Open Access Article
Open Access ArticleProcess modelling and life cycle assessment coupled with experimental work to shape the future sustainable production of chemicals and fuels
Iasonas
Ioannou
 a,
Sebastiano Carlo
D'Angelo
a,
Sebastiano Carlo
D'Angelo
 a,
Ángel
Galán-Martín
a,
Ángel
Galán-Martín
 a,
Carlos
Pozo
a,
Carlos
Pozo
 b,
Javier
Pérez-Ramírez
b,
Javier
Pérez-Ramírez
 a and
Gonzalo
Guillén-Gosálbez
a and
Gonzalo
Guillén-Gosálbez
 *a
*a
aInstitute for Chemical and Bioengineering, Department of Chemistry and Applied Biosciences, ETH Zürich, Vladimir-Prelog-Weg 1, 8093 Zürich, Switzerland. E-mail: gonzalo.guillen.gosalbez@chem.ethz.ch
bLEPAMAP Research Group, University of Girona, C/Maria Aurèlia Capmany 61, 17003, Girona, Spain
First published on 4th February 2021
Abstract
Meeting the sustainable development goals and carbon neutrality targets requires transitioning to cleaner products, which poses significant challenges to the future chemical industry. Identifying alternative pathways to cover the growing demand for chemicals and fuels in a more sustainable manner calls for close collaborative programs between experimental and computational groups as well as new tools to support these joint endeavours. In this broad context, we here review the role of process systems engineering tools in assessing and optimising alternative chemical production patterns based on renewable resources, including renewable carbon and energy. The focus is on the use of process modelling and optimisation combined with life cycle assessment methodologies and network analysis to underpin experiments and generate insight into how the chemical industry could optimally deliver chemicals and fuels with a lower environmental footprint. We identify the main gaps in the literature and provide directions for future work, highlighting the role of PSE concepts and tools in guiding the future transition and complementing experimental studies more effectively.
1. Introduction
At present, the chemical industry is mostly based on fossil-based feedstocks, consumes large amounts of energy and water and emits a myriad of substances into the soil, water and air with detrimental effects on the ecosystems, human health and resources.1 With the recent trend of moving towards a more sustainable economy, there is a strong motivation to defossilise and decarbonise the chemical sector to ensure a carbon-neutral production aligned with the Paris agreement climate goal and the sustainable development goals (SDGs).2–4 In this broad context, a wide range of emerging routes are being investigated, seeking to reduce the environmental footprint of chemicals via a combination of strategies. These include, among others, (i) the move to renewable carbon feedstocks, e.g., CO2 captured, biomass or recycled plastics, (ii) the minimisation of energy and water consumption and pollutant emissions, (iii) the adoption of circular economy principles to reduce waste generation and (iv) the electrification of the chemical industry.Experimental research should be underpinned by analytical tools to gain further insight into the technologies investigated and the phenomena dictating their behaviour. Analytical tools could also facilitate the screening of the most promising reaction pathways and catalytic materials. Notably, computer-aided process engineering methods are gaining wide acceptance in the early-stage evaluation, scale-up, and optimisation of processes.
Within this general context, here we discuss the role of process systems engineering (PSE), with emphasis on process modelling and optimisation and life cycle assessment (LCA), in the discovery, assessment, and optimisation of chemicals and fuels with a lower environmental footprint. Our article differs from recently published works in that it focuses on PSE applied to the chemical industry, as opposed to previous contributions focusing on PSE applied to general sustainability problems5 or dealing with sustainability problems within chemical engineering.6,7 It also goes beyond other studies focusing only on LCA8–10 to embrace process modelling and optimisation and discuss the most promising pathways to transform the chemical industry.
We start by motivating the need to transition towards an environmentally benign chemical industry and then introduce the main strategies to accomplish this goal. We then describe PSE's role in evaluating and optimising emerging technologies and finally draw some conclusions and outline future potential research directions.
2. The need to transition towards a more sustainable chemical industry
The stark economic growth of nations has led to the depletion of the Earth's resources and resulted in unprecedented pollution levels, with detrimental effects on the population and the terrestrial and marine ecosystems.11 Notably, the greenhouse gas (GHG) levels in the atmosphere will likely lead to an estimated average global temperature increase of 1.5 °C between 2030 and 2052,12 while mismanagement of waste, resulting in tons of plastic entering the oceans,13 and water scarcity14 pose severe threats to the planet.Several initiatives aim to reduce these high levels of anthropogenic impact. To mention a few, in 2015, the United Nations adopted the 2030 agenda for sustainable development, which considers 17 SDGs4 covering a set of areas deemed critical to ensure sustainable development. Similarly, focusing on the environmental pillar of sustainability, the Stockholm Resilience Centre introduced the planetary boundaries (PBs) concept,15,16 which defines a set of ecological thresholds on critical Earth system processes that should never be surpassed to ensure the safe operation of the planet. These biophysical limits include climate change and biosphere integrity, regarded as core boundaries, which are currently the focus of growing interest in research and policymaking.17
There is, therefore, a clear need to transition toward manufacturing practices consistent with the SDGs and lying within the safe operating space delimited by the PBs. Given its vital role in the global economy and its strong links to other industrial sectors, including the power and transport sectors, the chemical industry is expected to contribute decisively toward this goal. This will require deploying a range of emerging environmentally benign technologies, as discussed throughout this article.
3. The chemical industry in a nutshell
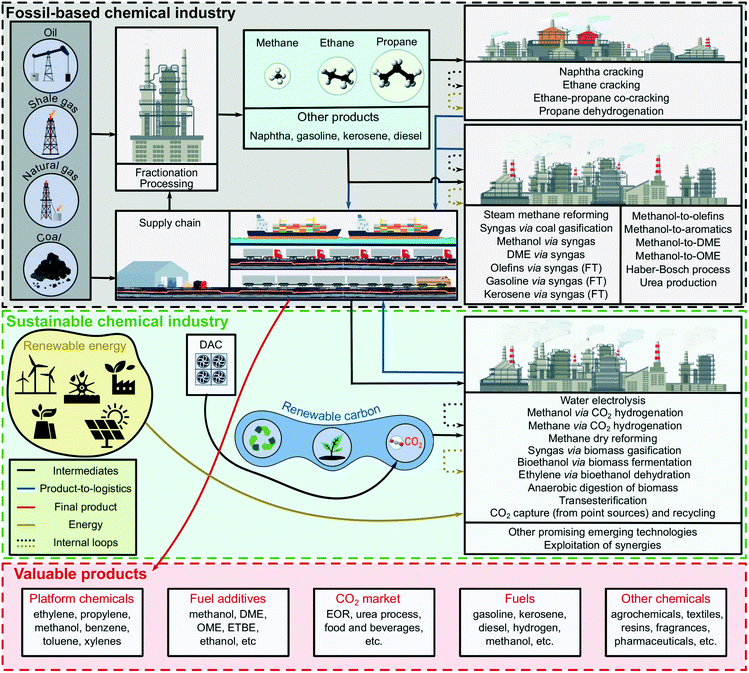
The chemical sector covers a wide range of products that support our daily lives and underpin numerous industries and businesses. Notably, chemical processes are linked to supply chains spanning multiple areas within the manufacturing industry, e.g., electrical equipment, motor vehicles and machinery, ultimately playing a pivotal role in the global economy.The chemical industry encompasses hundreds of chemical processes that transform a variety of feedstocks into a myriad of valuable products. These integrated production networks are highly interconnected due to the exchange of mass and energy flows and the consumption of shared resources (Fig. 1).18 Such networks have evolved opportunistically over time, driven mainly by the availability of resources, economic criteria, and market trends. Consequently, they are unable to cover the future demand for chemicals with lower environmental footprint effectively.
In essence, the backbone of the current chemical industry converts fossil carbon, mostly in the form of coal, naphtha or natural gas, into ethylene, propylene and aromatics, i.e., benzene, toluene and xylenes, via the cracking of hydrocarbons (Fig. 1). Together with methanol from syngas, these molecules are used as platform chemicals to synthesise a wide range of bulk, specialty and fine chemicals, including monomers used in plastic production. In addition to these petrochemicals and their derivatives, this sector produces a range of inorganic chemicals, including ammonia and urea used primarily to manufacture fertilizers and explosives.19 In 2010, the chemical and petrochemical industry emitted 1.24 Gt CO2eq. and consumed 10% of the global final energy demand,20 whereas the transport sector, in 2018, emitted almost 8.37 Gt CO2eq. and consumed around 2% of the global electricity.21 These high emissions and energy consumption levels raise concerns about the sustainability level of these industrial sectors.
With the need to transition towards a less polluting economy, the chemical industry faces the challenge of shifting to cleaner production patterns by reducing water and energy consumption, minimising waste generation, and moving to renewable feedstocks and energy sources (e.g., biomass, CO2, wind, and solar power). Furthermore, this transformation may create strong synergies with other industries, particularly with the energy sector, where cleaner chemicals and fuels can be synthesised to harness the excess of intermittent renewable power in the transition towards cleaner energy systems.22 Following this trend, a wide range of production technologies, most of them yet commercially immature, are under investigation to improve the sustainability level of chemicals and fuels, as described in detail next.
4. Emerging trends to transition toward a more sustainable chemical industry
4.1. Shift towards renewable carbon sources
The current petrochemical industry and transport sector are based on fossil carbon (grey box in Fig. 1). This fossil carbon, entering the chemical industry in various forms, is ultimately released after the product end-use phase, e.g., via incineration of plastics or the combustion of fuels, thereby contributing to global warming. The emissions linked to the use of fossil carbon as feedstock are accompanied by other emissions associated with the whole range of activities in the chemicals' life cycle. These activities include the extraction of raw materials, production and storage, transport of intermediate and final products and waste management (after the use-phase).One option to reduce the chemical industry's (life cycle) carbon footprint would be to decarbonise its energy inputs while keeping its main reaction pathways based on fossil carbon unaltered. The decarbonisation of the energy inputs could be accomplished in several ways. One alternative is to deploy carbon capture and storage (CCS) in the highly emitting process units, mainly thermal energy generation systems (e.g., furnaces and boilers).23,24 Alternatively, these units could be electrified using renewable sources.25 None of these approaches, however, solves the fundamental problem of relying on fossil carbon as feedstock, which would ultimately accumulate in the atmosphere after the use-phase of chemicals and fuels.
