 Open Access Article
Open Access ArticleOxygen-enriched surface modification for improving the dispersion of iron oxide on a porous carbon surface and its application as carbon molecular sieves (CMS) for CO2/CH4 separation
Nur Indah Fajar Mukti abc,
Teguh Ariyantoac,
Wahyudi Budi Sediawana and
Imam Prasetyo
abc,
Teguh Ariyantoac,
Wahyudi Budi Sediawana and
Imam Prasetyo *ac
*ac
aDepartment of Chemical Engineering, Faculty of Engineering, Universitas Gadjah Mada, Yogyakarta, 55281, Indonesia. E-mail: imampras@ugm.ac.id
bDepartment of Chemical Engineering, Faculty of Industrial Technology, Universitas Islam Indonesia, Yogyakarta, 55584, Indonesia
cCarbon Material Research Group, Department of Chemical Engineering, Universitas Gadjah Mada, Yogyakarta, 55281, Indonesia
First published on 16th November 2021
Abstract
The separation of CO2/CH4 can be enhanced by impregnating porous carbon with iron oxide. Dispersion of iron oxide is one of the critical factors which supports the separation process performance. Iron oxide dispersion can be enhanced by enriching the oxygen functional groups on the carbon surface. This study investigates three distinct oxidation processes: oxidation with a 10% H2O2 solution, ozonation with distilled water, and ozonation with a 10% H2O2 solution. The research steps included the following: (i) oxidation, (ii) impregnation of iron oxide followed by calcination, (iii) material characterization, and (iv) material performance analysis. Materials were characterized using N2 sorption analysis, X-ray diffraction analysis (XRD), scanning electron microscopy-energy dispersive X-ray spectroscopy analysis (SEM-EDX), and Fourier transform infrared analysis (FT-IR). Iron oxide was well dispersed on the carbon surface, as evidenced by the elemental mapping of materials. In addition, the oxygen functional groups increased significantly in the range of 28.6–79.7% following the oxidation process, as indicated by the elemental component using SEM-EDX analysis. The impregnation of iron oxide on oxidized carbon ozonated with distilled water (COA–Fe) obtained a maximum CO2 uptake capacity of 3.0 mmol g−1 and CO2/CH4 selectivity increased by up to 190% at a temperature of 30 °C and pressure of 1 atm. Furthermore, the enhancement of CO2/CH4 separation up to 1.45 times was the best performance achieved by COA–Fe. Thus, improving iron oxide dispersion on oxidized carbon surfaces has a potential application in CO2/CH4 separation.
1 Introduction
Biogas is a renewable energy source that can be substituted for fossil fuels and natural gas. A typical biogas mixture contains 50–70% CH4 and 30–49% CO2.1,2 Carbon dioxide removal from CO2/CH4 gas mixtures is critical since it results in a more energy-dense product, due to the high calorific value of methane. In contrast, carbon dioxide has no heating value.3 A number of technologies are currently available to remove carbon dioxide, including absorption, membrane separation, and cryogenic separation.4,5 However, these technologies are energy-intensive and costly. Adsorption-based separation is considered promising for CO2/CH4 separation because it produces high-purity methane (>98 percent vol), is relatively inexpensive, easy to operate, and energy-efficient.6 Adsorption-based separation occurs due to the difference in affinity and diffusivity of CO2 and CH4.7 Several types of molecular sieves, including zeolite molecular sieve (ZMS),8,9 carbon molecular sieve (CMS),10–13 and metal–organic framework (MOF),14,15 are widely used for adsorption-based separation. Molecular sieves made from carbon are relatively stable long-term due to the low heat of adsorption and can be synthesized from a variety of materials, including coal,16,17 biomass,10,18 and polymer.12,19 As a consequence, it is simple to regenerate.CMS material derived from palm kernel shells (PKS) was investigated for CO2/CH4 separation. This material achieved separation ratios of up to 2.10 However, the adsorption capacity of CO2 is quite limited, which will require further investigation. Metal oxide impregnation may enhance carbon dioxide uptake.20 In contrast, metal oxide agglomerations are common due to the fact that carbon is non-polar and hydrophobic, whereas metals are polar and hydrophilic. To enhance dispersion, reduced metal oxide agglomeration is employed. In recent years, several authors have discussed the use of highly dispersed iron oxide for a wide variety of applications.21–23
The addition of oxygen groups to the surface chemistry of carbon can increase its hydrophilic character.24 As a consequence, it becomes wettable, and metal oxide dispersion is enhanced. Oxygen-enriched surfaces have been widely used in a variety of modification processes, including gaseous or aqueous oxidation, ozonation, and gamma irradiation, etc. Aqueous oxidation enhanced carboxylic acid functional groups significantly, whereas gaseous oxidation enhanced carbonyl and hydroxyl functional groups significantly. While ozonation increases the number of acid surface groups, it alters the surface area and porosity.25
In this study, porous carbon impregnated with iron oxide was utilized to fabricate a molecular sieve for CO2/CH4 separation. Palm kernel shell biomass was a precursor to porous carbon. Three different oxidation processes were evaluated in order to increase the hydrophilicity of carbon: oxidation with 10% H2O2, ozonation with distilled water, and ozonation with 10% H2O2 solution. In the CMS preparation, the oxidation processes are followed by iron oxide impregnation and calcination. An investigation of the effect of three different oxidation processes on the dispersion of iron oxide impregnated porous carbon on separation performance is conducted, which has not been previously described. In this study, the characterization of materials is extended to ensure the feasibility of impregnation, adsorption isotherms, and breakthrough separation for the purpose of separation.
