Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ammonium-to-sodium ion-exchange process at the interlayer of octacalcium phosphate†
Yuki Sugiura *,
Yoji Makita and
Masanori Horie
*,
Yoji Makita and
Masanori Horie
Health and Medical Research Institute, National Institute of Advanced Industrial Science and Technology (AIST), 2217-14, Hayashi-cho, Takamatsu, Kagawa, Japan 761-0395. E-mail: yuki-sugiura@aist.go.jp
First published on 13th December 2021
Abstract
Octacalcium phosphate (OCP) has been considered as the layer component of calcium phosphate, but whether it achieves the ionic-exchange ability of conventional layer components is unclear. As OCP is highly biocompatible, understanding its ionic-exchange properties would potentially expand its pharmaceutical and medical applications. Herein, we demonstrate that the substituted cations in ammonium (NH4)-substituted octacalcium phosphate (OCP-NH4) and sodium (Na)-containing ammonium phosphate solutions undergo ion exchanges with OCP interlayers. Replacing NH4+ with Na+ did not alter the crystal structure of OCP, confirming that a substituted cation exchange process similar to that in other layered compounds occurs in OCP.
Introduction
Octacalcium phosphate [OCP: Ca8H2(PO4)6·5H2O] is an attractive material in biomedical applications because its components—calcium (Ca), phosphate (PO4), and water (H2O)—are partially found in biological tissues and especially in bone.1–3 OCP has a layered crystal structure with relatively high stability and low environmental loading. Moreover, multiple ions and molecules, such as cations, dicarboxylates, and tris(hydroxymethyl)amino methane, can be substituted into precipitating OCP crystals without changing their crystallinity or crystal structure.1,4–10 These guest ions and molecules are mainly substituted in the hydrous layer of OCP, where they displace the Ca, PO4, and H2O constituents.4,11–13 In our previous studies on cation substitution under weakly basic solutions, we found that monovalent cations such as alkali metal ions and silver (mixture of states of Ag0 and Ag+) were substituted at the conjugated sites of P5 PO4, the root of the hydrous layer of OCP. When OCP was synthesized in the presence of NH4 and Na, the Na ions (rather than NH4) were preferentially substituted because their ionic radius is similar to that of Ca (Na+: 0.102 nm, NH4+: 0.175 nm, Ca2+: 0.100 nm).14–16Many examples of coexisting ions and molecules being substituted into the OCP unit lattice during the precipitation process have been reported. However, the process by which ions or molecules are substituted into the OCP unit lattice remains unknown. Considering the OCP crystal structure, the ion-exchange process of guest ions substituted in the interlayers of the OCP unit lattice probably mimics that of other layered materials such as clay minerals and micas (Scheme 1).17–20 In a case study of ammonium-substituted OCP (OCP-NH4) and Na-containing solutions, the present study examines the ionic-exchange process of guest ions substituted in the interlayers of OCP.
Materials and methods
Preparation of OCP-NH4
All reagents were purchased from FUJIFILM Wako Pure Chemical Industry Inc., Japan. The preparation method of OCP-NH4 was described in ref. 21. Briefly, 12.5 g monocalcium hydrogen phosphate monohydrate [MCPM: Ca(H2PO4)2·H2O] was immersed in 1 L of 1 mol L−1 diammonium hydrogen phosphate ((NH4)2HPO4) solution for three days at 40 °C. The treated samples were washed with distilled water several times, then dried in a dry oven at 40 °C overnight.The reference material was OCP-Na, a conventional OCP formed under weak basic phosphate solutions as described in ref. 15. Briefly, 2.39 g calcium hydrogen phosphate dihydrate [DCPD: CaHPO4·2H2O] was immersed in 20 mL of 1 mol L−1 disodium hydrogen phosphate (Na2HPO4) solution for 1 day at 60 °C. The treated samples were washed with distilled water several times, then dried in a dry oven at 40 °C overnight.
