Examining learning of atomic level ideas about precipitation reactions with a resources framework
Resa M.
Kelly
 *a,
Sevil
Akaygun
*a,
Sevil
Akaygun
 b,
Sarah J. R.
Hansen
b,
Sarah J. R.
Hansen
 c,
Adrian
Villalta-Cerdas
d and
Jonathan
Adam
a
c,
Adrian
Villalta-Cerdas
d and
Jonathan
Adam
a
aChemistry Department, San José State University, San José, CA 95192, USA. E-mail: resa.kelly@sjsu.edu
bDepartment of Mathematics and Science Education, Bogazici University, Istanbul 34342, Turkey
cDepartment of Chemistry, Columbia University, New York, NY 10027, USA
dDepartment of Chemistry, Sam Houston State University, Huntsville, TX 77340, USA
First published on 30th June 2021
Abstract
One particular challenge in chemistry learning is developing students’ atomic level understanding of chemical processes. It is necessary to help students learn how to critique atomic models rather than accept them as “truth.” In this study, we used a resources-based framework to examine how students made sense of macroscopic level information to account for what was happening at the atomic level. We interviewed 20 students enrolled in the first semester of general chemistry. Each student completed three exercises. The first exercise involved a card sorting task and the second exercise involved constructing an atomic model to learn how students made sense of the atomic level of a reaction involving the mixing of aqueous silver nitrate and aqueous sodium chloride to produce a precipitate. Next, students engaged in an exercise in which they were shown three conflicting atomic level animations of the same experiment and they were charged with selecting the animation that was most scientifically accurate. We analyzed the general patterns of characterization that emerged when students engaged in the card sorting and modeling exercise and the conflicting animation exercise using a resources framework. We contend that students apply and sometimes misapply knowledge resources to make sense of the atomic level. The process affects decisions that they make and stances that they develop about the accuracy of atomic level models.
Introduction
It is not uncommon for introductory general chemistry students to have misconceptions about the particulate nature of precipitation reactions commonly referred to as double replacement reactions (Yarroch, 1985; Ben-Zvi et al., 1987; Garnett et al., 1995; Ahtee and Varjola, 1998; Kelly et al., 2010). Kelly et al. (2010) studied the nature of first-semester general chemistry students’ misconceptions about the atomic level of three molecular equations. Their findings revealed that many students tended to map their submicroscopic level beliefs onto symbolic equations for specified reaction equations even after receiving traditional instruction and laboratory investigations. Students who are taught about precipitation reactions with a symbolic emphasis may have difficulty understanding how the molecular equation relates to the total ionic equation. From studies of salt dissolution, it was observed that students struggle with the dissolution of salts and how to represent the solute and solvent substances (Kelly and Jones, 2007). Another common misconception is that students depict aqueous reactants as molecular pairs before mixing and reason that upon mixing the compounds break apart to exchange partners (Kelly and Jones, 2007; Kelly et al., 2010). Students also conclude that the precipitate is composed of molecular pairs that somehow settle while the aqueous product is also made of molecular pairs (Kelly et al., 2010). In some cases, students believe that aqueous product molecules form but then dissociate. It is apparent that students hold a wide range of ideas that are not entirely consistent with what we want them to know about the atomic level of precipitation reactions. To facilitate learning and get students to consider what they know about the atomic level, our research explores the resources students use to make sense of the atomic level of a precipitation reaction.Learning and the benefits of comparing incorrect and correct examples
In cognitive psychology, learning is broadly viewed as an internal mental process that involves interaction between what the student is taught and their current ideas or concepts. How students respond to a piece of information presented to them depends both on what they know already and on the information they are cued to access. Learning complex abstract information requires substantial repetition and practice, and it also involves consideration of the learning environment or context that fosters appropriate patterns of association. To explicitly define learning, Posner et al. (1982) stated that “learning is a rational activity,” and it is best viewed as a process of conceptual change. To further elucidate learning as a construct, Posner et al. (1982) stated that “learning is fundamentally coming to comprehend and accept ideas because they are seen as intelligible and rational.” It is an inquiry in which students make sense of evidence. However, a limitation of this learning theory is that it focuses on conceptions as the basic unit of cognitive structure, which leads to identifying incorrect conceptions as misconceptions (Hammer et al., 2005). When students form explanations, it is assumed that the ideas stem from “precompiled” knowledge and misconceptions that are simply wrong, robust conceptions. We next review research that examined ways to challenge misconceptions through a comparison of incorrect and correct examples that is relevant to our treatment involving contrasting animations.One way to directly examine how students’ current ideas interact with incompatible ideas is through comparisons of correct and incorrect models. Models that conflict or contrast encourage students to reflect on and reorganize their cognitive structures and experience conceptual change. Several lines of research across a variety of disciplines have examined the importance of instructional support that explicitly compare and contrast between erroneous models and correct models (Eryilmaz, 2002; Huang et al., 2008; Van den Broek and Kendeou, 2008; Durkin and Rittle-Johnson, 2011; Asterhan and Dotan, 2018). For example, in physics education, Eryilmaz (2002) studied how discussion, in which teachers shared students’ ideas, both correct and incorrect, then monitored discussion, encouraged confrontation about their different ideas before introducing them to a discrepant event designed to challenge incorrect beliefs. This activity helped students to reduce their misconceptions and improved their physics achievement as measured via pre- and posttest measures on the Force Achievement Test. In mathematics learning, contrasting incorrect examples (comparing one correct and one incorrect example) assisted students (4th and 5th Graders), even those with limited prior knowledge, to learn correct concepts and procedures above and beyond the benefits of comparing only correct examples (Durkin and Rittle-Johnson, 2011). Van den Broek and Kendeou (2008) investigated the effects of readers’ incorrect knowledge on the on-line comprehension process during the reading of science texts to encourage revision of knowledge. In their refutation textbook, they positioned examples of some common misconceptions through presenting the correct scientific explanations to simultaneously activate correct and incorrect conceptions to promote conceptual change. They concluded that a crucial first step in achieving a conceptual change of students with misconceptions was to create a situation in which students had to confront misconceptions and correct conceptualizations simultaneously. They referred to this as co-activation. Co-activation enhanced the chance that students recognized a conflict, an important first step toward conceptual change. In biology education, a study by Asterhan and Dotan (2018) examined the effect of feedback that corrected and contrasted a student's wrong solutions with a canonical, correct one on a conceptual change task. Their study revealed that giving students detailed corrective feedback that explicitly contrasted correct explanations with an erroneous, student-generated explanation improved students’ conceptual understanding as measured by an assessment of conceptual understanding on transfer items. In these studies, students were taught what is correct and incorrect through the feedback they received or the explicit identification of the conflict, but they were not empowered to ascertain the nature of the conflict on their own.
Multiple external representations
Research on learning from multiple representations as synthesized by Ainsworth (2006) reports four fundamental aspects of learning that should be considered when students are tasked with external representations such as videos, simulations, and animations: First, learners must consider how a representation connects to and presents information; second, learners must examine the connection between the representation and the concepts it represents; third, students should have the agency to select an appropriate representation and they should be mindful of their reasons; and fourth, students should then have the agency to construct or invent an appropriate representation to communicate their understanding.Chemistry concepts that reflect the particulate nature of matter, as it is connected to the macroscopic domain it represents, provide the context for this investigation. Specifically, learners should understand how a representation such as an atomic level animation is connected to or presents information about macroscopic behavior. Often, educators assume that the macroscopic level is the more obvious and easier to understand of the components of chemistry first introduced by Johnstone(1993). However, researchers have reported that students can lack understanding of the macroscopic level too, and this makes it challenging to connect to this level (Talanquer, 2011; Taber, 2013; Kelly, 2014). The complexity of learning from multiple external representations can be beneficial, such as providing students with the opportunity to connect abstract concepts to more concrete ideas and building inferences (Ainsworth, 2006). However, studies also report the challenges and misconceptions that students can develop as they work to make sense of what they view (Rosenthal and Sanger, 2012, 2013; Kelly et al., 2017). It is recommended that learners understand individual representations and then consider the relationships between representations as they learn to integrate information from more than one source.
Ainsworth (1999) shared three key functions of multiple external representations (MERs): to complement, constrain, and construct. Complementary processes are those representations that “theoretically contain the same information but differ in their advantages for learning in certain situations due to the extent to which they support computational offloading, re-representation, or graphical constraining. In this study, the animations are re-representations of the macroscopic event focused on the atomic level. In this sense, the videos of evidence and the animations serve to complement each other because they present different information about the same event. In addition, they serve to constrain how learners think about the atomic level of a macroscopic event as the macroscopic evidence can help students make sense of the more abstract atomic level of moving ions, atoms and molecules. Lastly, multiple representations support the construction of deeper understanding as learners integrate information from the different representations to make sense of the phenomenon. This enhanced cognitive process is referred to as abstraction as learners create mental entities that serve as the basis for new concepts (Ainsworth, 1999).
