Open Access Article
Open Access ArticleEffects of urease inhibitors on enzymatic activities and fungal communities during the biosolids composting
Jishao Jiang *,
Yang Wang,
Dou Yu,
Jingyu Li,
Jin Han,
Huilin Cui,
Ronghui Cheng,
Xing Yao,
Guangxuan Yan,
Yunbei Li and
Guifen Zhu
*,
Yang Wang,
Dou Yu,
Jingyu Li,
Jin Han,
Huilin Cui,
Ronghui Cheng,
Xing Yao,
Guangxuan Yan,
Yunbei Li and
Guifen Zhu
School of Environment, Henan Normal University, Key Laboratory for Yellow River and Huai River Water Environmental and Pollution Control, Ministry of Education, Henan Key Laboratory for Environmental Pollution Control, Xinxiang, Henan 453007, PR China. E-mail: jiangjishao@163.com
First published on 23rd November 2021
Abstract
This study evaluated the influences of urease inhibitors (UIs) on nitrogen conversion, enzyme activities, and fungal communities during aerobic composting. Results showed that UI addition reduced NH3 emissions by 22.2% and 21.5% and increased the total nitrogen (TN) content by 9.7% and 14.3% for the U1 (0.5% UI of the dry weight of the mixture) and U2 (1% UI of the dry weight of the mixture) treatments, respectively. The addition of UI inhibited the enzyme activity during thermophilic stage while increased enzyme activity during the cool and maturity stages. Ascomycota, Basidiomycota and unclassified fungi were the main phyla, and Ascomycota increased significantly during the maturity period. Network analysis showed that Aspergillus, Penicillium, Trichoderma, Talaromyces, Peseudeurotium, and Exophiala were the main “connecting” genera. The redundancy analysis (RDA) showed that the fungal community was mainly influenced by temperature, DOC, pH, and urease. The results suggested that UI was an effective additive for nitrogen conservation and the increase of enzyme activity reduce nitrogen loss and promote enzyme activity during biosolids composting.
1. Introduction
Composting is the biotransformation process mediated by microbial extracellular enzymes, which play critical roles in organic matter degradation, substances transformation, and energy flow during the composting.1 Hydrolytic and oxidation–reduction enzymes are the main enzymes during the whole composting process. Hydrolytic enzymes are critical components in the initial phase, while oxidation–reduction enzymes become important later in the process. During the earlier stage, the hydrolytic enzymes, consisting of cellulase (CL), neutral protease (NPT), urease (UE),2,3 and sucrase, decomposed the organic matter of the mixture and enabled the conversion of carbon (C) and nitrogen (N) into small molecules, such as carbohydrates, amino acids, and organic acids, which supplied enough nutrient substance for microbial growth during later stage.4,5 Unfortunately, large amounts of gases are emitted when hydrolytic enzymes decompose organic matter, such as NH3, CO2, nitrous oxide, and methane.6,7 These gas emissions not only lead to secondary pollution but also result in the loss of nutrient and reduce the compost quality.8 Therefore, hydrolytic enzymes should be reasonably regulated to effectively reduce gas emissions and nutrient loss.Urease inhibitors (UIs) are usually used for slowing down urea hydrolysis, and reducing NH3 volatilization of in farm production, thus more available NH3/NH4-N be reserved for crop growth.9 In recent years, based on the experiences in agriculture, UIs have been used during composting. Hagenkamp-Korth et al.10 reported that three UIs, phenyl phosphorodiamidate, cyclohexylphosphoric triamide and N-(n-butyl) thiosphosphoric triamide (NBPT), could inhibit NH3 volatilization and retain more NH4-N of the end compost during the composting of pia manure and cornstalk. This result corroborates the result found by Yin et al.,11 who studied chicken manure and mushroom residue composting. UI addition could affect not only UI activity but also the activity of other hydrolytic enzymes and regulate subsequent substance transformations, ultimately improving compost quality during the composting process. However, few studies were conducted the effects of UIs on hydrolytic enzymes during composting process.
Fungi are the main decomposers of organic C and N, and the evolution of fungal community diversity and abundance during composting is closely related to enzyme activity.12 Therefore, the main purpose of this study was to investigated explore the influences of the addition of UI on enzyme activities and fungal composition during composting process. Furthermore, the correlation analysis among physicochemical indexes, hydrolytic enzyme activity, and fungal composition was also determined.
