge GTAs bring while also supporting the development of content knowledge and PCK they may be lacking.
Conflicts of interest
There are no conflicts to declare.Appendix 1: codebook
| Code | Definition | Example from the substitution reaction question |
|---|---|---|
| Learner's prior knowledge | Any mention of knowledge students have or do not have that is relevant to learning a new concept. | “I think the student probably doesn't know that it's going to be an ionic force what's going to be holding them together.” – incoming GTA |
| Assumptions of student knowledge, thinking, or problem-solving strategies. | ||
| Common alternative conceptions about content. | ||
| What makes the topic difficult | The ability to identify gate-keeping concepts within a concept that are difficult to understand. | “The most difficult part would be that the way they've [the instructors] drawn the sodium chloride could be somewhat misleading to people who don't have a proper understanding of ionic bonds versus covalent bonds, which is something that CHEM 210 students often struggle with at the beginning.” – experienced GTA |
| Representations | Teachers’ knowledge of a range of subject matter representations, including examples, illustrations, analogies, simulations, and models used to teach content. | “One thing that I learned in teaching 210 is that the students constantly want to take their counterion and bind them to the molecule somehow. And so one thing that at least I tried to hit home to them was that if you see an ionic compound, keep it as an ionic compound and then you'll be able to better think about its ionic compoundness.” – experienced GTA |
| Knowledge of limitations of representations and how they might influence students’ reasoning. | ||
| Curricular saliency | Teachers’ knowledge of the learning of various topics relative to the curriculum as a whole. Teachers’ understanding of which topics are the most central and which are more peripheral. | “And maybe that's not reviewed a lot in my experience, always, in 210. So maybe by the time they got to the exam, they were not focused on that this would be an ionic bond.” – incoming GTA |
| Enables teachers to judge the depth to which a topic should be covered and hence the amount of time to spend on it. | ||
| Teaching strategy | Any method of supporting students in correcting or improving their thinking. | “I would ask them, I think because the only thing I think is actually wrong on here is the covalent bond of the sodium chloride that they're drawing with their arrows, is I would ask them what type of bond that is, and they would probably tell me it's ionic. And I would then ask them, why don't we draw an arrow to that, then? Or just to the chlorine?” – experienced GTA |
Appendix 2: answer keys to mechanism questions
 | ||
| Fig. 8 The answer key for the acid–base reaction question. This task includes a question that is flawed, and we caution readers not to use it in an actual assessment. | ||
References
- Anderson T. L., and Bodner G. M., (2008), What can we do about ‘Parker’? A case study of a good student who didn’t ‘get’ organic chemistry, Chem. Educ. Res. Pract., 9(2), 93–101.
- Aydin S., Friedrichsen P. M., Boz Y. and Hanuscin D. L., (2014), Examination of the topic-specific nature of pedagogical content knowledge in teaching electrochemical cells and nuclear reactions, Chem. Educ. Res. Pract., 15(4), 658–674.
- Bhattacharyya G., (2006), Practitioner development in organic chemistry: How graduate students conceptualize organic acids, Chem. Educ. Res. Pract., 7(4), 240–247.
- Bhattacharyya G., (2013), From source to sink: Mechanistic reasoning using the electron-pushing formalism, J. Chem. Educ., 90(10), 1282–1289.
- Bhattacharyya G., (2014), Trials and tribulations: Student approaches and difficulties with proposing mechanisms using the electron-pushing formalism, Chem. Educ. Res. Pract., 15(4), 594–609.
- Bhattacharyya G. and Bodner G. M., (2005), “It gets me to the product”: How students propose organic mechanisms, J. Chem. Educ., 82(9), 1402–1407.
- Bhattacharyya G. and Bodner G. M., (2014), Culturing reality: How organic chemistry graduate students develop into practitioners, J. Res. Sci. Teach., 51(6), 694–713.
- Bode N. E. and Flynn A. B., (2016), Strategies of successful synthesis solutions: Mapping, mechanisms, and more, J. Chem. Educ., 93, 593–604.
- Bodner G. M., (1986), Constructivism: A theory of knowledge, J. Chem. Educ., 63(10), 873–878.
- Bodner G. M. and Domin D. S., (2000), Mental models: The role of representations in problem solving in chemistry, Univ. Chem. Educ., 4(1), 24–30.
- Bond-Robinson J., (2005), Identifying pedagogical content knowledge (PCK) in the chemistry laboratory, Chem. Educ. Res. Pract., 6(2), 83–103.
- Bond-Robinson J. and Rodriques R. A. B., (2006), Catalyzing graduate teaching assistants’ laboratory teaching through design research, J. Chem. Educ., 83(2), 313.
- Caspari I., Weinrich M. L., Sevian H. and Graulich N., (2018), This mechanistic step is “productive”: Organic chemistry students’ backward-oriented reasoning, Chem. Educ. Res. Pract., 19, 42–59.