Another alternative to reduce carbon emissions would be to deploy CCS in the incineration of chemicals to capture the fossil carbon they store chemically. This approach, unsuitable for fuels, would require a high geological storage capacity to permanently store the fossil carbon. Likewise, enhanced oil recovery (EOR), that is, the use of CO2 in oil extraction, could reduce CO2 emissions by retaining high amounts of CO2 underground.26 EOR, however, would also attempt to close the carbon loop in an, arguably, unsustainable manner, i.e., ground–use–ground vs. the more sustainable air–use–air (as discussed next). In addition to this, the large-scale implementation of CCS and EOR would further reduce the proven fossil reserves. Finally, notwithstanding the value of CCS and EOR as interim solutions to meet the climate goals, even if the CO2 was stored permanently in a neutral environment, it could raise ethical intergenerational concerns.26
A paradigm shift implying the substitution of fossil- by renewable-based carbon, either as (i) CO2 captured, (ii) biomass feedstock or (iii) carbon from the mechanical and chemical recycling of products (e.g., existing plastics or monomers), is required to enable the defossilisation of the chemical industry. The shift to renewable carbon could reduce the environmental footprint of chemicals (middle green box in Fig. 1), mainly in terms of climate change, perceived today as a major environmental threat.
Biomass represents the main alternative to the direct use of captured CO2 as renewable feedstock. Biomass can be used to produce a wide range of products,27 including bioplastics and biofuels,28via thermochemical and biochemical routes. In particular, it can be gasified to produce syngas (thermochemical route), which can then be used for synthesizing methanol (used as a platform chemical or fuel) and dimethyl ether (DME) as well as fuels (via Fischer–Tropsch (FT) synthesis).27,29–31 Biomethanol could then be further converted into olefins, aromatics and DME, replacing their conventional fossil-based analogues. Alternatively, biomass could also be used for the production of biofuels such as bioethanol and biodiesel via biological routes, mostly through fermentation, anaerobic digestion (AD), and organic waste transesterification. As an example, biogas, produced via AD, can be processed towards biomethane and further used in combined heat and power (CHP) plants. An LCA study, based on five real AD-CHP plants, indicated that this electricity generation pathway could provide environmental benefits compared to a fossil-dominated electricity production mix. Nevertheless, the environmental impacts of other protection areas may increase.32 It was also shown that biogas-based electricity cannot compete environmentally with other renewable power sources, such as hydropower, wind, and geothermal. Alternatively, biomethane can be transformed into hydrocarbons or used in ammonia production. However, ammonia based on biomethane may lead to lower environmental benefits compared to other utilisation pathways, e.g., methanol and DME production.33 With regard to ammonia production from biomass, it was shown that the economic performance of biogas as the hydrogen source (via steam reforming) lies in between that of woody biomass gasification and electrolytic hydrogen from renewable sources.34 Finally, ethylene, one of the most valuable petrochemicals, can also be produced from biomass through dehydration of bioethanol derived via fermentation.35
On the downside, certain types of biomass (i.e., first-generation biomass), e.g., starch, sugar, corn and vegetable oil, compete for agricultural land and water, thus leading to increasing food prices or even hindering biodiversity via deforestation.36 This limitation does not apply to non-edible biomass (i.e., second-generation biomass) such as residues from forest and agricultural activities, municipal solid waste, or dedicated crops, which can be cultivated in non-arable or abandoned land (e.g., Miscanthus, poplar, switchgrass, willow). The interest has recently shifted toward the so-called third-generation biomass resources related to algal biomass, which avoid the competition with both agricultural food and land.36 These technologies, however, are not yet competitive due to their low efficiencies. The complexity and diversity of biomass resources, which can show very different physical and chemical properties (e.g., lignin composition, density and moisture contents), make their assessment challenging.
CO2 captured, preferentially from the air (or at point sources, e.g., power plants and industries), could also be used as feedstock to produce chemicals.37,38 The advantage of CO2 utilisation, which could potentially lead to an estimated annual consumption of 0.6–3.7 and 1.0–4.2 Gt CO2eq. for the production of chemicals26,39 and fuels,26 respectively, is twofold: (i) it can enable a short-term anthropogenic carbon cycle (carbon would be released after being stored temporarily) and (ii) it reduces the emissions (not only of CO2) linked to the fossil-based feedstocks. However, this approach could promote further the use of fossil carbon when relying on point sources rather than air as the carbon source. Furthermore, long-term sequestration of CO2 could only be achieved via products with a long carbon storage life, e.g., construction materials, through CO2 mineralization, with an estimated annual utilisation of 0.1–1.4 Gt CO2eq. in 2050 (e.g., concrete and carbon fibre).26,40
Four main approaches are available to transform CO2 into valuable chemicals and fuels: (i) electrocatalytic routes; (ii) thermocatalytic routes; (iii) photo-electrocatalytic routes; and (iv) plasma-assisted routes.41 These technologies require substantial energy to activate the inert CO2 molecule. For example, they often consume large amounts of electricity (e.g., electroreduction toward ethylene38 and formic acid42) or rely on energy-dense reagents (e.g., thermocatalytic hydrogenation towards methanol43). Thus, the environmental footprint and economic profitability of the final products are closely linked to the amount and origin of the required energy or energy carrier. In particular, synthetic natural gas (SNG) can be produced via CO2 and hydrogen, but is at present economically unappealing.44 Bardow and co-workers39 investigated the large-scale adoption of carbon capture and utilisation (CCU) in the chemical industry for both low and high technology readiness level (TRL) pathways, providing an excellent overview of such a transition. In particular, an annual utilisation of 2.8–3.7 Gt CO2eq. in the chemical industry, via either low- or high-TRL technologies, respectively, could result in reductions of global warming impacts by as much as 3.5–3.4 Gt CO2eq.. However, such a transition would require vast amounts of low-carbon electricity, i.e., from 55% up to 97% of the projected global electricity production in 2030, for the low- and high-TRL scenarios, respectively.39 Thus, CCU at a large scale may require the massive installation of renewable power technologies, which could exacerbate other environmental areas of protection.45
In terms of the level of maturity, some CCU processes already reached TRLs of 3–4 or higher, including methanol,43,46–49 SNG,50–52 formic acid,53–55 olefins via methanol,56 aromatics via methanol,57 ethanol,46,53 DME,47 and polyols.46,58 Direct CO2 electroreduction38 processes tend to be less mature (TRLs of 2–4), including methanol,48 formic acid,54,55 SNG,51 carbon monoxide, and ethylene.59 The assessment of CCU routes should consider their different TRLs; along these lines, Roh et al.59 proposed a systematic procedure for the early-stage evaluation of emerging CCU technologies with low TRLs.
CCU can also find applications in the transport sector, where electrofuels chemically storing energy can be used in various transport modes, including road, rail, shipping, and aviation.60 The energy needed should be ideally sourced from the excess of renewable sources linked to the intermittent operation of the power facility.40 Harnessing the excess of intermittent renewable energy for electrofuels production is particularly appealing because their energy embodied often exceeds their energy content.61 Hydrogen, methanol, DME and oxymethylene ethers (OMEs) are among the most investigated electrofuels.60 In particular, OMEs have been the focus of intense research in recent years.62,63 Specifically, two different routes involving intermediate production of formaldehyde are particularly promising.64,65 New synthesis pathways for OMEs have been welcome with enthusiasm by industry as well, with Chinese and German companies already testing some of these routes in pilot plants.66 An alternative pathway relies on the production of syngas via either the reverse water gas shift (RWGS) reaction in tandem with water splitting or the co-electrolysis of water and CO2.67 Subsequently, syngas can be transformed via FT into fuels exhibiting similar properties to those of their fossil counterparts.
Bio- and CO2-based fuels or electric vehicles (EVs), perceived as greener alternatives for the passenger transport, may not necessarily contribute effectively to the sector's decarbonisation. Notably, greener transport alternatives may face social–economic barriers, such as public acceptability and poor economic performance,68,69 which should be considered in their assessment. It was highlighted that the public acceptance of EVs is affected by factors such as driving range, price, and charging infrastructure.69 A recent work also highlighted the importance of evaluating the social acceptance of CO2-based fuels along with their techno-economic and LCA performance, identifying as main public concerns the capture and transport of CO2.68 Furthermore, the competition for biomass between biofuels, power generation and food production, due to the water–energy–food nexus, and the associated impact on ecosystems should not be overlooked.36 Finally, a proper assessment should also consider the links between the electrification of the transport sector and the production of CO2-based fuels and biofuels. In this realm, covering all possible counterfactual scenarios for transportation is not straightforward. Thus, there is a need to enlarge the scope of integrated assessment models (IAMs) to provide a more comprehensive analysis of technologies while exploiting the benefits of combining them into integrated portfolios.
The use of fossil methane as feedstock constitutes a promising alternative to oil.70 The shale gas revolution, brought about by advances in hydraulic fracturing and horizontal drilling, has increased methane and natural gas availability. These feedstocks could replace oil as the carbon source for chemicals. Indeed, light olefins could be produced by selective thermocatalytic dehydrogenation, hydrocarbon halogenation and plasma-assisted non-oxidative methane coupling as well as through syngas from steam methane reforming (SMR) and its combination with the methanol-to-olefins or Fischer–Tropsch-to-olefins process.71–75 Shale gas could lower carbon emissions (relative to oil),76 yet it is still a fossil carbon feedstock suffering from the limitations described above. Furthermore, leakages of methane remain an issue due to its very high global warming potential. Indeed, the optimal methane utilisation route will depend on its end-use phase and the environmental impact of its supply chain.77 Thus, it might be regarded as a short-term interim solution in the transition of the chemical industry. Notably, optimization-based assessments suggested that hybrid plants consuming biomass and natural or shale gas feedstock reduce the environmental footprint and the dependency on fossil sources.78,79 Such hybridization concepts could facilitate the chemical industry's transition towards decarbonised and defossilised manufacturing practices.
Hydrogen80 and ammonia81 are also attracting substantial interest as energy carriers, energy storage alternatives, and fuels. Despite not chemically storing carbon, both chemicals could also lower their carbon footprint by replacing the SMR with water electrolysis or by coupling SMR with CCS systems.81 At present, the CO2 generated in ammonia production is used to produce urea in integrated plants. Therefore, substituting fossil-based hydrogen with electrolytic hydrogen would pose challenges for urea production, creating a demand for CO2 for fertilizers that could be covered with CO2 from the air (direct air capture, DAC), thereby closing the carbon loop. Even though 0.73 kg of CO2 are consumed for each kg of urea produced, it should not be overlooked that the embodied carbon is released into the atmosphere within days after the use of the fertilizer.26
In the last century, ammonia production via the Haber–Bosch process achieved significant improvements in energy efficiency due to (i) the shift from coal to natural gas, (ii) improvements in the efficiency of the compressors, (iii) heat integration, and (iv) catalyst formulation.82 Further efficiency gains are forecasted to occur mainly in the generation of syngas, since the synthesis of ammonia represents only 14% of the theoretical energy loss, here intended as the difference between the process actual energy consumption and the lower heating value of ammonia.83 Advances in the latter stage could be achieved via catalysis engineering and more efficient separation methods for ammonia removal in the process. Careful tuning of the catalyst functionalities and the introduction of optimized separation technologies, such as membranes or efficient absorption units, could increase the process yield and consistently reduce the recompression needs associated with the recycle streams.19,83 In terms of CO2 emissions, the current ammonia production accounts for about 1.2% of the global anthropogenic activities. A shift to electrolytic hydrogen from SMR could lead to a decrease of roughly 71–79% of the total CO2 emissions.19 Finally, pathways that replace the Haber–Bosch synthesis, e.g., using water as a proton source in the direct electrocatalytic reduction of nitrogen84 or in plasma-driven ammonia production from air,85 have been the focus of intense experimental research. A shift to the latter technologies could theoretically improve the energy efficiency,19 yet both alternatives still suffer from very low conversions.