Ion-exchange experiment of OCP-NH4
The fabricated OCP-NH4 (0.4 g) was immersed in 40 mL of 1 mol L−1 (NH4)2HPO4 and 0–5 mol L−1 sodium nitrate (NaNO3) solution placed in 50 mL polypropylene centrifugation tubes at 40 °C. After closely packing, the vessels were shaked for 3 days at 150 rpm and 40 °C in a thermostatic shaker (BioShaker BR-23FP, Taitec Co., Japan).The pH values of the suspensions were measured using a pH electrode (LAQUA ToupH 9615S-10D) connected to a pH meter (Horiba Co. D-72, Kyoto, Japan). The samples were then washed several times with distilled water to remove any residual immersion solution before being dried in a 40 °C oven overnight.
Characterization
The crystallographic information of the samples was obtained in an X-ray diffraction (XRD) analysis (MiniFlex600, Rigaku Co., Japan) at an acceleration voltage and amplitude of 40 and 15 kV, respectively. The diffraction angle was continuously scanned over 2θ values ranging from 3° to 70° at a scanning rate of 5° min−1 for characterization and from 2° to 12° at 1° min−1 for crystallographic parameter analysis.The chemical bonding scheme of the samples was characterized through Fourier transform infrared spectroscopy (FT-IR: Nicolet NEXUS670, Thermofisher Scientific Co., USA) using a triglycine sulfate detector (32 scans, resolution 2 cm−1) with an attenuated total-reflection GeSe prism. All measurements were conducted in the atmosphere.
After dissolving the samples in 2% HNO3 solution, the Ca, P(PO4), and Na concentrations in the samples were determined using inductively coupled plasma atomic emission spectroscopy (ICP-OES: 5110VDV, Agilent Technology Co., Japan).
Results and discussion
The main aim of this study was to investigate the ionic-exchange phenomena of NH4 and Na in OCP crystals. Both the initial and final phases were required to be OCP. When OCP is immersed in aqueous solutions, it may convert to other phases such as hydroxyapatite [HAp: Ca10(PO4)6(OH)2] and/or DCPD via different phase-conversion processes such as dissolution and precipitation reactions. The dissolution and precipitation kinetics are mainly controlled by the ionic products and solubilities of the samples. The solubility difference between OCP and HAp was minimized under weak basic conditions. In addition, coexisting Ca and/or PO4 in the solution reduced the dissolving reaction of OCP, consistent with the ionic product hypothesis. We thus chose a weakly basic PO4 solution (1 mol L−1 (NH4)2HPO4) as the reaction solution.The pH value of the reacting solution mainly determined the process of the reaction. Fig. 1 plots the initial and final pH values of the reacting solutions as functions of Na concentration. Although both the initial and final pH values decreased slightly with increasing Na concentration, they remained in the weakly basic region.
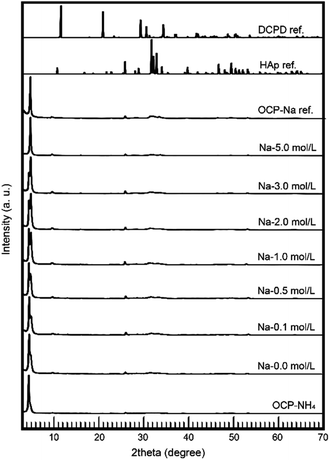
The most important factors of the OCP ionic-exchange process are the phases during the reactions. In this study, the phases were determined by XRD. Fig. 2 shows the wide-range XRD patterns of the samples. At all Na concentrations, the treated samples were monophasic OCP with no HAp or DCPD phases. The crystallographic phenomena in the OCP unit lattice were then subjected to further evaluation.
