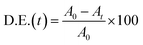DOI:
10.1039/D1RA06101A
(Paper)
RSC Adv., 2021,
11, 40100-40111
Magnetic Lu2Cu2O5-based ceramic nanostructured materials fabricated by a simple and green approach for an effective photocatalytic degradation of organic contamination
Received
12th August 2021
, Accepted 2nd December 2021
First published on 16th December 2021
Abstract
Designing and fabricating an efficient photocatalytic compound with an appropriate band gap to eliminate toxic contaminants is necessary to remediate the environment. This article presents the development of a new type of nanostructure, Lu2Cu2O5–Lu2O3 nanocomposites to photo-catalytically degrade different kinds of toxic pollutants under sunlight. The oxide nanocomposites were fabricated via a quick and eco-friendly approach. In order to fabricate oxide nanostructures with appropriate features in terms of morphology and particle size, the effects of the kind of green reactant and its quantity were examined. Amylum was an appropriate and green reactant for the efficient synthesis of Lu2Cu2O5–Lu2O3 nanobundles with the most organized morphology. The features of Lu2Cu2O5-based nanostructures were carefully investigated utilizing multiple characterization methods. Then, the catalytic role of the fabricated nanobundles was evaluated for the removal of various kinds of toxic contaminants. The effects of the quantity of photocatalytic nanostructure, the concentration of the contaminant compound, and the type of light source in the catalytic degradation process were screened. The findings of this research demonstrated that utilizing 0.05 g of Lu2Cu2O5–Lu2O3 nanobundles, 98.5% of the contaminant with a concentration of 10 ppm can be degraded in 2 h under ultraviolet light irradiation. The experimental results also certified that, during the photocatalytic pathway, superoxide radicals play a meaningful role in the elimination of toxic pollutants. To our knowledge, this is the first report of the fabrication of Lu2Cu2O5–Lu2O3 nanocomposite through a facile and eco-friendly approach and its photocatalytic efficiency.
1. Introduction
Wastewater effluents have been reported to originate from domestic use as well as from various industries and are often comprised of significant quantities of chemical and toxic compounds, including dyes, which can trigger serious environmental problems.1–3 The presence of organic and toxic contaminants in the environment can be a great danger to both human life and wildlife.4–6 Various approaches have been utilized to remediate toxic contaminants.7–9 In the process of photocatalysis, a completely environmentally friendly method utilizing free sunlight in the presence of photocatalytic compounds, poisonous contaminants can be degraded or oxidized.10–12 Features such as non-toxicity, superior efficiency, cost-effectiveness, and the creation of safe secondary compounds have made photocatalysis an appealing method for many scientists for the remediation of environmental contaminants.13–15 So far, a wide variety of photocatalysts have been utilized to remove these toxic residues.16–18 However, there is still the challenge of designing and fabricating a photocatalytic compound with an appropriate band gap to eliminate a wide range of toxic contaminants. Thus, there is great interest in producing an efficient photocatalytic compound that can remove a broad spectrum of toxic pollutants to remediate the environment. The photocatalysis technique has undergone ultra-rapid development during the past several decades, exhibiting unique merits including stable redox ability, excellent renewability, and a green and sustainable nature, and has been widely applied in many fields such as photo-synthesis, pollutant treatment, and other chemical or energy-related processes.19–21 Recently, photocatalysis has attracted much interest for using solar energy to convert chemical energy, such as in H2 production,22 degradation of various kinds of pollution,23 H2O2 generation, and CO2 reduction.