4.2. Moving to renewable energy sources
As already mentioned, an alternative strategy to lower the chemical industry's impact consists of decarbonising its energy inputs, i.e., heat and power. The potential role of industrial heating based on solar energy was discussed in an excellent review by Schoeneberger et al.86 Notably, in 2014, the industrial heating requirements, mainly based on fossil fuels, amounted to 50% of all industrial energy inputs. In the US, the top 14 industries in terms of GHG emissions and energy consumption require heat at temperatures below 300 °C.86,87 At present, the installed solar systems provide temperatures below 250 °C,86 while geothermal heating systems cannot operate above 150 °C.87 Hence, at the current state, renewable-based heating can be used to some extent in industrial applications. Nonetheless, renewable-based heat at higher temperatures could be generated with advances and the adoption of enhanced geothermal systems and heliostats,86,87 allowing industrial processes to shift from fossil combustion- to renewable-based heating. Heat based on renewable carbon, mainly biomass, could also reduce impacts, especially when coupled with CCS systems. Finally, the paradigm of a circular economy should be prioritized by exploiting waste heat from nearby plants.The chemical industry is also expected to benefit (environmentally speaking) from the global transition to cleaner power systems. Notably, customized isolated (off-grid) renewable energy technologies powering chemical clusters could further reduce environmental impacts. In particular, electrolysers coupled with renewable generation (decoupled from existing power networks) could harvest the excess of renewable power (when generation exceeds demand). This technology would produce hydrogen and oxygen with a lower carbon footprint and fossil-resource dependency, which could then be used either as chemical feedstock or for electricity generation in a polymeric membrane fuel cell (to improve the network reliability).22,88
Advances in processes powered by intermittent electricity, like plasma-assisted (i) ethylene production from methane-rich streams,75,89 (ii) ammonia production from air and water,85 and (iii) production pathways based on CO2,41 could help to reduce the environmental footprint of the chemical sector by replacing fossil fuel combustion with renewable electricity as the energy source. A recent work evaluated a hybrid SNG production process from coal and biomass integrating CO2 hydrogenation based on renewable power.44 The CO2 from the SNG is here combined with electrolytic hydrogen, to increase the process yield, and partially stored in geological formations. Therefore, greener production pathways, such as SNG and methanol based on CO2 hydrogenation and biomass, could be combined with methanol-to-olefins and methanol-to-aromatics plants to attain better environmental performance by focusing on the main platform chemicals (Fig. 1). However, the production and use of olefins and aromatics in tandem with CO2-based methanol production, at the current state, is unlikely to be able to compete economically with conventional technologies.56 Nonetheless, similar to the fossil and biomass hybridization concepts mentioned above, a methanol-based facility could flexibly operate with methanol synthesised from fossil-, bio- or CO2-based feedstocks combined with renewable electrolytic hydrogen (Fig. 2).30,56 As a short-term interim solution, hybridization can exploit pathways bringing substantial environmental benefits at a marginal increase in cost. Attaining more ambitious environmental targets, however, will most likely require more costly production pathways. In addition, the use of renewables in the chemical industry competes with the power grid decarbonisation.40
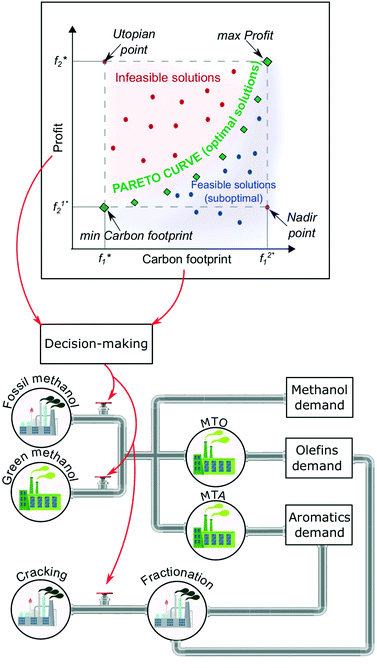 | ||
| Fig. 2 Schematic representation of the chemical industry's smooth and optimal decarbonisation to satisfy the global demand of platform chemicals via hybridization56 of flexible methanol, MTO, and MTA production plants. | ||
4.3. Circular economy
The application of circular economy principles to the production of chemicals and fuels still merits further attention.90 Notably, waste treatment should optimally reuse and recycle materials for further processing as feedstock in the chemical industry and beyond. Iaquaniello et al.91 provided an excellent overview of this area. According to Geyer et al.,13 8300 Mt of plastics were produced as direct resin from fossil resources up until 2017. Also, they estimated that 407 Mt of plastics were produced from virgin materials in 2015, whereas 302 Mt of plastic wastes were generated. The carbon loop could be closed, for instance, by using recycled plastics and other recycled organic materials, mainly valuable chemical monomers, as feedstock, which could even become economically appealing. Within this spirit, Meys et al.92 carried out an environmental assessment of 26 chemical recycling technologies and compared them with 18 conventional waste treatment technologies (e.g., energy recovery in municipal solid waste incinerators or cement kilns, mechanical recycling). In particular, they developed a lower bound LCA analysis of chemical recycling technologies for five types of plastic packaging waste, representing approximately half of the global plastic waste, and analysed the most promising pathways. Finally, Avraamidou et al.93 discussed the role of PSE in the development of optimal circular economy supply chains.5. The role of process modelling and LCA in guiding experimental research on sustainable chemical technologies: a conceptual framework
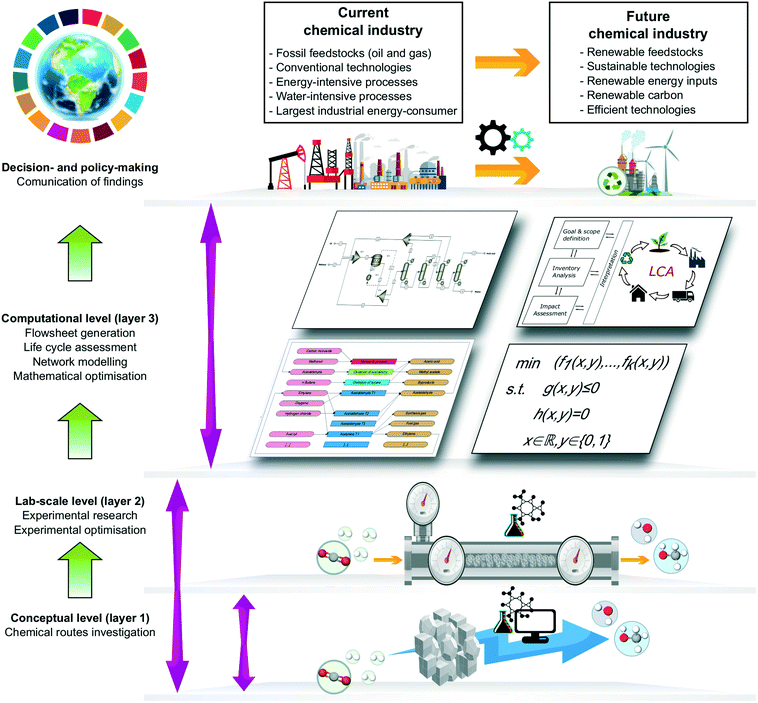
PSE provides a conceptual and methodological framework to improve the chemical supply chain across multiple temporal and spatial scales (from the molecular level to the business level). Notably, PSE can underpin process intensification, material recycling, and energy recovery (e.g., through pinch analysis), all of which could reduce the annual carbon footprint of the chemical industry by as much as 346 Mt CO2eq..20 Tian et al.94 provide an extensive review of state-of-the-art PSE approaches for process intensification. Further improvements could be realised via process optimisation and enterprise-wide optimisation.95 Here, however, we focus on the role of PSE in guiding experimental research on sustainable technologies more effectively, rather than on the optimisation of existing chemical facilities (Fig. 3).Developing technologies with a lower environmental footprint is a challenging task that encompasses various research activities often carried out by separate communities. These steps are taken before technologies are commercialised and decision- and policy-makers can promote their deployment. At the lab scale, after a preeliminary analysis (layer 1 in Fig. 3), experimentalists investigate promising reaction routes, catalytic systems and materials (layer 2 of Fig. 3). Experimental work is often underpinned by molecular models, including density functional theory simulations at the molecular level, which help interpret the results and guide further experiments more sensibly (layer 1 in Fig. 3).96,97 As an example, promising solvents can be identified using mixed-integer programming (MIP) based on quantum mechanical computer-aided molecular design.98
Models of a different nature, i.e., process simulations, LCA studies, and network models, are built next to elucidate whether technologies can add value at an industrial scale (layer 3 of Fig. 3). In preliminary assessments, reaction pathways at an early stage of development (TRLs 1–2), can be screened based on various selection criteria (e.g., environmental and economic potential).37,99 Lack of data at this stage, however, represents a major bottleneck in the assessment, in terms of both accuracy and implementation. This evaluation can still be carried out by considering ideal stoichiometric reactions, full separation of products, general chemical engineering principles (e.g., thermodynamic feasibility, enthalpy of reaction, gross operating margin), and proxy data.59 This preliminary assessment can guide experimental research by identifying potential hotspots and quantifying alternative pathways' benefits. Promising technologies can then be further investigated and optimised experimentally (layer 2 of Fig. 3) (TRLs 3–4) to attain better performance.
These early assessments can be complemented by process simulations relying on well-established first principles (e.g., mass and energy balances and thermodynamics) and ideally validated with experimental data. These models provide the mass and energy flows needed to quantify key techno-economic and environmental performance metrics.59,99 Financial metrics require estimates of the capital (CAPEX) and operational (OPEX) expenditures of the process. The former term is estimated from the equipment sizes provided by the simulation using appropriate correlations.100 Likewise, OPEX expenditures can be obtained from the raw materials and energy requirements retrieved from a simulation model. Based on the obtained results, critical barriers and limitations can be pinpointed. Finally, the operating conditions in process simulations can be optimised to improve the process performance.
