Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
One-pot synthesis of new alkyl 1-naphthoates bearing quinoline, pyranone and cyclohexenone moieties via metal-free sequential addition/oxidation reactions†
Seyedeh Hekmat Mousavia,
Mohammad Reza Mohammadizadeha,
Samira Poorsadeghib,
Satoru Arimitsub,
Fatemeh Mohammadsaleh *a,
Genta Kojyac and
Shinichi Gimac
*a,
Genta Kojyac and
Shinichi Gimac
aDepartment of Chemistry, Faculty of Nano and Bioscience and Technology, Persian Gulf University, Bushehr 75169, Islamic Republic of Iran. E-mail: f.mohammadsaleh@gmail.com; Fax: +98 7731 223348
bDepartment of Chemistry, Biology and Marine Science, Faculty of Science, University of the Ryukyus, 1-Senbaru, Nakagami, Nishihara, Okinawa 903-0213, Japan
cCenter for Research Advancement and Collaboration, University of the Ryukyus, Senbaru 1, Nishihara, Okinawa 903-0213, Japan
First published on 16th November 2021
Abstract
A mild and one-pot synthetic pathway was successfully developed for the synthesis of new naphthoate-based scaffolds containing quinoline, pyranone and cyclohexenone moieties via a multistep reaction between acenaphthoquinone and various 1,3-diketones in the presence of different primary aliphatic and benzylic alcohols. This reaction proceeds via a sequential addition/oxidation mechanistic process including a metal-free addition step of acenaphthoquinone and 1,3-diketones followed by the H5IO6-mediated C–C oxidative cleavage of the corresponding vicinal diols at room temperature. The alcohols play a dual role, as the reaction solvent as well as the nucleophile, to conduct the reaction process toward naphthoate formation. All alkyl naphthoate derivatives prepared in this work are new compounds and were definitively characterized using 1H-NMR, 13C-NMR and HRMS analysis, while X-ray crystallography was carried out for one of the products. The synthesis of a naphthalene-based nucleus attached to heterocyclic moieties is noteworthy to follow in the near future for diverse applications in biology, medicine, metal complex design, and semiconductor and optical materials.
Introduction
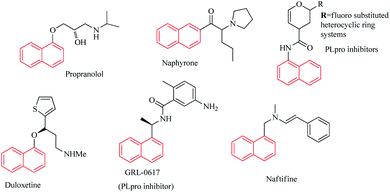
Naphthalene and its derivatives are important organic platforms which are used extensively in industrial chemical compounds and in the chemistry of various pharmacology agents including anticancer,1,2 antiviral3 and anti-inflammatory4 agents and the production of synthetic plastics,5 organic semiconductor materials6 and optics.7Heterocyclic compounds are of very much interest to medicinal chemists and many heterocyclic scaffolds have demonstrated unique biological properties.8–10 Due to their applications in various fields of chemistry and material sciences,4 naphthalene-based heterocyclic compounds have attracted many attentions. Large number of heterocyclic naphthalene-based scaffolds has shown significant and satisfactory biological action.11–13 Several naphthalene-based structures have been marketed as medicine and approved by FDA14,15 (Fig. 1). However, much studies need to be conducted to the synthesis of new naphthalene scaffolds and the potential of these compounds should be more discovered through extensive research.
Bhati has recently designed a series of naphthalene based SARS-CoV PLpro inhibitors by linking naphthalene scaffold to the 3,4-dihydro-2H-pyran moiety via –NHCO functional group.16 PLpro is an essential enzyme for coronaviruses to express and replicate their genomic and it is very important for targeting PLpro to treat coronavirus infections. The ongoing global pandemic of coronavirus disease 2019 (COVID-19) has influenced all the sections of the society and as the most immediate problem of the world has created an excessive social economic and health care challenge.
