Student-generated PowerPoint animations: a study of student teachers’ conceptions of molecular motions through their expressed models†
Guspatni
Guspatni

Department of Chemistry, Universitas Negeri Padang, West Sumatra, Indonesia. E-mail: patni@fmipa.unp.ac.id
First published on 8th December 2020
Abstract
Student-generated drawings are known to be effective in building and revealing students’ conceptions of chemistry. Some chemistry concepts, moreover, include changes and processes that cannot be merely represented by static drawings. Computer-based animations are needed to represent the dynamics. In this study, 25 chemistry student teachers, who had studied the concept of molecular motions and had taken the course of Chemistry Instructional Media and Technology, were assigned to make expressed models of water molecules’ motions in the form of animations with PowerPoint, the most familiar program and installed on students’ computers. Students were also assigned to give written explanations of the three molecular motions. Within one month, both tasks were due simultaneously. Students’ expressed models were analysed based on Custom Animation features used for the animations, while students’ written explanations were analysed based on the typology of the sentences. It was found that all students appeared to hold correct conceptions of translation; many students appeared to hold correct conceptions of rotation; and almost all students appeared to hold misconceptions of vibration. There was no substantial difference between PowerPoint Animations and written explanations in revealing students’ conceptions of molecular motions. However, there were several inconsistencies of students’ conceptions that occurred in both tasks. For example, several students who incorrectly explained rotation as circular movements displayed a spinning of the particle on its own axis in the animation. Students’ expressed models in PowerPoint Animations provided other information unrevealed in their written explanations. These pieces of information included types of molecular motion in different phases, simultaneous motions, and deflections of molecules after collisions. The analysis of students’ expressed models in PowerPoint Animations can be an effective approach to reveal students’ conceptions of molecular dynamics if accompanied by adequate tutorials on the animation program, clear instructions, and guidance to get learning resources.
Introduction
Students’ conceptions are usually assessed from their explanations in a written test (Gharibyan, 2005). Some practitioners and researchers ask students to draw static visuals of chemistry concepts. The analysis of student-generated drawings allows us to probe students’ understandings of chemistry concepts (Davidowitz et al., 2010) and is claimed to be more explicit, coherent and complete in revealing students’ mental models (Harle and Towns, 2013; Cooper et al., 2017). Student-generated drawings show the underlying struggles with fundamental chemistry concepts that students face (Nyachwaya et al., 2011). In fact, asking students to draw visuals does not only function as an assessment but also serves as a model-based learning which contributes to the construction of mental models (van Joolingen et al., 2015; Ainsworth et al., 2016). Drawing helps students integrate information (Zhang and Linn, 2011), and it helps students overcome common misconceptions associated with atomic-scale processes in chemistry (Dickson et al., 2016).Some chemistry concepts, however, cannot merely be represented by static visuals. Concepts such as molecular kinetics (Stern et al., 2008), molecular dynamics and many other concepts describing processes and changes in time and space need other modes of communication such as dynamic visualizations. Asking students to simply draw representations of these concepts on paper hardly gives complete descriptions of their understandings. Fortunately, with the advances of computer-based animation programs, asking students to make dynamic visuals or animations of chemistry concepts becomes feasible (Albert, 2012; Chang et al., 2014). Studies (Chang and Quintana, 2006; Chang et al., 2009; Hoban et al., 2011; Akaygun, 2016) found that student-generated animations show more descriptions of their mental models. Engaging students in generating animations helps them develop more integrated understandings (Chang et al., 2014). The construction of a computational modelling engages students in a meaningful learning and thus fosters the development of mental models (Jonassen and Strobel, 2006).
Several studies have used the approach of assigning students to generate animations of dynamic chemistry concepts. Chang and Quintana (2006) studied molecular chemical phenomena; Williamson et al. (2013) studied physical and chemical equilibriums; Chang et al. (2014) studied chemical reaction processes; Akaygun (2016) studied the atomic structure; Berg et al. (2019) studied sub-micro processes in chemistry laboratories. All of the studies used animation programs that are not pre-installed on most computers. The programs are either developed by the researchers or downloaded from the Internet. Media players are needed to open the animation files produced; such a thing that is not practical for many users.
In order to use student-generated animations for learning and assessment, the animation program should be available without challenges. In other words, the program is already installed when the computer is first used. In this study, students were assigned to generate animations with PowerPoint. PowerPoint was chosen because it is installed on millions of computers worldwide (Kealey, 2000; Lowenthal, 2009); it is the most predominant technology platform in learning (Berk, 2011); and it is most familiar to students (O’Day, 2006). The dynamics of concepts can be represented by the use of PowerPoint Custom Animation features (Bertea, 2012; Yang and Chen, 2013).
In this study, students were asked to express models of molecular motions concept in PowerPoint Animations in order to study their conceptions. It is hoped that this study contributes to existing literatures about model-based learning. Also, it is hoped that the approach becomes a practical alternative for all practitioners to study students’ conceptions in chemistry.
Literature review
Model-based learning
Model-based learning is the formation and subsequent development of mental models by a learner (Buckley, 2012). It involves constructing and transforming models (Khan, 2008). Model construction relates to direct experiences with phenomena or interactions with representations and expressed models in learning (Buckley and Boulter, 2000). Model development is the result of many learning processes (Nunez-Oveido et al., 2008) and diverse educational experiences (Buckley and Boulter, 2000). Model development is mediated by a series of expressed models (Gilbert et al., 2000). Expressed models provide pieces of information about the structure, behavior and mechanisms of phenomena (Buckley and Boulter, 2000). An expressed model is a version of mental models that can be placed in a public domain by the use of five modes including visuals such as models (Lohr, 2008) and verbal, symbolic, concrete and gestural ones (Gilbert, 2004). Expressed models, along with mental and consensus models as well as curricular, hybrid, and teaching models play a key role in the teaching and learning of science and technology (Gilbert and Boulter, 2000).Model-based learning emphasizes the development of conceptual understandings through graphical and diagrammatic representations (Jackson et al., 2008). The goal is that students have the ability to construct complex mental models that are functional and structural (Rea-Ramirez and Núñez-Oviedos, 2008). Structural properties relate to visual features of how something looks, while functional properties relate to what something does or how something works (Else et al., 2008). Thus, model-based learning involves means of visualizations that include graphical representations (e.g., drawings and diagrams) or more advanced, computer-based animations, simulations, and virtual realities (Seel, 2017). The integration of 3D animations in a model-based learning enhances students’ understandings and their abilities to transfer across different levels of chemistry understandings (Barak and Hussein-Farraj, 2013).
Models and modelling
Models are used to facilitate visualizations of what is happening at macroscopic and sub-microscopic worlds of chemistry. Models may be static or dynamic ones expressed in concrete (three-dimensional), visual (two-dimensional and pseudo-three-dimensional/computerized model), and/or verbal modes of representations (Justi and Gilbert, 2006). Chemical models are thinking tools that have been used by scientists to understand chemistry concepts. Likewise, chemical models also help students to understand the complexity of chemistry concepts (Coll, 2006).The key to achieve a comprehensive understanding of chemistry is the act of modelling (Justi and Gilbert, 2006). Modelling is a core skill in the scientific enquiry that consists of dynamic processes of producing, testing, and revising a model (Justi et al., 2009) and metaknowledge that guides and motivates the practices (Schwarz et al., 2009). Modelling is an essential skill for engaging students in a meaningful learning (Jonassen and Strobel, 2006). Modelling should be taught to students rather than be an incidental consequence of a chemistry teaching (Chittleborough and Treagust, 2007). Thus, students need to have opportunities to develop the capability to produce and test their own models (Gilbert, 2004).