Recently, the use of green chemistry-based approaches to prepare a variety of nanoscale oxide compounds has become an important research topic.24–26 This environmentally friendly technology utilizes non-hazardous reactants that do not lead to the problem of environmental contamination. The qualities of energy-saving, reproducibility and simplicity are also prominent features of this efficient technology.24
Binary lanthanide cuprates (Ln2Cu2O5) are known for their distinctive features that make them viable for a variety of uses, such as superconductors, magnetocaloric compounds, and sensors.27–29 Ln2Cu2O5 has been reported to crystallize as an orthorhombic phase with space group Pna2.30–32 The reported fabrication approaches of binary lanthanide cuprates have been limited to co-precipitation and solid-state reactions.29 The use of these approaches cannot lead to the fabrication of oxide compounds with control of their particle morphology and dimensions. Thus, it is indispensable to design easy, reproducible, energy-saving, and fast approaches for the production of Ln2Cu2O5 with controlled features. To our knowledge, no research work has reported on the use of a simple, quick, and environmentally friendly approach to fabricate Lu2Cu2O5-based nanostructured materials by employing green reactants. Here, employing soluble carbohydrates as green fuel, we report the fabrication, through a quick and eco-friendly approach, of a new type of nanostructure, Lu2Cu2O5–Lu2O3 nanocomposites, and their use for the photocatalytic elimination of toxic contaminants in water.
2. Experimental
2.1. Materials
Analytical reagent grade fructose, lutetium(III) nitrate, amylum, copper nitrate, and maltobiose were purchased from Merck. The reagents were employed directly without any purification. The Lu2Cu2O5–Lu2O3 nanocomposite was investigated utilizing a variety of techniques.
2.2. Physical instrument
The morphologies of the samples were obtained using a TESCAN BRNO-Mira3 LMU field emission scanning electron microscope. The samples were covered by a thin layer of gold (Au) before taking images to achieve better contrast and bypass charge accretion. An X-ray diffractogram (Philips) with X'Pert Pro sieved by copper Ka radiation (λ = 15.4 nm) was used to record the XRD patterns. X'Pert HighScore Plus software was applied to distinguish the compounds. The FT-IR spectra of the samples were obtained on a Shimadzu Varian 4300 spectrometer in the range of 400–4000 cm−1 in KBr pellets. Energy-dispersive spectroscopy (EDS) with a 20 kV exciting charge was utilized for the elemental analysis of the products. The UV-visible spectrum was registered utilizing a JASCO UV-Vis scanning spectroscope (V-670). The GC-2550TG (Teif Gostar Faraz Company, Iran) was used for each alchemical agent. The surface areas (BET) were characterized via nitrogen adsorption at −196 °C employing automated gas adsorption analysis equipment (Tristar 3000, Micromeritics). Transmission electron microscopy (TEM) was executed on a JEM-2010 UHR JEOL transmission electron microscope (UHR-TEM) for the first batch, a FEI Tecnai G2 F20 for the second batch, and a Thermo Fischer Talos F200X for the last batch of pictures.
2.3. Synthesis of Lu2Cu2O5–Lu2O3 nanocomposite
An eco-friendly approach was utilized to fabricate Lu2Cu2O5–Lu2O3 nanocomposites. First, an aqueous solution containing 5 mmol of carbohydrate (amylum) was slowly added to a solution containing 1 mmol of lutetium(III) nitrate as well as 2 mmol of copper nitrate and the mixture was stirred for half an hour at about 50 °C. By heating the mixture to a temperature above the evaporation temperature, a puffy precipitate was created. The nanocomposite sample was prepared by calcination of the precipitate at 900 °C for 4 hours (Scheme 1). The effects of the type of green reactant and its quantity on the features of the oxide nanocomposites in terms of morphology and particle size were examined (Table 1).
