Open Access Article
Open Access ArticleRibose conversion with amino acids into pyrraline platform chemicals – expeditious synthesis of diverse pyrrole-fused alkaloid compounds†
Soohyeon Cho‡
a,
Lina Gu‡ab,
Ik Joon INa,
Bo Wuc,
Taehoon Leed,
Hakwon Kimd and
Sangho Koo *ab
*ab
aDepartment of Energy Science and Technology, Department of Chemistry, Myongji University, Myongji-Ro 116, Cheoin-Gu, Yongin, Gyeonggi-Do 17058, Korea. E-mail: sangkoo@mju.ac.kr
bSchool of Pharmacy, East China University of Science and Technology, Meilong Road 130, Shanghai, 200237, China
cSchool of Forensic Medicine, China Medical University, Puhe Road 77, Shenyang, 110122, China
dGlobal Center for Pharmaceutical Ingredient Materials, Department of Applied Chemistry, Kyung Hee University, Yongin, Gyeonggi-Do 17104, Korea
First published on 23rd September 2021
Abstract
One-pot conversion of sustainable D-ribose with L-amino acid, methyl esters produced pyrrole-2-carbaldehydes 5 in reasonable yields (32–63%) under pressurized conditions of 2.5 atm at 80 °C. The value-added pyrraline compounds 5 as platform chemicals were utilized for quick installation of poly-heterocyclic cores for the development of pyrrole-motif natural and artificial therapeutic agents. A pyrrole-fused piperazin-2-one scaffold 6 was prepared by reductive amination of pyrralines 5 with benzylamine. While further cyclization of pyrralines 5 with ethane-1,2-diamine produced pyrrolo-piperazin-2-ones 7 with an extra imidazolidine ring, the reaction with 2-amino alcohols derived from natural L-amino acids, alanine, valine, and phenylalanine, respectively provided pyrrolo-piperazin-2-ones 8, 9, and 10 with oxazolidine as the third structural core. Cell viability and an anti-inflammatory effect of the synthesized compounds were briefly tested by the MTT method and the Griess assay, among which 8h and 10g exhibited significant anti-inflammatory effects with negligible cell toxicity.
Introduction
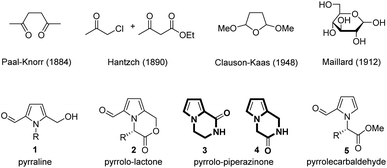
Pyrrole is an interesting five-membered heterocyclic compound with the nitrogen lone-pair electrons being delocalized within the ring for aromaticity. It is therefore non-basic contrary to the six-membered ring homologue, pyridine. Alkaloid natural products containing pyrrole as a pharmacophore exhibit various biological activities such as sedative, anti-inflammatory, anti-biotic, anti-cancer, anti-hypertensive, anti-convulsant, anti-malarial, and many more.1–6 The organic synthesis of pyrrole was reported in the late 19th century,7–9 among which the Paal–Knorr method utilizing a 1,4-dicarbonyl compound and primary amine was the first (Fig. 1).10–12 The Hantzsch method by the reaction of α-haloketone and β-ketoester with a primary amine is useful for the preparation of 3-carboxylated pyrroles.13,14 In 1948, Clauson-Kaas reported furan conversion into 2,5-dimethoxytetrahydrofuran for the efficient preparation of pyrroles.15 Many others then modified the above original methods for the syntheses of various pyrrole compounds.16–33Transformation of D-glucose as sustainable biomass into 5-hydroxymethyl-2-furfural (5-HMF) as a value-added platform chemical has been well documented in the literature in recent years.34–36 Utilization of reducing sugars for the preparation of pyrroles would also be beneficial and providential. In 1912, Maillard reported the reaction between D-glucose and amino acids at high temperature (>140°) to produce hundreds of small chemicals for flavouring in food chemistry,37 which was later known to include pyrraline 1, the nitrogen analogue of 5-HMF, in a very small quantity.38 Pyrraline 1 would be a useful platform chemical for the development of pyrrole-motif natural and artificial therapeutic agents by further reactions.39–43
The first chemical synthesis of pyrralines 1 by Kato from the reaction of D-glucose and primary amines either at 100 °C in H2O for 1 h or at 70 °C in MeOH for 3 h both with acetic acid just confirmed the formation of pyrralines 1.44 Monnier reported the synthesis of butyl pyrraline 1 (R = Bu) in only 3.4% yield by the reaction of D-glucose and butylamine in aqueous acetic acid at 100 °C for 2 h.38 We believed that the low yield of pyrraline 1 was ascribed to the unstable imine intermediates under the aqueous acidic condition. We recently studied the reaction conditions of D-glucose and primary amines, and significantly improved the yield of pyrralines 1 (20–50%) under non-aqueous condition by use of oxalic acid in DMSO at 90 °C for 30 min.45 Anti-inflammatory and pain-relieving pyrrolo-lactones 2, which could be isolated from Celastrus orbiculatus (R = Bn) and Capparis spinosa (R = Me), were synthesized in 27% and 32% yields, respectively when methyl esters of amino acids, phenylalanine and alanine, were used as primary amines in the above one-pot reaction condition.45 Nonetheless, it was still necessary to improve the one-pot reaction conditions of sugars and amino acids for reasonable yields of the pyrrole platform chemicals, which might be realized by reducing the formation of tar by-product under a pressurized reaction condition.
Piperazin-2-one is a privileged structural unit in drug discovery because many of the biologically active natural products and the approved drug molecules contain the above scaffold,46–49 which exhibit opiate,50 antiviral,51 anti-cancer,52 and anti-diabetes activities53 as well as treatment effects for immunological disorder54 and non-Hodgkin's lymphoma.55 Pyrrole-fused piperazin-2-ones (3 and 4 in Fig. 1) would thus be a very promising structural unit for the discovery of new therapeutic agents. In fact, the structure of pyrrolo-piperazinone 3 has been found in numerous antibiotic and cytotoxic alkaloid natural products, such as agelastatins,56,57 longamide,58 hanishin,59 and agesamides.60
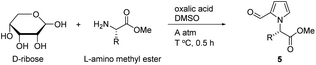
We envisioned that the structure of pyrrolo-piperazinone 4 would be obtainable from pyrrole-2-carbaldehyde 5, which might be prepared by the one-pot “Maillard-type” reaction between D-ribose and α-amino acids. Amination of the formyl group and subsequent lactam formation with the carboxylic ester in pyrrole-2-carbaldehyde 5 would form the core structure of pyrrolo-piperazinone 4. In this paper, we described one-pot conversion of D-ribose with various α-amino acids under a pressurized condition to obtain reasonable yields of pyrrole-2-carbaldehydes 5 as platform chemicals and their further cyclization reactions to pyrrolo-piperazinone derivatives 6–10 as new potential therapeutic agents. Finally, cell viability and anti-inflammatory effect of the synthesized pyrrolo-piperazinones were briefly tested for RAW264.7 cells by the MTT method and the Griess assay.
Results and discussion
One-pot ribose conversion with amino acid
D-Ribose was first reacted with L-valine methyl ester in DMSO with oxalic acid at 90 °C for 30 min to produce pyrraline 5 (R = i-Pr) in 24% yield, which was the previous optimized condition for D-glucose.45 Unlike D-glucose case providing pyrrololactones, pyrrole-2-carbaldehyde 5 with intact methyl ester was obtained. Pyrroles as the glycosylation end products were believed to be formed through 3-deoxyglucosone intermediate after N-glycosylation and Amadori reaction.38,61,62 The reaction of D-ribose was thus speculated to follow N-glycosylation (A) and Amadori reaction (B) in sequence (Scheme 1). Second amino ester was introduced to give the structure C by imine tautomerization and dehydration, which is the adduct of amino esters to the 3-deoxyglucosone homologue from D-ribose. Cyclization of enamine and dehydration produced pyrrole ring D. Finally, hydrolysis of the imine provided pyrraline 5 and regenerated amino ester. D-Ribose reaction with other amino esters from L-phenylalanine and L-methionine also produced the corresponding pyrralines 5 in 37% (R = Bn) and 30% (R = CH2CH2SMe) yields, respectively, but it was imperative to improve the ribose conversion reaction to utilize pyrralines 5 as sustainable platform chemicals for the discovery of potent therapeutic agents.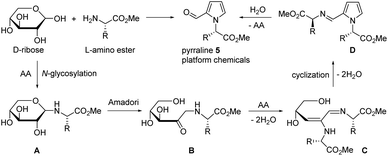 | ||
| Scheme 1 Ribose conversion with amino ester through N-glycosylation, Amadori reaction, and cyclization to produce pyrraline 5 as platform chemicals. | ||
The ribose conversion reaction with α-amino methyl esters under the above condition produced pyrralines 5 in 24–37% yields as the only isolable product together with dark-brown tarry materials, which suggested that reducing the amount of tar would improve the yield of pyrralines 5. The ribose conversion was thus studied for the above three amino esters under pressurized conditions (1–4 atm) at 90 °C to reduce the tar formation (Table 1). We found gradual yield improvements of 5 as pressure was increased with the maximum value at 2.5 atm. The yield of 5 was maintained or slowly fell after 3.0 atm.
Reaction temperature was then screened from 60 °C to 90 °C at the optimal pressure of 2.5 atm (Table 2). The yield of 5 was progressively improved as temperature was raised up to 80 °C and fell slowly down since then. The best yields of pyrralines 5 were obtained at 80 °C (2.5 atm) for all three amino esters, which were approximately 150% of those at the previous optimal condition for glucose (90 °C, 1 atm).45
Generality of the ribose conversion with α-amino methyl esters and improvement of the yields of pyrralines 5 under the pressure condition (2.5 atm, 80 °C) compared to the original one (1 atm, 90 °C) were summarized in Table 3, in which 150–300% yield increases for pyrralines 5 were notified for eleven natural L-α-amino acids. Amino esters reacted with D-ribose as nucleophile and the yields of pyrralines 1 were reflected by the nucleophilicity of each amino ester, which was differentiated by the α-substituent R in this case. It was initially predicted that R would provide steric hinderance to the amino group, thereby lower yield of 1 could be obtained as the size of R was increased. Surprisingly, it was L-glycine (R = H), however, which gave the lowest yield (32%) of pyrraline 1, and the highest yield of 1 (63%) was obtained for L-leucine (R = i-Bu).
| Amino Acida | Compound 5 | Yield of 5 (%) | |
|---|---|---|---|
| 1.0 atm, 90 °C | 2.5 atm, 80 °C | ||
| a Amino acid (R): glycine (H), alanine (Me), valine (i-Pr), leucine (i-Bu), isoleucine (s-Bu), phenylalanine (CH2Ph), benzylalanine (CH2CH2Ph), aspartic acid (CH2CO2Me), glutamic acid (CH2CH2CO2Me), methionine (CH2CH2SMe), tryptophane (3-indole). | |||
| Gly | 5a | 10 | 32 |
| Ala | 5b | 23 | 38 |
| Val | 5c | 24 | 42 |
| Leu | 5d | 43 | 63 |
| Ile | 5e | 20 | 40 |
| Phe | 5f | 37 | 54 |
| Bn | 5g | 20 | 53 |
| Asp | 5h | 18 | 47 |
| Glu | 5i | 16 | 37 |
| Met | 5j | 30 | 46 |
| Trp | 5k | 34 | 55 |
It seemed that the hydrogen bonding between primary amino and ester groups would reduce the nucleophilicity of amino esters. L-Glycine with no α-substituent may participate in the hydrogen bonding, which reduces the nucleophilicity of the amino group. Primary alkyl or aryl substituents R (e.g., Leu, Phe, Bn) would be good enough to maintain the nucleophilicity of amino group by interrupting the hydrogen bonding without providing steric hinderance to the amino group. Pyrralines 5 can now be obtained in 32–60% yield by one-pot conversion from D-ribose with L-amino acid methyl esters and certainly be flatform chemicals for the syntheses of pyrrole-based poly heterocyclic compounds as potential therapeutic agents.
Cyclization of the platform pyrralines 5 to pyrrolo-piperazinones 6–10
We demonstrated three different methods for further cyclization of pyrralines 5, derived from eight different L-amino acids – glycine (a), alanine (b), valine (c), isoleucine (d), phenylalanine (e), benzylalanine (f), aspartic acid (g), and methionine (h) – with five different nitrogen reagents to produce the 2-piperazin-2-one skeleton as the second pharmacophore with the basic pyrrole scaffold (Scheme 2 and Table 4). The 1,5-relationship between the carbonyl carbons of formyl and ester groups in pyrralines 5 allowed δ-lactam formation with primary amines by reductive amination and cyclization. Benzylamine was utilized for the reductive amination with pyrralines 5, which was carefully carried out first by imine formation in MeOH at 25 °C, followed by selective NaBH4 reduction at 0 °C due to the presence of the sensitive α-amino ester group. The resulting secondary amine underwent lactam formation with the ester group in the presence of DBU at the reflux temperature of toluene to produce pyrrolo-piperazin-2-ones 6 in 36–84% yields (Table 4).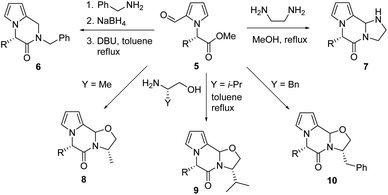 | ||
| Scheme 2 Further cyclization of pyrraline platform chemicals 5 to pyrrolo-piperazin-2-one derivatives 6–10. | ||
| Entry | R | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|
| a | H | 36 | 58 | 12 | 4 | 6 |
| b | Me | 66 | 87 | 41 | 27 | 32 |
| c | i-Pr | 73 | 94 | 73 | 61 | 41 |
| d | s-Bu | 86 | 91 | 77 | 50 | 31 |
| e | PhCH2 | 41 | 86 | 52 | 71 | 62 |
| f | PhCH2CH2 | 64 | 88 | 22 | 51 | 57 |
| g | MeO2CCH2 | 84 | 72 | 27 | 10 | 14 |
| h | MeSCH2CH2 | 47 | 87 | 24 | 24 | 22 |
α,ω-Alkanediamine (e.g., 1,2-ethanediamine) can be utilized in lactam formation with pyrralines 5, where initially formed imine may react with the second amine by cyclization.40,41 One of the resulting two symmetrical secondary amines participated in further cyclization with the ester group to form pyrrolo-piperazinones 7 with an imidazolidine ring as the third core in 58–94% yields (Table 4). The conversion of pyrralines 5 with norephedrine was reported,42 where the initially formed imine was reacted with the alcohol group by cyclization to form an oxazolidine unit, which underwent further cyclization with the ester group to produce pyrrolo-piperazinones with the oxazolidine unit. In a similar manner, 2-amino alcohols derived from natural α-amino acids, alanine (Y = Me), valine (i-Pr), and phenylalanine (Bn), can be utilized for the syntheses of oxazolidine-fused pyrrolo-piperazinones 8, 9, and 10, respectively. Even though somewhat lower yields were notified for the pyrralines 5 from Gly (a, R = H), Asp (g, CH2CO2CH3), and Met (h, CH2CH2SCH3), fare yields of poly heterocyclic products 8–10 were obtained for the pyrralines 5 derived from α-amino acids with a simple alkyl substituent (Table 4).