2. Materials and methods
2.1 Raw materials and the additive
Fresh biosolids and sawdust, as raw materials, were all obtained from a Xinxiang sewage disposal works and a furniture factory, China. The UI used in this study was NBPT (C4H14N3PS), which was purchased on the Taobao platform (https://www.taobao.com/). The basic properties of the biosolids and sawdust were shown in Table 1.| Raw material | pH | Moisture (%) | TOC (g kg−1) | TN (g kg−1) | C/N |
|---|---|---|---|---|---|
| a The values are the mean of three measurement replicates ± standard deviation. | |||||
| Biosolids | 7.12 ± 0.04 | 81.0 ± 0.23 | 286.1 | 43.3 | 6.61 |
| Saw dust | 7.52 ± 0.06 | 8.38 ± 0.04 | 625.6 | 6.80 | 92.0 |
2.2 Composting design and sample collection
Composting was conducted in three separate but identical reactors with an active volume of 14 L for 20 days. The mixed ratio of fresh biosolids and sawdust for the three treatments was 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in order to obtain appropriate the C/N (∼25) and the moisture content (∼55–65%) of the mixture. U1 and U2 are designated as the addition of UI with contents of 0.3% and 0.6% of the mixture, respectively (with dry weight basis). The CK treatment was originally mixed without the addition of UI. Because of the small volume of the composting pile, the three reactors were heated through water bath to maintain heat, and the temperature of the water bath was given 1–2 °C lower than the minimum temperature of three reactors.13 Set the speed of the air pumps for ventilation at the bottom of the reactor to 0.4 L min−1. Temperature sensors were embedded and placed at a height of 25 cm. Three homogeneous samples were collected by hands on 0, 4, 8, 12, 16 and 20 days, and divide each one into two parts. One portion was placed at 4 °C, and the other was air-dried and mashed for the total organic carbon (TOC) and total nitrogen (TN) analysis. In addition, the samples on 0, 4, 12 and 20 days were frozen at −80 °C for molecular experiments.
1 in order to obtain appropriate the C/N (∼25) and the moisture content (∼55–65%) of the mixture. U1 and U2 are designated as the addition of UI with contents of 0.3% and 0.6% of the mixture, respectively (with dry weight basis). The CK treatment was originally mixed without the addition of UI. Because of the small volume of the composting pile, the three reactors were heated through water bath to maintain heat, and the temperature of the water bath was given 1–2 °C lower than the minimum temperature of three reactors.13 Set the speed of the air pumps for ventilation at the bottom of the reactor to 0.4 L min−1. Temperature sensors were embedded and placed at a height of 25 cm. Three homogeneous samples were collected by hands on 0, 4, 8, 12, 16 and 20 days, and divide each one into two parts. One portion was placed at 4 °C, and the other was air-dried and mashed for the total organic carbon (TOC) and total nitrogen (TN) analysis. In addition, the samples on 0, 4, 12 and 20 days were frozen at −80 °C for molecular experiments.
2.3 Physical–chemical and enzymes activity analyses
The pH and the contents of ammonia N (NH4-N), nitrate N (NO3-N), nitrite N (NO2-N), and dissolved organic carbon (DOC) of the compost were determined using the methods described previously.14 TOC and TN were determined using the colorimetry method by automatic elemental analysis (Elemental Vario EL, Germany). NH3 was absorbed in a solution of boric acid and then determined by titration with HCl. The concentration of CO2 was measured by gas chromatography in 24 h after sampled with the 50 mL injection syringe.The activity of CL was determined by the anthranone colorimetric method. UR activity was determined by the indophenol blue colorimetric method. NPT was determined by spectrophotometry to express the amount of tyrosine released by the hydrolysis of casein.
2.4 Microbial analysis
DNA of the fungal community was extracted from 0.15 g of each sample using the MoBio PowerSoil™ DNA Isolation Kit (MoBio, USA), according to the manufacturer's instructions. 18S rRNA gene sequencing was performed using the Illumina MiSeq paired-end 300 bp protocol. The ITS region was amplified using the ITS1F/ITS2 primer.15 QIIME 1.7.0 was used for quality optimization of raw sequences, and the quality filtered sequences were analysed at 97% similarity to cluster into operational taxonomic units (OTUs). The details of primers for present genome amplification and data processing are already provided in our previously published paper.62.5 Data analysis
Principal component analysis (PCA) was performed using CANOCO 5.0 to identify the variation in the activity of the 3 hydrolytic enzymes among the different sampling stages. Correlation analyses between NH3, CO2 and physical–chemical indexes were conducted using SPSS 22 (SPSS Inc., USA). Network analysis of the fungal compositions at the genus level based on Pearson's correlation coefficients using Cytoscope 3.7.1. The relationships among the first 15 genera, enzyme activity and environmental indexes was performed by a heat map illustrator (HemI-1.0).3. Results and discussion
3.1 Temperature, pH, DOC and TOC
Temperature is a key parameter that influences microbial activity and composting efficiency.16 On day 3, the maximum if the pile temperature of CK, U1 and U2 were 56.4, 54.0, and 54.9 °C, respectively (Fig. 1a). During the thermophilic phase, the average temperatures for the U1 (52.2 °C) and U2 treatments (53.2 °C) were lower than that in the CK (54.1 °C), which was mainly due to that UI addition inhibited microbial activity and slowed down the rise in temperature. After that, it gradually dropped and reached room temperature on day 16.As shown in Fig. 1b, the pH value of all treatments expanded within the first 8 days and reached maxima of 8.45, 8.40 and 8.41, respectively. The initial rise in pH might be caused by organic nitrogen hydrolysis and decomposed NH4-N.17 Then, the pH in the later composting process decreased, which may be ascribed to NH3 volatilization.18 During days 16–20, there was a small increase in pH in all treatments. Chen et al.19 reported a similar phenomenon in composting using vegetable waste and chicken manure mixed with wheat straw and corn stalk, which was mainly due to unresolved organic nitrogen of prophase continuing to decompose.
