Open Access Article
Open Access ArticleOne-pot synthesis of α-aminophosphonates by yttrium-catalyzed Birum–Oleksyszyn reaction†
Davide Ceradini and
Kirill Shubin
and
Kirill Shubin *
*
Latvian Institute of Organic Synthesis, 21 Aizkraukles St., Riga, LV-1006, Latvia. E-mail: kir101@osi.lv
First published on 8th December 2021
Abstract
For the first time, yttrium triflate was used as an efficient green catalyst for the synthesis of α-aminophosphonates through a one-pot three-component Birum–Oleksyszyn reaction. Under the action of this Lewis acid, enhancement of the yield and reaction chemoselectivity was provided by the achievement of an appropriate balance in the complex network of reactions.
Multicomponent reactions are commonly used to achieve molecular complexity. In particular, one-pot three-component reactions have been exploited for the synthesis of α-aminophosphonates. These organophosphorus compounds have attracted the attention of medicinal chemists due to their similarity to α-amino acids. They find application in agriculture as plant growth regulators1 and herbicides,2 in medicinal chemistry as antibacterial,3,4 antiviral5 and antitumor agents,6 activity-based probes,7 and building blocks for peptides and proteins.8 Since the first preparation of α-aminophosphonates, reported in 1952 by Fields,9 various methods for their synthesis have been proposed.10–16 Nowadays, one-pot three-component condensation of aldehyde, amine, and phosphite, catalyzed by an excess of acetic acid, is the most common method due to its simplicity and, in general, high yields of products.4 Application of Lewis acids as the catalyst instead of Brønsted acids was first reported in 1973 by Birum.17 The advantage of Lewis acids is their compatibility with acid-sensitive functional groups that, under typical conditions (glacial acetic acid) would be degraded.
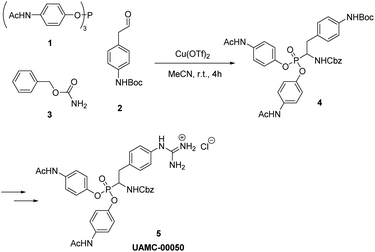
Condensation of aldehyde, carbamate, and triaryl phosphite has been named as Birum–Oleksyszyn reaction.18 Recently, a new biologically active α-aminophosphonate (UAMC-00050) was developed at the University of Antwerp.19–21 This diarylphosphonate shows good inhibitory activity against urokinase plasminogen activator (uPA), an enzyme involved in several physiological processes, such as tissue remodeling.22 uPA can be also involved in the development of different diseases, for example, thrombolytic disorder,23 cancer,24 and eye diseases.25 UAMC-00050 is currently under investigation for the treatment of irritable bowel syndrome26 and dry eye disease.21 The key step of the synthesis of UAMC-00050, proposed by Joossens et al.,19,21 involves Birum–Oleksyszyn reaction, catalyzed by Cu(OTf)2 in acetonitrile, which, on a small scale, provides 20% yield of the product (Scheme 1). In current work, a new catalyst has been introduced for the Birum–Oleksyszyn reaction. For the first time, we report the use of yttrium salt in a one-pot three-component synthesis of α-aminophosphonates, which provides a remarkable improvement of the yield of product for the key step in the synthesis of UAMC-00050. The scope of the protocol has been demonstrated on a variety of aldehydes, phosphites, and carbamates.
In the frame of the dry eye disease drug development (IT-DED3) project,27 we have worked toward upscaling of the synthetic route and providing larger amounts of UAMC-00050 for more detailed research of its biological activity.
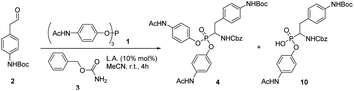
During our attempts to upscale the key step to 3.0 g scale, we noticed a considerable decrease of the yield of product from 20% to 11%. In our view, the poor outcome of the process cannot be attributed to the Birum–Oleksyszyn reaction alone. The nature of substituents in the target molecule affects the overall efficiency of the transformation through both the main and a number of side reactions (Scheme 2). This three-component reaction involves unstable paracetamol phosphite 1, aliphatic aldehyde 2 bearing Boc-protected amino group, and benzyl carbamate 3 (Scheme 1). In general, aliphatic aldehydes are less reactive in Birum–Oleksyszyn reaction, which consequently requires a stronger catalyst or longer reaction time to achieve satisfactory yield of product.15,28 The imine generated from the condensation of carbamate and aldehyde is highly reactive and can add a second molecule of carbamate forming aminal 7.29 Compound 7 can participate in Arbuzov-type reactions,30 if, as hypothesized, it is converted into reactive cation 13 by Brønsted29 or Lewis acid.31 However, Lewis acid-catalyzed reactions of aminal 7 or its analogs and triaryl phosphites are not known.