PowerPoint as an animation program
PowerPoint is used as a presentation tool in the classroom. PowerPoint presentations involving multimedia have the potentials to increase students’ attention, comprehension, memory, and deep learning (de Wet, 2006; Berk, 2011). Yet, if used incorrectly, PowerPoint can have a negative impact on the teaching and learning processes. Some improper practices of PowerPoint include excessive use of text, graphics and animations; the use of slides that fail to follow visual design principles; and the use of multimedia options that are not needed (Jones, 2003). It is an excellent aid for presentations when created with pedagogical principles (Jones, 2003; Rowcliffe, 2003).PowerPoint provides users options to create animations of a concept (done by Carmichael and Pawlina, 2000; Stith, 2004; DeAntonio et al., 2006; O’Day, 2006; Takahashi, 2011; Bertea, 2012; Zanin, 2015) with Effects and Timings features in Custom Animation. Effects of the animations are grouped into Entrance, Emphasis, Exit, and Motion Path; Timings of the animations include Start, Speed, Delay and Repeat (Zimmerman et al., 2010; Yang and Chen, 2013). These features are useful to make representations of dynamic chemistry concepts. With its simplicity, both students and teachers can use PowerPoint to implement a model-based approach in learning.
Molecular motions
Molecular motions consist of translation, rotation and vibration. Gas and liquid phase particles have all three types of motion, while solid phase particles have only one type of motion namely vibration (Atkins and Paula, 2006). However, secondary school students taught about the states of matter in the traditional way failed to understand that particles in all three phases are in the motion (Pallant and Tinker, 2004). While secondary school students knew that solid particles perform vibrational motions, (Yaseen, 2016), they struggled to imagine such situations at the particulate level (Adbo and Taber, 2009). In view of the dynamic nature of molecular motions, dynamic representations are necessary to foster students’ understandings of these concepts (Adadan, 2013). With dynamic representations, students were able to transfer their knowledge of molecular motions in the different states (Pallant and Tinker, 2004).Rational for this study
Understanding chemistry involves the interconnection of its multiple representations; the macroscopic, sub-microscopic and symbolic levels (Johnstone, 1991; Tan et al., 2009; Treagust and Chandrasegaran, 2009). Yet, Stojanovska et al. (2014) found that students had misconceptions due to the incorrect transferring of the macroscopic into the sub-microscopic world. Students had difficulties moving across or connecting multiple representations (Kozma, 2003; Tsaparlis, 2009).One way to help students deepen understandings of representations is by asking them to model their own representations (Ainsworth et al., 2011). Through the modelling or drawing, students can reveal what they know and understand (Dikmenli, 2010). In modelling practices, students are engaged in constructing and revising increasingly accurate models and applied these models to make predictions for closely related phenomena (Schwarz et al., 2009). Asking students to make dynamic drawings or animations will help them transform information and build understandings of chemistry concepts.
How students build understandings of a concept through the modelling practices is worth studying (Hoban et al., 2011). In this study, chemistry student teachers used PowerPoint to model dynamic representations of molecular motions. Generating representations can be a learning strategy and serves as diagnostic, formative and summative assessments as well (Ainsworth et al., 2011). With its multimedia features (Bartsch and Cobern, 2003; Stephen, 2006; Zimmerman et al., 2010; Yang and Chen, 2013), the use of PowerPoint to make representations can be an authentic (Cox et al., 2010) and a valid form of assessments for learning (Dobson, 2006).
Research questions in this study are:
• How do student teachers express their conceptions of molecular motions using PowerPoint Animations as a model-based learning tool?
• How do student teachers explain their conceptions of molecular motions in a written task?
• How consistent are students’ conceptions of molecular motions as expressed in PowerPoint Animations and explained in the written task?
Methodology
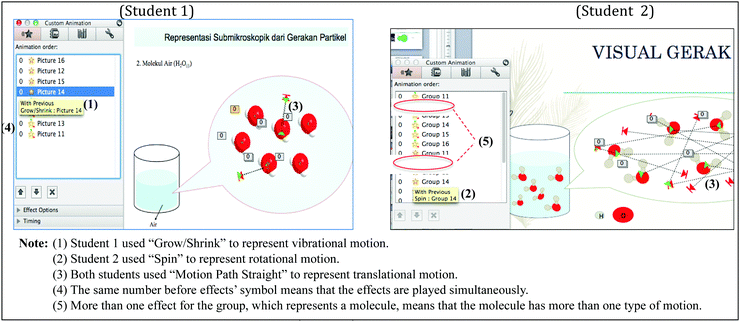
This is a descriptive research study using a cross-sectional design. Using model-based learning as the theoretical framework, the main goal of this research was to characterize students’ conceptions of molecular motions through expressed models in the form of animations that they generated with PowerPoint and students’ explanations in a written task. Based on inclusion and exclusion criteria set for this cross-sectional study (Setia, 2016), chemistry student teachers were selected as the participants for reasons including (1) chemistry student teachers should be able to design and develop computer-assisted media for their future teaching and (2) chemistry student teachers should master multiple representations for their own learning and future teaching.All 25 students who enrolled on the course of Chemistry Instructional Media and Technology in a university in Indonesia became subjects of this study. These second-year undergraduate students had studied the concept of molecular motions in General Chemistry course (a first-year course) and Physical Chemistry 1 course (a second-year course). They also studied chemical multiple representations and received 5 × 150 minute tutorials where they practiced designing instructional media with PowerPoint in the course of Chemistry Instructional Media and Technology. In the tutorials, students learnt and practiced about PowerPoint Effects (for example, Motion Path Effect to move a ball, Spin Effect to represent a roll of the ball); PowerPoint Timings (for example, With Previous Timing to make two or more given Effects to the ball play at the same time, After Previous Timing to make the second-ordered Effect appear after the first Effect is completed); and PowerPoint shapes (for example, Oval shape to represent an atom).
There was no Institutional Review Board at the university at the time the research was conducted. However, ethical considerations were met. Research data were not shared with other parties, students’ identities were disguised, and students’ permission that their data would be analysed and published in an academic journal was obtained. To avoid burdening the students, they were assigned to make the written and animation tasks after completing all the courses’ semester examinations. Both tasks could be done simultaneously at home and were submitted within one month. The purpose of enabling students to do this was to give them time and opportunities to get learning resources. The instruction for the animation task was “make animations of molecular motions for water (H2O)”, while the instruction for the written task was “Explain three motions that a particle can have”. Animations of molecular motions were analysed based on PowerPoint Custom Animation Effects and Timings that students used. Students’ written explanations were analysed based on the typology of the sentences (keywords indicating the critical attribute and meanings of the sentences).
The number of students who met assessment criteria in the rubrics was calculated. The rubric used to assess student-generated animations was set based on animation Effects used to represent the motions. Effects that represent molecular motions are Spin in Emphasis, Swivel in Entrance, and Motion Paths. Fig. 1 depicts how Custom Animation Effects and Timings can be extracted from students’ animations. An overview over the resulting system of categories is given in Appendix 1.”
The rubric used to assess students’ written explanations was suited to the principle or theory stated in the literature (university textbook). Students’ written explanations were transcribed and tabulated in an excel file. A correct explanation was determined to be one that had the critical attribute of the concept. The critical attribute of translation is the change in position; the critical attribute of rotation is the spinning around its own axis; and the critical attribute of vibration is the shaking in the form of changes in bond length or bond angle (Atkins and Paula, 2006). The results of the typology analysis of students’ explanations were categorized into correct conceptions (ones that contained the critical attribute of the concept and the meanings were correct) and misconception (ones that either missed or contained incomplete critical attribute of the concept which made the meanings incorrect).