 |
| | Scheme 1 Schematic diagram illustrating the production of Lu2Cu2O5–Lu2O3 nanobundles and their photocatalytic degradation performance. | |
Table 1 The production conditions for all samples
2.4. Photocatalysis experiments
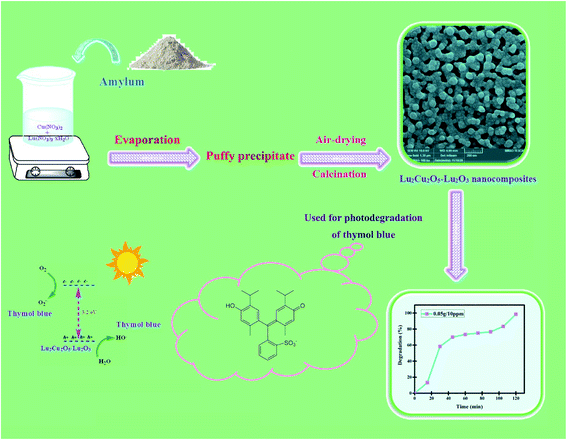
The catalytic role of the Lu2Cu2O5–Lu2O3 nanobundles was evaluated in the removal of various kinds of toxic contaminants. In each test, 0.04 g of the nanoscale photocatalyst was mixed with a solution containing 0.001 g of toxic contaminant and then stirred in the dark for half an hour to achieve equilibrium between adsorption and desorption. After that, the light source (400 W mercury lamp) was turned on.18 The degradation efficiency of the toxic contaminant was estimated by employing the following equation12,18| |
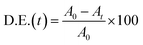 | (1) |
where A0 and At denote the absorption of the contaminant solution before and after irradiation. The effectiveness of the quantity of nanocatalyst, the concentration of the contaminant compound and the type of light source in the catalytic degradation process were carefully examined (Table 2).
Table 2 Photocatalytic degradation conditions of toxic pollutant by Lu2Cu2O5–Lu2O3 nanocomposites
| Type of pollutant |
Quantity of nanocatalyst (g) |
Contaminant concentration (ppm) |
Scavenger |
Removal (%) |
| Rhodamine B |
0.03 |
10 |
— |
25.9 |
| Eriochrome Black T |
0.03 |
10 |
— |
32.0 |
| Thymol blue |
0.03 |
10 |
— |
70.0 |
| Acid yellow |
0.03 |
10 |
— |
25.0 |
| Methyl orange |
0.03 |
10 |
— |
8.7 |
| Acid red |
0.03 |
10 |
— |
50.0 |
| Thymol blue |
0.03 |
15 |
— |
96.6 |
| Thymol blue |
0.03 |
20 |
— |
75.0 |
| Thymol blue |
0.05 |
10 |
— |
98.5 |
| Thymol blue |
0.05 |
15 |
— |
21.4 |
| Thymol blue |
0.05 |
20 |
— |
33.3 |
| Thymol blue |
0.07 |
10 |
— |
70.4 |
| Thymol blue |
0.07 |
15 |
— |
96.6 |
| Thymol blue |
0.07 |
20 |
— |
50.0 |
| Thymol blue |
0.05 |
10 |
Benzoquinone |
25.2 |
| Thymol blue |
0.05 |
10 |
Benzoic acid |
80.0 |
| Thymol blue |
0.05 |
10 |
EDTA |
75.7 |
2.5. Scavenging investigation
Scavenging experiments were undertaken separately to detect the reactive radical species that are mainly responsible for decomposing the thymol blue. One mmol of each scavenger was employed. Benzoquinone, EDTA, and benzoic acid were employed to remove O2−˙, h+, and OH˙, respectively.12
3. Results and discussion
3.1. Microstructure and morphology
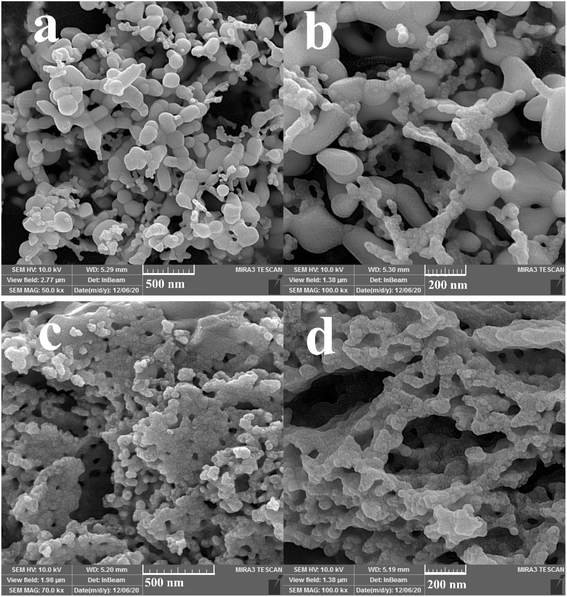