Contrasting animations
Our research team first began using contrasting animations of a reduction–oxidation reaction to examine how the experience affected students’ understanding of the redox process involving the reaction between solid copper and aqueous silver nitrate (Kelly, 2017; Kelly and Hansen, 2017; Kelly et al., 2017). In this body of research, we made an animation to contrast with a well-known and very accurate and detailed animation designed by VisChem. While our animation had a similar color scheme, it was noticeably more simplistic (fewer moving species) and shorter in duration lasting approximately 21 seconds compared to the nearly four-minute VisChem animation. There was one caveat, our animation was designed to include a common misconception. Findings revealed that nearly half of the students were unable to determine that the VisChem animation was the more scientifically accurate animation, in spite of the huge differences in the complexity of the models, which would seem to direct students’ attention to favor the longer more complex animation, in this case, the VisChem animation. Meaningful learning from contrasting animations required learners to articulate connections between their ideas, the experimental evidence, and the information depicted in the animations. Students who had better recall of basic chemistry concepts and could make sense of macroscopic experimental evidence had greater success in selecting the best animation and in adapting their explanations to fit with the animation. Students who exhibited less ability to recall basic chemistry as related to redox or were unable to relate to the macroscopic evidence were enticed by the simplistic animation that was easier for them to explain. However, the animation models were substantially different from each other and even though students described how the animations were similar or different to their understanding, many students were unable to discern that the animations conveyed different reaction mechanisms (Kelly et al., 2017). The work called attention to the need to examine how students make sense of contrasting animations and how this affects their ability to select the most scientifically accurate model.More recently, we used eye-tracking and qualitative analysis to investigate the impact of viewing contrasting animations with structured variation and eye-tracking feedback on visual attention (Hansen et al., 2019). We reported that students’ visual attention shifted when chemically relevant features differed in accuracy and they reconsidered the link between macroscopic experimental evidence and submicroscopic representations after viewing these variations. More importantly, students who were presented with contrasting animations needed to confront the contradicting ideas presented to decide which animation was most scientifically accurate, and this resulted in more accurate drawings concerning the chemically relevant features emphasized in the animations. While this study provided evidence that students were focusing on the chemically relevant features in the animation, further study is needed to examine how students reconcile with their prior beliefs to make sense of animation features and what they do when their understanding is not an exact fit.
Theoretical framework
Resources-based framework of cognitive structure
The framework we have selected as the lens for analyzing our data is the resources-based framework introduced by Hammer et al. (2005). We chose this framework because we are particularly interested in how students activate resources, applying knowledge gained in other contexts to reason about the atomic level of a precipitation reaction. The resources framework is founded on a manifold ontology of mind, knowledge and reasoning abilities made from many fine-grained components, known as resources, that may or may not be activated in a particular context (Hammer et al., 2005; Elby and Hammer, 2010). The resources-based framework views learning as a cognitive state the learner enters or forms that involves the activation of multiple resources (Hammer and Elby, 2002; Elby and Hammer, 2010). The structure of the cognitive state provides meaning and organization to experiences, helping students make sense of new information and retrieve stored information. However, when we are first learning, it is not uncommon for beginning students to develop naïve epistemologies, meaning that they draw on context dependent resources that can be activated appropriately or inappropriately. In this manner, students may develop mini-generalizations when the context of the event triggers a resource or activates an intuitive idea that may or may not be an appropriate fit or could be misapplied. The resources framework predicts that students’ epistemologies are not necessarily stable structures, and there will be shifts in thinking and reasoning during learning. Our research shines a lens on students’ naïve epistemology as they examine their beliefs about the atomic level.In a resource-based framework learning is best viewed as a cognitive state the learner enters or forms as a local or momentary activation and it involves multiple resources that may be applied correctly or incorrectly (Hammer et al., 2005). Hammer and Elby (2002) posited the existence of four categories of epistemological resources that include: (1) resources for understanding the nature and sources of knowledge, such as what knowledge is and how it arises. Resources within this category include knowledge as propagated stuff, knowledge as fabricated stuff, knowledge as free creation. (2) Resources for understanding epistemological activities; employed to understand and engage in activities. These are resources students may use to answer the question: “What are you doing?” Resources within this category include accumulation, formation, checking, application, comparing, sorting, naming, counting, and adding. (3) Resources for understanding epistemological forms; resources that are engaged while doing activities to promote understanding. Resources within this category include Stories, rules, rule systems, songs, lists, pictures, categories, statements, words, names, and numbers). (4) Resources for understanding epistemological stances, stances include belief and disbelief, doubting, puzzlement, understanding and acceptance). In our research we are focusing on resources that were used and students’ epistemological stances, including belief and disbelief. In particular, we will show how a student's belief or disbelief appears with their review and critique of the accuracy of the animations that stems from their trust or distrust of their understanding.
Research question
In our study, we recognized inductively that we manipulated and constrained the context of learning by intentionally guiding students to draw on a few productive resources. We specifically asked students to compare the atomic level cards and to use evidence from an entry video of the experiment to make their card selections. In addition, we asked them to construct a dynamic atomic model (modeling) to assist them in thinking more deeply about the atomic level, and to learn what knowledge resources students invoked to explain the atomic level of the precipitation reaction before they performed the animation activity. Upon completing the card sort and modeling exercise, students were guided to compare the animations to the model they constructed and they were asked to judge the accuracy of the animation components revealing their epistemological stance.We investigated the following research question:
What epistemological resources do students invoke and what stances do students engage with during the exercises in the study? In other words, we were interested in the forms of knowledge that students accessed to make sense of the exercises and how this affected students’ beliefs toward the model they constructed and their position toward the animations.
Methods
Participants and the context of the study
After obtaining approval to conduct the study from the university's Institutional Review Board, an instructor's approval was obtained to solicit the participation of students from two sections of first semester General Chemistry by oral announcement. Students who expressed interest were selected based on their availability and their responsiveness to email requests to participate. Twenty students consisting of fourteen males and six females of diverse ethnicity: 40% Asian, 20% Caucasian, 15% Hispanic, 15% Asian Indian, 10% multi-race were each interviewed by the researcher (first author) face-to-face over six weeks from February 2018 to April 2018. All students received a small stipend for their participation. Most of the students were interviewed individually, apart from two students (identified as K16 and K17), who participated together as it was the only time that worked for their schedules. Most of the students were enrolled in the laboratory that accompanied the General Chemistry course. There were two participants, K2 and K18, that had completed and passed General Chemistry before spring 2018. K2, a chemistry major, completed the course and lab three years prior to the study and served as a mentor for the students in the course. K18 completed the course with the laboratory one semester before the study and learned of the study from friends in the course. At the beginning of each approximately two-hour session, the consent form was reviewed with the participant. They were assured that their participation would have no bearing on their course grade, and they were informed of how their anonymity would be protected and how information from the study could be shared. All participants agreed to the conditions.Prior knowledge and experiences are important to help us understand how students build new knowledge and how students respond to the research exercises, thus we present key lecture and lab background experiences. All students had completed a lab on conductivity that emphasized how conductivity could be useful for identifying strong and weak electrolytes, and how to represent solutions symbolically based on the evidence. For example, aqueous sodium chloride is a strong electrolyte that conducts well in solution and can be represented as NaCl(aq) or Na+(aq) + Cl−(aq) while a weak acid, such as acetic acid conducts weakly and would best be represented as molecules of HC2H3O2(aq). Also, the students had completed a Mystery Bottles lab in which the objective was to identify aqueous salt solutions in five unlabeled bottles based on a series of precipitation reactions via a qualitative scheme (Singmaster, 2018). Students received instruction on how to use solubility rules to identify whether a substance was soluble or insoluble and predict whether a precipitate would form from mixing two aqueous salt solutions in a double replacement reaction. They also were required to write molecular equations, total ionic equations, and net ionic equations for all reactions.
Interview sessions
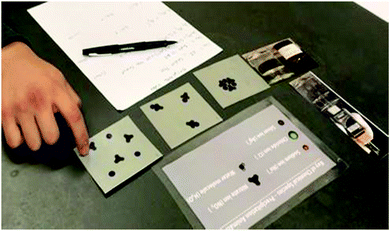
Each interview session consisted of three parts: (1) video of experimental evidence, (2) card sort and modeling exercise, (3) contrasting animations exercise.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 58 min long) that showed how four solutions of silver nitrate, copper(II)nitrate, sodium sulfide nonahydrate, and sodium chloride were made (Fig. 1) (Kelly et al., 2018a, 2018b, 2018c, 2018d). The concentrations were unknown. Initially, the conductivity of pure water for each solution was tested and shown to be zero in every case. After making each solution, the conductivity was measured again and noted to be at the maximum value of 10 on a 10-level conductivity tester for all four solutions. Next, two solutions were mixed at a time until six possible combinations were completed. In three cases, a precipitate formed and in three cases, no reaction was noticed. The resulting solutions were transferred to beakers and the conductivities of the product solutions were tested. Students were encouraged to view the video as many times as they wanted, and they were invited to take notes if they wished. Following the video, students were asked to describe the familiar and unfamiliar processes in the video. They were also asked to share anything that they knew about precipitation reactions based on their educational experiences.