The DOC contents of the three treatments sharply expanded during the first 4 days of composting (Fig. 1c), which was largely caused by the rapid degradation of organic matter.20 The DOC content for CK, U1, and U2 treatments reached maximum values on day 4, with values of 34.09, 29.45 and 31.06 g kg−1, respectively. The peak values for the U1 and U2 treatments were lower than that in CK due to that UI inhibited the decomposition of TOC (Fig. 1d). Then, the DOC contents for all treatments began to decrease until the end of the experiment. However, the DOC contents in the U2 treatment during days 8–20 were higher than those in the CK. The reason may be that with the progress of composting, the inhibitory effect of UI is weakened or eliminated due to its own decomposition in the early stage, and the unresolved TOC continues to decompose to produce DOC.21
TOC gradually decreased in all treatments during the whole composting process (Fig. 1d), probably due to mineralization of the substrate and loss of C in the exemplar of CO2. The degradation rates of TOC during the first 8 days for the three treatments were 28.5%, 22.2%, 21.5%, respectively. Throughout the experiment, the degradation rates of the CK, U1 and U2 treatments were 34.43%, 34.94% and 37.28%, respectively. In addition, a kinetic degradation model was also conducted to evaluate the degradation rate of TOC during the whole process. The kinetic degradation constants for the CK, U1, and U2 treatments were 0.0181, 0.0205 and 0.0225 d−1, respectively, which also fell within the range of 0.005–0.37 d−1. The degradation rate of organic matter treated with U1 and U2 satisfied the first-order degradation kinetic model (R2 = 0.96). The degradation rate of organic matter treated with U1 and U2 met the first-order degradation kinetic model (R2 = 0.97), but the degradation rate of organic matter treated with CK did not meet the first-order kinetic model (R2 = 0.78).22 This phenomenon indicated that UI only inhibits the degradation of TOC during the early stage of composting and promotes TOC degradation throughout the entire process.
3.2 NH4-N, NO2-N, NO3-N and TN
Typically, NH4-N is positively correlated with NH3 emissions,23 and its concentration influences N conversion during composting.24 NH4-N in the three treatments drastically expanded during the top 4 days (Fig. 2a), possibly owing to the conversion of organic N to NH4-N via the ammonification process.25 The peak values for the CK, U1, and U2 treatments on day 4 were 1504.2, 1080.6 and 1209.8 mg kg−1, respectively. The peak values of U1 and U2 were 28.16% and 19.57% lower than that of CK, which may be because the UI content in U2 is higher than U1, which greatly inhibits the decomposition of organic nitrogen. Then, it gradually dropped until the end of the composting process as a result of nitrification. Until the end of composting, the NH4-N content of the three treatments was lower than the safety standard (<0.4 g kg−1). It is worth noting that the NH4-N contents in the U1 and U2 treatments were higher than those in the CK from 16–20 days, and the changes in DOC and TOC from 16–20 days (Fig. 1c and d) could support this phenomenon.N2O-N as the intermediate product of N conversion accumulates to a relatively small degree, which exists for a short time.26 N2O-N increased during the earliest 12 days and then dropped down until the end of composting (Fig. 2b), which was mainly due to rapid nitrification. Guo et al.27 also found a similar trend in compost using pig manure and straw as raw materials. During the first 8 days, the N2O-N content in U1 and U2 was lower than control (Fig. 2b) because the addition of UI reduced the NH4-N content (Fig. 2a), thereby reducing the substrate of nitrification.
As shown in Fig. 2c, the variation in NO3-N hold a low level at the early stage of composting, which was mainly because nitrification was limited by higher temperatures and pH values.26 After 12 days, the contents of NO3-N increased rapidly. Zeng et al.28 also reported that a similar phenomenon appeared during composting with straw, vegetables, bran and soil. At the end of composting, the NO3-N concentrations in the CK, U1 and U2 treatments were 187.6, 291.7 and 268.9 mg kg−1 on day 20, respectively. It also indicated that UI addition promoted nitrification and increased the NO3-N content of the end compost.
The TN concentrations in all three treatments decreased rapidly (Fig. 2d) due to NH3 emissions during thermophilic stage.29 The minimum values were all recorded on day 8, and the reduction rates for the CK, U1 and U2 treatments relative to the initial values were 28.5%, 22.2%, and 21.5%, respectively. Thereafter, the TN contents sharply increased due to the concentration effect of TOC. The TN contents of the end compost were 21.7, 23.8 and 24.8 g kg−1 in CK, U1, and U2, respectively, and TN contents of the end composting in U1 and U2 increased by 9.7% and 14.3%, respectively, relative to the control. These results indicated UI inhibited the ammonization of TN during thermophilic phase (Fig. 2a), reduced the release of NH3, and therefore increased the TN content during the entire period.