Cell viability (MTT test) and anti-inflammation assays (Griess method)
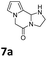
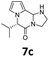
All the synthesized pyrrolo-piperazinones 6–10 (a–h) were briefly screened for cytotoxicity to RAW264.7 cells (Murine macrophages) at a fixed concentration at 20 μg mL−1. Four compounds 7a, 7c, 8h, and 10g showed 80% or higher cell viability by Formazan formation in MTT {3-(4,5-dimetnythiazol-2-yl)-2,5-diphenyl-thetazolium bromide} assay (see ESI†).63 The other regents might still be good candidates for antibiotics.Anti-inflammatory activities to RAW264.7 cells were then tested for the above four samples at five different concentrations (0, 1, 5, 10, 20 μg mL−1 each) in 24 h after triggering by 1 μg mL−1 of LPS (lipopolysaccharides). Nitric oxide concentration, which is correlated with the inflammation level, was determined by the Griess method.64 The measurements were repeated three times. The mean and standard deviation values of the NO concentration (μg mL−1) for the cell treated by each compound at each concentration are listed in Table 5. Pyrrolo-piperazinones 8h and 10g containing oxazolidine as the third core exhibited significant anti-inflammation activities, in which IC50 values were estimated to be 20 μg mL−1 and 10 μg mL−1, respectively. For comparison, IC50 of NIL {L-N6-(1-iminoethyl)lysine} as a positive control was measured as 10.17 mM, which is converted as 2.64 μg mL−1 (see ESI†).
| Conc. (μg mL−1) | ||||
|---|---|---|---|---|
| a Without LPS treatment. | ||||
| 0a | 3.21 ± 0.15 | 3.21 ± 0.15 | 3.21 ± 0.15 | 3.21 ± 0.15 |
| 0 | 25.54 ± 2.60 | 25.54 ± 2.60 | 25.54 ± 2.60 | 25.54 ± 2.60 |
| 1 | 26.23 ± 2.60 | 25.03 ± 2.13 | 22.23 ± 2.13 | 17.31 ± 1.31 |
| 5 | 22.31 ± 1.65 | 24.32 ± 1.31 | 21.32 ± 1.31 | 15.32 ± 2.60 |
| 10 | 20.30 ± 2.65 | 23.51 ± 1.10 | 16.31 ± 1.10 | 14.31 ± 2.11 |
| 20 | 20.02 ± 3.64 | 21.65 ± 2.51 | 14.32 ± 2.51 | 11.10 ± 1.31 |
Experimental
General experimental
Reactions were performed in a well-dried flask under argon atmosphere unless noted otherwise. A mini-clave steel reactor (100 mL, up to 15 bar) made from büchiglasuster (Switzerland) was used for the pressurized reactions. The pressure was controlled by argon gas (see ESI† for picture demonstration). Solvents used as reaction media were dried over pre-dried molecular sieve (4 Å) by microwave oven. Solvents for extraction and chromatography were reagent grade and used as received. The flash column chromatography was performed by the method of Still (J. Org. Chem., 1978, 43, 2923) with silica gel 60 (70–230 mesh) using a mixture of EtOAc/hexane as gradient eluent. 1H and 13C NMR spectra were respectively recorded on a 400 MHz and 100 MHz NMR spectrometer in deuterated chloroform (CDCl3) with tetramethylsilane (TMS) as an internal reference unless noted otherwise.Cell viability assay (MTT method)63
RAW264.7 cells (Murine macrophages) were cultured using Dulbecco's modified Eagle medium (DMEM) (Welgene, Seoul, Korea) containing 10% fetal bovine serum (FBS), 2 mM glutamine, and 100 unit/mL of antibiotics (Gibco BRL, Rockville, MD). Cells were incubated at 37 °C incubators, which maintained a humidified atmosphere of 5% (v/v) air/CO2. The incubated RAW264.7 cells (5 × 103/well) were seeded to a 96-cell culture plate to conduct a cell viability assay. The RAW264.7 cells prepared for the viability assay went through 18 h of attachment and stabilization. The growth medium was removed and replaced by a fresh medium without FBS. The synthetic compounds were treated to the cells at a concentration of 20 μg mL−1 and the resulting cells were incubated for 24 h. The sample-treated culture medium was removed and 100 μg mL−1 of 3-(4,5-dimetnythiazol-2-yl)-2,5-diphenyl-thetazolium bromide (MTT) was added to each well. In 1 h, purple formazan produced by cellular respiration was dissolved in a 200 μL DMSO solution and the absorbance at 560 nm was measured using a multi-plate reader. The analyses were repeated three times. The results were expressed as means of three independent experiments.Measurement of nitric oxide concentration (Griess assay)64
RAW264.7 cells were transferred into 3 × 105 cells per well in a 96-cell culture plate and incubated for 24 h in a 5% CO2 incubator at 37 °C. Four different concentrations (1, 5, 10, and 20 μg mL−1) of synthetic compounds was treated to the incubated RAW264.7 cells. Simultaneously, 1 μg mL−1 of lipopolysaccharides (LPS) (Sigma-Aldrich, St. Louis, MO) was added and the resulting cells were incubated for 24 h. The same amount of Griess reagent (Sigma-Aldrich) equal to 100 μL of the culture solution was added according to the manufacturer's recommendations, and the absorbance was measured at 540 nm with a multi-plate reader. The NO concentration in the culture medium was determined using a standard curve for each concentration of sodium nitrite.General procedure for D-ribose conversion with L-amino acids for the synthesis of pyrraline 5
Under ambient pressure. To a stirred solution of the above glycine methyl ester, ammonium chloride salt (8.35 g, 66.5 mmol, 1 equiv.) in DMSO (20 mL) were added Et3N (18.4 mL, 0.133 mol, 2 equiv.) and D-ribose (10.0 g, 66.6 mmol, 1 equiv.). After complete dissolution, oxalic acid (12.0 g, 0.133 mol, 2 equiv.) was added and the resulting mixture was heated at 90 °C for 30 min. Upon cooling to room temperature, the mixture was filtered through a short pad of SiO2 with EtOAc rinsing. The filtrate was concentrated and purified by SiO2 flash column chromatography to give 5a (1.08 g, 6.45 mmol) in 10% yield as light-yellow liquid.
Under pressure bottle at 2.5 bar. A mixture of glycine methyl ester, ammonium chloride salt (2.00 g, 15.9 mmol) and Et3N (2.18 mL, 15.9 mmol) in dry DMSO (10 mL) was stirred in a mini-clave steel reactor and then D-ribose (4.73 g, 31.8 mmol) and oxalic acid (1.41 g, 15.9 mmol) were added. The mixture was pressurized to 2.5 bar with argon and heated at 80 °C for 0.5 h. The mixture was then cooled to room temperature and depressurized. The resulting viscous mixture was filtered through a short pad of SiO2 rinsing with EtOAc. The filtrate was concentrated and purified by SiO2 flash column chromatography (eluent 7
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 EtOAc/hexane) to give 5a (0.85 g, 5.09 mmol) in 32% yield as light-yellow liquid.
1 EtOAc/hexane) to give 5a (0.85 g, 5.09 mmol) in 32% yield as light-yellow liquid.
Data for 5a. Rf = 0.50 (4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 EtOAc/hexane); 1H NMR δ = 3.77 (s, 3H), 5.08 (s, 2H), 6.31 (dd, J = 2.4, 1.6 Hz, 1H), 6.92 (dd, J = 4.0, 2.4 Hz, 1H), 7.00 (dd, J = 4.0, 1.6 Hz, 1H), 9.54 (s, 1H) ppm; 13C NMR δ = 50.0, 52.5, 110.2, 124.6, 124.6, 132.0, 168.7, 179.8 ppm; IR ν = 3111, 2941, 2904, 2840, 1740, 1650, 1528, 1480, 1403, 1359, 1321, 1216, 1081, 1031, 999, 761 cm−1; HRMS (EI) calcd for C8H9NO3 167.0582, found 167.0582.
6 EtOAc/hexane); 1H NMR δ = 3.77 (s, 3H), 5.08 (s, 2H), 6.31 (dd, J = 2.4, 1.6 Hz, 1H), 6.92 (dd, J = 4.0, 2.4 Hz, 1H), 7.00 (dd, J = 4.0, 1.6 Hz, 1H), 9.54 (s, 1H) ppm; 13C NMR δ = 50.0, 52.5, 110.2, 124.6, 124.6, 132.0, 168.7, 179.8 ppm; IR ν = 3111, 2941, 2904, 2840, 1740, 1650, 1528, 1480, 1403, 1359, 1321, 1216, 1081, 1031, 999, 761 cm−1; HRMS (EI) calcd for C8H9NO3 167.0582, found 167.0582.
Data for 5b. Rf = 0.53 (4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 EtOAc/hexane); 1H NMR δ =1.73 (d, J = 7.6 Hz, 3H), 3.71 (s, 3H), 5.86 (q, J = 7.6 Hz, 1H), 6.30 (dd, J = 4.0, 2.0 Hz, 1H), 6.98 (dd, J = 4.0, 1.6 Hz, 1H), 7.18 (ddd, J = 2.0, 1.6, 1.2 Hz, 1H), 9.50 (d, J = 1.2 Hz, 1H) ppm; 13C NMR δ = 17.6, 52.2, 55.1, 110.0, 125.1, 128.7, 131.3, 171.2, 179.3 ppm; IR ν = 3116, 2999, 2954, 2851, 2809, 1729, 1608, 1517, 1470, 1406, 1364, 1339, 1313, 1211, 1090, 1066, 1033, 983, 743 cm−1; HRMS (ESI) calcd for C9H11NO3 + Na 204.0631, found 204.0636.
6 EtOAc/hexane); 1H NMR δ =1.73 (d, J = 7.6 Hz, 3H), 3.71 (s, 3H), 5.86 (q, J = 7.6 Hz, 1H), 6.30 (dd, J = 4.0, 2.0 Hz, 1H), 6.98 (dd, J = 4.0, 1.6 Hz, 1H), 7.18 (ddd, J = 2.0, 1.6, 1.2 Hz, 1H), 9.50 (d, J = 1.2 Hz, 1H) ppm; 13C NMR δ = 17.6, 52.2, 55.1, 110.0, 125.1, 128.7, 131.3, 171.2, 179.3 ppm; IR ν = 3116, 2999, 2954, 2851, 2809, 1729, 1608, 1517, 1470, 1406, 1364, 1339, 1313, 1211, 1090, 1066, 1033, 983, 743 cm−1; HRMS (ESI) calcd for C9H11NO3 + Na 204.0631, found 204.0636.
Data for 5c. Rf = 0.76 (4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 EtOAc/hexane); 1H NMR δ = 0.78 (d, J = 6.4 Hz, 3H), 1.01 (d, J = 6.4 Hz, 3H), 2.40 (m, 1H), 3.75 (s, 3H), 5.99 (d, J = 10.4 Hz, 1H), 6.31 (ddd, J = 4.0, 2.8, 1.2 Hz, 1H), 6.93 (ddd, J = 4.0, 1.6, 1.6 Hz, 1H), 7.39 (ddd, J = 2.8, 1.6, 1.2 Hz, 1H), 9.54 (dd, J = 1.6, 1.2 Hz, 1H) ppm; 13C NMR δ = 18.5, 19.2, 33.0, 52.2, 63.8, 110.7, 125.3, 129.9, 131.9, 171.2, 179.9 ppm; IR ν = 3126, 2952, 2874, 2846, 2813, 1729, 1655, 1523, 1460, 1408, 1373, 1338, 1258, 1201, 1147, 1062, 1033, 1000, 877, 825, 743 cm−1; HRMS (ESI) calcd for C11H15NO3 + Na 232.0944, found 232.0942.
6 EtOAc/hexane); 1H NMR δ = 0.78 (d, J = 6.4 Hz, 3H), 1.01 (d, J = 6.4 Hz, 3H), 2.40 (m, 1H), 3.75 (s, 3H), 5.99 (d, J = 10.4 Hz, 1H), 6.31 (ddd, J = 4.0, 2.8, 1.2 Hz, 1H), 6.93 (ddd, J = 4.0, 1.6, 1.6 Hz, 1H), 7.39 (ddd, J = 2.8, 1.6, 1.2 Hz, 1H), 9.54 (dd, J = 1.6, 1.2 Hz, 1H) ppm; 13C NMR δ = 18.5, 19.2, 33.0, 52.2, 63.8, 110.7, 125.3, 129.9, 131.9, 171.2, 179.9 ppm; IR ν = 3126, 2952, 2874, 2846, 2813, 1729, 1655, 1523, 1460, 1408, 1373, 1338, 1258, 1201, 1147, 1062, 1033, 1000, 877, 825, 743 cm−1; HRMS (ESI) calcd for C11H15NO3 + Na 232.0944, found 232.0942.