On the other hand, life cycle assessment is the primary approach to evaluate the environmental impact. In essence, the LCA methodology allows quantifying all the environmental burdens associated with a product or service across all the stages in its life cycle.101 LCA follows four phases, as described in the ISO 14040 and 14044 standards:102,103 (i) the definition of the goal and scope of the analysis; (ii) the life cycle inventory (LCI) phase, which quantifies the feedstock, emissions and waste associated with the functional unit, i.e., the basis for the calculations; (iii) the life cycle impact assessment step, which translates the inventory flows into environmental impacts on human health, ecosystems and resources; and, finally, (iv) the interpretation phase of the LCA results. LCA is data-intensive; data of the main technologies can be retrieved from process simulations (foreground data),104 whereas data of raw materials and energy inputs are often available in environmental databases (background data) (e.g., Ecoinvent105 and GREET106). The latter data can be used under the assumption that the technologies modelled therein resemble those under study. Unfortunately, sometimes the LCA primary data might not be readily available from databases or other sources. This situation often arises when dealing with new chemicals and bio-based products, for which proxy data estimated using streamlined LCA methods could be used instead.107 Nonetheless, LCAs performed in the absence of a process model by retrieving the foreground data from scientific articles, technical reports, experiments, or existing facilities38,39,63,77,108–120 often overlook the potential for process integration and optimisation, thereby compromising accuracy. Furthermore, relying on a process model is more convenient because it allows analysing more in-depth the process variables' economic and environmental role, generating valuable insight into how to improve technologies.
The scale-up is considered next to elucidate whether higher production capacities can improve, mainly economically,65,78,121 the performance at an industrial scale. Moreover, certain technologies may prove more appropriate than others at different scales, which needs to be investigated before their eventual deployment. For example, pressure swing adsorption and membranes for air separation units perform economically better at smaller scales, while cryogenic distillation is more appealing at larger scales.122 In cases with large OPEX terms and little potential efficiency gains at larger scales, e.g., biogas-based ammonia production,34 the role of scale-up becomes less relevant. In terms of environmental performance, the scale-up could affect the LCIs for the construction of the production site and transportation infrastructure. In the absence of detailed LCIs for the latter activities, the influence of scale-up on the environmental impacts could be modelled with the six-tenths rule,32 as done in economic assessments. Other factors that may influence the environmental performance during the scale-up are the need for feedstock storage, the storage capacity of final products, and the feedstock nature (e.g., liquid or gas, leakages) as well as its regional availability. Furthermore, larger plants are usually associated with a centralized transportation infrastructure, leading to a higher environmental footprint compared to a distributed network of smaller plants.32 As a matter of fact, economies of scale may play a minor role when the environmental impact associated with feedstock transportation and infrastructure construction is negligible compared with the total impact across the product life cycle.121
In a pioneering work, LCA was coupled with optimisation, giving rise to the life cycle optimisation (LCO) approach,123 which has found numerous applications in PSE. In essence, LCO couples an optimisation model with LCA to automate the search for alternatives leading to environmental benefits. The LCO framework was applied to various systems, including hybrid biomass–shale gas plants,79 biofuel and bioproduct networks,124 biofuel production125 and supply chain management.126 Furthermore, LCO was used to study inherent economic and environmental trade-offs in the optimisation of process flowsheets.127 Also, consequential oriented LCO is gaining wide attention.128 Here, changes in physical flows of other sectors are considered when evaluating emerging technologies. More details on the LCO approach can be found elsewhere.5,129,130
We note that further improvements at the plant level in water and energy consumption (and, therefore, in environmental performance) can also be attained through process integration.131 An excellent review of heat and water integration can be found in Ahmetović et al.132 Despite the progress made, most of these methods are very seldom applied to those processes evaluated via LCA, as discussed further in the next sections, which results in less accurate estimates.
The processes of the chemical industry are highly interconnected due to the exchange of mass and energy flows and the consumption of shared resources, e.g., heat, electricity and water. Therefore, the fair and insightful evaluation of emerging technologies should consider the synergistic effects between chemical processes (within a chemical cluster and beyond). To this end, network models based on superstructures should also be developed to screen numerous interlinked processes (layer 3 of Fig. 3). A superstructure is a mathematical representation that embeds all possible combinatorial solutions to a problem. In our context, each solution would represent an alternative pathway/technology to synthesize one or more products.133 Mathematical models based on superstructures identify the best alternatives (i.e., process topology in a process design problem or portfolio of technologies in network models) among many options, considering intermediate material and energy flows, waste, and emissions. In a subsequent step, because the superstructure is often based on shortcut/simplified models, more detailed simulation models can be developed to improve the most promising solutions further and obtain better environmental and economic performance estimates.134 Some network models were developed for biomass28 and petrochemicals.18,39,135 However, a unified model of the chemical industry is still lacking.
The assessment of technologies is affected by various uncertainty sources,103,136 whose omission could lead to inaccurate results and less robust conclusions. The lack of experimental data, the modeling choices, and the wide range of parameters needed, among others, significantly influence the assessment at the early process design stages.137,138 Uncertainties affecting the economic evaluation mainly stem from (i) the market, e.g., feedstock cost and availability, product price, and demand and (ii) process-related factors, e.g., investment cost, yield, and energy consumption. Finally, in the environmental assessment, uncertainties are mostly related to the scope definition, assumptions, data quality, spatial and temporal scope of the LCI analysis, and the LCIA model of choice.139,140 Within this spirit, several articles studied uncertainties in (i) the evaluation of the economic competitiveness of processes,79,136 (ii) the sustainable management of supply chains,141 (iii) the coupling of LCA with process simulation, focusing on uncertainties related to both the foreground and the background system,138 (iv) the quantification of the environmental efficiency of power technologies,140 and (v) the comparison of pathways (with respect to their carbon footprint) based on lab-scale experimental data and simulated models.89 The development and evaluation of process flowsheets under uncertainty were also investigated by Steimel et al.,137 who identified the most critical process parameters from experiments. Finally, failures during the scale-up linked to uncertainties were also studied for processes proven at a pilot-scale.136,142 Sensitivity analysis can also be used to handle uncertainties and quantify their impact; this approach has been widely applied to evaluate technologies and set improvement targets.104,121
Bearing the above in mind, it becomes clear that close collaboration between experimental and computational research groups could facilitate the design and optimisation of emerging, more sustainable pathways and their industrial-scale implementation. Focusing on the multi-scale modeling of processes with complex reaction mechanisms, Zhuang et al.143 also stressed the need for close collaboration between modeling communities and decision-makers. Using a biochemical industry as an example, they introduced a framework for analysing systems holistically at different scales, e.g., from the metabolic model to the economic sector level (via economic input–output, EIO, modeling) and also considering the surrounding ecosystem. Another multi-scale LCA approach was applied to a novel methane-cracking process based on lab-scale experimental data. The scope of the study was expanded to (i) a hypothetical industrial scale-up scenario and (ii) the stoichiometric limit scenario.144 The early stage of development of the process precluded the consideration of the co-product (solid carbon) separation process and its quality in the analysis, leading to uncertainties in the separation burdens and the co-product utilisation benefits. Even though the latter factors bring about uncertainties, early-stage assessments of lab-scale technologies allow guiding future research and development efforts more sensibly. Notably, by screening alternatives and identifying their main hotspots, better experiments could be designed to improve the most promising technologies and overcome their main technical barriers. Ideally, this iterative enhancement loop should be carried out until the desired environmental and economic performance levels are attained. As an example, a model based on thermodynamic equilibrium was suggested to investigate the direct synthesis of OMEs from methanol and formaldehyde.145 The model, after being validated with experimental data, was used to propose further improvements. Namely, they identified the need (i) to adjust the reactor's inlet composition, (ii) to improve the process yield via an efficient downstream separation, and (iii) to increase productivity via catalyst development to enhance the interaction between the acid catalyst and the OME chain.
6. Applications of process modelling and LCA to emerging technologies toward sustainable chemicals and fuels
This section covers PSE studies relevant to the emerging trends discussed in section 4. We focus on the role of process simulation, LCA and network modelling in guiding research on more sustainable technologies. The molecular level is only discussed briefly, as it falls outside the scope of this work.6.1. Molecular level (layer 1 of Fig. 3)
Building process models of emerging technologies can be a difficult task, mainly when dealing with substances with scarce thermodynamic data or complex unit operations. Bearing these limitations in mind, some authors developed streamlined LCA methods to predict the impact embodied in chemicals from information readily available in practice, thereby avoiding the need for process models.146–148 These approaches work well for specific chemicals, mainly petrochemicals, while their application to a broader set of molecules is yet to be studied. Another topic that remains largely unexplored is the multi-objective molecular design of chemicals with minimum environmental footprint.149 The goal here would be to minimise the life cycle impact of molecules meeting some specifications. The scope of this assessment could be enlarged further to cover the use-phase of the chemical, which would require combining process simulation and molecular design constraints within a single model; this approach would be particularly appealing for the assessment of solvents.6.2. Process simulation, life cycle assessment and network modelling (layer 3 of Fig. 3)
Concerning the application domains, biomass-based chemicals and fuels have been the focus of numerous environmental and economic assessments.43,79,115,116,118,121,150–154 Most of the studies focused on stand-alone systems, evaluating a specific biomass type and a limited number of conversion technologies.118,150,152,154 Indeed, the performance of biomass routes strongly depends on the biomass source, so it should be evaluated on a case-by-case basis. For example, methanol from switchgrass via gasification, and its use as intermediate feedstock towards OMEs, was shown to outperform its biogas- and renewable-based CO2 hydrogenation production counterparts.65 Notably, different biomass types exhibit various performance trade-offs, e.g., ethylene via bioethanol from corn stover shows lower GHG emissions but is more expensive than ethylene from corn grain.121 Hybrid plants were also investigated that use renewable electrolytic hydrogen (i) to utilise the CO2 captured during the production of syngas or to improve its quality,30,150 (ii) for biogas upgrading via CO2 hydrogenation towards methane116 and methanol,115 or (iii) to minimise the fossil feedstocks.79 Using biomass as feedstock can find numerous applications, making it the perfect candidate for process integration paradigms. In particular, many biomass processes are multi-functional,115,118,150–152 meaning that electricity, heat, or other co-products can provide revenue or reduce the environmental impact by replacing the fossil-based analogues. These synergies can be exploited within the biorefinery concept via process optimisation.36In the area of CCU, most of the efforts focused on C1-chemicals, e.g., methanol, formic acid, syngas, and methane.47,49,55,109,155 Similarly, as with biomass, most studies limit the scope to single processes, overlooking potential synergies with other technologies. Nevertheless, some CCU studies exploited synergies between the main process and the CO2 capture step via process integration,31,156–158e.g., meeting the heating requirements for CO2 capture with surplus heat from the primary process.