Some recent literatures have reported the naphthalene-based PLpro inhibitors and confirmed their potential as COVID-19 therapeutics.15–20 Naphthalene based PLpro inhibitors were found to be effective at blocking SARS-CoV-2 (COVID-19) PLpro activity as well as SARS-CoV-2 replication.21
Ersan reported the synthesis of new naphthalene utilizing heterocyclic moieties and studied their antimicrobial activity.11 These compounds showed high potential in designing new inhibitors of E. coli topoisomerase I. Naphthoates as important derivatives of naphthalene exist in many natural and synthetic biologically active materials and their derivatives demonstrate various applications including biological activity,22 optoelectronic properties,23 ligand and metal-chelator.3,24 Liou prepared several methyl naphthalene carboxylates (naphthoates) and evaluated their anti-inflammatory activities by superoxide anion generation and elastase release.25 New derivatives of naphthoates have been widely used as ligands and efficient chelators for metal-complex formation with a variety of metals including Cu,26,27 Cd,28,29 Pb24 and etc. Dai reported the synthesis and X-ray single-crystal structure analysis of new naphthoate-based cadmium(II) and lead(II) complexes.24,29 Ahmad Irfan7 synthesized Schiff based naphthalene compounds and studied the effect of electron donor/acceptor groups on the electro-optical, charge transfer and NLO properties of products by DFT and TDDFT. Due to the electron injection, electronic coupling constant and light harvesting efficiency they concluded that the studied Schiff base compounds would be good contestants to be used in dye-sensitized solar cells. Based on these results, the heterocyclic scaffolds containing naphthalene ring as fully π-conjugated materials are good candidate for optoelectronic applications and organic semiconductor materials. Chen26 prepared the naphthoate-modifying Cu2+-detective Bodipy sensors with the fluorescent ON–OFF performance. Their results reveal the effect of the naphthoated esterification on the fluorescence emission of the products.
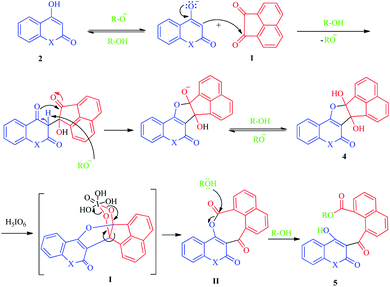
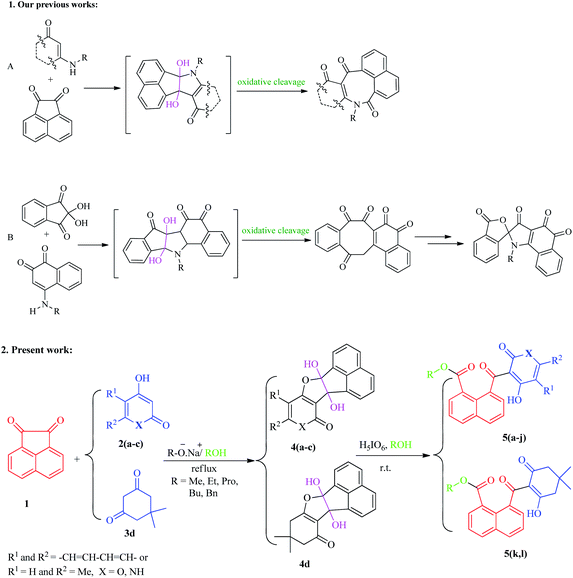
With these considerations in mind, and in the continuation of our efforts to develop new pathways toward the chemical synthesis30–36 and as part of our current study on the oxidative cleavages of cyclic vicinal diols to synthesize new potentially biologically active heterocycles,31 we have herein focused on the development of a facile and effective method for the synthesis of new naphthoate derivatives (Scheme 1). In this study, we report the results of our efforts on the synthesis of various new alkyl 1-naphthoates containing 2,4-dihydroxyquinoline, 4-hydroxy-2-pyranone, and 2-hydroxycyclohexenones, starting from acenaphthoquinone and various 1,3-diketones in the presence of different primary alcohols (ROH, R: aliphatic and benzylic groups) as solvent and reagent followed by metal-free H5IO6-mediated oxidative cleavage of the corresponding vicinal diols. The different naphthoate derivatives bearing N- or O-heterocyclic and non-heterocyclic parts were synthesized with good to excellent yields under mild reaction conditions.