Data for 5d. Rf = 0.76 (4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 EtOAc/hexane); 1H NMR δ = 0.90 (d, J = 6.8 Hz, 3H), 0.93 (d, J = 6.8 Hz, 3H), 1.40 (m, 1H), 1.99 (dd, J = 8.0, 6.8 Hz, 2H), 3.72 (s, 3H), 6.10 (t, J = 8.0 Hz, 1H), 6.32 (dd, J = 4.0, 2.8 Hz, 1H), 6.96 (dd, J = 4.0, 1.6 Hz, 1H), 7.21 (ddd, J = 2.8, 1.6, 1.2 Hz, 1H), 9.52 (d, J = 1.2 Hz, 1H) ppm; 13C NMR δ = 21.3, 22.8, 24.6, 41.3, 52.4, 57.4, 110.4, 125.5, 129.5, 131.6, 171.5, 179.6 ppm; IR ν = 3119, 2963, 2873, 2810, 2773, 2729, 1750, 1657, 1534, 1473, 1411, 1373, 1348, 1273, 1203, 1164, 1132, 1080, 1029, 1001, 911, 878, 832, 778, 753 cm−1; HRMS (EI) calcd for C12H17NO3 223.1208, found 223.1207.
6 EtOAc/hexane); 1H NMR δ = 0.90 (d, J = 6.8 Hz, 3H), 0.93 (d, J = 6.8 Hz, 3H), 1.40 (m, 1H), 1.99 (dd, J = 8.0, 6.8 Hz, 2H), 3.72 (s, 3H), 6.10 (t, J = 8.0 Hz, 1H), 6.32 (dd, J = 4.0, 2.8 Hz, 1H), 6.96 (dd, J = 4.0, 1.6 Hz, 1H), 7.21 (ddd, J = 2.8, 1.6, 1.2 Hz, 1H), 9.52 (d, J = 1.2 Hz, 1H) ppm; 13C NMR δ = 21.3, 22.8, 24.6, 41.3, 52.4, 57.4, 110.4, 125.5, 129.5, 131.6, 171.5, 179.6 ppm; IR ν = 3119, 2963, 2873, 2810, 2773, 2729, 1750, 1657, 1534, 1473, 1411, 1373, 1348, 1273, 1203, 1164, 1132, 1080, 1029, 1001, 911, 878, 832, 778, 753 cm−1; HRMS (EI) calcd for C12H17NO3 223.1208, found 223.1207.
Data for 5e. Rf = 0.80 (4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 EtOAc/hexane); 1H NMR δ = 0.83 (t, J = 7.2 Hz, 3H), 0.98 (d, J = 6.8 Hz, 3H), 1.00–1.24 (m, 2H), 2.18 (m, 1H), 3.74 (s, 3H), 6.06 (d, J = 9.6 Hz, 1H), 6.31 (dd, J = 3.2, 2.4 Hz, 1H), 6.93 (dd, J = 3.2, 1.6 Hz, 1H), 7.41 (ddd, J = 2.4, 1.6, 1.2 Hz, 1H), 9.53 (s, 1H) ppm; 13C NMR δ = 10.5, 15.3, 24.7, 38.8, 52.0, 62.6, 110.5, 125.2, 129.7, 131.8, 171.0, 179.6 ppm; IR ν = 3116, 2961, 2862, 2703, 1735, 1655, 1530, 1468, 1410, 1375, 1343, 1250, 1202, 1151, 1070, 1026, 991, 750 cm−1; HRMS (EI) calcd for C12H17NO3 223.1208, found 223.1207; HRMS (ESI) calcd for C12H17NO3 + Na 246.1101, found 246.1105.
6 EtOAc/hexane); 1H NMR δ = 0.83 (t, J = 7.2 Hz, 3H), 0.98 (d, J = 6.8 Hz, 3H), 1.00–1.24 (m, 2H), 2.18 (m, 1H), 3.74 (s, 3H), 6.06 (d, J = 9.6 Hz, 1H), 6.31 (dd, J = 3.2, 2.4 Hz, 1H), 6.93 (dd, J = 3.2, 1.6 Hz, 1H), 7.41 (ddd, J = 2.4, 1.6, 1.2 Hz, 1H), 9.53 (s, 1H) ppm; 13C NMR δ = 10.5, 15.3, 24.7, 38.8, 52.0, 62.6, 110.5, 125.2, 129.7, 131.8, 171.0, 179.6 ppm; IR ν = 3116, 2961, 2862, 2703, 1735, 1655, 1530, 1468, 1410, 1375, 1343, 1250, 1202, 1151, 1070, 1026, 991, 750 cm−1; HRMS (EI) calcd for C12H17NO3 223.1208, found 223.1207; HRMS (ESI) calcd for C12H17NO3 + Na 246.1101, found 246.1105.
Data for 5f. Rf = 0.88 (4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 EtOAc/hexane); 1H NMR δ = 3.27 (dd, J = 14.0, 10.0 Hz, 1H), 3.54 (dd, J = 14.0, 5.6 Hz, 1H), 3.74 (s, 3H), 6.06 (br s or dd, J = 10.0, 5.6 Hz, calcd. 1H), 6.21 (dd, J = 4.0, 2.8 Hz, 1H), 6.91 (dd, J = 4.0, 1.6 Hz, 1H), 6.98–7.03 (m, 3H), 7.15–7.23 (m, 3H), 9.43 (d, J = 0.8 Hz, 1H) ppm; 13C NMR δ = 39.0, 52.6, 61.1, 110.2, 125.6, 126.9, 128.4, 128.9, 130.6, 131.2, 136.0, 170.3, 179.5 ppm; IR ν = 3114, 3067, 3029, 2955, 2845, 2812, 1740, 1654, 1530, 1500, 1472, 1409, 1373, 1343, 1277, 1215, 1079, 1032, 1002, 744, 698 cm−1; HRMS (EI) calcd for C15H15NO3 + Na 280.0944, found 280.0948.
6 EtOAc/hexane); 1H NMR δ = 3.27 (dd, J = 14.0, 10.0 Hz, 1H), 3.54 (dd, J = 14.0, 5.6 Hz, 1H), 3.74 (s, 3H), 6.06 (br s or dd, J = 10.0, 5.6 Hz, calcd. 1H), 6.21 (dd, J = 4.0, 2.8 Hz, 1H), 6.91 (dd, J = 4.0, 1.6 Hz, 1H), 6.98–7.03 (m, 3H), 7.15–7.23 (m, 3H), 9.43 (d, J = 0.8 Hz, 1H) ppm; 13C NMR δ = 39.0, 52.6, 61.1, 110.2, 125.6, 126.9, 128.4, 128.9, 130.6, 131.2, 136.0, 170.3, 179.5 ppm; IR ν = 3114, 3067, 3029, 2955, 2845, 2812, 1740, 1654, 1530, 1500, 1472, 1409, 1373, 1343, 1277, 1215, 1079, 1032, 1002, 744, 698 cm−1; HRMS (EI) calcd for C15H15NO3 + Na 280.0944, found 280.0948.
Data for 5g. Rf = 0.71 (4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 EtOAc/hexane); 1H NMR δ = 2.26–2.36 (m, 1H), 2.45–2.60 (m, 3H), 3.70 (s, 3H), 5.89 (br d, J = 8.0 Hz, 1H), 6.34 (dd, J = 4.0, 2.8 Hz, 1H), 6.98 (dd, J = 4.0, 1.6 Hz, 1H), 7.07–7.12 (m, 2H), 7.15–7.20 (m, 2H), 7.22–7.28 (m, 2H), 9.51 (d, J = 1, 2 Hz, 1H) ppm; 13C NMR δ = 31.8, 34.0, 52.5, 58.9, 110.5, 125.5, 126.2, 128.2, 128.4, 129.7, 131.6, 140.0, 170.8, 179.6 ppm; IR ν = 3114, 3067, 3027, 2955, 2847, 2810, 1735, 1644, 1532, 1502, 1472, 1413, 1375, 1340, 1254, 1209, 1179, 1087, 1031, 1008, 734, 686 cm−1; HRMS (FAB) calcd for C16H18NO3 272.1287, found 272.1284.
6 EtOAc/hexane); 1H NMR δ = 2.26–2.36 (m, 1H), 2.45–2.60 (m, 3H), 3.70 (s, 3H), 5.89 (br d, J = 8.0 Hz, 1H), 6.34 (dd, J = 4.0, 2.8 Hz, 1H), 6.98 (dd, J = 4.0, 1.6 Hz, 1H), 7.07–7.12 (m, 2H), 7.15–7.20 (m, 2H), 7.22–7.28 (m, 2H), 9.51 (d, J = 1, 2 Hz, 1H) ppm; 13C NMR δ = 31.8, 34.0, 52.5, 58.9, 110.5, 125.5, 126.2, 128.2, 128.4, 129.7, 131.6, 140.0, 170.8, 179.6 ppm; IR ν = 3114, 3067, 3027, 2955, 2847, 2810, 1735, 1644, 1532, 1502, 1472, 1413, 1375, 1340, 1254, 1209, 1179, 1087, 1031, 1008, 734, 686 cm−1; HRMS (FAB) calcd for C16H18NO3 272.1287, found 272.1284.
Data for 5h. Rf = 0.45 (4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 EtOAc/hexane); 1H NMR δ = 3.06 (dd, J = 17.2, 8.4 Hz, 1H), 3.39 (dd, J = 17.2, 4.8 Hz, 1H), 3.66 (s, 3H), 3.74 (s, 3H), 5.42 (d, J = 14.8 Hz, 1H), 5.87 (br s, 1H), 6.29 (dd, J = 4.0, 2.8 Hz, 1H), 7.01 (dd, J = 4.0, 1.6 Hz, 1H), 7.13 (ddd, J = 2.8, 1.6, 1.2 Hz, 1H), 9.48 (d, J = 1.2 Hz, 1H) ppm; 13C NMR δ = 37.1, 52.1, 52.9, 57.5, 110.2, 125.9, 131.0, 131.9, 169.2, 170.7, 179.3 ppm; IR ν = 3116, 3002, 2952, 2848, 2817, 1737, 1652, 1533, 1474, 1433, 1413, 1370, 1339, 1276, 1221, 1166, 1084, 1039, 1008, 755 cm−1; HRMS (EI) calcd for C11H13NO5 239.0794, found 239.0797.
6 EtOAc/hexane); 1H NMR δ = 3.06 (dd, J = 17.2, 8.4 Hz, 1H), 3.39 (dd, J = 17.2, 4.8 Hz, 1H), 3.66 (s, 3H), 3.74 (s, 3H), 5.42 (d, J = 14.8 Hz, 1H), 5.87 (br s, 1H), 6.29 (dd, J = 4.0, 2.8 Hz, 1H), 7.01 (dd, J = 4.0, 1.6 Hz, 1H), 7.13 (ddd, J = 2.8, 1.6, 1.2 Hz, 1H), 9.48 (d, J = 1.2 Hz, 1H) ppm; 13C NMR δ = 37.1, 52.1, 52.9, 57.5, 110.2, 125.9, 131.0, 131.9, 169.2, 170.7, 179.3 ppm; IR ν = 3116, 3002, 2952, 2848, 2817, 1737, 1652, 1533, 1474, 1433, 1413, 1370, 1339, 1276, 1221, 1166, 1084, 1039, 1008, 755 cm−1; HRMS (EI) calcd for C11H13NO5 239.0794, found 239.0797.
Data for 5i. Rf = 0.46 (4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 EtOAc/hexane); 1H NMR δ = 2.19–2.37 (m, 3H), 2.55–2.65 (m, 1H), 3.65 (s, 3H), 3.74 (s, 3H), 5.95 (br s, 1H), 6.33 (dd, J = 4.0, 2.8 Hz, 1H), 6.99 (dd, J = 4.0, 1.6 Hz, 1H), 7.15 (ddd, J = 2.8, 1.6, 1.2 Hz, 1H), 9.51 (d, J = 1.2 Hz, 1H) ppm; 13C NMR δ = 27.8, 30.0, 51.8, 52.7, 58.6, 110.7, 125.6, 130.1, 131.6, 170.4, 172.6, 179.7 ppm; IR ν = 3120, 2996, 2960, 2927, 2857, 2811, 2727, 1744, 1664, 1535, 1478, 1443, 1417, 1368, 1337, 1268, 1211, 1181, 1097, 1036, 1017, 887, 849, 834, 765 cm−1; HRMS (EI) calcd for C12H15NO5 253.0950, found 253.0948.
6 EtOAc/hexane); 1H NMR δ = 2.19–2.37 (m, 3H), 2.55–2.65 (m, 1H), 3.65 (s, 3H), 3.74 (s, 3H), 5.95 (br s, 1H), 6.33 (dd, J = 4.0, 2.8 Hz, 1H), 6.99 (dd, J = 4.0, 1.6 Hz, 1H), 7.15 (ddd, J = 2.8, 1.6, 1.2 Hz, 1H), 9.51 (d, J = 1.2 Hz, 1H) ppm; 13C NMR δ = 27.8, 30.0, 51.8, 52.7, 58.6, 110.7, 125.6, 130.1, 131.6, 170.4, 172.6, 179.7 ppm; IR ν = 3120, 2996, 2960, 2927, 2857, 2811, 2727, 1744, 1664, 1535, 1478, 1443, 1417, 1368, 1337, 1268, 1211, 1181, 1097, 1036, 1017, 887, 849, 834, 765 cm−1; HRMS (EI) calcd for C12H15NO5 253.0950, found 253.0948.
Data for 5j. Rf = 0.59 (4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 EtOAc/hexane); 1H NMR δ = 2.07 (s, 3H), 2.21–2.36 (m, 2H), 2.36–2.44 (m, 1H), 2.48–2.58 (m, 1H), 3.74 (s, 3H), 5.91 (br s, 1H), 6.33 (dd, J = 4.0, 2.8 Hz, 1H), 7.00 (dd, J = 4.0, 1.6 Hz, 1H), 7.16 (dd, J = 2.8, 1.6 Hz, 1H) ppm; 13C NMR δ = 16.2, 30.1, 31.4, 52.7, 58.7, 110.5, 110.0, 125.7, 130.5, 131.5, 170.5, 179.5 ppm; IR ν = 3112, 2946, 2918, 2843, 2817, 1741, 1650, 1532, 1472, 1407, 1373, 1342, 1272, 1226, 1208, 1085, 1031, 1003, 750 cm−1; HRMS (EI) calcd for C11H15NO3S 241.0773, found 241.0771.
