Open Access Article
Open Access ArticlePhosphine-catalyzed [3 + 2] annulation of β-sulfonamido-substituted enones with trans-α-cyano-α,β-unsaturated ketones for the synthesis of highly substituted pyrrolidines†
Zhenzhen Gao *a,
Lei Xiea,
Lusha Jia,
Xin Maa,
Xiaojing Li
*a,
Lei Xiea,
Lusha Jia,
Xin Maa,
Xiaojing Li a,
Honglei Liu
a,
Honglei Liu *b and
Hongchao Guo
*b and
Hongchao Guo c
c
aSchool of Pharmacy, Liaocheng University, Liaocheng 252000, Shandong, P. R. China. E-mail: gaozhenzhen@lcu.edu.cn
bCollege of Materials Science and Engineering, Qingdao University, Qing dao 266071, Shandong, P. R. China. E-mail: tjslhl@126.com
cDepartment of Applied Chemistry, China Agricultural University, Beijing 100193, P. R. China. E-mail: hchguo@cau.edu.cn
First published on 17th December 2021
Abstract
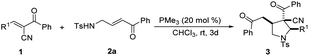
To synthesize highly substituted pyrrolidines, we developed a phosphine-catalyzed [3 + 2] annulation of β-sulfonamido-substituted enones with trans-α-cyano-α,β-unsaturated ketones. We prepared a series of pyrrolidines under mild conditions with high yields and moderate-to-good diastereoselectivities. A catalytic mechanism for this reaction is suggested.
Nucleophilic phosphine catalysis is a practical and powerful synthetic approach to obtain heterocyclic compounds using various annulation reactions, the advantages of which are it being mild and metal-free, ecologically friendly, and inexpensive.1 Phosphine-catalyzed intermolecular [3 + 2],2 [4 + 1],3 [2 + 2 + 1]4 and intramolecular annulations are often used to obtain pyrrole derivatives. Intermolecular [3 + 2] annulations of imines and phosphorus ylides formed in situ from allenoates, alkynes, or Morita–Baylis–Hillman carbonates under the presence of phosphine catalysts are especially the most widely used approach to synthesize pyrrolidine derivates. In these reactions, phosphorus ylides act as C–C–C synthons for the [3 + 2] annulations with a C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond converting to a pyrrolidine ring (Scheme 1). However, literature reports on exploring new activation modes, namely, phosphorus ylides acting as C–C–N synthons for the [3 + 2] annulations, are rare.
N bond converting to a pyrrolidine ring (Scheme 1). However, literature reports on exploring new activation modes, namely, phosphorus ylides acting as C–C–N synthons for the [3 + 2] annulations, are rare.
 | ||
| Scheme 1 Pyrrolidine ring formation through reaction of phosphorus ylides act as C–C–C and C–C–N synthons. | ||
β-Sulfonamido-substituted enones could be used as C–C–N synthons to form various N-based heterocycles. Catalytically activated (by amines) β-sulfonamido-substituted enones act as nucleophiles towards electron-deficient olefins or imines during [3 + 2] annulation reactions. Du's5 and Pan's groups6 have made outstanding contributions to this field.7 In 2018, Guo's group developed a Bu3P-catalyzed [5 + 1] annulation of γ-sulfonamido-substituted enones with N-sulfonyl-imines to obtain chiral 2,4-di-substituted imidazolidines. They also synthesized γ-sulfonamido-substituted enones attacked by phosphine catalyst and acting as C–C–C–C–N synthon (see Scheme 2).8 Recently, Guo et al.9 used β-sulfonamido-substituted enone as a phosphine acceptor as well as a C–C–N synthon for the [3 + 2] annulation with sulfamate-derived cyclic imines (see Scheme 2). Using of β-sulfonamido-substituted enone as a novel phosphine acceptor is very promising for phosphine-catalyzed reactions. Inspired by Guo's work, we further extended the substrate scope of this reaction from sulfamate-derived cyclic imines to unsaturated ketones for the construction of pyrrolidine rings. Therefore, in this work, we report phosphine-catalyzed [3 + 2] annulation of β-sulfonamido-substituted enones and trans-α-cyano-α,β-unsaturated ketones, to synthesize highly substituted pyrrolidines (see Scheme 2), which are among the primary building blocks and the core structures of natural and bioactive compounds.10
 | ||
| Scheme 2 Phosphine-catalyzed annulation of γ-sulfonamido-substituted enones and β-sulfonamido-substituted enones. | ||
We first used trans-α-cyano-α,β-unsaturated ketone 1a and β-sulfonamido-substituted enone 2a as model substrates to obtain optimum reaction conditions. Tertiary phosphine catalysts were screened with 1,2-dichloroethane (DCE) as solvent at room temperature (see Table 1, entries 1–6). After 8 h, the desired pyrrolidine products (3aa) were obtained. Among them, MePPh2, Me2PPh, and PMe3 promoted the [3 + 2] cycloaddition reactions with 85%, 82%, and 84% yields and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3
1, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 6
