Open Access Article
Open Access ArticleCharacteristics of poly-silicate aluminum sulfate prepared by sol method and its application in Congo red dye wastewater treatment†
Yunlong Zhaoa,
Yajie Zheng *a,
Yinglin Pengb,
Hanbing Hea and
Zhaoming Suna
*a,
Yinglin Pengb,
Hanbing Hea and
Zhaoming Suna
aSchool of Metallurgy and Environment, Central South University, Changsha 410083, China. E-mail: zyj@csu.edu.cn
bSchool of Materials and Chemical Engineering, Hunan City University, Yiyang 413099, China
First published on 29th November 2021
Abstract
A novel method for synthesizing poly-silicate aluminum sulfate coagulant (PSAS) using a silica-alumina sol was reported. Herein, two modalities (nSiO2/nNa2O: 1.11 and 3.27) of self-made water glasses were used as the silica source for synthesizing the sol precursor. Then, the PSAS1.11 and PSAS3.27 with different basicity were obtained by controlling the Al molar ratio of precursor to aluminum sulfate. The results showed that the PSAS1.11 coagulant prepared with low modulus water glass (LMWS, 1.11) has low turbidity and good stability. Using low modulus water glass, the effect of the Al molar ratio of precursor to aluminum sulfate on the basicity and stability of PSAS1.11 with Al/Si of 20 and the effect of the molar ratio of aluminum to silicon on the basicity and stability of PSAS1.11 were studied, respectively. Based on XRD and Fourier infrared (FTIR) characterization of the sol precursor and PSAS1.11, the synthesis mechanism of PSAS by the silica-alumina sol method was discussed. Al species distribution of PSAS1.11 was determined using the Al-Ferron timed spectrophotometric method. Moreover, the performance of PSAS1.11 coagulant was examined, regarding its efficiency towards color removal of Congo red. The results showed that PSAS1.11 coagulant with Al/Si of 20 and Al molar ratio of 1/12 exhibits excellent performance, and the color removal rate reached 98.6% at an initial pH of 11 and coagulant dosage of 40 mg L−1 (Al mg L−1). Finally, the PSAS coagulant mechanism was discussed in detail through infrared characterization, 27Al NMR, Raman, morphology and mapping of the flocs.
1. Introduction
Synthetic dyes are a necessity in various significant industries such as the paper, fiber, leather as well as textile industries for their colour-giving properties.1 Currently, more than 7000 types of synthetic dyes have been reported. It is estimated that 7 × 105 to 1 × 106 tonnes of various colourings are manufactured from about 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 commercially accessible dyes each year.2,3 However, during the production and use of dyes, approximately 10% of the dyes are released into the ecosystem as wastewater. Synthetic dyes, as the common pollutant in wastewater, are often toxic and carcinogenic, posing a great threat to the survival of terrestrial and marine life. Dyeing wastewater is characterized by deep color, high toxicity, high chemical oxygen demand (COD) and biochemical oxygen demand (BOD) values, complex composition, large discharge, wide distribution, and difficult degradation.4,5 Therefore, treatment of dye wastewater has been widely concerned. Several methods include biological approach, sedimentation, adsorption, oxidation, coagulation and flocculation (CF) have been reported.6–9 Biological methods are effective in reducing the chemical oxygen demand (COD) required for wastewater treatment, however, non-completely decolorize. Oxidation methods are complex, and it often needs to be used in combination with other methods. The adsorption method is difficult to apply in industry because of the difficulty of regenerating the adsorbent and the long adsorption time. CF methods are widely recognized and widely used because of its simple operation, large processing capacity and low investment cost.10,11
000 commercially accessible dyes each year.2,3 However, during the production and use of dyes, approximately 10% of the dyes are released into the ecosystem as wastewater. Synthetic dyes, as the common pollutant in wastewater, are often toxic and carcinogenic, posing a great threat to the survival of terrestrial and marine life. Dyeing wastewater is characterized by deep color, high toxicity, high chemical oxygen demand (COD) and biochemical oxygen demand (BOD) values, complex composition, large discharge, wide distribution, and difficult degradation.4,5 Therefore, treatment of dye wastewater has been widely concerned. Several methods include biological approach, sedimentation, adsorption, oxidation, coagulation and flocculation (CF) have been reported.6–9 Biological methods are effective in reducing the chemical oxygen demand (COD) required for wastewater treatment, however, non-completely decolorize. Oxidation methods are complex, and it often needs to be used in combination with other methods. The adsorption method is difficult to apply in industry because of the difficulty of regenerating the adsorbent and the long adsorption time. CF methods are widely recognized and widely used because of its simple operation, large processing capacity and low investment cost.10,11
For coagulation process, the coagulant with excellent performance is the core technology and research concerns. With the improvement of human living standards, conventional coagulation, such as aluminum and iron salts, can no longer meet the demand due to their high dosage and high residual metal concentration. They are gradually replaced by organic and inorganic polymer coagulants developed in recent years. Organic polymer coagulant is very variety and excellent performance. However, due to the high price and non-completely eliminating toxicity, it is mainly used as coagulation auxiliaries. Inorganic polymer flocculants have stronger electrical neutralization and net flutter bridging functions than traditional coagulation. They are also rapidly developed and used because of their cost-effective.12,13 The use of iron-base inorganic polymer coagulants is far inferior to that of aluminum inorganic polymer coagulants owing to low adjustable basicity and deep color of wastewater treated. So, aluminum inorganic polymer coagulants, such as polymeric aluminum chloride (PAC), poly-silicate aluminum chloride (PSAC), poly-silicate aluminum sulfate (PSAS) and poly-silicate aluminum ferric sulfate (PSAF) etc., have good performance in the coagulation of various wastewaters. Among of Si-containing coagulants, the introduction of silicic acid can enhance the coagulation bridging ability and complement the deficiency of original molecular weight and particle size. So, those coagulation has received particular attention.14
