Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dalpulapans A–E from the roots of Dalbergia stipulacea†
Priyapan Posria,
Thurdpong Sribuhoma,
Sookkawath Walunchaprukb,
Thanaset Senawong b,
Sarawut Tontapha
b,
Sarawut Tontapha c,
Vittaya Amornkitbamrung
c,
Vittaya Amornkitbamrung c and
Chavi Yenjai
c and
Chavi Yenjai *a
*a
aNatural Products Research Unit, Center of Excellence for Innovation in Chemistry, Department of Chemistry, Faculty of Science, Khon Kaen University, Khon Kaen 40002, Thailand. E-mail: chayen@kku.ac.th; Tel: +66-4320-2222-41 ext. 12243
bNatural Products Research Unit, Department of Biochemistry, Faculty of Science, Khon Kaen University, Khon Kaen 40002, Thailand
cIntegrated Nanotechnology Research Centre, Department of Physics, Faculty of Science, Khon Kaen University, Khon Kaen 40002, Thailand
First published on 23rd November 2021
Abstract
Five new compounds, dalpulapans A–E (1–5), were isolated from the hexane extract of the roots of Dalbergia stipulacea Roxb. Five new compounds, dalpulapans A–E (1–5), were isolated from the hexane extract of the roots of Dalbergia stipulacea Roxb. An evaluation of cytotoxic activity against HeLa, A549 and normal cell lines using MTT assay was performed. The results showed that R,R-velucarpin A (6) was the most active against HeLa cells with an IC50 value of 10.9 ± 0.42 μM, while fortunately this compound exhibited weak cytotoxicity against normal cells (29.20 ± 1.16 μM). Structures of all isolates were identified from their 1D and 2D NMR spectroscopic data and MS analysis. Experimental and calculated ECD spectra were studied to define the absolute configurations.
1. Introduction
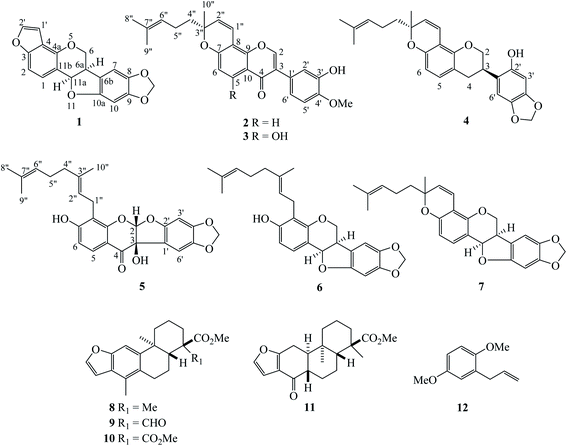
Dalbergia stipulacea Roxb., which belongs to the family Fabaceae, is found throughout southern China, eastern India, Myanmar, Thailand, Vietnam and Laos and is known as “Ma Kham Tao” in Thai. This plant has been used as an emmenagogue and for abortion when taken in moderate amounts. It is believed that this plant can be used for gonorrhea, syphilis and mouth ulcers.1 Moreover, the roots of this plant are poisonous to fish.2 The chemical constituents from this plant which include isoflavonoids, chalcone, pterocarpan and phenylpropene have been reported.2,3 Antifungal activity against Pythium insidiosum, a fungus-like microorganism for which at present there is no effective agent for treatment, was evaluated and shows interesting results.4 Further investigation of compounds from the roots of this plant and testing for cytotoxicity against A549 (lung cancer cells), HeLa (cervical cancer cells) and Vero cells (kidney of African green monkey cells; normal cells) was made. In this work, five new compounds (dalpulapans 1–5) and seven known compounds were reported. The absolute configurations of chiral carbons were determined using experimental electronic circular dichroism (ECD) analysis and comparing the specific rotations with those previously reported.2. Discussion
