Open Access Article
Open Access ArticleMatrix-dispersed magnetic molecularly-imprinted polyaniline for the effective removal of chlorpyrifos pesticide from contaminated water
Hadeel Saad†
a,
F. A. Nour El-Dien *a,
Nadia E. A. El-Gamela and
Ahmed S. Abo Dena
*a,
Nadia E. A. El-Gamela and
Ahmed S. Abo Dena *bc
*bc
aChemistry Department, Faculty of Science, Cairo University, Giza 12613, Egypt
bPharmaceutical Chemistry Department, National Organization for Drug Control and Research (NODCAR), Giza, Egypt
cFaculty of Oral and Dental Medicine, Future University in Egypt (FUE), New Cairo, Egypt
First published on 14th December 2021
Abstract
We report a new adsorbent nanocomposite material based on matrix-dispersed superparamagnetic iron oxide nanoparticles (SPIONs) in molecularly-imprinted polyaniline for the removal of chlorpyrifos (CPF), a hazardous organophosphate pesticide, from water. The synthesized magnetic molecularly-imprinted polymer (MMIP) was characterized by FTIR spectroscopy, XRD, magnetic susceptibility, DLS, zeta potential measurement, SEM and high-resolution TEM imaging. The average size of the naked SPIONs ranges from 15 to 30 nm according to the high-resolution TEM analysis. Moreover, the adsorption kinetics, thermodynamic parameters (ΔG, ΔH and ΔS), adsorption isotherms and rebinding conditions were investigated in detail. The proposed MMIP has an imprinting factor of 1.64. In addition, it showed a high experimental adsorption capacity of 1.77 mg g−1 and a removal efficiency of nearly 80%. The fabricated MMIP material demonstrated excellent magnetic susceptibility allowing for easy separation using an external magnetic field. The adsorption mechanism of CPF onto the MMIP adsorbent followed the second-order kinetics model and fitted to the Temkin adsorption isotherm. By studying the adsorption thermodynamics, negative ΔG values (−1.955 kJ mol−1 at room temperature) were obtained revealing that the adsorption process is spontaneous. Furthermore, the maximum adsorption capacity was obtained at room temperature (ca. 303 K), neutral pH and using a high CPF concentration.
Introduction
Water pollution has become one of the most serious issues facing people at the present time, especially with the huge population increase that requires a corresponding increase in drinking and irrigation water resources. The global water crisis is not only due to the scarcity of water resources, but also due to the progressive deterioration of water quality that leads to noticeable reduction in the quantity of water suitable for safe use. There is a tight link between the global water-quality crisis and agriculture due to the heavy use of pesticides/herbicides for agricultural purposes, causing serious contamination to a large amount of municipal/surface water. In addition, agricultural causalities of water pollution have overtaken industrial and/or settlement-based contaminations. On the other hand, the increasing food demand intensifies the agricultural pressure on the global water quality. Nutrients/fertilizers, pesticides, herbicides, fungicides, salts, sediments, metals, organic matter, pathogens (e.g. bacteria), drug residues, hormones and feed additives are the major agricultural sources of surface municipal water pollution.Acute poisoning with highly hazardous pesticides, used by poor farmers in developing countries, can cause significant human/animal mortality and morbidity worldwide.1 From these pesticides, chlorpyrifos (CPF; IUPAC name: O,O-diethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate; molar mass: 350.6 g mol−1) is one of the most hazardous organophosphate pesticides (Scheme 1). Although CPF has low solubility in water at room temperature (6.36 ± 0.58 mg L−1),2 it is considered one of the most hazardous water contaminants due to its very high toxicity. Therefore, due to the sustained discharge of CPF from soil to water, it is necessary to develop new selective, efficient and cost-effective sorbents for the removal of CPF from water.
The emergence of enzyme-analog built polymers, nowadays known as molecularly-imprinted polymers (MIPs), in the 1970s introduced a new kind of target-specific polymeric materials.3–7 These materials have found a very wide range of applications in different areas, including chemical sensing,8–13 adsorption,14–16 catalysis,17 biology,18 etc. Combining MIPs with superparamagnetic iron oxide nanoparticles (SPIONs) enables the control of the produced nanocomposites using an external magnetic field.19 SPIONs also provide the advantages of being biocompatible and easy-to-synthesize.20 Recently, the attention of many researchers has been grasped by the use of magnetic nanomaterials for the removal of hazardous contaminants, including pesticides, from water.21–24 The removal of CPF from water via bioremediation,25–28 Fenton reaction,29–33 graphitic carbon nitride-incorporated chitosan34 and metal nanoparticles35 was reported in the literature. CPF-selective MIPs and magnetic MIPs (MMIPs) were also reported using different kinds of polymers either for extraction/pre-concentration or removal of CPF.36–39 Most of these MIPs/MMIPs are based on functional monomers that require a high-temperature medium and a very long polymerization time. In addition, crosslinking agents and inert atmospheres are often required. Some studies reported the use of molecularly-imprinted polyaniline (PANI) for various applications including water treatment,40–42 sensor applications,43,44 etc. The recent interest in PANI applications is due to its unique properties which include, but are not limited to, the ease of fabrication, low cost, high stability, high electrical conductivity, etc. Besides, PANI preparation does not require a high-temperature polymerization medium nor a crosslinking agent.
The objective of the present study is to prepare a MMIP-based nanocomposite adsorbent for the effective and selective removal of CPF from contaminated water. The proposed MMIP nanomaterial is based on SPIONs dispersed in an imprinted PANI matrix. Bulk polymerization of aniline is very rapid and takes place at low temperature; thus allowing for facile scaling up of the MMIP-fabrication process. In the following sections, the MMIP preparation procedure, characterization techniques and CPF adsorption experiments will be discussed.