58 min long) that showed how four solutions of silver nitrate, copper(II)nitrate, sodium sulfide nonahydrate, and sodium chloride were made (Fig. 1) (Kelly et al., 2018a, 2018b, 2018c, 2018d). The concentrations were unknown. Initially, the conductivity of pure water for each solution was tested and shown to be zero in every case. After making each solution, the conductivity was measured again and noted to be at the maximum value of 10 on a 10-level conductivity tester for all four solutions. Next, two solutions were mixed at a time until six possible combinations were completed. In three cases, a precipitate formed and in three cases, no reaction was noticed. The resulting solutions were transferred to beakers and the conductivities of the product solutions were tested. Students were encouraged to view the video as many times as they wanted, and they were invited to take notes if they wished. Following the video, students were asked to describe the familiar and unfamiliar processes in the video. They were also asked to share anything that they knew about precipitation reactions based on their educational experiences.
Following the reactants, students were shown two pictures of the resulting product solution, as seen in the video. One picture showed the results of the mixed solutions, and another showed the same solution being tested for electrical conductivity. Three pictures illustrating possible atomic level representations of the products were also presented along with a key of atomic level species. Each student was asked to select the picture that best represented the atomic level of the solution. A semi-structured interview accompanied the card selection task. Students were asked why they selected their card and how closely it matched with their ideas. They were also asked: (1) Why did you not select the other cards? (2) Did you use the conductivity test information to make your selection? and (3) Did you think about the chemical formulas or equations to make your selection?
For each animation, each participant was instructed to critique each animation and ultimately decide which one was the most scientifically accurate of the three based on the animation's fit with the video of experimental evidence. They were asked to rate each animation on its depiction of the same four attributes that they were presented with in the card sort and reaction modeling exercises: reactants, mechanism, and products. Specifically, they rated the accuracy of the representations of silver nitrate and sodium chloride reactant solutions before reacting, the mechanism for how reactants changed into products and the product solution on a scale of 1 to 5 (1 being inaccurate and 5 being accurate). In the semi-structured interview that followed each animation, students were asked to explain their ratings and to reflect on how the animation was similar to and different from their card sort and modeling exercise. Ultimately, students were asked to choose the animation that they felt was best or most scientifically accurate of the three animations.
Data analysis
Upon completion of the video-recorded interviews, the interviews were transcribed verbatim. The transcripts were analyzed using a constant comparison methodology to find answers to the research question (Merriam, 2001). The analysis involved separating participants’ transcripts into three major groups based on the animation they selected as being scientifically accurate (animations 1, 2 or 3). Next, each transcript was divided into subsections that coincided with the methodology stages: (1) card sort exercise, (2) reaction modeling exercise, and (3) contrasting animations exercise. We reviewed the subsections of each interview to map the resources and stances that were activated in accordance with Hammer and Elby's (2002) four categories of epistemological resources (Appendix A). Next, we examined each of the exercises.In order to ensure the dependability of the codes, an inquiry audit was used to authenticate the application of the codes (Lincoln and Guba, 1985). The auditor examined the assignment of codes for their accuracy, discussion ensued when there were disagreements, and consensus was reached on all codes reviewed.
Results and discussion
An initial stage of our analysis was to examine the video and transcribed interviews to identify the epistemological resources students exercised during the card sort and contrasting animations exercise based on Hammer and Elby's (2002) framework consisting of four categories of epistemological resources. We observed all twenty students engaged in comparing during the interviews, indicating that students drew comparisons between two or more representations (i.e., cards and animations). This was not surprising as the context of the study was intended to invoke the comparing resource as a way to help students think more deeply about the animations, and we specifically asked students why they selected one card or animation and not another. In this regard, we manipulated the context of learning or constrained it and emphasized the use of comparing, a productive resource that students have likely used in other contexts. Our reason for invoking comparing was for the specific purpose of critiquing pictures and animations and promoting students’ awareness of why they were selecting one card or animation and not another. Another manipulation of context was to engage all students in the model building activity, such that they had to model (a resource for understanding epistemological form) how the reactants as illustrated in the cards they selected transitioned to become the products represented in their card selection. While the nature of the exercises constrained the context and the entry and exit conditions, students brought their previous knowledge and ideas to make their card and animation selections and constructed a model.Card sort exercise
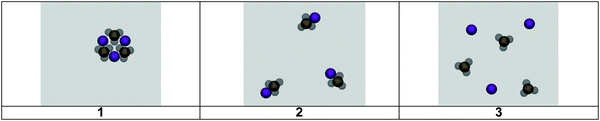
Students were asked to compare the atomic level cards, but they were also asked to consider how their card selection fit with the experimental evidence. The card they selected cued particular resources and stances and guided the progression of inquiry. We now examine the card sorting task: selecting a card that represents the atomic level of two reactants aqueous silver nitrate and aqueous sodium chloride and the products (aqueous sodium nitrate and solid silver chloride).Resources applied during the reactant card sorting exercise
As students engaged in the card selection tasks, a constraint of the exercise was to direct students to relate macroscopic evidence to the submicroscopic level; this was done by presenting pictorial reminders of the macro-level solutions from the video to show the salt dissolution and electrical conductivity evidence. Students were also asked to explain the reason behind the card they selected and why they did not select the other cards. This served as an additional constraint that caused students to compare the representations. We now share two cases: Students K4 and K11 to demonstrate how an analysis of resources reveal what students do to make sense of the atomic level when guided to consider the fit between experimental evidence and atomic level and to compare representations.K4: (takes a while to choose) I'm thinking about the charges what I'm going to pick is based on what are the charges of the nitrate and the silver and then I have to figure out what these are representing. The nitrate ion says NO3− so there is one nitrogen and three oxygens and oxygens are going to be negative because there's the minus, and for the silver ion that's going to be positive so the silver ion is going to attract with the oxygen, so based on that (pauses) also I’m thinking about how close or far apart they are with each other. I know that liquids tend to have the molecules not as condensed as you know with the solid the atoms are usually rigid and really close together and liquids, the atoms tend to be like, not as close to each other, but not super far away, and just kind of moving around each other as with gases that are far and moving all over the place. For that reason I'm choosing two.
R: Why did you select card 2?
K4: I know it wasn't three for sure.
R: How do you know it's not three?
K4: Because if it's silver nitrate they are supposed to be bonded together and it's clear they are not bonded at all together. With one, I was going to pick one, but I'm not sure if they're connected right, the NO3−, the oxygens are like the lighter grey and they are connecting with each other and like charges are supposed to repel so I'm thinking that that wouldn't make any sense because they wouldn't be attracted to each other because they are the same charge. With number two you have the positive silver ion and you have the negative oxygen and you see here that they attract to each other, and also because it's a liquid solution they are going to be near each other but they're not going to be like condensed and like next to each other.
R: Why did you not select the other cards?
K4: One, it is like too close to each other indicating it being a solid and the oxygens are negative and they wouldn't be attracted to each other, they would be repulsed and the atoms are not connected to each other at all so that wouldn't make any sense because they are supposed to be one compound.
K4 drew upon a few resources for understanding the epistemological activity. Initially, he considered symbolic information, recalling rules for constructing formulas, and the rule that opposite charges attract while like charges repel. He also considered what he had learned about states of matter, applying knowledge of how the spacing of molecules in the liquid state compared to molecules in the solid state. He eliminated card 3 because it went against the rule that opposite charges should attract. K4 seemed to ignore the presence of water molecules in spite of the instructions that water molecules were not included to simplify the pictures. It is important to note what K4 did not do. He did not seem to consider the conductivity evidence, and he was asked about this. He replied, “I don’t think it was relevant in this particular case. I just thought you could tell by the atoms and what the charges are.” K4 did not use his experience from the lab in which he had to write a net ionic equation for salt solutions that were strong conductors. He did not see the relevance and shared that he could select a card without the conductivity information.
R: Why did you select card three?
K7: I selected card three because I know all nitrates, oh that's nitrite, no it's nitrate. So I know that all nitrate ions are soluble like when they combine with silver and I’m pretty sure it's soluble so I know that they would dissolve and dissociate. I’m pretty sure. So having them all separated is what I think about when it's dissolving and dissociating, I believe. And I picked, obviously there is blue(refers to silver ion) and there's nitrate ion. I knew it wasn’t this one (card 1) because I feel like that one would form a solid.
R: That's my next question: why did you not choose the other two cards?
K7: I’m pretty sure this is a solid (card 1) because it's all staying together. In the video I wrote down I think it made a solid, but I don’t know I thought it made a solid? But you said it dissolved so that made me go back over here (card 3). And then I didn’t pick this one (card 2) because I was between this (card 2) and this one (card 3). I didn’t know actually yeah because the nitrate ion and the silver ion I thought they would break up because they were soluble. That's why I picked this one (card 3).