6 EtOAc/hexane); 1H NMR δ = 2.07 (s, 3H), 2.21–2.36 (m, 2H), 2.36–2.44 (m, 1H), 2.48–2.58 (m, 1H), 3.74 (s, 3H), 5.91 (br s, 1H), 6.33 (dd, J = 4.0, 2.8 Hz, 1H), 7.00 (dd, J = 4.0, 1.6 Hz, 1H), 7.16 (dd, J = 2.8, 1.6 Hz, 1H) ppm; 13C NMR δ = 16.2, 30.1, 31.4, 52.7, 58.7, 110.5, 110.0, 125.7, 130.5, 131.5, 170.5, 179.5 ppm; IR ν = 3112, 2946, 2918, 2843, 2817, 1741, 1650, 1532, 1472, 1407, 1373, 1342, 1272, 1226, 1208, 1085, 1031, 1003, 750 cm−1; HRMS (EI) calcd for C11H15NO3S 241.0773, found 241.0771.
Data for 5k. Rf = 0.40 (4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 EtOAc/hexane); 1H NMR δ = 3.47 (dd, J = 14.4, 9.2 Hz, 1H), 3.71 (dd, J = 14.4, 5.6 Hz, 1H), 3.73 (s, 3H), 6.09 (br s, 1H), 6.17 (dd, J = 3.6, 2.4 Hz, 1H), 6.71 (d, J = 2.4 Hz, 1H), 6.93 (dd, J = 3.6, 1.6 Hz, 1H), 7.01 (br s, 1H), 7.11 (ddd, J = 8.4, 6.8, 0.8 Hz, 1H), 7.18 (ddd, J = 8.4, 6.8, 1.2 Hz, 1H), 7.31 (d, J = 8.4 Hz, 1H), 7.54 (d, J = 8.4 Hz, 1H), 7.99 (br s, 1H), 9.48 (d, J = 1, 2 Hz, 1H) ppm; 13C NMR δ = 28.6, 52.6, 60.5, 110.0, 110.3, 111.1, 118.3, 119.6, 122.1, 122.7, 125.7, 127.0, 130.8, 131.2, 135.9, 170.6, 179.5 ppm; IR ν = 3017, 2872, 1743, 1658, 1457, 1402, 1372, 1345, 1223, 1151, 1092, 1079, 1034, 997, 977, 912, 758, 733
6 EtOAc/hexane); 1H NMR δ = 3.47 (dd, J = 14.4, 9.2 Hz, 1H), 3.71 (dd, J = 14.4, 5.6 Hz, 1H), 3.73 (s, 3H), 6.09 (br s, 1H), 6.17 (dd, J = 3.6, 2.4 Hz, 1H), 6.71 (d, J = 2.4 Hz, 1H), 6.93 (dd, J = 3.6, 1.6 Hz, 1H), 7.01 (br s, 1H), 7.11 (ddd, J = 8.4, 6.8, 0.8 Hz, 1H), 7.18 (ddd, J = 8.4, 6.8, 1.2 Hz, 1H), 7.31 (d, J = 8.4 Hz, 1H), 7.54 (d, J = 8.4 Hz, 1H), 7.99 (br s, 1H), 9.48 (d, J = 1, 2 Hz, 1H) ppm; 13C NMR δ = 28.6, 52.6, 60.5, 110.0, 110.3, 111.1, 118.3, 119.6, 122.1, 122.7, 125.7, 127.0, 130.8, 131.2, 135.9, 170.6, 179.5 ppm; IR ν = 3017, 2872, 1743, 1658, 1457, 1402, 1372, 1345, 1223, 1151, 1092, 1079, 1034, 997, 977, 912, 758, 733![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 703, 666, 651 cm−1; HRMS (FAB) calcd for C17H17N2O3 297.1239, found 297.1237.
703, 666, 651 cm−1; HRMS (FAB) calcd for C17H17N2O3 297.1239, found 297.1237.
General procedure for reductive amination of pyrralines 5 with benzylamine
Data for 6a. 1H-NMR δ = 4.43 (s, 2H), 4.70 (s, 2H), 4.75 (s, 2H), 5.91 (dd, J = 2.4, 1.6 Hz, 1H), 6.21 (dd, J = 3.6, 2.4 Hz, 1H), 6.61 (dd, J = 2.4, 1.6 Hz, 1H), 7.26–7.37 (m, 5H) ppm; 13C-NMR δ = 44.2, 48.4, 50.0, 103.2, 109.6, 118.0, 121.9, 127.9, 128.2, 128.8, 135.8, 164.6 ppm; IR (neat) ν = 3034, 2926, 2848, 1715, 1651, 1543, 1491, 1450, 1424, 1323, 1256, 1204, 1182, 1073, 1025, 950, 902, 816, 742, 697, 608 cm−1; HRMS (ESI) calcd for C14H14N2O + Na 249.0998, found 249.1001.
Data for 6b. 1H-NMR δ = 1.68 (d, J = 7.2 Hz, 3H), 4.36 (A of ABq, J = 15.2 Hz, 1H), 4.42 (B of ABq, J = 15.2 Hz, 1H), 4.72 (s, 2H), 4.74 (q, J = 7.2 Hz, 1H), 5.89 (m, 1H), 6.20 (dd, J = 3.6, 2.8 Hz, 1H), 6.64 (dd, J = 2.8, 2.0 Hz, 1H), 7.24–7.36 (m, 5H) ppm; 13C-NMR δ = 20.2, 43.8, 50.2, 54.7, 103.1, 109.4, 117.0, 121.6, 127.7, 128.0, 128.7, 135.9, 168.1 ppm; IR (neat) ν = 3068, 3030, 2974, 2926, 2848, 1711, 1651, 1543, 1483, 1450, 1319, 1256, 1204, 1144, 1073, 1029, 965, 932, 896, 738, 697, 611 cm−1; HRMS (ESI) calcd for C15H16N2O + Na 263.1155, found 263.1158.
Data for 6c. 1H-NMR δ = 0.84 (d, J = 7.2 Hz, 3H), 1.06 (d, J = 7.2 Hz, 3H), 2.39 (m, 1H), 4.27 (A of ABq, J = 15.6 Hz, 1H), 4.39 (B of ABq, J = 15.6 Hz, 1H), 4.52 (d, J = 4.4 Hz, 1H), 4.59 (A of ABq, J = 14.8 Hz, 1H), 4.82 (B of ABq, J = 14.8 Hz, 1H), 5.89 (dd, J = 3.6, 1.6 Hz, 1H), 6.16 (dd, J = 3.6, 2.8 Hz, 1H), 6.57 (dd, J = 2.8, 1.6 Hz, 1H), 7.22–7.34 (m, 5H) ppm; 13C-NMR δ = 17.6, 19.9, 34.7, 44.2, 50.2, 65.2, 103.0, 108.6, 119.1, 122.5, 127.7, 128.2, 128.7, 136.0, 167.4 ppm; IR (neat) ν = 3094, 3027, 2967, 2933, 2870, 1655, 1543, 1495, 1469, 1454, 1387, 1353, 1290, 1275, 1216, 1152, 1070, 991, 957, 891, 861, 790, 749, 716, 696, 662, 615 cm−1; HRMS (ESI) calcd for C17H20N2O + Na 291.1468, found 291.1470.
Data for 6d. 1H-NMR δ = 0.90 (t, J = 7.2 Hz, 3H), 1.00 (d, J = 6.8 Hz, 3H), 1.05–1.17 (m, 1H), 1.49–1.60 (m, 1H), 2.05–2.16 (m, 1H), 4.30 (A of ABq, J = 15.2 Hz, 1H), 4.43 (B of ABq, J = 15.2 Hz, 1H), 4.62 (d, J = 4.4 Hz, 1H), 4.67 (A of ABq, J = 14.8 Hz, 1H), 4.78 (B of ABq, J = 14.8 Hz, 1H), 5.89 (dd, J = 3.2, 2.0 Hz, 1H), 6.18 (dd, J = 3.2, 2.8 Hz, 1H), 6.59 (dd, J = 2.8, 2.0 Hz, 1H), 7.26–7.36 (m, 5H) ppm; 13C-NMR δ = 11.5, 15.9, 24.9, 41.7, 44.3, 50.3, 64.3, 103.0, 108.9, 118.8, 122.6, 127.8, 128.3, 128.7, 136.1, 167.2 ppm; IR (neat) ν = 3019, 2963, 2926, 2874, 1707, 1651, 1543, 1487, 1454, 1323, 1215, 1141, 1073, 1029, 950, 928, 898, 742, 697, 667, 634, 611 cm−1; HRMS (ESI) calcd for C18H22N2O + Na 305.1624, found 305.1628.
Data for 6e. 1H-NMR δ = 3.09 (d, J = 15.2 Hz, 3H), 3.26 (d of A of ABq, JAB = 13.6, Jd = 4.4 Hz, 1H), 3.41 (d of B of ABq, JAB = 13.6, Jd = 4.4 Hz, 1H), 3.93 (d, J = 15.2 Hz, 1H), 4.39 (A of ABq, JAB = 14.8 Hz, 1H), 4.65 (B of ABq, JAB = 14.8 Hz, 1H), 5.03 (t, J = 4.4 Hz, 1H), 5.71 (dd, J = 3.6, 1.6 Hz, 1H), 6.22 (dd, J = 3.6, 2.8 Hz, 1H), 6.62 (dd, J = 2.8, 1.6 Hz, 1H), 6.66–6.70 (m, 2H), 7.00–7.06 (m, 2H), 7.13–7.17 (m, 3H), 7.24–7.32 (m, 3H) ppm; 13C-NMR δ = 41.5, 43.6, 50.1, 60.0, 102.4, 109.8, 116.9, 122.8, 127.2, 127.7, 128.1, 128.5, 128.6, 129.6, 134.9, 135.6, 166.6 ppm; IR (neat) ν = 3068, 3027, 2933, 2844, 1647, 1483, 1439, 1394, 1327, 1252, 1204, 1170, 1077, 1029, 965, 898, 861, 742, 693, 663, 630, 611 cm−1; HRMS (ESI) calcd for C21H20N2O + Na 339.1468, found 339.1470.
Data for 6f. 1H-NMR δ = 2.25–2.40 (m, 2H), 2.44–2.53 (m, 1H), 2.56–2.65 (m, 1H), 4.31 (A of ABq, JAB = 15.6 Hz, 1H), 4.39 (B of ABq, JAB = 15.6 Hz, 1H), 4.61 (A of ABq, JAB = 14.4 Hz, 1H), 4.74 (B of ABq, JAB = 14.4 Hz, 1H), 4.75 (dd, J = 5.6, 5.2 Hz, 1H), 5.89 (dd, J = 3.6, 1.6 Hz, 1H), 6.21 (dd, J = 3.6, 2.8 Hz, 1H), 6.60 (dd, J = 2.8, 1.6 Hz, 1H), 7.09–7.18 (m, 3H), 7.21–7.33 (m, 7H) ppm; 13C-NMR δ = 30.6, 36.2, 43.9, 50.1, 58.7, 103.1, 109.4, 117.6, 121.9, 126.0, 127.7, 128.0, 128.2, 128.3, 128.6, 135.9, 140.2, 167.2 ppm; IR (neat) ν = 3067, 3027, 2922, 2856, 1652, 1485, 1452, 1328, 1256, 1078, 1026, 746, 698 cm−1; HRMS (ESI) calcd for C22H22N2O + Na 353.1624, found 353.1626.
Data for 6g. 1H-NMR δ = 3.14 (d of A of ABq, JAB = 16,8, Jd = 4.8 Hz, 1H), 3.18 (d of B of ABq, JAB = 16,8, Jd = 5.2 Hz, 1H), 3,62 (s, 3H), 4.37 (d of A of ABq, JAB = 15.2, Jd = 0.8 Hz, 1H), 4.68 (B of ABq, JAB = 15.2 Hz, 1H), 4.70 (A of ABq, JAB = 14.8 Hz, 1H), 4.79 (B of ABq, JAB = 14.8 Hz, 1H), 5.05 (dd, J = 5.2, 4,8 Hz, 1H), 5.88 (dd, J = 3.6, 1.6 Hz, 1H), 6.19 (dd, J = 3.6, 2.8 Hz, 1H), 6.63 (dd, J = 2.8, 1.6 Hz, 1H), 7.26–7.37 (m, 5H) ppm; 13C-NMR δ = 38.3, 44.0, 50.4, 52.0, 55.0, 103.3, 109.7, 117.3, 122.2, 127.7, 128.1, 128.7, 135.7, 166.4, 170.2 ppm; IR (neat) ν = 3031, 2923, 2854, 1735, 1654, 1487, 1437, 1370, 1330, 1249, 1201, 1168, 1077, 1018, 987, 896, 744, 699 cm−1; HRMS (ESI) calcd for C17H18N2O3 + Na 321.1210, found 321.1214.
Data for 6h. 1H-NMR δ = 2.06 (s, 3H), 2.23–2.33 (m, 2H), 2.34–2.49 (m, 2H), 4.33 (A of ABq, J = 15.6 Hz, 1H), 4.41 (B of ABq, J = 15.6 Hz, 1H), 4.67 (A of ABq, J = 14.8 Hz, 1H), 4.73 (B of ABq, J = 14.8 Hz, 1H), 4.87 (t, J = 5.6 Hz, 1H), 5.89 (dd, J = 2.8, 1.6 Hz, 1H), 6.19 (dd, J = 3.6, 2.8 Hz, 1H), 6.63 (dd, J = 2.8, 1.6 Hz, 1H), 7.24–7.36 (m, 5H) ppm; 13C-NMR δ = 15.2, 29.2, 33.6, 44.0, 50.4, 58.0, 103.4, 109.6, 117.9, 122.1, 127.9, 128.2, 128.8, 136.0, 167.3 ppm; IR (neat) ν = 3008, 2915, 2837, 1647, 1483, 1431, 1331, 1286, 1252, 1159, 1073, 1025, 950, 898, 820, 742, 697, 626, 611 cm−1; HRMS (ESI) calcd for C17H20N2OS + Na 323.1189, found 323.1192.