Three different types of green reagents, namely fructose, maltobiose and amylum, were employed to prepare nanocomposite samples 1, 2, and 3, respectively, to select the option which best leads to the fabrication of an oxide nanostructure with desirable features in terms of morphology and particle size (Fig. 1 and 2). As can be seen, the use of the monosaccharide carbohydrate fructose resulted in the production of irregular micro/nanostructures (Fig. 1a and b). Bundle-like nanostructures with low uniformity and high aggregation were prepared in the presence of the disaccharide carbohydrate maltobiose (Fig. 1c and d), while the use of the polysaccharide carbohydrate amylum resulted in nanobundles with the most organized morphology (Fig. 1e and f). In the process of preparing the oxide samples, all three kinds of carbohydrates played simultaneous roles as reductant and fuel and the available nitrate played the role of oxidant. All three green reactants have hydroxyl groups that can form a chelating complex with the metal ions in the reaction mixture, affecting the crystalline facets of the oxide sample and resulting in the creation of different oxide structures.33 It seems that amylum, which has more hydroxyl groups, is more effective in fabricating an oxide nanostructure with a more uniform morphology.33
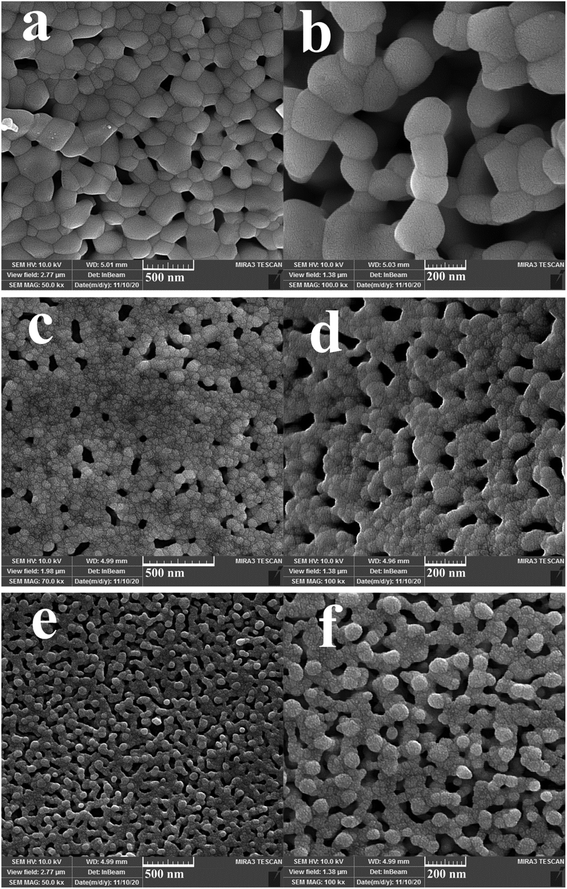 |
| | Fig. 1 FESEM images of samples 1, 2 and 3 produced with fructose (a and b), maltobiose (c and d) and amylum (e and f). | |
 |
| | Fig. 2 FESEM images of samples 4 and 5 produced with 7 (a and b) and 3 (c and d) mmol of amylum. | |
In addition, other doses of amylum, including 7 and 3 mmol, were employed to fabricate nanocomposite samples 4 and 5 to select the most appropriate dose that could lead to a more homogeneous nanostructure. It is observed that when increasing the amylum dose to 7 mmol (Fig. 2a and b), irregular oxide microstructures are formed. Changing the amylum dose to 3 mmol (Fig. 2c and d) results in particle-like structures with high aggregation. Based on the above results, it can be concluded that the use of 5 mmol of amylum is more suitable for the creation of a uniform oxide nanocomposite in terms of morphology (Fig. 1e and f). The presence of all expected elements in the EDS study of samples prepared in this research verifies the production of oxide nanocomposites. It can be seen in Fig. 3 that elements including lutetium, oxygen, and copper are present in all prepared samples.