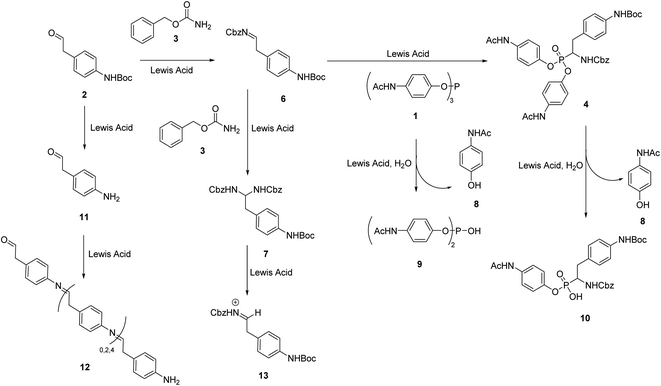 | ||
| Scheme 2 Birum–Oleksyszyn reaction as the key step and possible side reactions in the synthesis of UAMC-00050. | ||
The presence of paracetamol moiety complicates the synthesis due to the lower stability of its phosphorus esters both in starting triaryl phosphite and in the formed α-aminophosphonate. In the reaction environment, they readily hydrolyze with one equivalent of water formed in the condensation of aldehyde 2 and the carbamate 3 generating diarylphosphite 9 and monoaryl side product 10.
As previously reported, product 4 is stable in the presence of water.21 However, under the reaction conditions, hydrolysis proceeds with the help of the Lewis acid.32 With prolonged reaction time (i.e., 24 h) we noticed a decrease in the yield of product 4 almost in half compared to the yield obtained in the reaction performed for 4 h and formation of acid 10 as the major side product.
Application of N-Boc-protected starting material is also challenging, since, in the presence of a Lewis acid catalyst, partial removal of the Boc group takes place and unprotected amino aldehyde 11 forms black-brown polymer 12 (Scheme 2).
Improving the overall efficiency of the synthesis of α-aminophosphonate 4 requires simultaneous promotion of imine formation and Birum–Oleksyszyn reaction and suppression of unwanted hydrolysis and Boc group removal. In search for optimal conditions, we initiated screening of various acidic catalysts using equimolar ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of aldehyde 2, carbamate 3, and phosphite 1 with 10 mol% load of the catalyst in MeCN performing the reaction at room temperature for 4 h.19 Acetonitrile proved to be an optimal solvent for the preparation of 4, it is polar enough to dissolve all the starting materials, it is not a concern for the environment, like DCM, and it does not hydrolyze the product like protic solvents: MeOH, EtOH and H2O.
1) of aldehyde 2, carbamate 3, and phosphite 1 with 10 mol% load of the catalyst in MeCN performing the reaction at room temperature for 4 h.19 Acetonitrile proved to be an optimal solvent for the preparation of 4, it is polar enough to dissolve all the starting materials, it is not a concern for the environment, like DCM, and it does not hydrolyze the product like protic solvents: MeOH, EtOH and H2O.
In Table 1, a group of 18 catalysts (16 Lewis acids and 2 Brønsted acids) is reported and yields of isolated product 4 have been indicated. The reaction occurs spontaneously, but it needs a catalyst to proceed in an acceptable speed to avoid the formation of impurities. Reaction in AcOH did not provide any product, since a complete decomposition of starting materials took place. In contrast, TfOH showed a much better performance.