The extraction and isolation of hexane extract of the roots of D. stipulacea by chromatographic methods led to five new compounds named dalpulapans A–E (1–5) (Fig. 1) and seven known compounds (6–12) including R,R-velucarpin A (6),5 R,R-velucarpin C (7),5,6 taepeenin A (8),7 taepeenin E (9),7 pteroloterin A (10),8 nortaepeenin A (11)8 and 2-allyl-1,4-dimethoxybenzene (12) (Fig. 1). All chemical structures were identified by spectroscopic methods, HRESIMS and ECD data.Compound 1 showed the molecular formula C18H12O5 identified from its HRESIMS ion at m/z 331.0580 [M + Na]+ (calcd 331.0582). It contains five oxygenated aromatic carbons at δC 156.4 (C-3), 154.4 (C-10a), 149.6 (C-4a), 148.3 (C-9) and 141.9 (C-8) from the 13C NMR data (Table 1). The 1H NMR displayed two doublet signals (J = 8.4 Hz) at δH 7.42 and δH 7.22 of aromatic protons H-1 and H-2, respectively. This molecule contains a furan moiety, shown by proton signals at δH 6.85 (J = 2.0 Hz, H-1′) and δH 7.56 (J = 2.0 Hz, H-2′); in addition, these protons were linked to carbons at δC 144.5 and δC 104.2, respectively. The HMBC correlations between H-1 (δH 7.42) and C-3 (δC 156.4), C-4a (δC 149.6) and C-11a (δC 78.8), and between H-2 (δH 7.22) and C-4 (δC 117.4) and C-11b (δC 113.1), and between H-2′ (δH 7.56) and C-3 (δC 156.4) and C-4 (δC 117.4) confirmed the connectivity of a furan moiety at the C-3 and C-4 positions (Fig. 2). Two singlet signals of aromatic protons H-7 (δH 6.86) and H-10 (δH 6.46) were evident. Methylenedioxy protons were observed at δH 5.90 and δH 5.92 in the 1H NMR spectrum and connected to the same carbon at δC 101.6. A doublet of doublets signal at δH 4.37 (J = 10.8, 4.8 Hz) was assigned to H-6α, while a triplet signal at δH 3.76 (J = 10.8 Hz) was given as H-6β. The coupling constant of oxymethine proton H-11a was 7.2 Hz, confirming the cofacial orientation to H-6a.9 Long-range couplings between H-7 (δH 6.86) and C-6a (δC 40.3) and C-10a (δC 154.4), and between H-10 (δH 6.46) and C-6b (δC 118.1) and C-10a (δC 154.4) were detected in the HMBC spectrum (Fig. 2). The absolute configuration was confirmed by comparison with the ECD spectrum and specific rotation with pterocarpan (Chem. Rev. 2013).10 The compound 1 showed a negative optical rotation value ([α]27D −104.7) and displayed negative Cotton effect at 238 nm (Δε −41.14). Kaennakam and coworkers reported the specific rotation of velucarpin A as [α]20D −83.8 and also showed negative Cotton effect at 247 (Δε −8.71).5 Thus, the absolute configuration of compound 1 was 6aR,11aR and it was named dalpulapan A.
| Position | 1 | Position | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | ||
| 1 | 7.42, d (8.4) | 126.9 | 1 | — | — | — | — | — | — | — | |
| 2 | 7.22, d (8.4) | 105.9 | 2 | 7.93, s | 152.0 | 7.87, s | 152.6 | 4.33, dd (10.4, 1.4) | 70.1 | 6.33, s | 110.3 |
| 3 | 156.4 | — | — | 3.99, t (10.4) | — | ||||||
| 4 | 117.4 | 3 | — | 124.8 | — | 123.6 | 3.51, m | 32.0 | — | 80.4 | |
| 4a | 149.6 | 4 | — | 175.9 | — | 180.9 | 2.92, dd (15.7, 10.3) | 30.8 | — | 189.4 | |
| 6α | 4.37, dd (10.8, 4.8) | 66.7 | — | — | 2.83, dd (15.7, 5.2) | — | |||||
| 6β | 3.76, t (10.8) | 5 | 8.05, d (9.0) | 126.9 | — | 162.4 | 6.81, d (8.2) | 129.3 | 7.62, d (8.8) | 127.1 | |
| 6a | 3.55, m | 40.3 | 6 | 6.84, d (9.0) | 115.1 | 6.28, s | 100.2 | 6.36, d (8.2) | 108.8 | 6.55, d (8.8) | 112.2 |
| 6b | 118.1 | 7 | — | 157.7 | — | 160.0 | — | 152.3 | — | 162.9 | |
| 7 | 6.86, s | 104.9 | 8 | — | 109.1 | — | 101.0 | — | 109.9 | — | 117.9 |
| 8 | 141.9 | 9 | — | 152.4 | — | 152.2 | — | 149.8 | — | 158.1 | |
| 9 | 148.3 | 10 | — | 118.4 | — | 106.0 | — | 114.0 | — | 115.3 | |
| 10 | 6.46, s | 94.0 | 1′ | — | 125.3 | — | 124.0 | — | 128.1 | — | 110.3 |