Furthermore, studies often restrict the scope to environmental impact(s) tightly linked to the use of biomass and CO2 as feedstock, focusing on global warming and fossil depletion metrics.38,42,48,77,78,109,120,126,159–163 However, the large-scale adoption of emerging technologies could unintendedly shift burdens across environmental categories or echelons in the product's supply chain. It was stressed that LCA studies on CCU products are not harmonized in terms of assumptions, scope, system boundaries and impact metrics, which makes comparisons challenging.164 A meta-analysis of recent peer-reviewed articles showed that environmental burden-shifting might occur after harmonizing the LCA studies,164 most of which differ in the methodological approach and background data (e.g., CO2 feedstock origin). An increasing number of more recent studies, however, started to embrace environmental impacts beyond climate change and fossil depletion.31,43,47,52,55,56,58,63,108,110,111,113–117,151–153,158,165
An open issue in CCU concerns how to deal with the multi-functionality arising in LCAs,24,166 on which the lack of a generalized consensus has led to significant disparities across studies.24 In essence, there are four modelling choices to address multi-functionality, e.g., (i) subdivision, (ii) system expansion, (iii) substitution, and (iv) allocation. There is, therefore, still a clear need to establish a common ground for future assessments, as analysed in detail elsewhere.24
At the process modelling side, modelling the continuous operation of processes using intermittent renewable power remains challenging. Nonetheless, some studies investigated how the intermittent nature of renewable sources affects the production of electrolytic hydrogen167,168 and also identified suitable locations for the integrated facility.30 The standard approach here is to rely on simplifications with various accuracy levels to avoid the explicit dynamic modelling of the fluctuating nature of renewable energy sources such as wind and solar. The latter behaviour was omitted in many studies,42,63,112 which may result in optimistic economic and environmental estimates. Other studies assumed an ideal intermittent power generation by considering the average operational hours of the non-dispatchable source, which results in less accurate predictions.50,155 Alternatively, some studies considered storage modules to close the gap between the fluctuating production of feedstocks and the plant's continuous operation to satisfy a constant demand. In particular, focusing on CO2 hydrogenation routes, a constant supply of hydrogen was assumed by considering seasonal storage in pressurized vessels or salt caverns.56,156,157,162,169–172 Along these lines, other studies also considered energy storage in batteries.156
The studies above found that at the current state, only a few CCU applications are economically appealing compared to their fossil counterparts.58,160 Fernández-Dacosta et al. investigated, both environmentally and economically, the CO2-based (i) production of polyols as a stand-alone system58 and (ii) co-production of DME (via syngas from dry methane reforming) and polyols.160 The CO2 feedstock was assumed to be captured by a hydrogen unit at a refinery. They showed that these emerging CCU alternatives are economically feasible and lead to lower environmental impact than their fossil counterparts. However, most CCU chemicals and fuels are economically unappealing due to their high energy requirements.56,155,157 Nonetheless, CCU technologies could exploit regional advantages to lower the cost, e.g., through their deployment in suitable locations with cleaner and cheaper renewable energy and CO2.30,170
A chemical industry based on CO2 feedstock would require large amounts of water to produce electrolytic hydrogen or for the co-electrolysis step. Some studies proposed to obtain this water from water desalination,156 while others suggested the separation, treatment, and recycling of water to the electrolyser.167,168 Several works investigated the economic and environmental potential of the oxygen co-product in water electrolysis.42,157,162,168 However, the standard approach has been to omit it in the environmental assessment due to the uncertain oxygen demand linked to the large-scale water splitting economy and the need for liquefaction to meet its commercial specifications.56,162,165
With regard to network models, optimisation-based approaches have proved useful to investigate networks encompassing multiple emerging technologies. The goal is to find the optimal combination of biomass and CCU processes to meet a given demand for chemicals and fuels while meeting some constraints. Network models have been long considered in the literature,173–175e.g., the production of methanol via the combination of technologies was already investigated in 1984.175 However, their application to the analysis of biomass28,124,126,176,177 and CCU routes53,56,178–182 is scarce to date.
A wide range of biomass sources (e.g., Miscanthus, switchgrass, corn woodchips, sewage sludge, manure) can be converted into valuable products (e.g., electricity, heat, chemicals, and fuels) through multiple routes (e.g., combustion, gasification, fermentation, anaerobic digestion). Therefore, the analysis of biomass conversion pathways leads to complex optimisation problems due to the vast amount of options available.36 In this context, optimisation tools can automate the search for the best alternatives (from an economic and environmental viewpoint).176 Since natural resources constrain the use of biomass (e.g., water, land, and agrochemicals), a proper analysis should be region-specific and consider the competition for resources with other technologies176,177 while covering the whole biomass supply chain.126 Finally, motivated by the limited availability and price of fossil- and biomass-based feedstocks, several studies investigated the integration of conventional fossil-based production technologies with biomass routes via hybrid feedstock facilities.78,177 As an example, the combination of different feedstocks, e.g., biomass, coal, and natural gas, was considered to produce liquid fuels within the framework of simultaneous process synthesis, heat, power, and water integration.183
Moving to CCU, most of the network models focused mainly on supply chain problems with only economic objectives, where reductions in CO2 emissions if at all attained were considered as an advantageous side effect rather than a pursued objective.53,178,180,181,184 In contrast, only a few studies incorporated (via LCA) environmental metrics others than climate-related impacts in their optimisation frameworks.39,56,179,182 Furthermore, some studies developed carbon capture utilisation and storage (CCUS) supply chain network optimisation problems, mostly for supplying the CO2 for EOR, thereby avoiding its release to the atmosphere and the associated adverse environmental impacts.178,180,181,184 Finally, recent studies highlighted that the success of energy-intensive CCU pathways is strongly dependent on the economic performance of hydrogen production.56,179,180,182
The combined use of LCA and network modeling still needs to address some challenges associated with CCU. First, network models should consider integrating emerging technologies within the same production site to reduce the mass and energy inputs, e.g., via heat and mass integration.185 Notably, CCU technologies can be highly energy-intensive. Hence, overlooking heat integration could result in pessimistic estimates. Furthermore, as occurred at the plant level, future studies should embrace environmental impacts beyond climate change to identify the potential occurrence of environmental burden-shifting. The planetary boundaries framework, which defines a set of critical biophysical limits that should never be transgressed for the planet's safe operation, could help quantify these trade-offs precisely and identify appealing technologies from a worldwide sustainability perspective. This approach holds good promise in the environmental assessment of chemicals and fuels with large production volumes, as shown in recent studies on methanol43 and hydrogen.186
7. Conclusions
The successful transition towards an environmentally friendly chemical industry will require shifting away from fossil resources. In this context, the PSE community can underpin this transition by evaluating and optimising emerging technologies (e.g., biomass and CCU).The proper assessment of chemicals and fuels still requires further methodological developments. At the modelling side, and particularly in CCU, process models should be harmonized in areas such as the use of intermittent renewable energy and the seasonal storage of electrolytic hydrogen. Notably, assuming a smooth discrete operation driven by intermittent power sources, or full availability of an excess of renewable energy at all times, might result in too optimistic estimates.
With regard to LCA, the multi-functionality problem linked to the use of CO2 as feedstock needs to be tackled adequately by following LCA-based standards to ensure a fair comparison of alternatives.24,187 Furthermore, impacts beyond climate change should be considered to identify the potential occurrence of burden-shifting on ecosystems and human health. Planetary boundaries could help to understand the global implications of burden-shifting and quantify the absolute sustainability level of technologies precisely. The social dimension of sustainability should also be evaluated, which requires addressing challenges related to data scarcity as well as methodological gaps.188 Indeed, combining life cycle costing and environmental LCA with social LCA could help to uncover the real potential of emerging technologies. These assessments should always be based on sound process models validated with experimental data and accounting for the synergistic effects of integrating technologies.
Notably, the integrated design of renewable-based co-production networks for chemicals, fuels, and electricity with a detailed dynamic and regionalized modelling of resource availability seems the way forward. This holistic analysis could be performed via network modelling and superstructure optimisation with explicit consideration of operational constraints and the time-varying availability of renewable resources.189,190
A chemical industry based on CO2 as the carbon feedstock will demand a vast amount of clean energy, which can worsen several environmental categories. On the other hand, the large-scale conversion of biomass into chemicals and biofuels might pose threats to land and water use, which call for regionalized assessments. Given these limitations, integrating fossil, biomass and CCU technologies could smooth the transition towards a more sustainable chemical industry. Holistic network models covering a wide range of alternative routes and their potential synergies could assist in the design of these hybrid facilities.
The interplay between economic sectors will ultimately determine the best use of renewable feedstocks (e.g., biomass and CO2) and the optimal combination of technologies (e.g., wind plants, solar panels, CO2 storage and batteries) to produce chemicals and fuels with a lower environmental footprint. In this context, PSE could assist in identifying the best pathways toward sustainable development by enlarging the scope of IAMs and, in so doing, enabling a more accurate assessment of chemicals and fuels.
All in all, we identified the need of integrating process modelling, life cycle assessment and optimisation following general guidelines and agreed standards and apply them to guide experimental work more sensibly. These analyses should consider other sectors, including the power and transport sector (sector coupling), and the option of hybridizing existing and emerging technologies to exploit their complementary strengths. Therefore, the PSE, industrial ecology and experimental communities should interact more closely to integrate multiple scales (from the molecular to the planet level) and develop the technologies needed to underpin sustainable development.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This publication was created as part of NCCR Catalysis, a National Centre of Competence in Research funded by the Swiss National Science Foundation.References
- OECD, Introduction to the OECD Environment, Health and Safety Programme, Paris, 2019 Search PubMed.
- C. Blum, D. Bunke, M. Hungsberg, E. Roelofs, A. Joas, R. Joas, M. Blepp and H.-C. Stolzenberg, Sustain. Chem. Pharm., 2017, 5, 94–104 CrossRef CAS.
- S. Axon and D. James, Curr. Opin. Green Sustain. Chem., 2018, 13, 140–145 CrossRef.
- G. A. UN, Transforming our world: The 2030 agenda for sustainable development, A/RES/70/1, 21 October, 2015 Search PubMed.
- G. Guillén-Gosálbez, F. You, Á. Galán-Martín, C. Pozo and I. E. Grossmann, Curr. Opin. Chem. Eng., 2019, 26, 170–179 CrossRef.
- B. R. Bakshi, Annu. Rev. Chem. Biomol. Eng., 2019, 10, 265–288 CrossRef PubMed.
- A. Bazzanella and F. Ausfelder, Low carbon energy and feedstock for the European chemical industry, DECHEMA, Gesellschaft für Chemische Technik und Biotechnologie eV, 2017 Search PubMed.