General procedure for cyclization of pyrralines 5 with ethane-1,2-diamine
Data for 7a. 1H-NMR δ = 2.14 (br s, 1H), 3.18–3.30 (m, 1H), 3.48–3.62 (m, 3H), 4.55 (A of ABq, J = 16.8 Hz, 1H), 4.60 (B of ABq, J = 16.8 Hz, 1H), 5.25 (br s, 1H), 6.22–6.26 (m, 2H), 6.66 (dd, J = 2.0, 1.6 Hz, 1H) ppm; 13C-NMR δ = 43.9, 45.0, 49.0, 70.4, 104.2, 109.5, 119.1, 124.8, 162.1 ppm; IR (KBr) ν = 3226, 3115, 3098, 2976, 2960, 2885, 2857, 1636, 1470, 1441, 1305, 1216, 1173, 1159, 1126, 1070, 1004, 968, 951, 932, 906, 856, 770, 756, 729, 681 cm−1; HRMS (ESI) calcd for C9H11N3O + Na 200.0794, found 200.0797.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of stereoisomers) as white solid after purification by SiO2 column chromatography.
1 mixture of stereoisomers) as white solid after purification by SiO2 column chromatography.
Data for 7b. 1H-NMR (major) δ = 1.61 (d, J = 7.2 Hz, 3H), 2.14 (br s, 1H), 3.20–3.31 (m, 1H), 3.47–3.61 (m, 3H), 4.68 (q, J = 7.2 Hz, 1H), 5.26 (br s, 1H), 6.22–6.28 (m, 2H), 6.68 (dd, J = 2.4, 2.0 Hz, 1H) ppm; 13C-NMR (major) δ = 21.2, 43.8, 44.8, 55.8, 69.7, 103.8, 109.3, 117.9, 124.2, 165.7 ppm; (minor) δ = 14.6, 44.2, 44.8, 53.4, 69.7, 103.9, 108.7, 116.9, 125.9, 164.6 ppm; IR (KBr) ν = 3065, 3030, 2946, 2920, 2872, 2844, 1648, 1543, 1486, 1460, 1431, 1357, 1329, 1252, 1221, 1140, 1073, 1025, 962, 766, 739, 700 cm−1; HRMS (ESI) calcd for C10H13N3O + Na 214.0951, found 214.0954.
Data for 7c. 1H-NMR δ = 0.93 (d, J = 6.4 Hz, 3H), 1.08 (d, J = 6.8 Hz, 3H), 2.10 (br s, 1H), 2.22–2.34 (m, 1H), 3.17–3.28 (m, 1H), 3.47–3.60 (m, 3H), 4.39 (d, J = 5.2 Hz, 1H), 5.25 (s, 1H), 6.22 (dd, J = 3.6, 2.0 Hz, 1H), 6.24 (dd, J = 3.6, 1.2 Hz, 1H), 6.64 (dd, J = 2.0, 1.2 Hz, 1H) ppm; 13C-NMR δ = 18.1, 19.6, 34.3, 44.2, 45.0, 66.3, 70.4, 103.8, 108.7, 120.0, 125.6, 165.1 ppm; IR (KBr) ν = 3278, 2971, 2891, 1654, 1567, 1463, 1430, 1395, 1372, 1325, 1303, 1251, 1221, 1178, 1153, 1112, 1075, 950, 922, 903, 855, 750, 717, 667 cm−1; HRMS (ESI) calcd for C12H17N3O + Na 242.1264, found 242.1265.
Data for 7d. 1H-NMR δ = 0.90 (t, J = 7.2 Hz, 3H), 0.98 (d, J = 7.2 Hz, 3H), 1.05–1.17 (m, 1H), 1.49–1.61 (m, 1H), 1.92–2.04 (m, 1H), 2.25 (br s, 1H), 3.13–3.24 (m, 1H), 3.42–3.56 (m, 3H), 4.42 (d, J = 5.6 Hz, 1H), 5.21 (br s, 1H), 6.17–6.21 (m, 2H), 6.62 (dd, J = 2.0, 2.0 Hz, 1H) ppm; 13C-NMR δ = 11.1, 15.3, 24.7, 40.8, 44.0, 44.7, 65.0, 70.2, 103.5, 108.6, 119.3, 125.4, 164.6 ppm; IR (KBr) ν = 3292, 3103, 2962, 2931, 2880, 2860, 1638, 1464, 1430, 1349, 1323, 1277, 1247, 1221, 1189, 1108, 1078, 1025, 958, 902, 877, 772, 710 cm−1; HRMS (ESI) calcd for C13H19N3O + Na 256.1420, found 256.1421.
Data for 7e. 1H-NMR δ = 1.94 (br s, 1H), 2.90–2.99 (m, 1H), 3.24 (d of A of ABq, JAB = 13.6, Jd = 4.4 Hz, 1H), 3.30 (d of B of ABq, JAB = 13.6, Jd = 4.8 Hz, 1H), 3.25–3.39 (m, 2H), 3.43–3.52 (m, 1H), 3.79 (s, 1H), 4.90 (dd, J = 4.8, 4.4 Hz, 1H), 6.07 (dd, J = 3.6, 1.2 Hz, 1H), 6.23 (dd, J = 3.6, 2.8 Hz, 1H), 6.59 (dd, J = 2.8, 1.2 Hz, 1H), 6.74–6.79 (m, 2H), 7.12–7.18 (m, 2H), 7.19–7.24 (m, 1H) ppm; 13C-NMR δ = 41.4, 43.6, 44.7, 61.3, 69.6, 103.5, 109.7, 118.2, 125.6, 127.5, 128.2, 129.5, 134.8, 164.0 ppm; IR (KBr) ν = 3276, 3064, 3031, 2951, 2885, 2857, 1644, 1469, 1459, 1436, 1328, 1306, 1217, 1173, 1119, 1076, 942, 914, 773, 728, 696 cm−1; HRMS (ESI) calcd for C16H18N3O [M + 1]+ 268.1444, found 268.1448.
Data for 7f. 1H-NMR δ = 2.08 (br s, 1H), 2.14–2.24 (m, 1H), 2.25–2.35 (m, 1H), 2.58–2.67 (m, 1H), 2.68–2.77 (m, 1H), 3.17–3.28 (m, 1H), 3.47–3.60 (m, 3H), 4.63 (dd, J = 6.8, 6.4 Hz, 1H), 5.24 (s, 1H), 6.23–6.27 (m, 2H), 6.66 (dd, J = 2.4, 1.6 Hz, 1H), 7.13–7.21 (m, 3H), 7.24–7.30 (m, 2H) ppm; 13C-NMR δ = 31.3, 36.7, 44.2, 45.0, 60.1, 70.2, 104.2, 109.4, 119.1, 125.1, 126.2, 128.3, 128.5, 140.0, 165.3 ppm; IR (KBr) ν = 3276, 3028, 2950, 2885, 2859, 1652, 1458, 1429, 1334, 1305, 1221, 1158, 1118, 1073, 912, 771, 752, 698 cm−1; HRMS (ESI) calcd for C17H20N3O [M + 1]+ 282.1601, found 282.1604.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of stereoisomers) as white solid after purification by SiO2 column chromatography.
1 mixture of stereoisomers) as white solid after purification by SiO2 column chromatography.
Data for 7g. 1H-NMR (major) δ = 2.31 (br s, 1H), 2.91 (d of A of ABq, JAB = 16.8, Jd = 8.0 Hz, 1H), 3.02 (d of B of ABq, JAB = 16.8, Jd = 4.4 Hz, 1H), 3.17–3.48 (m, 1H), 3.45–3.59 (m, 3H), 3.66 (s, 3H), 4.99 (dd, J = 8.0, 4.4 Hz, 1H), 5.26 (s, 1H), 6.18–6.23 (m, 2H), 6.72 (br s, 1H) ppm; (minor) δ = 2.31 (br s, 1H), 3.17–3.38 (m, 3H), 3.45–3.58 (m, 3H), 3.67 (s, 3H), 4.91 (dd, J = 5.2, 4.8 Hz, 1H), 5.23 (s, 1H), 6.22–6.27 (m, 2H), 6.62 (br s, 1H) ppm; 13C-NMR (major) δ = 38.9, 44.0, 44.8, 52.0, 56.1, 70.0, 104.1, 109.5, 119.1, 124.7, 163.7, 169.9 ppm; (minor) δ = 35.1, 44.3, 44.8, 51.9, 54.5, 70.0, 104.7, 109.3, 116.8, 125.1, 163.7, 170.5 ppm; IR (KBr) ν = 3287, 2999, 2941, 2878, 1734, 1653, 1461, 1438, 1376, 1330, 1307, 1214, 1173, 1118, 1072, 909, 750, 725 cm−1; HRMS (ESI) calcd for C12H16N3O3 [M + 1]+ 250.1186, found 250.1188.
Data for 7h. 1H-NMR δ = 2.00–2.11 (m, 1H), 2.07 (s, 3H), 2.15–2.25 (m, 1H), 2.38–2.46 (m, 1H), 2.50–2.58 (m, 1H), 3.14–3.24 (m, 1H), 3.42–3.52 (m, 3H), 4.71 (dd, J = 8.0, 5.2 Hz, 1H), 5.20 (s, 1H), 6.17–6.21 (m, 2H), 6.67 (dd, J = 2.8, 1.6 Hz, 1H) ppm; 13C-NMR δ = 14.7, 29.1, 33.6, 43.7, 44.6, 58.5, 69.7, 103.7, 108.9, 118.7, 124.6, 164.6 ppm; IR (KBr) ν = 3272, 3108, 2959, 2922, 2885, 2851, 1643, 1454, 1412, 1335, 1298, 1220, 1159, 1156, 1073, 948, 923, 897, 761, 708 cm−1; HRMS (ESI) calcd for C12H18N3OS [M + 1]+ 252.1165, found 252.1168.
General procedure for cyclization of pyrralines 5 with (S)-2-aminopropan-1-ol
Data for 8a. 1H-NMR δ = 1.41 (d, J = 6.0 Hz, 3H), 3.63 (ddd, J = 6.8, 6.4, 1.2 Hz, 1H), 4.40 (dd, J = 7.2, 6.8 Hz, 1H), 4.44 (ddq, Jd = 7.2, 6.4, Jq = 6.0 Hz, 1H), 4.60 (A of ABq, JAB = 16.8 Hz, 1H), 4.65 (d of B of ABq, JAB = 16.8, Jd = 0.8 Hz, 1H), 5.88 (br s, 1H), 6.29 (dd, J = 4.0, 2.4 Hz, 1H), 6.33 (ddd, J = 4.0, 1.6, 0.8 Hz, 1H), 6.64 (dd, J = 2.4, 1.6 Hz, 1H) ppm; 13C-NMR δ = 18.0, 48.5, 51.0, 72.2, 81.7, 106.2, 110.3, 119.1, 122.2, 162.7 ppm; IR (neat) ν = 2928, 2870, 1712, 1660, 1542, 1463, 1428, 1345, 1310, 1229, 1168, 1075, 1046, 976, 747, 720 cm−1; HRMS (ESI) calcd for C10H12N2O2 + Na, 215.0791, found 215.0791.
Data for 8b. 1H-NMR δ = 1.39 (d, J = 6.8 Hz, 3H), 1.63 (d, J = 6.8 Hz, 3H), 3.59–3.67 (m, 1H), 4.34–4.44 (m, 2H), 4.67 (q, J = 6.8 Hz, 1H), 5.79 (br s, 1H), 6.26–6.31 (m, 2H), 6.60 (dd, J = 2.4, 2.0 Hz, 1H) ppm; 13C-NMR δ = 16.3, 17.9, 51.4, 53.5, 72.1, 81.5, 106.3, 110.0, 117.7, 122.9, 165.7 ppm; IR (neat) ν = 3129, 2989, 2931, 2872, 1714, 1655, 1539, 1460, 1422, 1367, 1302, 1206, 1176, 1142, 1018, 978, 920, 820, 746, 719 cm−1; HRMS (ESI) calcd for C11H14N2O2 + Na, 229.0947, found 229.0952.
Data for 8c. 1H-NMR δ = 0.92 (d, J = 6.8 Hz, 3H), 1.10 (d, J = 6.8 Hz, 3H), 1.40 (d, J = 6.0 Hz, 3H), 2.27 (m, 1H), 3.60–3.68 (m, 1H), 4.33–4.43 (m, 3H), 5.80 (s, 1H), 6.26 (dd, J = 4.0, 2.8 Hz, 1H), 6.30 (ddd, J = 4.0, 1.6, 0.8 Hz, 1H), 6.62 (dd, J = 2.8, 1.6 Hz, 1H) ppm; 13C-NMR δ = 17.8, 18.0, 19.3, 34.9, 51.1, 65.8, 72.3, 82.1, 105.2, 109.4, 119.7, 123.8, 165.0 ppm; IR (neat) ν = 3116, 2963, 2920, 2857, 1714, 1666, 1564, 1449, 1417, 1381, 1370, 1301, 1229, 1197, 1100, 1057, 973, 867, 814, 766, 703 cm−1; HRMS (ESI) calcd for C13H18N2O2 + Na 257.1260, found 257.1262.
Data for 8d. 1H-NMR δ = 0.90 (d, J = 6.8 Hz, 3H), 0.93 (t, J = 7.6 Hz, 3H), 1.08–1.20 (m, 1H), 1.38 (d, J = 6.8 Hz, 3H), 1.54–1.65 (m, 1H), 1.91–2.02 (m, 1H), 3.57–3.65 (m, 1H), 4.31–4.40 (m, 2H), 4.51 (d, J = 4.8 Hz, 1H), 5.79 (br s, 1H), 6.24 (dd, J = 3.6, 2.8 Hz, 1H), 6.27 (ddd, J = 3.6, 1.6, 0.8 Hz, 1H), 6.61 (dd, J = 2.8, 1.6 Hz, 1H) ppm; 13C-NMR δ = 11.4, 15.0, 17.6, 25.0, 41.7, 50.9, 64.3, 72.0, 81.9, 104.9, 109.4, 119.0, 123.6, 164.5 ppm; IR (neat) ν = 3099, 2958, 2925, 2867, 1709, 1666, 1563, 1539, 1460, 1430, 1386, 1295, 1216, 1195, 1068, 1055, 978, 871, 817, 766, 708 cm−1; HRMS (ESI) calcd for C14H20N2O2 + Na 271.1417, found 271.1419.