In order to make her selection, K7 draws on her knowledge of solubility rules, all nitrates are soluble, to select card 3. She also compared cards 1 and 3 and identified card 3 as representative of the solid state of matter. She applied her intuitive knowledge of states of matter. K7 focused on the nature of the aqueous solution, and she did not consider the conductivity evidence. When asked if she would like to consider electrical conduction she responded:
K7: I didn't even think about that, conductivity means, oh conductivity means when something is soluble, conductivity is high, I believe. So knowing that these two break up, the conductivity is going to be high. So I guess it just reassured me that this picture (card 3) is the answer.
Once K7 was reminded of the conductivity evidence, she was able to recall the connection to conductivity which reinforced her confidence in her card selection.
To provide a sense of the information the participants considered as they compared the representations and reasoned about which card best represented the atomic level, we observed that the following concepts were applied to make sense of the context: (1) dissolution, a process that involves breaking up on a macroscopic level and dissociation of ions/atoms/molecules at an atomic level; (2) states of matter, identification or connection to three previously learned atomic level representations as they represent physical states of matter – solid, liquid and gas; (3) macroscopic measures of high electrical conductance has connections to the atomic level; (4) symbolic formula representations were used to think about charge attraction and the submicroscopic representation.
K3: In order for a solution to be conductive it has to have free ions. I remembered that in order to be conductive it should have the ions so I went through it really quick and it helped me to choose the card I chose.
There were a few students that incorrectly recalled that conductivity reminded them of “something that would “stay together”. K1 explained, “Since it's all clustered together that might make energy more transferable or easier to pass through.” This recollection caused K1 to pick the solid-like representations for each card, which did not fit with his understanding of dissolution. He rationalized that dissolution happened after the solid dissolved, while conductivity was measured when undissolved solid was still present.
A condition of this exercise was that students were asked to compare the card representations to select the one that best represented the atomic level. More than half of the students were able to narrow their selection to two cards: the paired representation and the separated ion representation. They articulated a strong connection to the formula and consequently the paired representation, but they were conflicted in knowing strong electrical conductivity was associated with free ions. A few students wavered between the solid representation and the paired representation expressing that both represented liquid states. Students compiled their explanations from resources that were fundamentally correct, but often applied incorrectly or misapplied. This is consistent with the findings of Hammer et al. (2005) in studies of physics learning.
Products: aqueous sodium nitrate and solid silver chloride
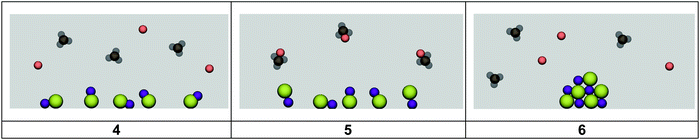
As a reminder, students were presented with macroscopic level images from the video showing the formation of a milky solution after mixing the aqueous solutions of silver nitrate and sodium chloride. They were provided with three atomic level representations from which to select the image that most accurately represented the atomic level of the product solution (Fig. 5).Resources applied during the product card sorting exercise
As students engaged in this task, the same constraints applied. Students were directed to select an atomic level card that best represented the macroscopic level observed in the pictures. Students were also asked to explain the reason behind the card they selected and why they did not select the other cards. This served as an additional constraint that caused students to compare the representations. We now share three cases:Reactant – Card sort exercise: Which card best represents the atomic level of the product solution?
R: What features do you like about card 4?
K5: Well the thing is the conductivity level is also low, also I believe one of the solutions can't break up but then I suddenly just realized that I forgot what the solution is. Is it okay if I look up the formulas? I just want to see the formulas (goes back and reviews the video so that she can see the formulas and equations). …I remember most of the chlorides breakup go but then Na is from (group)1A so they both break up. Okay, this kind of changes my opinion a little bit since I saw the formula. I feel like I want to go with, let's see, is it okay if I write the formulas (R: sure)
K5: (She writes the equation) This should break up. Does it? Okay, I'm going to select card 5 because…I believe silver is one of those things that chloride can't break up with.
R: Could you recap why you picked card 5?
K5: Well the thing is, actually I keep changing my mind, I accidentally mixed up. Okay I think I will change my mind to six, because…I remember agonizing in lab that silver chloride didn't break up and then I remembered that nitrate breaks up with anything although sodium is within the group 1a category, also there's a lot of free ions in here which explains the conductivity also it's cloudy which explains the silver chloride, which is why I am going to choose six.
In the case of K5, we see that she uses several resources to make her card selection. Initially she tries to recall and apply a connection between conductivity and solution opacity, but it is unclear how she is using this information to inform her card selection. She works to retrieve information from her mastery of formula construction and associates it with the solubility rules, which allows her to check on whether the product compounds dissociate. From here, she goes back to the conductivity evidence, she is able to recall that the presence of free ions is responsible for the conductivity, and she also recognizes that the cloudiness is due to the silver chloride precipitate or solid state. Later, when asked about her use of the conductivity evidence she provides evidence of using this knowledge to compare the cards:
“Yes, I actually got it right on the latest quiz, which is if it's soluble there are free ions that meet. It's conductible which means card 6 is most likely right. Card 4 is possibly right, but card 5 is definitely wrong.”
Thus she seems uncertain about the connection between a cloudy solution and whether the ion pair model would be better than the aggregate, but she selects card 6 which represents the solid precipitate as an aggregate.
K11: I think it would be this picture here, number five.
R: Tell me why you selected that picture.
K11: I selected that picture because, since in this case, it would be a double replacement reaction, the metals would switch places with the ions that they would be attached to, for example the silver instead of being with the nitrate it would be with the chloride in the finished product, in that case this(points to the macro level solution picture) is what we are talking about. So when we see the silver and the chloride, that would be a blue plus a green (referring to the atomic level card), so yes that makes sense and that would mean immediately eliminate number six and then the next part would be the sodium and the nitrate. So we have to look for the sodium and the nitrate together. This (card 4) does not have that picture however this one (card 5) does. So in this case number five would be the correct answer.
R: Why not the other two cards?
K11: I did not pick the other two pictures because they do not illustrate the ions in which they were, in which the metals combine and with the other respective ions. For example, instead of silver with the nitrate it would be silver with the chloride and instead of sodium with the chloride it would be sodium and the nitrate. And the other two pictures do not illustrate both of these together.
When K11 was asked to select the best atomic level card for the reaction, the context activated those resources he associated with double replacement reaction equations. K11's resources about double replacement reactions were actually correct although it was applied incorrectly to serve as a reflection of the atomic level of the reaction. However, applying this resource in another context such as solving a stoichiometry problem, could be very useful. In addition to activating his application resources, K11 also compared the three representations and was very certain of how they differed. K11 did not mention the conductivity evidence on his own accord, but he was asked if he thought about it to make his selection. He replied:
K11: No I didn't think of it as I made my selection however it further validates my selection as a high conductivity just because since both of these samples were, they would almost completely, the ions would completely split apart like upon a reaction that they would have because there is a lot of ions it would be high conductivity but obviously since you have instead of just one sample but you have two samples and no sample is perfect there is no such thing as a 100% complete like splitting apart of the ions so if it is only nine instead of 10 that's probably the reason why unless the actual conductivity tester is somewhat outdated, but that can also account for why it's nine and not 10.
Once again K11 was able to apply resources associated with his understanding that substances with free ions conduct, however he misapplied it to develop an explanation that allowed him to retain his belief that the reaction happened by switching ions and at the moment of switching the ions would be free, which fits with both of his resources about conductivity and resources about balancing double replacement reaction equations.
In summary, the card sort exercise tasked students with drawing comparisons among the atomic level representations for their fit with the macroscopic experiment. The exercise also resulted in all twenty students activating the resource – application. Application involved using a piece of existing knowledge to make a decision or in this case select a card (Hammer and Elby, 2002); however, there were instances of struggle and misapplication of resources. For example, students applied their symbolic level understanding of chemical equations (molecular, total ionic, net ionic), double replacement patterns, and solubility rules to make decisions about the representations. The value of this resource application was that it helped students identify the product that was responsible for the cloudy appearance of the solution; however, often students were compelled to retain the paired look of the formulas in the equation in conceptualizing the atomic level. Students justified the formulaic look by applying knowledge that opposite charges attract while seeming to ignore the role of water in the reaction. Another resource that students accessed was their knowledge of the atomic level of physical states of matter (solids, liquids, and gases), although there was a struggle to represent an aqueous solution and a cloudy solution as this did not work with their rigid beliefs about solids and liquids. It seems that their knowledge resource regarding states of matter was fundamentally correct, but they were unable to apply this knowledge to fit the reaction they observed. Students’ more recent experience with electrical conduction tests was also applied by some students, but some had difficulty recalling whether conductivity was influenced by ions that were free or that were positioned close together, this sometimes affected the application of the resource. Ultimately, students applied intuitive knowledge resources to make their atomic level card selection.