3.3 UE, NPT and CL activities
The change trend of UE in CK, U1, and U2 was the alike (Fig. 3a), with a first increase and succedent decrease. In addition, the trend of UE was in accordance with the variation in NH4-N (Fig. 2a). The UE activity in CK, U1 and U2 reached maxima of 1708.6, 1398.264 and 1230.65 μg NH4-N g−1 h−1, respectively, on day 4. The UE activity for the U1 and U2 treatments on day 4 reduced by 64.8% and 48.0% compared to control, which was probably due to the addition of UI. Then, the UE activity began to decline mainly because of the reduction in organic matter.As shown in Fig. 3b, the NPT activities of U1 and U2 during initial stage of composting were significantly smaller than control. With the progress of composting, the activity of NPT was the same as that of UE and CL, showing a trend of first increasing and then decreasing. After 12 days, the NPT activity in the two treatments with UI added was greater than that in the CK.
The variation of CL activity of the three treatments (Fig. 3c) were same, first gradually increased and then reduced. The increase in the early stage is due to the proliferation of microorganisms, which secrete more enzymes; the decrease in the later stage is due to the decrease in organic matter and the weakening of the growth of microorganisms. The activity of CL in U1 and U2 was higher than that in CK during the first 12 days, mainly because of the inhibition of UI for the growth of microorganisms, especially cellulolytic microorganisms.30 After 20 days, the CL activity of the treatment with added UI was higher than that of the CK, which was consistent with the change in the later TOC (Fig. 1d).
In general, UI not only inhibited the activity of UE but also inhibited the activity of CL and NPT during the thermophilic phase period, thus inhibiting the degradation of organic C and N (Fig. 1 and 2). In the late stage of composting, the enzyme activity was more active than control because the added UI was degraded and the inhibitory effect was reduced or eliminated.
Furthermore, PCA showed that the sampling time explained 96.7% of the variation in different enzyme activities (Fig. 3d), and it showed that the distances of 4 days and 20 days among the three treatments were large, indicating that the enzyme activity was significantly different at this time. Therefore, samples of 4 days and 20 days were selected for high-throughput sequencing of fungi to analyse the impact of adding UI on microbial evolution.
3.4 NH3 and CO2 emission
Organic N generates NH4-N and NH3 under the action of UE and NPT,4 NH3 emissions increased drastically after the start of the trial, and the increase lasted until day 12 of composting because of the rapid hydrolysis of organic N, the high temperature and the pH (Fig. 4a).31 After that, the release of NH3-N was kept at a low level. At the end of composting, the cumulative NH3-N in CK, U1, and U2 was 2727.2, 2216.7, and 2008.6 mg, respectively (Fig. 4b). Compared with CK, the U1 and U2 treatments significantly reduced NH3 emissions by 18.72% and 26.35%, respectively, during the composting process. There was a significant correlation between NH3 and NH4-N, with a correlation coefficient of 0.93 (P < 0.001) (Table 2). In addition, UE and CL were also significantly positively correlated with NH3, with correlation coefficients of 0.844 (P < 0.001) and 0.868 (P < 0.001), respectively. | ||
| Fig. 4 Variations of NH3-N (a), cumulative NH3-N (b), CO2 (c), and cumulative CO2 (d) during biosolids composting. | ||
| NH3 | CO2 | T | pH | DOC | TOC | NH4-N | NO2-N | NO3-N | TN | CL | NPT | UE | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Significant correlation.b Extremely significant correlation. | |||||||||||||
| NH3 | 1 | ||||||||||||
| CO2 | 0.493 | 1 | |||||||||||
| T | 0.511 | 0.459 | 1 | ||||||||||
| pH | 0.736b | 0.152 | −0.174 | 1 | |||||||||
| DOC | 0.908b | 0.48 | 0.728b | 0.453 | 1 | ||||||||
| TOC | −0.165 | 0.175 | 0.757b | −0.751b | 0.16 | 1 | |||||||
| NH4-N | 0.930b | 0.600a | 0.683a | 0.538 | 0.890b | 0.062 | 1 | ||||||
| NO2-N | 0.181 | 0.149 | −0.335 | 0.351 | 0.178 | −0.477 | 0.017 | 1 | |||||
| NO3-N | −0.074 | −0.138 | −0.723b | 0.463 | −0.425 | −0.803b | −0.193 | 0 | 1 | ||||
| TN | −0.666a | −0.627a | −0.735b | −0.162 | −0.856b | −0.381 | −0.701a | −0.319 | 0.682a | 1 | |||
| CL | 0.868b | 0.660a | 0.312 | 0.688a | 0.687a | −0.304 | 0.834b | 0.331 | 0.139 | −0.523 | 1 | ||
| NPT | 0.434 | 0.677a | 0.571 | 0.087 | 0.361 | 0.288 | 0.618a | −0.48 | 0.026 | −0.243 | 0.533 | 1 | |
| UE | 0.844b | 0.639a | 0.781b | 0.368 | 0.839b | 0.237 | 0.925b | −0.227 | −0.228 | −0.655a | 0.724a | 0.764b | 1 |
Organic C releases CO2 under the decomposition of CL, which is an important indicator for assessing enzyme activity during composting.13 The evolution of CO2 emissions in the three treatments is shown in Fig. 4c. The CO2 emission rates in CK, U1, and U2 raised sharply during the initial stage of composting and then gradually decreased over time. The maximum value was observed on the second day, and the maximum values were 41.46, 29.96 and 28.00 g d−1. During the entire composting process, the release of CO2 was mainly concentrated in the first 12 days, and its CO2 emissions accounted for 91.11%, 79.05% and 84.05% of the cumulative emissions, respectively. Hwang et al.32 found that CO2 emissions were mainly concentrated in the first 4 days. During the whole process, the cumulative emissions of CO2 were 251.4, 209.3 mg and 177.7 mg in the CK, U1 and U2 treatments, respectively (Fig. 4d). As shown in Table 2, CO2 was significantly positively correlated with CL, NPT, and UE, with correlation coefficients of 0.66 (P < 0.01), 0.68 (P < 0.01) and 0.64 (P < 0.01), respectively.