| a Reaction conditions: 1.0 equiv. of aldehyde 2, 1.0 equiv. of phosphite 1, 1.0 equiv. of benzyl carbamate 3 and 10 mol% of Lewis acid, anhydrous MeCN, under argon, RT, 4 h.b Isolated yield.c Selectivity expressed as a percent ratio of compounds 4 and 10.d AcOH as a solvent.e 1.0 equiv. trimethyl orthoformate (TMOF).f 1.5 g of 4 Å MS. | ||||
|---|---|---|---|---|
| Entry | Catalyst | Yield of product 4, %b | Ratio 4/10 | Selectivity, %c |
| 1 | AcOHd | 0 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
0 |
| 2 | TiCl4 | 8 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 66 66 |
44 |
| 3 | ZrCl4 | 8 | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
81 |
| 4 | Cu(OTf)2 | 11 | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24 |
76 |
| 5 | BiCl3 | 13 | 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26 |
74 |
| 6 | TfOH | 13 | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
82 |
| 7 | Mg(OTf)2 | 14 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
50 |
| 8 | FeCl3 | 15 | 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 32 |
68 |
| 9 | LiOTf | 15 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
85 |
| 10 | Sc(OTf)3 | 16 | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
81 |
| 11 | Et2O·BF3 | 16 | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24 |
76 |
| 12 | Bi(NO3)3·5H2O | 19 | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24 |
76 |
| 13 | Yb(OTf)3 | 20 | 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 33 |
67 |
| 14 | SnCl4 | 22 | 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 43 43 |
57 |
| 15 | ZnCl2 | 22 | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
81 |
| 16 | La(OTf)3 | 25 | 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 31 |
69 |
| 17 | Bi(OTf)3 | 31 | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24 |
76 |
| 18 | AcOH | 35 | 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46 46 |
54 |
| 19 | Y(OTf)3 | 42 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
80 |
| 20 | Y(OTf)3e | 17 | 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
84 |
| 21 | Y(OTf)3f | 31 | 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34 34 |
66 |
Although the yield of product 4 was low (13%), TfOH provided a much better stability of compound 4 toward the hydrolysis (82% of it survived vs. only 18% of the hydrolyzed 10). Lewis acids like TiCl4, Cu(OTf)2, ZnCl2, and FeCl3 are commonly used in three-component synthesis of α-aminophosphonates.18,33–35 Triflate salts were included in the list because of their enhanced stability in the presence of water generated after the condensation of aldehyde 2 and carbamate 3.36,37
As reported in literature, phosphodiesters can be hydrolyzed by Lewis acids.32,38 Paracetamol-containing phosphorus esters are much more sensitive to the presence of water compared to their alkyl analogs. Therefore, analysis of the catalyst efficiency can be carried out by using an additional parameter – selectivity for the formation of the target diester 4 compared to the proportion of the hydrolyzed monoester side product 10. This parameter is expressed as a percentage ratio of compounds 4 and 10, obtained from HPLC-UV assay with internal standard. Diagram with yield of product 4 on the horizontal axis and selectivity of the formation of compound 4 on the vertical axis is shown on Fig. 1.
For example, Lewis acids like TiCl4, Mg(OTf)2, and SnCl4 hydrolyzed up to a half of diester 4 comprising the group with the lowest overall efficiency. On the other hand, LiOTf provided the best compatibility with diester 4 and the lowest degree of hydrolysis (85% of diester 4 preserved), but only a moderate yield of 15%. The two elements of group 1 and group 2 in catalysts LiOTf and Mg(OTf)2 both provided low yield of product 4 (15% and 14%, respectively) but different selectivity, quite high for lithium (85%) and moderate for magnesium (50%).
Three different salts of bismuth were tested, for all of which similar selectivity was registered (74–76%). However, quite large difference in yields of compound 4 was observed – 13% for BiCl3, 19% for Bi(NO3)3·5H2O, and 31% for Bi(OTf)3. Eight triflate salts were screened and provided a wide range of yield and selectivity. Cu(OTf)2 was the most ineffective in terms of yield, while Mg(OTf)2 was the least selective toward the formation of product 4. Among four chloride salts, group 4 elements in catalysts TiCl4 and ZrCl4 ensured the same low yield of product 4 (8%) but differed in selectivity two-fold (44 and 81%, respectively). At the same time, SnCl4 and ZnCl2 afforded product 4 in three times higher yield (22%), but selectivity of SnCl4 was much lower (57 vs. 81% for ZnCl2). The poor performance of TiCl4 and SnCl4 can be explained by their hydrolysis with liberation of HCl under the reaction conditions. FeCl3 was reported as an excellent catalyst for the synthesis of α-aminophosphonates from alkylphosphites,39 but, in the case of triaryl phosphite 1, it was able to provide product 4 in only 15% yield with 68% selectivity.