Reaction modeling exercise
After the card sort exercise, students were given the reactant cards and product card they selected and a set of magnetic atom pieces that matched with the species in the atomic level card representations. Students were asked to provide a model and a description of the movement and interaction that must occur to progress from reactants to products. We examined the knowledge resources students applied and found that the most common resource exercised was knowledge of molecular and total ionic equations. Students also applied knowledge of opposite charge attraction and like charge repulsion.Students’ description of the reaction mechanism was classified into three groups (Table 2): (1) accurate model – representing the attraction between silver and chloride ions to form an aggregate of ions while maintaining sodium and nitrate ions as separate species in solution. (2) Double replacement model – representing that the products formed ionic pairs. (3) Unorthodox model – representing interactions that did not fit in the previous categories.
| Models | Classification features | Students |
|---|---|---|
| Accurate | AgCl forms aggregate, aqueous product remains separate ions in solution (minor imperfections – like charges near each other) | K3, K5, K18 |
| Double replacement | Both products form pairs | K11, K12, K13, K15, K16, K20 |
| AgCl pairs, aqueous product (NaNO3) separates | K6, K7, K10, K17, K19 | |
| AgCl aggregate, aqueous (NaNO3) product separates | K1, K8, K9 | |
| AgCl aggregate, NaNO3 pairs | K14 | |
| Unorthodox | Chloride ion instigator attacks the Ag+ to form AgCl pairs, NaNO3 pair up, then separate | K2 |
| Reactant pairs orient end-to-end, AgCl formed in the middle, aqueous ions go free | K4 |
We now consider two cases that provide examples of a student who demonstrated an accurate model (K3) and a student who demonstrated a double replacement model (K11).
R: What did you think about in order to do this task?
K3: I thought about the features of each atom in here. This one will magically combine with this one because they are different charges to make up this compound over here (aggregate). And also, I thought of the solubility because this compound (silver chloride) is not soluble in water which indicates it stayed as a solid here however these ones (sodium nitrate) are soluble in water which indicates that they are free ions.
K3 applied knowledge of solubility rules and charge attraction to account for his model and the reason the reactants form products. When asked about his confidence, he initially expressed a little uncertainty. He was confident of the silver chloride aggregate that formed, but he wasn’t completely sure if it was due only to the attraction and formation of ionic bonds. He then reasoned about the involvement of water in the process:
“Both of them are already stable compounds or if they are ions they are not completely stable, what forced them to, why this one was attracted to this one (referring to silver and chloride ions) and not this one as an anion to the sodium as it was there? But I believe the reason behind that, they are soluble, so if they are attracted together, they would also be separated by water because it's soluble so, it's only way to stabilize it is by hitting this one. Now I am more confident.”
At this point in the semester, students had not yet learned about intermolecular forces of attraction, but K3 seems to have a sense that some ions are more stable when surrounded by water while others may be more stable when they attract. He applied these ideas about stability to account for the model he constructed.
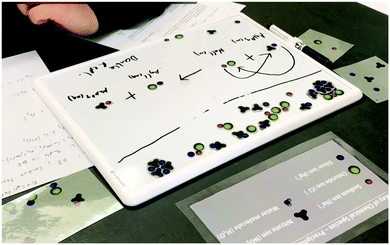 | ||
| Fig. 6 Screenshot of K11's model of the reaction showing emphasis on double replacement reaction equation. | ||
R: I noticed that you put the reactants together here but they're separated here(on the reactant card he chose). Do they come back together to look like this or how does that work?
K11: Well since you are reacting them together for the sake of showing why they react, how they react, I put them together because they were grouped in like that type of sample beforehand, in reality they all look like a bunch of free-floating ions because they had already dissociated with the water you can't exactly match both of them together and solid form is just not going to do anything because it can't dissolve or react.
R: How would they move?
K11: I don't really know how they would move. I would just know that any bond that they had would not be very strong. Since it already was in water it would split apart and they would be free-floating ions and again they would have to find another they would have to find like the silver is a positive it would have to find another negative and the sodium is positive it would have to find another negative. …Yeah so the picture would indicate that they would be closer to each other. They wouldn't exactly bond into something because if they were to bond into let's say silver chloride then that would indicate precipitate or some kind of solid but if we show I believe it's the net ionic equation of this reaction forming, you will realize that all of these are spectator ions and therefore no reaction would have occurred, meaning that they would've just mixed together. There's just a bunch of ions there is no real, there is nothing that formed out of it so that's why you don't really see, you wouldn't really see how anything combines.
R: Is there anything in this model that you think is making the solution white?
K11: I can't really tell because I don't know if it's like – I can't really tell if it's like the sodium or the chloride that mixes that would make the solution white so to speak. For why it's white I couldn't tell you
K11 constructed his model from the resources he used to write a double replacement reaction equation. However, we learned that K11 actually did not believe a precipitate was formed because he did not see the presence of a solid. His implicit knowledge of a solid is that it must be rigid with a distinct form. The solution he saw in the video and the one in the pictures looked like a liquid. His resources about states of matter were not wrong. Solids do hold their shape and have a more rigid structure, but he could use practice with his sense of scale or to consider that very small aggregates could exist and cause the solution to appear cloudy. However, K11 held firmly that the reaction did not result in a solid. He returned to his resources for representing double replacement reactions and told us that he modeled a molecular equation, but since both products were aqueous, they would really split apart as happens in the total ionic equation.
Evidence of doubt
During the modeling exercise, students rated their confidence in their model. When students were asked about their confidence, it revealed the stance students took toward their model. A pervasive stance we noticed when students described their confidence during the reaction modeling exercise was doubting. Doubting is a stance one adopts toward a piece of information one has neither accepted nor rejected it (Hammer and Elby, 2002). Example of how K10 expressed doubting follow:K10: I just think that the way that I described it didn't really make sense. I don't think they all break apart. I feel like there's something to it…I just felt that the way I was describing it would make a little bit more sense to me but I'm not too sure that it's really the way or not. I'm confident about the product. It's more about the procedure itself. I'm sure the finished product was like that (AgCl) but I'm not completely certain that is completely due to the ionic bond or the attraction.
When students are asked to try out their ideas by constructing models, it may be helpful for them to know that expressing doubt is a very normal resource to experience. All scientists experience doubting, but we need to be metacognitive or mindful of why we experience this stance. For example, if it implies we are uncertain of information based on our knowledge resources, we must consider what we can do to address our doubt and reach clarity or understanding.
Conflicting animations exercise
In the last exercise, students viewed three conflicting animations that depicted the atomic level reaction between aqueous silver nitrate and aqueous sodium chloride in different ways. Students were cautioned that the animations may or may not be scientifically accurate, and they were asked to critique four components of the animations: Each reactant solution (aqueous silver nitrate and aqueous sodium chloride), the mechanism, and the products to ultimately decide which animation was the most scientifically accurate. A constraint for guiding students’ review of the animations involved asking them to judge the accuracy of the animation components and to reflect on how the animation compared to their model. Students’ judgment of accuracy involved comparing their model of the reaction, consisting of the cards they selected and the reaction mechanism, to the animations they observed. As a consequence of this comparison, students exhibited an understanding of Belief or Disbelief toward the information in the animations and sometimes Puzzlement when their experiences of the idea did not make sense.The results revealed that slightly more than half of the students correctly chose animation 1, the most scientifically accurate animation. This outcome was different from what might be expected based on the mechanism students modeled during the modeling exercise, in which only a few students modeled mechanisms consistent with the accurate animation and most modeled a double replacement mechanism (Table 3).
| a Animation 1 was consistent with representations illustrated in cards marked with (also shown in teal color). b Animation 2 was consistent with representations illustrated in cards marked with (also shown in tan color). c Animation 3 was consistent with representations illustrated in cards marked with (also shown in pink color). d D-Double replacement model, A-Accurate Model, U-Unusual model. |
|---|
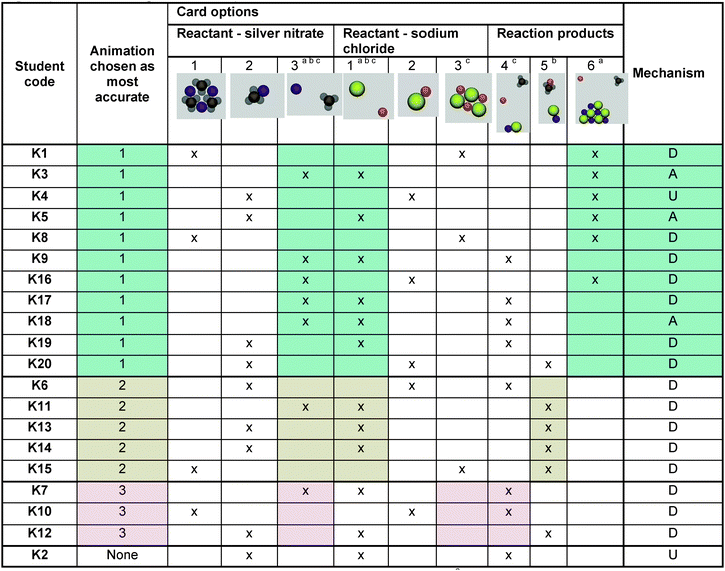
|
We begin with a review of the animation components and how students rated their accuracy. Reactant silver nitrate was accurately represented as separated ions in all three animations, while reactant sodium chloride was depicted accurately in two of the three animations. Students sometimes disbelieved the separated ion representations because it differed from their belief, but when they recognized that it was in all three of the animations, sometimes they accepted the depiction or they were puzzled by it and continued to disbelieve it but had to move forward to make a selection. Students were intrigued by the mechanisms they observed, but it was difficult for them to see the switching action that they had focused on in their models because the reactants were not initially paired. This caused students to focus on the representation of the products, as these were unique to each animation, yet they also fit with each of the representations they selected in the card sort exercise. We observed that when students’ product card selection was in alignment with the depiction of products in an animation, students often chose that animation (Table 4). In general, when students used resources for comparing, they observed consistency between their selected cards from the card sort exercise and the depiction of reactants and products in an animation. This reinforced their trust in their animation selection. Examples follow:
K1: But for the product that forms, the solid, the silver chloride that's 100%. I think that's spot on….I'm going to go with one. The first one I saw. It just matches what I put down, pretty much 100%. There's no differences in what I had and the animation one. And there's nothing really that stood out to me that made it like that I could definitely say that doesn't look right.