- J. Kleinekorte, L. Fleitmann, M. Bachmann, A. Kätelhön, A. Barbosa-Póvoa, N. von der Assen and A. Bardow, Annu. Rev. Chem. Biomol. Eng., 2020, 11, 203–233 CrossRef PubMed.
- Z. Yuan, M. R. Eden and R. Gani, Ind. Eng. Chem. Res., 2016, 55, 3383–3419 CrossRef CAS.
- P. Gabrielli, M. Gazzani and M. Mazzotti, Ind. Eng. Chem. Res., 2020, 59, 7033–7045 CrossRef CAS.
- W. Steffen, W. Broadgate, L. Deutsch, O. Gaffney and C. Ludwig, Anthr. Rev., 2015, 2, 81–98 Search PubMed.
- IPCC, Global Warming of 1.5°C. An IPCC Special Report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty, Intergovernmental Panel on Climate Change, 2018 Search PubMed.
- R. Geyer, J. R. Jambeck and K. L. Law, Sci. Adv., 2017, 3, e1700782 CrossRef PubMed.
- M. M. Mekonnen and Y. A. Hoekstra, Sci. Adv., 2016, 2, e1500323 CrossRef PubMed.
- J. Rockström, W. Steffen, K. Noone, Å. Persson, F. S. Chapin, E. Lambin, T. M. Lenton, M. Scheffer, C. Folke, H. J. Schellnhuber, B. Nykvist, C. A. de Wit, T. Hughes, S. van der Leeuw, H. Rodhe, S. Sörlin, P. K. Snyder, R. Costanza, U. Svedin, M. Falkenmark, L. Karlberg, R. W. Corell, V. J. Fabry, J. Hansen, B. Walker, D. Liverman, K. Richardson, P. Crutzen and J. Foley, Ecol. Soc., 2009, 14, 1–22 Search PubMed.
- W. Steffen, K. Richardson, J. Rockström, S. E. Cornell, I. Fetzer, E. M. Bennett, R. Biggs, S. R. Carpenter, W. de Vries, C. A. de Wit, C. Folke, D. Gerten, J. Heinke, G. M. Mace, L. M. Persson, V. Ramanathan, B. Reyers and S. Sorlin, Science, 2015, 347, 1259855 CrossRef PubMed.
- T. Sterner, E. B. Barbier, I. Bateman, I. van den Bijgaart, A.-S. Crépin, O. Edenhofer, C. Fischer, W. Habla, J. Hassler and O. Johansson-Stenman, Nat. Sustain., 2019, 2, 14 CrossRef.
- S. E. DeRosa and D. T. Allen, in Encyclopedia of Sustainable Technologies, ed. M. A. Abraham, Elsevier, Oxford, 2017, pp. 339–347 Search PubMed.
- C. Smith, A. K. Hill and L. Torrente-Murciano, Energy Environ. Sci., 2020, 13, 331–344 RSC.
- IEA, Technology Roadmap - Energy and GHG Reductions in the Chemical Industry via Catalytic Processes, IEA, Paris, 2013 Search PubMed.
- IEA, CO2 Emissions from Fuel Combustion: Overview, IEA, Paris, 2020 Search PubMed.
- A. Sternberg and A. Bardow, Energy Environ. Sci., 2015, 8, 389–400 RSC.
- D. Leeson, N. Mac Dowell, N. Shah, C. Petit and P. S. Fennell, Int. J. Greenhouse Gas Control, 2017, 61, 71–84 CrossRef CAS.
- L. Müller, A. Kätelhön, S. Bringezu, S. McCoy, S. Suh, R. Edwards, V. Sick, S. Kaiser, R. Cuellar-Franca and A. El Khamlichi, Energy Environ. Sci., 2020, 13, 2979–2992 RSC.
- K. M. van Geem, V. V. Galvita and G. B. Marin, Science, 2019, 364, 734–735 CrossRef CAS PubMed.
- C. Hepburn, E. Adlen, J. Beddington, E. A. Carter, S. Fuss, N. Mac Dowell, J. C. Minx, P. Smith and C. K. Williams, Nature, 2019, 575, 87–97 CrossRef CAS PubMed.
- A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411–2502 CrossRef CAS PubMed.
- J. Kim, S. M. Sen and C. T. Maravelias, Energy Environ. Sci., 2013, 6, 1093–1104 RSC.
- G. W. Huber, S. Iborra and A. Corma, Chem. Rev., 2006, 106, 4044–4098 CrossRef CAS PubMed.
- M. Martín and I. E. Grossmann, Comput. Chem. Eng., 2017, 105, 308–316 CrossRef.
- W. Schakel, G. Oreggioni, B. Singh, A. Strømman and A. Ramírez, J. CO2 Util., 2016, 16, 138–149 CrossRef CAS.
- A. Fusi, J. Bacenetti, M. Fiala and A. Azapagic, Front. Bioeng. Biotechnol., 2016, 4, 26 Search PubMed.
- E. A. Moghaddam, S. Ahlgren and Å. Nordberg, Front. Bioeng. Biotechnol., 2016, 4, 89 Search PubMed.
- P. Tunå, C. Hulteberg and S. Ahlgren, Environ. Prog. Sustainable Energy, 2014, 33, 1290–1297 CrossRef.
- A. Mohsenzadeh, A. Zamani and M. J. Taherzadeh, ChemBioEng Rev., 2017, 4, 75–91 CrossRef.
- H. R. Ghatak, Renewable Sustainable Energy Rev., 2011, 15, 4042–4052 CrossRef CAS.
- A. Otto, T. Grube, S. Schiebahn and D. Stolten, Energy Environ. Sci., 2015, 8, 3283–3297 RSC.
- P. De Luna, C. Hahn, D. Higgins, S. A. Jaffer, T. F. Jaramillo and E. H. Sargent, Science, 2019, 364, eaav3506 CrossRef CAS PubMed.
- A. Kätelhön, R. Meys, S. Deutz, S. Suh and A. Bardow, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 11187–11194 CrossRef PubMed.
- J. C. Abanades, E. S. Rubin, M. Mazzotti and H. J. Herzog, Energy Environ. Sci., 2017, 10, 2491–2499 RSC.
- A. George, B. Shen, M. Craven, Y. Wang, D. Kang, C. Wu and X. Tu, Renewable Sustainable Energy Rev., 2021, 135, 109702 CrossRef CAS.
- M. Rumayor, A. Dominguez-Ramos and A. Irabien, Appl. Sci., 2018, 8, 914 CrossRef.
- A. González-Garay, M. S. Frei, A. Al-Qahtani, C. Mondelli, G. Guillén-Gosálbez and J. Pérez-Ramírez, Energy Environ. Sci., 2019, 12, 3425–3436 RSC.
- C. Bassano, P. Deiana, G. Vilardi and N. Verdone, Appl. Energy, 2020, 263, 114590 CrossRef CAS.
- I. M. Algunaibet and G. Guillén-Gosálbez, J. Cleaner Prod., 2019, 229, 886–901 CrossRef.
- S. Schemme, J. L. Breuer, M. Köller, S. Meschede, F. Walman, R. C. Samsun, R. Peters and D. Stolten, Int. J. Hydrogen Energy, 2020, 45, 5395–5414 CrossRef CAS.
- M. Matzen and Y. Demirel, J. Cleaner Prod., 2016, 139, 1068–1077 CrossRef CAS.
- M. Rumayor, A. Dominguez-Ramos and A. Irabien, J. Cleaner Prod., 2019, 225, 426–434 CrossRef CAS.
- M. Pérez-Fortes, J. C. Schöneberger, A. Boulamanti and E. Tzimas, Appl. Energy, 2016, 161, 718–732 CrossRef.
- S. Chiuta, N. Engelbrecht, G. Human and D. G. Bessarabov, J. CO2 Util., 2016, 16, 399–411 CrossRef CAS.
- A. Dominguez-Ramos and A. Irabien, Sustain. Prod. Consum., 2019, 17, 229–240 CrossRef.
- D. Parra, X. Zhang, C. Bauer and M. K. Patel, Appl. Energy, 2017, 193, 440–454 CrossRef.
- A. Xu, S. Indala, T. A. Hertwig, R. W. Pike, F. C. Knopf, C. L. Yaws and J. R. Hopper, Clean Technol. Environ. Policy, 2005, 7, 97–115 CrossRef CAS.
- M. Rumayor, A. Dominguez-Ramos, P. Perez and A. Irabien, J. CO2 Util., 2019, 34, 490–499 CrossRef CAS.
- N. Thonemann and A. Schulte, Environ. Sci. Technol., 2019, 53, 12320–12329 CrossRef CAS PubMed.
- I. Ioannou, S. C. D'Angelo, A. J. Martín, J. Pérez-Ramírez and G. Guillén-Gosálbez, ChemSusChem, 2020, 13, 6370–6380 CAS.
- D. Zhang, M. Yang and X. Feng, Comput. Chem. Eng., 2019, 126, 178–188 CrossRef CAS.
- C. Fernández-Dacosta, M. Van Der Spek, C. R. Hung, G. D. Oregionni, R. Skagestad, P. Parihar, D. T. Gokak, A. H. Strømman and A. Ramirez, J. CO2 Util., 2017, 21, 405–422 CrossRef.
- K. Roh, A. Bardow, A. Bardow, A. Bardow, A. Bardow, D. Bongartz, J. Burre, W. Chung, S. Deutz, D. Han, M. Heßelmann, Y. Kohlhaas, A. König, J. S. Lee, R. Meys, S. Völker, M. Wessling, M. Wessling, J. H. Lee, A. Mitsos, A. Mitsos and A. Mitsos, Green Chem., 2020, 22, 3842–3859 RSC.
- S. Brynolf, M. Taljegard, M. Grahn and J. Hansson, Renewable Sustainable Energy Rev., 2018, 81, 1887–1905 CrossRef.
- R. McGinnis, Joule, 2020, 4, 509–511 CrossRef.
- M. Held, Y. Tönges, D. Pélerin, M. Härtl, G. Wachtmeister and J. Burger, Energy Environ. Sci., 2019, 12, 1019–1034 RSC.
- S. Deutz, D. Bongartz, B. Heuser, A. Kätelhön, L. S. Langenhorst, A. Omari, M. Walters, J. Klankermayer, W. Leitner and A. Mitsos, Energy Environ. Sci., 2018, 11, 331–343 RSC.
- P. Bokinge, S. Heyne and S. Harvey, Energy Sci. Eng., 2020, 8, 2587–2598 CrossRef.
- M. Mart, J. Redondo, I. E. Grossmann, M. Martín, J. Redondo and I. E. Grossmann, ACS Sustainable Chem. Eng., 2020, 8, 6496–6504 CrossRef.