A cross tabulation was performed to see if there were inconsistencies of students’ conceptions between those expressed in PowerPoint Animations and those explained in the written task. This study was also prepared for an interview to investigate any discrepancy in students’ conceptions of molecular motions as revealed in both tasks. A short confirmative interview was carried out with one student to investigate why the student failed to express correct animations of vibrational motion but explained it correctly in the written task. The interview that had been informed to the student was done for about 15 minutes. As the interview was intended as a confirmation, student’ answers had been expected and could be marked.
Findings
Animation task
The analysis of student-generated PowerPoint Animations is summarized in Table 1. All students correctly represented translational motion in their animations by moving the molecules from one point to the other. Twenty-three students used Motion Path Effect with straight directions, while two students used Motion Path Effect with irregular directions (they drew the directions). Twenty-four students attempted to show rotational motion. Twenty-one students correctly animated rotation with Spin Effect (rotating the molecules on their own axis), while three students incorrectly represented rotation with Motion Path Circle as if the molecules moved in the circular path. Furthermore, 17 students attempted to show vibrational motion but failed to express the correct model of vibration. They used Teeter Effect; Wave, Bounce, Up and Down Motion Path Effects; and Grow and Shrink Effect to represent shakings of the particles.| Motion | Effect used | Criteria | N | Total |
|---|---|---|---|---|
| Translation | Motion path straight | Correct model | 23 | 25 |
| Motion path scribble | Correct model | 2 | ||
| Rotation | Spin | Correct model | 21 | 24 |
| Motion path circle | Incorrect model | 3 | ||
| Vibration | Teeter | Incorrect model | 8 | 17 |
| Motion path wave, bounce, up and down | Incorrect model | 8 | ||
| Grow/shrink | Incorrect model | 1 | ||
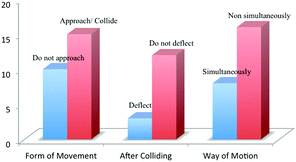
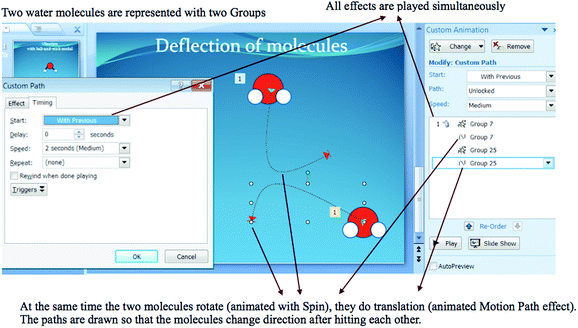
Fig. 2 shows other findings obtained from student-generated animations. In the animations, students represented at least three water molecules. The phase was either liquid or gas. Fifteen students displayed molecules approach and collide with other molecules along the way, while other 10 students did not. Only three out of these 15 students intentionally let the molecules deflect after colliding.
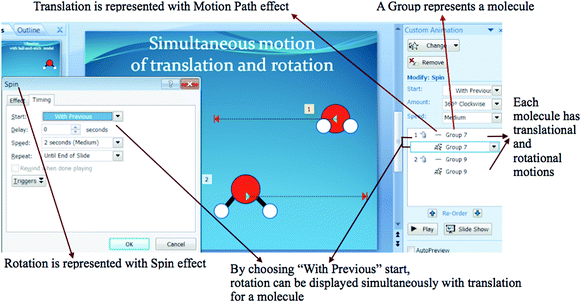
Furthermore, PowerPoint Animations showed students’ conceptions of how a molecule experiences the motions. Eight students made a molecule experience the motions simultaneously (simultaneous motions were represented by With Previous Timing in PowerPoint Custom Animation). These students let a molecule spin while it was moving and changing position. And for those who attempted to make three motions, they let a molecule undergo rotation and translation while it was vibrating (although the expressed model of vibration was theoretically incorrect; animated with Teeter, Wave or Bounce Effect). Sixteen students made non-simultaneous motions. They made several molecules undergo rotation, some undergo translation and some others undergo vibration independently.
Written task
All students correctly explained translation as a motion of a particle from one point to the other point. Fifteen students wrote straight directions, while 10 students did not. Seventeen students correctly explained rotation as the spinning of a particle around its own axis, while eight others incorrectly explained rotation as a circular movement of a particle. Furthermore, one student correctly explained vibration as the shaking of a particle in the form of changes in bond length or bond angle, 24 students misunderstood vibration either as simply the shaking of a particle or as an oscillation of a matter (see Table 2).| Motion | Criteria | Answer | N |
|---|---|---|---|
| Translation | Correct conception | Movement of a particle from one point to the other | 10 |
| Straight movement of a particle from one point to the other | 15 | ||
| Rotation | Correct conception | Circular movement of a particle around its own axis – spinning | 17 |
| Misconception | Circular movement of a particle | 8 | |
| Vibration | Correct conception | Shaking of a particle in the form of changes in bond length or angle | 1 |
| Misconception | Shaking of a particle | 17 | |
| Back and forth movement of a particle like an oscillation | 7 | ||
Comparisons between students’ expressed models and written explanations
A cross tabulation of students’ conceptions revealed in both tasks is presented in Table 3. There were seven students who incorrectly explained rotation but expressed its correct model in PowerPoint Animations. These students used Spin Effect that showed the spinning of a molecule on its own axis. There were three students who correctly explained rotation but incorrectly expressed the model in PowerPoint Animations. These few students used Motion Path Circle Effect. Instead of spinning around its own axis, the molecule moved around in the circular paths.| Written explanation | Animation | ||
|---|---|---|---|
| Incorrect | Correct | ||
| Translation | Incorrect | 0 | 0 |
| Correct | 0 | 25 | |
| Rotation | Incorrect | 1 | 7 |
| Correct | 3 | 14 | |
| Vibration | Incorrect | 24 | 0 |
| Correct | 1 | 0 | |
One student showed inconsistencies of concept on vibration. The student could explain vibration correctly but failed to express its correct model in the animations. More than one object (oval shapes as the atoms and lines as the bonds) and repeating Effects (Motion Path) should be used to represent molecular vibration in PowerPoint Animations. Due to possible difficulties to express the animations for vibration, the student was interviewed with English translations of all dialogues given below.
“Interviewer: You are the only student who explained vibration as the shaking of a particle due to changes in its bond length or bond angle in the written task. Do you still have the same explanation on vibration?”
“Student: Yes. It is the shaking of a particle. The particle's bond length or angle changes”
“Interviewer: Do you still remember the animation Effect that you used to represent vibration in PowerPoint?”
“Student: [silent while trying to remember the name]”
“Interviewer: You used Grow and Shrink Effect like this [by showing the student's animation]. Why?”
“Student: Yes. I wanted to show the shaking. I did not know what else could represent it”
“Interviewer: You used the space-filling model to represent H2O molecules. Why did not you use the ball-and-stick model?”
“Student: Yes, I used the space-filling model. I did not consider of making the ball-and-stick model”
“Interviewer: What are other motions that a particle can have?
“Student: Rotation and translation”
“Interviewer: How do the motions occur in a liquid water molecule?”
“Student: [Keep silent]”
“Interviewer: Do they occur at the same time?”
“Student: [silent while thinking] I do not know about it”
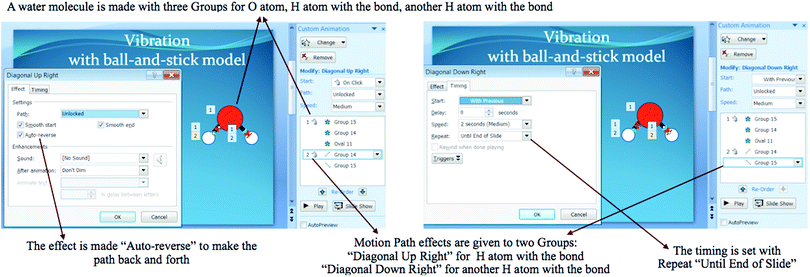
As can be seen in the dialogue, the student appeared to hold a correct conception of vibration in the written task. Yet the student could not make a correct animation due to the use of space-filling model to represent water molecules. That was why the only way for the student to show vibration was by using Grow and Shrink Effect.