K3: They are similar by they have the same reactants and the same products and like similar mechanisms that those ions: chloride and silver are attracted together forming this precipitate over here and these ones remain free so it's like that's a similarity yeah.
K5: Despite the mechanisms for the second one and the third one being kind of closer to my brain, the product and the solution were very similar for the first one. I would say the first one is most accurate for me. It was more similar to the image I had in my brain.
K16: It (animation) matches the ones (cards) that I chose originally.
Epistemological stance of students who selected animation 1
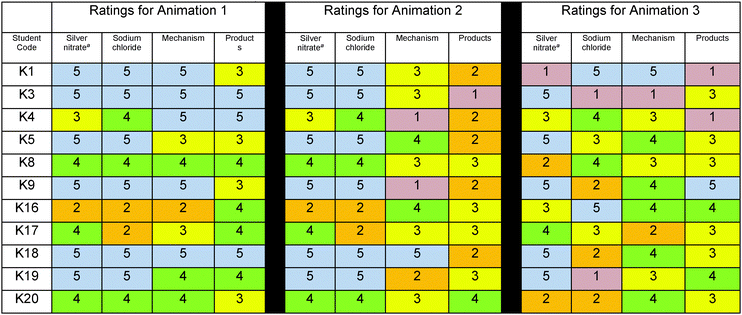
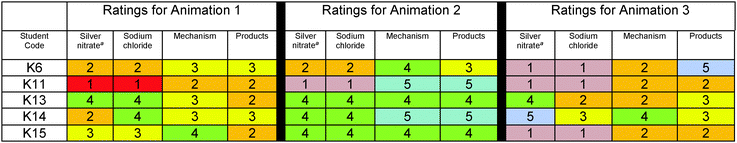
To examine how students, who selected animation 1 as the most scientifically accurate animation, rated the accuracy of the animation components of the three contrasting animations, a table was created (Table 4). The ratings were both numerical and color-coded in a heat map in which cool colors blue (5) and green (4) represented ratings of accuracy; yellow (3) represented a non-committal stance and warm colors orange (2) and red (1) represented ratings of inaccuracy. The ratings revealed that in spite of selecting animation 1, students had Doubts about the accuracy of its animation components; however, these students’ ratings for animation 1 were generally more favorable than their ratings for the other animations (Table 4) reflecting that the animation fit best with their understanding and resulted in the stance – Belief.To examine how students, who selected animation 2 and 3 as the most scientifically accurate animation, rated the animation components of the three contrasting animations, similar tables were created (Tables 5 and 6). The ratings followed the same color-coding scheme that we used for students who chose animation 1.
| a The silver nitrate was represented identically in each animation. |
|---|
|
| a The silver nitrate was represented identically in each animation. |
|---|
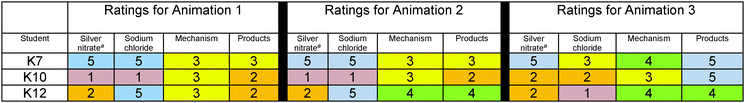
|
We contend that students’ accuracy ratings reflected their epistemological stance (Belief/Disbelief) toward the information presented in the animations and was important in their animation selection. According to Hammer and Elby (2002), when we are presented with something that seems unreasonable, it reflects an understanding of Disbelief. Belief is the alternative stance and reflects accepting information. When students judged an animation component to be accurate, they were telling us that they believed the animation and when they judged an animation to be inaccurate, they were telling us that they disbelieved the representation. Students learned to use their ratings to make their animation selection. For example in the case of K2, he justified why he did not select any of the animations.
K2: Oh man! Just looking at the grading here, a lot of the scores are below a three for every single scenario like statistically wise it would be all of more inaccurate just using the numbers. Scientifically, I chose those numbers for a reason so yeah, I would say they are all inaccurate. I wouldn't pick one of them.
Students expressed doubt as an epistemological stance providing insight that they were using resources to compare the animation to their model of the reaction, and the animation was not comparing well. As expected, for the animation students selected, they generally tended to have higher ratings of accuracy than the ratings they gave for the non-selected animations. We infer that they had less doubt about the animation they selected. To support this inference, we coded the transcripts of each student's response to their final animation selection using Hammer and Elby's resources for understanding epistemological stances: belief/disbelief, doubting, and puzzlement. (Hammer and Elby, 2002). Our analysis revealed that most students (90%) expressed Belief in the animation they selected and described. Despite their beliefs, many also expressed doubt (50%) and puzzlement (45%) as the animations typically never perfectly represented what they hoped to see or had modeled initially.
Examples that reflect students’ epistemological stance – belief follow:
K3: I believe it demonstrated more accurately then the others because it demonstrated the reactants as they should be in the aqueous state as free ions and both products and it display the mechanism in a more accurate procedure with free ions attracted to each other to form an ionic bond or ionic compound, each by itself as a molecule, the molecules attracted together to form the compound as a whole and the product also indicates that the whole is held together not separated molecules of silver chloride and also there all floated in the sodium nitrate.
K5: I would say the first one is most accurate for me. It was more similar to the image I had in my brain. It gave me confirmation number three was correct (for silver nitrate solution). I know this one should be right (for NaCl), technically it's highly conductive and it's separated and salt so that's what you use in everyday life.
K7: I liked how the animation stole the chloride and I don't know the silver is kind of near the sodium no nitrate is near the sodium that's what it's supposed to be like I believe.
Examples that reflect students’ epistemological stance – Doubting follow:
K1: Because when they are solid there are no barriers, nothing else in it, it's just the compound and also it just makes more sense because when you combine them you know these are all separated yet because it's all a cluster makes a little bit less sense to me. How can they all separate as soon as, because they're already in water? Why didn’t they separate when they were in the water? Why are they going to separate when you combine two clusters together?
K3: Oddly because I've never thought about it that way so it got me thinking and questioning my ability to understand.
K8: It's confusing because you don't know exactly if what you're seeing is right or not. What you don't know is if it's fake or not. I'm not entirely sure that the one that I picked here is real, but that's what I'm going with….Yeah, it is frustrating. Extremely frustrating because I don't know if this is right or not. I want to be right. I do. At the same time there's just this doubt that is lingering.
Examples that reflect students’ epistemological stance – Puzzlement follow:
K2: I would just say the way that the silver ion kind of attacked that chloride in this last step was kind of confusing just because it goes against everything that I kind of learned before so that I would say is confusing.
K5: Yeah, they gave me more input on my products which means I'm technically still thinking between six and four, but even though I know I chose six sometimes I feel like it's four because the animations are like this could be a possible thing. So they kind of made me debate on these two.
K15: I guess for all the animations how the ions looked like at first like how, I forgot, some of the ions are separated in the beginning and in another animation the sodium chloride is all clumped up and that made it confusing because I don't understand which ions would clump up together and which ions don't clump up together.
To triangulate observations of how students made their selection of the most scientifically accurate animation, the participants were interviewed to uncover their reasoning for their animation selection. Specifically, during the semi-structured interview, students were asked why and how they made the selection and, then students were asked to elaborate more deeply on their responses. A few trends emerged from the analysis of the transcripts. In general, students were purposeful in their intent to find the animation that matched their beliefs. For example, when a feature in the animation fit with their understanding, students made comments such as “It's familiar”. It reinforces or confirms what I thought. It matches what I have learned. Nearly every student commented on animation features that matched what they pictured when they thought about the atomic level of the reaction.
K2: … I would say for the things that I was more confident about I was looking for information to back it up, and for things that I wasn't as confident about I was looking for things to kind of shed a light on that.
K4: It's kind of reinforced my own ideas about how it works.
K5: It wasn’t confusing because it was similar to what was in my head, then these animations I just saw were all the possible solutions in my head that could have happened, so it didn’t really confuse me.