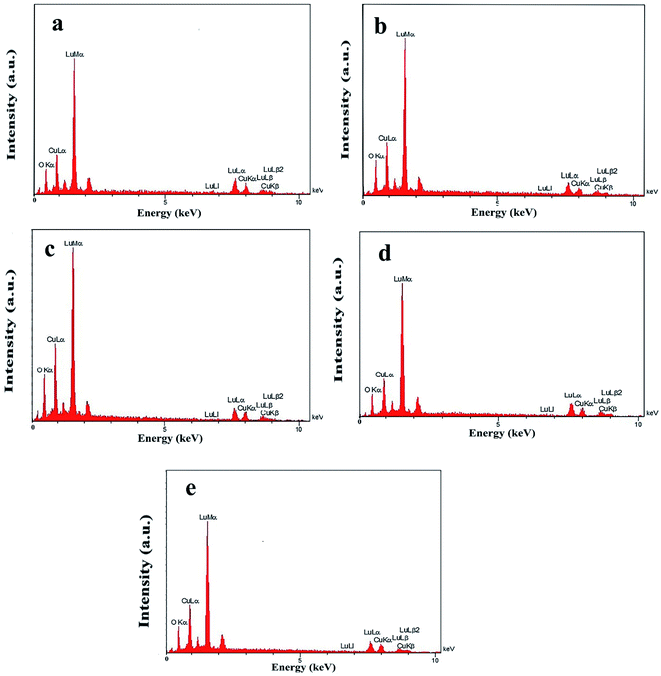 |
| | Fig. 3 EDS patterns of samples 1 (a), 2 (b), 3 (c), 4 (d), and 5 (e). | |
The oxide sample fabricated with 5 mmol of amylum was selected for further studies. TEM images of sample 3 display its microstructure in more detail (Fig. 4). It is observed that the oxide sample is comprised of sphere-shaped particles with a size of about 20 to 40 nanometers.
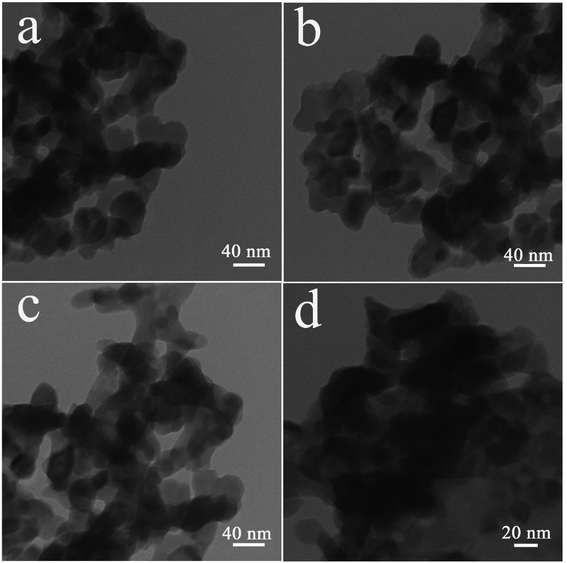 |
| | Fig. 4 TEM images of Lu2Cu2O5–Lu2O3 nanobundles fabricated with 5 mmol of amylum. | |
3.2. XRD and FTIR studies
The crystal structures of samples 1 and 3 fabricated utilizing environmentally friendly reactants, including fructose and amylum, were identified by their corresponding XRD patterns (Fig. 5). In the diffractograms of both oxide samples, the characteristic diffraction lines of Lu2O3 (JCPDS no. 01-086-2475) and Lu2Cu2O5 (JCPDS no. 01-083-1255) can be found, which clearly verifies the fabrication of a Lu2Cu2O5–Lu2O3 binary composite. No diffraction lines indicating impurities were observed. The mean crystalline sizes of both oxide samples were calculated employing the well-known Scherrer formula.26 It was observed that the mean crystalline sizes for the fabricated oxide samples were about 36 nm (sample 1) and 34 nm (sample 3). Thus, when the green reactant is changed from fructose to amylum, the mean size of the crystals diminishes.