2 Experimental
2.1. Materials
Porous carbon from palm kernel shell (20–25 mesh) was obtained from PT Home System Indonesia. Hydrogen peroxide with a purity of 50% from PT Indonesia Inti Pratama. Iron(III) nitrate nonahydrate for analysis EMSURE® ACS, ISO, Reag. Ph Eur from Merck. Nitrogen with a purity of 99.95% was used as inert gas in the calcination process. Methanol for analysis EMSURE® ACS, ISO, Reag. Ph Eur from Merck as a solvent for metal impregnation. CO2 and CH4 with a purity of 99.9% were obtained from PT. Aneka Gas Industri Indonesia as adsorbate. A mixed gas CH4/CO2 (55/45% v/v) as a biogas representative.2.2. Preparation of oxidized carbon
Oxidized carbon was obtained by oxidizing porous carbon with H2O2 (10% v/v), ozonating it with distilled water, and ozonating it with an H2O2 (10% v/v) solution. To oxidize a porous carbon with a 10% H2O2 solution, a 15 g porous carbon was mixed with 150 mL 10% H2O2 solution at room temperature for two hours, followed by filtering and heating to 60 °C for 24 hours. As part of the ozonation process, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (w/v) mixture of porous carbon and distilled water was placed in the ozone contact reactor (IONTECH QLA-3G ozone generator). Ozone gas was fed into the system at a flow rate of 3000 mg h−1 and a power output of 60 watts for 120 minutes. The ozone–H2O2 reaction was initiated by substituting distilled water for a 10% H2O2 solution.
10 (w/v) mixture of porous carbon and distilled water was placed in the ozone contact reactor (IONTECH QLA-3G ozone generator). Ozone gas was fed into the system at a flow rate of 3000 mg h−1 and a power output of 60 watts for 120 minutes. The ozone–H2O2 reaction was initiated by substituting distilled water for a 10% H2O2 solution.
2.3. Impregnation of iron oxide
The iron oxide was dispersed on porous carbon by an incipient wetness method, followed by calcination. At first, pristine 15 g carbon was degassed at a temperature of 150 °C for 2 hours in order to clean the pores. Carbon pores were then slowly filled with a salt solution of 5.4 g iron(III) nitrate nonahydrate in 12 mL methanol. The sample was heated under nitrogen flow for 6 hours at 500 °C during the calcination process. Iron as iron oxide was targeted at 5% by weight. The procedure was also applied to oxidized carbon. A schematic of the CMS preparation is presented in Fig. 1.2.4. CMS characterization
Fourier transforms infrared (FTIR) analysis was carried out for the functional group's characterization of the material by using Nicolet Avatar 360 IR. In this study, samples were analyzed at a wavelength of 400–4000 cm−1. The morphology of the CMSs was characterized by SEM-EDX instrument using JEOL JSM-6510 LA at a voltage of 10 kV. The structure of crystalline materials was characterized by XRD using Bruker D2 Phaser. The diffractogram was obtained by using Cu-Kα radiation (λ = 1.5406 Å) in the range 10° < 2θ < 90°.2.5. Adsorption isotherm measurement
The adsorption capacity of CO2 and CH4 was measured by the volumetric method from 0 to 1.2 atm at a temperature of 30 °C. An ultrahigh vacuum adsorption apparatus rig was constructed using Swagelok® VCR valves and fittings. First, CMS samples were degassed for 6 hours at 150 degrees celsius until a static pressure of at least 0.01 torr was reached. These results are presented as an adsorption isotherm curve. The schematic diagram and procedure for measuring adsorption isotherms have been published in the literature.262.6. Separation performance with a breakthrough analysis
The composition of mixed gases (CH4 and CO2) was determined using a portable gas analyzer (Biogas Analyzer Gas Board 3200plus, Hubei Cubic-Ruiyi Instrument Co., Ltd.). The material was first placed in the column with fixed bed dimensions (D = 9.5 mm and L = 300 mm), followed by a 200 mL min−1 nitrogen flush until no gas content of CH4, CO2, or O2 could be detected on the gas detector. The mixed gas mixture, comprised of CH4 and CO2, was then introduced into the system at a flow rate of 50 mL min−1 at room temperature and 1.2 bar pressure. The separation cycle was continued until the gas composition returned to the initial concentrations of the gas inlet.133 Results and discussion
3.1. CMS characteristics
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching)27–30 and C–O group (stretching), respectively.29 It is remarkable that there is an increase in the intensity of the O–H stretching, C
O stretching)27–30 and C–O group (stretching), respectively.29 It is remarkable that there is an increase in the intensity of the O–H stretching, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching, and C–H stretching when compared to pristine carbon. As a result, the oxygen groups on all oxidized carbons increase.