Materials and methods
Materials
Chlorpyrifos, a product of Excel Crop Care Limited, was provided by El Nasr for Intermediate Chemicals (Egypt) and was used as a template. For the synthesis of the molecularly imprinted polyaniline, aniline (Al-Alamia for Chemical Industries, Egypt), dimethyl sulphoxide (Oxford Lab Fine Chem LLP, India) and ammonium persulphate (APS, Veb-Laborchemie Apolda, Germany) were used. Ethylene glycol (Honeywell International Inc., USA), ferric chloride hexahydrate (FeCl3·6H2O, Daejung Chemicals and Metals, South Korea) and anhydrous sodium acetate (ADWIC, Egypt) were utilized in the solvothermal synthesis of SPIONs. Ethyl alcohol was obtained from the International Company for Medical Industries (Egypt), methyl alcohol and hydrochloric acid were purchased from Alpha Chemika (India), and glacial acetic acid was provided by Piochem (Egypt). All reagents were of analytical grade and were used without any further purification.Instrumentation
An evolution 60 Thermo Scientific Spectrophotometer (USA) was used to detect/quantify the concentration of CPF at 290 nm using a 1 cm quartz cell. To study the surface morphology and particle size, field-emission scanning electron microscopy (FE-SEM) and high-resolution transmission electron microscopy (HRTEM) were used with the aid of a VEGA3 TESCAN (Czech Republic) and JEOL JEM-2100 (Japan), respectively. The particle size and zeta potential (ZP) of naked SPIONs and MMIPs were determined by a Malvern Panalytical instrument (UK). Fourier transform infrared spectra (FTIR) were recorded using a Nicolet 6700 ATR-FTIR spectrometer (Thermo Scientific, Germany). The magnetic properties were investigated with a vibrating-sample magnetometer (VSM) (Lakeshore, model 7410). Magnetite crystallographic structure was confirmed by measuring the X-ray diffraction (XRD) spectra (Discover-D8, Bruker, USA).Preparation of SPIONs
Superparamagnetic Fe3O4 nanoparticles were prepared via the modified solvothermal method.45,46 Briefly, 2 g of FeCl3·6H2O and 6 g of anhydrous sodium acetate were dissolved in 65 mL of ethylene glycol. This solution was transferred to a Teflon-lined solvothermal reactor and then heated to 200 °C in the oven (Heraeus, Thermo Electron Corporation, Germany) for 12 h. The prepared black magnetite nanoparticles were left to cool at room temperature, separated with an external magnet, washed several times using distilled water and ethanol to effectively remove the solvent, and then vacuum dried at room temperature for 24 h. The solvothermal reactor must be completely dry to prevent the formation of any iron oxide but magnetite.Preparation of MMIPs
First, 1 mL (ca. 10.95 mmol) of aniline as a functional monomer and 1 g of SPIONs were mixed in 25 mL of HCl (1 mol L−1) and stirred constantly at 4 °C. Thereafter, 0.96 g (ca. 2.74 mmol) of CPF – as a template – were dissolved in 20 mL of DMSO and added to the above mixture in a dropwise manner with constant stirring.47 After mixing, 1.21 g (ca. 5.48 mmol) of APS – as an initiator – were dissolved in 5 mL of 1 mol L−1 HCl and dropped onto the mixture for about 1 h with constant stirring. The solution was stirred continuously for 6 h in an ice bath. The appearance of a dark green color indicates the formation of emeraldine salt of polyaniline (PANI), Fig. 1. Magnetic non-imprinted polymer (MNIP) was prepared by following the same procedure but without the addition of the template, CPF.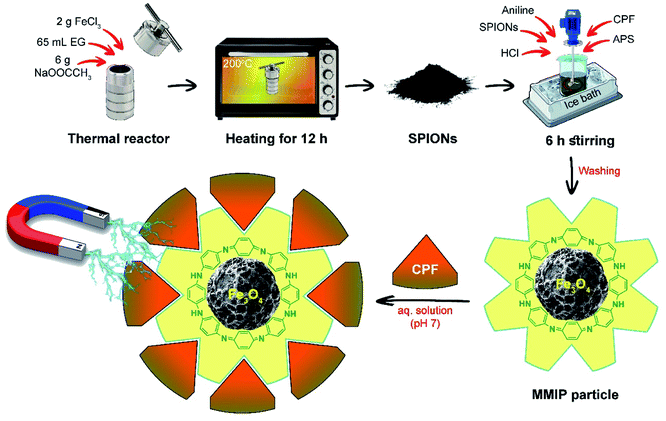 | ||
| Fig. 1 Schematic illustration summarizing the synthetic steps of the MMIP and its use in the treatment of CPF-contaminated water at neutral pH. | ||
The obtained PANI/SPIONs nanocomposite was collected from the suspension with a strong magnet, and then washed thrice with distilled water. Subsequently, CPF was removed from the PANI/SPIONs nanocomposite via repeated washing with a mixture of methanol and 1% acetic acid solution (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) until no CPF could be detected spectrophotometrically at 290 nm, and then the resultant solid was washed several times with methanol to remove the excess acid. The resulting MMIP was left overnight to dry at room temperature. It is worth mentioning that the prepared MNIP was treated similarly to minimize any systematic errors.
1, v/v) until no CPF could be detected spectrophotometrically at 290 nm, and then the resultant solid was washed several times with methanol to remove the excess acid. The resulting MMIP was left overnight to dry at room temperature. It is worth mentioning that the prepared MNIP was treated similarly to minimize any systematic errors.
Adsorption experiments
The adsorption of CPF in aqueous solution was studied by the batch-mode adsorption technique. All the adsorption experiments were carried out in 50 mL glass-stoppered Erlenmeyer flasks containing 0.2 g of the adsorbent and 10 mL of CPF solution of the appropriate concentration at room temperature. The suspension was placed in a temperature-controlled shaker (Clifton, UK) with a shaking speed of 400 rpm. Working CPF solutions (15–45 mg L−1) were prepared shortly before each experiment by dilution of a 1000 mg L−1 CPF stock solution with a methanol/water mixture (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, v/v).
7, v/v).
The effect of pH on CPF adsorption was studied by using working solutions of different pH values (pH 2–10) adjusted by 0.1 mol L−1 hydrochloric acid and sodium hydroxide solutions. The pH of the prepared solutions was measured using a JENCO 6173 pH meter.
After reaching the adsorption equilibrium, the adsorbent was separated by an external magnetic field. The remaining supernatant was filtered and the concentration of CPF was determined using a standard curve by measuring the absorbance at 290 nm. The %removal of CPF was calculated from eqn (1).
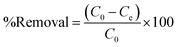 | (1) |
The amount of adsorbed CPF on the MMIP (a.k.a. adsorption capacity) was calculated according to eqn (2):
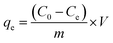 | (2) |
Thereafter, the imprinting factor (IF) was calculated as the ratio between the binding capacity of MMIP and that of the corresponding MNIP according to eqn (3).
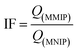 | (3) |
Adsorption kinetics
The pseudo-first-order, PFO, (eqn (4)),46,48 pseudo-second-order, PSO, (eqn (5))46,48 and the intraparticle diffusion, IPD, (eqn (6))48,49 kinetic models were implemented to study the adsorption process of CPF on the surface of the MMIP particles.| q = qe(1 − e−k1t) | (4) |
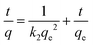 | (5) |
| qt = kidt1/2 + I | (6) |
Adsorption isotherms
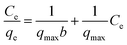 | (7) |
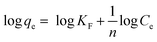 | (8) |
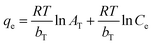 | (9) |
In Langmuir isotherm, a linear regression of Ce/qe vs. Ce was plotted to calculate qmax and b constants. Meanwhile, a plot of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe versus log
qe versus log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce can be used to determine 1/n and KF constants from Freundlich isotherm. Similarly, the slope and intercept of a plot of qe versus ln
Ce can be used to determine 1/n and KF constants from Freundlich isotherm. Similarly, the slope and intercept of a plot of qe versus ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce in Temkin equation can be used to calculate bT and AT constants.
Ce in Temkin equation can be used to calculate bT and AT constants.
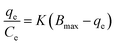 | (10) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C, where C is the concentration of the adsorbate CPF (eqn (11)). A slope of 0.1 < Q < 0.9 is the Hill coefficient, h, and is an index of cooperativity.
C, where C is the concentration of the adsorbate CPF (eqn (11)). A slope of 0.1 < Q < 0.9 is the Hill coefficient, h, and is an index of cooperativity.