Discussion
In principle, a molecule has three types of molecular motion. These motions relate to internal energy and entropy of the molecule, concepts discussed in thermodynamics topic. Molecular motions also relate to molecular spectroscopy (Atkins and Paula, 2006; Rayner-Canham and Overton, 2010), a topic discussed in advanced chemistry courses. Therefore, students’ conceptions of molecular motions should be addressed so that students can easily study and have a correct understanding of these related concepts.This study addressed students’ conceptions of molecular motions through an animation task accompanied by a written task for a comparison. If we analysed students’ written explanations, it was found that all students appeared to hold correct conceptions of translation. More than half students appeared to hold correct conceptions of rotation (they explained rotation as the circular movement—spinning—of a particle around its own axis, which was stated as “gerakan berputar suatu partikel pada sumbunya” in Indonesian). Several students appeared to hold misconceptions of rotation (they explained rotation as the circular movement of a particle, which was stated as “gerakan berputar suatu partikel” in Indonesian). Almost all students appeared to hold misconceptions of vibrational motion.
If we analysed students’ expressed models as shown in their PowerPoint Animations, it was found that more students created correct model of rotational motion using animations as compared to using written explanations. The phrase of “pada sumbunya” (English: around its own axis) in the explanations had substantial meaning. When students did not include the phrase, their explanations became incorrect. The circular movements of a particle can be expressed as a translation in circular paths, while the circular movement of a particle around its own axis is the same with the spinning of a particle around its own axis – rotation. Interestingly, though missed in the students’ written explanations, what was meant by the phrase was finally shown in their PowerPoint Animations. In line with Taber (2019), students seemed to have multifaceted and inconsistent conceptions of rotation. When these students were asked to provide written explanations, it was concluded that they misunderstood rotation. However, when compared to their expressed models in PowerPoint Animations, the conclusion changed. The animations helped them to show correct expressed models of the concept. In line with Chang et al. (2014), asking students to create visualizations provides chances for them to examine or express their conceptions. Student-generated PowerPoint Animations complemented students’ explanations by showing more information or descriptions about rotation.
On the other hand, it was also found that some of the students who correctly explained rotation as the spinning of a particle around its own axis showed incorrect expressed models of the concept in their PowerPoint Animations. These students chose a wrong animation Effect to model rotation (Motion Path Effect with circular path). Asking students to make expressed models with this type of technology could address their performances in thinking skills and abilities to use resources (Ravitz, 2002). When creating animations of molecular representations, students are challenged (Bucat and Mocerino, 2009) as they are engaged in ways that encourage them to refer to the science behind the concept being modelled (Berg et al., 2019) and evaluate and transform their understandings (Ainsworth et al., 2016). These students had troubles expressing their conceptions of rotation with PowerPoint Animations. If they used Spin effect, they would be able to represent the phrase “on its own axis” in the animation.
Furthermore, vibration was the most difficult molecular motions concept for students in this study. Many students incorrectly explained vibration either as simply the shaking of a particle or the oscillation of a matter. In agreement to that, these students could not express a correct model of vibration with PowerPoint Animations. Many of them used Teeter Effect and few of them used Wave, Bounce, Up and Down Effects to show the shaking or oscillation of the particle. In line with Davidowitz and Chittleborough (2009), asking students to express, at the particulate level, the models with PowerPoint Animations showed their misconceptions of vibration.
Only one student could correctly explain vibration as the shaking of a particle due to changes in its bond length or angle. Yet, the student failed to express its correct model in PowerPoint Animations. The inconsistency of students’ conceptions in the animation and written tasks was the result of troubles expressing the model in PowerPoint. As proven by the confirmative interview, the student chose to show growing and shrinking of H2O molecules to mean the stretching of their bond length or bending of their bond angle. The student used space-filling models for H2O instead of ball-and-stick models. In fact, the choice of the analog (the space filling model or the ball-and-stick model) influences the model produced and hence its explanatory power (Justi and Gilbert, 2006). The ball-and-stick representation uses ‘spheres’ to depict atoms and ions and ‘sticks’ to notice spatial distributions, bond length and angles to produce clear angular relationships (Gilbert, 2010). If the ball-and-stick model was chosen, the student could have made correct vibrational motion for water molecules; symmetric and asymmetric stretching to show changes in the bond length and bending in-and-out to show changes in the bond angle (Atkins and Paula, 2006; Rayner-Canham and Overton, 2010). In fact, by choosing the right shape or model, students could make a vivid animation with Motion Path Effects in PowerPoint (Bertea, 2012; Yang and Chen, 2013). The approach to make vibrational motion with Motion Path Effects in PowerPoint can be seen in Appendix 2.
This study also showed that student-generated animations gave more information about students’ conceptions when compared to their written explanations on the paper (Akaygun, 2016). The information includes the number of motions experienced by a molecule, how a molecule experiences the motions, and what happens when a molecule collides with other molecules. In their animations, students represented H2O exist in gas or liquid phase. Every water molecule – in gas and liquid phases – has vibrational, rotational and translational motions that occur simultaneously (McMurry and Fay, 2003: p. 730; Jespersen et al., 2012: p. 257). Yet, not all students modelled the three motions for liquid and gas phase water molecules. In fact, one student modelled only translational motion for all molecules. More than half students did not express simultaneous motions for every water molecule. They expressed that translation occurred and was possessed by certain water molecules; vibration occurred and was possessed by other water molecules, and vibration occurred and was possessed by some other water molecules.
An object in PowerPoint can be given as many animation Effects as needed. Likewise, a water molecule can be given animation Effects to represent the three motions. By using “With Previous” Timing in PowerPoint, students could make many effects given for an object play at the same time. For example, if students made two different motion Effects for a water molecule, they could set the second Effect play simultaneously with the first Effect by choosing “With Previous” in the Start option. The approach to make simultaneous motions can be seen in Appendix 3.
Many animations displayed colliding of H2O molecules when they moved. However, only a few students included the deflection of molecules after they collided with the others in their animations. Molecules are continually exchanging energy with each other during collisions (Jespersen et al., 2012: p. 257). The change of energy will change the orientation of molecules to other directions. There are two ways of modelling the deflection with PowerPoint Animation Effects. Firstly, we need to give additional Motion Path Effects with different direction to each molecule. Secondly, we can draw the paths so that they are changed to other directions after the molecules collide. The approach to make a molecules’ deflection with the second way can be seen in Appendix 4.
PowerPoint has been the most used presentation program by both lecturers and students in the university where the study was done. Informal observation during the study and during teaching in the university showed that students were familiar with PowerPoint Effects (O’Day, 2006). Most of them were capable of using PowerPoint to make presentations that displayed Animation Effects for texts and pictures. However, students were not accustomed to combining two or more shapes (as models to represent chemistry concepts or objects) and giving many effects to the combined shapes to represent dynamics of chemistry concepts. Some lecturers had been using PowerPoint as a presentation tool to display texts and pictures of chemistry concepts in their courses. Lecturers (including the author) who teach Instructional Media and Technology course could use PowerPoint Animation Effects to represent the dynamic chemistry concepts.