O stretching, and C–H stretching when compared to pristine carbon. As a result, the oxygen groups on all oxidized carbons increase.
As shown in Fig. 2(b), the FTIR spectrum and absorption bands of iron oxide impregnated porous carbon. There is an addition at the peak of 590 cm−1 in comparison to the pristine and oxidized carbon prior to iron oxide impregnation. This peak is thought to be caused by Fe–O bonds. A peak at 576.3 cm−1 was also observed in a composite of activated carbon and iron oxide (Fe3O4).31
SEM images of each material reveal the oxidized carbon's morphological structure (CH, COA, and COH). Fig. 3 illustrates the results of SEM images of C (a), CH (b), COA (c), and COH. SEM images reveal an increase in the size of oxidized carbon pores.25 This increase has been modified by either oxidation treatment using 10% H2O2 solution, ozonation using distilled water, or ozonation using 10% H2O2 solution. According to the SEM images, the pore size of the carbon can become irregular as a result of the ozonation process, whether using distilled water (COA) or a 10% H2O2 solution. In comparison to carbon, which is oxidized with a 10% H2O2 solution (CH). Despite the increased size of the CH pore cavities, the carbon structure remains regular.
The composition of C and O was determined via SEM-EDX analysis. Table 1 summarizes the percentage increase in oxygen groups for CH, COA, and CH. It can be concluded that each treatment increases the oxygen groups in the following order: COA > COH > CH.
| Oxidation method | Sample | Element (%) | Addition of oxygen group (%) | |
|---|---|---|---|---|
| C | O | |||
| Pristine carbon | C | 93.06 | 6.94 | — |
| Oxidation using 10% H2O2 solution | CH | 91.07 | 8.93 | 28.67 |
| Ozonation using distilled water | COA | 87.77 | 12.33 | 79.74 |
| Ozonation using 10% H2O2 solution | COH | 89.95 | 10.05 | 38.43 |
The SEM micrographs of C–Fe (a), CH–Fe (b), COA–Fe (c), and COH–Fe (d), as well as the elemental mapping of iron oxide on C–Fe (e), CH–Fe (f), COA–Fe (g), and COH–Fe (h), are shown in Fig. 4. Based on Fig. 4(a–d), it can be seen that there are some small aggregates of iron oxide visible from the lateral view of the CMS. These aggregates are brighter in colour and are supported on the darker surface of the porous carbon.
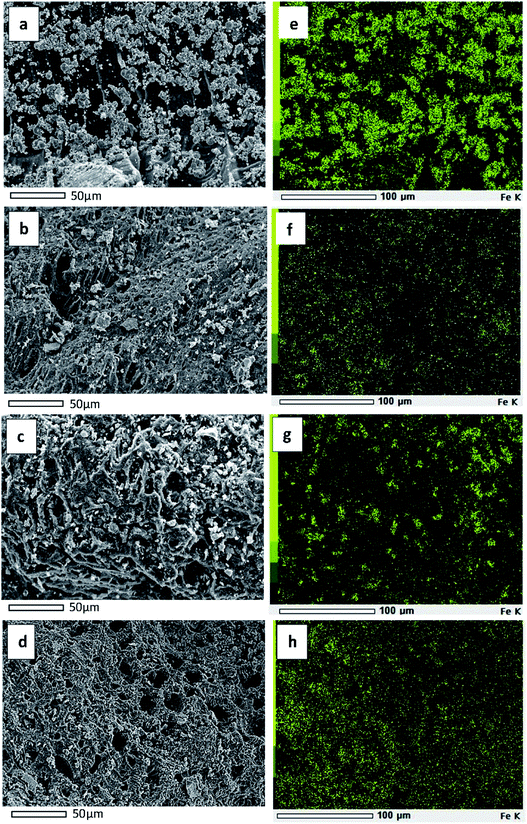 | ||
| Fig. 4 SEM images of C–Fe (a), CH–Fe (b), COA–Fe (c), COH–Fe (d) and their elemental mapping of Fe on C–Fe (e), CH–Fe (f), COA–Fe (g), COH–Fe (h). | ||
As shown in Fig. 4(a) and its elemental mapping in Fig. 4(e), an agglomeration of iron oxide exists on the pristine carbon surface. This demonstrates that the iron salt solution is incapable of spreading uniformly across the pristine carbon surface, resulting in the formation of iron oxide agglomerates. This is because the iron salt solution is unable to wet the surface of the pristine carbon due to its hydrophobic nature. While iron oxide is widely dispersed on the surface of oxidized carbon, as illustrated in Fig. 4(b–d) and as indicated by the elemental mapping in Fig. 4(f–h). This indicates that the addition of oxygen functional groups increases the wettability of carbon while decreasing its hydrophobicity. The composition of iron oxide impregnated porous carbon was determined via SEM-EDX analysis. The results are presented in Table 2.