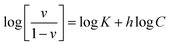 | (11) |
Adsorption thermodynamics
Adsorption thermodynamics was studied by incubating 0.2 g of MMIP in 10 mL of 20 mg L−1 CPF solution for the equilibration time while keeping mechanical shaking at 400 rpm. The experiment was repeated at different test solution temperatures (ca. 303–333 K). Thereafter, the adsorption equilibrium constant (Ka) was calculated at the studied temperatures from eqn (12); Liu reported that at low concentrations the distribution constant (Kc) is equal to the adsorption equilibrium constant.50 The obtained Ka values were used to calculate the adsorption thermodynamic parameters (ΔH and ΔS) from Van't Hoff equation (eqn (13)) via plotting ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka vs. 1/T. Moreover, the change in Gibbs free energy (ΔG) was calculated from eqn (14).51
Ka vs. 1/T. Moreover, the change in Gibbs free energy (ΔG) was calculated from eqn (14).51| Kc = Cad/Ce | (12) |
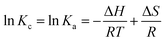 | (13) |
| ΔG = ΔH − TΔS | (14) |
Results and discussion
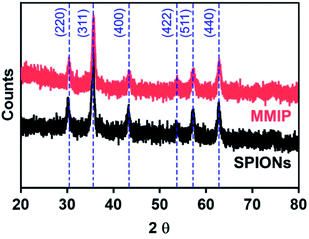
Characterization of MMIP
The materials synthesized in the present work were characterized by means of the following characterization techniques to confirm the formation of crystalline cubic magnetite crystals and to prove the efficient synthesis of the desired nano MMIP.The FTIR spectrum of naked SPIONs (Fig. 2) demonstrated a characteristic band at about 580 cm−1 corresponding to the Fe–O bond stretching. A sharp band appearing at about 1628 cm−1 indicates the presence of a carboxylic-metal (Fe–COO) linkage which may be responsible for the surface negative charges of SPIONs. In addition, the presence of hydroxyl groups was confirmed with the broad band appearing at 3650–3000 cm−1.52
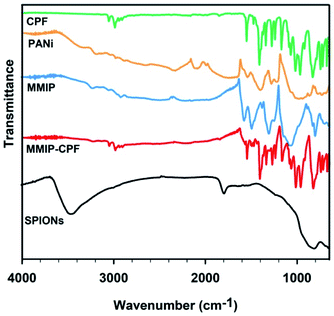 | ||
| Fig. 2 FTIR spectra of dry solid CPF, SPIONs, PANI, washed MMIP and unwashed MMIP (MMIP-CPF) samples over the spectral window of 4000–400 cm−1. | ||
Confirming the association of PANI with SPIONs necessitates the investigation of the FTIR spectrum of PANI. The synthesis of emeraldine salt of PANI was confirmed via the presence of some characteristic bands.53 For instance, the band at 800–700 cm−1 was assigned to C–H in-plane bending and 1,4-disubstituted benzene. The strong broad band positioned around 1000 cm−1 is attributed to the bond vibrations of –NH+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) , Q
, Q![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH+–B, or B–NH+˙–B, where B is the benzenoid unit and Q is the quinonoid unit.53 This band also confirms the dihedral angles distribution between B and Q rings as well as the presence of positively charged moieties in the PANI chain; thus indicating the emeraldine salt structure. In the paper published by Prashar and Nihal,54 the two absorption bands appearing at 1375 and 1635 cm−1 were assigned to the quinonoid and benzenoid rings, respectively. The absorption band demonstrated at 1225 cm−1 is due to the C–N+˙ bond. Moreover, the bands at 1400 and 1545 cm−1 are attributed to C
NH+–B, or B–NH+˙–B, where B is the benzenoid unit and Q is the quinonoid unit.53 This band also confirms the dihedral angles distribution between B and Q rings as well as the presence of positively charged moieties in the PANI chain; thus indicating the emeraldine salt structure. In the paper published by Prashar and Nihal,54 the two absorption bands appearing at 1375 and 1635 cm−1 were assigned to the quinonoid and benzenoid rings, respectively. The absorption band demonstrated at 1225 cm−1 is due to the C–N+˙ bond. Moreover, the bands at 1400 and 1545 cm−1 are attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations, respectively. The broad absorption band higher than 2000 cm−1 is a typical band of conducting PANI.53 Aromatic C–H stretching was observed via the several weak-to-moderate bands appearing at 3300–2880 cm−1. The absence of the hydroxyl band at 3500–3300 cm−1 negates the presence of bound water; indicating that the PANI sample was well dried prior to the FTIR measurement. Coming to the FTIR of MMIP, the presence of the absorption band at 580 cm−1 is attributed to SPIONs. Moreover, all the above-mentioned absorption bands characteristic to PANI were observed in MMIP spectrum, indicating the successful synthesis of MMIP.
C stretching vibrations, respectively. The broad absorption band higher than 2000 cm−1 is a typical band of conducting PANI.53 Aromatic C–H stretching was observed via the several weak-to-moderate bands appearing at 3300–2880 cm−1. The absence of the hydroxyl band at 3500–3300 cm−1 negates the presence of bound water; indicating that the PANI sample was well dried prior to the FTIR measurement. Coming to the FTIR of MMIP, the presence of the absorption band at 580 cm−1 is attributed to SPIONs. Moreover, all the above-mentioned absorption bands characteristic to PANI were observed in MMIP spectrum, indicating the successful synthesis of MMIP.
The FTIR spectrum of CPF (Fig. 2) shows characteristic absorption bands at 2986, 1551, 1413, 1272, 1169, 1019, 970, 828 and 674 cm−1 that can be assigned to C–H stretching, C–O stretching, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching, pyridine stretching, ring vibration, ring breathing, P–O–C, P
N stretching, pyridine stretching, ring vibration, ring breathing, P–O–C, P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S stretching and C–Cl stretching, respectively.55,56 The large similarity between the FTIR spectrum of CPF and that of unwashed MMIP (MMIP-CPF) confirms the successful binding of CPF to the PANI backbone. For instance, all the characteristic CPF absorption bands appear at 2986, 1551, 1409, 1276, 1165, 1019, 965, 828 and 674 cm−1 in the MMIP-CPF spectrum. However, after washing of MMIP with a mixture of methanol and 1% acetic acid solution (9
S stretching and C–Cl stretching, respectively.55,56 The large similarity between the FTIR spectrum of CPF and that of unwashed MMIP (MMIP-CPF) confirms the successful binding of CPF to the PANI backbone. For instance, all the characteristic CPF absorption bands appear at 2986, 1551, 1409, 1276, 1165, 1019, 965, 828 and 674 cm−1 in the MMIP-CPF spectrum. However, after washing of MMIP with a mixture of methanol and 1% acetic acid solution (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), these bands almost disappeared and the MMIP spectrum was found to be very similar to that of pure PANI (Fig. 2). The above results depict that MMIP was successfully synthesized and efficiently washed with the washing solution before applying it to the removal of CPF from contaminated water samples.
1, v/v), these bands almost disappeared and the MMIP spectrum was found to be very similar to that of pure PANI (Fig. 2). The above results depict that MMIP was successfully synthesized and efficiently washed with the washing solution before applying it to the removal of CPF from contaminated water samples.