1, and 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr diastereo-selectivities, respectively (entries 1, 4 and 6). Judging by the highest yield, PMe3 showed the highest catalytic activity. It also produced the product 3aa with the highest diastereoselectivity. Thus, for further tests, we used PMe3 as catalyst. Then, to further enhance the diastereoselectivity, we screened different solvents. THF behaved similarly to toluene, providing 3aa with 7
1 dr diastereo-selectivities, respectively (entries 1, 4 and 6). Judging by the highest yield, PMe3 showed the highest catalytic activity. It also produced the product 3aa with the highest diastereoselectivity. Thus, for further tests, we used PMe3 as catalyst. Then, to further enhance the diastereoselectivity, we screened different solvents. THF behaved similarly to toluene, providing 3aa with 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (entries 7 and 8). The EtOAc was not as efficient as other solvents, and its usage resulted in the formation of 3aa with 5
1 dr (entries 7 and 8). The EtOAc was not as efficient as other solvents, and its usage resulted in the formation of 3aa with 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (entry 9). The best solvent was CHCl3 because the yield of 3aa compound was 88%, and the diastereoselectivity was a little higher (8
1 dr (entry 9). The best solvent was CHCl3 because the yield of 3aa compound was 88%, and the diastereoselectivity was a little higher (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, entry 10). When we used 3 Å and 4 Å molecular sieves as additives, the diastereo-selectivities could not be further enhanced (entries 11 and 12). A significantly enhanced diastereoselectivity was obtained at lower concentrations (entries 13–15). Both the yield and diastereoselectivity were excellent (86% yield and 14
1 dr, entry 10). When we used 3 Å and 4 Å molecular sieves as additives, the diastereo-selectivities could not be further enhanced (entries 11 and 12). A significantly enhanced diastereoselectivity was obtained at lower concentrations (entries 13–15). Both the yield and diastereoselectivity were excellent (86% yield and 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, respectively) with a concentration of 0.02 mol L−1(see entry 16 in Table 1), albeit requiring a longer reaction time of 72 h. Further screening of temperatures and additives are listed in the ESI.† Thus, the optimum reaction conditions were determined as follows: using 20 mol% of PMe3 as catalyst, CHCl3 as solvent at room temperature.
1 dr, respectively) with a concentration of 0.02 mol L−1(see entry 16 in Table 1), albeit requiring a longer reaction time of 72 h. Further screening of temperatures and additives are listed in the ESI.† Thus, the optimum reaction conditions were determined as follows: using 20 mol% of PMe3 as catalyst, CHCl3 as solvent at room temperature.
| a Unless otherwise indicated, all reactions were carried out at room temperature using 0.12 mmol of 1aa and 0.1 mmol of 2aa in a solvent containing 20 mol% of the catalyst.b Isolated yield.c Determined by 1H NMR.d 100 mg 3 Å molecular sieves were used.e 100 mg 4 Å molecular sieves were used. | ||||||
|---|---|---|---|---|---|---|
| Entry | PR3 | Solvent | t/h | Con./mol L−1 | Yieldb (%) | drc |
| 1 | MePPh2 | DCE | 8 | 0.1 | 85 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | EtPPh2 | DCE | 8 | 0.1 | 74 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | n-PrPPh2 | DCE | 8 | 0.1 | 76 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | Me2PPh | DCE | 8 | 0.1 | 82 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | PBu3 | DCE | 8 | 0.1 | 78 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | PMe3 | DCE | 8 | 0.1 | 84 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | PMe3 | THF | 8 | 0.1 | 85 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | PMe3 | Toluene | 8 | 0.1 | 75 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | PMe3 | EtOAc | 8 | 0.1 | 78 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | PMe3 | CHCl3 | 8 | 0.1 | 88 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 11d | PMe3 | CHCl3 | 8 | 0.1 | 84 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 12e | PMe3 | CHCl3 | 8 | 0.1 | 86 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 13 | PMe3 | CHCl3 | 24 | 0.05 | 85 | 9.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 14 | PMe3 | CHCl3 | 48 | 0.033 | 85 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 15 | PMe3 | CHCl3 | 24 | 0.02 | 65 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 16 | PMe3 | CHCl3 | 72 | 0.02 | 86 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Under the optimum conditions, the performance of various trans-α-cyano-α,β-unsaturated ketone 1 with β-sulfonamido-substituted enones 2a in the cycloaddition reactions was analyzed (see Table 2). The reactions proceeded well in the presence of a wide range of substituted unsaturated ketones (1a–1p) acting as substrates and capable of producing pyrrolidines with good yieldsand diastereoselectivities. However, the presence of electron-deficient or -rich substituents on the benzene ring affected the reaction outcome strongly. When unsaturated ketones with electron-donating groups on the benzene ring were used, only moderate yields (up to 80%) were obtained, however the diastereo-selectivities were excellent (10.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–14
