Open Access Article
Open Access ArticleUnderstanding the metal free alginate gelation process†
Ornella Ursini *a,
Roberta Angelinib,
Silvia Francob and
Barbara Cortesea
*a,
Roberta Angelinib,
Silvia Francob and
Barbara Cortesea
aNanotechnology Institute -National Research Council (CNR-NANOTEC), C/o Sapienza University of Rome, Piazzale A. Moro 2, 00185 Rome, Italy. E-mail: ornella.ursini@cnr.it
bInstitute for Complex Systems, National Research Council (CNR-ISC), C/o Sapienza University of Rome, Piazzale A. Moro 2, 00185 Rome, Italy
First published on 25th October 2021
Abstract
Alginate is a natural polysaccharide that has been recently gaining increasing attention as a biomaterial in the field of tissue engineering due to its favourable biocompatibility and gelation properties. Alginate hydrogels are commonly made by ionic crosslinking in the presence of divalent cations. Only a few studies have attempted to prepare alginate hydrogels without the presence of metal cations. Here the formation of metal free alginate hydrogels in the presence of the amino-acid glutamine is investigated. The transition from sol to gel is monitored by rheological measurements in the viscoelastic regime that reveal how the charged or neutral form of glutamine induces deep differences in the gelling ability. In particular, we show that the storage, G′, and loss, G′′, moduli differ significantly by shifting the glutamine zwitterionic equilibrium. Protonated amino acid could induce a shielding effect of the electrostatic repulsion of the alginate chains. Stable gels are obtained in the presence of a larger amount of free organic acid that gives rise to chain crosslink junctions and chain–chain stabilization. This opens up the possibility of preparing metal-free alginate hydrogels based on amino acid equilibria being pH sensitive.
1. Introduction
The field of regenerative tissue engineering is an interdisciplinary field that requires the interaction among a biomaterial and living cells to develop, augment or replace any tissue, organ or function of the body. The design of new biomaterials focuses on the mimicking of many functions of the extracellular matrices of body tissues, because the host responds to external stimuli in a well-defined manner.1–3 Three-dimensional (3D) scaffolds in particular play a crucial role in promoting and guiding tissue regeneration.4,5 Usually, they are biocompatible and biodegradable and display a highly appropriately connected pore structure with a large surface area. Naturally derived materials (i.e. alginate gels) have recently gained attention regarding their chemical properties and the geometrical features owing to their inherent biocompatibility.6–11The chemical nature and the structural feature of the binding process of this copolymer have been widely investigated.12–14
The most extensively studied crosslinking method is based on metal–polysaccharide coordination.
Alginate gelling by ionic cross-linking using multivalent ions has been known and exploited for several decades. In particular, calcium chloride is one of the most frequently used agents for ionic cross linking of alginate. Calcium ions (Ca2+) induce a sol/gel transition in mild gelling conditions.15 Recently new preparation approaches have been studied to improve the properties of the ionic alginate hydrogels.16,17
These gels have proven compatible with living cells and tissues, and alginates have therefore gained high popularity as an immobilization matrix in such systems.3,18,19
Up to now, few works have reported on alginate hydrogels prepared without the presence of metal cations. Perez-Madrigal and co-workers investigated the effect of some common organic solvents as agents to induce the gelation of alginate, observing the following gel stability.20
In particular, attention has been dedicated to understand the role of dimethylsulfoxide (DMSO) and of some dicarboxylic acids to act as crosslinking agent in the gelation process.
By computational simulations, the authors revealed a difference between the DMSO and oxalic acid in the coordination geometry of the alginate cage, specifying the role of water in the stability of these alginate gels.
In this paper we focus on the possibility to obtain alginate hydrogels without using metal cations. Encouraged by the aforementioned aspects underlining the influence of dicarboxylic groups as crosslinking agents for the alginate gelation process, we investigated the role of special amino acids as possible gelation agents in order to obtain new metal-free alginate.21
In particular we aim to understand the effect of carboxylic groups, namely the presence or not of a synergic effect, on the alginate gelation process, induced by suitable amino acids. The selected amino acids, glutamine and glutamic acid, were chosen to have structural features which contain a single carboxylic group chain joined with a carbonylic group and two carboxylic groups in the same molecule. Thus, we report the behavior and the viscoelastic properties of the amino-acid glutamine system. These structural features allow us to discriminate the role of each single group and to understand the occurrence of a possible joining effect.