| Sample | Element (%) | ||
|---|---|---|---|
| C | O | Fe | |
| C–Fe | 83.47 | 10.57 | 5.96 |
| CH–Fe | 80.97 | 13.78 | 5.25 |
| COA–Fe | 73.23 | 20.96 | 5.81 |
| COH–Fe | 79.71 | 14.79 | 5.50 |
An estimation of the magnetite Fe3O4 crystallite size was performed using Debye–Scherrer's equation. The mean crystallite size calculated from the main diffraction peak (311) around 35.9° was listed in Table 3.
| Sample | Crystallite size, nm |
|---|---|
| C–Fe | 18.34 |
| CH–Fe | 9.65 |
| COA–Fe | 16.93 |
| COH–Fe | 14.57 |
Fig. 6(b) shows a nitrogen sorption isotherm for oxidized carbon impregnated with iron oxide. As can be seen from the isotherm, the CMS also has a type I isotherm. Interestingly, COA–Fe has a narrow and sharp pore size distribution compared to other materials, which is advantageous for CMS applications.
Table 4 summarizes the specific surface area (SSA), micropore surface area (Smic), total pore volume (V), micropore volume (Vmic), and mean pore diameter (Davg). Pristine carbon has a high surface area of 708 m2 g−1, but its specific surface area decreases by ca. 15–25% after oxidation and iron oxide loading. The reduction is most likely the result of cavities formed during the oxidation process33 and the pore being occupied during the impregnation process.13,21,34 All materials exhibit a predominance of microporous structure, both in terms of surface area and pore volume.
| Parameter | C | CH | COA | COH | CH–Fe | COA–Fe | COH–Fe |
|---|---|---|---|---|---|---|---|
| SSA, m2 g−1 | 708 | 602 | 561 | 528 | 649 | 612 | 622 |
| Smic, m2 g−1 | 651 | 560 | 520 | 491 | 603 | 573 | 573 |
| % Smic | 92.0 | 93.0 | 92.7 | 93.0 | 93.0 | 93.6 | 92.1 |
| V, cm3 g−1 | 0.33 | 0.28 | 0.27 | 0.26 | 0.31 | 0.29 | 0.30 |
| Vmic, cm3 g−1 | 0.25 | 0.22 | 0.21 | 0.20 | 0.23 | 0.22 | 0.22 |
| % Vmic | 75.8 | 78.6 | 77.8 | 76.9 | 74.2 | 75.9 | 73.3 |
| Davg, nm | 1.88 | 1.90 | 1.95 | 1.96 | 1.89 | 1.86 | 1.95 |
3.2. CO2 and CH4 adsorption isotherm
The CO2 and CH4 adsorption isotherm curves in. Fig. 7(a) were determined at a temperature of 30 °C and pressure of up to 1 atm. As shown in Fig. 7(a), the CO2 uptake capacity was significantly greater than the CH4 uptake capacity. It was consistent with the other outcomes.13 Furthermore, impregnation with iron oxide can increase CO2 uptake capacity while decreasing CH4 uptake capacity. The uptake capacity of CH4 on the impregnated carbon is lower than that of pristine carbon. The increased capacity for CO2 uptake is most likely due to the iron oxide, as the active site has a higher affinity for CO2 than the pristine carbon surface. However, impregnation of carbon with iron oxide resulted in a decrease in the affinity of CH4. It will improve CO2/CH4 selectivity, which is beneficial for CO2/CH4 separation. The pristine carbon has a CO2 uptake capacity of 1.85 mmol g−1 at a pressure of 1 bar, whereas the CH–Fe, COA–Fe, and COH–Fe have capacities of 2.94 mmol g−1, 3.00 mmol g−1, and 2.94 mmol g−1, respectively. Meanwhile, the CH4 uptake capacities of pristine carbon, CH–Fe, COA–Fe, and COH–Fe are respectively 1.08 mmol g−1, 0.85 mmol g−1, 0.92 mmol g−1, and 0.89 mmol g−1. In comparison to other studies in the literature (Table 5), the CO2 uptake capacity of oxidized carbon impregnated with iron oxide is attractive and can compete with other impregnation of metal oxide. | ||
| Fig. 7 Adsorption of CO2 and CH4 on pristine carbon, CH–Fe, COA–Fe and COH–Fe (a) and selectivity of CO2/CH4 (b) at temperature of 30 °C. | ||