1–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, entries 2–5). The unsaturated ketone 1f bearing a CF3 group at 4- position of benzene ring was also compatible with the reaction, and product 3fa was obtained with 66% yield and 10.5
1 dr, entries 2–5). The unsaturated ketone 1f bearing a CF3 group at 4- position of benzene ring was also compatible with the reaction, and product 3fa was obtained with 66% yield and 10.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (entry 6). However, using of unsaturated ketones with halogen-substitutions on the corresponding phenyl groups produced relatively lower diastereoselectivities (5
1 dr (entry 6). However, using of unsaturated ketones with halogen-substitutions on the corresponding phenyl groups produced relatively lower diastereoselectivities (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–9.5
1–9.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr) and only moderate yields (entries 7–13). All 1-naphthyl, 2-naphthyl- and 2-thienyl-substituted unsaturated ketones (1n, 1o and 1p, respectively) performed well, and the corresponding products 3na, 3oa and 3pa were obtained with 81%, 80% and 78% yields and 14
1 dr) and only moderate yields (entries 7–13). All 1-naphthyl, 2-naphthyl- and 2-thienyl-substituted unsaturated ketones (1n, 1o and 1p, respectively) performed well, and the corresponding products 3na, 3oa and 3pa were obtained with 81%, 80% and 78% yields and 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 8
1, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 7
1, and 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereoselectivities, respectively (entries 14–16). In addition, the 2-furyl derived unsaturated ketones 1q also underwent the reaction, providing the product 3qa in 80% yield and 14
1 diastereoselectivities, respectively (entries 14–16). In addition, the 2-furyl derived unsaturated ketones 1q also underwent the reaction, providing the product 3qa in 80% yield and 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (entry 17). The absolute configuration of the product 3aa was verified by single-crystal X-ray diffraction.11
1 dr (entry 17). The absolute configuration of the product 3aa was verified by single-crystal X-ray diffraction.11
| Entry | R1 | 3 | Yieldb (%) | drc |
|---|---|---|---|---|
| a Unless otherwise indicated, all reactions were conducted at room temperature for 3 days using 0.12 mmol of compound 1 and 0.1 mmol of compound 2 in 5 ml CHCl3 in the presence of 20 mol% of PMe3.b Isolated yield.c Determined by 1H NMR. | ||||
| 1 | Ph (1a) | 3aa | 86 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | 2-MeC6H4 (1b) | 3ba | 75 | 10.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | 3-MeC6H4(1c) | 3ca | 77 | 12.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | 4-MeC6H4 (1d) | 3da | 78 | 10.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | 4-OMeC6H4 (1e) | 3ea | 80 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | 4-CF3-C6H4 (1f) | 3fa | 66 | 10.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | 2-FC6H4 (1g) | 3ga | 72 | 9.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | 3-FC6H4 (1h) | 3ha | 74 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | 4-FC6H4 (1i) | 3ia | 76 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | 2-ClC6H4 (1j) | 3ja | 74 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 11 | 3-ClC6H4(1k) | 3k | 76 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 12 | 4-ClC6H4 (1l) | 3la | 82 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 13 | 4-BrC6H4 (1m) | 3ma | 85 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 14 | 1-Naphthyl (1n) | 3na | 81 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 15 | 2-Naphthyl (1o) | 3oa | 80 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 16 | 2-thienyl (1p) | 3pa | 78 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 17 | 2-furyl (1q) | 3qa | 80 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
We also tested various substituted enones containing different R groups under the optimal reaction conditions (see Table 3). Benzene-sulfonyl-protected enone 2b produced the desired product 3ab with 84% yield and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (entry 2). However, the using of p-nitro-benzene-sulfonyl-protected enone 2c resulted in lower yield and diastereoselectivity (equal to 81% yield and 4.5