Data for 8e. 1H-NMR δ = 1.21 (d, J = 6.0 Hz, 3H), 3.23 (dd, J = 13.6, 4.4 Hz, 1H), 3.30–3.37 (m, 2H), 4.18–4.30 (m, 2H), 4.35 (s, 1H), 4.93 (t, J = 4.4 Hz, 1H), 6.10 (ddd, J = 3.6, 1.6, 0.8 Hz, 1H), 6.31 (dd, J = 3.6, 2.8 Hz, 1H), 6.66–6.72 (m, 3H), 7.11–7.16 (m, 2H), 7.20–7.25 (m, 1H) ppm; 13C-NMR δ = 17.2, 41.8, 50.6, 60.4, 72.1, 80.8, 104.7, 110.5, 117.6, 124.0, 127.5, 128.0, 129.8, 134.3, 163.8 ppm; IR (neat) ν = 3027, 2962, 2923, 2861, 1704, 1659, 1561, 1456, 1437, 1404, 1361, 1311, 1234, 1165, 1077, 1041, 972, 822, 744, 695 cm−1; HRMS (ESI) calcd for C17H18N2O2 + Na 305.1260, found 305.1264.
Data for 8f. 1H-NMR δ = 1.37 (d, J = 6.4 Hz, 3H), 2.24–2.33 (m, 2H), 2.47–2.55 (m, 1H), 2.60–2.68 (m, 1H), 3.59–3.66 (m, 1H), 4.35–4.44 (m, 2H), 4.68 (t, J = 6.0 Hz, 1H), 5.78 (s, 1H), 6.30 (dd, J = 3.6, 2.8 Hz, 1H), 6.32 (ddd, J = 3.6, 1.6, 0.8 Hz, 1H), 6.66 (dd, J = 2.8, 1.6 Hz, 1H), 7.12–7.16 (m, 2H), 7.16–7.22 (m, 1H), 7.24–7.30 (m, 2H) ppm; 13C-NMR δ = 17.8, 30.9, 37.4, 51.1, 59.5, 72.3, 81.7, 105.7, 110.2, 118.7, 122.9, 126.3, 128.3, 128.5, 140.0, 165.6 ppm; IR (neat) ν = 3016, 2966, 2930, 2857, 1714, 1666, 1561, 1449, 1434, 1406, 1359, 1302, 1227, 1168, 1076, 1058, 977, 826, 766, 725, 693 cm−1; HRMS (ESI) calcd for C18H20N2O2 + Na, 319.1417, found 319.1419.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of stereoisomers) as light-yellow liquid after purification by SiO2 column chromatography.
1 mixture of stereoisomers) as light-yellow liquid after purification by SiO2 column chromatography.
Data for 8g. 1H-NMR (major) δ = 1.42 (d, J = 6.4 Hz, 3H), 2.97 (dd, J = 16.4, 6.8 Hz, 1H), 3.06 (dd, J = 16.4, 4.4 Hz, 1H), 3.60–3.70 (m, 1H), 3.65 (s, 3H), 4.35–4.46 (m, 2H), 4.99 (dd, J = 6.7, 4.4 Hz, 1H), 5.82 (br s, 1H), 6.26 (dd, J = 3.6, 1.6 Hz, 1H), 6.30 (dd, J = 3.6, 2.4 Hz, 1H), 6.70 (dd, J = 2.4, 1.6 Hz, 1H) ppm; (minor) δ = 1.40 (d, J = 6.4 Hz, 3H), 3.21 (dd, J = 17.2, 5.6 Hz, 1H), 3.36 (dd, J = 17.2, 5.6 Hz, 1H), 3.60–3.70 (m, 1H), 3.71 (s, 3H), 4.35–4.48 (m, 2H), 5.00 (t, J = 5.6 Hz, 1H), 5.79 (br s, 1H), 6.27–6.36 (m, 2H), 6.64 (m, 1H) ppm; 13C-NMR (major) δ = 17.9, 39.5, 51.2, 52.2, 54.5, 72.4, 81.5, 106.8, 110.4, 118.8, 122.9, 164.1, 169.9 ppm; (minor) δ = 17.6, 36.2, 51.7, 52.1, 55.8, 72.0, 81.3, 105.8, 110.5, 117.5, 122.7, 164.7, 170.6 ppm; IR (neat) ν = 2957, 2926, 2856, 1737, 1671, 1563, 1461, 1442, 1379, 1336, 1310, 1272, 1220, 1203, 1172, 1077, 1055, 976, 914, 776, 733, 673 cm−1; HRMS (ESI) calcd for C13H16N2O4 + Na 287.1002, found 287.1002.
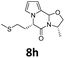
Data for 8h. 1H-NMR δ = 1.40 (d, J = 6.0 Hz, 3H), 2.09 (s, 3H), 2.12–2.22 (m, 1H), 2.22–2.31 (m, 1H), 2.33–2.41 (m, 1H), 2.47–2.55 (m, 1H), 3.61–3.69 (m, 1H), 4.34–4.44 (m, 2H), 4.78 (dd, J = 6.8, 5.6 Hz, 1H), 5.80 (s, 1H), 6.28 (dd, J = 4.0, 2.8 Hz, 1H), 6.31 (ddd, J = 4.0, 1.6, 0.8 Hz, 1H), 6.68 (dd, J = 2.8, 1.6 Hz, 1H) ppm; 13C-NMR δ = 15.2, 17.7, 29.2, 34.9, 51.1, 58.6, 72.3, 81.7, 105.8, 110.2, 118.8, 122.9, 165.4 ppm; IR (neat) ν = 2967, 2910, 2862, 1645, 1556, 1465, 1412, 1398, 1354, 1307, 1228, 1168, 1041, 975, 830, 766, 719 cm−1; HRMS (ESI) calcd for C13H18N2O2S + Na, 289.0981, found 289.0985.
General procedure for cyclization of pyrralines 5 with (S)-2-amino-3-methylbutan-1-ol (L-valinol)
Data for 9a. 1H-NMR δ = 0.98 (d, J = 6.8 Hz, 3H), 1.01 (d, J = 6.4 Hz, 3H), 2.14 (m, 1H), 3.83 (dd, J = 8.8, 6.8 Hz, 1H), 4.21 (dd, J = 8.8, 8.0 Hz, 1H), 4.29 (ddd, J = 8.0, 7.2, 6.8 Hz, 1H), 4.63 (A of ABq, JAB = 17.2 Hz, 1H), 4.72 (B of ABq, JAB = 17.2 Hz, 1H), 5.74 (s, 1H), 6.30 (dd, J = 3.6, 2.4 Hz, 1H), 6.33 (ddd, J = 3.6, 1.6, 0.8 Hz, 1H), 6.65 (dd, J = 2.4, 1.6 Hz, 1H) ppm; 13C-NMR δ = 17.5, 19.2, 30.8, 48.4, 60.2, 67.7, 82.5, 106.5, 110.4, 119.1, 121.9, 163.9 ppm; IR (neat) ν = 2952, 2929, 2872, 1666, 1561, 1460, 1429, 1367, 1322, 1268, 1227, 1189, 1081, 1048, 973, 870, 825, 761, 713 cm−1; HRMS (ESI) calcd for C12H16N2O2 + Na, 243.1104, found 243.1107.
Data for 9b. 1H-NMR δ = 0.97 (d, J = 7.2 Hz, 3H), 1.01 (d, J = 6.8 Hz, 3H), 1.79 (d, J = 7.2 Hz, 3H), 2.11 (m, 1H), 3.83 (dd, J = 8.8, 6.8 Hz, 1H), 4.17 (dd, J = 8.8, 7.6 Hz, 1H), 4.26 (ddd, J = 8.0, 7.6, 6.8 Hz, 1H), 4.68 (q, J = 7.2 Hz, 1H), 5.74 (s, 1H), 6.30 (dd, J = 3.6, 2.8 Hz, 1H), 6.33 (ddd, J = 3.6, 1.6, 0.8 Hz, 1H), 6.76 (dd, J = 2.8, 1.6 Hz, 1H) ppm; 13C-NMR δ = 17.2, 17.7, 19.3, 30.8, 53.4, 60.7, 67.6, 82.2, 106.6, 110.1, 117.9, 122.4, 167.1 ppm; IR (neat) ν = 2958, 2942, 2872, 1703, 1661, 1562, 1544, 1460, 1422, 1365, 1301, 1216, 1111, 1085, 1058, 984, 950, 857, 825, 751, 708 cm−1; HRMS (ESI) calcd for C13H18N2O2 + Na, 257.1260, found 257.1263.
Data for 9c. 1H-NMR δ = 0.95 (d, J = 6.8 Hz, 3H), 0.99 (d, J = 7.2 Hz, 3H), 1.02 (d, J = 6.8 Hz, 3H), 1.03 (d, J = 6.8 Hz, 3H), 2.17–2.33 (m, 2H), 3.86 (dd, J = 8.8, 6.8 Hz, 1H), 4.17 (dd, J = 8.8, 7.6 Hz, 1H), 4.28 (ddd, J = 7.6, 7.2, 6.8 Hz, 1H), 4.46 (d, J = 5.2 Hz, 1H), 5.77 (s, 1H), 6.26 (dd, J = 3.6, 2.8 Hz, 1H), 6.28 (ddd, J = 3.6, 1.6, 0.8 Hz, 1H), 6.63 (dd, J = 2.8, 1.6 Hz, 1H) ppm; 13C-NMR δ = 17.5, 18.2, 19.2, 19.2, 30.3, 35.0, 60.1, 65.9, 67.2, 83.0, 105.2, 109.5, 119.4, 123.8, 165.9 ppm; IR (neat) ν = 3100, 2958, 2857, 1702, 1661, 1561, 1455, 1429, 1396, 1375, 1306, 1227, 1189, 1105, 1058, 967, 867, 825, 765, 708 cm−1; HRMS (ESI) calcd for C15H22N2O2 + Na, 285.1573, found 285.1575.
Data for 9d. 1H-NMR δ = 0.90 (d, J = 7.2 Hz, 3H), 0.95 (t, J = 7.6 Hz, 3H), 0.98 (d, J = 6.0 Hz, 3H), 1.00 (d, J = 6.8 Hz, 3H), 1.08–2.22 (m, 1H), 1.57–1.68 (m, 1H), 1.93–2.03 (m, 1H), 2.14–2.26 (m, 1H), 3.85 (dd, J = 8.8, 6.8 Hz, 1H), 4.17 (dd, J = 8.8, 7.6 Hz, 1H), 4.28 (ddd, J = 7.6, 7.2, 6.8 Hz, 1H), 4.56 (d, J = 5.2 Hz, 1H), 5.77 (s, 1H), 6.26–6.28 (m, 2H), 6.63 (dd, J = 2.8, 1.6 Hz, 1H) ppm; 13C-NMR δ = 11.6, 15.2, 17.6, 19.2, 25.4, 30.4, 42.0, 60.2, 64.6, 67.3, 83.0, 105.2, 109.7, 119.0, 123.8, 165.6 ppm; IR (neat) ν = 2958, 2939, 2878, 1709, 1655, 1568, 1544, 1460, 1431, 1381, 1295, 1232, 1200, 1077, 1058, 967, 879, 833, 766, 713 cm−1; HRMS (ESI) calcd for C16H24N2O2 + Na, 299.1730, found 299.1734.
Data for 9e. 1H-NMR δ = 0.76 (d, J = 7.2 Hz, 3H), 0.88 (d, J = 6.8 Hz, 3H), 2.09 (m, 1H), 3.29 (d of A of ABq, JAB = 13.6, Jd = 4.8 Hz, 1H), 3.34 (d of B of ABq, JAB = 13.6, Jd = 4.4 Hz, 1H), 3.68 (dd, J = 8.8, 6.8 Hz, 1H), 4.04 (dd, J = 8.8, 7.6 Hz, 1H), 4.17 (ddd, J = 7.6, 7.2, 6.8 Hz, 1H), 4.86 (s, 1H), 4.96 (t, J = 4.8 Hz, 1H), 6.16 (ddd, J = 3.6, 1.6, 0.8 Hz, 1H), 6.31 (dd, J = 3.6, 2.8 Hz, 1H), 6.64 (dd, J = 2.8, 1.6 Hz, 1H), 6.66–6.70 (m, 2H), 7.12–7.23 (m, 3H) ppm; 13C-NMR δ = 17.1, 19.0, 30.0, 41.6, 60.2, 60.6, 66.6, 82.2, 105.1, 110.4, 117.7, 123.6, 127.2, 128.2, 129.4, 134.5, 165.3 ppm; IR (neat) ν = 3032, 2962, 2926, 2872, 1708, 1661, 1556, 1455, 1430, 1403, 1350, 1306, 1297, 1237, 1175, 1113, 1079, 1041, 967, 853, 816, 729, 693 cm−1; HRMS (ESI) calcd for C19H22N2O2 + Na, 333.1573, found 333.1575.
Data for 9f. 1H-NMR δ = 0.99 (d, J = 7.2 Hz, 3H), 1.02 (d, J = 7.2 Hz, 3H), 2.19 (m, 1H), 2.25–2.32 (m, 2H), 2.41–2.50 (m, 1H), 2.63–2.72 (m, 1H), 3.86 (dd, J = 8.8, 6.8 Hz, 1H), 4.20 (dd, J = 8.8, 7.6 Hz, 1H), 4.30 (ddd, J = 7.6, 7.2, 6.8 Hz, 1H), 4.71 (t, J = 5.6 Hz, 1H), 5.79 (s, 1H), 6.29–6.33 (m, 2H), 6.63 (dd, J = 2.4, 1.6 Hz, 1H), 7.11–7.15 (m, 2H), 7.16–7.22 (m, 1H), 7.24–7.30 (m, 2H) ppm; 13C-NMR δ = 17.5, 19.2, 30.6, 31.0, 38.0, 59.6, 60.1, 67.6, 82.6, 105.9, 110.3, 118.6, 122.8, 126.3, 128.3, 128.5, 140.1, 166.6 ppm; IR (neat) ν = 3015, 2963, 2925, 2878, 1709, 1666, 1549, 1455, 1422, 1403, 1343, 1302, 1232, 1158, 1058, 983, 876, 828, 713, 698 cm−1; HRMS (ESI) calcd for C20H24N2O2 + Na, 347.1730, found 347.1732.