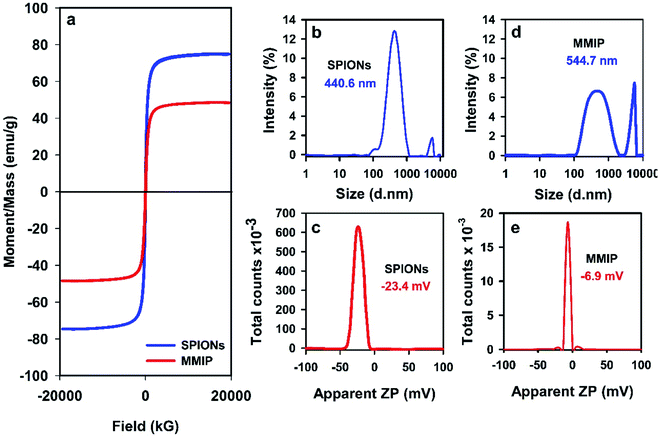
SPIONs usually bear negative charges on their surfaces due to the presence of carboxylate and hydroxyl functional groups (mentioned above in the FTIR results). Coating of SPIONs with a cationic polymer such as PANI is supposed to increase the positive charges on the surface of the particles, and thus shifts the apparent ZP to a more positive (less negative) value. That is why, the apparent ZP of both SPIONs and the MMIP was measured and the results were plotted as shown in Fig. 4(c) and (e), respectively. Naked SPIONs showed a negative ZP of −23.4 mV, and MMIP revealed a more positive ZP of about −6.9 mV indicating the entrapment of SPIONS into the CPF-imprinted PANI matrix.
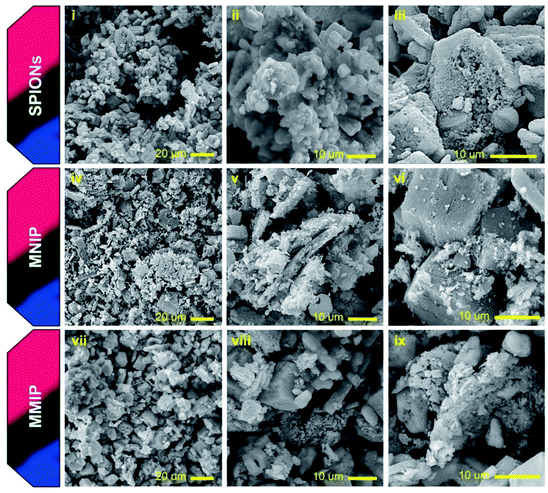
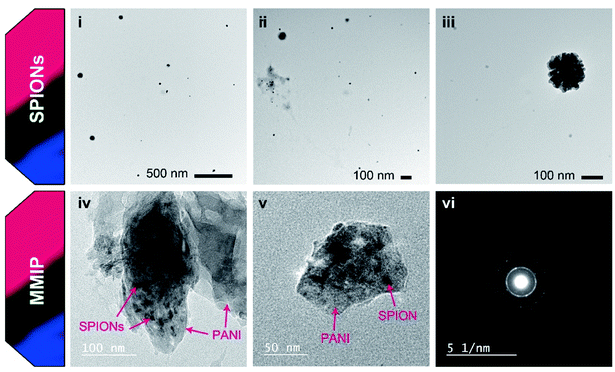
HR-TEM imaging was also used to further confirm the incorporation of SPIONs into the PANI matrix. Naked SPIONs and MMIP samples were imaged using HR-TEM and the obtained images are depicted in Fig. 6. The images show that the fabricated SPIONs are spherical and their size ranges from 15 to 30 nm. Fig. 6(iii) shows a group of aggregated SPIONs of a diameter slightly larger than 100 nm, thus confirming the aforementioned DLS results. HR-TEM images of MMIP (Fig. 6(iv) and (v)) demonstrate irregular flakes of PANI embedded with SPIONs of the same size shown in (i), (ii), and (iii), a structure known in the literature as matrix-dispersed structure.20,46 The images also depict that at least one of the dimensions of the PANI flakes is less than 100 nm in size; hence the name nanocomposite. Fig. 6(vi) depicts the selected area electron diffraction (SAED) pattern of MMIP showing a ring pattern indicating that the fabricated MMIP is a polycrystalline material. The single spots in the SAED pattern may indicate that the MMIP flakes are few nanometers thick. Both SEM and HR-TEM analyses confirm the formation of very small sized SPIONs and SPIONs/PANI nanocomposite.
Adsorption kinetics
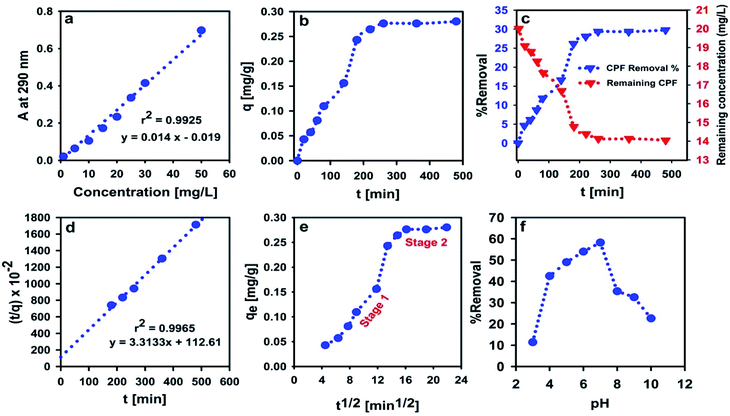
The influence of contact time on the CPF removal from spiked water samples is shown in Fig. 7. It is obvious that the adsorption capacity of CPF onto the synthesized MMIP composite nanomaterial increases by increasing the contact time and reaches a plateau after 200 min (Fig. 7b). The mean adsorption capacity of MMIP was found to be 0.27 mg g−1. In other words, about 30% of the CPF content was removed when 10 mL of CPF solution (20 mg L−1) were treated with 0.2 g of MMIP for ≥200 min.The experiments of adsorption kinetics are usually used to simply supplement the adsorbent evaluation via fitting the obtained experimental data to either the PFO or the PSO kinetic models. In addition, studying adsorption kinetics plays a very important role in understanding the adsorption mechanism. The most common empirical models of adsorption kinetics used in studying liquid adsorption are PFO and PSO models. The PSO model often fits adsorption data better than the PFO model as revealed by least-square discrimination. However, the experimental value of qe is usually higher than the value calculated from the PSO kinetic model, yet very close to it. In the PSO model, the rate of adsorption is mathematically expressed in terms of the occupied adsorption sites (i.e. adsorbed amount, q) rather than in terms of the adsorbate concentration. Thus, the changes in the initial bulk concentration of the adsorbate are assumed to be small enough such that they do not influence the kinetic relationship.65 Interestingly, qe value calculated from the PSO kinetic model was found to be 0.30 mg g−1 which is almost identical to the aforementioned experimental value. In addition, the calculated adsorption rate constant was found to be 9.7 × 10−2 g mg−1 min−1. Fitting of experimental data to the PSO kinetic model indicates that CPF chemisorption is the rate-limiting step, and the removal of CPF molecules from bulk solution is due to the physicochemical interactions between CPF and the MMIP surface.66
The IPD model further clarifies the removal mechanism of contaminants by newly designed materials, in addition to the PFO and the PSO models. Fig. 7e shows that the adsorption follows a two-stage scenario. Stage 1 represents film diffusion/rapid adsorption which results from the diffusion of CPF through the liquid film surrounding the MMIP surface, whereas the plateau at stage 2 represents the equilibrium stage. The calculated rate constant (kid) was 0.021 and 0.002 mg g−1 min−1/2 for stage 1 and stage 2, respectively, indicating that the rate of adsorption is faster in case of the former. This can be also confirmed by comparing the values of the intercept (the intercept represents the external resistance to the diffusion of CPF from bulk to the MMIP surface through the boundary layer). Larger intercept values imply a greater effect of the boundary layer. The obtained intercept values are −0.073 mg g−1 and 0.243 mg g−1 for stage 1 and stage 2, respectively.