In addition, it was found that strong Lewis acids (e.g., TiCl4 and BF3) generate a large amount of a brown-black side product (polymer 12) formed by the removal of Boc protecting group and subsequent reaction of amine and aldehyde (Scheme 2).
Less strong Lewis acids (e.g., Mg(OTf)2 and LiOTf) generated significantly less polymer 12, however, most of aldehyde 2 was left unreacted providing poor conversion of the starting materials.
An interesting comparison can be made between elements of group 3 – Y(OTf)3 provided the highest yield (42%) and good selectivity (80%) of diaryl product 4, Sc(OTf)3 showed similar selectivity (81%) but afforded much lower yield (16%) of product 4. Yb(OTf)3 and La(OTf)3 are located in the middle of Fig. 1, since they ensured formation of product 4 in 20 and 25% yield and with selectivity of 67 and 69%, respectively.
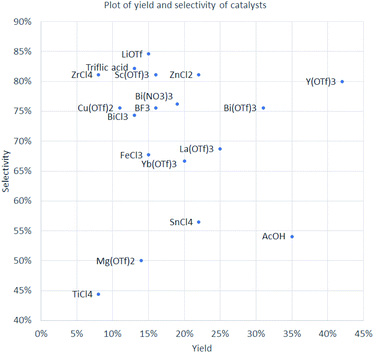 | ||
| Fig. 1 Yield of product 4 (horizontal axis, percent) and selectivity of the formation of diaryl product 4 compared to monoaryl product 10 (vertical axis, percent ratio). | ||
The group of best-performing catalysts includes ZnCl2, La(OTf)3, Bi(OTf)3, and Y(OTf)3. On the scale of 3 g of target compound 4, the best performance was achieved with Y(OTf)3, which provided the highest yield (42%), almost 4 times higher than in the case of Cu(OTf)2 (11%). The stability of the paracetamol diester group was also one of the highest – only 20% of diester was hydrolyzed.
This catalyst was used for elucidation of the behavior of aminal 7 in the reaction system. Y(OTf)3-catalyzed reaction of aldehyde 2 and 2.0 equiv. of carbamate 3 after 2 h provided compound 7, which proved to be bench-stable and easily isolable. Aminal 7 was then reacted with tri(paracetamol) phosphite 1 under standard conditions in the presence of Y(OTf)3. After 4 h, HPLC-UV assay with an internal standard showed only half amount of phosphonate 4 compared to that obtained in three-component Birum–Oleksyszyn reaction.
According to these data, it can be concluded that aminal 7 can react with phosphite 1 but is twice less reactive than imine 6. Therefore, one-pot three-component reaction with in situ formation of imine is a preferable strategy for the synthesis of aryl α-aminophosphonates. Dehydrating agents like 4 Å MS and trimethyl orthoformate (TMOF) were not able to increase the yield over the standard protocol. A small increase in selectivity from 80% to 84% was noted when TMOF was used but with a much lower yield of 4 (17%).
With the optimized conditions in hand, the efficiency of Y(OTf)3 catalyst in the Birum–Oleksyszyn reaction was investigated using various aldehydes, carbamates, and aryl phosphites (Fig. 2). The highest yields of products were obtained for activated phosphites, for example, tris(p-methoxyphenyl) phosphite and the tris(p-acetamidophenyl) phosphite, combined with benzyl carbamate 3 and aromatic aldehydes (18, 22). The electron-donating substituent in phosphite facilitates the nucleophilic attack of phosphorus atom on the imine. Aliphatic aldehydes represented by t-butyl [4-(2-oxoethyl)phenyl]carbamate and 2-phenylacetaldehyde afforded lower yields (38–48%) of products (4, 29, 33) compared to the yields (72–92%) of products derived from benzaldehyde (14, 15, 17, 18). Among aromatic aldehydes, interesting results were obtained demonstrating how different halogen substituents in the para-position affect the yield of the respective product: 4-chlorobenzaldehyde (21) and 4-bromobenzaldehyde (26) provided higher yields (55 and 54%, respectively) compared to 4-fluorobenzaldehyde (20, 36%) and 4-iodobenzaldehyde (27, 25%). For product 27 the poor yield might be caused by its diminished solubility of starting aldehyde in MeCN. Compared to the standard conditions (application of AcOH) of Birum–Oleksyszyn reaction, the selected Lewis acid catalyst allows to perform the synthesis of α-aminophosphonates using acid-labile compounds, as demonstrated by the examples with t-butyl carbamate (34–36) and Boc-protected amine (4, 33). While yields of products 34 and 35 (43 and 41%, 34 and 35) were lower compared to those obtained for CbzNH2 analogs (82 and 64%, 14 and 19), there were no elevated amounts of hydrolysis products. The lower conversion of starting materials might be explained by the higher steric hindrance of the tert-butyl group.18 Similarly, tri(o-tolyl)phosphite provided lower yields of products (34 and 26%, 16 and 25) when compared to the analogous reaction with triphenyl phosphite (82 and 55%, 14 and 21) and tri(p-tolyl)phosphite (75 and 43%, 15 and 24). These differences in yields also might be attributed to the increased steric hindrance near the phosphorus atom, which obstructs the nucleophilic attack of phosphite toward imine.