3.5 Fungal diversity and community composition
After quality filtering, 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 497–73
497–73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 602 reads per sample and a total of 1095 high-quality read clustered OTUs were obtained for data analysis. The layout of OTU numbers (Table 3) confirmed that among all the composting samples, the compost of day 4 had the highest numbers of ITS OTUs. This was mainly because the compost pile screened the microorganisms in the compost via the high temperature. The coverages of the 6 samples were all higher than 0.99, indicating that sufficient sequences of fungi were achieved. The Chao and Ace exponent of U1 and U2 on day 4 and 24 both lower than that of CK, indicating that the addition of UI could inhibit the activity of microorganisms. However, the Shannon index indicated that the fungal community diversity of U1 and U2 increased during the maturity stage.
602 reads per sample and a total of 1095 high-quality read clustered OTUs were obtained for data analysis. The layout of OTU numbers (Table 3) confirmed that among all the composting samples, the compost of day 4 had the highest numbers of ITS OTUs. This was mainly because the compost pile screened the microorganisms in the compost via the high temperature. The coverages of the 6 samples were all higher than 0.99, indicating that sufficient sequences of fungi were achieved. The Chao and Ace exponent of U1 and U2 on day 4 and 24 both lower than that of CK, indicating that the addition of UI could inhibit the activity of microorganisms. However, the Shannon index indicated that the fungal community diversity of U1 and U2 increased during the maturity stage.
| Sobs | Chao | Ace | Shannon | Simpson | Coverage | |
|---|---|---|---|---|---|---|
| CK (4) | 623.0 | 637.6 | 642.1 | 3.454 | 0.0912 | 0.9993 |
| U1 (4) | 568.0 | 603.3 | 599.9 | 2.905 | 0.1620 | 0.9991 |
| U2 (4) | 580.0 | 605.6 | 602.6 | 3.587 | 0.0834 | 0.9993 |
| CK (20) | 382.0 | 388.7 | 388.4 | 3.033 | 0.1595 | 0.9997 |
| U1 (20) | 184.0 | 185.5 | 185.9 | 3.634 | 0.0742 | 0.9999 |
| U2 (20) | 245.0 | 249.0 | 248.9 | 3.493 | 0.0833 | 0.9998 |
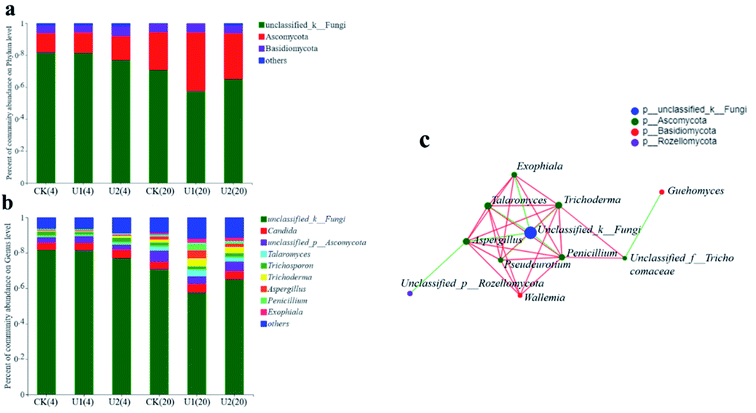
Fig. 5a shows that a majority of the OTUs were assigned to the phyla unclassified_k_Fungi, Ascomycota, and Basidiomycota, and the three dominant fungal phyla accounted for 4.19–81.73% of the total sequences. The results also reported by Jiang et al.6 for SS composting. From the thermophilic to maturity stages, the abundance of unclassified fungi gradually decreased for the three treatments, while the abundance of Ascomycota showed an increasing trend. On day 20, its abundance in the U1 (36.76%) and U2 (28.71%) treatments was apparently higher than that in the control (23.67%) (P < 0.001). Ascomycota as the important decomposers during composting, its abundance is advantageous for the organic matter degradation.33 Basidiomycota, another major phylum of the fungi, had a comparative abundance of 5.23, 5.65 and 4.79% for CK, U1 and U2 on day 20, respectively. Wang et al.34 also reported that the phylum Ascomycota and Basidiomycota could degrade lignin and cellulose during the composting process. This result also supported the changes in TOC (Fig. 1d), which showed that UI inhibits the degradation of TOC during the early stage of composting but promotes it during the later stage.