Usually, coexisting anions in the hydrolyzed aluminum solution are an important factor affecting the coagulation effect of aluminum-based coagulants, while affect the Al species distribution and structure of the coagulants. Coordination affinity of OH− to Al3+ is far greater than other anions such as Cl−, NO3− and SO42−. The order of coordination affinity between anion and Al3+ is: SO42− > Cl− > NO3−. Therefore, compared with PSAC, PSAS has a lower content of Al in the form of monomer or dimer (i.e., Ala), while the content of polymers (Alb) and Al(OH)3–solid (Alc) is higher.15–18 Meanwhile, the source of aluminum sulfate is more extensive than that of aluminum chloride, and it has reliable industrial production and is cheaper. Therefore, PSAS has higher research value than PSAC. Qiu et al., reported that banknote printing wastewater was treated using poly-silicate ferro-aluminum sulfate (PSFA). The maximal colour removal efficiency of 98% and COD removal efficiency of 85% could be achieved at the optimal dosage of 30.33 g L−1.19 The treatment of arsenic-containing wastewater by using PSFA and PSAS coagulants was reported by Li et al. The removal efficiency of arsenic was 98% and 93% respectively, when the molar ratio of (Fe3+ + Al3+)/SiO2 was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 using PSFA coagulant.20 In another study, polysilicate aluminum magnesium (PSAM) and cationic polyacrylamide (cPAM) were stepwise used for drinking water treatment. Under suitable conditions, the removal efficiency was over 98% for turbidity and color, respectively.21 To the best of the author's limited knowledge, the synthesis of PSAS is mainly divided into compound method and copolymerization two methods.12,22 The treatment of oil-contaminated water using the poly-silicate aluminum ferric sulfate prepared by compound method was reported by You et al.23 Compound method needs to obtain a poly-silicate acid solution in advance. In the compounding method, poly-silicate acid was prepared and used immediately to prevent the solution from gelation and thus loss of coagulation performance, its polymerization degree is difficult to control, as well insufficient combination of silicon and aluminum.24 Copolymerization method firstly mix sodium silicate and aluminum sulfate, and then alkalize and polymerize by using alkaline substances such as sodium aluminate, sodium carbonate or sodium hydroxide to obtain PSAS. Although the copolymerization method can avoid this phenomenon, silicon is easy to precipitate during the alkalization polymerization process. Moreover, the polymerization process requires strong agitation i.e. high speed shear force.12 Therefore, to overcome the drawbacks of the above methods, this work proposes a sol precursor method for the preparation of PSAS.
1 using PSFA coagulant.20 In another study, polysilicate aluminum magnesium (PSAM) and cationic polyacrylamide (cPAM) were stepwise used for drinking water treatment. Under suitable conditions, the removal efficiency was over 98% for turbidity and color, respectively.21 To the best of the author's limited knowledge, the synthesis of PSAS is mainly divided into compound method and copolymerization two methods.12,22 The treatment of oil-contaminated water using the poly-silicate aluminum ferric sulfate prepared by compound method was reported by You et al.23 Compound method needs to obtain a poly-silicate acid solution in advance. In the compounding method, poly-silicate acid was prepared and used immediately to prevent the solution from gelation and thus loss of coagulation performance, its polymerization degree is difficult to control, as well insufficient combination of silicon and aluminum.24 Copolymerization method firstly mix sodium silicate and aluminum sulfate, and then alkalize and polymerize by using alkaline substances such as sodium aluminate, sodium carbonate or sodium hydroxide to obtain PSAS. Although the copolymerization method can avoid this phenomenon, silicon is easy to precipitate during the alkalization polymerization process. Moreover, the polymerization process requires strong agitation i.e. high speed shear force.12 Therefore, to overcome the drawbacks of the above methods, this work proposes a sol precursor method for the preparation of PSAS.
Inspired by the preparation of zeolite from silica alumina sol, this research proposes a novel method for preparing PSAS from silica alumina sol. Herein, two modulus water glass extracted from bauxite reaction residue was used as silica source25 and mixed it with sodium aluminate to prepare sol precursor. Then, the PSAS1.11 and PSAS3.27 with different basicity were obtained by controlling Al molar ratio of precursor to aluminum sulfate. Later, a detailed study of synthesis of PSAS1.11 by using low modulus water glass was carried out, including the Al molar ratio of precursor to aluminum sulfate and the silica–aluminum ratio in PSAS1.11. The process and mechanism of the preparation of PSAS1.11 are discussed in detail based on the XRD and FTIR characterization of the precursor and PSAS. Moreover, the removal mechanism of Congo red was discussed by Al species analysis and flocs (combination of Congo red and PSAS1.11 coagulant) infrared analysis.