Data for 9g. 1H-NMR δ = 0.99 (d, J = 6.8 Hz, 3H), 0.99 (d, J = 7.2 Hz, 3H), 2.26 (m, 1H), 3.00 (d of A of ABq, JAB = 16.4, Jd = 6.8 Hz, 1H), 3.08 (d of B of ABq, JAB = 16.4, Jd = 4.4 Hz, 1H), 3.65 (s, 3H), 3.89 (dd, J = 8.0, 6.0 Hz, 1H), 4.21 (dd, J = 8.0, 7.6 Hz, 1H), 4.26 (ddd, J = 7.6, 6.8, 6.0 Hz, 1H), 5.01 (dd, J = 6.4, 4.4 Hz, 1H), 5.79 (s, 1H), 6.27 (dd, J = 4.0, 2.8 Hz, 1H), 6.29 (ddd, J = 4.0, 1.6, 0.8 Hz, 1H), 6.70 (dd, J = 2.8, 1.6 Hz, 1H) ppm; 13C-NMR δ = 17.2, 19.0, 30.4, 39.7, 52.1, 55.9, 60.3, 67.5, 82.5, 106.0, 110.5, 118.6, 122.8, 165.0, 169.8 ppm; IR (neat) ν = 2958, 2931, 2862, 1740, 1655, 1570, 1449, 1439, 1399, 1371, 1317, 1237, 1191, 1168, 1063, 978, 862, 822, 772, 713 cm−1; HRMS (ESI) calcd for C15H20N2O4 + Na, 315.1315, found 315.1316.
Data for 9h. 1H-NMR δ = 0.96 (d, J = 6.8 Hz, 3H), 0.97 (d, J = 6.8 Hz, 3H), 2.06 (s, 3H), 2.13–2.36 (m, 4H), 2.46–2.55 (m, 1H), 3.84 (dd, J = 8.8, 6.8 Hz, 1H), 4.17 (dd, J = 8.8, 7.6 Hz, 1H), 4.25 (ddd, J = 7.6, 7.2, 6.8 Hz, 1H), 4.78 (dd, J = 6.4, 5.6 Hz, 1H), 5.74 (s, 1H), 6.26 (dd, J = 4.0, 2.8 Hz, 1H), 6.28 (ddd, J = 4.0, 1.6, 0.8 Hz, 1H), 6.67 (dd, J = 2.8, 1.6 Hz, 1H) ppm; 13C-NMR δ = 15.3, 17.5, 19.2, 29.2, 30.5, 35.3, 58.7, 60.1, 67.5, 82.6, 106.0, 110.3, 118.7, 122.8, 166.4 ppm; IR (neat) ν = 2958, 2910, 2872, 1703, 1661, 1550, 1544, 1449, 1422, 1406, 1345, 1307, 1237, 1163, 1114, 1053, 978, 870, 831, 777, 715 cm−1; HRMS (ESI) calcd for C15H22N2O2S + Na, 317.1294, found 317.1296.
General procedure for cyclization of pyrralines 5 with (S)-2-amino-2-phenylethan-1-ol (l-phenylalaninol)
Data for 10a. 1H-NMR δ = 2.98 (dd, J = 13.6, 8.8 Hz, 3H), 3.29 (dd, J = 13.6, 4.0 Hz, 1H), 3.85 (dd, J = 9.2, 8.0 Hz, 1H), 4.21 (dd, J = 9.2, 7.6 Hz, 1H), 4.60–4.68 (m, 1H), 4.64 (A of ABq, JAB = 17.2 Hz, 1H), 4.69 (B of ABq, JAB = 17.2 Hz, 1H), 5.54 (s, 1H), 6.26–6.30 (m, 2H), 6.65 (dd, J = 2.4, 1.6 Hz, 1H), 7.22–7.28 (m, 3H), 7.29–7.36 (m, 2H) ppm; 13C-NMR δ = 37.7, 48.6, 55.7, 69.6, 82.5, 106.1, 110.3, 119.1, 122.3, 126.9, 128.7, 129.5, 136.2, 163.0 ppm; IR (neat) ν = 3026, 2925, 2877, 1719, 1666, 1547, 1498, 1465, 1429, 1347, 1317, 1269, 1216, 1079, 1047, 973, 750, 693 cm−1; HRMS (ESI) calcd for C16H16N2O2 + Na, 291.1104, found 291.1107.
Data for 10b. 1H-NMR δ = 1.65 (d, J = 7.2 Hz, 3H), 3.08 (dd, J = 13.6, 8.0 Hz, 3H), 3.18 (dd, J = 13.6, 4.0 Hz, 1H), 3.88 (dd, J = 8.8, 8.0 Hz, 1H), 4.24 (dd, J = 8.8, 8.0 Hz, 1H), 4.62 (dddd, J = 8.0, 8.0, 8.0, 4.0 Hz, 1H), 4.71 (q, J = 7.2 Hz, 1H), 5.41 (s, 1H), 6.22 (ddd, J = 4.0, 1.6, 0.8 Hz, 1H), 6.26 (dd, J = 4.0, 2.8 Hz, 1H), 6.65 (dd, J = 2.8, 1.6 Hz, 1H), 7.20–7.28 (m, 3H), 7.29–7.34 (m, 2H) ppm; 13C-NMR δ = 22.4, 37.2, 55.3, 55.7, 69.4, 82.3, 105.6, 110.3, 118.1, 122.1, 127.0, 128.6, 129.8, 136.1, 166.7 ppm; IR (neat) ν = 3027, 2973, 2920, 2857, 1714, 1655, 1566, 1449, 1422, 1402, 1370, 1307, 1221, 1153, 1114, 1074, 1031, 973, 819, 751, 698 cm−1; HRMS (ESI) calcd for C17H18N2O2 + Na, 305.1260, found 305.1264.
Data for 10c. 1H-NMR δ = 0.89 (d, J = 6.8 Hz, 3H), 1.00 (d, J = 6.4 Hz, 3H), 2.25 (m, 1H), 2.92 (dd, J = 13.2, 9.2 Hz, 1H), 3.34 (dd, J = 13.2, 4.0 Hz, 1H), 3.85 (dd, J = 8.8, 7.2 Hz, 1H), 4.17 (dd, J = 8.8, 7.6 Hz, 1H), 4.44 (d, J = 4.8 Hz, 1H), 4.60 (dddd, J = 9.2, 7.6, 7.2, 4.0 Hz, 1H), 5.64 (s, 1H), 6.24 (dd, J = 3.6, 2.4 Hz, 1H), 6.26 (ddd, J = 3.6, 1.6, 0.8 Hz, 1H), 6.62 (dd, J = 2.4, 1.6 Hz, 1H), 7.22–7.28 (m, 3H), 7.29–7.34 (m, 2H) ppm; 13C-NMR δ = 18.1, 19.3, 34.8, 37.7, 56.0, 65.9, 69.8, 82.7, 105.2, 109.4, 119.6, 123.8, 126.9, 128.6, 129.5, 136.4, 165.4 ppm; IR (neat) ν = 3032, 2958, 2925, 2878, 1666, 1560, 1465, 1425, 1399, 1361, 1302, 1227, 1194, 1158, 1063, 1058, 1030, 967, 861, 756, 703 cm−1; HRMS (ESI) calcd for C19H22N2O2 + Na, 333.1573, found 333.1576.
Data for 10d. 1H-NMR δ = 0.91 (d, J = 6.8 Hz, 3H), 0.95 (t, J = 7.2 Hz, 3H), 1.00–1.14 (m, 1H), 1.50–1.62 (m, 1H), 1.92–2.04 (m, 1H), 2.93 (dd, J = 13.2, 8.8 Hz, 1H), 3.33 (dd, J = 13.2, 4.4 Hz, 1H), 3.85 (dd, J = 8.8, 7.6 Hz, 1H), 4.17 (dd, J = 8.8, 7.6 Hz, 1H), 4.55 (d, J = 5.2 Hz, 1H), 4.60 (dddd, J = 8.8, 7.6, 7.6, 4.4 Hz, 1H), 5.64 (s, 1H), 6.23–6.26 (m, 2H), 6.62 (dd, J = 2.8, 1.6 Hz, 1H), 7.22–7.28 (m, 3H), 7.29–7.35 (m, 2H) ppm; 13C-NMR δ = 11.6, 15.4, 25.1, 37.8, 41.8, 56.1, 64.7, 69.8, 82.7, 105.1, 109.6, 119.2, 123.8, 126.9, 128.6, 129.5, 136.4, 165.2 ppm; IR (neat) ν = 3020, 2958, 2920, 2872, 1705, 1655, 1561, 1455, 1431, 1398, 1366, 1290, 1227, 1189, 1151, 1068, 1052, 1026, 978, 862, 735, 703 cm−1; HRMS (ESI) calcd for C20H24N2O2 + Na, 347.1730, found 347.1732.
Data for 10e. 1H-NMR δ = 2.41 (dd, J = 13.2, 10.4 Hz, 1H), 3.28 (d of A of ABq, JAB = 13.6, Jd = 4.8 Hz, 1H), 3.32 (d of B of ABq, JAB = 13.6, Jd = 4.8 Hz, 1H), 3.44 (dd, J = 13.2, 4.4 Hz, 1H), 3.56 (dd, J = 9.2, 7.6 Hz, 1H), 3.99 (dd, J = 9.2, 7.6 Hz, 1H), 4.45 (dddd, J = 10.4, 7.6, 7.6, 4.4 Hz, 1H), 4.52 (s, 1H), 4.95 (t, J = 4.8 Hz, 1H), 6.12 (ddd, J = 4.0, 1.6, 0.8 Hz, 1H), 6.28 (dd, J = 4.0, 2.8 Hz, 1H), 6.63 (dd, J = 2.8, 1.6 Hz, 1H), 6.71–6.76 (m, 2H), 7.15–7.32 (m, 8H) ppm; 13C-NMR δ = 37.6, 41.8, 55.7, 60.6, 70.1, 81.4, 104.9, 110.4, 117.9, 123.7, 126.8, 127.5, 128.2, 128.7, 129.1, 129.7, 134.5, 136.5, 164.2 ppm; IR (neat) ν = 3016, 2915, 2862, 1655, 1544, 1460, 1438, 1405, 1361, 1316, 1206, 1158, 1079, 1049, 1029, 975, 851, 740, 698 cm−1; HRMS (ESI) calcd for C23H22N2O2 + Na, 381.1573, found 381.1577.
Data for 10f. 1H-NMR δ = 2.19–2.36 (m, 2H), 2.47–2.58 (m, 1H), 2.63–2.73 (m, 1H), 2.99 (dd, J = 13.6, 8.4 Hz, 1H), 3.21 (dd, J = 13.6, 4.0 Hz, 1H), 3.86 (dd, J = 8.8, 7.6 Hz, 1H), 4.21 (dd, J = 8.8, 7.6 Hz, 1H), 4.62 (dddd, J = 8.4, 7.6, 7.6, 4.0 Hz, 1H), 4.69 (t, J = 6.0 Hz, 1H), 5.51 (s, 1H), 6.26 (ddd, J = 3.6, 1.6, 0.8 Hz, 1H), 6.28 (dd, J = 3.6, 2.8 Hz, 1H), 6.65 (dd, J = 2.8, 1.6 Hz, 1H), 7.14–7.19 (m, 2H), 7.19–7.33 (m, 8H) ppm; 13C-NMR δ = 31.0, 37.5, 37.5, 55.6, 59.5, 69.6, 82.4, 105.7, 110.2, 118.7, 122.9, 126.3, 127.0, 128.3, 128.6, 128.6, 129.6, 136.2, 140.0, 165.9 ppm; IR (neat) ν = 3025, 2964, 2920, 2862, 1709, 1666, 1544, 1443, 1431, 1338, 1325, 1211, 1074, 1026, 973, 772, 687 cm−1; HRMS (ESI) calcd for C24H24N2O2 + Na, 395.1730, found 395.1733.
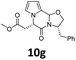
Data for 10g. 1H-NMR δ = 2.91 (dd, J = 16.4, 7.6 Hz, 1H), 3.02 (dd, J = 13.6, 8.8 Hz, 1H), 3.07 (dd, J = 16.4, 4.0 Hz, 1H), 3.29 (dd, J = 13.6, 4.0 Hz, 1H), 3.69 (s, 3H), 3.89 (dd, J = 9.2, 7.6 Hz, 1H), 4.22 (dd, J = 9.2, 7.6 Hz, 1H), 4.59 (dddd, J = 8.8, 7.6, 7.6, 4.0 Hz, 1H), 5.05 (dd, J = 7.6, 4.0 Hz, 1H), 5.54 (s, 1H), 6.22–6.27 (m, 2H), 6.70 (dd, J = 2.4, 1.6 Hz, 1H), 7.22–7.36 (m, 5H) ppm; 13C-NMR δ = 37.2, 39.7, 52.2, 55.8, 55.9, 69.7, 82.3, 105.8, 110.4, 118.9, 122.8, 127.0, 128.7, 129.6, 136.2, 164.4, 169.9 ppm; IR (neat) ν = 3032, 2947, 2927, 2867, 1735, 1666, 1555, 1455, 1438, 1442, 1400, 1365, 1317, 1221, 1168, 1084, 1026, 1029, 973, 898, 858, 830, 766, 703 cm−1; HRMS (ESI) calcd for C19H20N2O4 + Na, 363.1315, found 363.1319.