 | ||
| Scheme 1 Our synthetic methods on the preparation of various heterocyclic and non-heterocyclic compounds including the C–C oxidative cleavage of cyclic vicinal diols intermediates. | ||
Results and discussion
As shown in the Scheme 1, the various naphthoate derivatives (5a–l) were synthesized in two sequential steps involving addition step resulting to the formation of vicinal cyclic dihydroxy intermediates 4 and C–C oxidative cleavage process as a one-pot reaction. In the addition step, acenaphthoquinone 1 reacted with various derivatives of β-diketones 2 and 3 in different aliphatic and benzylic alcohols under reflux conditions and in the presence of sodium alkoxide (conjugate base of each alcohol).As described in our previous works, vicinal diols are produced as the main intermediates in the addition reactions between different dinucleophiles and diketones.31,37 The C–C bond oxidative cleavage of vicinal diols has been studied in many literatures.38–40 Vicinal diols are cleaved by oxo-donor reagents such as periodic acid to yield carbonyl-containing derivatives via oxo-transfer mechanism, depending on the reaction conditions, reagents, and the number of groups substituted on the carbon atoms bearing the hydroxyl groups. In this study, no attempts were carried out for separation of diols intermediates 4, thus, after the addition step, they directly treated with H5IO6 as the oxidant reagent and the reaction was allowed to continue at room temperature.
Both reaction steps were performed as one-pot in the presence of various alcohols, in which the alcohol plays a dual role as the reaction solvent as well as nucleophiles after the oxidative cleavage stage to produce the final naphthoate derivatives. Both aliphatic and benzylic alcohols were found to be successful in this reaction process and resulted in the formation of different naphthoate products (5a–l) (Scheme 2).
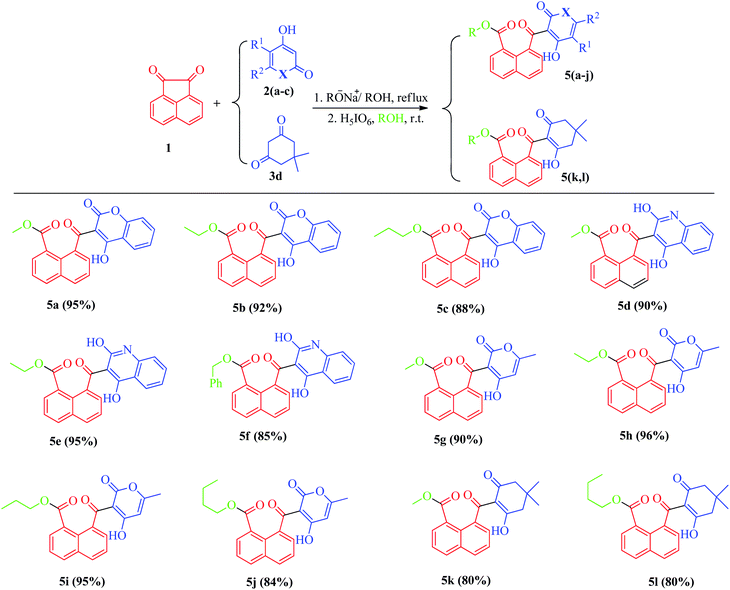 | ||
| Scheme 2 Reaction conditions: β-diketones (1 mmol), acenaphthoquinone (1 mmol); step 1: Na (0.3 mmol), ROH (3 ml), reflux, 12 h; step 2: H5IO6 (1.1 mmol), r.t., ROH, 30–60 min. | ||
We also studied the scope and limitation of this synthetic methodology using different cyclic β-dicarbonyls such as dimedone (5k, l), 4-hydroxycoumarin (5a–c), 4-hydroxyquinoline (5d–f) and 4-hydroxy-6-methyl-2-pyrone (5g–j). As shown in Scheme 3, the corresponding naphthoate derivatives were successfully obtained in good to excellent yields, indicating that the type and structure of β-dicarbonyls had no significant effect on the reaction rates, however, under the same reaction conditions, the use of heterocyclic β-dicarbonyls 4-hydroxycoumarin, 4-hydroxyquinoline and 4-hydroxy-6-methyl-2-pyrone gave higher yields of the corresponding products (5a–j) than those for dimedone (5k, l). All the final products were purified by recrystallization and fully characterized by recording their spectral data using 1H-NMR, 13C-NMR as well as high-resolution mass (HRMS) analysis.