Although the PSO model fits the most with the experimental results, diffusion from the bulk must take place in order to bring out CPF to the liquid film (i.e. thin layer) adjacent to the MMIP surface. Indeed, it is not necessary that this diffusion process is the rate-determining step; hence, the rate-determining step is the physico-chemical process/interaction occurring at the MMIP surface as indicated above from the PSO model.
Effect of solution pH
Studying the effect of solution pH on the rate of removal of water contaminants is very crucial, especially for ionizable adsorbates. Changes in solution pH may enhance or even hinder molecular interactions occurring at the surface of the adsorbent particles due to its significant influence on surface charges. Fig. 7f shows that the highest sorption efficiency is attained at pH 7, while it dropped significantly at higher and lower pH values. In acidic media, emeraldine salt is normally protonated, and thus bearing a large number of positive charges.67–69 CPF molecule is capable of accepting a proton at pH ≤ 10.76, as indicated from its pKa value. However, the percentage of protonated nitrogen atoms in the PANI chains decreases gradually at pH = 5.5–7.0 giving the partially protonated emeraldine base. Meanwhile, at pH > 7, the number of deprotonated nitrogen atoms in the emeraldine base increases abruptly, leading to the observed decrease in CPF removal shown in Fig. 7f.69These facts suggest that at neutral pH (pH 7), PANI is in the form of the neutral chains of emeraldine base and CPF is protonated bearing a single positive charge per molecule. Indeed, this is the best scenario for adsorption of CPF onto PANI from the electrostatic point of view because it provides the least electrical repulsion forces between CPF molecules and PANI chains. Similar results were obtained by Hamadeen et al. in treating CPF-contaminated water with Moringa oleifera seed waste.70 The gradual decrease in the removal percent at pH < 7 confirms that PANI is the main player because it was found that emeraldine salt is a perfect pH-sensitive material which was used for fabricating solid-state pH sensors.71
Adsorption isotherms
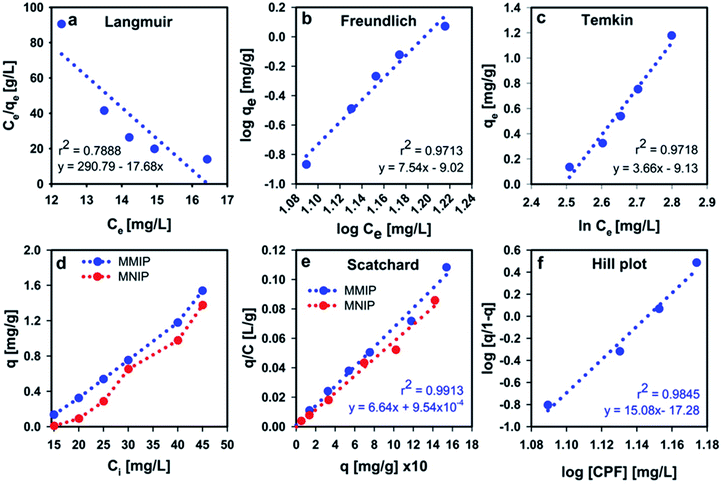
Adsorption equilibrium takes place when a solution of an adsorbate is brought to contact with the adsorbent particles for a sufficient time until attaining a dynamic balance between the adsorbate bulk concentration and that on the surface of the adsorbent. Adsorption isotherm, a plot that describes the adsorption equilibrium at constant conditions of pH and temperature, is an important tool to predict and/or describe the retention (or release) of an adsorbate to (or from) the surface of an adsorbent.48 In the present work, Langmuir, Freundlich and Temkin isotherms were used to describe the adsorption of CPF on the surface of the MMIP in aqueous media.Langmuir isotherm assumes that: (i) adsorption takes place at certain homogeneous sites on the surface of the adsorbent, (ii) the molecules of the adsorbed species do not interact significantly, and (iii) adsorbent saturation occurs after the formation of a single layer of the adsorbate (i.e. monolayer coverage).72 When Ce/qe was plotted versus Ce and the data was regressed linearly, the obtained values of r2, qmax and b were found to be 0.7888, −0.057 mg g−1 and −0.06, respectively. The negative values of qmax and b indicate the inadequacy of the Langmuir isotherm to describe CPF adsorption onto the MMIP surface.
Freundlich isotherm best fits experimental data resulting from multilayer adsorption to heterogeneous surfaces.72 The linear correlation coefficient of Freundlich isotherm plot (r2 = 0.9713) suggests that the isotherm fits the experimental data better than Langmuir model (Fig. 8). The calculated n and KF values are 0.13 and 9.55 × 10−10, respectively. The small value of n indicates that the adsorption of more CPF molecules becomes difficult/unfavorable as the amount of adsorbed CPF increases on the MMIP surface, thus interpreting the relatively long time required for attaining equilibrium.
Temkin isotherm (Fig. 8c) assumes that the heat of adsorption of all molecules in the layer decreases linearly as a result of increase in surface coverage.73 The value of the constant b determines the type of the sorption process. When bT value is <80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, this indicates physical adsorption. However, the value of the term (RT/bT), also termed as B, gives information about the heat of adsorption. Values <8 kJ mol−1 indicate a weak interaction between the adsorbate and adsorbent molecules at the adsorbent surface.73 The obtained bT value is about 677 whereas the B value was found to be 3.66 J mol−1. These values were obtained from a linear plot between qe and ln
000, this indicates physical adsorption. However, the value of the term (RT/bT), also termed as B, gives information about the heat of adsorption. Values <8 kJ mol−1 indicate a weak interaction between the adsorbate and adsorbent molecules at the adsorbent surface.73 The obtained bT value is about 677 whereas the B value was found to be 3.66 J mol−1. These values were obtained from a linear plot between qe and ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce with r2 = 0.9718. From regression coefficient values, it is obvious that Temkin isotherm is the equation which best describes the adsorption of CPF onto the MMIP surface. Meanwhile, both Freundlich and Temkin isotherms confirm the involvement of physical adsorption in the governing mechanism of the removal of CPF by the proposed MMIP particles.
Ce with r2 = 0.9718. From regression coefficient values, it is obvious that Temkin isotherm is the equation which best describes the adsorption of CPF onto the MMIP surface. Meanwhile, both Freundlich and Temkin isotherms confirm the involvement of physical adsorption in the governing mechanism of the removal of CPF by the proposed MMIP particles.
Effect of CPF initial concentration
By investigating the influence of initial CPF concentration on the amount of adsorbed CPF onto the surface of MMIP particles (Fig. 8d) it was found that the amount of adsorbed CPF increased from 0.14 to 1.54 mg g−1 when the initial concentration of CPF increased from 15 to 45 mg L−1. Indeed, this concentration range is very far from the real concentration present in surface water samples which does not exceed 6.36 mg L−1 (i.e. CPF solubility in water). These results indicate that the proposed MMIP can be efficiently used for the removal of high dissolved amounts of CPF from environmental water. On the other hand, although the amount of adsorbed CPF on the surface of MNIP particles increases from 0.05 to 1.42 mg g−1 by increasing the initial CPF concentration from 15 to 45 mg L−1, it remains below the amount of adsorbed CPF in case of MMIP. This finding confirms the involvement of the selective sites on the surface of MMIP particles in the adsorption process. These sites are not available in case of MNIP.In order to make a quantitative comparison between CPF-removal efficiency of MMIP and MNIP particles from aqueous solution, the IF was calculated from the adsorption capacity values as mentioned in the Materials and methods section. The obtained IF was found to be 1.64 indicating that the efficiency/selectivity of MMIP in removing CPF from water is 1.64 folds higher than that of the MNIP counterpart.