Fig. 5b showed the differences of the fungi succession at the genus level. The main fungal genera, Candida, Talaromyces, Trichoderma, Aspergillus, Penicillium, Exophiala and Pseudeurotium, belong to the Ascomycota phylum. The main fungal genera, Trichosporon, Guehomyces and Wallemia, belong to Basidiomycota. Fig. 5b also indicated greater diversity in the maturity phase than the high-temperature stage, especially in the U1 and U2 treatments, which was also supported by the changes in the Shannon and Simpson indexes (Table 3). Microorganisms usually form complex networks through neutral, positive and negative interactions. The network between species regulates the structure and function of ecological communities to a large extent. In order to evaluate the similarity of fungal communities in different treatments in different periods, the network diagram was operated. As shown in Fig. 5c, the Aspergillus, Penicillium, Trichoderma, Talaromyces, Peseudeurotium, and Exophiala genera were the main “connecting” nodes in the network in the present study. The first five genera all belong to the phylum Ascomycota. It was reported that Aspergillus could secrete the glycoside hydrolase, and had closely related to the degradation of lignocellulose, which was usually observed during the composting process.34 Mansour and Salem35 collected leguminous bark in different locations in Alexandria, Egypt, and found that Trichoderma often appeared on wood. In addition, some particular Trichoderma species in charge of the enzyme produced belong to Ascomycota, which can degrade various organic matter, not just lignin. Many studies have found that Thermomyces is the superior genus during the experiment process.6,36 Exophiala can degrade hydrophobic compounds and has been extensively studied in biological filtration systems for processing VOCs.37
3.6 Relationships among environmental factors, enzyme activity, and fungal composition
The relationships among the top 15 fungal genera, the 3 enzyme activities and 11 environmental factors is shown in Fig. 6. UE and pH were significantly correlated with 6 genera, while temperature and DOC were significantly related to 8 genera, which was more than other environmental factors. Temperature, DOC, UE, and pH all showed a significant negative correlation with Penicillium, Trichoderma, Talaromyces, and Peseudeurotium, which were the 4 main “connecting” nodes from the network analysis, as shown in Fig. 5c. In addition, temperature and DOC had an extremely significant positive correlation with unclassified_p_Rozellomycota (R2 = 0.938) and an extremely significant negative correlation with Aspergillus (R2 = −0.944). UE was significantly negatively correlated with Wallemia (R2 = −0.999) and Aspergillus (R2 = −0.928). pH had an tremendously significant positive correlation with unclassified_k_Fungi (R2 = −0.919) and an highly significant positive correlation with Exophiala (R2 = −0.941). Jiang et al.6 and Wang et al.38 also discovered that temperature and pH significantly affected the fungal community during composting. Almost all microbes, especially fungi, need DOC to supply nutrition for growth, thus need secrete a great deal of lignocellulolytic enzymes to degrade cellulose and lignin.38 The UE also played a main role and could catalyse urea into CO2 and NH4-N during the composting process, which are also substances used for the growth of fungi.39 Thus, temperature, DOC, pH, and UE mainly influenced the fungal succession during SS composting in the present study. | ||
| Fig. 6 Heat map among the environmental indexes, enzyme activities, and fungal compositions at the genera level. R is given in different colours. *0.01 < P < 0.05, **0.001 < P < 0.001, ***P < 0.001. | ||
4. Conclusions
UI could ameliorate the composting process and facilitate the degradation rate of TOC. Compared with the CK, the U1 and U2 treatments reduced NH3 emissions by 22.2% and 21.5% and increased the TN content of the end compost by 9.7% and 14.3%, respectively. Moreover, UI addition inhibited the activities of UE, CL and NPT during the high-temperature phase, while the activities significantly reduced during maturity stage. The HTS data indicated showed that UI significantly added the abundance of Ascomycota during the maturity period, which was beneficial for the degradation of TOC. The redundancy analysis showed that temperature, DOC, pH, and urease mainly affected the fungal composition during biosolids composting.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was funded by the National Natural Science Foundation of China (No. 41805123), the Program for Innovative Research Team in Science and Technology in University of Henan Province (No. 20IRTSTHN011), and the Science Fund for Distinguished Young Scholars of Henan Normal University (No. 2020JQ05).References
- M. Karwal and A. Kaushik, Co-composting and vermicomposting of coal fly-ash with press mud: changes in nutrients, micro-nutrients and enzyme activities, Environ. Technol. Innovation, 2020, 18, 100708 CrossRef.
- G. Zeng, M. Yu, Y. Chen, D. Huang, J. Zhang and H. Huang, et al., Effects of inoculation with Phanerochaete chrysosporium at various time points on enzyme activities during agricultural waste composting, Bioresour. Technol., 2010, 101(1), 222–227 CrossRef PubMed.
- G. Zhou, X. Qiu, J. Zhang and C. Tao, Effects of seaweed fertilizer on enzyme activities, metabolic characteristics, and bacterial communities during maize straw composting, Bioresour. Technol., 2019, 286, 121375 CrossRef PubMed.