This work can be of interest in shedding further insight on the preparation and behaviour of metal free-alginate hydrogels.
2. Materials and methods
2.1. Preparation of alginate solution
1.96008 g of Na-alginate, weighted by an analytical balance, is dissolved in 50 ml of pure deionized water obtaining the experimental concentration of 3.9% w/v.2.2. Preparation of formic acid (HCOOH) solution [1.0] M
377.2 μL of pure formic acid is dissolved in 10 ml of fresh deionized water obtaining a solution [1.0] M.2.3. Preparation of formic acid solutions
Three different solutions of pure HCOOH containing exactly the same amount of formic acid present in the acidified glutamine solutions were prepared. The details were described in the ESI.†2.4. Preparation of aqueous glutamine solution [0.175] M
0.25586 g of pure glutamine was dissolved with deionized water into 10 ml volumetric flask obtaining a solution [0.175] M. The pH value, measured by pH-meter, is 6.8 (mean value of five acquisitions).2.5. Preparation of glutamine solutions acidified with formic acid
We prepared three different solution of glutamine containing suitable volumes of HCOOH in order to obtain different molar ratios HCOOH/glutamine. The details were described in the ESI.†2.6. Preparation of alginate gels
The alginate gels were obtained as described in the ESI.†2.7. Characterization of gels: rheological measurement
Rheological measurements were performed on aqueous alginate solution 4.0% w/w (control sample), on alginate–glutamine solution, alginate–formic acid solutions (0.5H+, 1.0H+, 2.0H+) and on alginate–glutamine–formic acid solutions (0.5H+, 1.0H+, 2.0H+).The viscoelastic properties were probed using a rotational rheometer Anton Paar MCR102 with a cone–plate geometry.22,23 The plate diameter was of 49.97 mm, the cone angle of 2.006° and the truncation of 212 μm. The latter value represents the gap between the cone and plate within which the sample is contained during measurements. The temperature was controlled by a Peltier system on the bottom plate connected to a water bath and kept constant at T = (25.0 ± 0.1) K. To prevent sample evaporation the plates were covered with a rheometer integrated hood system designed for this aim. To probe the linear response, rheological measurements were carried out within the linear viscoelastic region that is achieved at sufficiently small values of the applied strain when the loss (G′) and storage (G′′) moduli are not dependent on strain. Frequency sweeps were performed in the range f = (10−2 to 102) Hz.
3. Results
3.1. Measure of viscoelastic properties
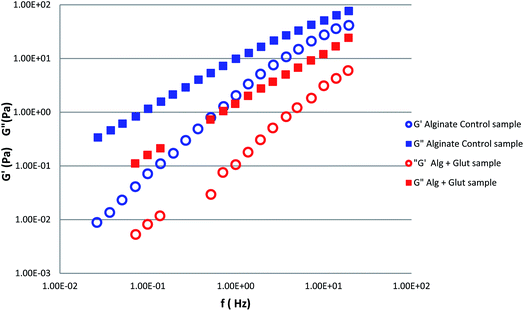
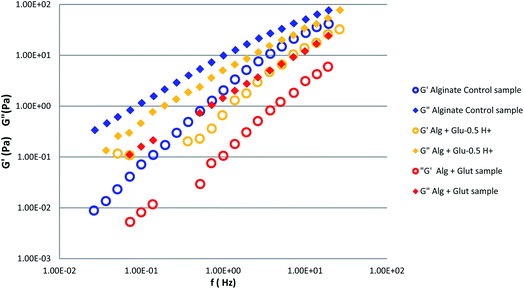
To investigate the alginate gelation process induced by glutamine, we measured the frequency dependence of the storage (G′) and loss (G′′) moduli for alginate aqueous solutions with and without the organic crosslinker (Fig. 1). G′ and G′′ moduli display a power low dependent frequency behavior over the probed frequency range, with the loss modulus G′′ higher than the storage modulus indicating that the system is in a liquid like state. The addition of aqueous glutamine solution to alginate 4% w/v, led to a decrease of the values of G′ and G′′ to almost one order of magnitude.In order to understand the glutamine gelation properties when the amino acid is present in a zwitterions or charged form, we measured G′ and G′′ moduli of samples obtained by adding the glutamine acidified solutions into the aqueous alginate. The presence of formic acid determines an increase of both moduli with respect to the case of alginate–glutamine sample (Fig. 2).