Scatchard and Hill plots
The Scatchard and Hill analyses are used to study the adsorption processes occurring at the surface of adsorbents. In addition, the Scatchard analysis is often used to determine the affinity of the adsorbate molecules to the adsorbent as well as the number of active biding sites on the surface of the adsorbent. The calculated association constants (K) from Scatchard analysis (Fig. 8e) are −0.066 and −0.058 g L−1 for MMIP and MNIP, respectively. Theoretically speaking, the slope of the Scatchard plot should be negative in order to give a positive value for K. However, in 1978, Bowmer and Lindup reported Scatchard plots with positive slopes.74 Later in 1984, they published another paper attributing the positive value of the slope to the dependence of binding on the concentration of the adsorbate, albumin in their work.75 They suggested that, increasing the concentration of the adsorbate to a certain extent may reverse the sign of the slope from positive to negative. Henis and Levitzki claimed: “the meaning of the slope in such cases is a complex function of the binding parameters and its exact interpretation depends on the particular model used to analyze the binding data”.76 They analyzed the meaning of the slope of the Scatchard plot in terms of the different allosteric models.76 The aforementioned information indicates that the dependence of CPF adsorption at the adsorbent surface on the amount of adsorbate is the reason behind the obtained positive Scatchard plots.Hill plots (Fig. 8f) were first used to interpret the interaction/binding of oxygen to hemoglobin. Although Hill plots are usually drawn only when Scatchard plots are not linear, the slope of Hill plot, h, is an index of cooperativity. The obtained value of h was found to be 15.08 indicating positive cooperativity (occurs when h > 1.0). Positive cooperativity means that binding of CPF molecules makes their further binding easier. This is usually attributed to the interaction between the active binding sites at the adsorbent surface such that the binding at one site affects the binding at another.
Adsorption thermodynamics and the effect of temperature
To have a closer insight into the CPF adsorption mechanism at the surface of the MMIP particles, the adsorption equilibrium constant (Ka) was calculated at different test solution temperatures, and then the thermodynamic parameters were calculated. The calculated negative values of ΔG at the studied temperatures indicate that CPF adsorption onto the MMIP particles is a spontaneous process and that CPF molecules tend to stay in the stationary phase. Normally, physisorption is indicated by standard free energy change values (ΔG°) ranging from 0 to −20 kJ mol−1, whereas that of chemisorption ranges from −80 to −400 kJ mol−1.51,77 The calculated values of ΔG (Table 1) confirm that the adsorption process takes place by physisorption. Therefore, the involvement of the imprinted active sites on the MMIP surface is highly acceptable which coincides with the calculated IF.| T (K) | Ka | Slope | Intercept | ΔH (kJ mol−1) | ΔS (J mol−1 K−1) | ΔG (kJ mol−1) |
|---|---|---|---|---|---|---|
| 303 | 2.17 | 1773.6 | −5.07 | −14.746 | −42.191 | −1.955 |
| 308 | 1.99 | −1.745 | ||||
| 313 | 1.80 | −1.534 | ||||
| 318 | 1.65 | −1.323 | ||||
| 323 | 1.07 | −1.112 | ||||
| 328 | 0.85 | −0.901 | ||||
| 333 | 0.57 | −0.690 |
Moreover, the values of ΔH, ΔS and TΔS can be used for expecting the type of interaction taking place at the adsorbent surface. For instance, non-covalent interactions usually include hydrophobic interactions, van der Waals interactions, electrostatic interactions and/or hydrogen bonding. Hydrophobic interactions among nonpolar molecules were proved to be entropy-driven, with ΔH > 0, ΔS > 0 and ΔH < TΔS. However, enthalpy-driven processes such as van der Waals interactions and hydrogen bonding usually have ΔH < 0, ΔS < 0 and |ΔH| > |TΔS|. Electrostatic interactions-governed adsorption is often characterized by a minor enthalpy and a positive favorable entropy (ΔH ∼ 0 or ΔH > 0 and ΔS > 0).78 The thermodynamic parameters (Table 1) suggest an enthalpy-driven adsorption mechanism via van der Waals interactions and hydrogen bonding. These results agree with the results of FTIR spectroscopy. It is worth mentioning that increasing the test solution temperature above 318 K leads to shifting ΔG towards more positive values, thus making the adsorption process unfavorable and significantly affecting the adsorption efficiency. Moreover, the highest removal efficiency was obtained at room temperature (Fig. 9a), making the synthesized MMIP material a promising candidate for removal of CPF from contaminated water at ambient conditions of temperature and pH. This thermal behavior of the adsorption process was observed because the rate-determining step (i.e. physico-chemical interaction as indicated from the PSO model) is very sensitive to elevations in solution temperature; unlike covalent interactions which usually show higher thermal stability.
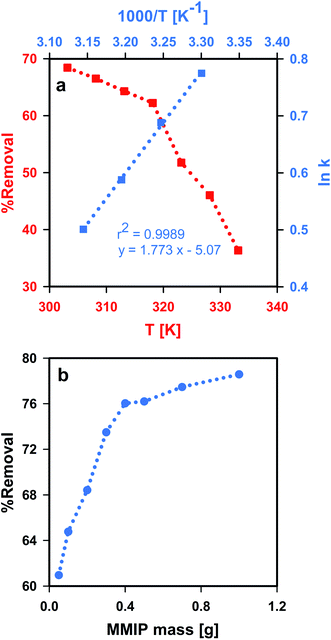 | ||
| Fig. 9 Thermodynamics and the effect of aqueous solution temperature (a), and the influence of MMIP dose (b) on the adsorption of CPF onto the synthesized MMIP. | ||
Effect of MMIP dose
The amount of MMIP adsorbent (i.e. adsorbent dose) was found to significantly influence the removal efficiency of CPF (Fig. 9b). The removal efficiency increased from 61.0 to 78.6% upon increasing the MMIP dose from 0.05 g to 1.0 g. This indicates that the addition of a larger amount of MMIP provides new available active sites for CPF adsorption.Conclusions
The use of CPF in agriculture represents a serious danger to the environment, especially the freshwater resources, which may act as a possible life threat for both humans, terrestrial and aquatic organisms. Magnetic adsorbents based on MIPs are promising adsorbent materials on account of their selectivity, efficiency, facile large-scale production, safety and ease of separation/control via external magnetic field. The present work suggested a novel magnetic molecularly-imprinted polymer material for the efficient removal of CPF from contaminated water. The use of SPIONs in the proposed matrix-dispersed material provided excellent magnetic susceptibility. The suggested MMIP adsorbent was found to attract CPF molecules from bulk via van der Waals interactions and hydrogen-bond formation. The adsorption experimental results fitted well to the PSO kinetics model and Temkin adsorption isotherm. Moreover, the most important advantage of this MMIP adsorbent is its selectivity towards CPF in aqueous solution due to the high IF obtained from the adsorption experiments. However, the relatively long time required for adsorption can be shortened by adding more functional groups to the MMIP surface to enhance the interaction with CPF molecules in the bulk. For now, we are trying to investigate the influence of surface functionalization of the proposed MMIP adsorbent, via copolymerization, on the equilibration time required for CPF adsorption.Author contributions
Hadeel Saad: methodology, investigation, formal analysis, writing – original draft, visualization. F. A. Nour El-Dien: supervision, project administration, resources, writing – review and editing. Nadia E. A. El-Gamel: supervision, resources, writing – review and editing. Ahmed S. Abo Dena: conceptualization, methodology, investigation, resources, formal analysis, data curation, visualization, writing – original draft, writing – review and editing.Conflicts of interest
There are no conflicts to declare.Notes and references
- S. M. Maliyekkal, T. S. Sreeprasad, D. Krishnan, S. Kouser, A. K. Mishra, U. V Waghmare and T. Pradeep, Small, 2013, 9, 273–283 CrossRef CAS PubMed.