- C. J. Baranowski, A. M. Bahmanpour and O. Kröcher, Appl. Catal., B, 2017, 217, 407–420 CrossRef CAS.
- G. Herz, E. Reichelt and M. Jahn, Appl. Energy, 2018, 215, 309–320 CrossRef CAS.
- J. Offermann-van Heek, K. Arning, A. Sternberg, A. Bardow and M. Ziefle, Energy Policy, 2020, 143, 111586 CrossRef CAS.
- L. Noel, G. Zarazua de Rubens, J. Kester and B. K. Sovacool, Energy Policy, 2020, 138, 111292 CrossRef.
- J. Gao and F. You, Comput. Chem. Eng., 2017, 106, 699–718 CrossRef CAS.
- E. McFarland, Science, 2012, 338, 340–342 CrossRef CAS PubMed.
- J. J. H. B. Sattler, J. Ruiz-Martinez, E. Santillan-Jimenez and B. M. Weckhuysen, Chem. Rev., 2014, 114, 10613–10653 CrossRef CAS.
- R. Lin, A. P. Amrute and J. Pérez-Ramírez, Chem. Rev., 2017, 117, 4182–4247 CrossRef CAS PubMed.
- G. Zichittella, N. Aellen, V. Paunović, A. P. Amrute and J. Pérez-Ramírez, Angew. Chem., Int. Ed., 2017, 56, 13670 CrossRef CAS PubMed.
- E. Delikonstantis, M. Scapinello and G. Stefanidis, Processes, 2019, 7, 68 CrossRef CAS.
- K. Feng, S. J. Davis, L. Sun and K. Hubacek, Nat. Commun., 2015, 6, 1–8 Search PubMed.
- W. Hoppe, S. Bringezu and N. Thonemann, J. Cleaner Prod., 2016, 121, 231–237 CrossRef CAS.
- O. Onel, A. M. Niziolek, J. A. Elia, R. C. Baliban and C. A. Floudas, Ind. Eng. Chem. Res., 2015, 54, 359–385 CrossRef CAS.
- C. He and F. You, AIChE J., 2015, 61, 1209–1232 CrossRef CAS.
- I. Staffell, D. Scamman, A. Velazquez Abad, P. Balcombe, P. E. Dodds, P. Ekins, N. Shah and K. R. Ward, Energy Environ. Sci., 2019, 12, 463–491 RSC.
- D. R. MacFarlane, P. V. Cherepanov, J. Choi, B. H. R. Suryanto, R. Y. Hodgetts, J. M. Bakker, F. M. Ferrero Vallana and A. N. Simonov, Joule, 2020, 4, 1186–1205 CrossRef CAS.
- K. H. R. Rouwenhorst, P. M. Krzywda, N. E. Benes, G. Mul and L. Lefferts, in Techno-Economic Challenges of Green Ammonia as an Energy Vector, ed. A. Valera-Medina and R. Banares-Alcantara, Academic Press, 2021, pp. 41–83 Search PubMed.
- S. E. Nielsen, in Innovations in Industrial and Engineering Chemistry, American Chemical Society, 2008, vol. 1000, pp. 2–15 Search PubMed.
- A. J. Martín, T. Shinassssgawa and J. Pérez-Ramírez, Chem, 2019, 5, 263–283 Search PubMed.
- J. Sun, D. Alam, R. Daiyan, H. Masood, T. Zhang, R. Zhou, P. J. Cullen, E. C. Lovell, A. R. Jalili and R. Amal, Energy Environ. Sci., 2021, 14, 865–872 RSC.
- C. A. Schoeneberger, C. A. McMillan, P. Kurup, S. Akar, R. Margolis and E. Masanet, Energy, 2020, 206, 118083 CrossRef.
- C. McMillan, R. Boardman, M. McKellar, P. Sabharwall, M. Ruth and S. Bragg-Sitton, Generation and Use of Thermal Energy in the U.S. Industrial Sector and Opportunities to Reduce its Carbon Emissions, United States, 2016 Search PubMed.
- J. Kotowicz, D. Węcel and M. Jurczyk, Appl. Energy, 2018, 216, 45–59 CrossRef.
- E. Delikonstantis, E. Igos, M. Augustinus, E. Benetto and G. D. Stefanidis, Sustainable Energy Fuels, 2020, 4, 1351–1362 RSC.
- T. Keijer, V. Bakker and J. C. Slootweg, Nat. Chem., 2019, 11, 190–195 CrossRef CAS PubMed.
- G. Iaquaniello, G. Centi, A. Salladini, E. Palo and S. Perathoner, Chem. – Eur. J., 2018, 24, 11831–11839 CrossRef CAS PubMed.
- R. Meys, F. Frick, S. Westhues, A. Sternberg, J. Klankermayer and A. Bardow, Resour., Conserv. Recycl., 2020, 162, 105010 CrossRef.
- S. Avraamidou, S. G. Baratsas, Y. Tian and E. N. Pistikopoulos, Comput. Chem. Eng., 2020, 133, 106629 CrossRef CAS.
- Y. Tian, S. E. Demirel, M. M. F. Hasan and E. N. Pistikopoulos, Chem. Eng. Process., 2018, 133, 160–210 CrossRef CAS.
- I. Grossmann, AIChE J., 2005, 51, 1846–1857 CrossRef CAS.
- A. Klamt, F. Eckert and W. Arlt, Annu. Rev. Chem. Biomol. Eng., 2010, 1, 101–122 CrossRef CAS PubMed.
- T. A. Young, J. J. Silcock, A. J. Sterling and F. Duarte, Angew. Chem., Int. Ed., 2021, 60, 4266–4274 CrossRef CAS PubMed.
- H. Struebing, Z. Ganase, P. G. Karamertzanis, E. Siougkrou, P. Haycock, P. M. Piccione, A. Armstrong, A. Galindo and C. S. Adjiman, Nat. Chem., 2013, 5, 952–957 CrossRef CAS PubMed.
- G. Thomassen, M. Van Dael, S. Van Passel and F. You, Green Chem., 2019, 21, 4868–4886 RSC.
- G. P. Towler and R. K. Sinnott, Chemical engineering design: principles, practice, and economics of plant and process design, Butterworth-Heinemann, Oxford, 2012 Search PubMed.
- S. Hellweg and L. Milà i Canals, Science, 2014, 344, 1109–1113 CrossRef CAS PubMed.
- ISO, ISO 14040 International Standard, in Environmental Management –Life Cycle Assessment – Principles and Framework, International Organisationfor Standardization, Geneva, Switzerland, 2006 Search PubMed.
- ISO, ISO 14044 International Standard, in Environmental Management –Life Cycle Assessment – Requirements and Guidelines, International Organisation for Standardisation, Geneva, Switzerland, 2006 Search PubMed.
- M. Yang and F. You, Ind. Eng. Chem. Res., 2017, 56, 4038–4051 CrossRef CAS.
- G. Wernet, C. Bauer, B. Steubing, J. Reinhard, E. Moreno-ruiz and B. Weidema, Int. J. Life Cycle Assess., 2016, 3, 1218–1230 CrossRef.
- Argonne National Laboratory, Greenhouse gases, Regulated Emissions, and Energy use in Transportation (GREET) Model Version 1.3.0.13520, 2019 Search PubMed.
- R. G. Hunt, T. K. Boguski, K. Weitz and A. Sharma, Int. J. Life Cycle Assess., 1998, 3, 36 CrossRef.
- R. Meys, A. Kätelhön and A. Bardow, Green Chem., 2019, 21, 3334–3342 RSC.
- A. Sternberg, C. M. Jens and A. Bardow, Green Chem., 2017, 19, 2244–2259 RSC.
- N. von der Assen and A. Bardow, Green Chem., 2014, 16, 3272–3280 RSC.
- W. Hoppe, N. Thonemann and S. Bringezu, J. Ind. Ecol., 2018, 22, 327–340 CrossRef CAS.
- C. van der Giesen, R. Kleijn and G. J. Kramer, Environ. Sci. Technol., 2014, 48, 7111–7121 CrossRef CAS PubMed.
- Y. Ahn, J. Byun, D. Kim, B.-S. Kim, C.-S. Lee and J. Han, Green Chem., 2019, 21, 3442–3455 RSC.
- M. Rumayor, A. Dominguez-Ramos and A. Irabien, Sustain. Prod. Consum., 2019, 18, 72–82 CrossRef.
- L. Eggemann, N. Escobar, R. Peters, P. Burauel and D. Stolten, J. Cleaner Prod., 2020, 122476 CrossRef CAS.
- P. Collet, E. Flottes, A. Favre, L. Raynal, H. Pierre, S. Capela and C. Peregrina, Appl. Energy, 2017, 192, 282–295 CrossRef CAS.
- R. Cuéllar-Franca, P. García-Gutiérrez, I. Dimitriou, R. H. Elder, R. W. K. Allen and A. Azapagic, Appl. Energy, 2019, 253, 113560 CrossRef.
- C. Liptow, A.-M. M. Tillman and M. Janssen, Int. J. Life Cycle Assess., 2015, 20, 632–644 CrossRef CAS.
- M. Li, W. Zhao, Y. Xu, Y. Zhao, K. Yang, W. Tao and J. Xiao, Ind. Eng. Chem. Res., 2019, 58, 19179–19188 CrossRef CAS.
- R. Aldaco, I. Butnar, M. Margallo, J. Laso, M. Rumayor, A. Dominguez-Ramos, A. Irabien and P. E. Dodds, Sci. Total Environ., 2019, 663, 738–753 CrossRef CAS PubMed.
- M. Yang, X. Tian and F. You, Ind. Eng. Chem. Res., 2018, 57, 5980–5998 CrossRef CAS.
- A. Sánchez and M. Martín, Sustain. Prod. Consum., 2018, 16, 176–192 CrossRef.
- A. Azapagic and R. Clift, Comput. Chem. Eng., 1999, 23, 1509–1526 CrossRef CAS.
- D. J. Garcia and F. You, ACS Sustainable Chem. Eng., 2015, 3, 1732–1744 CrossRef CAS.
- D. F. Rodríguez-Vallejo, Á. Galán-Martín, G. Guillén-Gosálbez and B. Chachuat, AIChE J., 2018, 65, e16480 CrossRef.
- D. Yue, M. A. Kim and F. You, ACS Sustainable Chem. Eng., 2013, 1, 1003–1014 CrossRef CAS.
- G. Guillen-Gosalbez, J. A. Caballero and L. Jimenez, Ind. Eng. Chem. Res., 2008, 47, 777–789 CrossRef CAS.
- J. Gong and F. You, ACS Sustainable Chem. Eng., 2017, 5, 5887–5911 CrossRef CAS.