2. Materials and methods
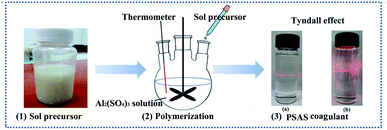
2.1 Preparation of poly-silicate aluminum sulfate by sol method
2.2 Analysis and methods
| Ala = Ca/CT × 100% | (1) |
| Alb = Cb/CT × 100% | (2) |
| Alc = 1 − (Ala + Alb) | (3) |
| η% = (A0 − At)/A0 × 100 | (4) |
3. Results and discussion
3.1 The structure and morphology of the precursor
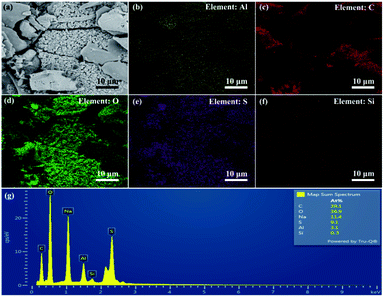
XRD pattern of the sol precursor1.11 solid powder is shown in Fig. 2(a). The precursor has no obvious diffraction peaks, which indicate that the precursors1.11 is no long-range crystalline order. In the sol formed by the combination of sodium aluminate and sodium silicate, silicon is preferentially combined with silicon or aluminum to form a Si–O–Si or Al–O–Si tetrahedral structure. The colloids formed during this process are almost the same as those formed before zeolite trans-crystallization, so the colloidal particles show no long-range crystalline order structure.27 Meanwhile, it can be seen from Fig. 2(c) that the precursor is formed by agglomeration of regular-shaped particles. Fig. 2(d) shows the energy spectra of the relevant elements in the precursors. Oxygen is the most abundant element in the precursor. In addition, the aluminum content is greater than that of silicon, which facilitates the adequate combination of aluminum and silicon. PSAS coagulants prepared by using this precursor may have good performance.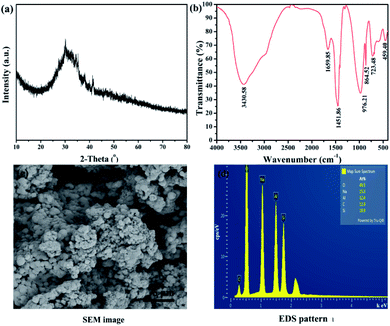 | ||
| Fig. 2 Precursor characterization; (a) XRD diffraction pattern, (b) FTIR spectra and (c) SEM images (d) EDS pattern. | ||
Fig. 2(b) presents the FT-IR spectra of sol precursor1.11. The peak at 3430.58 cm−1 can be attributed to the intermolecular association of the stretching vibration of –OH.28 The stronger absorption band located at 976 cm−1 is associated with aluminosilicate skeleton vibration.27 This indicates that sodium silicate and sodium aluminate do form soluble silica–aluminum colloids. The characteristic peak at 1655.85 cm−1 is the stretching vibration of water absorbed. The peak at about 948 cm−1 corresponds to the symmetric stretching vibrational structure of Si–O–Al. The peaks at 603–605 and 459–460 cm−1 are associated with the single bond vibrations of Si–O and Al–O, respectively. Two peaks at 723.48 cm−1 and 632 cm−1 are attributed to the antisymmetric bending and stretching vibrations of Al–OH, respectively.
3.2 Stability and basicity of PSAS coagulants
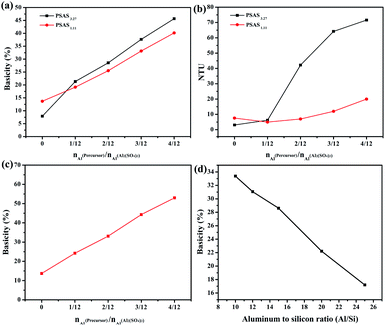
As shown in Fig. 3(b), the turbidity of PSAS coagulant increases with the increase in basicity.29 Especially, the turbidity increases significantly when the high modulus water glass is used. In terms of amorphous silica dissolved in strong alkali to form water glass, usually, there are three important areas in the amorphous silicon concentration pH diagram: (1) the insoluble domain is the precipitation zone of amorphous silicon; (2) the multimeric domain where silicon polyanions are stable; and (3) the monomeric domain where mononuclear Si species [Si(OH)4, SiO(OH)3−, and SiO2(OH)22−] prevail thermodynamically.30 Silica is mainly stabilized in water glasses (3.27) as polyanions and mononuclear. In contrast, a small amount of Si polyions is present in the low modulus water glass.