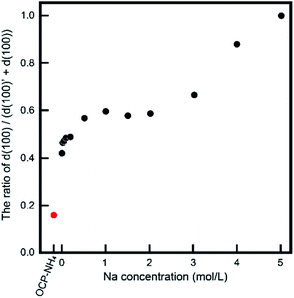
The behavior of NH4-substituted OCP was easily detected as an extra peak at ∼4.2° [d(100)′] in the XRD spectrum. When Na was replaced at the same site (the P5 PO4 conjugating site of the OCP unit lattice), no significant peak shifts or extra peaks were observed and the usual OCP d(100) peak appeared at ∼4.7°. By estimating these peaks in the XRD pattern, we can elucidate the NH4 substitution mechanism and the Na-exchange degree in the OCP unit lattice. Fig. 3 magnifies the XRD patterns of the samples to highlight the main peaks and Fig. 4 plots the relative peak intensity ratio of d(100)′/(d(100)′ + d(100)) versus Na concentration. After immersion, the intensities of the d(100) peak of OCP-NH4 increased in all treated samples, indicating the development of a typical OCP unit lattice in the samples. Note that the d(100)′/(d(100)′ + d(100)) ratio of OCP-NH4 was significantly decreased even after immersion in the solution containing 0 mol L−1 Na. The d(100)′/[d(100)′ + d(100)] ratios in the treated samples increased with Na content up to 0.2 mol L−1 and then plateaued at ∼0.50 until the Na concentration reached 2.0 mol L−1. At this concentration, the d(100)′/(d(100)′ + d(100)) ratios of the treated samples began increasing again and the d(100)′ peak could not be detected at 5 mol L−1 Na.
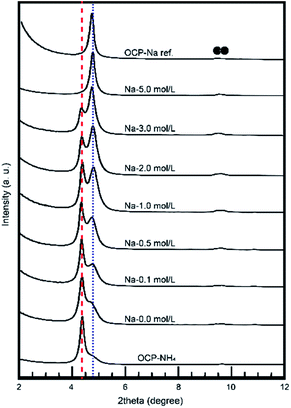 | ||
| Fig. 3 Small-angle XRD patterns of treated samples. ●: OCP, blue dot line: conventional OCP d(100) and, red broken line: OCP-NH4 d(100)′. | ||
The decreased d(100)′/(d(100)′ + d(100)) ratio of OCP-NH4 in Na-free solution was attributed to instability of OCP-NH4. When OCP-NH4 was immersed in different concentrations of (NH4)2HPO4 solution (0–1 mol L−1) as same manner of 1 mol L−1 (NH4)2HPO4 with NaNO3 solutions, its d(100)′ structure gradually decomposed over time. The decomposability of the d(100)′ structure of OCP-NH4 was an inversely proportional to (NH4)2HPO4 concentration (Fig. S1†).
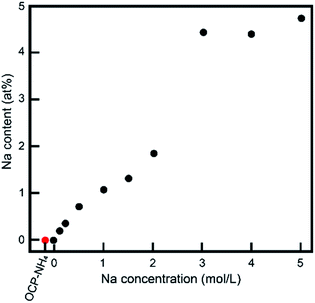
The XRD measurements imply an ionic exchange of NH4 with Na in the OCP unit lattice. The XRD data were validated in other chemical compositional analyses. Fig. 5 shows the Na contents in the samples determined by ICP-OES. The Na content in the treated sample increased as the Na concentration in the treatment solution increased up to 3 mol L−1, and plateaued thereafter. We also measured the Ca/P and (Ca + Na)/P ratios of the samples. Because the chemical composition might alter through dissolution and/or development of OCP processes other than NH4-to-Na ionic exchange, these ratios provide vital information about the crystallographic alternations during treatments. Before treatment, the Ca/P ratio of OCP-NH4 was 1.38. Fig. 6 plots the Ca/P and (Ca + Na)/P ratios of the samples versus Na concentration in the treatment solution. The Ca/P ratios of the treated samples trended nearly identically to those of OCP-NH4, but the (Ca + Na)/P ratios steadily increased with increasing Na concentration in the treated solution.