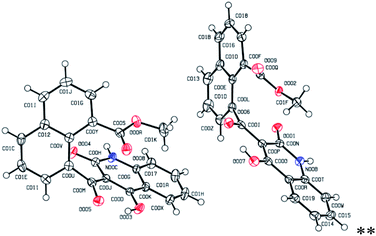
For a typical example, the 1H-NMR spectrum of 5d in CDCl3 displayed three singlets, at 3.64, 11.52, and 15.92 ppm, which were attributed to the methoxy and the OH groups of quinolone ring, respectively. The aromatic protons were also characterized by the presence of two doublets (δ = 6.48, J = 8.4 Hz; δ = 7.68, J = 7.2 Hz), two triplets (δ = 7.19, J = 7.6 Hz; δ = 7.41, J = 8.0), one doublet–doublet (δ = 7.97, J = 7.2 Hz, J = 1.6 Hz) and two multiplet peaks at δ = 7.52–7.57 and δ = 8.03–8.13 ppm. The 13C-NMR spectrum of 5d exhibited 22 characteristic signals, whereas methoxy carbon at δ = 52.9 and carbonyl carbons were observed at δ 169.4 and δ 201.8. The aromatic carbons were appeared at the region of 114.6–140.7. The mass analysis of 5d displayed an [M + H]+ peak at m/z = 374.0964, which is in agreement with the proposed structure. We also recorded a single crystal X-ray crystallographic analysis of compound 5d for unambiguous structure determination, which demonstrated the exact structure of this product (Fig. 2).
According to our results and the other reports,31,41 we have proposed a plausible mechanism for this synthetic process as depicted in Scheme 3, in which the alkoxide ion acts as the base to activate the 1,3-diketone 2, which subsequently attacks acenaphthoquinone 1 to produce the vicinal diol intermediate 4. Next, the vicinal hydroxyl containing intermediate 4 is oxidatively cleaved by periodic acid to form the intermediate II passed from furo[2,3-c][1,2,5]iodadioxole-2,2,2-triol intermediate I.42 Due to sp2 hybridization of all carbon atoms, specially, existence of rigid acenaphthylene moiety, the intermediate II suffers from significant strain and prone to ring-opening reactions. Thus, it undergoes solvolysis with ROH to produce the final naphthoate derivatives 5. Due to the growing importance of naphthalene-containing compounds in many areas of chemistry and biology, the development of high-yielding and selective synthetic procedures for the synthesis of these versatile platforms will be significantly considered by researchers.
Conclusions
A variety of new naphthoate derivatives was synthesized via a mild, eco-friendly, high-yielding and efficient methodology, in which the various 1,3-diketones reacted with acenaphthoquinone by the sequential addition/oxidation mechanistic reaction process in the presence of RO−/ROH. The reaction processes were conducted as metal-free one-pot reaction in two steps. After the addition stage which performed in alcoholic solvents under reflux, the oxidative cleavage step was carried out in the presence of periodic acid at room temperature. The ROH acts as a solvent as well as O-nucleophiles and conducts the reaction to the formation of the products. The new naphthoates were purified and fully characterized. In general, reaction rate, reaction condition and selectivity of the products are important advantages that must be considered in this transformation.Experimental
General information
The chemicals used in this work were purchased from Merck and Sigma-Aldrich chemical companies and were used without further purification. Melting points were determined using an Electro thermal 9100 apparatus. 1H-NMR and 13C-NMR spectra were recorded by using a Bruker DRX-400 AVANCE spectrometer in CDCl3 as solvent. IR spectra were recorded using a Shimadzu IR-470 spectrometer with KBr plates.General procedure for the synthesis of naphthoate derivatives (5a–l)
To a mixture of sodium (0.3 mmol) and alcohol (3 ml) in a round-bottom flask, 1,3-diketone (1 mmol) and acenaphthoquinone (1 mmol) were added and the mixture was refluxed with stirring for 12 hours. The reaction process was monitored by performing TLC using n-hexane/EtOAc. After completion, the reaction mixture was cooled to room temperature and periodic acid (1.1 mmol) was added to the reaction flask and the mixture was stirred at room temperature for about 30–60 min. The final products 5 were formed as the precipitates in the reaction mixture and separated by filtration and recrystallized from their corresponding alcohols to yield pure naphthoate derivatives 5.Conflicts of interest
All authors declare that they have no conflict of interest associated with this publication.Acknowledgements
The authors gratefully acknowledge the financial support received for this work from the Persian Gulf University of Bushehr (PGU), IR, Iran.Notes and references
- L. Luo, J. J. Jia, Q. Zhong, X. Zhong, S. Zheng, G. Wang and L. He, Eur. J. Med. Chem., 2021, 213, 113039 CrossRef CAS.