| 10a | 154.4 | 2′ | 7.11, s | 115.6 | 7.07, d (2.0) | 115.3 | — | 148.0 | — | 155.6 | |
| 11a | 5.61, d (7.2) | 78.8 | 3′ | — | 145.7 | — | 145.8 | 6.37, s | 98.5 | 6.48, s | 94.2 |
| 11b | 113.1 | 4′ | — | 146.7 | — | 147.0 | — | 146.6 | — | 150.5 | |
| 1′ | 6.85, d (2.0) | 144.5 | 5′ | 6.92, d (8.0) | 110.8 | 6.91, d (8.0) | 110.9 | — | 142.0 | — | 143.4 |
| 2′ | 7.56, d (2.0) | 104.2 | 6′ | 7.11, d (8.0) | 121.3 | 7.05, dd (2.0,8.0) | 121.1 | 6.60, s | 107.1 | 6.66, s | 103.7 |
| OCH2O | 5.92, d (1.2) | 101.6 | 1′′ | 6.84, d (10.0) | 115.3 | 6.71, d (10.2) | 115.2 | 6.67, d (10.0) | 117.4 | 3.46, d (6.8) | 22.3 |
| 5.90, d (1.2) | |||||||||||
| 2′′ | 5.66, d (10.0) | 129.4 | 5.53, d (10.2) | 126.4 | 5.51, d (10.0) | 128.1 | 5.23, t (6.8) | 120.3 | |||
| 3′′ | — | 80.3 | — | 80.7 | — | 78.1 | — | 140.3 | |||
| 4′′ | 1.77, m | 41.6 | 1.73, m | 41.7 | 1.71, m | 41.0 | 2.08, m | 39.8 | |||
| 5′′ | 2.12, m | 22.8 | 2.10, m | 22.7 | 2.10, m | 22.8 | 2.08, m | 26.4 | |||
| 6′′ | 5.10, t (6.8) | 123.8 | 5.09, t (7.2) | 123.8 | 5.09, t (7.0) | 124.4 | 5.04, t (6.8) | 123.7 | |||
| 7′′ | — | 132.2 | — | 132.1 | — | 131.7 | — | 132.3 | |||
| 8′′ | 1.66, s | 25.8 | 1.66, s | 25.8 | 1.66, s | 25.8 | 1.66, s | 25.8 | |||
| 9′′ | 1.57, s | 17.8 | 1.58, s | 17.8 | 1.58, s | 17.8 | 1.59, s | 17.9 | |||
| 10′′ | 1.46, s | 27.0 | 1.44, s | 27.1 | 1.39, s | 26.3 | 1.81 | 16.4 | |||
| OCH2O | — | — | — | — | 5.89, d (1.2) | 101.3 | 5.92, d (1.2), | 102.0 | |||
| 5.88, d (1.2) | 5.89, d (1.2) | ||||||||||
| –OH | 5.65, s | — | 5.71, s | — | 4.74, s | — | 4.16, s | — | |||
| 12.95, s | 6.16, s | ||||||||||
| –OCH3 | 3.92, s | 56.2 | 3.91, s | 56.2 | — | — | — | — | |||
Isoflavone derivative 2, dalpulapan B, showed the molecular formula of C26H26O5 identified from its HRESIMS ion at m/z 419.1858 [M + H]+ (calcd for C26H27O5, 419.1858). The singlet signal at δH 7.93 was located at oxygenated carbon C-2 (δC 152.0); in addition, this proton correlated with C-3 (δC 124.8), C-4 (δC 175.9) and C-9 (δC 152.4) in the HMBC spectrum, indicating the isoflavone core structure. The protons H-5 (δH 8.05) and H-6 (δH 6.84) showed two doublet signals with a coupling constant of 9.0 Hz (Table 1). The HMBC correlations were found between H-5 (δH 8.05) and C-4 (δC 175.9), C-7 (δC 157.7) and C-9 (152.4). The protons on the B-ring displayed an ABX system at δH 7.11 (d, J = 8.0 Hz, H-6′), δH 6.92 (d, J = 8.0 Hz, H-5′) and δH 7.11 (s, H-2′). Cross-peaks between H-5′ (δH 6.92) to C-1′ (δC 125.3) and C-3′ (δC 145.7) and between methoxy proton (δH 3.92) and C-4′ (δC 146.7) were observed in the HMBC data. The presence of a pyran ring and prenyl group were detected in the 1H and 13C NMR spectra. The olefinic protons in the pyran ring resonated at δH/δC 6.84/115.3 of 1′′ position and at δH/δC 5.66/129.4 of 2′′ position, in addition, an oxygenated quaternary carbon, C-3′′ exhibited at δC 80.3. The 1H NMR data displayed a triplet signal of an olefinic proton H-6′′ (δH 5.10, t, J = 6.8 Hz) which coupled with H-5′′ (δH 2.12, m). Cross-peaks between CH3-8′′ and CH3-9′′ and C-6′′ were observed in the HMBC data. The experimental ECD spectrum displayed negative Cotton effects at 233 nm (Δε −11.50) and positive Cotton effects at 265 nm (Δε +7.89) which was similar to the calculated spectrum for the (3′R) configuration confirming the structure of 2 as shown in Fig. 1.