- Chan K. K. H. and Hume A., (2019), Towards a consensus model: Literature review of how science teachers’ pedagogical content knowledge is investigated in empirical studies, in Hume A., Cooper R. and Borowski A. (ed.), Repositioning pedagogical content knowledge in teachers’ knowledge for teaching science, Singapore: Springer, pp. 3–76.
- Charalambous C. Y., Hill H. C. and Ball D. L., (2011), Prospective teachers’ learning to provide instructional explanations: how does it look and what might it take? J. Math. Teach. Educ., 14(6), 441–463.
- Coll R. K. and Treagust D. F., (2002), Exploring tertiary students’ understanding of covalent bonding, Res. Sci. Tech. Educ.20(2), 241–267.
- Connor M. C. and Shultz G. V., (2018), Teaching assistants’ topic-specific pedagogical content knowledge in 1H NMR spectroscopy, Chem. Educ. Res. Pract., 19(3), 653–669.
- Davis E. A. and Krajcik J. S., (2005), Designing educative curriculum materials to promote teacher learning, Educ. Res., 34(3), 3–14.
- de Arellano D. C.-R. and Towns M. H., (2014), Students’ understanding of alkyl halide reactions in undergraduate organic chemistry, Chem. Educ. Res. Pract., 15, 501–515.
- Decocq V. and Bhattacharyya G., (2019), TMI (Too much information!) Effects of given information on organic chemistry students’ approaches to solving mechanism tasks, Chem. Educ. Res. Pract., 20, 213–228.
- De Jong O., Van Driel J. H. and Verloop N., (2005), Preservice teachers’ pedagogical content knowledge of using particle models in teaching chemistry, J. Res. Sci. Teach., 42(8), 947–964.
- Ekiz-Kiran B., Boz Y. and Oztay E. S., (2021), Development of pre-service teachers' pedagogical content knowledge through a PCK-based school experience course, Chem. Educ. Res. Pract., 22, 415–430.
- Ericsson K. A. and Simon H. A., (1980), Verbal Reports as Data, Psych. Rev., 87(3), 215–251.
- Ferguson R. and Bodner G. M., (2008), Making sense of the arrow-pushing formalism among chemistry majors enrolled in organic chemistry, Chem. Educ. Res. Pract., 9(2), 102–113.
- Galloway K. R., Leung, M. W. and Flynn A. B., (2019), Patterns of reactions: a card sort task to investigate students’ organization of organic chemistry reactions, Chem. Educ. Res. Pract., 20(1), 30–52.
- Galloway K. R., Stoyanovich C. and Flynn A. B., (2017), Students’ interpretations of mechanistic language in organic chemistry before learning reactions, Chem. Educ. Res. Pract., 18(2), 353–374.
- Graulich N., (2015), The tip of the iceberg in organic chemistry classes: how do students deal with the invisible? Chem. Educ. Res. Pract., 16(1), 9–21.
- Grossman P. L., (1990), The making of a teacher: Teacher knowledge and teacher education, Columbia University, Teachers College Press.
- Grove N. P. and Bretz S. L., (2012), A continuum of learning: From rote memorization to meaningful learning in organic chemistry, Chem. Educ. Res. Pract., 13, 201–208.
- Grove N. P., Cooper M. M. and Rush K. M., (2012), Decorating with arrows: Toward the development of representational competence in organic chemistry, J. Chem. Educ., 89(7), 844–849.
- Hale L. V. A., Lutter J. C. and Shultz G. V., (2016), The development of a tool for measuring graduate students’ topic specific pedagogical content knowledge of thin layer chromatography, Chem. Educ. Res. Pract., 17(4), 700–710.
- Houseknecht J. B., (2010), Topic sequence and emphasis variability of selected organic chemistry textbooks, J. Chem. Educ., 87(6), 592–597.
- Howell H., Lai Y. and Suh H., (2017), Questioning assumptions about the measurability of subdomains of mathematical knowledge for teaching, Proceedings of the 20th annual conference on research in undergraduate mathematics education, pp. 413–427.
- Hume A., Cooper R. and Borowski A. (ed.), (2019), Repositioning Pedagogical Content Knowledge in Teachers’ Knowledge for Teaching Science, Singapore: Springer.
- Kirilenko A. P. and Stepchenkova S., (2016), Inter-coder agreement in one-to-many classification: Fuzzy kappa, PLoS One, 11(3), e0149787.
- Kozma R., (2000), The use of multiple representations and the social construction of understanding in chemistry, in Jacobson M. and Kozma R. (ed.), Innovations in science and mathematics education: Advanced designs for technologies of learning, Mahwah, NJ: Erlbaum, pp. 11–46.
- Kozma R., (2003), The material features of multiple representations and their cognitive and social affordances for science understanding, Learn. Instr., 13(2), 205–226.