| Sorbent type | Metal/metal oxide | Uptake capacity of CO2, mmol g−1 | Temp., °C | Ref. |
|---|---|---|---|---|
| Oxidized AC (CH) | Fe3O4 | 2.95 | 30 | This work |
| Oxidized AC (COA) | Fe3O4 | 3.01 | 30 | This work |
| Oxidized AC (COH) | Fe3O4 | 2.94 | 30 | This work |
| Mesoporous carbon | NiO | 2.00 | 30 | 37 |
| AC | MgO | 2.72 | 0 | 38 |
| AC | Cu/Zn | 2.25 | 30 | 39 |
| AC | CuO | 0.30 | 25 | 40 |
| Unmodified AC | NiO | 3.02 | 30 | 41 |
The selectivity of CO2/CH4 is a critical factor in the separation of CO2/CH4. Fig. 7(b) illustrates the selectivity of CO2/CH4. The data indicate that as pressure increases, the selectivity value of pristine carbon decreases, which is consistent with previous literature.13,35 On the pressure range investigated, the selectivity of CO2/CH4 for pristine carbon was ca. 3.75–1.7. Interestingly, for iron oxide-impregnated carbon, the selectivity increased as the pressure was increased. This is most likely because CO2 chemisorption occurs at active sites such as iron oxide. Iron oxide's active sites may have a strong affinity for CO2. As a result, the intermolecular interaction between CO2 and iron oxide is much stronger than the interaction between CH4 and iron oxide, resulting in a significant increase in the uptake capacity of CO2 as the gas's pressure increases. As a result, CO2 has a greater selectivity than CH4.36 Meanwhile, physisorption occurs as a result of CO2 adsorption onto pristine carbon. When iron oxide impregnated oxidized carbon was compared to pristine carbon, the selectivity of CO2/CH4 increased up to 190%.
3.3. Performance of CO2/CH4 separation
The breakthrough analysis was conducted to determine the CO2/CH4 separation performance of the materials. A 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55% mixture of CO2/CH4 was flowed into a packed bed column containing the material, and the gas composition at the outlet was monitored over time. The CO2 breakthrough curve is depicted in Fig. 8. The breakthrough curves plot the outlet concentration of gas species at a certain time (Ct)/the initial inlet concentration (C0) versus time. As illustrated in Fig. 8(a), there was no difference in the time required to achieve CO2 breakthrough between pristine and oxidized carbon. Thus, oxidation enhances the oxygen functional group on the carbon surface but does not transform the time of the breakthrough. Based on the data, iron oxide impregnated-oxidized carbon has an increase in CO2 breakthrough time (see Fig. 8(b)). Pristine carbon achieved a CO2 breakthrough time of 400 s, while iron oxide impregnated-pristine carbon increased slightly (425 s). However, iron oxide impregnated-oxidized carbon resulted in a 25–45% increase. The breakthrough time for CO2 was defined as when the concentration of CO2 reaches 5%, whereas the requirement for biomethane is at least 95%. CH–Fe, COA–Fe, and CH–Fe had CO2 breakthrough times of 500 s, 570 s, and 530 s, respectively. The CO2 breakthrough curve may indicate that iron oxide impregnated oxidized carbon prepared via ozonation with distilled water has a superior iron oxide dispersion. The order of the CO2 breakthrough time is CH–Fe < COH–Fe < COA–Fe. The results of this study indicate that the addition of oxygen groups to carbon surfaces can increase iron oxide dispersion on the surface, which can enhance CO2/CH4 separation.