The ionic-exchange process of OCP-NH4 to OCP-Na was also evaluated by the spectroscopic method. Within the lowly symmetric crystal structure of OCP (P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ), the vibrations of each component can be detected.21,22 The six major states of PO4 (P1–P6 PO4) in OCP all contain highly conjugated Ca ions.15,23–25 Our previous evaluations indicated that monovalent cations such as NH4 and Na can be substituted at the Ca site conjugated to P5 PO4, the root of the hydrous layer.16,21 Fig. 7 shows the FT-IR spectra of the samples. Before immersion, the NH4 absorption band ranging from 1400 to 1500 cm−1 appeared in the spectrum of OCP-NH4. In the treatment solutions, increasing the Na concentration decreased the intensity of the NH4 absorption band. Above 3 mol L−1 NaOH, the NH4 absorption band disappeared. This observation was consistent with the XRD analysis. However, at the P5 PO4 site, numerous bands related to Na-substituted OCP emerged with increasing Na concentration in the treatment solution, but the band corresponding to OCP-NH4 remained visible at high Na concentrations.
), the vibrations of each component can be detected.21,22 The six major states of PO4 (P1–P6 PO4) in OCP all contain highly conjugated Ca ions.15,23–25 Our previous evaluations indicated that monovalent cations such as NH4 and Na can be substituted at the Ca site conjugated to P5 PO4, the root of the hydrous layer.16,21 Fig. 7 shows the FT-IR spectra of the samples. Before immersion, the NH4 absorption band ranging from 1400 to 1500 cm−1 appeared in the spectrum of OCP-NH4. In the treatment solutions, increasing the Na concentration decreased the intensity of the NH4 absorption band. Above 3 mol L−1 NaOH, the NH4 absorption band disappeared. This observation was consistent with the XRD analysis. However, at the P5 PO4 site, numerous bands related to Na-substituted OCP emerged with increasing Na concentration in the treatment solution, but the band corresponding to OCP-NH4 remained visible at high Na concentrations.
The obtained results provided clear evidence of the ion-exchange process at the NH4-substituted sites of OCP-NH4 in Na solution. The NH4-exchange degree of OCP-NH4 was controlled by varying the Na concentration in the treatment solution. However, the ionic-exchange process in the OCP unit lattice required a higher Na concentration than direct precipitation.16 The above chemical composition analysis indicated that the threshold value of Na intercalation into OCP-NH4 depended on the Na concentration in solution. Below 2 mol L−1 Na, the ion-exchange process of OCP-NH4 dominated and the d(100)′/(d(100)′ + d(100)) of OCP-NH4 reached ∼0.6. Above 3 mol L−1 Na in solution, the ionic-exchange process apparently reached the threshold in simple exchange and the Na contents in the samples no longer increased. Thus, it was suggested that Na adsorption simultaneously occurred on the sample surfaces with ion exchange process. It was also suggested that Na adsorption onto OCP-NH4 influences further ion-exchange processes. The expected exchange phenomena are summarized in Scheme 2.
 | ||
| Scheme 2 Schematic of the ion-exchange process of OCP-NH4 in systems with different Na concentrations. | ||
Heretofore, although various molecules and cations can be substituted in the OCP unit lattice, ordinary ionic-exchange properties of OCP have not been reported because OCP exists in a metastable phase.2,26 However, we verified that OCP, like other layered components (clay minerals and layered double hydrates), possesses ionic-exchange properties under optimal settings.17–20 This phenomenon can be exploited in new clinical usages of OCP, especially, in combinations of medical products. For example, a controlled burst of medical drugs could be released from substituted interlayers of the OCP unit lattice.
Conclusions
We demonstrated the ionic-exchange properties of OCP in OCP-NH4- and Na-containing solutions, which are weakly basic phosphate buffer solutions. NH4-substituted OCP was gradually replaced by Na as the ionic exchange proceeded. The degree of Na exchange was comparable with the Na concentration in the reaction solution.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The Health Research Institute, National Institute of Advanced Industrial Science and Technology (AIST), and KAKENHI for Young Researchers, JP19K19081, funded this research. Dr Y. Suezawa and T. Nakanishi assisted with the FT-IR measurements. This study is partially supported by the Research Center for Industrial Science & Technology, Kagawa Industry Support Foundation (RIST Kagawa).Notes and references
- E. Davies, K. H. Müller, W. C. Wong, C. J. Pickard, D. G. Reid, J. N. Skepper and M. J. Duer, Proc. Natl. Acad. Sci. U. S. A., 2014, E1354 CrossRef CAS PubMed.
