Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Metal-free oxidative coupling of arylmethylamines with indoles: a simple, environmentally benign approach for the synthesis of 3,3′-bis(indolyl)methanes
Vikas D. Kadu*a,
Sankala Naga Chandrudub,
Mahesh G. Hublikara,
Dattatraya G. Rauta and
Raghunath B. Bhosalea
aSchool of Chemical Sciences, Punyashlok Ahilyadevi Holkar Solapur University, Solapur-413255, Maharashtra, India. E-mail: vikaskadu1@gmail.com
bDepartment of Chemistry, College of Engineering, Rayalseema University, Kurnool-518002, Andhra Pradesh, India
First published on 16th April 2021
Abstract
Correction for ‘Metal-free oxidative coupling of arylmethylamines with indoles: a simple, environmentally benign approach for the synthesis of 3,3′-bis(indolyl)methanes” by Vikas D. Kadu et al., RSC Adv., 2020, 10, 23254–23262, DOI: 10.1039/D0RA03221B.
The authors regret several errors throughout this RSC Advances manuscript. These include an incorrect description of ref. 23 (ref. 1) which starts at line 11 of paragraph 3 in the Introduction and should read as: “Previously, the Gopalaiah research group reported the synthesis of 3,3′-benzylidenebis(1H-indole), using an iron(II) triflate catalyst, from benzylamine with indoles.23” as opposed to “Previously, the Sankala research group reported the synthesis of 3,3′-benzylidenebis(1H-indole), using an iron(II) triflate catalyst, from benzylamine with indoles.23”
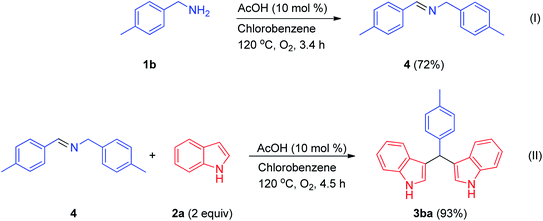
The authors regret an error in Scheme 2 where 4-methylbenzylamine was incorrectly labelled as compound 1a and should have been labelled as compound 1b. The correct version of Scheme 2 is shown below.
The authors regret the inclusion of the following sentence starting at line 3 of paragraph 1 in the Experimental section: “Iron salts were purchased from Sigma-Aldrich and were used as received.” This sentence should be removed as it does not relate to the experimental procedures in this RSC Advances article.
The authors also regret that the wrong temperature was stated in line 4 of paragraph 2 in the Experimental section, it should read as “The round bottom flask was equipped with an O2 balloon, and the reaction mixture was stirred at 120 °C until the complete consumption of indole 2 occurred, as monitored via TLC.”
The authors regret that 4-methyl-N-(4-methylbenzylidene)benzylamine was incorrectly described in lines 10 and 11 of paragraph 3 in the Experimental section. 4-Methylbenzylamine was also incorrectly labelled as compound 1a in line 2 and the wrong temperature was stated in line 5. Paragraph 3 in the Experimental section should therefore read as: “4-Methylbenzylamine (1b) (350 mg, 2.89 mmol), AcOH (10 mol%), and dry chlorobenzene (2 mL) were added to a 25 mL round bottom flask. The round bottom flask was equipped with an O2 balloon, and the reaction mixture was stirred at 120 °C for 3.4 h. The reaction mixture was cooled to room temperature and adsorbed on basic alumina. It was purified via column chromatography over basic alumina using a hexane/ethyl acetate (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture as the eluent to afford 4-methyl-N-(4-methylbenzylidene)benzylamine (4) (232 mg, 72% yield) as a pale yellow semisolid.”
1) mixture as the eluent to afford 4-methyl-N-(4-methylbenzylidene)benzylamine (4) (232 mg, 72% yield) as a pale yellow semisolid.”