55% mixture of CO2/CH4 was flowed into a packed bed column containing the material, and the gas composition at the outlet was monitored over time. The CO2 breakthrough curve is depicted in Fig. 8. The breakthrough curves plot the outlet concentration of gas species at a certain time (Ct)/the initial inlet concentration (C0) versus time. As illustrated in Fig. 8(a), there was no difference in the time required to achieve CO2 breakthrough between pristine and oxidized carbon. Thus, oxidation enhances the oxygen functional group on the carbon surface but does not transform the time of the breakthrough. Based on the data, iron oxide impregnated-oxidized carbon has an increase in CO2 breakthrough time (see Fig. 8(b)). Pristine carbon achieved a CO2 breakthrough time of 400 s, while iron oxide impregnated-pristine carbon increased slightly (425 s). However, iron oxide impregnated-oxidized carbon resulted in a 25–45% increase. The breakthrough time for CO2 was defined as when the concentration of CO2 reaches 5%, whereas the requirement for biomethane is at least 95%. CH–Fe, COA–Fe, and CH–Fe had CO2 breakthrough times of 500 s, 570 s, and 530 s, respectively. The CO2 breakthrough curve may indicate that iron oxide impregnated oxidized carbon prepared via ozonation with distilled water has a superior iron oxide dispersion. The order of the CO2 breakthrough time is CH–Fe < COH–Fe < COA–Fe. The results of this study indicate that the addition of oxygen groups to carbon surfaces can increase iron oxide dispersion on the surface, which can enhance CO2/CH4 separation.
 | ||
| Fig. 8 Carbon dioxide-breakthrough curves of mixed gas CO2/CH4 for (a) C, CH, COA and COH; (b) C–Fe, CH–Fe, COA–Fe and COH–Fe. | ||
Fig. 9 displays the breakthrough curves of CO2 and CH4 for the materials. As shown in Fig. 9, no CH4 or CO2 gas is present initially until approximately 250 seconds, after which CH4 appears with a purity of >98 percent until a certain time, and finally, CO2 emerges until the final concentration in the outlet equals the inlet. A similar CH4 breakthrough time was obtained for all materials, but with a different CO2 breakthrough time. A delayed flow of CH4 at the outlet could indicate a slower diffusion of gas through the bed, caused by the interaction of carbon surface with gas species. Additionally, a larger curve between the CH4 and CO2 signals indicated that CO2 and CH4 had a better separation performance. COA–Fe generates the largest curve.
 | ||
| Fig. 9 Breakthrough curves of mixed gas CO2/CH4 (♦C, ▲CH–Fe, ●COA–Fe, ■COH–Fe for CO2 and ◊C, ΔCH–Fe, □COA–Fe, ○COA–Fe for CH4). | ||
Generally, the separation process resembles that of chromatographic separation. The chromatography separation occurs when the components with a faster rate of movement are separated first. In the separation of CO2/CH4, molecule types with higher diffusivity values or those that diffuse more rapidly are separated first. The illustration mechanism of separation is depicted in Fig. 10. In a column, as shown in Fig. 10(a) and (b), CH4 diffuses more rapidly than CO2.13 Therefore, CH4 and CO2 can be separated. CO2 diffuses more slowly over iron oxide impregnated carbon in Fig. 10(b) than it does over pristine carbon (Fig. 10(a)). Iron oxide exhibits a stronger affinity for CO2. This can result in increased interaction between CO2 and the iron oxide active surface, resulting in a slower rate of CO2 diffusion in the CMS column. As a result, the process of separation lengthens.
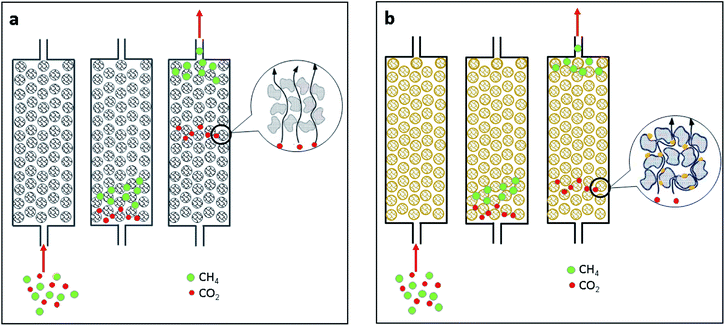 | ||
| Fig. 10 Separation mechanism using (a) pristine carbon and (b) oxidized carbon impregnated with iron oxide. | ||
3.4. Performance comparison on CH–Fe2O3 and CH–Fe3O4