1 dr (entry 2). However, the using of p-nitro-benzene-sulfonyl-protected enone 2c resulted in lower yield and diastereoselectivity (equal to 81% yield and 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, entry 3). Substituted enones 2 bearing a halogen (2d–2h), or two halogen groups (2i) on the phenyl ring were also used in this cycloaddition reaction. Yet, only moderate yields of product 3 were obtained (77–85%) but the diastereoselectivities were good (8
1 dr, entry 3). Substituted enones 2 bearing a halogen (2d–2h), or two halogen groups (2i) on the phenyl ring were also used in this cycloaddition reaction. Yet, only moderate yields of product 3 were obtained (77–85%) but the diastereoselectivities were good (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–10
1–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, entries 4–9). The using of substituted enones 2j bearing a 4-CNC6H4 group also produced good results with 86% yield of product 3aj, possessing good diastereoselectivity (11
1 dr, entries 4–9). The using of substituted enones 2j bearing a 4-CNC6H4 group also produced good results with 86% yield of product 3aj, possessing good diastereoselectivity (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, entry 10). Substituted enones bearing electron-rich methoxy group at the 3- and 4-positions of benzene ring supported the formation of products 3ak and 3al with 79% and 80 yields, 10
1 dr, entry 10). Substituted enones bearing electron-rich methoxy group at the 3- and 4-positions of benzene ring supported the formation of products 3ak and 3al with 79% and 80 yields, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 8.5
1 and 8.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, respectively (entries 11, 12). In addition, the 4-Ph- and 2-naphthyl-modified enones underwent the [3 + 2] annulation reaction and produced the desired compounds in high yields (86 and 81%) with excellent diastereoselectivities (12.5
1 dr, respectively (entries 11, 12). In addition, the 4-Ph- and 2-naphthyl-modified enones underwent the [3 + 2] annulation reaction and produced the desired compounds in high yields (86 and 81%) with excellent diastereoselectivities (12.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 8
1 and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, entries 13 and 14, respectively).
1 dr, entries 13 and 14, respectively).
| Entry | R2/R3 | 3 | Yieldb (%) | drc |
|---|---|---|---|---|
| a Unless otherwise noted, all reactions were performed at room temperature for 3 days using 0.12 mmol of compound 1 and 0.10 mmol of compound 2 in 5 ml CHCl3 under the presence of 20 mol% PMe3.b Isolated yield.c Determined by 1H NMR. | ||||
| 1 | Ph/Ts (2a) | 3aa | 86 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | Ph/Bs (2b) | 3ab | 84 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | Ph/Ns (2c) | 3ac | 81 | 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | 2-FC6H4/Ts (2d) | 3ad | 77 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | 3-FC6H4/Ts (2e) | 3ae | 79 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | 2-ClC6H4/Ts (2f) | 3af | 82 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | 3-BrC6H4/Ts(2g) | 3ag | 74 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | 4-BrC6H4/Ts (2h) | 3ah | 85 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | 3,4-Cl2C6H3/Ts (2i) | 3ai | 74 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | 4-CNC6H4/Ts (2j) | 3aj | 86 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 11 | 3-OMeC6H4/Ts (2k) | 3ak | 79 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 12 | 4-OMeC6H4/Ts (2l) | 3al | 80 | 8.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 13 | 4-PhC6H4/Ts (2m) | 3am | 86 | 12.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 14 | 2-naphthyl/Ts (2n) | 3an | 81 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
To demonstrate the synthetic potential of the cycloaddition reaction, a scale-up preparation of 3aa and the derivatization of 3am were performed (Scheme 3). The unsaturated ketone 1a (699 mg, 3.0 mmol) reacted with substituted enone 2a (788 mg, 2.5 mmol) under the standard condition to give 3aa in 81% yield with 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. In comparison with the reaction at 0.1 mmol of scale, no significant loss of yield and diastereoselectivity was observed. Reduction of the carbonyl group of 3ma with NaBH4 in MeOH/CH2Cl2 led to the formation of compound 4 in 85% yield and 5.5
1 dr. In comparison with the reaction at 0.1 mmol of scale, no significant loss of yield and diastereoselectivity was observed. Reduction of the carbonyl group of 3ma with NaBH4 in MeOH/CH2Cl2 led to the formation of compound 4 in 85% yield and 5.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr.