To clarify the role played by formic acid in alginate gelation, we measured G′ and G′′ moduli of alginate formic acid gels, at the different concentration of formic acid (Fig. 3).
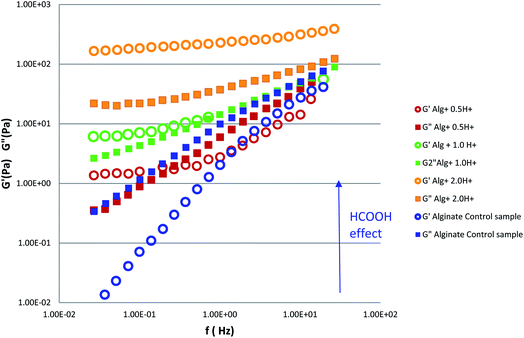 | ||
| Fig. 3 Storage (G′) and loss (G′′) moduli of alginate control sample (1 ml of aqueous solution alginate 4.0% w/w mixed with 1 ml H2O) compared with the samples HCOOH alginate gels. | ||
A significant change of the storage and loss moduli behaviour was observed.
The system passed from a liquid like status (G′′ > G′) in absence of formic acid to the situation in which the elastic modulus G′ is always bigger (one order of magnitude) than the storage modulus G′′. This feature indicates the passage to a solid like state for the sample containing the higher concentration of formic acid (sample Alg + 2.0H+ of Table 3 details in ESI†).
At intermediate molarities, a gradual transition is observed with a dominance of liquid like or solid like nature of the system depending on the frequency region.
To understand the gelation process induced by glutamine in relation to the pH, we measured the viscoelastic properties of the glutamine–alginate sample at different glutamine/formic acid molar ratios (Fig. 4).
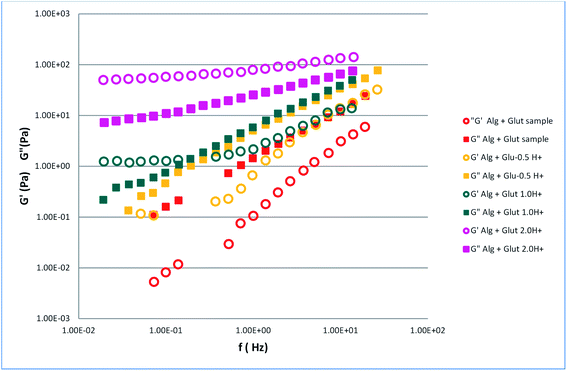 | ||
| Fig. 4 Storage (G′) and loss (G′′) moduli of glutamine–alginate sample and glutamine acidified solutions alginate gels. | ||
The acidified solutions of glutamine containing different glutamine/formic acid molar ratios, seems to produce a combined effect between the fluidization effect of glutamine and the opposite effect due to the presence of formic acid. The combined effect gave rise to the passage from a liquid to a solid like state. The moduli of alginate acidified glutamine samples (Fig. 4) were lower than the values observed for the alginate–formic acid gels (Fig. 3). These results seem to suggest a fluidization effect due to the presence of glutamine.
To clarify the fluidization effect, it is useful to compare the storage and loss moduli for the samples [Alg + glutamine + HCOOH 1.0H+] from Table 4 (details in ESI†) and the sample [Alg + HCOOH 0.5H+] from Table 3 (details in ESI†) (Fig. 5).