- A. R. Kulkarni, K. S. Soppimath, A. M. Dave, M. H. Mehta and T. M. Aminabhavi, J. Hazard. Mater., 2000, 80, 9–13 CrossRef CAS PubMed.
- G. Wulff, A. Sarhan and K. Zabrocki, Tetrahedron Lett., 1973, 14, 4329–4332 CrossRef.
- J. J. BelBruno, Chem. Rev., 2019, 119, 94–119 CrossRef CAS PubMed.
- A. S. Abo Dena, A. M. Ali and I. M. El-sherbiny, J. Nanotechnol. Adv. Mater., 2020, 8, 1–19 Search PubMed.
- J. Xu, H. Miao, J. Wang and G. Pan, Small, 2020, 16, 1906644 CrossRef CAS PubMed.
- E. Nazarzadeh Zare, A. Mudhoo, M. Ali Khan, M. Otero, Z. M. A. Bundhoo, M. Patel, A. Srivastava, C. Navarathna, T. Mlsna, D. Mohan, C. U. Pittman Jr, P. Makvandi and M. Sillanpää, Small, 2021, 2007840 CrossRef CAS PubMed.
- J. Wang, J. Dai, Y. Xu, X. Dai, Y. Zhang, W. Shi, B. Sellergren and G. Pan, Small, 2019, 15, 1803913 CrossRef PubMed.
- R. B. Pernites, S. K. Venkata, B. D. B. Tiu, A. C. C. Yago and R. C. Advincula, Small, 2012, 8, 1669–1674 CrossRef CAS PubMed.
- A. G. Ayankojo, J. Reut, V. Ciocan, A. Öpik and V. Syritski, Talanta, 2020, 209, 120502 CrossRef CAS PubMed.
- T. Beduk, A. Ait Lahcen, N. Tashkandi and K. N. Salama, Sens. Actuators, B, 2020, 314, 128026 CrossRef CAS.
- M. Sundhoro, S. R. Agnihotra, B. Amberger, K. Augustus, N. D. Khan, A. Barnes, J. BelBruno and L. Mendecki, Food Chem., 2021, 344, 128648 CrossRef CAS PubMed.
- J. Wang, R. Liang and W. Qin, TrAC, Trends Anal. Chem., 2020, 130, 115980 CrossRef CAS.
- H. Y. Hijazi and C. S. Bottaro, J. Chromatogr. A, 2020, 1617, 460824 CrossRef CAS PubMed.
- Z. Zhang, X. Cao, Z. Zhang, J. Yin, D. Wang, Y. Xu, W. Zheng, X. Li, Q. Zhang and L. Liu, Talanta, 2020, 208, 120385 CrossRef CAS PubMed.
- L. A. F. Dinali, H. L. de Oliveira, L. S. Teixeira, W. de Souza Borges and K. B. Borges, Food Chem., 2021, 345, 128745 CrossRef CAS PubMed.
- M. Guo, Y. Hu, R. Wang, H. Yu and L. Sun, Environ. Res., 2021, 194, 110684 CrossRef CAS PubMed.
- J. Li, M. Zhu, M. Wang, W. Qi, R. Su and Z. He, Soft Matter, 2020, 16, 7033–7039 RSC.
- L. Fang, Y. Miao, D. Wei, Y. Zhang and Y. Zhou, Chemosphere, 2021, 262, 128032 CrossRef CAS PubMed.
- O. A. Abdel Aziz, K. Arafa, A. S. Abo Dena and I. M. El-sherbiny, J. Nanotechnol. Adv. Mater., 2020, 8, 21–29 Search PubMed.
- D. S. Cardona, K. B. Debs, S. G. Lemos, G. Vitale, N. N. Nassar, E. N. V. M. Carrilho, D. Semensatto and G. Labuto, J. Environ. Manage., 2019, 242, 362–371 CrossRef CAS PubMed.
- A. Z. M. Badruddoza, Z. B. Z. Shawon, M. T. Rahman, K. W. Hao, K. Hidajat and M. S. Uddin, Chem. Eng. J., 2013, 225, 607–615 CrossRef CAS.
- M. Soylak, O. Ozalp and F. Uzcan, Trends Environ. Anal. Chem., 2021, 29, e00109 CrossRef CAS.
- K. Shrivas, A. Ghosale, N. Nirmalkar, A. Srivastava, S. K. Singh and S. S. Shinde, Environ. Sci. Pollut. Res., 2017, 24, 24980–24988 CrossRef CAS PubMed.
- P. Wang, Y. Yin, Y. Guo and C. Wang, RSC Adv., 2015, 5, 72572–72578 RSC.
- C. Anudechakul, A. S. Vangnai and N. Ariyakanon, Int. J. Phytorem., 2015, 17, 678–685 CrossRef CAS PubMed.
- P. Prasertsup and N. Ariyakanon, Int. J. Phytorem., 2011, 13, 383–395 CrossRef PubMed.
- M. M. Jacob, M. Ponnuchamy, A. Kapoor and P. Sivaraman, J. Environ. Chem. Eng., 2020, 8, 103904 CrossRef CAS.
- Y. Samet, E. Hmani and R. Abdelhédi, Water SA, 2012, 38, 537–542 CrossRef CAS.
- H. Liu, J. Chen, N. Wu, X. Xu, Y. Qi, L. Jiang, X. Wang and Z. Wang, Ecotoxicol. Environ. Saf., 2019, 170, 259–266 CrossRef CAS PubMed.
- K. Naddafi, S. S. Martinez, R. Nabizadeh, K. Yaghmaeian, S. J. Shahtaheri and H. Amiri, Water Sci. Technol., 2020, 83, 212–222 CrossRef PubMed.
- J. Femia, M. Mariani, C. Zalazar and I. Tiscornia, Water Sci. Technol., 2013, 68, 2279–2286 CrossRef CAS PubMed.
- M. Rani and U. Shanker, Environ. Sci. Pollut. Res., 2018, 25, 10878–10893 CrossRef CAS PubMed.
- S. Vigneshwaran, J. Preethi and S. Meenakshi, Int. J. Biol. Macromol., 2019, 132, 289–299 CrossRef CAS PubMed.
- A. S. Nair and T. Pradeep, J. Nanosci. Nanotechnol., 2007, 7, 1871–1877 CrossRef CAS PubMed.
- N. Kumar, N. Narayanan and S. Gupta, React. Funct. Polym., 2019, 135, 103–112 CrossRef CAS.