The authors have also updated some 1H and 13C NMR spectra in the ESI to differentiate them from the NMR spectra in their previous Synthesis article.1 The updated spectra in the revised ESI are the 1H and 13C NMR spectra for compounds 3ba, 3da, 3ea, 3fa, 3ga, 3ha, 3ia, 3ja, 3na, 3oa, 3pa, 3qa, 3ra and 3sa. The 1H NMR and 13C NMR signal values given in the Experimental section in the main article have been corrected to reflect the updated NMR spectra. An expert viewed the corrected data and was satisfied that the conclusions are not affected. The corrected NMR data are given below:
Synthesis of 3,3′-(4-methylbenzylidene)bis(1H-indole) (3ba)
1H NMR (400 MHz, CDCl3): δ 7.88 (br s, 2H, NH), 7.40 (d, J = 8.0 Hz, 2H), 7.36–7.34 (m, 2H), 7.24 (d, J = 8.4 Hz, 2H), 7.20–7.16 (m, 2H), 7.09 (d, J = 8.0 Hz, 2H), 7.04–7.00 (m, 2H), 6.64 (d, J = 2.4 Hz, 2H), 5.86 (s, 1H), 2.33 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 140.9, 136.7, 135.5, 128.9, 128.6, 127.1, 123.6, 121.9, 119.9, 119.8, 119.2, 111.0, 39.7, 21.1 ppm.Synthesis of 3,3′-(4-hydroxybenzylidene)bis(1H-indole) (3da)
1H NMR (400 MHz, CDCl3): δ 8.68 (br s, 2H, NH), 8.17 (br s, 1H, OH), 7.32–7.24 (m, 4H), 7.10–7.04 (m, 4H), 6.90–6.87 (m, 2H), 6.70–6.67 (m, 2H), 6.59 (d, J = 1.2 Hz, 2H), 5.72 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3): δ 154.1, 136.7, 135.2, 129.5, 127.0, 123.7, 121.3, 119.8, 119.7, 118.6, 115.1, 111.1, 39.3 ppm.Synthesis of 3,3′-(4-tert-butylbenzylidene)bis(1H-indole) (3ea)
1H NMR (400 MHz, CDCl3): δ 8.04 (br s, 2H, NH), 7.41 (d, J = 8.0 Hz, 2H), 7.35 (d, J = 8.0 Hz, 2H), 7.27–7.26 (m, 4H), 7.18–7.13 (m, 2H), 7.01–6.97 (m, 2H), 6.90 (d, J = 2.0 Hz, 2H) 5.86 (s, 1H), 1.29 (s, 9H) ppm; 13C NMR (100 MHz, CDCl3): δ 148.7, 140.8, 136.6, 128.2, 127.1, 125.0, 123.5, 121.7, 119.9, 119.8, 119.0, 111.0, 39.6, 34.4, 31.4 ppm.Synthesis of 3,3′-(4-fluorobenzylidene)bis(1H-indole) (3fa)
1H NMR (400 MHz, CDCl3): δ 7.94 (br s, 2H, NH), 7.38–7.28 (m, 6H), 7.19 (t, J = 7.6 Hz, 2H), 7.04–6.95 (m, 4H), 6.47 (d, J = 1.6 Hz, 2H), 5.88 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3): δ 139.7, 136.7, 130.1, 130.0, 126.9, 123.5, 122.0, 119.9, 119.6, 119.3, 115.1, 114.9, 111.1, 39.4 ppm.Synthesis of 3,3′-((4-chlorophenyl)methylene)bis(1H-indole) (3ga)
1H NMR (400 MHz, CDCl3): δ 7.95 (br, s, 2H, NH), 7.38–7.23 (m, 8H, Ar-H), 7.20–7.16 (m, 2H, Ar-H), 7.04–6.99 (m, 2H, Ar-H), 6.66 (t, J = 1.2 Hz, 2H, Ar-H), 5.87 (s, 1H, Ar-CH) ppm;13C NMR (100 MHz, CDCl3): δ 142.5, 136.7, 131.8, 130.1, 128.4, 126.9, 123.6, 122.1, 119.8, 119.3, 119.2, 111.1, 39.6 ppm.Synthesis of 4-(di(1H-indol-3-yl)methyl)benzonitrile (3ha)
1H NMR (400 MHz, CDCl3): δ 8.86 (br s, 2H, NH), 7.26–7.11 (m, 7H, Ar-H), 6.98–6.81 (m, 5H, Ar-H), 6.71 (t, J = 7.6 Hz, 2H, Ar-H), 6.09 (s, 1H, CH) ppm; 13C NMR (100 MHz, CDCl3): δ 144.2, 135.1, 132.4, 129.3, 128.7, 128.2, 126.6, 120.2, 118.8, 118.6, 111.1, 110.2, 38.3 ppm.Synthesis of 3,3′-(2-methoxybenzylidene)bis(1H-indole) (3ia)
1H NMR (400 MHz, CDCl3): δ 7.79 (br s, 2H, NH), 7.43 (d, J = 7.6 Hz, 2H), 7.31 (d, J = 8.0 Hz, 2H), 7.21–7.14 (m, 4H), 7.03–6.83 (m, 4H), 6.60 (s, 2H), 6.37 (s, 1H), 3.82 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 156.9, 136.7, 132.3, 129.7, 127.2, 127.1, 123.7, 123.5, 122.3, 121.7, 120.4, 120.0, 119.8, 119.6, 119.0, 110.9, 110.6, 55.7, 32.0 ppm.Synthesis of 3,3′-(2-chlorobenzylidene)bis(1H-indole) (3ja)