- Kozma R. and Russell J., (1997), Multimedia and understanding: expert and novice responses to different representations of chemical phenomena, J. Res. Sci. Teach., 43(9), 949–968.
- Kozma R. and Russell J., (2005), in Gilbert J. K. (ed.), Visualization in science education, Springer, pp. 121–146.
- Kozma R., Chin E., Russell J. and Marx N., (2000), The roles of representations and tools in the chemistry laboratory and their implications for chemistry learning, J. Learn. Sci., 9(2), 105–143.
- Kraft A., Strickland A. M. and Bhattacharyya G., (2010), Reasonable reasoning: multi-variate problem-solving in organic chemistry, Chem. Educ. Res. Pract., 11(4), 281–292.
- Lederman N. G. and Gess-Newsome J., (1999), Reconceptualizing secondary science teacher education, Examining pedagogical content knowledge, Springer, Dordrecht, pp. 199–213.
- Lincoln Y. S. and Guba E. G., (1985), Naturalistic inquiry, Newbury Park, CA: Sage Publications, Inc.
- Lutter J. C., Hale L. V. A. and Shultz G. V., (2019), Unpacking graduate students’ knowledge for teaching solution chemistry concepts, Chem. Educ. Res. Pract., 20(1), 258–269.
- Magnusson S. J., Krajcik J. and Borko H., (1999), Nature, Sources, and Development of Pedagogical Content Knowledge for Science Teaching, Examin Pedagogical Content Knowledge, Dordrecht: Springer, pp. 95–132.
- Mavhunga E. and Rollnick M., (2013), Improving PCK of Chemical Equilibrium in Pre-service Teachers, Af. J. Res. Math. Sci. Tech. Educ., 17, 113–125.
- McClary L. M. and Bretz S. L. (2012) Development and assessment of a diagnostic tool to identify organic chemistry students' alternative conceptions related to acid strength, Int. J. Sci. Educ., 34, 2317–2341.
- McClary L. M. and Talanquer V., (2011a), College chemistry students’ mental models of acids and acid strength, J. Res. Sci. Teach., 48, 396–413.
- McClary L. M. and Talanquer V., (2011b), Heuristic reasoning in chemistry: Making decisions about acid strength, Int. J. Sci. Educ., 33, 1433–1454.
- McHugh M. L., (2012), Interrater reliability: The kappa statistic, Biochem. Med., 22(3), 276–282.
- Park S., & Chen Y. C., (2012), Mapping out the integration of the components of pedagogical content knowledge (PCK): Examples from high school biology classrooms, J. Res. Sci. Teach., 49(7), 922–941.
- Park S. and Oliver J. S., (2008), Revisiting the conceptualisation of pedagogical content knowledge (PCK): PCK as a conceptual tool to understand teachers as professionals, Res. High. Educ., 38, 261–284.
- Rollnick M., (2017), Learning about semi conductors for teaching—The role played by content knowledge in pedagogical content knowledge (PCK) development, Res. Sci. Educ., 47(4), 833–868.
- Rollnick M., Davidowitz B. and Potgieter M., (2017), Is topic-specific PCK unique to teachers? in Hahl K., Juuti K., Lampiselkä J., Uitto A. and Lavonen J. (ed.), Cognitive and affective aspects in science education research: Selected papers form the ESERA 2015 conference, Cham: Springer International Publishing, pp. 69–85.
- Saldaña J., (2016), The coding manual for qualitative researchers, Thousand Oaks, CA: Sage.
- Shulman L. S., (1986), Those who understand: Knowledge growth in teaching, Educ. Res., 15(2), 4–14.
- Shulman L., (1987), Knowledge and teaching: Foundations of the new reform, Harv. Educ. Rev., 57(1), 1–23.
- Taber K. S., (1998), An alternative conceptual framework from chemistry education, Int. J. Sci. Educ., 20(5), 597–608.
- Watts F. M., Schmidt-McCormack J. A., Wilhelm C. A., Karlin A., Sattar A., Thompson B. C., Gere A. R. and Shultz G. V., (2020), What students write about when students write about mechanism: Analysis of features present in students’ written descriptions of an organic reaction mechanism, Chem. Educ. Res. Pract., 21, 1148–1172.
- Webber D. M. and Flynn A. B., (2018), How are students solving familiar and unfamiliar organic chemistry mechanism questions in a new curriculum? J. Chem. Educ., 95(9), 1451–1467.
- Weinrich M. L. and Talanquer V., (2016), Mapping students’ modes of reasoning when thinking about chemical reactions used to make a desired product, Chem. Educ. Res. Pract., 17(2), 394–406.
- Zotos E. K., Moon A. C. and Shultz G. V., (2020), Investigation of chemistry graduate teaching assistants’ teacher knowledge and teacher identity, J. Res. Sci. Teach., 57(6), 943–967.
| This journal is © The Royal Society of Chemistry 2021 |