- L. Wang and G. H. Nancollas, Chem. Rev., 2008, 108, 4628 CrossRef CAS PubMed.
- S. V. Dorozhkin and M. Epple, Angew. Chem., Int. Ed., 2002, 41, 3130 CrossRef CAS PubMed.
- M. Markovic, B. O. Fowler and W. E. Brown, Chem. Mater., 1993, 5, 1401 CrossRef CAS.
- T. Yokoi, M. Kamitakahara and C. Ohtsuki, Dalton Trans., 2015, 44, 7943 RSC.
- H. Monma and M. Goto, Bull. Chem. Soc. Jpn., 1983, 56, 3843 CrossRef CAS.
- T. W. T. Tsai, F. C. Chou, Y. H. Tseng and J. C. C. Chan, Phys. Chem. Chem. Phys., 2010, 12, 6692 RSC.
- S. Kamakura, Y. Sasano, T. Shimizu, K. Hatori, O. Suzuki, M. Kagayama and K. Motegi, J. Biomed. Mater. Res., 2002, 59, 29 CrossRef CAS.
- A. Bigi, E. Boanini, B. Bracci, G. Falini and K. Rubini, J. Inorg. Biochem., 2003, 95, 291 CrossRef CAS.
- E. Boanini, M. Gazzano, K. Rubini and A. Bigi, Cryst. Growth Des., 2010, 10, 3612 CrossRef CAS.
- H. Shi, F. He and J. Ye, J. Mater. Chem. B, 2016, 4, 1712 RSC.
- Y. Sugiura and Y. Makita, Chem. Lett., 2019, 48, 1304 CrossRef CAS.
- I. Yamada and M. Tagaya, Colloid Interface Sci. Commun., 2019, 30, 100182 CrossRef CAS.
- Y. Sugiura, Y. Saito, T. Endo and Y. Makita, Cryst. Growth Des., 2019, 19, 4162 CrossRef CAS.
- Y. Sugiura and Y. Makita, Cryst. Growth Des., 2018, 18, 6165 CrossRef CAS.
- Y. Sugiura and Y. Makita, Dalton Trans., 2019, 48, 1386 RSC.
- R. Zhu, Q. Chen, Q. Zhou, Y. Xi, J. Zhu and H. He, Appl. Clay Sci., 2016, 123, 239 CrossRef CAS.
- T. Okumura, K. Tamura, E. Fujii, H. Yamada and T. Kogure, Microscopy, 2014, 63, 65 CrossRef CAS PubMed.
- G. M. Bowers, D. L. Bish and R. J. Kirkpatrick, Langmuir, 2008, 24, 10240 CrossRef CAS PubMed.
- K. Matsuo, K. Yoshihara, N. Nagaoka, Y. Makita, H. Obika, T. Okihara, A. Matsukawa, Y. Yoshida and B. V. Meerbeek, Acta Biomater., 2019, 100, 388 CrossRef CAS PubMed.
- Y. Sugiura and Y. Makita, Chem. Lett., 2018, 47, 1371 CrossRef CAS.
- E. E. Berry and C. B. Baddiel, Spectrochim. Acta, Part A, 1967, 23, 1781 CrossRef CAS.
- W. E. Brown, J. P. Smith, J. R. Lehr and A. W. Frazier, Nature, 1962, 196, 1050 CrossRef CAS.
- M. Mathew and W. E. Brown, Bull. Chem. Soc. Jpn., 1987, 60, 1141 CrossRef CAS.
- M. Mathew, W. E. Brown, L. W. Schroeder and B. Dickens, J. Crystallogr. Spectrosc. Res., 1988, 18, 235 CrossRef CAS.
- G. Vereecke and J. Lemaitre, J. Cryst. Growth, 1990, 194, 820 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra07939e |
| This journal is © The Royal Society of Chemistry 2021 |