However, it is important to note that nearly every student also recognized differences between their thoughts and beliefs and aspects depicted in the animations. Students reported features that conflicted with what they believed they had learned or that they did not expect to see. Differences that were counter to their beliefs caused several students to question their understanding, and as a consequence of this dissatisfaction, they expressed doubting when tasked with selecting the best animation. For some students, having to select an animation from the choices when they were uncertain was frustrating, especially because they were not “taught” the answer.
K8: It's confusing because you don't know exactly if what you're seeing is right or not, if it's fake or not. I'm not entirely sure that the one that I picked here is real, but that's what I'm going with… yeah, it is frustrating, extremely frustrating because I don't know if this is right or not. I want to be right. I do. At the same time there's just this doubt that is lingering.” It's like I'm supposed to be learning this right now, so I'm supposed to know what exactly is correct or not. I should really get the answer down, since I'm learning about this but, I think it's just that I am being bombarded with all of these other lessons, like I can't exactly get down what's right.
K10: I don’t know. These videos have kind of like not sold me onto their ideas, but like just them giving different ideas in my head like I said, just doubting myself. Like, am I right? Or are these videos wrong?
K14: I feel like it's just being kind of stubborn you know, like when you find an answer that you think could be right, then you go through anything to justify that one answer even if you have other clues and you just keep trying to justify that one answer that you think could be correct.
Conclusions
In our journey to learn how students would respond to contrasting atomic level animations connected to a video of experimental evidence, we endeavored to examine students’ prior understanding via two exercises: a card sort exercise and a reaction model exercise. To examine our data we used a resource-based framework, and we soon realized that each of these exercises afforded us an opportunity to examine how students activated and coordinated resources to think about the atomic level through three key tasks: selected cards (reactants and products), constructed magnetic model of the reaction and the animation selected as most scientifically accurate. We examined the knowledge resources students engaged with as they conducted each exercise and demonstrated how the constraints that were applied to the exercises affected the resources students activated and the stances that were elicited.In the case of the card sorting exercise, students were asked to explain why they chose a card and why they did not select the other cards. These guiding questions caused students to compare the card representations and apply their knowledge to defend their selection. Our findings revealed that students sometimes shifted between their resources and sometimes they misapplied them. The resources they applied or misapplied were connected to their understanding of dissolution, states of matter, electrical conductivity and formula construction and charge attraction/repulsion. In the second exercise, constructing a reaction model was the epistemic form of the exercise and students most often misapplied their knowledge of double replacement reactions to construct their model. Many students expressed doubt about their model revealing that they were uncertain of their construction. Helping students understand their stance represents a teaching opportunity as we could invite students to connect back to the evidence to consider what they could do experimentally to reach resolution and shift their application of resources and their stance.
After students completed the card sort and model building task, they had static models of the reactants and products and a mechanism for the reaction. As they viewed each animation, they were guided to rate the accuracy of the atomic level representation of four components: each reactant, the mechanism, and the products. Students were also guided to compare each animation to their model. Since students had already applied or misapplied resources to construct their models, when they reviewed the animations, they developed a stance toward the animation either believing the animation or disbelieving it and sometimes they were quite puzzled. Ultimately, they made their selection usually focusing on the fit of the products to their model. Students thought deeply about the animations as they compared what they saw to their own models and considered what they believed. They were able to try out ideas and decide for themselves which animation was best. With this empowered independence, students experienced frustration as they doubted their ideas or were puzzled by how to make their selection. Regardless of whether a student was able to select the most scientifically accurate animation or not, it was apparent that every student critiqued the animations and made decisions about whether the features of the animation were consistent with their beliefs. This is an exciting shift from using animations as atomic-level facts to viewing them as models with recognizable limitations.
Implications
In our study we show that students struggled with how to select the most scientifically accurate animation because the representations were not an exact match with their ideas. Some students even questioned whether an activity in which they are not told the answer was learning, reflecting the epistemological belief that chemistry knowledge comes from authority. Of course, we do not want instructors to stop teaching students how to balance equations or how to use solubility rules, but we do want to explore how to invite students to think about the atomic level and most importantly how to use their resources. We guided our exercises by introducing comparing as a constraint. The importance of this constraint is that every single student, regardless of their prior chemistry experiences, was able to compare. This has importance for our teaching practice as lessons that are framed to ask students to compare will encourage all students to participate and try out ideas connected to their experiences and interests. It also provides an opportunity for the instructor to learn what students think about. We deem that card sorting exercises, model construction, and contrasting animations are useful exercises, and with the conditions we invoked we were able shed insight into how they applied and misapplied resources. If students can learn how to recognize their epistemological stance toward information, such as what to do when they feel disbelief or doubt, then they may be better able to engage resources for action to help them learn and grow. A few suggestions would be to partner the exercises with inquiry lab practices to teach students how to turn their doubts into actionable experiments. For example, based on our research we noticed that students struggled with the nature of the solid precipitate, K11 did not believe that a solid had formed. Thus, we might encourage students to explore the nature of the precipitate that is formed by allowing the solution to settle or using a centrifuge to separate the solid from the solution. Some students struggle with the conductivity evidence, we might invite them to monitor the conductivity throughout the process or invite them to extract the precipitate, dry it and test it for electrical conductance and compare this to how the remaining extract conducts. It could be very exciting to have students conducting multiple experiments, where they could share their findings and draw new conclusions.Lastly, we want to acknowledge that once students completed the animation exercise, we actually shared one more animation, an animation that was available online and deemed to be “scientifically accurate”. We asked students which of the three animations it resembled and all students were able to identify the animation it matched even though stylistically the animations were quite different. Since many students look for answers online, this was a way to debrief students while also engaging their comparing resource and could be a useful addition to teaching practice.
Ultimately, we believe that students, just like well-trained scientists, will make good and poor decisions as they apply and misapply resources, but the process of making those decisions and reflecting on one's stance is what is most important in developing students’ ability to think critically.
Limitations
One limitation resided in students’ educational training. Most of the students had little practice solving un-modeled problems or making sense of atomic level representations. Also, most of the students had not yet learned about intermolecular forces of attraction in their chemistry studies. Thus, students found it difficult to process why ions of opposite charge would not attract in an aqueous water environment. This may have made the animation activity difficult for students leading to amplified frustration.Conflicts of interest
There are no conflicts to declare.Appendix A
A summary of four categories of epistemological resources adapted from Hammer and Elby (2002) that were used to map resources during exercises.| aModified from Redish (2004). | |
|---|---|
| I. Resources for Understanding the Nature and Sources of Knowledge – “How do you know?” | |
| Knowledge as propagated stuff | Treat knowledge as a kind of stuff, passed from one person to the next. Knowledge has a source and a recipient. |
| Ex. No one has told me how to think about the atomic level. How can I know this if no one has ever told me? | |
| Knowledge as free creation | Knowledge does not have any source other than the person's own mind, where it arose spontaneously. |
| Ex. How do you know, that atoms behave this way? I don’t know, I just made it up | |
| Knowledge as fabricated stuff | Knowledge that is inferred or developed from other knowledge. This is not a free creation; it is constrained by the nature of the material. |
| Ex. Solutions that conduct electricity possess mobile ions, an aqueous solution of silver nitrate conducts so it must possess mobile ions | |
| Knowledge as direct perception | Knowledge from seeing, hearing, touching |
| Ex. How do you know a precipitate formed? Because I see it. I see a cloudy solution, so I know a precipitate formed | |
| Knowledge as inherent | Knowing without being able to explain why or how |
| Ex. How do you know a precipitate formed? I just do | |
| Knowledge by phenomenological primitive (p-prim)a | Knowing a phenomenological primitive – a cognitive resource corresponding to a basic statement about the structure and function of the physical and chemical world that a user considers obvious or irreducible |
| Ex. Why do ions attract? Because opposite charges attract, like charges repel | |
| II. Resources for Understanding Epistemological Activities – “What are you doing?” | |
| Accumulation | Reflects an understanding of “finding out” as a simple activity, the retrieval of information. Reflected in language when we speak of “gathering” or “retrieving” information |
| Formation | Reflects an understanding of constructing ideas for themselves, whether in writing stories, composing songs, devising rules or inventing games. Can also be viewed as a collection of more specific primitives. For example: Forming rules is a distinct primitive from Forming stories; Guessing and Brainstorming is distinct from Crafting and Adjusting |
| Checking | Reflects an understanding of “making sure” as an epistemological activity. Checking may evolve in response to early experiences of error, in conjunction with Doubting as a stance. It may be invoked in conjunction with Accumulation – re-retrieve information, or in conjunction with Formation, checking the conclusion. For example, looking up whether a substance is soluble or insoluble to determine the nature of a precipitate that forms in conjunction with experimental evidence that verifies precipitate formation |
| Application | A set of resources invoked in situations that involve using a piece of existing knowledge, such as singing a song, telling information, or in following or enforcing a rule |
| Comparing | Reflects drawing comparisons between two or more representations |
| Sorting/Listing | Reflects organizing information to enhance understanding |
| III. Resources for Understanding Epistemological Forms(external structures or representations that guide inquiry) | |
| Stories | The activity of writing a story requires resources for understanding the activity of writing. For example using the resource Formation in conjunction with the resource Free Creation or Fabricated Stuff. There are also resources for understanding the activity, listening to the story done in conjunction with the resources: Application and Propagated Stuff |
| Rule System | Is a resource for understanding a coherent set of rules that define a game or a process, like solubility rules for predicting the phase/state of a matter for substances based on their chemical composition |
| Games | A coherent activity that uses particular kinds of knowledge and the processes associated with that knowledge to create knowledge or solve a problem |
| Diagrams/graphs/pictures/models | Examples of epistemic forms that require using and interpreting the results of manipulating the structures |
| IV. Resources for Understanding Epistemological Stances | |
| Belief/Disbelief | A stance one can adopt toward a piece of information. For example, I don’t believe that. |
| Doubting | A stance toward a piece of information one has neither accepted nor rejected |
| Understanding | A resource for understanding experiences of an idea seeming right or making sense |
| Puzzlement | A resource for experiences of an idea not making sense |
| Acceptance | Experience of believing an idea |
Additional codes
Card sorting exercise:
We examined ways that students drew comparisons between the three levels: macroscopic, submicroscopic and symbolic (Johnstone, 1993) – for example if a student made observable connections between the macroscopic level of the experiment (such as referencing: the state of matter (solid precipitate or liquid solution, electrical conductivity evidence, mixing solutions), submicroscopic level (use of descriptors: atoms, ions, molecules); symbolic level (formula discussion, equations like double replacement) we coded this using the software Invivo.