The type of iron oxide formed in this study is Fe3O4. CH–Fe3O4 was mentioned previously in conjunction with CH–Fe, which can improve CO2/CH4 separation performance by up to 125%. Its performance will be compared to that of other types of iron oxide. Hematite (Fe2O3) was chosen as a representative of other iron oxides due to its high affinity for CO2.20 Fig. 11 compares the CO2 breakthrough for CH, CH–Fe3O4, and CH–Fe2O3. The CO2 breakthrough curves for all samples are ideal, indicating that mass transfer occurs instantaneously.13 The order of breakthrough time is CH–Fe2O3 (260 s) < CH (400 s) CH–Fe3O4 (500 s).4 Conclusions
The feasibility of preparing iron oxide impregnated porous carbon was investigated. In this study, increased dispersion of iron oxide on the carbon surface can be achieved by adding oxygen via the three oxidation processes used. The impregnation of iron oxide on oxidized carbon can be characterized as CMS for CO2/CH4 separation. CMS, which was obtained by aqueous ozonation with distilled water, followed by impregnation with iron oxide and labeled COA–Fe, exhibited superiority. The COA–Fe can provide an enhancement of CO2 uptake capacity up to ca. 1.7 times at 30 °C and 1 atm, while the enhancement of CO2/CH4 separation up to ca. 45% compared to pristine carbon. Furthermore, the iron oxide of Fe3O4 is more favorable for CO2/CH4 separation than that of Fe2O3.Author contributions
Nur Indah Fajar Mukti: conceptualization, investigation, methodology, formal analysis, funding acquisition, writing-original draft. Teguh Ariyanto: supervision, funding acquisition, writing-review and editing. Wahyudi Budi Sediawan: supervision, writing-review and editing. Imam Prasetyo: conceptualization, supervision, funding acquisition, writing-review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by Indonesia Endowment Fund for Education (LPDP), Indonesian Ministry of Finance through Dissertation Research Grant (Beasiswa BUDI-DN). The authors wish thanks to PT Home System for the gift of porous carbon produced from palm kernel shell.References
- I. U. Khan, M. H. D. Othman, H. Hashim, T. Matsuura, A. F. Ismail, M. Rezaei-DashtArzhandi and I. W. Azelee, Energy Convers. Manage., 2017, 150, 277–294 CrossRef
.
- I. Angelidaki, L. Treu, P. Tsapekos, G. Luo, S. Campanaro, H. Wenzel and P. G. Kougias, Biotechnol. Adv., 2018, 36, 452–466 CrossRef CAS
.
- M. T. Kallo and M. J. Lennox, Langmuir, 2020, 36, 13591–13600 CrossRef CAS PubMed
.
- O. W. Awe, Y. Zhao, A. Nzihou, D. P. Minh and N. Lyczko, Waste Biomass Valorization, 2017, 8, 267–283 CrossRef CAS
.
- X. Y. Chen, H. Vinh-Thang, A. A. Ramirez, D. Rodrigue and S. Kaliaguine, RSC Adv., 2015, 5, 24399–24448 RSC
.
- I. Durán, N. Álvarez-Gutiérrez, F. Rubiera and C. Pevida, Chem. Eng. J., 2018, 353, 197–207 CrossRef
.
- I. Prasetyo, R. Rochmadi and E. Wahyono, Reaktor, 2010, 13, 24–30 Search PubMed
.
- N. K. Jensen, T. E. Rufford, G. Watson, D. K. Zhang, K. I. Chan and E. F. May, J. Chem. Eng. Data, 2012, 57, 106–113 CrossRef CAS
.
- M. Younas, M. Sohail, L. L. Kong, M. J. K. Bashir and S. Sethupathi, Int. J. Environ. Sci. Technol., 2016, 13, 1839–1860 CrossRef CAS
.
- I. Prasetyo, N. I. F. Mukti, R. B. Cahyono, A. Prasetya and T. Ariyanto, Waste Biomass Valorization, 2020, 11, 5599–5606 CrossRef CAS
.
- I. Prasetyo and D. D. Do, Chem. Eng. Sci., 1998, 53, 3459–3467 CrossRef CAS
.
- I. Prasetyo, R. Rochmadi, E. Wahyono and T. Ariyanto, Eng. J., 2017, 21, 83–94 CrossRef CAS
.
- T. Ariyanto, K. Masruroh, G. Yunita, S. Pambayun, N. Indah, F. Mukti, R. B. Cahyono, A. Prasetya and I. Prasetyo, ACS Omega, 2021, 6, 19194–19201 CrossRef CAS
.
- W. Lou, J. Yang, L. Li and J. Li, J. Solid State Chem., 2014, 213, 224–228 CrossRef CAS
.
- D. Britt, H. Furukawa, B. Wang, T. G. Glover and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 20637–20640 CrossRef CAS PubMed
.
- J. Alcañiz-Monge, J. P. Marco-Lozar and D. Lozano-Castelló, Fuel Process. Technol., 2012, 95, 67–72 CrossRef
.
- Z. Yang, D. Wang, Z. Meng and Y. Li, Sep. Purif. Technol., 2019, 218, 130–137 CrossRef CAS
.