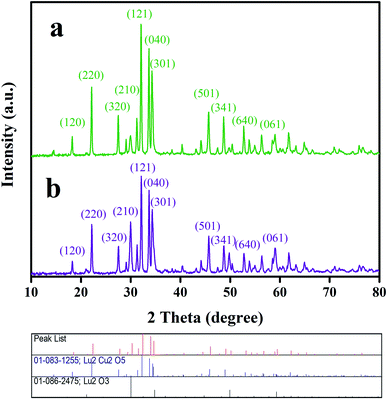 |
| | Fig. 5 XRD patterns of samples 1 and 3 produced with fructose (a) and amylum (b), respectively. | |
Fig. 6a exhibits the FTIR spectrum of the Lu2Cu2O5–Lu2O3 nanobundles. The two peaks located around 576 and 451 cm−1 are related to the metal–oxygen bonds.34,35 There are two peaks near 3436 and 1631 cm−1 which demonstrate the presence of surface adsorbed water molecules.18 These results verified the successful fabrication of Lu2Cu2O5–Lu2O3 nanocomposites utilizing an eco-friendly approach.
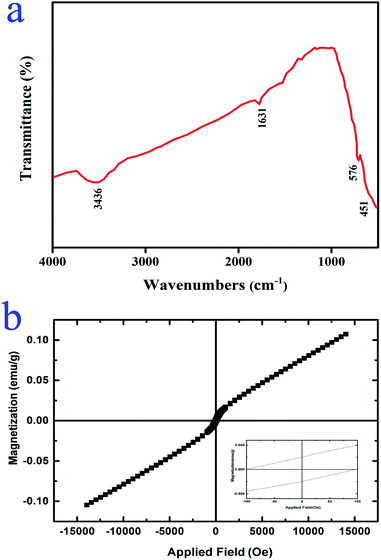 |
| | Fig. 6 (a) FT-IR spectrum and (b) VSM results of Lu2Cu2O5–Lu2O3 nanobundles fabricated with 5 mmol of amylum. | |
3.3. VSM and BET studies
The magnetic features of Lu2Cu2O5–Lu2O3 nanobundles were examined at 300 K. It is observed that the nanocomposite sample displays dual behavior. In low fields, it has weak ferromagnetic behavior, while in high fields, it exhibits paramagnetic behavior (Fig. 6b).36 Having magnetic properties is an advantage for nanoscale photocatalytic compounds for environmental usage because it allows for easy recycling.37,38
The specific surface area and porosity of the photocatalytic compounds are also significant features because they can greatly affect performance.39 The BET–BJH profiles of the Lu2Cu2O5–Lu2O3 nanobundles are exhibited in Fig. 7. It is clear that the nanocomposite sample has a structure containing both meso- and macropores. Lu2Cu2O5–Lu2O3 nanobundles exhibit a surface area of ∼1.76 m2 g−1, with a mean pore size of ∼40 nm and a total pore volume of 0.0176 cm3 g−1. Thus, the nanocomposite sample has different pores that can be active areas for the adsorption–desorption of contaminant molecules.