Table 1 presents the physical properties of PSAS coagulant. PSAS3.27 coagulant is very unstable and usually shows significant gelation after 2–3 days of storage at ambient temperature. Due to the poor stability, we did not consider subsequent dye performance tests on this material. However, the obtained PSAS1.11 coagulant is a clear colloidal solution with good stability, and its stability is enhanced significantly. Meanwhile, the PSAS1.11 coagulant solution has Tyndall effect as showing in Fig. S1.†
| nAl(precursor)/nAl(aluminum sulfate) | 0 | 1/12 | 2/12 | 3/12 | 4/12 |
|---|---|---|---|---|---|
| Al/Si(PSAS3.27) | 20 | 21.6 | 23.3 | 24.8 | 26.7 |
| Al/Si(PSAS1.11) | 20 | 21.6 | 23.3 | 24.8 | 26.7 |
| Al/Si(PSAS1.11) | 20 | 20 | 20 | 20 | 20 |
| Gel (day) (PSAS3.27) | 30 | 2 | 3 | 3 | 3 |
| Gel (day) (PSAS1.11) | 60 | 23 | 34 | Ungelled | Ungelled |
| Gel (day) (PSAS1.11, Al/Si of 20) | 60 | 20 | 75 | Ungelled | Ungelled |
| pH value (PSAS3.27) | 2.53 | 3.15 | 3.16 | 3.19 | 3.25 |
| pH value (PSAS1.11) | 2.86 | 3.28 | 3.33 | 3.34 | 3.36 |
| pH value (PSAS1.11, Al/Si of 20) | 2.86 | 3.29 | 3.38 | 3.45 | 3.47 |
Above-mentioned PSAS1.11, its basicity and ratio of Al/Si increase with the increase in the Al molar ratio of precursor to aluminum sulfate. Therefore, it is difficult to determine whether it is the aluminum to silicon ratio or the salinity that affects the performance of PSAS1.11 coagulant. Therefore, PSAS1.11 coagulants with aluminum–silica ratio of 20 were synthesized. Variation of coagulant basicity is shown in Fig. 2(c). It can be seen from Fig. 3(c) that the basicity of PSAS1.11 coagulant is linearly increasing in relation to the aluminum molar ratio of the precursor to aluminum sulfate. Therefore, the PSAS with different basicity prepared by precursor has obvious advantages, example controllable basicity. The physical properties of PSAS1.11 with aluminum to silica ratio of 20 are shown in Table 1.
The pH and gel time data for PSAS1.11 solution are given in Table 2. The results showed that the pH value and basicity of PSAS1.11 increased with the decrease of aluminum to silicon. Increase of PSAS basicity means that hydroxyl groups are consumed during the polymerization process of aluminum sulfate. If the hydroxyl group is provided by water causing the coagulant basicity increases, the pH of the solution will inevitably decrease. However, the interpretation is contrary to the experimental results. Since the precursors have the same Al content, the increased alkalinity of the coagulant could only be caused by the water glass.
| Al/Si | 25/1 | 20/1 | 15/1 | 12/1 | 10/1 |
|---|---|---|---|---|---|
| pH value (PSAS1.11) | 3.11 | 3.15 | 3.24 | 3.29 | 3.31 |
| Gel time (day) | 30 | 20 | 16 | 14 | 10 |
3.3 Analysis of the structure and morphology of PSAS coagulant
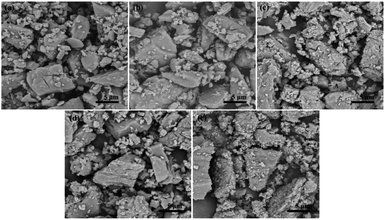 | ||
| Fig. 4 SEM images of different Al molar ratio of PSAS1.11 powder sample with an Al/Si ratio of 20, (a) 0, (b) 1/12, (c) 2/12, (d) 3/12 and (e) 4/12. | ||
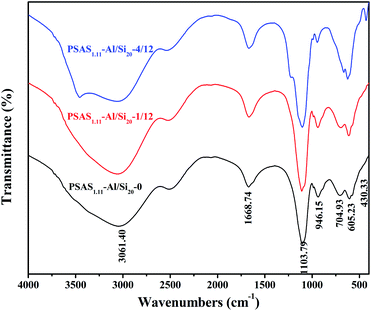
FTIR analysis of the PSAS1.11 powder samples with Al/Si ratio of 20 is shown in Fig. 5. Comparison with the precursor IR shows that some of the functional groups in the coagulant undergo obvious changes. The aluminosilicate skeleton peak of the alkaline intermediate located at 976 cm−1 was not significantly observed in the PSAS, indicating involvement of silica–aluminosols in the polymerization of the PSAS, the result that we expected. The stretching vibration of Si–O–Al bond is obvious at the peak of 948 cm−1. The peaks at 603–605 cm−1 are associated with Si–O bonds. Peaks of 723.48 cm−1 and 632 cm−1 are the tensile and bending vibrations of Al–OH in sodium aluminate, respectively. However, this structure was not detected in PSAS, indicating that sodium aluminate was completely involved in the polymerization reaction of PSAS. A broader peak appears at 3061 cm−1, which is characteristic of the C–O–H in stabilizers.31 The peak at 1103.79 cm−1 is the stretching vibration of the Al–OH–Al structure. –OH– group combined with different metal produces a slight shift in the position of the characteristic peak of this structure.23
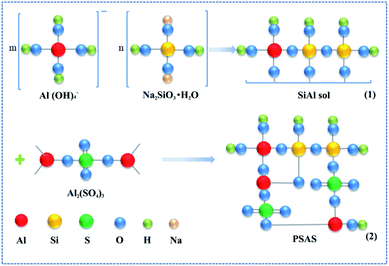
| NaAlO2 + 2H2O = Na+ + Al(OH)4− | (5) |
| xNa2[H2SiO4] + 2NaAl(OH)4 → Na2O·Al2O3·xSiO2·2H2O + 2xNaOH | (6) |
| Na2O·Al2O3·xSiO2·2H2O + NaAl(OH)4 + Al2(SO4)·18H2O + NaOH → Ala(OH)b(SO4)c(SiOx)d(H2O)e | (7) |
The molecular formula of PSAS: Ala(OH)b(SO4)c(SiOx)d(H2O)e, wherein, a = 1.0, b = 0.75–2.0, c = 0.3–1.12, d = 0.005–0.1, e > 4, x = 2.0–4. According to the above reactions, the schematic of preparing PSAS from the precursor is shown in Fig. 6.