- P. Castaldi, G. Garau and P. J. W. M. Melis, Maturity assessment of compost from municipal solid waste through the study of enzyme activities and water-soluble fractions, Waste Manage., 2008, 28(3), 534–540 CrossRef PubMed.
- C. Qiao, C. Ryan Penton, C. Liu, Z. Shen, Y. Ou and Z. Liu, et al., Key extracellular enzymes triggered high-efficiency composting associated with bacterial community succession, Bioresour. Technol., 2019, 288, 121576 CrossRef PubMed.
- J. Jiang, Y. Pan, X. Yang, J. Liu, H. Miao, Y. Ren, C. Zhang, G. Yan, J. Lv and Y. Li, Beneficial influences of pelelith and dicyandiamide on gaseous emissions and the fungal community during sewage sludge composting, Environ. Sci. Pollut. Res., 2019, 26(9), 8928–8938 CrossRef CAS PubMed.
- H. Mao, H. Zhang, Q. Fu, M. Zhong, R. Li and B. Zhai, et al., Effects of four additives in pig manure composting on greenhouse gas emission reduction and bacterial community change, Bioresour. Technol., 2019, 292, 121896 CrossRef CAS PubMed.
- Z. Han, D. Sun, H. Wang, R. Li, Z. Bao and F. Qi, Effects of ambient temperature and aeration frequency on emissions of ammonia and greenhouse gases from a sewage sludge aerobic composting plant, Bioresour. Technol., 2018, 270, 457–466 CrossRef CAS PubMed.
- S. H. Chien, L. I. Prochnow and H. Cantarella, Chapter 8 Recent Developments of Fertilizer Production and Use to Improve Nutrient Efficiency and Minimize Environmental Impacts, Adv. Agron., 2009, 102, 267–322 CAS.
- F. Hagenkamp-Korth, S. Ohl and E. Hartung, Effects on the biogas and methane production of cattle manure treated with urease inhibitor, Biomass Bioenergy, 2015, 75, 75–82 CrossRef CAS.
- Y. Yin, C. Yang, J. Tang, J. Gu, H. Li and M. Duan, et al., Bamboo charcoal enhances cellulase and urease activities during chicken manure composting: roles of the bacterial community and metabolic functions, J. Environ. Sci., 2021, 108, 84–95 CrossRef PubMed.
- Q. He, Y. Wu, H. Bing, J. Zhou and J. Wang, Vegetation type rather than climate modulates the variation in soil enzyme activities and stoichiometry in subalpine forests in the eastern Tibetan Plateau, Geoderma, 2020, 374, 114424 CrossRef CAS.
- L. Meng, W. Li, S. Zhang, C. Wu and L. Lv, Feasibility of co-composting of sewage sludge, spent mushroom substrate and wheat straw, Bioresour. Technol., 2017, 226, 39–45 CrossRef CAS PubMed.
- J. Jiang, K. Kang, C. Wang, X. Sun, S. Dang and N. Wang, et al., Evaluation of total greenhouse gas emissions during sewage sludge composting by the different dicyandiamide added forms: mixing, surface broadcasting, and their combination, Waste Manage., 2018, 81, 94–103 CrossRef CAS PubMed.
- D. B. Holman, X. Hao, E. Topp, H. E. Yang and T. W. Alexander, Effect of Co-Composting Cattle Manure with Construction and Demolition Waste on the Archaeal, Bacterial, and Fungal Microbiota, and on Antimicrobial Resistance Determinants, PLoS One, 2016, 11(6), e0157539 CrossRef PubMed.
- X. Zhao, X. He, B. Xi, R. Gao, W. Tan and H. Zhang, et al., The evolution of water extractable organic matter and its association with microbial community dynamics during municipal solid waste composting, Waste Manage., 2016, 56, 79–87 CrossRef CAS PubMed.
- Y. Li, W. Li, C. Wu and K. Wang, New insights into the interactions between carbon dioxide and ammonia emissions during sewage sludge composting, Bioresour. Technol., 2013, 136, 385–393 CrossRef PubMed.
- J. Pan, R. Li, L. Zhai, Z. Zhang, J. Ma and H. Liu, Influence of palygorskite addition on biosolids composting process enhancement, J. Cleaner Prod., 2019, 217, 371–379 CrossRef.
- M. Chen, C. Wang, B. Wang, X. Bai, H. Gao and Y. Huang, Enzymatic mechanism of organic nitrogen conversion and ammonia formation during vegetable waste composting using two amendments, Waste Manage., 2019, 95, 306–315 CrossRef PubMed.
- Z. Zeng, X. Guo, P. Xu, R. Xiao, D. Huang and X. Gong, et al., Responses of microbial carbon metabolism and function diversity induced by complex fungal enzymes in lignocellulosic waste composting, Sci. Total Environ., 2018, 643, 539–547 CrossRef PubMed.
- L. Zhang, Y. Zhu, J. Zhang, G. Zeng, H. Dong and W. Cao, et al., Impacts of iron oxide nanoparticles on organic matter degradation and microbial enzyme activities during agricultural waste composting, Waste Manage., 2019, 95, 289–297 CrossRef PubMed.