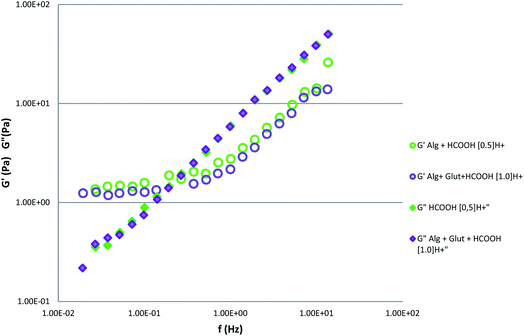 | ||
| Fig. 5 Storage (G′) and loss (G′′) moduli of sample containing alginate 4% w/v + 8.83 × 10−5 mol of HCOOH (sample code: Alg + 0.5H+ of Table 3†) and the sample containing alginate + 1.786 × 10−4 mol of glutamine + 1.780 × 10−4 mol of formic acid (sample code: Alg + Glu-1.0H+ of Table 4†). | ||
It should be noted that both the G′ and G′′ curves become completely overlapped for the two samples.
To clarify the effect of this overlapping, we applied the Henderson–Hasselbach (HH) equation, which describes the relation between the pH and the concentration of the amino acid using its pKa. To this aim, we take into account that:
• In the sample denoted as [Alg + glutamine + HCOOH 1.0H+] the moles of formic acid in contact with alginate solution are 1.780 × 10−4.
• In the sample denoted as Alg + HCOOH 0.5H+, the moles of formic acid are 8.830 × 10−5.
Moreover, the glutamine pKa value is = 2.1.
At a pH value = pKa, the concentration of [H3N+–X– COO−] is equal to [H3N+–X– COOH].
This means that the COOH groups of amino acid molecules present in dissociated/protonated form, are in the ratio half/half. Following the HH model, half moles of formic acid are used to give protonation of amino acid, shielding the negative charge of glutamine. Thus, the remaining amount of HCOOH is free to interact directly with alginate chains, producing the gelling effect.
Simple calculations, in the sample containing alginate + glutamine (1.786 × 10−4 mol) + HCOOH (1.780 × 10−4 mol), showed that the mole number of formic acid that remains free after the protonation of glutamine, is 8.87 × 10−5 mol.
This value is comparable with the mole number used in the {HCOOH – alginate} gels, where the formic acid moles are 8.83 × 10−5.
To reinforce our theory, we compared the trend curve of the storage (G′) and loss (G′′) moduli for the samples [Alg + glutamine + HCOOH 2.0H+] to the [Alg + HCOOH 1.0H+] (Fig. 6)
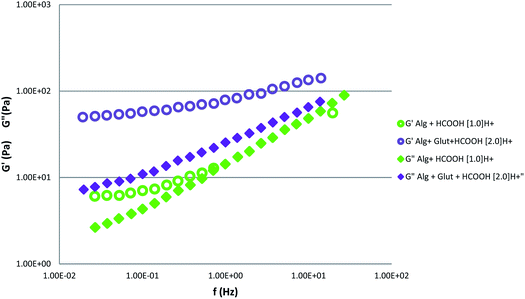 | ||
| Fig. 6 Storage (G′) and loss (G′′) moduli of sample contained alginate 4% w/v + 1.720 × 10−4 mol of HCOOH (sample code: Alg + 1.0H+ of Table 3†) and the sample contained alginate + 1.721 × 10−4 mol of glutamine + 3.44 × 10−4 mol of formic acid (sample code: Alg + Glu-2.0H +of Table 4†). | ||
In the sample Alg + Glu-2.0H+ containing alginate + glutamine + HCOOH, the mole number of formic acid that remains free after the protonation of the glutamine is 2.58 × 10−4, in fact as glutamine starting moles are 1.721 × 10−4 mol and the formic acid starting moles are 3.44 × 10−4 mol, following the HH model, the half amount of glutamine is protonated. Thus, HCOOH free = (3.44 × 10−4 − 0.86 × 10−4) was 2.58 × 10−4 mol.
This denotes that the amount of formic acid (2.58 × 10−4 mol) available is free to interact directly with alginate chains, producing the gelling effect. It is about twice the amount of formic acid contained in the sample Alg + HCOOH 1.0H+ (1.72 × 10−4).
The higher availability of [H+] pushes up the gelling process, and G′ becomes larger than G′′ for the whole range of frequencies corresponding to the formation of a gel-like system.