- G. Wang, J. Qiu, X. Xiao, A. Cao and F. Zhou, Bioorg. Chem., 2018, 76, 249–257 CrossRef CAS PubMed.
- R. Perrone, F. Doria, E. Butovskaya, I. Frasson, S. Botti, M. Scalabrin, S. Lago, V. Grande, M. Nadai and M. Freccero, J. Med. Chem., 2015, 58, 9639–9652 CrossRef CAS.
- M. A. Abozeid, A. A. El-Sawi, M. Abdelmoteleb, H. Awad, M. M. Abdel-Aziz, A.-R. H. Abdel-Rahman and E.-S. I. El-Desoky, RSC Adv., 2020, 10, 42998–43009 RSC.
- E. Hu, S. Shang, Z. Fu, X. Zhao, X. Nan, Y. Du and X. Chen, Chemosphere, 2020, 245, 125602 CrossRef CAS PubMed.
- N. Kobayashi, H. Kuwae, J. Oshima, R. Ishimatsu, S. Tashiro, T. Imato, C. Adachi, S. Shoji and J. Mizuno, J. Lumin., 2018, 200, 19–23 CrossRef CAS.
- A. Irfan, A. Kalam, A. R. Chaudhry, A. G. Al-Sehemi and S. Muhammad, Optik, 2017, 132, 101–110 CrossRef CAS.
- M. Kidwai, R. Venktaramanan, R. Mohan and P. Sapra, Curr. Med. Chem., 2002, 9, 1209–1228 CrossRef CAS PubMed.
- A. Mermer, T. Keles and Y. Sirin, Bioorg. Chem., 2021, 105076 CrossRef CAS.
- S. Rani, K. Raheja, V. Luxami and K. Paul, Bioorg. Chem., 2021, 105017 CrossRef CAS.
- R. H. Ersan, A. Yuksel, T. Ertan-Bolelli, A. Dogen, S. Burmaoglu and O. Algul, J. Chin. Chem. Soc., 2021, 68, 374–383 CrossRef CAS.
- H. J. Park, C. W. Song, S. Sarkar, Y. W. Jun, Y. J. Reo, M. Dai and K. H. Ahn, Chem. Commun., 2020, 56, 7025–7028 RSC.
- G. Chinnasamy, K. Subramani and V. Srinivasan, Orient. J. Chem., 2017, 33, 1856–1863 CrossRef CAS.
- S. Makar, T. Saha and S. K. Singh, Eur. J. Med. Chem., 2019, 161, 252–276 CrossRef CAS.
- A. K. Ghosh, M. Brindisi, D. Shahabi, M. E. Chapman and A. D. Mesecar, ChemMedChem, 2020, 15, 1–27 CrossRef.
- S. Bhati, Heliyon, 2020, 6, e05558 CrossRef PubMed.
- E. Pitsillou, J. Liang, K. Ververis, K. W. Lim, A. Hung and T. C. Karagiannis, Front. Chem., 2020, 8, 1171 Search PubMed.
- P. Rao, R. Patel, A. Shukla, P. Parmar, R. M. Rawal, M. Saraf and D. Goswami, Mol. Diversity, 2021, 1–21 CAS.
- Z. Shen, K. Ratia, L. Cooper, D. Kong, H. Lee, Y. Kwon, Y. Li, S. Alqarni, F. Huang and O. Dubrovskyi, bioRxiv, 2021, 1–13 Search PubMed.
- M. U. Mirza, S. Ahmad, I. Abdullah and M. Froeyen, Comput. Biol. Chem., 2020, 89, 107376–107383 CrossRef CAS.