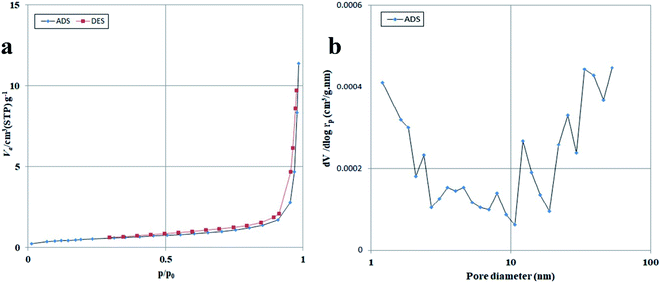 |
| | Fig. 7 N2 adsorption/desorption isotherm (a) and pore size distribution curve (b) of Lu2Cu2O5–Lu2O3 nanobundles fabricated with 5 mmol of amylum. | |
3.4. Optical properties
In photocatalytic reactions, the optical band gap of the oxide compound can play a meaningful role in the ability to harvest light. In the DRS spectral plot, Lu2Cu2O5–Lu2O3 nanobundles display a peak of about 276 nm (Fig. 8). The band gap can be found from the Tauc plot drawn from DRS absorbance results.12 The Tauc plot of Lu2Cu2O5–Lu2O3 nanobundles exhibits a direct band gap near 3.2 eV (Fig. 8). Due to this feature, the nanocomposite sample can be employed as a photocatalytic compound for environmental uses because it can be easily activated by light.
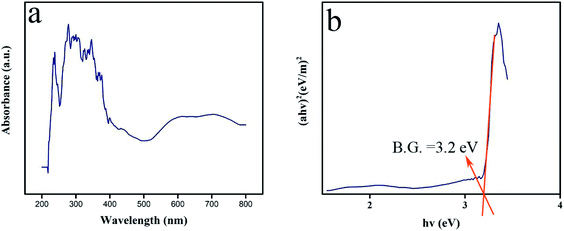 |
| | Fig. 8 DRS spectrum (a) and plot to determine the band gap (b) of Lu2Cu2O5–Lu2O3 nanobundles fabricated with 5 mmol of amylum. | |
3.5. Photocatalytic degradation evaluation
A nanocomposite sample prepared by an environmentally friendly approach (sample 3) was employed as a nanophotocatalyst to evaluate its ability to degrade toxic contaminants. The presence of organic and toxic contaminants such as dyes in the environment can be a great danger to human life and wildlife. Purifying water of these toxic contaminants is a great challenge. Hence, we selected a variety of organic dyes (including rhodamine B, Eriochrome Black T, thymol blue, acid yellow, methyl orange, and acid red) and evaluated the photocatalytic performance of Lu2Cu2O5–Lu2O3 nanobundles to purify water of these contaminants under ultraviolet light (Fig. 9 and Table 2). In each test, 0.03 g of the nanocomposite sample was employed for the purification of a solution with a concentration of 10 ppm of each contaminant. It is interesting to note that without light irradiation or without the presence of Lu2Cu2O5–Lu2O3 nanobundles, almost no dye contaminants were removed after 120 minutes, which confirms an insignificant self-destructive contribution.18 It was observed that the nanocomposite sample works best for the elimination of thymol blue and can remove 70% of it in 120 minutes.