Conclusions
All students appeared to hold correct conceptions of translation. The students explained and modelled translation by moving water molecules from one point to the other. However, many of them did not show changes of directions after the molecules collided with the others. Many students appeared to hold correct conceptions of rotation by explaining and modelling the spinning of a molecule around its own axis. Yet, few students appeared to hold misconceptions and modelled rotation as a translation in circular paths. Furthermore, almost all students appeared to hold misconceptions of vibration. Instead of modelling vibration as the shaking of a particle by changing its bond length or bond angle, students modelled vibration as simply shaking the particle as a whole object.Students’ conceptions of molecular motions expressed in PowerPoint Animations were not substantially different from those revealed in written explanations. Yet some inconsistencies of students’ conceptions occurred between the two modes of assessment. There were few students who appeared to hold correct conceptions but displayed incorrect model of rotation in PowerPoint Animations. In several cases, PowerPoint Animations turned out to be a complement because the students appeared to hold incorrect explanation but displayed correct model of rotation in the animations. One student, who correctly explained vibration as the shaking of a particle due to changes in its bond length or bond angle, failed to correctly express this conception in the PowerPoint Animations. This stems from troubles using PowerPoint, since the student chose the wrong molecular model for H2O.
Student-generated animations yielded other information about students’ conceptions. These pieces of information include, that (1) only a few students modelled that the three motions were possessed by gas and liquid-phase molecules (2) most students displayed that the three motions did not simultaneously occur in a gas or liquid phase water molecule (3) students did not correctly make their animations as they did not show deflections of molecules after they collided with others.
Limitations of the study
Data about students’ skill of PowerPoint was not gathered for this study. This information could be useful to decide if more supports (such as duration of the tutorial) should have been given to students. This study was done with a small sample without considering factors such as spatial thinking skill (Chang and Tzeng, 2018), students’ skill of and experience in PowerPoint, students’ attitude toward PowerPoint, etc. Further studies in this area could investigate a larger sample, consider factors that may affect students’ performance in generating animations of chemistry concepts with PowerPoint, and categorize students’ performance based on those factors.Assignment given in this study was one that could be done outside the class within one-month duration. There might be possibility of intervention from other people. Further researchers could do study as in-class test so what the result conveys is unquestionably from the students.
This study also revealed some limitations due to limited Effect and Timing options in PowerPoint as well as students’ use of the program. In the case of molecular motions, PowerPoint Custom Animation that can be used include Spin and Swivel Effects for rotational motion; Motion Path Effect for translational and vibrational motions; and With Previous Timing for simultaneous motions. Simultaneous motions of translation and rotation can be displayed by manipulating Timing in Custom Animation, but simultaneous motions of all three motions cannot be. Vibrational motion cannot be displayed at the same time the molecule undergoes translation and rotation. This is because PowerPoint only displays one Motion Path (that is Motion Path put in the last order) out of many Motion Paths when they are set to be started With Previous. This probably also induced students to choose non-simultaneous motions. However, this assumption will be true to the only student who had the concept of the shaking of a particle due to changes in its bond length or bond angle for vibration. From the responses given by the student in the interview, it was concluded that the student did not consider of making H2O molecule with ball-and-stick model in PowerPoint. With this model, the student could have represented the stretching of bond length or bending of bond angle of the molecule.
In comparisons to other methods such as student-generated drawings, the diagnostic interview and think-aloud-protocols, asking students to generate animations with PowerPoint has some weakness. Generating animations is more challenging than drawing models on the paper with hands. It took considerable amount of time for students to express models with the animation program (Berg et al., 2019). There are many PowerPoint Animation Effects that can be used to represent dynamics of a concept. If not accompanied by exploration interview – which was not done in this study – data about students’ conceptions could be less precise and comprehensive. Working with computer program might be problematic. Students might become frustrated with the technical problems that hinder them to express models of dynamic concepts as they actually could.
In the case of the written task, this study used the word “explain” as the instruction. Yet, students’ explanations were like definitions stated in the textbook glossary. They did not really explain neither gave more details on the explanation. Many more questions should have been asked to get students’ conceptions of molecular motions in the written test. A question stated as “explain three types of molecular motion a particle can have” could have been followed by questions including “are all motions possessed by molecules in different phases (gas, liquid and solid)” and “how do those motions occur in a molecule”. Alternatively, higher order thinking (HOT) questions could have asked students to explain their answer in more complete way.
Implications for teaching and learning
PowerPoint Animations created by students can be a versatile assessment tool for learning evaluation (done by Dobson, 2006; Basturk, 2008; Song, 2012; Pieketaleyee and Bazargani, 2018; Balagiu et al., 2019) and an interesting teaching strategy in model-based learning. In this study, student-generated PowerPoint Animations helped them show complete expressed models of the concept by complementing their explanations in the written task. For student teachers who would use and design media for their future teaching, this task would not be very demanding. Instructors can adapt this use of technology as a form of assessment (Eyal, 2012) as well as a teaching strategy to help students experience a model-based learning. By assigning students to generate animations, we indirectly teach them problem solving, science process and modelling skills that can help them learn chemistry concept (Williamson et al., 2013), get better understandings and construct mental models (Jonassen and Strobel, 2006; Chittleborough and Treagust, 2007; Quillin and Thomas, 2015).Some implications in assigning students to generate animations are given. First, teachers have to make sure that students have enough skill and support on the animation program (Chang and Quintana, 2006; Quillin and Thomas, 2015) including how long the tutorials should be given. Second, teachers should give clear instruction on the task. For example, teachers inform students of the models required, whether space-filling models or ball-and-stick models, to get better representations (Justi and Gilbert, 2006). As stated by Pallant and Tinker (2004), teachers may guide students to explore computational models that represent dynamics of molecular motions. Third, teachers inform students what learning resources they should refer to do the task.
Several suggestions are recommended to solve problems related to students’ misconceptions of molecular motions. First, teachers should emphasize the integration of chemical multiple representations in learning (Chittleborough and Treagust, 2007; Davidowitz and Chittleborough, 2009; Tan et al., 2009; Treagust and Chandrasegaran, 2009). With their central functions (Gilbert, 2005), visuals on the molecular or sub-microscopic level should be given while explaining translation, rotation and vibration. With their dynamic nature, teachers are recommended to use animations or computer simulations to increase students’ understanding of the concepts (Stern et al., 2008). Secondly, teachers ask students to draw static visuals of the molecular motions concept either through note takings, in class exercises, or assignments. Drawing can be an instructional practice serving as model-based learning for students to overcome misconceptions and construct correct mental models (Zhang and Linn, 2011; van Joolingen et al., 2015; Ainsworth et al., 2016; Dickson et al., 2016). If possible, teachers can ask students to make animations of molecular motions by choosing appropriate animation program and giving support and guidance on that animation task as well.
Implications for research
There are many descriptions of students’ expressed models that can be revealed through student-generated animations with PowerPoint, yet they are not discussed in this article. Other researchers may replicate this study by (1) asking students to make animations of translation, rotation and vibration in different slides in order to get descriptions of students’ expressed models of each motion, and (2) asking students to make animations of molecular motions for several molecules in one slide to determine if students had expressed models of simultaneous motions as well as deflections of molecules after colliding. By doing so, we can be sure of any limitation of PowerPoint Animations that may significantly affect expressed models that students make.Researchers can study other related concepts such as degrees of freedom or modes of molecular motions (translational motion in x, y, z Cartesian; rotational motion about two or three perpendicular axes; and vibrational motion in the form of stretching-compressing, symmetric stretching, asymmetric stretching or bending-scissoring) in relation to whether the molecule has a linear or nonlinear structure.