1 dr.
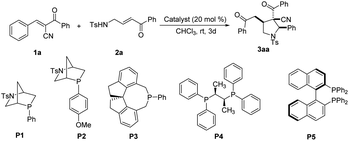
The asymmetric variant of the present reaction had also been investigated (Table 4). Unfortunately, most commercial chiral phosphines did not work. To our delight, with the use of chiral phosphine P3 as the catalyst, the [3 + 2] annulation of unsaturated ketone 1a with substituted enone 2a worked at rt for 72 h to give chiral product 3aa in 50% yield with up to 31% ee and >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr.
1 dr.
| Entry | Catalyst | t/h | Yieldb (%) | drc | eec |
|---|---|---|---|---|---|
| a Unless otherwise indicated, all reactions were carried out at room temperature using 0.06 mmol of 1aa and 0.05 mmol of 2aa in a solvent containing 20 mol% of the catalyst in 2.5 ml of CHCl3.b Isolated yield.c Determined by HPLC on chiral column.d No reaction. | |||||
| 1 | P1 | 72 | Trace | — | — |
| 2 | P2 | 72 | 20 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
5 |
| 3 | P3 | 72 | 50 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
31 |
| 4 | P4 | 72 | NRd | — | — |
| 5 | P5 | 72 | NRd | — | — |
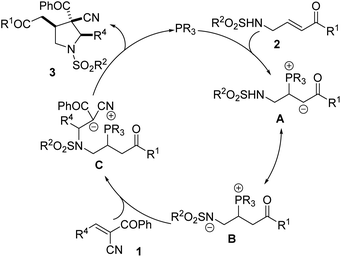
All these results allowed us to propose a catalytic cycle (see Scheme 4). Nucleophilic addition of the phosphine-based catalysts to β-sulfonamido-substituted enones yields phosphonium intermediate A, which converts into an intermediate B by proton transferation. The intermediate B undergoes intramolecular aza-Michael addition to an alkene yielding an intermediate compound C, followed by intramolecular nucleophilic substitution and the producing of product 3, during which the phosphine regenerates.
In conclusion, we developed a synthesis method (under mild conditions) for highly substituted pyrrolidines through phosphine-catalyzed [3 + 2] annulation of β-sulfonamido-substituted enones with trans-α-cyano-α,β-unsaturated ketones. A series of pyrrolidine derivates were obtained in good yields with moderate-to-good diastereoselectivities. In this reactions, using of β-sulfonamido-substituted enone as a novel phosphine acceptor, the formed phosphorus ylides act as C–C–N synthons for annulations. Further investigations on the application of β-sulfonamido-substituted enones in the asymmetric phosphine-catalyzed reactions are in progress in our laboratory.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for the financial support Natural Science Foundation of Shandong Province (ZR2019BB054, ZR2021MB110, ZR2018BB028), State Key R & D projects (SQ2020YFF0422322). Natural Science Foundation of China (81903504), and the Natual Science Foundation of Liaocheng University (318011908).Notes and references
-
(a) H. Ni, W.-L. Chan and Y.-X. Lu, Chem. Rev., 2018, 118, 9344–9411 CrossRef CAS PubMed
; (b) H.-C. Guo, Y.-C. Fan, Z.-H. Sun, Y. Wu and O. Kwon, Chem. Rev., 2018, 118, 10049–10293 CrossRef CAS PubMed
; (c) Y.-F. Huang, J.-N. Liao, W. Wang, H.-L. Liu and H.-C. Guo, Chem. Commun., 2020, 56, 15235–15281 RSC
.