Reaction modeling exercise:
Constant comparative method of data analysis was employed to examine the way students modeled their understanding of the reaction mechanism. It became obvious that most students were applying resources that they learned from balancing double replacement reactions, and as a consequence we examined how students connected to symbolic level representations of the reaction.
Contrasting animations:
We recognized that students were engaged in comparing due to this being a constraint of the exercise and we examined how the students drew comparisons. We recognized the connection between their judgements of accuracy and their epistemic stances. As a result, we applied Hammer and Elby's (2002)Resources for understanding epistemological stance (Table 1, part IV) to guide our coding work.
Acknowledgements
This material is based upon work supported by the National Science Foundation under grant no. 1525557, grant no. 1525475, and TUBITAK under grant no. 115K025.References
- Ahtee M. and Varjola I., (1998), Students’ understanding of chemical reaction, Int. J. Sci. Educ.20(3), 305–316.
- Ainsworth S., (1999), The functions of multiple representations, Comput. Educ.33(2–3), 131–152.
- Ainsworth S., (2006), DeFT: A conceptual framework for considering learning with multiple representations, Learn. Instruct.16, 183–198.
- Asterhan C. S. C. and Dotan A., (2018), Feedback that corrects and contrasts students’ erroneous solutions with expert ones improves expository instruction for conceptual change, Instr. Sci., 46, 337–355.
- Ben-Zvi R., Eylon B., Silberstein J., (1987), Students’ visualization of a chemical reaction, Educ. Chem., 24, 117–120.
- Durkin K. and Rittle-Johnson B., (2011), The effectiveness of using incorrect examples to support learning about decimal magnitude, Learn. Instruct., 22, 206–2014.
- Elby A. and Hammer D., (2010), Epistemological resources and framing: A cognitive framework for helping teachers interpret and respond to their students' epistemologies, in Bendixen L. D. and Feucht F. C. (ed.), Personal epistemology in the classroom: Theory, research, and implications for practice, Cambridge University Press, pp. 409–434.
- Eryilmaz A., (2002), Effects of conceptual assignments and conceptual change discussions on students’ misconceptions and achievement regarding force and motion, J. Res. Sci. Teach., 39, 1001–1015.
- Garnett P. J., Garnett P. J. and Hackling M. W., (1995), Students’ alternative conceptions in chemistry: A review of research and implications for teaching and learning, studies, Sci. Educ.25(1), 69–96.
- Hammer D. and Elby A., (2002), On the form of a personal epistemology, in Hofer B. K. and Pintrich P. R. (ed.), Personal epistemology: The psychology of beliefs about knowledge and knowing, Mahwah, NJ: Erlbaum, pp. 169–190.
- Hammer D., Elby A., Scherr R. E. and Redish E. F., (2005), Resources, framing, and transfer, in Mestre J. P. (ed.), Transfer of learning from a modern multidisciplinary perspective, IAP, pp. 89–119.
- Hansen S. J. R., Hu B., Riedlova D., Kelly R. M., Akaygun S. and Villalta-Cerdas A., (2019), Critical consumption of chemistry visuals: Eye tracking structured variation and visual feedback of redox and precipitation reactions. Chem. Educ. Res. Pract., 20, 837–850.
- Huang T. H., Liu Y. C. and Shiu C. Y., (2008), Construction of an online learning system for decimal numbers through the use of cognitive conflict strategy, Comput. Educ., 50, 61–76.
- Johnstone A., (1993), The development of chemistry teaching: A changing response to changing demand, J. Chem. Educ.70(9), 701–705.
- Kelly R. M., (2014), Using variation theory with metacognitive monitoring to develop insights into how students learn from molecular visualizations, J. Chem. Educ., 91(8), 1152–1161.
- Kelly R. M., (2017), Learning from contrasting molecular animations with a metacognitive monitoring activity, Educ. Quím., 181–194.
- Kelly R. M. and Hansen S. J. R., (2017), Exploring the design and use of molecular animations that conflict for understanding chemical reactions, Quim. Nova, 40(4), 476–481.
- Kelly R. M. and Jones L. L., (2007), Exploring how different features of animations of sodium chloride dissolution affect students’ explanations, J. Sci. Educ. Technol., 16(5), 413–429.
- Kelly R. M., Barrera J. H. and Mohamed S. C., (2010), An analysis of undergraduate general chemistry students’ misconceptions of the submicroscopic level of precipitation reactions, J. Chem. Educ., 87(1), 113–118.
- Kelly R. M., Akaygun S., Hansen S. J. R. and Villalta-Cerdas A., (2017), The effect that comparing molecular animations of varying accuracy has on students' submicroscopic explanations, Chem. Educ. Res. Pract., 582–600.
- Kelly R., Brunmeier J. and Evans M., (2018a), Precipitation Reaction Experiment, https://youtu.be/Vs_jb5teNaY. Last accessed March 2, 2020.
- Kelly R., Brunmeier J. and Evans M., (2018b), Conflicting Animation Series – Animation 1, https://youtu.be/F5btSzlTsNg. Last accessed March 2, 2020.
- Kelly R., Brunmeier J. and Evans M., (2018c), Conflicting Animation Series – Animation 2, https://youtu.be/SG1nVSXZnfo. Last accessed March 2, 2020.
- Kelly R., Brunmeier J. and Evans M., (2018d), Conflicting Animation Series – Animation 3, https://youtu.be/9hqEBFq8UAM. Last accessed March 2, 2020.
- Lincoln Y. S. and Guba E. G., (1985), Naturalistic Inquiry, Newbury Park: SAGE Publications, Inc.
- Merriam S. B., (2001), Qualitative Research and Case Study Applications in Education, San Francisco: Jossey-Bass.
- Posner G. J., Strike K. A., Hewson P. W. and Gertzog W. A., (1982), Accommodation of a scientific conception: Toward a theory of conceptual change, Sci. Educ., 66, 211–227.
- Redish E. F. (2004), A theoretical framework for physics education research: Modeling student thinking, in Redish E. F. and Vicentini M. (ed.), Proceedings of the International School of Physics, ‘‘Enrico Fermi’’ Course CLVI, Amsterdam: IOS Press.
- Rosenthal D. P. and Sanger M. J., (2012), Student misinterpretations and misconceptions based on their explanations of two computer animations of varying complexity depicting the same oxidation–reduction reaction, Chem. Educ. Res. Pract., 13, 471–483.
- Rosenthal D. P. and Sanger M. J., (2013), How does viewing one computer animation affect students’ interpretations of another animation depicting the same oxidation–reduction reaction? Chem. Educ. Res. Pract., 14, 286–296.
- Singmaster K., (2018), Chemistry 1A Lab Manual and Seminar Notebook, San Jose: SJSU.
- Taber K. S., (2013), Revisiting the chemistry triplet: Drawing upon nature of chemical knowledge and the psychology of learning to inform chemistry education, Chem. Educ. Res. Pract., 14, 156–168.
- Talanquer V., (2011), Macro, submicro, and symbolic: The many faces of the chemistry “triplet”, Int. J. Sci. Educ., 33(2), 179–195.
- Van den Broek P. and Kendeou P., (2008), Cognitive processes in comprehension of science texts: The role of co-activation in confronting misconceptions, Appl. Cog. Psych., 22, 335e351 DOI:10.1002/acp.1418.
- Yarroch W. L., (1985), Student understanding of chemical equation balancing, J. Res. Sci. Teach., 22, 449–459.
| This journal is © The Royal Society of Chemistry 2021 |