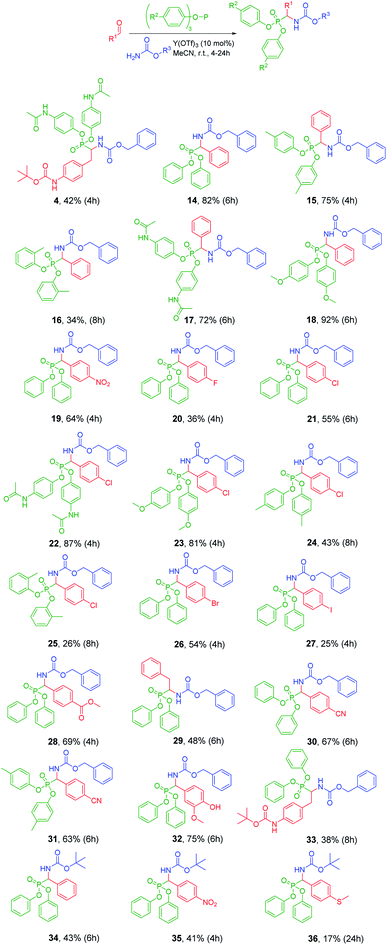 | ||
| Fig. 2 Scope of Birum–Oleksyszyn reaction catalyzed by Y(OTf)3 in anhydrous MeCN under argon. All the %yield presented is isolated yield. | ||
In general, the developed catalytic conditions using Y(OTf)3 are well compatible with a variety of functional groups in both aldehyde and phosphite. When comparing series with benzaldehyde (15, 17, and 18) and 4-chlorobenzaldehyde (22, 23, and 24), the hydrolyzed side product was detected only in case of product 17 and 22 testifying the lower stability of the paracetamol ester.
Conclusions
The Birum–Oleksyszyn step for the synthesis of UAMC-00050 showed to be a complex reaction with many side products. Optimization of the conditions with achieving of a proper balance between main and side reactions was necessary to increase the yield and provide a product with a better purity profile. Among 18 tested catalysts, Y(OTf)3 was selected as the most suitable and was used for the first time in the Birum–Oleksyszyn reaction. This Lewis acid, not only allowed us to avoid the use of the environment unfriendly copper but is capable to provide good conversion of the starting materials and selective formation of product ensuring increased stability of hydrolytically unstable aryl phosphonates under the reaction conditions.40 On a 3.0 g scale, it provided α-aminophosphonate 4, a key intermediate in the synthesis of uPA inhibitor UAMC-00050, in 42% isolated yield. Y(OTf)3 is compatible with various functional groups, such as Boc, cyano, hydroxy, methylthio, and nitro group, and is suitable for the Birum–Oleksyszyn reaction under very mild conditions. On the contrary to the conditions using acetic acid, application of Y(OTf)3 as a catalyst allows to conduct the synthesis of α-aminophosphonates with acid-labile substrates. The new methodology opens up the opportunity for a more green,41 and cost-effective synthesis of UAMC-00050 or other analogous α-aminophosphonates and is suitable for further upscale.Conflicts of interest
“There are no conflicts to declare”.Acknowledgements
This project has received funding from the European Union's Horizon 2020 research and innovation program under the Marie Skłodowska-Curie Initial Training Network (ITN) “IT-DED3” (H2020-MSCA-ITN-2017) grant agreement No 765608.Notes and references
- M. R. Nadiveedhi, P. Nuthalapati, M. Gundluru, M. R. Yanamula, S. V. Kallimakula, V. R. Pasupuleti, V. K. R. Avula, S. Vallela, G. V. Zyryanov, S. K. Balam and S. R. Cirandur, ACS Omega, 2021, 6, 2934–2948 CrossRef CAS PubMed.