Data for 10h. 1H-NMR δ = 2.07–2.17 (m, 1H), 2.11 (s, 3H), 2.20–2.31 (m, 1H), 2.32–2.40 (m, 1H), 2.48–2.57 (m, 1H), 3.04 (dd, J = 13.6, 8.4 Hz, 1H), 3.22 (dd, J = 13.6, 4.0 Hz, 1H), 3.88 (dd, J = 9.2, 7.6 Hz, 1H), 4.21 (dd, J = 9.2, 7.6 Hz, 1H), 4.61 (dddd, J = 8.4, 7.6, 7.6, 4.0 Hz, 1H), 4.79 (dd, J = 7.2, 5.6 Hz, 1H), 5.49 (s, 1H), 6.23–6.27 (m, 2H), 6.67 (dd, J = 2.4, 2.0 Hz, 1H), 7.20–7.35 (m, 5H) ppm; 13C-NMR δ = 15.2, 29.3, 35.0, 37.3, 55.6, 58.6, 69.5, 82.4, 105.7, 110.1, 118.9, 122.9, 127.0, 128.6, 129.6, 136.2, 165.7 ppm; IR (neat) ν = 3015, 2915, 2846, 1709, 1655, 1570, 1539, 1478, 1455, 1426, 1347, 1323, 1302, 1211, 1200, 1147, 1075, 1032, 977, 854, 818, 739, 701 cm−1; HRMS (ESI) calcd for C19H22N2O2S + Na, 365.1294, found 365.1296.
Conclusions
We demonstrated a practically efficient transformation method of D-ribose as sustainable reducing sugar with various α-amino acids into pyrralines 5 as platform chemicals. Up to 300% yield increasement of pyrralines 5 (32–63% yield) were realized by one-pot pressurized conversion of D-ribose with various α-amino esters at 2.5 atm and 80 °C. The pyrrole-based platform chemicals 5 containing formyl and ester groups as linchpin units were further cyclized to form the piperazin-2-one scaffold as the second pharmacophore. Reductive amination of the formyl group with benzylamine, followed by intramolecular amination with the ester group provided pyrrolo-piperazinones 6 in reasonable yields. 1,2-Ethanediamine reacted with the formyl group of pyrralines 5 by double amination, and the resulting secondary amine underwent subsequent amination with the ester group to produce pyrrolo-piperazinones 7 with an imidazolidine ring as the third structural unit in high yields. Likewise, 2-amino alcohols derived from natural α-amino acids, alanine, valine, and phenylalanine, respectively reacted with the formyl group of pyrralines 5 to give intermediate oxazolidines, which underwent further cyclization with the ester group to produce triply fused heterocycles 8–10 of pyrrole, piperazin-2-one, and oxazolidine in acceptable yields. Pyrrolo-piperazinones 8h and 10g with an oxazolidine motif exhibited significant anti-inflammation activities with high cell viability. The practical synthetic method of platform chemicals 5 from sustainable biomass would open a paved road to the discovery of new therapeutic agents and value-added functional materials.Author contributions
S. Cho: synthesis (lead); L. Gu: synthesis (equal); I. J. In: synthesis (supporting); B. Wu: investigation (equal), synthesis (supporting); T. Lee: bioassays (lead); H. Kim: bioassay (equal), funding acquisition (equal); S. Koo: project administration (lead), funding acquisition (lead), investigation (lead), formal analysis (lead), data curation (lead), writing manuscript (lead).Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by basic science research program and international cooperation program through the National Research Foundation of Korea (NRF, 2020R1A6A1A03038817 and 2017K2A9A2A06016784) and by the Ministry of Science and ICT (2020R1A2C1010724), and partly by the GRRC Program of Gyeonggi province [GRRC-KyungHee2018(B01)], Republic of Korea. This paper is dedicated to my dear Professor Lanny S. Liebeskind.Notes and references
- V. Bhardwaj, D. Gumber, V. Abbot, S. Dhiman and P. Sharma, RSC Adv., 2015, 5, 15233–15266 RSC.
- A. Domagala, T. Jarosz and M. Lapkowski, Eur. J. Med. Chem., 2015, 100, 176–187 CrossRef CAS PubMed.
- D. O'Hagan, Nat. Prod. Rep., 2000, 17, 435–446 RSC.
- S. Thirumalairajan, B. M. Pearce and A. Thompson, Chem. Commun., 2010, 46, 1797–1812 RSC.
- H. Fan, J. Peng, M. T. Hamann and J.-F. Hu, Chem. Rev., 2008, 108, 264–287 CrossRef CAS PubMed.
- S. S. Gholap, Eur. J. Med. Chem., 2016, 110, 13–31 CrossRef CAS PubMed.
- H. Li, H. Guo, Z. Fang, T. M. Aida and R. L. Smith Jr, Green Chem., 2020, 22, 582–611 RSC.
- H. Hoffmann and T. Lindel, Synthesis, 2003, 1753–1783 CAS.
- R. Khajuria, S. Dhamb and K. K. Kapoor, RSC Adv., 2016, 6, 37039–37066 RSC.
- C. Paal, Ber. Dtsch. Chem. Ges., 1884, 17, 2756–2767 CrossRef.
- L. Knorr, Ber. Dtsch. Chem. Ges., 1884, 17, 2863–2870 CrossRef.
- C. Paal, Ber. Dtsch. Chem. Ges., 1885, 18, 367–371 CrossRef.
- A. Hantzsch, Ber. Dtsch. Chem. Ges., 1890, 23, 1474–1476 CrossRef.
- F. Feist, Ber. Dtsch. Chem. Ges., 1902, 35, 1537–1544 CrossRef CAS.
- N. Clauson-Kaas, F. Limborg and J. Fakstorp, Acta Chem. Scand., 1948, 2, 109–115 CrossRef CAS.
- A. Kornienko and J. J. La Clair, Nat. Prod. Rep., 2017, 34, 1051–1060 RSC.
- Y. Ju, D. Miao, R. Yu and S. Koo, Org. Biomol. Chem., 2015, 13, 2588–2599 RSC.
- X. Jiang, H. Jin, T. Wang, H. Yoo and S. Koo, Synthesis, 2019, 51, 3259–3268 CrossRef CAS.
- H. Jin, X. Jiang, H. Yoo, T. Wang, C. G. Sung, U. Choi, C.-R. Lee, H. Yu and S. Koo, ChemistrySelect, 2020, 5, 12421–12424 CrossRef CAS.
- R. U. Braun, K. Zeitler and T. J. J. Müller, Org. Lett., 2001, 3, 3297–3300 CrossRef CAS PubMed.
- G. Minetto, L. F. Raveglia and M. Taddei, Org. Lett., 2004, 6, 389–392 CrossRef CAS PubMed.
- B. Wang, Y. Gu, C. Luo, T. Yang, L. Yang and J. Suo, Tetrahedron Lett., 2004, 45, 3417–3419 CrossRef CAS.
- M. Leonardi, V. Estévez, M. Villacampa and J. C. Menéndez, Synthesis, 2018, 50, 816–828 Search PubMed.
- M. W. Roomi and S. F. MacDonald, Can. J. Chem., 1970, 48, 1689–1697 CrossRef CAS.
- V. Estévez, M. Villacampa and J. C. Menéndez, Chem. Soc. Rev., 2010, 39, 4402–4421 RSC.
- V. Estévez, M. Villacampa and J. C. Menéndez, Chem. Soc. Rev., 2014, 43, 4633–4657 RSC.
- Y. Fang, D. Leysen and H. C. J. Ottenheijm, Synth. Commun., 1995, 25, 1857–1861 CrossRef CAS.
- B. S. Gourlay, P. P. Molesworth, J. H. Ryan and J. A. Smith, Tetrahedron Lett., 2006, 47, 799–801 CrossRef CAS.
- B. Zuo, J. Chen, M. Liua, J. Ding, H. Wu and W. Su, J. Chem. Res., 2009, 14–16 CrossRef CAS.
- D. Bandyopadhyay, S. Mukherjee and B. K. Banik, Molecules, 2010, 15, 2520–2525 CrossRef CAS PubMed.
- G. Balme, Angew. Chem., Int. Ed., 2004, 43, 6238–6241 CrossRef CAS PubMed.
- S. Michlik and R. Kempe, Nat. Chem., 2013, 5, 140–144 CrossRef CAS PubMed.
- Y. Yamamoto, H. Hayashi, T. Saigoku and H. Nishiyama, J. Am. Chem. Soc., 2005, 127, 10804–10805 CrossRef CAS PubMed.
- A. A. Rosatella, S. P. Simeonov, R. F. M. Frade and C. A. M. Afonso, Green Chem., 2011, 13, 754–793 RSC.
- J. B. Binder and R. T. Raines, J. Am. Chem. Soc., 2009, 131, 1979–1985 CrossRef CAS PubMed.
- X. Tong, Y. Ma and Y. Li, Appl. Catal., A, 2010, 385, 1–13 CrossRef CAS.
- S. I. F. S. Martins, W. M. F. Jongen and M. A. J. S. von Boekel, Trends Food Sci. Technol., 2001, 11, 364–373 CrossRef.
- F. Hayase, R. H. Nagaraj, S. Miyata, F. G. Njoroge and V. M. Monnier, J. Biol. Chem., 1989, 263, 3758–3764 CrossRef.
- G. V. Mokrov, A. M. Likhosherstov, V. S. Troitskaya and T. A. Gudasheva, Russ. J. Org. Chem., 2009, 45, 1829–1833 CrossRef CAS.
- G. V. Mokrov, A. M. Likhosherstov, V. P. Lezina, T. A. Gudasheva, I. S. Bushmarinov and M. Yu. Antipin, Russ. Chem. Bull., 2010, 59, 1254–1266 CrossRef CAS.
- G. V. Mokrov, A. M. Likhosherstov, V. P. Lezina, T. A. Gudasheva, I. S. Bushmarinov and M. Yu. Antipin, Russ. Chem. Bull., 2011, 60, 1694–1702 CrossRef CAS.
- A. S. Demir, N. T. Subasia and E. Sahin, Tetrahedron: Asymmetry, 2006, 17, 2625–2631 CrossRef CAS.
- J. M. Wood, D. P. Fukert and M. A. Bimble, Nat. Prod. Rep., 2019, 36, 289–306 RSC.
- H. Kato and M. Fujimaki, Agric. Biol. Chem., 1970, 34, 1071–1077 CrossRef CAS.
- N. D. Adhikary, S. Kwon, W.-J. Chung and S. Koo, J. Org. Chem., 2015, 80, 7693–7701 CrossRef CAS PubMed.
- A. M. Valdivielso, P. Ventosa-Andrés, M. T. García-López, R. Herranz and M. Gutiérrez-Rodríguez, Eur. J. Org. Chem., 2013, 155–161 CrossRef CAS.
- C. De Risi, M. Pelà, G. P. Pollini, C. Trapella and V. Zanirato, Tetrahedron: Asymmetry, 2010, 21, 255–274 CrossRef CAS.
- H. Zhang, T. Cravillion, N.-K. Lim, Q. Tian, D. Beaudry, J. L. Defreese, A. Fettes, P. James, D. Linder and S. Malhotra, et. al., Org. Process Res. Dev., 2018, 22, 978–990 CrossRef CAS.
- C. Sandoval, N.-K. Lim and H. Zhang, Org. Lett., 2018, 20, 1252–1255 CrossRef CAS PubMed.
- T. Yamashita, E. Tsuru, E. Banjyo, M. Doe, K. Shibata, M. Yasuda and M. Gemba, Chem. Pharm. Bull., 1997, 45, 1940–1944 CrossRef CAS PubMed.
- J. Sanchez-Cespedes, C. L. Moyer, L. R. Whitby, D. L. Boger and G. R. Nemerow, Antiviral Res., 2014, 108, 65–73 CrossRef CAS PubMed.
- P. Z. Aviv, M. Shubely, Y. Moskovits, O. Viskind, A. Albeck, D. Vertommen, S. Ruthstein, M. Shokhen and A. Gruzman, ChemistrySelect, 2016, 1, 4658–4667 CrossRef.
- H. J. Kim, W. Y. Kwak, J. P. Min, J. Y. Lee, T. H. Yoon, H. D. Kim, C. Y. Shin, M. K. Kim, S. H. Choi and H. S. Kim, et al., Bioorg. Med. Chem. Lett., 2011, 21, 3809–3812 CrossRef CAS PubMed.
- Q. Liu, Q. Shi, D. Marcoux, D. G. Batt, L. Cornelius, L.-Y. Qin, Z. Ruan, J. Neels, M. Beaudoin-Bertrand and A. S. Srivastava, et al., J. Med. Chem., 2017, 60, 5193–5208 CrossRef CAS PubMed.
- W.-Q. Zuo, R. Hu, W.-L. Wang, Y.-X. Zhu, Y. Xu, L.-T. Yu, Z.-H. Liu and N.-Y. Wang, Bioorg. Chem., 2020, 105, 104344 CrossRef CAS PubMed.
- M. D'Ambrosio, A. Guerriero, C. Debitus, O. Ribes, J. Pusset, S. Leroy and F. Pietra, J. Chem. Soc., Chem. Commun., 1993, 1305–1306 RSC.
- T. W. Hong, D. R. Jímenez and T. F. Molinski, J. Nat. Prod., 1998, 61, 158–161 CrossRef CAS PubMed.
- F. Cafieri, E. Fattorusso, A. Mangoni and O. Taglialatela-Scafati, Tetrahedron Lett., 1995, 36, 7893–7896 CrossRef CAS.
- I. Mancini, G. Guella, P. Amade, C. Roussakis and F. Pietra, Tetrahedron Lett., 1997, 38, 6271–6274 CrossRef CAS.
- M. Tsuda, T. Yasuda, E. Fukushi, J. Kawabata, M. Sekiguchi, J. Fromont and J. Kobayashi, Org. Lett., 2006, 8, 4235–4238 CrossRef CAS PubMed.
- T. Henle and A. Bachmann, Z. Lebensm.-Unters. Forsch., 1996, 202, 72–74 CrossRef CAS PubMed.
- T. Niwa, J. Chromatogr. B: Biomed. Sci. Appl., 1999, 731, 23–36 CrossRef CAS.
- T. Mosmann, J. Immunol. Methods, 1983, 65, 55–63 CrossRef CAS PubMed.
- M. J. Moorcroft, J. Davis and R. G. Compton, Talanta, 2001, 54, 785–803 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Cell viability assays, nitric oxide concentration (Griess assay), picture demonstration for the pressure reaction, and 1H/13C NMR spectra for compounds 5, 6, 7, 8, 9, and 10. See DOI: 10.1039/d1ra06110k |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2021 |