- H. R. Nodeh, W. A. Wan Ibrahim, M. A. Kamboh and M. M. Sanagi, RSC Adv., 2015, 5, 76424–76434 RSC.
- S. Meseguer-Lloret, S. Torres-Cartas, M. Catalá-Icardo, E. F. Simó-Alfonso and J. M. Herrero-Martínez, Anal. Bioanal. Chem., 2017, 409, 3561–3571 CrossRef CAS PubMed.
- O. Aydın Urucu, A. Beyler Çiğil, H. Birtane, E. Kök Yetimoğlu and M. V. Kahraman, J. Ind. Eng. Chem., 2020, 87, 145–151 CrossRef.
- C. Ayadi, A. Anene, R. Kalfat, Y. Chevalier and S. Hbaieb, Colloids Surf., A, 2019, 567, 32–42 CrossRef CAS.
- T. E. Milja, V. S. Krupa and T. P. Rao, RSC Adv., 2014, 4, 30718–30724 RSC.
- F. Saadati, M. Rahmani, F. Ghahramani, F. Piri, H. Shayani-Jam and M. R. Yaftian, Desalin. Water Treat., 2017, 82, 210–218 CrossRef CAS.
- M. B. Regasa, T. R. Soreta, O. E. Femi, P. C. Ramamurthy and S. Kumar, J. Mol. Recognit., 2020, 33, e2836 CrossRef CAS PubMed.
- J. Luo, J. Huang, Y. Wu, J. Sun, W. Wei and X. Liu, Biosens. Bioelectron., 2017, 94, 39–46 CrossRef CAS PubMed.
- H. Zhao, C. Zhang, D. Qi, T. Lü and D. Zhang, J. Dispersion Sci. Technol., 2019, 40, 231–238 CrossRef CAS.
- A. S. Shair, A. S. Abo Dena and I. M. El-Sherbiny, Spectrochim. Acta, Part A, 2021, 249, 119301 CrossRef CAS PubMed.
- O. Melad, H. Alhendawi and M. Fayyad, Res. Rev.: J. Chem., 2014, 3, 40–47 CAS.
- M. Ateia, D. E. Helbling and W. R. Dichtel, ACS Mater. Lett., 2020, 2, 1532–1544 CrossRef CAS.
- G. D. Sheng, D. D. Shao, X. M. Ren, X. Q. Wang, J. X. Li, Y. X. Chen and X. K. Wang, J. Hazard. Mater., 2010, 178, 505–516 CrossRef CAS PubMed.
- Y. Liu, J. Chem. Eng. Data, 2009, 54, 1981–1985 CrossRef CAS.
- C. Wu, X. Lou, A. Huang, M. Zhang and L. Ma, Appl. Clay Sci., 2020, 190, 105566 CrossRef CAS.
- H. N. Nassar, H. R. Ali and N. S. El-Gendy, Fuel, 2021, 294, 120534 CrossRef CAS.
- V. Suendo, Y. Lau, F. Hidayat, M. Reza, A. Qadafi and A. Rochliadi, Phys. Chem. Chem. Phys., 2021, 23, 7190–7199 RSC.
- A. Prashar and Nihal, IOP Conf. Ser.: Mater. Sci. Eng., 2021, 1033, 12048 CAS.
- P. Goel and M. Arora, MRS Adv., 2020, 5, 2661–2667 CrossRef CAS.
- T. Visser, Infrared Spectra of Pesticides, CRC Press, London, 2019 Search PubMed.
- A. A. Qureshi, S. Javed, H. M. A. Javed, A. Akram, M. S. Mustafa, U. Ali and M. Z. Nisar, Mater. Sci. Semicond. Process., 2021, 123, 105545 CrossRef CAS.
- X. Yang, C. Chen, J. Li, G. Zhao, X. Ren and X. Wang, RSC Adv., 2012, 2, 8821–8826 RSC.
- C. F. Holder and R. E. Schaak, ACS Nano, 2019, 13, 7359–7365 CrossRef CAS PubMed.
- R. M. Ashour, R. El-sayed, A. F. Abdel-Magied, A. A. Abdel-khalek, M. M. Ali, K. Forsberg, A. Uheida, M. Muhammed and J. Dutta, Chem. Eng. J., 2017, 327, 286–296 CrossRef CAS.
- M. Mahmoudi, S. Sant, B. Wang, S. Laurent and T. Sen, Adv. Drug Delivery Rev., 2011, 63, 24–46 CrossRef CAS PubMed.
- A. Akbarzadeh, M. Samiei and S. Davaran, Nanoscale Res. Lett., 2012, 7, 144 CrossRef PubMed.
- H. Fatima and K.-S. Kim, Korean J. Chem. Eng., 2017, 34, 589–599 CrossRef CAS.
- M. A. Mohamady Hussein, F. G. D. Baños, M. Grinholc, A. S. Abo Dena, I. M. El-Sherbiny and M. Megahed, Int. J. Biol. Macromol., 2020, 162, 1760–1769 CrossRef CAS PubMed.
- M. A. Hubbe, S. Azizian and S. Douven, BioResources, 2019, 14, 7582–7626 CrossRef.
- D. Robati, J. Nanostruct. Chem., 2013, 3, 55 CrossRef.
- H. Wan and S. C. Yang, MRS Online Proc. Libr., 2007, 965, 1223 Search PubMed.
- P. M. McManus, S. C. Yang and R. J. Cushman, J. Chem. Soc., Chem. Commun., 1985, 1556–1557 RSC.
- E. Gill, A. Arshak, K. Arshak and O. Korostynska, Sensors, 2007, 7, 3329–3346 CrossRef CAS PubMed.
- H. M. Hamadeen, E. A. Elkhatib, M. E. I. Badawy and S. A. M. Abdelgaleil, J. Environ. Chem. Eng., 2021, 9, 105376 CrossRef CAS.
- H. D. Nguyen, T. H. Nguyen, N. V. Hoang, N. N. Le, T. N. N. Nguyen, D. C. T. Doan and M. C. Dang, Adv. Nat. Sci.: Nanosci. Nanotechnol., 2014, 5, 45001 CAS.
- V. Fierro, V. Torné-Fernández, D. Montané and A. Celzard, Microporous Mesoporous Mater., 2008, 111, 276–284 CrossRef CAS.
- T. Z. E. Lee, J. Zhang, Y. Feng, X. Lin and J. Zhou, IOP Conf. Ser. Earth Environ. Sci., 2021, 657, 12026 CrossRef.
- C. J. Bowmer and W. E. Lindup, J. Pharm. Sci., 1978, 67, 1193–1195 CrossRef CAS PubMed.
- L. S. Clegg and W. E. Lindup, J. Pharm. Pharmacol., 2011, 36, 776–779 CrossRef PubMed.
- Y. I. Henis and A. Levitzki, Eur. J. Biochem., 1976, 71, 529–532 CrossRef CAS PubMed.
- G. Gereli, Y. Seki, İ. Murat Kuşoğlu and K. Yurdakoç, J. Colloid Interface Sci., 2006, 299, 155–162 CrossRef CAS PubMed.
- P. D. Ross and S. Subramanian, Biochemistry, 1981, 20, 3096–3102 CrossRef CAS PubMed.
Footnote |
| † Present address: Egyptian Chemistry Administration, Ramses street, Cairo, Egypt. |
| This journal is © The Royal Society of Chemistry 2021 |