The 1H and 13C NMR data from dalpulapan C (3) were similar to 2, except for the presence of a hydroxy group at the C-5 position in compound 3. The molecular formula, C26H26O6, confirmed the additional oxygen atom compared to 2. An aromatic proton H-6 (δH 6.28, s) showed long-range coupling with C-5 (δC 162.4), C-7 (δC 160.0), C-8 (δC 101.0) and C-10 (δC 106.0) in the HMBC experiment (Table 1). Intramolecular H-bonding was detected at δH 12.95, confirming the presence of a hydroxy group at the C-5 position, in addition, this hydroxy proton showed long-range coupling with C-5, C-6 and C-10 in the HMBC data. The specific rotation of compound 3, [α]28D +142.5, was the same as compound 2 and the experimental ECD was match to calculated ECD of 3R′ configuration. Thus the structure of compound 3 was identified as shown in Fig. 1.
Dalpulapan D (4) possessed a protonated adduct ion at m/z 421.2006 corresponding to the molecular formula C26H28O5. This molecule was an isoflavan derivative and contained a pyran moiety, as characterized from 1D and 2D NMR data. An oxygenated methylene proton at δH 4.33 (dd, J = 10.4, 1.4 Hz, H-2a) and δH 3.99 (t, J = 10.4 Hz, H-2b) correlated with carbon at δC 70.1 in the HMQC data. From the multiplicity and coupling constant, it can be identified that H-2b was located at the axial position. Two doublet of doublet signals at δH 2.92 (J = 15.7, 10.3 Hz, H-4a) and δH 2.83 (J = 15.7, 5.2 Hz, H-4b) were located on the carbon at δC 30.8. The multiplet signal of a methine proton H-3 showed at δH 3.51. The connection of the H-2/H-3/H-4 system was observed in the 1H–1H COSY data. Two singlet signals at δH 6.37 and δH 6.60 were assigned as H-3′ and H-6′, respectively. This compound showed a methylenedioxy group at δH 5.89 and δH 5.88 and located on the same carbon at δC 101.3. Cross-peaks between H-6′ (δH 6.60) and C-3 (δC 32.0) and between H-3′ (δH 6.37) and C-1′ (δC 128.1) were observed in the HMBC spectrum. The 1H and 13C NMR spectra displayed the containing of pyran ring and prenyl side chain as compounds 2 and 3. Compound 4 showed positive Cotton effect at 218 nm (Δε +2.28) and 284 nm (Δε +5.75) and a negative positive Cotton effect at 243 nm (Δε −3.32), which corresponded with the calculated ECD spectrum of 3R,3′′R configuration.11,12 It should be note that the absolute configuration at C-3′′ was the same as compounds 2 and 3. All data concluded that the structure of 4 was shown in Fig. 1.