4. Discussion
Alginate is commonly available as sodium salt. Dissolving sodium alginate in water, one obtains an aqueous solution where the constitutive alginate units, (mannuronic and guluronic units) present their carboxylic groups in anionic form, as carboxylate negative ions. The behavior of alginate in aqueous solution depends on the pH of the medium.Our rheological measurements of alginate gels obtained by addition of formic acid in three different molar concentrations, described in Fig. 3, are in good agreement with the literature data, using different acid molecules.24
In particular, dynamic rheological measurements performed on alginate gel obtained by adding HCl have been previously described.25
The authors showed that the storage and loss moduli changed with free [H+] in solution. In particular, at the starting pH ≈6 they found G′(ω) < G′′(ω) (sol behaviour) in the whole frequency range while at pH = 3.05, G′(ω) ≈ G′′ (ω) for the whole frequency range. As the pH continues to decrease, G′ becomes larger than G′′ for the whole frequency range corresponding to the formation of a gel-like system.
In our work, we observe that the system passes to a solid like state for the highest concentrations of formic acid while at intermediate molarities, it undergoes a gradual transition with a dominance of liquid or solid like nature depending on the frequency region.
It is renowned that the alginate may undergo a sol/gel transition upon lowering the pH below the pKa value of the uronic acid residues forming alginic acid gels, although to the best of our knowledge, there is no any specific study using HCOOH as a gelling molecule.26,27
The study of the mechanism of alginic acid gel formation under acidic conditions reveals that the mannuronic acid blocks (M-blocks) support the formation of alginic acid gels.28
In the alginic acid gels, the guluronic acid blocks (G-blocks) along with the M-blocks support gel formation. In the alginate acidic gels both the M-blocks and G-blocks of alginate creates stable junctions and the gels. The acid gels are more of an equilibrium nature compared to ionic gels.29
The formation of the stable gel at higher concentrations of HCOOH is due to the shielding effect of the electrostatic repulsions between the carboxylic groups present in the alginate. Increasing the concentration of hydrogen ions, some dissociated carboxylic groups in alginate chains are gradually protonated. The hydrogen bonds involving –COOH and –OH groups give rise to chain crosslinking junctions and a stabilization occurs through the chain–chain interactions.
In the case of aqueous solution of sodium alginate, it presents a random coil conformation due to negative charges of carboxylate groups present along the alginate chains.24
With the progressive pH decrease, the hydrophobic segments of the alginate chains increase and the hydrophilic segments decrease, resulting in the substantial decrease in the size of the alginate assemblies.
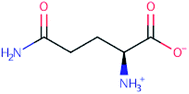
A singular behaviour was observed for the sample made up with alginate and glutamine solution 0.175 M when comparing G′ and G′′ with the alginate control sample since we observed a decrease of the moduli almost of one order of magnitude. This unexpected behaviour of the sample containing glutamine is related to the chemical feature of this amino acid that in solid state, presents a zwitterion form, i.e., as dipolar ion and the net charge of zwitterions is zero (Fig. 7).
When the amino acid is dissolved in water, its structure depends on the pH.
At pH = 5.65 (isoelectric value) glutamine is present as zwitterion with a net charge equal to zero.
If the pH value is higher than the isoelectric value (pH > 5.65), glutamine is present as an anion, with a net negative charge. This negative charge further increases the repulsive interactions due to the carboxylate groups of the alginate chains.
To understand if in our system the repulsive interactions between glutamine and alginate chains are present or not, we measured the pH of freshly prepared mixtures of alginate 4% w/v plus glutamine 0.175 M, resulting in a pH ≈ 6–7. At this pH value, glutamine probably reveals a net negative charge that leads to a fluidization effect due to the lack of shielding effect of the electrostatic repulsion between carboxylate groups of the alginate chains.
To verify this hypothesis and to understand better the viscoelastic properties, we prepared acidified glutamine solutions.
Adding different concentrations of HCOOH to the glutamine solutions, the equilibrium
| H3N+ –R–COO− + H3O+ ⇌ H3N+ –R–COOH + H2O |
The overlapping effect of G′ and G′′ observed in Fig. 5 confirmed that the addition of formic acid to the glutamine solution shifts the equilibrium reaction to the right, forming the positive charged amino acid.