- P. Kafarski, B. Lejczak, R. Tyka, L. Koba, E. Pliszczak and P. Wieczorek, J. Plant Growth Regul., 1995, 14, 199–203 CrossRef CAS.
- C. Moreno-Cinos, E. Sassetti, I. G. Salado, G. Witt, S. Benramdane, L. Reinhardt, C. D. Cruz, J. Joossens, P. Van der Veken, H. Brötz-Oesterhelt, P. Tammela, M. Winterhalter, P. Gribbon, B. Windshügel and K. Augustyns, J. Med. Chem., 2019, 62, 774–797 CrossRef CAS PubMed.
- A. A. Agbowuro, J. Hwang, E. Peel, R. Mazraani, A. Springwald, J. W. Marsh, L. McCaughey, A. B. Gamble, W. M. Huston and J. D. A. Tyndall, Bioorg. Med. Chem., 2019, 27, 4185–4199 CrossRef CAS PubMed.
- M. Skoreński, A. Milewska, K. Pyrć, M. Sieńczyk and J. Oleksyszyn, J. Enzyme Inhib. Med. Chem., 2019, 34, 8–14 CrossRef.
- X. Rao, Z. Song and L. He, Heteroat. Chem., 2008, 19, 512–516 CrossRef CAS.
- A.-C. Schulz-Fincke, M. Blaut, A. Braune and M. Gütschow, ACS Med. Chem. Lett., 2018, 9, 345–350 CrossRef CAS PubMed.
- P. Kafarski, RSC Adv., 2020, 10, 25898–25910 RSC.
- E. K. Fields, J. Am. Chem. Soc., 1952, 74, 1528–1531 CrossRef CAS.
- M. R. Saidi and N. Azizi, Synlett, 2002, 8, 1347–1349 CrossRef.
- B. C. Ranu, A. Hajra and U. Jana, Org. Lett., 1999, 1, 1141–1143 CrossRef CAS.
- B. R. P. Reddy, P. V. G. Reddy and B. N. Reddy, New J. Chem., 2015, 39, 9605–9610 RSC.
- C. Qian and T. Huang, J. Org. Chem., 1998, 63, 4125–4128 CrossRef CAS.
- H.-J. Ha and G.-S. Nam, Synth. Commun., 1992, 22, 1143–1148 CrossRef CAS.
- S. Bhagat and A. K. Chakraborti, J. Org. Chem., 2007, 72, 1263–1270 CrossRef CAS PubMed.
- H. Firouzabadi, Synthesis, 2004, 16, 2692–2696 CrossRef.
- G. H. Birum, J. Org. Chem., 1974, 39, 209–213 CrossRef.
- P. V. der Veken, I. E. Sayed, J. Joossens, C. V. Stevens, K. Augustyns and A. Haemers, Synthesis, 2005, 4, 634–638 Search PubMed.
- J. Joossens, O. M. Ali, I. El-Sayed, G. Surpateanu, P. Van der Veken, A.-M. Lambeir, B. Setyono-Han, J. A. Foekens, A. Schneider, W. Schmalix, A. Haemers and K. Augustyns, J. Med. Chem., 2007, 50, 6638–6646 CrossRef CAS PubMed.
- J. Joossens, P. Van der Veken, G. Surpateanu, A.-M. Lambeir, I. El-Sayed, O. M. Ali, K. Augustyns and A. Haemers, J. Med. Chem., 2006, 49, 5785–5793 CrossRef CAS PubMed.
- C. Joossen, A. Baán, C. Moreno-Cinos, J. Joossens, N. Cools, E. Lanckacker, L. Moons, K. Lemmens, A.-M. Lambeir, E. Fransen, P. Delputte, G. Caljon, P. Van Der Veken, L. Maes, I. De Meester, F. Kiekens, K. Augustyns and P. Cos, Sci. Rep., 2020, 10, 17268 CrossRef CAS PubMed.