Dalpulapan E (5) was given a molecular formula C26H26O7 characterized from the negative molecular ion peak [M − H]+ at m/z 449.1598. The signals of the aromatic protons H-5 and H-6 displayed as doublets (J = 8.8 Hz) at δH 7.62 and δH 6.55 and correlated with carbons at δC 127.1 and δC 112.2, respectively, in the HMQC experiment. Two singlet signals on the aromatic B-ring were evident (δH 6.48, H-3′ and δH 6.66, H-6′). Methylenedioxy protons showed two doublets (J = 1.2 Hz) at δH 5.92 and δH 5.89 and were located at carbon δC 102.0. This compound exhibited a geranyl group by showing two olefinic protons at δH 5.23 (t, J = 6.8 Hz, H-2′′) and δH 5.04 (t, J = 6.8 Hz, H-6′′), three methylene protons and three methyl protons. The HMBC cross-peaks showed correlation between H-1′′ (δH 3.46) and oxygenated carbons C-7 (δC 162.9) and C-9 (δC 158.1), which confirmed the hydroxy group at the C-7 position. A singlet signal proton at δH 6.33 (H-2) was located at C-2 (δC 110.3), which bears two oxygen atoms. This proton correlated with carbons C-9 (δC 158.1) and C-2′ (δC 155.6) in the HMBC spectrum, maintaining the presence of an acetal group. The broad singlet signal at δH 4.16 was assigned as a hydroxy proton, OH-3, and in addition the 13C NMR signal of the oxygenated quaternary carbon C-3 exhibited at δC 80.4. The experimental ECD data, shows a negative Cotton effect at 239 nm (Δε −32.31) and a positive Cotton effect at 315 nm (Δε +13.47) and possessed positive specific rotation at [α]27.5D +141.3. These information corresponded with the calculated ECD spectrum of 2S,3R configuration. In addition, both ECD data and the specific rotation of 5 are opposite to previous report, (2R,3S)-3,7,4′-trihydroxy-5-methoxycoumaronochromone.13 That compound showed negative specific rotation at [α]22D −184.6; and positive and negative Cotton effects at 211 nm (Δε +19.9) and 292 nm (Δε −19.9), respectively. Thus the absolute configuration at C-2 and C-3 were confirmed as 2S,3R as shown in Fig. 1.
All isolated compounds, except 12, were evaluated for cytotoxicity against A549 (lung cancer cells), HeLa (cervical cancer cells) and Vero cells using the MTT assay. The cytotoxicity results showed the most active compound was 6, which exhibited an IC50 value of 10.9 ± 0.42 μM against HeLa cells. In addition, IC50 values against A549 and Vero cells were 14.6 ± 1.31 and 29.2 ± 1.16 μM, respectively. The remaining compounds showed inactive (IC50 > 15 μM) to the test.
3. Experimental section
3.1. General experimental procedures
A Sanyo Gallenkamp (UK) melting point apparatus was used to find the melting points. Optical rotations were measured on a JASCO P-1020 digital polarimeter (Japan). The UV spectra were obtained on an Agilent 8453 UV-visible spectrophotometer (Germany). A PerkinElmer Spectrum One FT-IR spectrophotometer (USA) was used to acquire the IR spectra. NMR spectra were collected at 400 MHz (1H) and at 100 MHz (13C) using a Varian Mercury Plus spectrometer (USA). HRESIMS was performed on a Micromass Q-TOF 2 hybrid quadrupole time-of-flight (Q-TOF) mass spectrometer (Micromass, UK). Analytical thin-layer chromatography (TLC) was accomplished on Merck silica gel 60 F254. Column chromatography separations were carried out on silica gel less than 0.063 mm, 0.063–0.200 mm or RP-18.3.2. Plant material
The roots of D. stipulacea were collected in February 2018 at Ban Na Phaeng Village, Wiang Kao District, Khon Kaen Province, Thailand (16°41′53.5′′N, 102°20′24.1′′E). Plant material (voucher specimen KKU012018) was identified by Assoc. Prof. Suppachai Tiyaworanant, Faculty of Pharmaceutical Sciences, Khon Kaen University, Thailand.3.3. Extraction and isolation