Conflicts of interest
There are no conflicts to declare.Appendix 1. The analysis of student-generated PowerPoint animations
| No. | Motion | Translation | Rotation | Vibration | Simultaneous motions |
|---|---|---|---|---|---|
| Steps 1–7 are repeated for next students. | |||||
| 1 | Critical attribute | Change in position of a particle | Spinning of a particle around its own axis | Shaking of a particle due to changes in its bond length and/or bond angle | • All motions happen in a molecule |
| • Motions happen at the same time | |||||
| 2 | Appropriate custom animations | Motion path effect | Spin and swivel effects | Motion path effect | Start “with previous” timing |
| 1. Analysis of PowerPoint animation generated by student 1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Analysis | Conclusions | |||||||
| 1 | Number of molecules | 2 | 2 | 2 | 2 molecules had translation, 2 molecules had rotation, 2 molecules had vibration independently | ||||
| 2 | Analog to represent the molecules | Space filling model | |||||||
| 3 | Animation effects used | Molecule 1: | Motion path | Molecule 3: | Spin | Molecule 5: | Teeter | ||
| Molecule 2: | Motion path | Molecule 4: | Spin | Molecule 6: | Teeter | ||||
| 4 | Direction | Molecule 1: | Straight | Molecule 3: | Clockwise | Molecule 5: | – | ||
| Molecule 2: | Straight | Molecule 4: | Clockwise | Molecule 6: | – | ||||
| 5 | Timing settings | Start | Molecule 1: | With previous | Molecule 3: | With previous | Molecule 5: | With Previous | Motions happened at the same time |
| Molecule 2: | With previous | Molecule 4: | With previous | Molecule 6: | With Previous | ||||
| Speed | Molecule 1: | Fast | Molecule 3: | Fast | Molecule 5: | Fast | Motions had the same speed | ||
| Molecule 2: | Fast | Molecule 4: | Fast | Molecule 6: | Fast | ||||
| Repeat | Molecule 1: | Until end of slide | Molecule 3: | Until end of slide | Molecule 5: | Until end of slide | Motions always happened | ||
| Molecule 2: | Until end of slide | Molecule 4: | Until end of slide | Molecule 6: | Until end of slide | ||||
| Delay | Molecule 1: | 0 second | Molecule 3: | 0 second | Molecule 5: | 0 second | |||
| Molecule 2: | 0 second | Molecule 4: | 0 second | Molecule 6: | 0 second | ||||
| 6 | Effect settings | Auto reverse | Molecule 1: | Checked | Molecule 3: | Unchecked | Molecule 5: | No opition for teeter effect | |
| Molecule 2: | Checked | Molecule 4: | Unchecked | Molecule 6: | |||||
| Smooth start | Molecule 1: | Checked | Molecule 3: | Unchecked | Molecule 5: | ||||
| Molecule 2: | Checked | Molecule 4: | Unchecked | Molecule 6: | |||||
| Smooth end | Molecule 1: | Checked | Molecule 3: | Unchecked | Molecule 5: | ||||
| Molecule 2: | Checked | Molecule 4: | Unchecked | Molecule 6: | |||||
| 7 | The phase of molecules | Gas | |||||||
| The direction of molecules when moving | Approached other molecules | ||||||||
| The deflection of molecules (if approaching other molecules) | No deflection | ||||||||
| 2. Analysis of PowerPoint animation generated by student 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Analysis | Conclusions | |||||||
| 1 | Number of molecules | 3 (all molecules had translational, rotational and vibrational motions) | • The effects were applied to all molecules | ||||||
| 2 | Analog to represent the molecules | Space filling model | • All molecules had translation, rotation and vibration | ||||||
| 3 | Animation effects used | Motion path | Spin | Teeter | |||||
| 4 | Direction | Straight | Clockwise | – | |||||
| 5 | Timing settings | Start | Molecule 1: | With previous | Molecule 1: | With previous | Molecule 1: | With Previous | Motions happened at the same time |
| Molecule 2: | With previous | Molecule 2: | With previous | Molecule 2: | With Previous | ||||
| Molecule 3: | With previous | Molecule 3: | With previous | Molecule 3: | With Previous | ||||
| Speed | Molecule 1: | Slow | Molecule 1: | Slow | Molecule 1: | Slow | Motions had the same speed | ||
| Molecule 2: | Slow | Molecule 2: | Slow | Molecule 2: | Slow | ||||
| Molecule 3: | Slow | Molecule 3: | Slow | Molecule 3: | Slow | ||||
| Repeat | Molecule 1: | Until end of slide | Molecule 1: | Until end of slide | Molecule 1: | Until end of slide | Motions always happened | ||
| Molecule 2: | Until end of slide | Molecule 2: | Until end of slide | Molecule 2: | Until end of slide | ||||
| Molecule 3: | Until end of slide | Molecule 3: | Until end of slide | Molecule 3: | Until end of slide | ||||
| Delay | Molecule 1: | 0 second | Molecule 1: | 0 second | Molecule 1: | 0 second | |||
| Molecule 2: | 0 second | Molecule 2: | 0 second | Molecule 2: | 0 second | ||||
| Molecule 3: | 0 second | Molecule 3: | 0 second | Molecule 3: | 0 second | ||||
| 6 | Effect settings | Auto reverse | Molecule 1: | Checked | Molecule 1: | Unchecked | Molecule 1: | No opition for Teeter Effect | |
| Molecule 2: | Checked | Molecule 2: | Unchecked | Molecule 2: | |||||
| Molecule 3: | Checked | Molecule 3: | Unchecked | Molecule 3: | |||||
| Smooth start | Molecule 1: | Checked | Molecule 1: | Unchecked | Molecule 1: | ||||
| Molecule 2: | Checked | Molecule 2: | Unchecked | Molecule 2: | |||||
| Molecule 3: | Checked | Molecule 3: | Unchecked | Molecule 3: | |||||
| Smooth end | Molecule 1: | Checked | Molecule 1: | Unchecked | Molecule 1: | ||||
| Molecule 2: | Checked | Molecule 2: | Unchecked | Molecule 2: | |||||
| Molecule 3: | Checked | Molecule 3: | Unchecked | Molecule 3: | |||||
| 7 | The phase of molecules | Gas | |||||||
| The direction of molecules when moving | Did not approach other molecules | ||||||||
| The deflection of molecules (if approaching other molecules) | – | ||||||||
Appendix 2. Approach to make vibration with motion path in PowerPoint
Appendix 3. Approach to make two or more effects play simultaneously in PowerPoint
Appendix 4. Approach to make deflection of molecules with motion path in PowerPoint
Acknowledgements
Many thanks to Widya Kartika Sari, S.Pd and Rila Andriani, S.Pd who helped in the transcription of students’ written explanations. Many thanks to Department of Chemistry of Universitas Negeri Padang and to students who participated in this study as well.References
- Adadan E., (2013), Using multiple representations to promote grade 11 students’ scientific understanding of the particle theory of matter, Res. Sci. Educ., 43(3), 1079–1105.
- Adbo K. and Taber K. S., (2009), Learners’ mental models of the particle nature of matter: A study of 16-year-old Swedish science students, Int. J. Sci. Educ., 31(6), 757–786.
- Ainsworth S., Prain V. and Tytler R., (2011), Drawing to learn in Science, Science, 333(6046), 1096–1097.
- Ainsworth S., Stieff M., DeSutter D., Tytler R., Prain V., Panagiotopoulos D., … Puntambekar S., (2016), Exploring the value of drawing in learning and assessment, Proceedings of international Conference on Learning Sciences, 2016.
- Akaygun S., (2016), Is the oxygen atom static or dynamic? The effect of generating animations on students' mental models of atomic structure, Chem. Educ. Res. Pract., 17(4), 788–807.
- Albert J. L., (2012), Using Student-Generated Animations about Water Boiling to Impact Student Understanding of the Particulate Nature of Matter, North Carolina State University, pp. 1–157.
- Atkins P. and De Paula, J., (2006), Atkins' Physical Chemistry, New York: WH Freman.
- Balagiu A., Zechia D. and Patesan M., (2019), Students PowerPoint Presentations as Assessment Tool for Learning Evaluation, eLearn. Softw. Educ., 2, 170–176.