- B. T. Freitas, I. A. Durie, J. Murray, J. E. Longo, H. C. Miller, D. Crich, R. J. Hogan, R. A. Tripp and S. D. Pegan, ACS Infect. Dis., 2020, 6, 2099–2109 CrossRef CAS PubMed.
- M. Kurosu, P. Narayanasamy, K. Biswas, R. Dhiman and D. C. Crick, J. Med. Chem., 2007, 50, 3973–3975 CrossRef CAS.
- N. Arunadevi, M. Swathika, B. P. Devi, P. Kanchana, S. S. Sundari, S. J. Kirubavathy, P. Subhapriya and E. R. Kumar, Surf. Interfaces, 2021, 24, 101094 CrossRef CAS.
- P. Dai, Russ. J. Coord. Chem., 2016, 42, 129–136 CrossRef CAS.
- J.-R. Liou, M. El-Shazly, Y.-C. Du, C.-N. Tseng, T.-L. Hwang, Y.-L. Chuang, Y.-M. Hsu, P.-W. Hsieh, C.-C. Wu and S.-L. Chen, Phytochemistry, 2013, 88, 67–73 CrossRef CAS PubMed.
- Y. Chen, L. Zhao and J. Jiang, Spectrochim. Acta, Part A, 2017, 175, 269–275 CrossRef CAS.
- Y. Takasaki and S. Takamizawa, Chem. Commun., 2015, 51, 5024–5027 RSC.
- N. Arunadevi, P. Kanchana, V. Hemapriya, S. S. Sankaran, M. Mayilsamy, P. D. Balakrishnan, I.-M. Chung and P. Mayakrishnan, J. Dispersion Sci. Technol., 2021, 1–17 CrossRef.
- P. Dai, E. Yang and X. Zhao, Russ. J. Coord. Chem., 2015, 41, 16–24 CrossRef CAS.
- S. H. Mousavi, M. R. Mohammadizadeh, Z. Roshan, A. Jamaleddini and S. Arimitsu, ACS Omega, 2020, 5, 18273–18288 CrossRef CAS PubMed.
- S. H. Mousavi, M. R. Mohammadizadeh, S. Arimitsu, D. Saberi, S. Poorsadeghi and K. Genta, RSC Adv., 2020, 10, 20552–20557 RSC.
- S. A. Hashemi and M. R. Mohammadizadeh, ChemistrySelect, 2019, 4, 11995–12001 CrossRef CAS.
- A. Jamaleddini and M. R. Mohammadizadeh, Tetrahedron Lett., 2017, 58, 78–81 CrossRef CAS.
- M. R. Mohammadizadeh, D. Saberi and S. Z. Taghavi, Tetrahedron Lett., 2016, 57, 264–267 CrossRef CAS.
- S. A. Hashemi and M. R. Mohammadizadeh, ChemistrySelect, 2021, 6, 733–737 CrossRef CAS.
- N. Firoozi, Z. Roshan and M. R. Mohammadizadeh, Appl. Organomet. Chem., 2018, 32, e3963 CrossRef.
- A. Jamaledini and M. R. Mohammadizadeh, Heteroat. Chem., 2018, 29, e21417 CrossRef.
- T. Niu, S. Chen, M. Hong, T. Zhang, J. Chen, X. Dong and B. Ni, Green Chem., 2020, 22, 5042–5049 RSC.
- E. Amadio, J. González-Fabra, D. Carraro, W. Denis, B. Gjoka, C. Zonta, K. Bartik, F. Cavani, S. Solmi and C. Bo, Adv. Synth. Catal., 2018, 360, 3286–3296 CrossRef CAS.
- L. Meng, W. Li, P. Guo, S. Wang and X. Tong, Catal. Commun., 2021, 154, 106305 CrossRef CAS.
- H. Luo, L. Wang, S. Shang, J. Niu and S. Gao, Commun. Chem., 2019, 2, 1–10 CrossRef.
- E. L. Jackson, Org. React., 2004, 2, 341–375 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2110682. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1ra07092d |
| This journal is © The Royal Society of Chemistry 2021 |