The dried powdered roots (12 kg) of D. stipulacea were extracted with hexanes (15 L × 3), EtOAc (10 L × 3) and MeOH (10 L × 3) at ambient temperature. The solutions were concentrated in vacuo to give the crude hexane (162 g, 1.35%), EtOAc (368 g, 3.07%) and crude MeOH (649 g, 5.41%) extracts. The crude hexane extract (162 g) was subjected to column chromatography (silica gel 60) and obtained five fractions, F1–F5. Fraction F2 was purified by FCC (hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc) to give 7 (10.35 g) and 8 (0.12 g). Fraction F3 was further purified and gave four fractions, F3.1–F3.4. Compound 1 (7.5 mg) was obtained from F3.1 while compounds 4 (5.7 mg) and 12 (3.2 mg) were found from F3.2. Subfraction F3.4 was chromatographed on a silica gel column (90
EtOAc) to give 7 (10.35 g) and 8 (0.12 g). Fraction F3 was further purified and gave four fractions, F3.1–F3.4. Compound 1 (7.5 mg) was obtained from F3.1 while compounds 4 (5.7 mg) and 12 (3.2 mg) were found from F3.2. Subfraction F3.4 was chromatographed on a silica gel column (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 hexane
10 hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc) to obtain two subfractions, F3.4.1 and F3.4.2. Compounds 9 (5.5 mg) and 10 (9.9 mg) were found from F3.4.2. Fraction F4 was chromatographed out on silica gel FCC, eluting with hexane
EtOAc) to obtain two subfractions, F3.4.1 and F3.4.2. Compounds 9 (5.5 mg) and 10 (9.9 mg) were found from F3.4.2. Fraction F4 was chromatographed out on silica gel FCC, eluting with hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc (70
EtOAc (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) to obtain four subfractions, F4.1–F4.4. Compounds 3 (59.1 mg), 6 (0.40 g) and 11 (36.6 mg) were obtained from subfraction F4.1. Compound 5 (7.0 mg) was obtained from F4.2. Subfraction F4.4 was purified and gave 2 (3.2 mg).
30) to obtain four subfractions, F4.1–F4.4. Compounds 3 (59.1 mg), 6 (0.40 g) and 11 (36.6 mg) were obtained from subfraction F4.1. Compound 5 (7.0 mg) was obtained from F4.2. Subfraction F4.4 was purified and gave 2 (3.2 mg).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 249 (4.39), 311 (4.13) nm; ECD 205 nm (Δε +37.91), 215 nm (Δε −102.05); IR (neat) νmax 2884, 1602, 1476, 1372, 1142, 1057, 935, 766 cm−1; 1H NMR (400 MHz) and 13C NMR (100 MHz) data (CDCl3), see Table 1; HRESIMS m/z 331.0580 [M + Na]+ (calcd for C18H12O5Na, 331.0582).
ε) 249 (4.39), 311 (4.13) nm; ECD 205 nm (Δε +37.91), 215 nm (Δε −102.05); IR (neat) νmax 2884, 1602, 1476, 1372, 1142, 1057, 935, 766 cm−1; 1H NMR (400 MHz) and 13C NMR (100 MHz) data (CDCl3), see Table 1; HRESIMS m/z 331.0580 [M + Na]+ (calcd for C18H12O5Na, 331.0582).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 265 (4.80) nm; IR (neat) νmax 3367, 2929, 1626, 1576, 1511, 1438, 1395, 1274, 1196, 1134, 1080, 906, 807, 762 cm−1; 1H NMR (400 MHz) and 13C NMR (100 MHz) data (CDCl3), see Table 1; HRESIMS m/z 419.1858 [M + H]+ (calcd for C26H27O5, 419.1858).
ε) 265 (4.80) nm; IR (neat) νmax 3367, 2929, 1626, 1576, 1511, 1438, 1395, 1274, 1196, 1134, 1080, 906, 807, 762 cm−1; 1H NMR (400 MHz) and 13C NMR (100 MHz) data (CDCl3), see Table 1; HRESIMS m/z 419.1858 [M + H]+ (calcd for C26H27O5, 419.1858).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 274 (4.38) nm; IR (neat) νmax 3442, 2926, 1615, 1574, 1512, 1429, 1376, 1274, 1174, 1081, 1033, 954, 913, 819, 764 cm−1; 1H NMR (400 MHz) and 13C NMR (100 MHz) data (CDCl3), see Table 1; HRESIMS m/z 435.1808 [M + H]+ (calcd for C26H27O6, 435.1808).