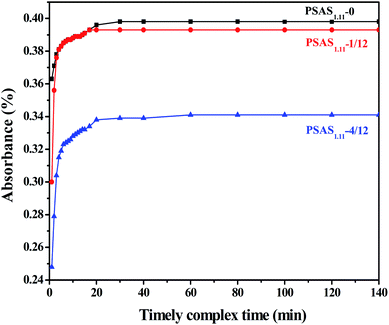
The complex reaction of PSAS1.11 and Ferron over time is shown in Fig. 7. With the increase of the aluminum molar ratio (precursor/aluminum sulfate), the basicity of PSAS1.11 coagulants gradually increases. This reflects the slow complexation reaction of Al and Ferron reagents. The PSAS1.11-0 solution showed the largest absorbance value, indicating the fastest complex reaction of Al and Ferron reagents. This result indicates that PSAS1.11-0/12 has the most mononuclear hydroxyaluminum complexes and primary aggregates. Polynuclear hydroxyl complexes of aluminum in PSAS1.11-1/12 and PSAS1.11-4/12 flocculants increased significantly. Especially, the result is more obvious when the basicity of the PSAS increases to more than 50%. Table S1† presents the Al species specific distribution of examined PSAS1.11 coagulants. Knowable, Al species in the coagulants prepared by sol method are mainly Ala and Alb. This result is attributable to the fact that the sol precursor first dissolves in aluminum sulfate and then polymerizes.
3.4 Decolorization performance of liquid PSAS1.11 on Congo red dye wastewater
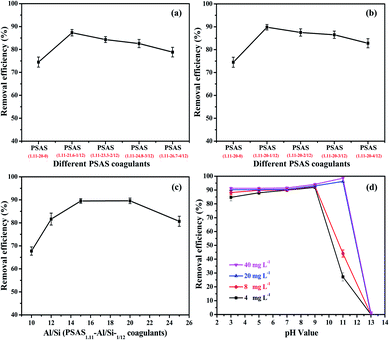
To clearly understand the reasons for the decline in coagulant performance, another group of PSAS1.11 coagulant with Al/Si of 20 prepared by different Al molar ratio was used. Fig. 8(b) shows the effect of coagulants with different Al molar ratios on the decolorization of dyeing wastewater. The same trend was observed compared to the above results. From the comparison of Fig. 8(a) and (b), the performance of PSAS1.11 (Al molar ratio of 12, Al/Si of 20) is improved, with decolorization rate of 89.9%, compared to PSAS1.11 (Al molar ratio of 12, Al/Si of 21.6). So, a certain degree of Al to Si ratio reduction can effectively improve the performance of coagulants. However, at the given condition (Al/Si of 20), the increase of coagulant basicity is not conducive to the decolorization of dyeing wastewater. It is not difficult to find the best performance of the PSAS coagulant prepared by using the Al molar ratio of 1/12. From the analysis of aluminum species distribution in PSAS1.11 (Al/Si of 20), increasing the basicity of coagulant caused the increase of Alb, so the performance of coagulant increased. However, when Al molar ratio is greater than 1/12, Alc increases, so the performance of coagulant shows a decreasing trend. Therefore, the suitable molar ratio of Al for the synthesis of PSAS1.11 coagulant is 1/12.
Fig. 8(c) shows the effect of different aluminum to silicon ratio of PSAS1.11 coagulants on the decolorization of dye wastewater. According to the performance test results, it can be seen that the decolorization ability of PSAS1.11 increases first and then decreases as the ratio of aluminum to silicon decreases. Suitable aluminum to silicon ratio of coagulants is between 15 and 20. The smaller the Al to Si ratio, the more pronounced the colloidal material observed in the coagulant, especially at an Al to Si ratio of 10. This result leads to a decrease in coagulant performance. Summarizing, PSAS1.11-1/12 coagulant with Al to Si ratio of 20 has the most excellent coagulation performance.
Well knows, coagulation is a physic-chemical process, which is highly dependent on solution pH, especially in the case of aluminum-based coagulants.34,35 So, in subsequent performance tests, we focus on PSAS1.11 (Al molar ratio of 12, Al/Si of 20) coagulant. The effects of the starting pH of the solution and the coagulant dose on the color removal rate are provided in Fig. 8(d). With below a dose of 8 mg L−1, the suitable working pH of PSAS1.11 coagulant is 9. At a relatively lower dosage of 4 mg L−1, the color removal efficiency of the coagulant of PSAS1.11 can reach 91.9%. However, with the increase of the dosage, the suitable working pH value of the coagulant increases. The suitable pH for dyeing wastewater treatment was 11 when the coagulant dose exceeded 20 mg L−1. In particular, PSAS1.11 shows excellent performance with color removal rate of 98.6% at the coagulant dosage of 40 mg L−1. Moreover, the coagulation performance of PSAS1.11 is compared against some coagulants reported references in Table S2.†36–39 This result clearly shows that as-prepared coagulant has high coagulation performance.