- A. R. Mohamed, M. Mohammadi and G. N. Darzi, Renew. Sustain. Energy Rev., 2010, 14, 1591–1599 CrossRef CAS
.
- S. J. Kim, Y. Kwon, D. Kim, H. Park, Y. H. Cho, S. E. Nam and Y. I. Park, Membranes, 2021, 11(482), 1–34 Search PubMed
.
- A. Hakim, T. S. Marliza, N. M. Abu Tahari, R. W. N. W. Isahak, R. M. Yusop, W. M. Mohamed Hisham and A. M. Yarmo, Ind. Eng. Chem. Res., 2016, 55, 7888–7897 CrossRef CAS
.
- B. G. H. Briton, L. Duclaux, Y. Richardson, K. B. Yao, L. Reinert and Y. Soneda, Appl. Water Sci., 2019, 9, 1–14 CrossRef CAS
.
- J. G. Mahy, L. Tasseroul, A. Zubiaur, J. Geens, M. Brisbois, M. Herlitschke, R. Hermann, B. Heinrichs and S. D. Lambert, Microporous Mesoporous Mater., 2014, 197, 164–173 CrossRef CAS
.
- I. Mazilu, C. Ciotonea, A. Chirieac, B. Dragoi, C. Catrinescu, A. Ungureanu, S. Petit, S. Royer and E. Dumitriu, Microporous Mesoporous Mater., 2017, 241, 326–337 CrossRef CAS
.
- A. Barroso-Bogeat, M. Alexandre-Franco, C. Fernández-González and V. Gómez-Serrano, Arab. J. Chem., 2019, 12, 3963–3976 CrossRef CAS
.
- A. Annisa, I. Prasetyo, D. Swantomo and T. Ariyanto, 4th Int. Semin. Chem., 2021, 2349, p. 020007 Search PubMed
.
- I. Prasetyo, N. I. F. Mukti, M. Fahrurrozi and T. Ariyanto, ASEAN J. Chem. Eng., 2018, 18, 09–16 CrossRef
.
- B. Lesiak, N. Rangam, P. Jiricek, I. Gordeev, J. Tóth, L. Kövér, M. Mohai and P. Borowicz, Front. Chem., 2019, 7(642), 1–16 Search PubMed
.
- V. Tucureanu, M. Alina and M. A. Andrei, Crit. Rev. Anal. Chem., 2016, 46, 502–520 CrossRef CAS PubMed
.
- W. Yang, Z. Du, Z. Ma, G. Wang, H. Bai and G. Shao, RSC Adv., 2016, 6, 3942–3950 RSC
.
- J. L. Figueiredo, M. F. R. Pereira, M. M. A. Freitas and J. J. M. Órfão, Carbon, 1999, 37, 1379–1389 CrossRef CAS
.
- P. L. Hariani, M. Faizal, R. Ridwan, M. Marsi and D. Setiabudidaya, Sustain. Environ. Res., 2018, 28, 158–164 CrossRef CAS
.
- S. Kamilah, C. Soh, A. Azzura, A. Rahman and M. Shamsuddin, Malaysian J. Anal. Sci., 2018, 22, 768–774 Search PubMed
.
- G. Lota, P. Krawczyk, K. Lota and A. Sierczy, J. Solid State Electrochem., 2016, 20, 2857–2864 CrossRef CAS
.
- S. H. Khalil, M. K. Aroua and W. M. A. W. Daud, Chem. Eng. J., 2012, 183, 15–20 CrossRef CAS
.
- W. Liang, Z. Liu, J. Peng, X. Zhou, X. Wang and Z. Li, Energy Fuels, 2019, 33, 493–502 CrossRef CAS
.
- M. Bokare, S. Bano, P. S. Antony, S. Pal and R. Biniwale, Adsorption, 2020, 26, 51–59 CrossRef CAS
.
- J. A. Schott, Z. Wu, S. Dai, M. Li, K. Huang and J. A. Schott, Microporous Mesoporous Mater., 2017, 249, 34–41 CrossRef
.
- S. Shahkarami, A. K. Dalai and J. Soltan, Ind. Eng. Chem. Res., 2016, 55, 5955–5964 CrossRef CAS
.
- S. Hosseini, I. Bayesti, E. Marahel and F. Eghbali, J. Taiwan Inst. Chem. Eng., 2015, 1–9 Search PubMed
.
- B. Kim, K. Cho and S. Park, J. Colloid Interface Sci., 2010, 342, 575–578 CrossRef CAS PubMed
.
- D. Jang and S. Park, Fuel, 2012, 102, 439–444 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2021 |