- F. Blasi and N. Sidenius, FEBS Lett., 2010, 584, 1923–1930 CrossRef CAS PubMed.
- H. Lin, L. Xu, S. Yu, W. Hong, M. Huang and P. Xu, Exp. Mol. Med., 2020, 52, 367–379 CrossRef CAS PubMed.
- M. Banys-Paluchowski, I. Witzel, B. Aktas, P. A. Fasching, A. Hartkopf, W. Janni, S. Kasimir-Bauer, K. Pantel, G. Schön, B. Rack, S. Riethdorf, E.-F. Solomayer, T. Fehm and V. Müller, Sci. Rep., 2019, 9, 2318 CrossRef PubMed.
- M. Cammalleri, M. Dal Monte, V. Pavone, M. De Rosa, D. Rusciano and P. Bagnoli, Cells, 2019, 8, 925 CrossRef CAS PubMed.
- H. Ceuleers, N. Hanning, J. Heirbaut, S. Van Remoortel, J. Joossens, P. Van Der Veken, S. M. Francque, M. De Bruyn, A.-M. Lambeir, J. G. De Man, J.-P. Timmermans, K. Augustyns, I. De Meester and B. Y. De Winter, Br. J. Pharmacol., 2018, 175, 3516–3533 CrossRef CAS PubMed.
- IT-DED3 Integrated Training in Dry Eye Disease Drug Development | Integrated Training in Dry Eye Disease Drug Development, University of Antwerp, https://www.uantwerpen.be/en/projects/dry-eye-disease-drug-development/, accessed 2 August 2021 Search PubMed.
- F. Xu, Y. Luo, J. Wu, Q. Shen and H. Chen, Heteroat. Chem., 2006, 17, 389–392 CrossRef CAS.
- M. E. Dmitriev and V. V. Ragulin, Tetrahedron Lett., 2012, 53, 1634–1636 CrossRef CAS.
- M. E. Dmitriev, S. R. Golovash, A. V. Borodachev and V. V. Ragulin, J. Org. Chem., 2021, 86, 593–600 CrossRef CAS PubMed.
- P. Kokkala, T. Rajeshkumar, A. Mpakali, E. Stratikos, K. D. Vogiatzis and D. Georgiadis, Org. Lett., 2021, 23, 1726–1730 CrossRef CAS PubMed.
- Q. Hu, V. M. Jayasinghe-Arachchige, J. Zuchniarz and R. Prabhakar, Front. Chem., 2019, 7, 195 CrossRef CAS PubMed.
- Y. T. Reddy, P. N. Reddy, B. S. Kumar, P. Rajput, N. Sreenivasulu and B. Rajitha, Phosphorus, Sulfur Silicon Relat. Elem., 2007, 182, 161–165 CrossRef CAS.
- A. S. Paraskar and A. Sudalai, Arkivoc, 2006, 10, 183–189 Search PubMed.
- Z. Rezaei, H. Firouzabadi, N. Iranpoor, A. Ghaderi, M. R. Jafari, A. A. Jafari and H. R. Zare, Eur. J. Med. Chem., 2009, 44, 4266–4275 CrossRef CAS PubMed.
- S. Kobayashi, S. Nagayama and T. Busujima, J. Am. Chem. Soc., 1998, 120, 8287–8288 CrossRef CAS.
- S. Kobayashi and I. Hachiya, J. Org. Chem., 1994, 59, 3590–3596 CrossRef CAS.
- R. A. Torres, F. Himo, T. C. Bruice, L. Noodleman and T. Lovell, J. Am. Chem. Soc., 2003, 125, 9861–9867 CrossRef CAS PubMed.
- B. Veeranjaneyulu and B. Das, Synth. Commun., 2017, 47, 449–456 CrossRef CAS.
- A. A. Mahamoud, E. Lyautey, C. Bonnineau, A. Dabrin and S. Pesce, Front. Microbiol., 2018, 9, 1852 CrossRef PubMed.
- P. Y. Cardon, G. Triffault-Bouchet, A. Caron, M. Rosabal, C. Fortin and M. Amyot, ACS Omega, 2019, 4, 13747–13755 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra07718j |
| This journal is © The Royal Society of Chemistry 2021 |