Moreover, the wastewater pH treated by coagulant was measured. With coagulant dosage of 4–8 mg L−1 and dyeing wastewater initial pH value of 3–9, the pH of the final treated solution is less than 5.0. However, working pH of 11, the pH value of the treated solutions are all greater than 10. When the coagulant dose was increased to 20 mg L−1, the pH of the treated solution decreased to below 6. Therefore, PSAS has a significant color removal rate for dyeing wastewater when the solution treated is acidic. A reasonable explanation that this result is relates to the Al species. Dissolved aluminum species Al(OH)4− > 6.8 can lead to the diminishing of Al(OH)3 precipitates,27 resulting in lower color uptake.
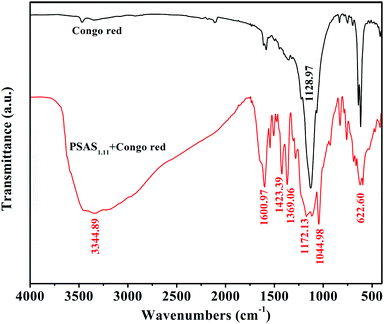
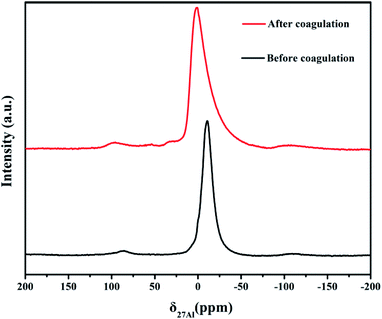
Fig. 10 shows the FTIR spectra of Congo red dye and flocculent. Black line is the transmittance curve of Congo red. The peak at 1128.97 cm−1 is the characteristic peak of R–SO3– structure. The peaks at 640 and 616 cm−1 are characteristic of C–H stretching vibrations of a disubstituted aromatic compound. After coagulant combined with the dye, the result of the infrared spectrum of the floc is shown in the red curve. Comparing the two curves, the intensity of the characteristic peak of R–SO3– structure at 1128.97 cm−1 is significantly weakened. The two R–SO3– groups were joined by Ala or Alb to form the R–SO3–Ala–SO3–R or R–SO3–Alb–SO3–R structures resulting in a decrease in the intensity of the characteristic peak of the R–SO3– structure. Suggesting that PSAS1.11 mainly reacts chemically with this structure, which is consistent with literature reports.38
Fig. S4† showed the Raman spectra of PSAS1.11 coagulant and floc. One typical feature is the result of scattering presentation of Al2SO4. The whole Raman spectral curve is not flat, which is caused by the no long-range crystalline order of polymers. In the red curve, the Raman shift is at 1442–1380 cm−1 for the –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– and aromatic ring conjugated structure.41 The peak of R–O–SO2– structure was found at about 1156 cm−1. The results indicate that the polymer of aluminum interacts with the sulfonic acid group in Congo red. This result is consistent with FTIR.
N– and aromatic ring conjugated structure.41 The peak of R–O–SO2– structure was found at about 1156 cm−1. The results indicate that the polymer of aluminum interacts with the sulfonic acid group in Congo red. This result is consistent with FTIR.
4. Conclusions
(1) Silica-alumina sol precursor was successfully used to synthesize poly-silicate aluminum sulfate coagulant. The prepared PSAS3.27 coagulant exhibits poor stability and high turbidity, with significant gelation typically occurring after 2–3 days of storage at ambient temperature. This makes it unsuitable for further testing and analysis. However, the PSAS1.11 coagulant solution prepared by low modulus (1.11) water glass had the advantages of low turbidity and good stability. With aluminum–silica ratio of 20 and the Al molar ratio (precursors to aluminum sulfate) of 3/12 or more, as-prepared PSAS1.11 coagulant had the long-term stability, no gel for several months. Therefore, it is of obvious practical significance to carry out further research on PSAS1.11 coagulant.(2) Al species distribution analysis of PSAS1.11 shown that the initial aggregation state Al (Ala) is gradually changes to Al (Alb and Alc) in the polymerization states and solids, with the increase of basicity.
(3) PSAS1.11 coagulant prepared with an aluminum-silica ratio of 20 and Al molar ratio of 1/12 between precursor to aluminum sulfate had good performance for dyeing wastewater treatment, and the color removal rate reached 98.6%, at solution initial pH of 11 and coagulant dosage of 40 mg L−1.
(4) The decolorization mechanism of PSAS1.11 coagulant suggested, charge neutralization reactions occur between the anionic Congo red molecules and the monomeric and polymeric metal ions. Two anionic Congo red molecules are bound together by positively charged aluminum polymers, thus completing the removal of the dye through a bridging process.
Author contributions
The contributions of all authors are now introduced as follows: the first author Yunlong Zhao completed the experiment and paper writing. Yajie Zheng mainly guided the paper direction and paper revision. Yinglin Peng participated in part of the experimental research and discuss. Hanbing He completed XRD and SEM data analysis. Zhaoming Sun completed part of the data analysis.Conflicts of interest
There are no conflicts to declare.References
- J. Abdi, M. Vossoughi, N. M. Mahmoodi and I. Alemzadeh, Synthesis of metal-organic framework hybrid nanocomposites based on GO and CNT with high adsorption capacity for dye removal, Chem. Eng. J., 2017, 326, 1145–1158 CrossRef CAS.
- V. Katheresan, J. Kansedo and S. Y. Lau, Efficiency of various recent wastewater dye removal methods: a review, J. Environ. Chem. Eng., 2018, 6(4), 4676–4697 CrossRef CAS.
- C. R. Holkar, A. J. Jadhav, D. V. Pinjari, M. Naresh, N. M. Mahamuni and A. B. Pandit, A critical review on textile wastewater treatments: possible approaches, J. Environ. Manage., 2016, 182, 351–366 CrossRef CAS PubMed.
- Z. Z. Sun, Z. H. Liu, L. Han, D. L. Qin, G. Yang and W. H. Xing, Study on the treatment of simulated azo dye wastewater by a novel micro-electrolysis filler, Water Sci. Technol., 2019, 79(11), 2279–2288 CrossRef CAS PubMed.
- H. Zazou, H. Afanga, S. Akhouairi, H. Ouchtak, A. A. Addi, R. A. Akbour, A. Assabbane, J. Douch, A. Elmchaour, J. Duplay, A. Jada and M. Handani, Treatment of textile industry wastewater by electrocoagulation coupled with electrochemical advanced oxidation process, J. Water. Process. Eng., 2019, 28, 214–221 CrossRef.
- N. Dafale, N. N. Rao, S. U. Meshram and S. R. Wate, Decolorization of azo dyes and simulated dye bath wastewater using acclimatized microbial consortium-biostimulation and halo tolerance, Bioresour. Technol., 2008, 99(7), 2552–2558 CrossRef CAS PubMed.
- H. P. Jia, H. Gang, Z. Rui and X. S. Zhao, Hierarchical N-doped TiO2 hollow microspheres consisting of nanothorns with exposed anatase 101 facets, Chem. Commun., 2011, 47(24), 6942–6944 RSC.
- H. Mittal, A. Maity and S. S. Ray, Gum karaya based hydrogel nanocomposites for the effective removal of cationic dyes from aqueous solutions, Appl. Surf. Sci., 2016, 364, 917–930 CrossRef CAS.
- H. Li, S. Liu, J. Zhao and N. Feng, Removal of reactive dyes from wastewater assisted with kaolin clay by magnesium hydroxide coagulation process, Colloids Surf., A, 2016, 494(5), 222–227 CrossRef CAS.
- T. Chen, B. Gao and Q. Yue, Effect of dosing method and pH on color removal performance and floc aggregation of polyferric chloride-polyamine dual-coagulant in synthetic dyeing wastewater treatment, Colloids Surf., A, 2010, 355(1–3), 121–129 CrossRef CAS.
- Y. J. Zheng, Z. Q. Gong, L. H. Liu and B. Z. Chen, Comparisons of species and coagulation effects of PFS solution and solid PFS from pyrite cinders, T. Nonferr. Soc., 2002, 12(005), 983–986 CAS.
- H. X. Tang, Inorganic polymer flocculation theory and coagulant, China Building Industry Press, 2006, ch 4 Search PubMed.
- J. Ma, R. Wang, X. Wang, H. Zhang, B. Zhu, L. L. Lian and D. W. Lou, Drinking water treatment by stepwise flocculation using polysilicate aluminum magnesium and cationic polyacrylamide, J. Environ. Chem. Eng., 2019, 7(3), 103049 CrossRef CAS.
- T. Sun, L. L. Liu, L. L. Wan and Y. P. Zhang, Effect of silicon dose on preparation and coagulation performance of poly-ferric-aluminum–silicate-sulfate from oil shale ash, Chem. Eng. J., 2010, 163(1–2), 48–54 CrossRef CAS.
- L. L. Cai, Y. Q. Xie, Q. P. Zhuang and M. Niu, Effects of polysilicate aluminum sulfate on fire resistance of ultra-low density materials, J. Beijing For. Univ., 2014, 36(5), 136–141 Search PubMed.
- Z. M. Qiu, W. T. Jiang and Z. J. He, Post-treatment of banknote printing wastewater using polysilicate ferro-aluminum sulfate (PSFA), J. Hazard. Mater., 2009, 166(2–3), 740–745 CrossRef CAS PubMed.
- S. Q. Li, P. J. Zhou, D. Ling and K. Feng, Treatment of oily wastewater using composite flocculant of polysilicate ferro-aluminum sulfate – rectorite, J. Water. Res. Prot., 2011, 3(4), 253–261 CrossRef CAS.
- J. Li, T. Xu, D. J. Bao, Z. L. Xu and Z. M. Liu, Study on the flocculation performance of poly-silicate aluminum and magnesium on domestic sewage, Appl. Mech. Mater., 2014, 7, 587–589 Search PubMed.
- Z. M. Qiu, J. W. Tiang and Z. J. He, Post-treatment of banknote printing wastewater using polysilicate ferro-aluminum sulfate (PSFA), J. Hazard. Mater., 2009, 166(2–3), 740–745 CrossRef CAS PubMed.
- Q. Li, S. Wang and L. Wang, Disposal of Arsenic Wastewater by Polysilicate Metal Sulfates in Power Plant, The 3rd International Conference on Bioinformatics and Biomedical Engineering (iCBBE), 2009, pp. 1–4 Search PubMed.