ε) 274 (4.38) nm; IR (neat) νmax 3442, 2926, 1615, 1574, 1512, 1429, 1376, 1274, 1174, 1081, 1033, 954, 913, 819, 764 cm−1; 1H NMR (400 MHz) and 13C NMR (100 MHz) data (CDCl3), see Table 1; HRESIMS m/z 435.1808 [M + H]+ (calcd for C26H27O6, 435.1808).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 209 (4.05), 282 (3.51) nm; IR (neat) νmax 3728, 3404, 2921, 1634, 1610, 1586, 1479, 1440, 1378, 1280, 1213, 1168, 1096, 1064, 1038, 935, 863, 772, 720 cm−1; 1H NMR (400 MHz) and 13C NMR (100 MHz) data (CDCl3), see Table 1; HRESIMS m/z 421.2006 [M + H]+ (calcd for C26H29O5, 421.2015).
ε) 209 (4.05), 282 (3.51) nm; IR (neat) νmax 3728, 3404, 2921, 1634, 1610, 1586, 1479, 1440, 1378, 1280, 1213, 1168, 1096, 1064, 1038, 935, 863, 772, 720 cm−1; 1H NMR (400 MHz) and 13C NMR (100 MHz) data (CDCl3), see Table 1; HRESIMS m/z 421.2006 [M + H]+ (calcd for C26H29O5, 421.2015).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 204 (4.58), 298 (4.06) nm; IR (neat) νmax 3352, 2923, 1663, 1598, 1466, 1445, 1280, 1216, 1148, 1034, 992, 938, 826 cm−1; 1H NMR (400 MHz) and 13C NMR (100 MHz) data (CDCl3), see Table 1; HRESIMS m/z 449.1598 [M − H]+ (calcd for C26H25O7, 449.1600).
ε) 204 (4.58), 298 (4.06) nm; IR (neat) νmax 3352, 2923, 1663, 1598, 1466, 1445, 1280, 1216, 1148, 1034, 992, 938, 826 cm−1; 1H NMR (400 MHz) and 13C NMR (100 MHz) data (CDCl3), see Table 1; HRESIMS m/z 449.1598 [M − H]+ (calcd for C26H25O7, 449.1600).3.4. Cytotoxic activity assay
MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay was performed to evaluate the cytotoxic effect of the compounds. The procedure has been explained in a previous report.14 In brief, all cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, penicillin (100 U mL−1) and streptomycin (100 μg mL−1) (Gibco-BRL, USA) and incubated at 37 °C in a humidified atmosphere of 5% CO2. For preliminary testing, cells were exposed to the selected compounds at a concentration of 100 μg mL−1 for 24, 48 and 72 hours. The compounds that caused less than 50% cell viability were further evaluated for their half maximal inhibitory concentration (IC50) values. Control groups were treated with a solvent (a mixture of DMSO and ethanol; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). After incubation, the medium was replaced with 110 μL of fresh medium containing MTT (0.5 mg mL−1 in PBS) (Sigma Chemical Co., St. Louis, MO, USA) and incubated for 2 h. The formazan formed after conversion of MTT was dissolved in DMSO. The absorbance of formazan was measured with a microplate reader (Bio-Rad Laboratories, USA) at the wavelength of 550 nm using 655 nm as a reference wavelength. Each assay was replicated four times. The percentage of viable cells which corresponds to the production of formazan was calculated as previously described (Kumnerdkhonkaen et al. 2018).
1). After incubation, the medium was replaced with 110 μL of fresh medium containing MTT (0.5 mg mL−1 in PBS) (Sigma Chemical Co., St. Louis, MO, USA) and incubated for 2 h. The formazan formed after conversion of MTT was dissolved in DMSO. The absorbance of formazan was measured with a microplate reader (Bio-Rad Laboratories, USA) at the wavelength of 550 nm using 655 nm as a reference wavelength. Each assay was replicated four times. The percentage of viable cells which corresponds to the production of formazan was calculated as previously described (Kumnerdkhonkaen et al. 2018).| % Cell viability = [sample (A550 − A655)/control (A550 − A655)] × 100 |
3.5. Calculation
The preliminary conformational analyses were evaluated using HyperChem software. These dominant conformers were further optimized at the B3LYP/6-311g (d,p) basis set by density functional theory.15 The GAUSSIAN 09 program was used to calculate the ECD spectra.16 The single point energy calculations were computed using time-dependent density functional theory (TD–DFT)17 at the CAM–B3LYP/6-311++g (d,p) level of theory.18 The CPCM polarizable conductor calculation model was used for bulk solvent effects.194. Conclusions
In this study, twelve compounds were isolated from hexane extract of the roots of Dalbergia stipulacea Roxb. They were five new compounds, dalpulapans A–E (1–5), and seven known compounds. The cytotoxicity evaluation against HeLa, A549 and normal cell lines using MTT assay was examined. It was found that 6 was the most active against HeLa cells with IC50 value of 10.9 ± 0.42 μM, and showed weak cytotoxicity against normal cells (29.20 ± 1.16 μM). The structures of all compounds were determined by 1D and 2D NMR spectroscopic studies. MS analysis and experimental and calculated ECD spectra were also studied to define the absolute configurations of new compounds.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
We are indebted the Thailand Research Fund (Grant No. RSA6280050) and Khon Kaen University for financial support. T. Sribuhom acknowledges The Post-Doctoral Training Program from the Research Affairs and Graduate School, Khon Kaen University (Grant No. 591471). We also thank The Center of Excellence for Innovation in Chemistry (PERCH-CIC), Office of the Higher Education Commission, Ministry of Education, Thailand.References
- R. Nongmaithem, S. D. Yumkham, N. P. Devi, S. Salam, A. K. Das and P. K. Singh, Indian J. Tradit. Knowl., 2018, 17(4), 754–762 Search PubMed.
- P. Bhatt and R. Dayal, Phytochemistry, 1992, 31(2), 719–721 CrossRef CAS.
- P. Borai and R. Dayal, Phytochemistry, 1993, 33(3), 731–732 CrossRef CAS.
- P. Posri, J. Suthiwong, Y. Thongsri and C. Yenjai, Nat. Prod. Res., 2019 DOI:10.1080/14786419.2019.1672068.
- S. Kaennakam, P. Siripong and S. Tip-pyang, Fitoterapia, 2015, 105, 165–168 CrossRef CAS PubMed.
- A. Vanangamudi, R. Gandhidasan and P. V. Raman, Fitoterapia, 1998, 69(2), 180 CAS.
- S. Cheenpracha, R. Srisuwan, C. Karalai, C. Ponglimanont, S. Chantrapromma, K. Chantrapromma, H. K. Fun, S. Anjum and R. Atta, Tetrahedron, 2005, 61(36), 8656–8662 CrossRef CAS.
- J. Suthiwong, S. Pitchuanchom, W. Wattanawongdon, C. Hahnvajanawong and C. Yenjai, J. Nat. Prod., 2014, 77(11), 2432–2437 CrossRef CAS PubMed.
- L. V. Puyvelde, N. D. Kimpe, J. P. Mudaheranwa, A. Gasiga, N. Schamp, J. P. Declercq and M. V. Meerssche, J. Nat. Prod., 1987, 50(3), 349–356 CrossRef.
- A. Goel, A. Kumar and A. Raghuvanshi, Chem. Rev., 2013, 113(3), 1614–1640 CrossRef CAS PubMed.
- D. Slade, D. Ferreira and J. Marais, Phytochemistry, 2005, 66(18), 2177–2215 CrossRef CAS PubMed.
- S. Cheenpracha, C. Karalai, C. Ponglimanont and A. Kanjana-Opas, J. Nat. Prod., 2009, 72(8), 1395–1398 CrossRef CAS PubMed.
- Y. H. Seo, J. H. Jeon, M. Jeong, S. M. Ryu, W. K. Jeon, D. S. Jang, S. H. Shim, D. Lee, J. H. Choi and J. Lee, J. Nat. Prod., 2018, 81(7), 1598–1603 CrossRef CAS PubMed.
- P. Kumnerdkhonkaen, S. Saenglee, M. Asgar, G. Senawong, K. Khongsukwiwat and T. Senawong, BMC Complementary Altern. Med., 2018, 18, 130 CrossRef PubMed.
- A. D. Becke, J. Chem. Phys., 1993, 98(7), 5648–5652 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. J. A. Montgomery, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision B.01, Gaussian, Inc., Wallingford, CT, 2010 Search PubMed.
- G. Pescitelli and T. Bruhn, Chirality, 2016, 28(6), 466–474 CrossRef CAS PubMed.
- T. Yanai, D. P. Tew and N. C. Handy, Chem. Phys. Lett., 2004, 393(1–3), 51–57 CrossRef CAS.
- M. Cossi, N. Rega, G. Scalmani and V. Barone, J. Comput. Chem., 2003, 24(6), 669–681 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra07041j |
| This journal is © The Royal Society of Chemistry 2021 |