- C. W. Higgins and L. P. Walker, Validation of a new model for aerobic organic solids decomposition: simulations with substrate specific kinetics, Process Biochem., 2001, 36(8), 875–884 CrossRef.
- Q. Wang, M. K. Awasthi, X. Ren, J. Zhao, R. Li, Z. Wang, M. Wang, H. Chen and Z. Zhang, Combining biochar, zeolite and wood vinegar for composting of pig manure: The effect on greenhouse gas emission and nitrogen conservation, Waste Manage., 2018, 74, 221–230 CrossRef CAS PubMed.
- B. Lv, D. Zhang, Q. Chen and Y. Cui, Effects of earthworms on nitrogen transformation and the correspond genes (amoA and nirS) in vermicomposting of sewage sludge and rice straw, Bioresour. Technol., 2019, 287, 121428 CrossRef CAS PubMed.
- X. Meng, J. Yan, B. Zuo, Y. Wang, X. Yuan and Z. Cui, Full-scale of composting process of biogas residues from corn stover anaerobic digestion: physical-chemical, biology parameters and maturity indexes during whole process, Bioresour. Technol., 2020, 302, 122742 CrossRef CAS PubMed.
- J. Zheng, J. Liu, S. Han, Y. Wang and Y. Wei, N2O emission factors of full-scale animal manure windrow composting in cold and warm seasons, Bioresour. Technol., 2020, 316, 123905 CrossRef CAS PubMed.
- H. Guo, J. Gu, X. Wang, J. Yu, M. Nasir and K. Zhang, et al., Microbial driven reduction of N2O and NH3 emissions during composting: effects of bamboo charcoal and bamboo vinegar, J. Hazard. Mater., 2019, 390, 121292 CrossRef PubMed.
- G. Zeng, J. Zhang, Y. Chen, Z. Yu, M. Yu and H. Li, et al., Relative contributions of archaea and bacteria to microbial ammonia oxidation differ under different conditions during agricultural waste composting, Bioresour. Technol., 2011, 102(19), 9026–9032 CrossRef CAS PubMed.
- R. Wang, Y. Zhao, X. Xie, T. A. Mohamed, L. Zhu and Y. Tang, et al., Role of NH3 recycling on nitrogen fractions during sludge composting, Bioresour. Technol., 2020, 295, 122175 CrossRef CAS PubMed.
- Z. Xu, F. Zhang, L. Zhang and J. Li, Effects of Indigenous and Exogenous Microbial Inocula on Dynamic Changes of Enzyme Activities during Composting in a Bioreactor, Adv. Mater. Res., 2011, 383–390, 4017–4023 Search PubMed.
- J. Du, Y. Zhang, M. Qu, Y. Yin, K. Fan and B. Hu, et al., Effects of biochar on the microbial activity and community structure during sewage sludge composting, Bioresour. Technol., 2019, 272, 171–179 CrossRef CAS.
- H. Y. Hwang, S. H. Kim, M. S. Kim, S. J. Park and C. H. Lee, Co-composting of chicken manure with organic wastes: characterization of gases emissions and compost quality, Appl. Biol. Chem., 2020, 63, 3 CrossRef.
- M. Yu, J. Zhang, Y. Xu, H. Xiao, W. An and H. Xi, et al., Fungal community dynamics and driving factors during agricultural waste composting, Environ. Sci. Pollut. Res., 2015, 22(24), 19879–19886 CrossRef CAS PubMed.
- X. Wang, Z. Kong, Y. Wang, M. Wang, D. Liu and Q. Shen, Insights into the functionality of fungal community during the large scale aerobic co-composting process of swine manure and rice straw, J. Environ. Manage., 2020, 270, 110958 CrossRef CAS PubMed.
- M. M. A. Mansour and M. Z. M. Salem, Evaluation of wood treated with some natural extracts and Paraloid B-72 against the fungus Trichoderma harzianum: wood elemental composition, in vitro and application evidence, Int. Biodeterior. Biodegrad., 2015, 100, 62–69 CrossRef CAS.
- M. K. Awasthi, J. Li, S. Kumar, S. K. Awasthi, Q. Wang and H. Chen, et al., Effects of biochar amendment on bacterial and fungal diversity for co-composting of gelatin industry sludge mixed with organic fraction of municipal solid waste, Bioresour. Technol., 2017, 246, 214–223 CrossRef CAS PubMed.
- Q. Liu, M. Li, R. Chen, Z. Li, G. Qian and T. An, et al., Biofiltration treatment of odors from municipal solid waste treatment plants, Waste Manage., 2009, 29(7), 2051–2058 CrossRef CAS PubMed.
- K. Wang, X. Yin, H. Mao, C. Chu and Y. Tian, Changes in structure and function of fungal community in cow manure composting, Bioresour. Technol., 2018, 255, 123–130 CrossRef CAS PubMed.
- M. Nikaeen, A. H. Nafez, B. Bina, B. F. Nabavi and A. Hassanzadeh, Respiration and enzymatic activities as indicators of stabilization of sewage sludge composting, Waste Manage., 2015, 39, 104–110 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |