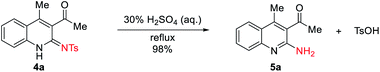Open Access Article
Open Access ArticleA simple and efficient copper-catalyzed three-component reaction to synthesize (Z)-1,2-dihydro-2-iminoquinolines†
Xiai Luoabc,
Yu Zhaoa,
Susu Taoa,
Zhong-Tao Yangab,
Hui Luo*abd and
Weiguang Yang *abd
*abd
aGuangdong Key Laboratory for Research and Development of Natural Drugs, The Marine Biomedical Research Institute, Guangdong Medical University, Zhanjiang, 524023, China. E-mail: luohui@gdmu.edu.cn; 09ywg@163.com
bThe Marine Biomedical Research Institute of Guangdong Zhanjiang, Zhanjiang, Guangdong 524023, China
cDepartment of Pharmacy, Hunan University of Medicine, Huaihua, 418000, China
dSouthern Marine Science and Engineering Guangdong Laboratory (Zhanjiang), Zhanjiang, Guangdong 524023, China
First published on 29th September 2021
Abstract
A operationally simple synthesis of (Z)-1,2-dihydro-2-iminoquinolines that proceeds under mild conditions is achieved by copper-catalyzed reaction of 1-(2-aminophenyl)ethan-1-ones, sulfonyl azides and terminal ynones. In particular, the reaction goes through a base-free CuAAC/ring-opening process to obtain the Z-configured products due to hydrogen bonding.
Nitrogen-containing polyheterocycles are present in a wide variety of bioactive natural products1 and biological molecules that may be good drug candidates.2 Specifically, quinoline-based compounds represent a medicinally and pharmaceutically important class of heterocyclic motifs that are found as the core structural skeletons in a variety of potential candidates.3 2-Aminoquinolines are found to be antagonists for the hormone 1-receptor (MCH1-R),4 as targets for JNK phosphorylation,5 as potent and selective neuronal nitric oxide synthase inhibitors,6 and as new inhibitors of protein kinase CK2.7 Therefore, the development of novel methods for the synthesis of these quinoline derivatives is important in the field of synthetic organic and pharmaceutical chemistry.
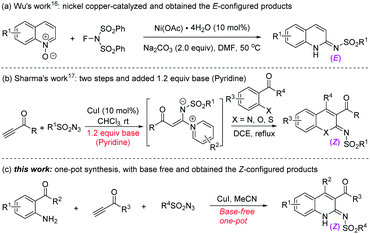
In the past few years, utilizing the annulation reactions of Cu,8 Pd,9 Ni,10 Ag,11 Ru,12 and a few other catalysts13–15 have provided attractive and valuable routes for the construction of 2-aminoquinolines. As an isomer of 2-aminoquinolines, the synthesis of 2-iminoquinoline skeletons has still rarely been investigated. To the best of our knowledge, only two examples of the nickel16 or copper-catalyzed17 cascade reaction have been developed, leading to 2-iminoquinolines. However, these two examples have been limited to the use of a base or obtained the E-configured products (Scheme 1a and b).
Previous studies reported on the copper-catalyzed multicomponent reactions (MCRs) of sulfonyl azides and terminal alkynes with other components that generated N-heterocycles and related compounds (CuAAC/ring-opening reaction),18,19 and have also been used in the synthesis of 2-aminoquinolines20 and 2-iminoquinolines.17 However, the reaction was generally carried out under strong basic conditions. This limited the application of some substrates, such as terminal ynones, which would undergo self-condensation under the basic conditions.21 Thus, neutral or weak acidic conditions have been developed in our previous study, and the terminal ynones were successfully used in the CuAAC/ring-opening reaction to form highly active intermediate α-acyl-N-sulfonyl ketenimines.22 Herein, we report the base-free copper-catalyzed reaction of 1-(2-aminophenyl)ethan-1-ones, sulfonyl azides and terminal alkynes, leading to Z-configured 2-iminoquinolines (Scheme 1c).
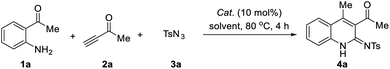
Our investigations began with an examination of the synthesis of the parent and previously unreported system, N-(3-acetyl-4-methylquinolin-2(1H)-ylidene)-4-methylbenzene sulfonamide (4a), from 1-(2-aminophenyl)ethan-1-one (1a), but-3-yn-2-one (2a) and p-tosyl azide (3a). Initial screenings involved using CuI as a catalyst in a range of standard solvents. These screenings revealed that the desired conversion could be achieved in many solvents (Table 1, entries 1–9), with MeCN delivering product 4a in highest yield (96%). The other solvents gave comparable yields, such as DCE, toluene, THF and 1,4-dioxane, while DMSO and DMF gave the lowest yield of 4a at 20% and 46%, respectively. Thus, the optimal solvent was determined to be MeCN. Encouraged by this promising result, a variety of catalysts were screened. Among the copper catalysts used, most Cu-catalysts exhibited high catalytic reactivity in this reaction, whether it was CuI-catalysts or CuII-catalysts (Table 1, entries 10–14). Other catalysts, such as AgOAc, failed to produce the desired product (Table 1, entries 15). Lastly, the effect of temperature was evaluated. Screening results revealed that a reaction temperature above or below 80 °C decreased the reaction yield and produced side-products (Table 1, entries 16 and 17).
| Entry | Cat. | Solvent | Yieldb (%) 4a |
|---|---|---|---|
| a Reaction conditions: 1a (0.5 mmol), cat. (10 mol%) in the solvent (3 mL) was added 2a (1.5 equiv.) and 3a (1.5 equiv.) stirring at 80 °C for 4 h.b Isolated yields.c nd = not detected the target product.d The reaction temperature was 70 °C.e The temperature was 90 °C. | |||
| 1 | CuI | CHCl3 | 72 |
| 2 | CuI | DCE | 81 |
| 3 | CuI | Toluene | 79 |
| 4 | CuI | MeCN | 96 |
| 5 | CuI | THF | 85 |
| 6 | CuI | 1,4-Dioxane | 94 |
| 7 | CuI | DMSO | 20 |
| 8 | CuI | DMF | 46 |
| 9 | CuI | EtOH | 40 |
| 10 | CuCl | MeCN | 88 |
| 11 | CuBr | MeCN | 84 |
| 12 | CuBr2 | MeCN | 78 |
| 13 | Cu(OAc)2 | MeCN | 80 |
| 14 | Cu(OTf)2 | MeCN | 22 |
| 15 | AgOAc | MeCN | ndc |
| 16 | CuI | MeCN | 90d |
| 17 | CuI | MeCN | 86e |
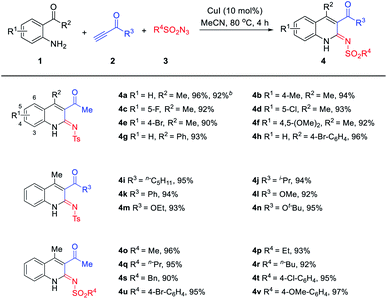
With optimized reaction conditions for the formation of the “parent reaction” having been defined, the capacity of these to affect the coupling of a range of different substrates was investigated. As shown in Table 2, the electron effects of the substituents R1 had slight influences for the substrates 1. For example, substrates bearing a 4-Me, 5-F, 4-Br and 4,5-(OMe)2 group were examined, and 90–96% yields of 4a–4f were isolated. The substrates R2 bearing the –Ph and 4-Br–C6H4 group also can obtain 4g–4h in good yield. Next, the scope and limitation of the substrate terminal ynones 2 were tested. When R3 was employed by the n-pentyl, isopropyl, –Ph, –OMe, –OEt and –Ot–Bu groups, it provided the corresponding iminoquinoline derivatives 4i–4n in good yields of 92–95%. It is noteworthy that the substrate sulfonyl azides also showed slight influences for the reaction. The R4 changed for aliphatic or aromatic substituents also can smoothly give the anticipated products (4o–4v) in excellent yields. From the above experimental results, this reaction is easy to operate and highly efficient.
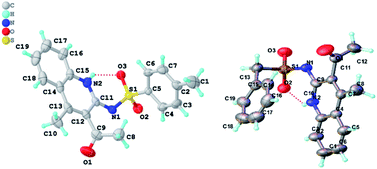
Except for 4m, none of the products 1,2-dihydro-2-iminoquinolines 4a–4v have been reported previously, which were subject to full spectroscopic characterization (see ESI for details†) and the derived data were in complete accordance with the assigned structures. Furthermore, 4a and 4s were confirmed by single-crystal X-ray analysis (Fig. 1). These analyses revealed that both incorporate Z-configured imine residues due to the hydrogen bonding (Fig. 1, the red dotted line). Thus, it has been assumed that all the other products formed during the course of this study possess the same geometry about the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond.
N bond.
In order to further explain the effect of hydrogen bonding on the spatial structure of products, we synthesized N1 substituted product 4w through 1-(2-(methylamino)phenyl)ethan-1-one (1i), but-3-yn-2-one (2a) and p-tosyl azide (3a) under the standard condition. The single crystal analyses revealed that the product 4w gave the E-configured imine residues without the hydrogen bonding (Fig. 2).
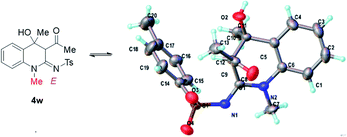 | ||
| Fig. 2 Single-crystal X-ray analysis of 4w (CCDC 2092350). | ||
Products 4a–4v are all relatively stable species that survive chromatographic purification under conventional conditions. However, the 2-iminoquinolines skeletons were easy hydrolysis to 2-aminoquinolines. For example, upon treatment with 1.5 equivalents of 30% H2SO4 in water under reflux for 6 h, compound 4a is converted into 2-iminoquinolines product 5a of yield 98% (Scheme 2).
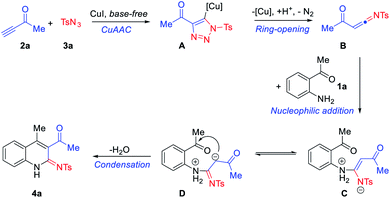
A possible reaction pathway for the formation of N-(3-acetyl-4-methylquinolin-2(1H)-ylidene)-4-methylbenzenesulfonamide (4a) from precursors 1a, 2a and 3a is shown in Scheme 3. Thus, in keeping with earlier proposals,22 substrates 2a and 3a are expected to react in the presence of the copper(I) catalyst, so as to form the metallated triazole A that fragments with the accompanying loss of nitrogen to form a highly active intermediate, α-acyl-N-sulfonyl ketenimine B. Then, B is captured by 1a to generate the adduct C, which can transfer to the isomer D that undergoes aldol condensation to deliver the observed product 4a.
In summary, we have developed an operationally simple and effective means for preparing (Z)-1,2-dihydro-2-iminoquinolines from a mixture of the corresponding 1-(2-aminophenyl)ethan-1-ones, sulfonyl azides and terminal ynones through the base-free CuAAC/ring-opening process, and obtain the Z-configured products. This methodology is quite flexible and offers the capacity to generate forms of the title products that will be particularly useful in, for example, building more 2-iminoquinolines block facility.
Experimental
General
All melting points were determined on a Yanaco melting point apparatus and were uncorrected. IR spectra were recorded as KBr pellets on a Nicolet FT-IR 5DX spectrometer. All spectra of 1H NMR (400 MHz) and 13C NMR (100 MHz) were recorded on a Bruker AVANCE NEO 400 MHz spectrometer in DMSO-d6 or CDCl3 (otherwise as indicated), with TMS used as an internal reference and the J values are given in Hz. HRMS were obtained on a Thermo Scientific Q Exactive Focus Orbitrap LC-MS/MS spectrometer. All 1-(2-aminophenyl)ethan-1-ones (1a–1i, see ESI Section 1†) were prepared by purchase, terminal ynones (2a–2g, see ESI Section 1†) were prepared by purchase or literature methods,23 and sulfonyl azides (3a–3i, see ESI Section 1†) were prepared by literature methods.24Preparation and characterizations of compounds 4a–4w and 5a
The products 4b–4w were prepared by the similar procedure.
All NMR spectra: please see ESI Section 3.†
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support was provided by the Foundation and Applied Basic Research Fund project of Guangdong Province of China (2019A1515110918); Medical Scientific Research Foundation of Guangdong Province (A2021037 and A2020202); Discipline Construction Project of Guangdong Medical University (4SG21004G); Funds for PhD researchers of Guangdong Medical University in 2021; and the Science and technology program of Guangdong Province (2019B090905011).Notes and references
- (a) L. Chen, Z. Deng and C. Zhao, ACS Chem. Biol., 2021, 16, 559 CrossRef CAS PubMed; (b) G. E. Chambers, A. E. Sayan and R. C. D. Brown, Nat. Prod. Rep., 2021 10.1039/D0NP00096E; (c) S. Bhambhani, K. R. Kondhare and A. P. Giri, Molecules, 2021, 26, 3374 CrossRef CAS PubMed; (d) A. Wang, P. Li, P. Han, G. Gu, T. Shan, D. Lai and L. Zhou, Nat. Prod. Res., 2021, 35, 272 CrossRef CAS PubMed; (e) M. A. Cinelli and A. D. Jones, Molecules, 2021, 26, 2629 CrossRef CAS PubMed; (f) S. Asamizu, Biosci., Biotechnol., Biochem., 2017, 81, 871 CrossRef CAS PubMed; (g) A. K. Chattopadhyay and S. Hanessian, Chem. Rev., 2017, 117, 4104 CrossRef CAS PubMed; (h) J. F. Hu, H. Fan, J. Xiong and S. B. Wu, Chem. Rev., 2011, 111, 5465 CrossRef CAS PubMed; (i) A. Padwa, Chem. Soc. Rev., 2009, 38, 3072 RSC; (j) H. Fan, J. Peng, M. T. Hamann and J. F. Hu, Chem. Rev., 2008, 108, 264 CrossRef CAS PubMed.
- (a) H. M. Hügel, N. H. de Silva, A. Siddiqui, E. Blanch and A. Lingham, Bioorg. Med. Chem., 2021, 43, 116270 CrossRef PubMed; (b) D. N. Huang, F. F. Wu, A. H. Zhang, H. Sun and X. J. Wang, Pharmacol. Res., 2021, 169, 105667 CrossRef CAS PubMed; (c) M. Ghanbari-Movahed, T. Kaceli, A. Mondal, M. H. Farzaei and A. Bishayee, Biomedicines, 2021, 9, 480 CrossRef CAS PubMed; (d) F. S. Youssef and J. Simal-Gandara, Biomedicines, 2021, 9, 485 CrossRef CAS PubMed.
- (a) A. Martorana, G. La Monica and A. Lauria, Molecules, 2020, 25, 4279 CrossRef CAS PubMed; (b) X. Nqoro, N. Tobeka and B. A. Aderibigbe, Molecules, 2017, 22, 2268 CrossRef PubMed; (c) S. Vandekerckhove and M. D'hooghe, Bioorg. Med. Chem., 2015, 23, 5098 CrossRef CAS PubMed; (d) P. Wadhwa, P. Jain, S. Rudrawar and H. R. A. Jadhav, Curr. Drug Discovery Technol., 2018, 15, 2 CrossRef CAS PubMed.
- R. J. DeVita, Curr. Top. Med. Chem., 2007, 7, 1433 CrossRef CAS PubMed.
- M. Zhu, D. Gong and A. Mouse, Med. Sci. Monit., 2020, 26, e920989 CAS.
- M. A. Cinelli, H. Li, G. Chreifi, P. Martásek, L. J. Roman, T. L. Poulos and R. B. Silverman, J. Med. Chem., 2014, 57, 1513 CrossRef CAS PubMed.
- A. R. Syniugin, O. V. Ostrynska, M. O. Chekanov, G. P. Volynets, S. A. Starosyla, V. G. Bdzhola and S. M. Yarmoluk, J. Enzyme Inhib. Med. Chem, 2016, 31, 160 CrossRef CAS PubMed.
- (a) Z. Chen and D. Ma, Org. Lett., 2019, 21, 6874 CrossRef CAS PubMed; (b) C. Wu, X. Qin, A. M. P Moeljadi, H. Hirao and J. S. Zhou, Angew. Chem., Int. Ed. Engl., 2019, 58, 2705 CrossRef CAS PubMed; (c) Y. Liang, H. Jiang, Z. Tan and M. Zhang, Chem. Commun., 2018, 54, 10096 RSC; (d) A. Behera, P. Sau, A. K. Sahoo and B. K. Patel, J. Org. Chem., 2018, 83, 11218 CrossRef CAS PubMed.
- (a) T. N. Ansari, A. Taussat, A. H. Clark, M. Nachtegaal, S. Plummer, F. Gallou and S. Handa, ACS Catal., 2019, 9, 10389 CrossRef CAS; (b) W. Wu, M. Li, J. Zheng, W. Hu, C. Li and H. Jiang, Chem. Commun., 2018, 54, 6855 RSC.
- (a) R. T. McGuire, A. A. Yadav and M. Stradiotto, Angew. Chem., Int. Ed. Engl., 2021, 60, 4080 CrossRef CAS PubMed; (b) R. T. McGuire, C. M. Simon, A. A. Yadav, M. J. Ferguson and M. Stradiotto, Angew. Chem., Int. Ed. Engl., 2020, 59, 8952 CrossRef CAS PubMed.
- (a) M. Li, S. Fang, J. Zheng, H. Jiang and W. Wu, Org. Lett., 2019, 21, 8439 CrossRef CAS PubMed; (b) R. Yi, X. Li and B. Wan, Adv. Synth. Catal., 2018, 360, 875 CrossRef CAS.
- (a) B. C. Roy, S. Debnath, K. Chakrabarti, B. Paul, M. Maji and S. Kundu, Org. Chem. Front., 2018, 5, 1008 RSC; (b) M. Maji, K. Chakrabarti, B. Paul, B. C. Roy and S. Kundu, Adv. Synth. Catal., 2018, 360, 722 CrossRef CAS.
- C. Zhou, T. Lei, X. Z. Wei, C. Ye, Z. Liu, B. Chen, C. H. Tung and L. Z. Wu, J. Am. Chem. Soc., 2020, 142, 16805 CrossRef CAS PubMed.
- K. Das, A. Mondal, D. Pal and D. Srimani, Org. Lett., 2019, 21, 3223 CrossRef CAS PubMed.
- S. Shee, K. Ganguli, K. Jana and S. Kundu, Chem. Commun., 2018, 54, 6883 RSC.
- S. Han, X. Gao, Q. Wu, J. Li, D. Zou, Y. Wu and Y. Wu, Org. Chem. Front., 2019, 6, 830 RSC.
- N. P. Massaro, A. Chatterji and I. Sharma, J. Org. Chem., 2019, 84, 13676 CrossRef CAS PubMed.
- (a) S. Bahadorikhalili, M. Divar, T. Damghani, F. Moeini, S. Ghassamipour, A. Iraji, M. A. Miller, B. Larijani and M. Mahdavi, J. Organomet. Chem., 2021, 939, 121773 CrossRef CAS; (b) S. H. Kim, S. H. Park, J. H. Choi and S. Chang, Chem.–Asian J., 2011, 6, 2618 CrossRef CAS PubMed; (c) I. Bae, H. Han and S. Chang, J. Am. Chem. Soc., 2005, 127, 2038 CrossRef CAS PubMed; (d) S. H. Cho, E. J. Yoo, I. Bae and S. Chang, J. Am. Chem. Soc., 2005, 127, 16046 CrossRef CAS PubMed.
- For examples of relevant recent work see: (a) Y. Zhao, Z. Zhou, L. Liu, M. Chen, W. Yang, Q. Chen, M. G. Gardiner and M. G. Banwell, J. Org. Chem., 2021, 86, 9155 CrossRef CAS PubMed; (b) Y. Zhao, Z. Zhou, M. Chen and W. Yang, Molecules, 2021, 26, 3700 CrossRef CAS PubMed; (c) W. Yang, Y. Zhao, Z. Zhou, L. Li, L. Cui and H. Luo, RSC Adv., 2021, 11, 8701 RSC; (d) C.-G. Wang, R. Wu, T.-P. Li, T. Jia, Y. Li, D. Fang, X. Chen, Y. Gao, H.-L. Ni, P. Hu, B.-Q. Wang and P. Cao, Org. Lett., 2020, 22, 3234 CrossRef CAS PubMed; (e) N. P. Massaro, A. Chatterji and I. Sharma, J. Org. Chem., 2019, 84, 13676 CrossRef CAS PubMed; (f) L. Xu, T. Zhou, M. Liao, R. Hu and B. Z. Tang, ACS Macro Lett., 2019, 8, 101 CrossRef CAS; (g) S. Guo, P. Dong, Y. Chen, X. Feng and X. Liu, Angew. Chem., Int. Ed., 2018, 57, 16852 CrossRef CAS PubMed; (h) K. Kacprzak, I. Skiera, M. Piasecka and Z. Paryzek, Chem. Rev., 2016, 116, 5689 CrossRef CAS PubMed.
- B. Reichart, G. Guedes de la Cruz, K. Zangger, C. O. Kappe and T. Glasnov, Adv. Synth. Catal., 2016, 358, 50 CrossRef CAS.
- (a) C. Nájera, L. K. Sydnes and M. Yus, Chem. Rev., 2019, 119, 11110 CrossRef PubMed; (b) M. Nallagangula and K. Namitharan, Org. Lett., 2017, 19, 3536 CrossRef CAS PubMed; (c) C. Shao, Q. Zhang and G. Cheng, Eur. J. Org. Chem., 2013, 6443 CrossRef CAS; (d) P. V. Ramachandran, M. T. Rudd and M. V. R. Reddy, Tetrahedron Lett., 2005, 46, 2547 CrossRef CAS.
- (a) Y. Zhao, L. Li, Z. Zhou, M. Chen, W. Yang and H. Luo, Org. Biomol. Chem., 2021, 19, 3868 RSC; (b) W. Yang, D. Huang, X. Zeng, J. Zhang, X. Wang and Y. Hu, Tetrahedron, 2019, 75, 381 CrossRef CAS; (c) W. Yang, D. Huang, X. Zeng, D. Luo, X. Wang and Y. Hu, Chem. Commun., 2018, 54, 8222 RSC.
- D. Chernyak, S. B. Gadamsetty and V. Gevorgyan, Org. Lett., 2008, 10, 2307 CrossRef CAS PubMed.
- D. Das and R. Samanta, Adv. Synth. Catal., 2018, 360, 379 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2092343, 2092351 and 2092350. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1ra06330h |
| This journal is © The Royal Society of Chemistry 2021 |