- Barak M. and Hussein-Farraj R., (2013), Integrating model-based learning and animations for enhancing students’ understanding of proteins structure and function, Res. Sci. Educ., 43(2), 619–636.
- Bartsch R. A. and Cobern k. M., (2003), Effectiveness of PowerPoint presentations in lectures, Comput. Educ., 41(1), 77–86.
- Basturk R., (2008), Applying the many-facet Rasch model to evaluate PowerPoint presentation performance in higher education, Assess. Eval. Higher Educ., 33(4), 431–444.
- Berg A., Orraryd D., Pettersson A. J. and Hultén M., (2019), Representational challenges in animated chemistry: self-generated animations as a means to encourage students’ reflections on sub-micro processes in laboratory exercises, Chem. Educ. Res. Pract., 20(4), 710–737.
- Berk R. A., (2011), Research on PowerPoint®: From basic features to multimedia, Int. J. Technol. Teach. Learn., 7(1), 24–35.
- Bertea A. F., (2012), Use of new features and add-ins in PowerPoint to create animations that stimulate learning, In Conference proceedings of eLearning and Software for Education (eLSE) (No. 01, pp. 428–433).” Carol I” National Defence University Publishing House.
- Bucat B. and Mocerino M., (2009), Learning at the sub-micro level: Structural representations, in Gilbert J. K. and Treagust D. (ed.), Multiple Representations in Chemical Education, Models and Modeling in Science Education, vol. 4, Scotland: Springer, Dordrecht, pp. 11–29.
- Buckley B. C., (2012), Model-based learning, Encycl. Sci. Learn., 5, 2300–2303.
- Buckley B. C. and Boulter C. J., (2000), Investigating the role of representations and expressed models in building mental models, Developing models in science education, Dordrecht: Springer, pp. 119–135.
- Carmichael S. W. and Pawlina W., (2000), Animated PowerPoint as a tool to teach anatomy, Anat. Rec., 261(2), 83–88.
- Chang H. Y. and Quintana C., (2006), Student-generated animations: Supporting middle school students' visualization, interpretation and reasoning of chemical phenomena, Proceedings of the 7th international Conference on Learning Sciences, International Society of the Learning Sciences, pp. 71–77.
- Chang H. Y., Quintana C. and Krajcik J. S., (2009), The impact of designing and evaluating molecular animations on how well middle school students understand the particulate nature of matter, Sci. Educ., 94(1), 73–94.
- Chang H. Y., Quintana C. and Krajcik J., (2014), Using drawing technology to assess students’ visualizations of chemical reaction processes, J. Sci. Educ. Technol., 23(3), 355–369.
- Chang H. Y. and Tzeng S. F., (2018), Investigating Taiwanese students’ visualization competence of matter at the particulate level, Int. J. Sci. Math. Educ., 16(7), 1207–1226.
- Chittleborough G. and Treagust D. F., (2007), The modelling ability of non-major chemistry students and their understanding of the sub-microscopic level, Chem. Educ. Res. Pract., 8(3), 274–292.
- Coll R. K., (2006), The role of models, mental models and analogies in chemistry teaching, in Aubusson P. J. et al. (ed.), Metaphor and Analogy in Science Education, Netherlands: Springer, Dordrecht, pp. 65–77.
- Cooper M. M., Stieff M. and DeSutter D., (2017), Sketching the Invisible to Predict the Visible: From Drawing to Modeling in Chemistry, Top. Cognit. Sci., 9 (2017), 902–920.
- Cox A.M., Vasconcelos A.C. and Holdridge P., (2010), Diversifying assessment through multimedia creation in a non-technical module: reflections on the MAIK project, Assess. Eval. Higher Educ., 35(7), 831–846.
- Davidowitz B. and Chittleborough G., (2009), Linking the macroscopic and sub-microscopic levels: Diagrams, in Gilbert J. K. and Treagust D. (ed.), Multiple Representations in Chemical Education, Models and Modeling in Science Education, vol. 4, Scotland: Springer, Dordrecht, pp. 169–191.
- Davidowitz B., Chittleborough G. and Murray E., (2010), Student-generated submicro diagrams: A useful tool for teaching and learning chemical equations and stoichiometry, Chem. Educ. Res. Pract., 11(3), 154–164.
- de Wet C. F., (2006), Beyond presentations: Using PowerPoint as an Effective Instructional Tool, Gifted Child Today, 29(4), 29–39.
- DeAntonio M., Sandoval L. M. and Arceo R., (2006), Work in progress: a quantitative study of the effectiveness of powerpoint in the classroom, In Proceedings. Frontiers in Education, 36th Annual Conference, IEEE, pp. 22–23.
- Dickson H., Thompson C. D. and O'Toole P., (2016), A picture is worth a thousand words: Investigating first year chemistry students’ ability to visually express their understanding of chemistry concepts, Int. J. Innovation Sci. Math. Educ., 24(1), 12–23.
- Dikmenli M., (2010), Misconceptions of cell division held by student teachers in biology: A drawing analysis, Sci. Res. Essay, 5(2), 235–247.
- Dobson S., (2006), The assessment of student PowerPoint presentations-attempting the impossible? Assess. Eval. Higher Educ., 31(1), 109–119.
- Else M. J., Clement J. and Rea-Ramirez M. A., (2008), Using analogies in science teaching and curriculum design: Some Guidelines, Model based learning and instruction in science, Springer, Dordrecht: pp. 215–231.
- Eyal L., (2012), Digital Assessment Literacy—the Core Role of the Teacher in a Digital Environment, Educ. Technol. Soc., 15 (2), 37–49.
- Gharibyan H., (2005), Assessing students' knowledge: oral exams vs. written tests, ACM SIGCSE Bull., 37(3), 143–147.
- Gilbert J. K., (2004), Models and modelling: Routes to more authentic science education, Int. J. Sci. Math. Educ., 2(2), 115–130.
- Gilbert J. K., (2005), Visualization: A metacognitive skill in science and science education, in Gilbert J. K. (ed.), Visualization in Science Education, Netherlands: Springer, Dordrecht, pp. 9–27.
- Gilbert J. K., (2010), The role of visual representations in the learning and teaching of science: An introduction, Asia-Pacific Forum on Science Learning & Teaching, vol. 11, no. 1.
- Gilbert J. K. and Boulter C. (ed.), (2000), Developing models in science education, The Netherlands: Kluwer Academic Publisher.
- Gilbert J. K., Boulter C. J. and Elmer R., (2000), Positioning models in science education and in design and technology education, Developing models in science education, Springer, Dordrecht, pp. 3–17.
- Harle M. and Towns M. H., (2013), Students' understanding of primary and secondary protein structure: Drawing secondary protein structure reveals student understanding better than simple recognition of structures, Biochem. Mol. Biol. Educ., 41(6), 369–376.
- Hoban G., Loughran J. and Nielsen W., (2011), Slowmation: Preservice elementary teachers representing science knowledge through creating multimodal digital animations, J. Res. Sci. Teach., 48(9), 985–1009.
- Jackson J., Dukerich L. and Hestenes D., (2008), Modeling Instruction: An Effective Model for Science Education, Sci. Educ., 17(1), 10–17.
- Jespersen N.D., Brady J.E. and Hyslop A., (2012), Chemistry the molecular nature of matter, 6th edn, New Jersey, USA: John Wiley & Sons, Inc.
- Johnstone A. H., (1991), Why is science difficult to learn? Things are seldom what they seem, J. Comput. Assisted Learn., 7(2), 75–83.
- Jonassen D. H. and Strobel J., (2006), Modeling for meaningful Learning, In Hung D and Khine M. S. (ed.), Engaged Learning with Emerging Technologies. Netherlands: Springer, Dordrecht.