The amino acid, as a positive charge molecule, shields the repulsive interactions existing between the alginate chains.
We could postulate that the overlapping effect of G′ and G′′ is a macroscopic behavior resulting from the combination of two molecular processes:
(a) The protonation reaction of the amino acid carboxyl group with the formation of positively charged molecules. The positive charge of amino acid molecule shields the repulsive interactions of the alginate chains;
(b) The direct protonation reaction of the alginate carboxyl groups.
The pristine electrostatic repulsions are screened allowing the stabilization of chain–chain interactions by a hydrogen bond network.
The slope of G′ and G′′ moduli observed in the Fig. 6 reinforces our hypothesis.
In fact, the comparison between these curves highlights the fact that formic acid acts as a protonation agent both for the glutamine and for the carboxylic groups of the alginate. Alginate is present in the aqueous solution as a negatively charged polymer generating an electrostatic repulsion between the polymer chains. When alginate is exposed to the acidic glutamine solution, and therefore to the protonated glutamine, the electrostatic repulsion between its chains is shielded by the positive charge of the positively charged amino acid. In addition to the protonation reaction of the amino acid, if formic acid is present in excess respect to the moles of the amino acid, it is free to interact directly with alginate chains, producing the gelling effect.
5. Conclusions
In the metal free alginate gelation, numerous factors need to be considered in order to understand the deep molecular interactions between the polysaccharide chains and the organic molecules.In our study, we investigated the possibility to use a special amino acid, glutamine, as organic molecule to induce the gelation process of alginate. We performed dynamic rheological measurement of the alginate–glutamine samples that reveal the important role of the zwitterionic form of the amino acid molecule. In aqueous medium the possibility to shift the zwitterionic equilibrium versus charged species is dominant. The addition of organic acid to the glutamine solution allows to distinguish the double role of proton free concentration. We investigated different ratios of concentration glutamine/formic acid as gelling systems. These investigations allowed us to empathize that the protonated amino acid induces a shielding effect to the alginate chains. The gels are also stabilized by a hydrogen bond network. The need to shift the glutamine zwitterionic equilibrium requires acidic conditions which may give rise to cytotoxic conditions. These results will allow us to understand the presence or not of a synergic effect when in the same molecules are simultaneously present two of carboxylic groups. This will set the basis to investigate the acid glutamic molecule and his gelling ability, shifting the zwitterionic equilibrium in different acid/base conditions. Finally our findings open the way to the design of new biomaterials using biocompatible alginate gels prepared without the presence of metal cations.
Author contributions
Conceptualization O. U.; data curation O. U.; methodology O. U.; rheological measurement O. U, R. A and S. F.; validation O. U.; writing—original draft preparation O. U; writing—review and editing O. U. and B. C.; funding acquisition O. U and B. C.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This research was partially supported by Progetto FISR—C. N. R project “Tecnopolo per la medicina di precisione” (TecnoMed Puglia) – Regione Puglia: DGR n.2117 del 21/11/2018, CUP: B84I18000540002 and “Tecnopolo di Nanotecnologia e Fotonica per la medicina di precisione” (TECNOMED) – FISR/MIUR-CNR: delibera CIPE n.3449 del 7-08-2017, CUP: B83B17000010001.References
- I. E. Palamà, S. D’Amone, P. Ratano, A. Donatelli, A. Liscio, G. Antonacci, M. Testini, S. Di Angelantonio, D. Ragozzino and B. Cortese, Cancers, 2019, 11(5), 643 CrossRef PubMed.
- M. Dalheim, J. Vanacker, M. Najmi, F. Aachmann, B. Strand and B. Christensen, Biomaterials, 2016, 80, 146–156 CrossRef CAS PubMed.
- K. Lee and D. Mooney, Prog. Polym. Sci., 2012, 37, 106–126 CrossRef CAS PubMed.
- I. E. Palamà, V. Arcadio, S. D'Amone, M. Biasiucci, G. Gigli and B. Cortese, Sci. Rep., 2017, 7, 12672 CrossRef PubMed.