- C. Pieragostini, M. C. Mussati and P. Aguirre, J. Environ. Manage., 2012, 96, 43–54 CrossRef PubMed.
- I. E. Grossmann and G. Guillen-Gosalbez, Comput. Chem. Eng., 2010, 34, 1365–1376 CrossRef CAS.
- L. S. Dias and M. G. Ierapetritou, Curr. Opin. Chem. Eng., 2019, 25, 82–86 CrossRef.
- E. Ahmetović, N. Ibrić, Z. Kravanja and I. E. Grossmann, Comput. Chem. Eng., 2015, 82, 144–171 CrossRef.
- R. R. Iyer and I. E. Grossmann, Ind. Eng. Chem. Res., 1998, 37, 474–481 CrossRef CAS.
- M.-O. Bertran, R. Frauzem, A.-S. Sanchez-Arcilla, L. Zhang, J. M. Woodley and R. Gani, Comput. Chem. Eng., 2017, 106, 892–910 CrossRef CAS.
- R. Calvo-Serrano and G. Guillén-Gosálbez, ACS Sustainable Chem. Eng., 2018, 6, 7109–7118 CrossRef CAS.
- E. Dheskali, A. A. Koutinas and I. K. Kookos, Chem. Eng. Res. Des., 2020, 163, 273–280 CrossRef CAS.
- J. Steimel, M. Harrmann, G. Schembecker and S. Engell, Comput. Chem. Eng., 2013, 59, 63–73 CrossRef CAS.
- A. D. Bojarski, G. Guillén-Gosálbez, L. Jiménez, A. Espuña and L. Puigjaner, Ind. Eng. Chem. Res., 2008, 47, 8286–8300 CrossRef CAS.
- M. A. J. Huijbregts, Int. J. Life Cycle Assess., 1998, 3, 273–280 CrossRef.
- A. Ewertowska, C. Pozo, J. Gavaldà, L. Jiménez and G. Guillén-Gosálbez, J. Cleaner Prod., 2017, 166, 771–783 CrossRef.
- G. Guillén-Gosálbez and I. Grossmann, Comput. Chem. Eng., 2010, 34, 42–58 CrossRef.
- K. Sanford, G. Chotani, N. Danielson and J. A. Zahn, Curr. Opin. Biotechnol., 2016, 38, 112–122 CrossRef CAS PubMed.
- K. Zhuang, B. R. Bakshi and M. J. Herrgård, Biotechnol. J., 2013, 8, 973–984 CrossRef CAS PubMed.
- S. Postels, A. Abánades, N. von der Assen, R. K. Rathnam, S. Stückrad and A. Bardow, Int. J. Hydrogen Energy, 2016, 41, 23204–23212 CrossRef CAS.
- M. Ouda, G. Yarce, R. J. White, M. Hadrich, D. Himmel, A. Schaadt, H. Klein, E. Jacob and I. Krossing, React. Chem. Eng., 2017, 2, 50–59 RSC.
- R. Calvo-Serrano, M. González-Miquel and G. Guillén-Gosálbez, ACS Sustainable Chem. Eng., 2018, 7, 3575–3583 CrossRef.
- R. Calvo-Serrano, M. González-Miquel, S. Papadokonstantakis and G. Guillén-Gosálbez, Comput. Chem. Eng., 2018, 108, 179–193 CrossRef CAS.
- G. Wernet, S. Hellweg, U. Fischer, S. Papadokonstantakis and K. Hungerbühler, Environ. Sci. Technol., 2008, 42, 6717–6722 CrossRef CAS PubMed.
- Y. S. Lee, E. J. Graham, A. Galindo, G. Jackson and C. S. Adjiman, Comput. Chem. Eng., 2020, 136, 106802 CrossRef CAS.
- H. Zhang, L. Wang, M. Pérez-Fortes, F. Maréchal and U. Desideri, Appl. Energy, 2020, 258, 114071 CrossRef CAS.
- Z. Navas-Anguita, P. L. Cruz, M. Martin-Gamboa, D. Iribarren and J. Dufour, Fuel, 2019, 235, 1492–1500 CrossRef CAS.
- K. Im-orb and A. Arpornwichanop, Energy, 2020, 193, 116788 CrossRef CAS.
- A. M. Parvez, T. Wu, S. Li, N. Miles and I. M. Mujtaba, Biomass Bioenergy, 2018, 110, 105–113 CrossRef CAS.
- E. Martinez-Hernandez, L. F. Ramírez-Verduzco, M. A. Amezcua-Allieri and J. Aburto, Chem. Eng. Res. Des., 2019, 146, 60–70 CrossRef CAS.
- W. L. Becker, R. J. Braun, M. Penev and M. Melaina, Energy, 2012, 47, 99–115 CrossRef CAS.
- M. Fasihi, D. Bogdanov and C. Breyer, Energy Procedia, 2016, 99, 243–268 CrossRef CAS.
- S. Michailos, S. McCord, V. Sick, G. Stokes and P. Styring, Energy Convers. Manage., 2019, 184, 262–276 CrossRef CAS.
- N. Meunier, R. Chauvy, S. Mouhoubi, D. Thomas and G. De Weireld, Renewable Energy, 2020, 146, 1192–1203 CrossRef CAS.
- C. M. Liu, N. K. Sandhu, S. T. McCoy and J. A. Bergerson, Sustainable Energy Fuels, 2020, 4, 3129–3142 RSC.
- C. Fernández-Dacosta, V. Stojcheva and A. Ramirez, J. CO2 Util., 2018, 23, 128–142 CrossRef.
- C. M. Jens, L. Müller, K. Leonhard, A. A. Bardow, L. Müller, K. Leonhard and A. A. Bardow, ACS Sustainable Chem. Eng., 2019, 7, 12270–12280 CAS.
- C. Fernández-Dacosta, L. Shen, W. Schakel, A. Ramirez and G. J. Kramer, Appl. Energy, 2019, 236, 590–606 CrossRef.
- K. Roh, H. Lim, W. Chung, J. Oh, H. Yoo, A. S. Al-Hunaidy, H. Imran and J. H. Lee, J. CO2 Util., 2018, 26, 60–69 CrossRef CAS.
- M. A. N. Thonemann, Appl. Energy, 2020, 263, 114599 CrossRef CAS.
- P. Biernacki, T. Röther, W. Paul, P. Werner and S. Steinigeweg, J. Cleaner Prod., 2018, 191, 87–98 CrossRef CAS.
- N. von der Assen, J. Jung and A. Bardow, Energy Environ. Sci., 2013, 6, 2721–2734 RSC.
- W. Davis and M. Martín, Energy, 2014, 69, 497–505 CrossRef CAS.
- M. Martín, J. CO2 Util., 2016, 13, 105–113 CrossRef.
- D. H. König, M. Freiberg, R.-U. Dietrich and A. Wörner, Fuel, 2015, 159, 289–297 CrossRef.
- M. Decker, F. Schorn, R. C. Samsun, R. Peters and D. Stolten, Appl. Energy, 2019, 250, 1099–1109 CrossRef.
- C. Hank, S. Gelpke, A. Schnabl, R. J. White, J. Full, N. Wiebe, T. Smolinka, A. Schaadt, H.-M. Henning and C. Hebling, Sustainable Energy Fuels, 2018, 2, 1244–1261 RSC.
- M. Reuß, T. Grube, M. Robinius, P. Preuster, P. Wasserscheid and D. Stolten, Appl. Energy, 2017, 200, 290–302 CrossRef.
- N. V. Sahinidis, I. E. Grossmann, R. E. Fornari and M. Chathrathi, Comput. Chem. Eng., 1989, 13, 1049–1063 CrossRef CAS.
- R. E. Fornari and I. E. Grossmann, in Progress Report, Camegie Mellon University, 1986 Search PubMed.
- G. Dobrowolski, J. A. Kopytowski, J. Wojtania and M. Zebrowski, Alternative Routes from Fossil Resources to Chemical Feedstocks, RR-84-019, Laxenburg, 1984 Search PubMed.
- R. Calvo-Serrano, M. Guo, C. Pozo, Á. Galán-Martín and G. Guillén-Gosálbez, ACS Sustainable Chem. Eng., 2019, 7, 10570–10582 CrossRef CAS.
- M. Martín and I. E. Grossmann, Energy, 2013, 55, 378–391 CrossRef.
- S. Zhang, L. Liu, L. Zhang, Y. Zhuang and J. Du, Appl. Energy, 2018, 231, 194–206 CrossRef CAS.
- A. Dutta, S. Farooq, I. A. Karimi and S. A. Khan, J. CO2 Util., 2017, 19, 49–57 CrossRef CAS.
- S. Zhang, Y. Zhuang, L. Liu, L. Zhang and J. Du, Comput. Chem. Eng., 2020, 139, 106885 CrossRef CAS.
- M. M. F. Hasan, E. L. First, F. Boukouvala and C. A. Floudas, Comput. Chem. Eng., 2015, 81, 2–21 CrossRef CAS.
- K. Roh, A. S. Al-Hunaidy, H. Imran and J. H. Lee, AIChE J., 2019, 65, e16580 CrossRef.
- R. C. Baliban, J. A. Elia and C. A. Floudas, Comput. Chem. Eng., 2012, 37, 297–327 CrossRef CAS.
- M. M. F. Hasan, F. Boukouvala, E. L. First and C. A. Floudas, Ind. Eng. Chem. Res., 2014, 53, 7489–7506 CrossRef CAS.
- R. Ahmed, S. Shehab, D. M. Al-Mohannadi and P. Linke, Chem. Eng. Sci., 2020, 227, 115922 CrossRef CAS.
- M. Ehrenstein, Á. Galán-Martín, V. Tulus and G. Guillén-Gosálbez, Appl. Energy, 2020, 276, 115486 CrossRef CAS.
- A. Zimmermann, L. J. Müller, A. Marxen, K. Armstrong, G. Buchner, J. Wunderlich, A. Kätelhön, M. Bachmann, A. Sternberg and S. Michailos, Techno-Economic Assessment & Life-Cycle Assessment Guidelines for CO2 Utilization, CO2Chem Media and Publishing Ltd, Sheffield, 2018 Search PubMed.
- I. Huertas-Valdivia, A. M. Ferrari, D. Settembre-Blundo and F. E. García-Muiña, Sustainability, 2020, 12, 6211 CrossRef.
- Q. Zhang, M. Martín and I. E. Grossmann, Comput. Chem. Eng., 2019, 122, 80–92 CrossRef CAS.
- S. I. Pérez-Uresti, M. Martín and A. Jiménez-Gutiérrez, ACS Sustainable Chem. Eng., 2020, 8, 4580–4597 CrossRef.
| This journal is © The Royal Society of Chemistry 2021 |