-
(a) Z.-R. Xu and X.-Y. Lu, J. Org. Chem., 1998, 63, 5031–5041 CrossRef CAS
; (b) Z.-R. Xu and X.-Y. Lu, Tetrahedron Lett., 1997, 38, 346–3464 Search PubMed
; (c) I. P. Andrews and O. Kwon, Org. Synth., 2011, 88, 138–151 CrossRef CAS
; (d) X.-F. Tang, B. Zhang, Z.-R. He, R.-L. Gao and Z.-J. He, Adv. Synth. Catal., 2007, 349, 2007–2017 CrossRef CAS
; (e) G.-L. Zhao and M. Shi, J. Org. Chem., 2005, 70, 9975–9984 CrossRef CAS PubMed
; (f) X.-F. Zhu, C. E. Henry and O. Kwon, Tetrahedron, 2005, 61, 6276–6282 CrossRef CAS
; (g) L.-G. Meng, P.-J. Cai, Q.-X. Guo and S. Xue, J. Org. Chem., 2008, 73, 8491–8496 CrossRef CAS PubMed
; (h) B. Zhang, Z.-R. He, S.-L. Xu, G.-P. Wu and Z.-J. He, Tetrahedron, 2008, 64, 9471–9479 CrossRef CAS
; (i) M. Sampath, P. Y. B. Lee and T. P. Loh, Chem. Sci., 2011, 2, 1988–1991 RSC
; (j) S. S. Kinderman, J. H. van Maarseveen and H. Hiemstra, Synlett, 2011, 12, 1693–1696 CrossRef
; (k) Z.-T. Zhu, Y.-W. Guo, X.-J. Wang, F.-H. Wu and Y.-M. Wu, J. Fluorine Chem., 2017, 195, 102–107 CrossRef CAS
; (l) S.-Q. Zheng and X.-Y. Lu, Org. Lett., 2008, 10, 4481–4484 CrossRef CAS PubMed
.
-
(a) Y. Lei, X.-N. Zhang, X.-Y. Yang, Q. Xu and M. Shi, RSC Adv., 2015, 5, 49657–49661 RSC
; (b) H.-Y. Li, J.-S. Luo, B.-J. Li, X.-Z. Yi and Z.-J. He, Org. Lett., 2017, 19, 5637–5640 CrossRef CAS PubMed
; (c) C.-X. Qian, P.-M. Zhang, W.-J. Li and P.-F. Li, Asian J. Org. Chem., 2019, 8, 242–245 CAS
; (d) V. Sriramurthy, G. A. Barcan and O. Kwon, J. Am. Chem. Soc., 2007, 129, 12928–12929 CrossRef CAS PubMed
.
- M. Shi and Y.-M. Xu, Eur. J. Org. Chem., 2002, 2002, 696–701 CrossRef
.
-
(a) B.-L. Zha and D.-M. Du, Asian J. Org. Chem., 2015, 4, 1120–1126 CrossRef
; (b) B.-L. Zhao, Y. Lin, H.-H. Yan and D.-M. Du, Org. Biomol. Chem., 2015, 13, 11351–11361 RSC
; (c) J.-H. Li, H.-L. Wen, L. Liu and D.-M. Du, Eur. J. Org. Chem., 2016, 14, 2492–2499 CrossRef
.
- S. Mukhopadhyay and S. C. Pan, Org. Biomol. Chem., 2018, 16, 9349–9353 RSC
.
-
(a) S. Mukhopadhyay and S. C. Pan, Chem. Commun., 2018, 54, 964–967 RSC
; (b) S. Mukhopadhyay and S. C. Pan, Adv. Synth. Catal., 2019, 361, 1028–1032 CAS
.
- L.-J. Zhou, C.-H. Yuan, Y. Zeng, H.-L. Liu, C. Wang, X. Gao, Q.-J. Wang, C. Zhang and H.-C. Guo, Chem. Sci., 2018, 9, 1831–1835 RSC
.
- W.-Y. Shi, L.-J. Zhou, B.-M. Mao, Q.-J. Wang, C. Wang, C. Zhang, X.-F. Li, Y.-M. Xiao and H.-C. Guo, J. Org. Chem., 2019, 84, 679–686 CrossRef CAS PubMed
.
-
(a) D. O'Hagan, Nat. Prod. Rep., 2000, 17, 435–446 RSC
; (b) X.-C. Cheng, Q. Wang, H. Fang and W.-F. Xu, Curr. Med. Chem., 2008, 15, 374–385 CrossRef CAS PubMed
; (c) I. V. Magedov, G. Luchetti, N. M. Evdokimov, M. Manpadi, W. F. A. Steelant, S. Van Slambrouck, P. Tongwa, M. Y. Antipin and A. Kornienko, Bioorg. Med. Chem. Lett., 2008, 18, 1392–1396 CrossRef CAS PubMed
; (d) X. Li and J. Li, Mini-Rev. Med. Chem., 2010, 10, 794–805 CrossRef CAS PubMed
.
- Crystallographic data for 3aa has been deposited with the Cambridge Crystallograohic Data Centre as deposition number CCDC 2081095.†.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental conditions and spectroscopic data of all new compounds. CCDC 2081095. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1ra07881j |
| This journal is © The Royal Society of Chemistry 2021 |