- I. E. Palamà, S. D'Amone and B. Cortese, Front. Bioeng. Biotechnol., 2018, 6, 131 CrossRef PubMed.
- K. Lee and D. Mooney, Chem. Rev., 2001, 101, 1869–1879 CrossRef CAS PubMed.
- J. Drury and D. Mooney, Biomaterials, 2003, 24, 4337–4351 CrossRef CAS PubMed.
- D. Gyles, L. Castro, J. Silva and R. Ribeiro-Costa, Eur. Polym. J., 2017, 88, 373–392 CrossRef CAS.
- K. Draget, G. Skjak-Braek, B. Tang and P. Williams, Renewable Resources For Functional Polymers and Biomaterials, 2011, vol. 1, pp. 186–209 Search PubMed.
- B. Slaughter, S. Khurshid, O. Fisher, A. Khademhosseini and N. Peppas, Adv. Mater., 2009, 21, 3307–3329 CrossRef CAS PubMed.
- A. Augst, H. Kong and D. Mooney, Macromol. Biosci., 2006, 6, 623–633 CrossRef CAS PubMed.
- P. Sikorski, F. Mo, G. Skjak-Braek and B. Stokke, Biomacromolecules, 2007, 8, 2098–2103 CrossRef CAS PubMed.
- M. Stewart, S. Gray, T. Vasiljevic and J. Orbell, Carbohydr. Polym., 2014, 102, 246–253 CrossRef CAS PubMed.
- C. Kuo and P. Ma, Biomaterials, 2001, 22, 511–521 CrossRef CAS PubMed.
- S. Vicini, M. Castellano, M. Mauri and E. Marsano, Carbohydr. Polym., 2015, 134, 767–774 CrossRef CAS PubMed.
- A. Dodero, L. Pianella, S. Vicini, M. Alloisio, M. Ottonelli and M. Castellano, Eur. Polym. J., 2019, 118, 586–594 CrossRef CAS.
- J. Hu, P. Seeberger and J. Yin, Org. Biomol. Chem., 2016, 14, 8648–8658 RSC.
- O. Smidsrod and G. Skjakbraek, Trends Biotechnol., 1990, 8, 71–78 CrossRef CAS PubMed.
- J. Sun and H. Tan, Materials, 2013, 6, 1285–1309 CrossRef CAS PubMed.
- M. Perez-Madrigal, J. Torras, J. Casanovas, M. Haring, C. Aleman and D. Diaz, Biomacromolecules, 2017, 18, 2967–2979 CrossRef CAS PubMed.
- P. Gurikov and I. Smirnova, Gels, 2018, 4(1), 14 CrossRef PubMed.
- S. Franco, E. Buratti, V. Nigro, E. Zaccarelli, B. Ruzicka and R. Angelini, Int. J. Mol. Sci., 2021, 22(8), 4032 CrossRef CAS PubMed.
- S. Franco, E. Buratti, B. Ruzicka, V. Nigro, N. Zoratto, P. Matricardi, E. Zaccarelli and R. Angelini, J. Phys. Condens. Matter, 2021, 33, 174004 CrossRef CAS PubMed.
- Y. Cao, X. Shen, Y. Chen, J. Guo, Q. Chen and X. Jiang, Biomacromolecules, 2005, 6, 2189–2196 CrossRef CAS PubMed.
- H. Andriamanantoanina and M. Rinaudo, Polym. Int., 2010, 59, 1531–1541 CrossRef CAS.
- K. Draget, G. Braek and O. Smidsrod, Carbohydr. Polym., 1994, 25, 31–38 CrossRef CAS.
- K. Draget, G. SkjakBraek, B. Christensen, O. Gaserod and O. Smidsrod, Carbohydr. Polym., 1996, 29, 209–215 CrossRef CAS.
- K. Draget, B. Stokke, Y. Yuguchi, H. Urakawa and K. Kajiwara, Biomacromolecules, 2003, 4, 1661–1668 CrossRef CAS PubMed.
- K. Draget, G. Skjak-Braek and B. Stokke, Food Hydrocolloids, 2006, 20, 170–175 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra06599h |
| This journal is © The Royal Society of Chemistry 2021 |