- Jones A. M. (2003) The use and abuse of PowerPoint in teaching and learning in the life sciences: A personal overview, Biosci. Educ., 2(1), 1–13.
- Justi, R. and Gilbert, J., (2006), The Role of Analog Models in the Understanging of the Nature of Models in Chemistry, in Aubusson P. J. et al. (ed.), Metaphor and Analogy in Science Education, Netherlands: Springer, Dordrecht, pp. 119–130.
- Justi R., Gilbert J. K. and Ferreira P. F. M., (2009), The application of a ‘model of modelling’to illustrate the importance of metavisualisation in respect of the three types of representation, in Gilbert J. K. and Treagust D. (ed.), Multiple Representations in Chemical Education, Models and Modeling in Science Education, vol. 4, Scotland: Springer, Dordrecht, pp. 285–307.
- Kealey R., (2000), Teaching animation using Microsoft PowerPoint, Aust. Screen Educ. Online, 23.
- Khan S., (2008), Co-construction and model evolution in chemistry, Model based learning and instruction in science, Springer, Dordrecht, pp. 59–78.
- Kozma R., (2003), Material and social affordances of multiple representations for science understanding, Learn. Instr., 13(2), 205–226.
- Lohr L. L., (2008), Creating graphics for learning and performance, 2nd edn, Ohio, USA: Merrill Prentice Hall.
- Lowenthal P. R., (2009), Improving the design of PowerPoint presentations, in Lowenthal P. R., Thomas D., Thai A. and Yuhnke B. (ed.), The CU online handbook. Teach differently: Create and collaborate, Raleigh, NC: LuLu Enterprises, pp. 61–66.
- McMurry J. E. and Fay R. C., (2003), Chemistry, 4th edn, New Jersey, USA: Prentice Hall.
- Núnez-Oveido M. C., Clement J. and Rea-Ramirez M. A., (2008), Developing complex mental models in biology through model evolution, Model based learning and instruction in science, Springer, Dordrecht, pp. 173–193.
- Nyachwaya J. M., Mohamed A. R., Roehrig G. H., Wood N. B., Kern A. L. and Schneider J. L., (2011), The development of an open-ended drawing tool: an alternative diagnostic tool for assessing students' understanding of the particulate nature of matter, Chem. Educ. Res. Pract., 12(2), 121–132.
- O'Day D. H., (2006), Animated cell biology: A quick and easy method for making effective, high-quality teaching animations, CBE–Life Sci. Educ., 5, 255–263.
- Pallant A. and Tinker R. F., (2004), Reasoning with atomic-scale molecular dynamic models, J. Sci. Educ. Technol., 13(1), 51–66.
- Pieketaleyee A. and Bazargani D. T., (2018), Exploring the Moves and Steps in TEFL MA Theses Introduction and Review of Literature PowerPoint Presentations: A Genre Analysis Approach, Theory Pract. Lang. Stud., 8(9), 1186–1194.
- Quillin K. and Thomas S., (2015), Drawing-to-learn: A framework for using drawings to promote model-based reasoning in biology, CBE–Life Sci. Educ., 14, 1–16.
- Ravitz J., (2002), CILT2000: Using technology to support ongoing formative assessment in the classroom, J. Sci. Educ. Technol., 11(3), 293–296.
- Rayner-Canham G. and Overton T., (2010), Descriptive inorganic chemistry, Macmillan.
- Rea-Ramirez M. A. and Núñez-Oviedo M. C., (2008), Model based reasoning among inner city middle school students, Model based learning and instruction in science, Springer, Dordrecht, pp. 233–253.
- Rowcliffe S., (2003), Using PowerPoint effectively in science education: Lessons from research and guidance for the classroom, School Sci. Rev., 84(309), 69–76.
- Schwarz C. V., Reiser B. J., Davis E. A., Kenyon L., Acher A., Fortus D., … Krajcik J., (2009), Developing a Learning Progression for Scientific Modeling: Making Scientific Modeling Accessible and Meaningful for Learners, J. Res. Sci. Teach., 46(6), pp. 632–654.
- Seel N. M., (2017), Model-based learning: a synthesis of theory and research, Educ. Technol. Res. Dev., 65(4), 931–966.
- Setia M. S., (2016), Methodology series module 3: Cross-sectional studies, Ind. J. Dermatol., 61(3), 261.
- Song J., (2012), Teaching Multiliteracies: A Research Based on Multimodality in a PPT Presentation, Theory Pract. Language Stud., 2(1), 113–117.
- Stephen M., (2006), Presentations with PowerPoint, Routledge.
- Stern L., Barnea N. and Shauli S., (2008), The effect of a computerized simulation on middle school students’ understanding of kinetic molecular theory, J. Sci. Educ. Technol., 17, 305–315.
- Stith B. J., (2004), Use of animation in teaching cell biology, Cell Biol. Educ., 3(3), 181–188.
- Stojanovska M., Petruševski V. M. and Šoptrajanov B., (2014), Study of the use of the three levels of thinking and representation, Contrib., Section Nat., Math. Biotech. Sci., 35(1), 37–46.
- Taber K. S., (2019), Alternative conceptions and the learning of chemistry, Isr. J. Chem., 59(6–7), 450–469.
- Takahashi L., (2011), Simple PowerPoint animation, Phys. Teach., 49(3), 189–189.
- Tan K. C. D., Goh N. K., Chia L. S. and Treagust D. F., (2009), Linking the macroscopic, sub-microscopic and symbolic levels: the case of inorganic qualitative analysis, in Gilbert J. K. and Treagust D. (ed.), Multiple Representations in Chemical Education, Models and Modeling in Science Education, vol. 4, Scotland: Springer, Dordrecht, pp. 137–150.
- Treagust D.F. and Chandrasegaran A. L., (2009), The Efficacy of an Alternative Instructional Programme Designed to Enhance Secondary Students’ Competence in the Triplet Relationship, in Gilbert J. K. and Treagust D. (ed.), Multiple Representations in Chemical Education, Models and Modeling in Science Education, vol. 4, Scotland: Springer, Dordrecht, pp. 151–168.
- Tsaparlis G., (2009), Learning at the macro level: The role of practical work, in Gilbert J. K. and Treagust D. (ed.), Multiple Representations in Chemical Education, Models and Modeling in Science Education, vol. 4, Scotland: Springer, Dordrecht, pp. 109–136.
- van Joolingen W. R., Aukes A. V., Gijlers H. and Bollen L., (2015), Understanding elementary astronomy by making drawing-based models, J. Sci. Educ. Technol., 24(2–3), 256–264.
- Williamson V. M., Watkins J. T. and Williamson III K. C., (2013), The effect of student-constructed animations versus storyboards on students’ mental rotation ability, equilibrium content knowledge, and attitudes. In Pedagogic roles of animations and simulations in chemistry courses, American Chemical Society, pp. 293–311.
- Yang W. C. and Chen L. H., (2013), A steganographic method via various animations in PowerPoint files, Multimedia Tools Appl., 74(3), 1003–1019.
- Yaseen Z., (2016), Student-generated animations and the teaching and learning of chemistry, Doctoral dissertation).
- Zanin M. K., (2015), Creating & Teaching with Simple Animation: Making Biology Instruction Come Alive, Am. Biol. Teach., 77(6), 463–466.
- Zhang Z. H. and Linn M. C., (2011), Can generating representations enhance learning with dynamic visualizations? J. Res. Sci. Teach., 48(10), 1177–1198.
- Zimmerman S. S., Zimmerman B. B. and Pinard K. T., (2010), New Perspectives on Microsoft PowerPoint 2010, Introductory, Cengage Learning.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0rp00229a |
| This journal is © The Royal Society of Chemistry 2021 |