- J. Ma, R. Wang and X. Wang, et al., Drinking water treatment by stepwise flocculation using polysilicate aluminum magnesium and cationic polyacrylamide, J. Environ. Chem. Eng., 2019, 7(3), 103049 CrossRef CAS.
- W. Zhang, T. Zhang, L. Xi, H. J. Gu, Y. H. Hu, C. Gu and Y. J. Zhang, Preparation of new type poly-silicate coagulant and its coagulation property, Energy Procedia, 2012, 17(1), 1627–1634 CrossRef CAS.
- Z. Y. You, L. Zhang, S. J. Zhang, Y. J. Sun and K. J. Shah, Treatment of oil-contaminated water by modified polysilicate aluminum ferric sulfate, Processes, 2018, 6(7), 95 CrossRef.
- R. H. Busey and R. E. Mesmer, Ionization equilibriums of silicic acid and polysilicate formation in aqueous sodium chloride solutions to 300 °C, Inorg. Chem., 1997, 16(10), 2444–2450 CrossRef.
- Y. L. Zhao, Y. J. Zheng, H. B. He, Z. M. Sun and A. Li, Silica extraction from bauxite reaction residue and synthesis water glass, Green. Process. Synth., 2021, 10, 268–283 CrossRef.
- A. K. Tolkou, M. Mitrakas, I. A. Katsoyiannis, M. Ernst and A. I. Zouboulis, Fluoride removal from water by composite Al/Fe/Si/Mg pre-polymerized coagulants: characterization and application, Chemosphere, 2019, 231, 528–537 CrossRef CAS PubMed.
- J. Singh and R. L. White, A variable temperature infrared spectroscopy study of NaA zeolite dehydration, Vib. Spectrosc., 2017, 94, 37–42 CrossRef.
- Y. Zeng and J. Park, Characterization and coagulation performance of a novel inorganic polymer coagulant-poly-zinc-silicate-sulfate, Colloids Surf., A, 2009, 334(1–3), 147–154 CrossRef CAS.
- D. Hasse, N. Spiratos and C. Jolicoeur, European Patent, no. 0372715A1, 1990, vol. 6, p. 13 Search PubMed.
- W. Stumm, H. Huper and R. L. Champlin, Formulation of polysilicates as determined by coagulation effects, Enviorn. Sci. Technol., 1967, 1(3), 221–227 CrossRef CAS.
- Q. Lin, H. Peng, S. Zhong and J. X. Xiang, Synthesis, characterization, and secondary sludge dewatering performance of a novel combined silicon–aluminum–iron–starch flocculant, J. Hazard. Mater., 2015, 285, 199–206 CrossRef CAS PubMed.
- W. Liu, Z. L. Yin and Z. Y. Ding, Low-temperature phase transitions of sodium aluminate solutions, T. Nonferr. Soc., 2019, 29, 194–199 CrossRef CAS.
- H. Wang, Q. M. Feng and K. Liu, The dissolution behavior and mechanism of kaolinite in alkali-acid leaching process, Appl. Clay Sci., 2016, 132–133, 273–280 CrossRef CAS.
- S. Aoudj, N. Drouiche, M. Hecini, T. Ouslimane and B. Palaouane, Coagulation as a post-treatment method for the defluoridation of photovoltaic cell manufacturing wastewater, Proc. Eng., 2012, 33, 111–120 CrossRef CAS.
- Z. He, R. Liu, Z. Hu, H. Liu and J. Qu, Defluoridation by Al-based coagulation and adsorption: species transformation of aluminum and fluoride, Separ. Purif. Technol., 2015, 148, 68–75 CrossRef CAS.
- K. Prajapati, L. G. Sorokhaibam, V. M. Bhandari, D. J. Killedar and V. V. Ranade, Differentiating process performance of various coagulants in removal of Congo Red and Orange G Dyes, Int. J. Chem. React. Eng., 2016, 14(1), 195–211 CrossRef CAS.
- H. Patel and R. T. Vashi, Removal of Congo Red dye from its aqueous solution using natural coagulants, J. Saudi Chem. Soc., 2012, 16(2), 131–136 CrossRef CAS.
- Y. X. Wei, Q. Z. Ji, L. Chen, J. W. Hao, C. L. Yao and X. Z. Dong, Preparation of an inorganic coagulant-polysilicate–magnesium for dyeing wastewater treatment: effect of acid medium on the characterization and coagulation performance, J. Taiwan. Inst. Chem., 2017, 72, 142–148 CrossRef CAS.
- J. Garvasis, A. R. Prasad, K. O. Shamsheera, P. K. Jaseela and A. Joseph, Efficient removal of Congo red from aqueous solutions using phytogenic aluminum sulfate nano coagulant, Mater. Chem. Phys., 2020, 251, 123040 CrossRef CAS.
- P. Zhang, H. Hahn, E. Hoffmann, E. Hoffmann and G. Zeng, Influence of some additives to aluminium species distribution in aluminium coagulants, Chemosphere, 2004, 57(10), 1489–1494 CrossRef CAS PubMed.
- J. A. Dean, Lang's Handbook of Chemistry, Science Press, Beijing, 15th edn, 2003, ISBN: 7-03-010409-9.05 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra06343j |
| This journal is © The Royal Society of Chemistry 2021 |