1H NMR (400 MHz, CDCl3): δ 7.90 (br s, 2H), 7.44–7.35 (m, 5H), 7.24–7.01 (m, 7H), 6.63 (dd, J = 2.4 & 0.8 Hz, 2H), 6.35 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3): δ 141.3, 136.7, 133.9, 130.3, 129.4, 127.5, 127.0, 126.6, 123.7, 122.0, 119.8, 119.3, 118.3, 111.0, 36.6 ppm.Synthesis of 3,3′-[3,5-bis(trifluoromethyl)benzylidene]bis(1H-indole) (3na)
1H NMR (400 MHz, CDCl3): δ 7.98 (br s, 2H, NH), 7.83 (s, 2H), 7.78 (s, 1H), 7.40–7.35 (m, 4H), 7.23 (t, J = 7.6 Hz, 2H), 7.06 (t, J = 7.2 Hz, 2H), 6.62 (d, J = 2.4 Hz, 2H), 6.04 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3): δ 146.7, 136.7, 131.6, 131.2, 128.8, 126.5, 124.8, 123.7, 122.4, 122.1, 120.5, 119.7, 119.4, 117.9, 111.3, 40.0 ppm.Synthesis of 3,3′-(1-naphthylmethylene)bis(1H-indole) (3oa)
1H NMR (400 MHz, CDCl3): δ 8.85 (br s, 2H), 8.11 (d, J = 8.4 Hz, 1H), 7.80 (d, J = 8.0 Hz, 1H), 7.65 (d, J = 7.2 Hz, 1H), 7.38–7.19 (m, 8H), 7.09–705 (m, 2H), 6.91–6.87 (m, 2H), 6.58 (s, 1H), 5.51 (s, 2H) ppm; 13C NMR (100 MHz, CDCl3): δ 136.8, 128.5, 126.7, 126.1, 125.6, 125.4, 125.1, 124.5, 121.4, 119.5, 118.7, 111.2, 35.7 ppm.Synthesis of 3,3′-(4-pyridylmethylene)bis(1H-indole) (3pa)
1H NMR (400 MHz, CDCl3): δ 8.45 (d, J = 6.0 Hz, 2H), 8.00 (br s, 2H, NH), 7.40 (d, J = 6.0 Hz, 2H), 7.31 (d, J = 8.4 Hz, 2H), 7.05 (t, J = 7.2 Hz, 2H), 6.93–6.90 (m, 5H), 6.74 (t, J = 7.2 Hz, 2H) ppm; 13C NMR (100 MHz, CDCl3): δ 174.1, 148.9, 136.7, 127.1, 124.5, 121.7, 121.6, 119.2, 111.0, 36.9 ppm.Synthesis of 3,3′-(pyridin-3-ylmethylene)bis(1H-indole) (3qa)
1H NMR (400 MHz, CDCl3): δ 8.67 (d, 1H, J = 2.4 Hz, ArH), 8.48 (dd, 1H, J = 4.8 & 1.6 Hz, ArH), 8.26 (brs, 2H, NH), 7.62–7.59 (m, 1H, ArH), 7.38–7.34 (m, 4H, ArH), 7.21–7.16 (m, 3H, ArH), 7.04–7.00 (m, 2H, ArH), 6.62 (d, 2H, J = 2.0 Hz, ArH), 5.92 (s, 1H, Ar-CH) ppm; 13C NMR (100 MHz, CDCl3): δ 150.3, 147.5, 139.5, 136.7, 136.1, 126.7, 123.7, 123.3, 122.2, 119.7, 119.4, 118.5, 111.2, 60.4 ppm.Synthesis of 3,3′-(2-thienylmethylene)bis(1H-indole) (3ra)
1H NMR (400 MHz, CDCl3): δ 7.92 (br s, 2H, NH), 7.48 (d, J = 8.0 Hz, 2H), 7.37 (d, J = 8.4 Hz, 2H), 7.21–7.16 (m, 3H), 7.06–7.02 (m, 2H), 6.94–6.91 (m, 2H), 6.84 (dd, J = 2.4 & 0.8 Hz, 2H), 6.17 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3): δ 148.6, 136.6, 126.7, 126.4, 125.1, 123.6, 123.1, 122.0, 119.8, 119.7, 119.6, 119.4, 111.1, 35.3 ppm.Synthesis of 3,3′-benzylidenebis(5-bromo-1H-indole) (3sa)
1H NMR (400 MHz, CDCl3): δ 9.23 (br s, 2H, NH), 7.38 (d, J = 1.6 Hz, 2H), 7.25–7.13 (m, 9H), 6.59 (d, J = 2.0 Hz, 2H), 5.68 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3): δ 143.5, 135.5, 128.6, 128.5, 128.3, 126.3, 125.2, 124.3, 121.9, 118.4, 112.9, 112.0, 39.8 ppm.The authors also regret errors and the mispelling of names in ref. 1, 2, 3, 10 and 12 in the original article which are given correctly as ref. 1–5 below, respectively.
References
- K. Gopalaiah, S. Naga Chandrudu and A. Devi, Synthesis, 2015, 47, 1766–1774 CrossRef CAS.
- R. B. Tjalkens and S. Safe, US Pat., 8580843, 2013 Search PubMed.
- A. Ramirez and S. Garcia-Rubio, Curr. Med. Chem., 2003, 10, 1891–1915 CrossRef CAS PubMed.
- M. A. Zeligs, J. Med. Food., 1998, 1, 67–85 CrossRef CAS.
- C. H. Thomas, US Pat., 4500689, 1985 Search PubMed.
| This journal is © The Royal Society of Chemistry 2021 |