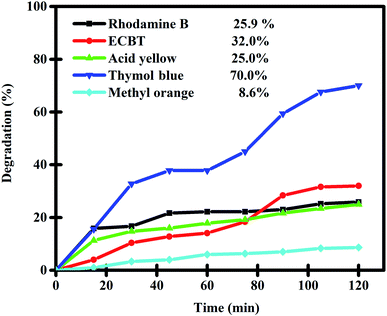 |
| | Fig. 9 Photocatalytic degradation of various pollutants in the presence of Lu2Cu2O5–Lu2O3 nanobundles under UV light irradiation. | |
To discover the most appropriate conditions for the highest degradation efficiency of thymol blue contaminant by the nanocomposite sample, we evaluated the effects of various factors, such as the quantity of photocatalytic nanostructure, the concentration of the contaminant compound and the kind of light source (Fig. 10 and 11 and Table 2). Fig. 10a–c reveals the effects of the Lu2Cu2O5–Lu2O3 catalyst dosage (0.03, 0.05, and 0.07 g) and the concentration of the contaminant compound in the photocatalytic degradation of thymol blue under UV light irradiation. It can be seen that, to purify a 10 ppm thymol blue contaminant solution, the use of 0.05 g of Lu2Cu2O5–Lu2O3 nanobundles leads to the highest degradation rate of 98.5% (Fig. 10b). To purify thymol blue solution with a concentration higher than 10 ppm (15 ppm), the use of 0.03 g and 0.07 g of the nanocomposite sample brings the same photocatalytic efficiency (96.6%). When increasing the concentration of thymol blue solution to 20 ppm, the use of 0.03 g of nanocomposite sample leads to the highest percentage of degradation (75%). Thus, the above outcomes indicate that the use of 0.05 g of Lu2Cu2O5–Lu2O3 nanobundles is the best option to purify thymol blue solution with a concentration of 10 ppm and achieve the highest degradation efficiency (Fig. 10) because, in these conditions, the adsorption of contaminant molecules on the surface of Lu2Cu2O5–Lu2O3 nanobundles is better and the penetration of light into the contaminant solution is more appropriate.12,40
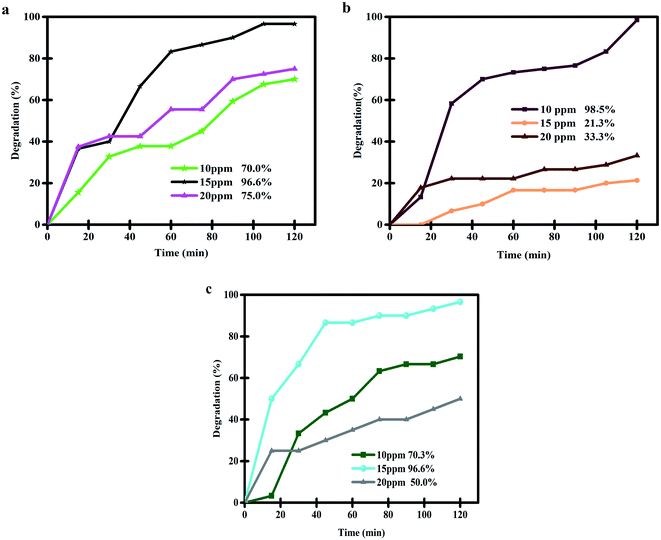 |
| | Fig. 10 Effects of the quantity of photocatalytic nanostructure (0.03, 0.05, and 0.07 g) and the concentration of contaminant compound in the photocatalytic degradation of thymol blue under UV light irradiation. | |
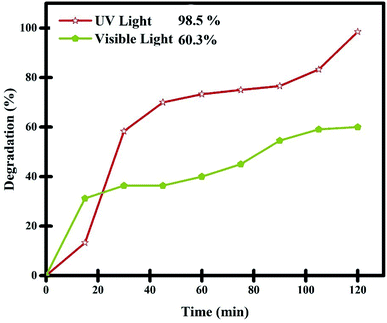 |
| | Fig. 11 Comparison of the photocatalytic performance of Lu2Cu2O5–Lu2O3 nanobundles under UV and Vis irradiation for the degradation of thymol blue. | |
The performance of the nanocomposite sample in the purification of aqueous thymol blue contaminant under visible light was also evaluated (Fig. 11). In this experiment, 0.05 g of Lu2Cu2O5–Lu2O3 nanobundles was employed to purify a solution with a concentration of 10 ppm thymol blue. It was observed that about 59.1% of thymol blue contaminant can be removed by the nanocomposite sample within 120 minutes of visible light irradiation. The performance of the Lu2Cu2O5–Lu2O3 nanobundles in the removal of thymol blue contaminant under visible light is lower than that under ultraviolet light, which confirms the DRS results.
3.6. Photocatalytic mechanism for the removal of thymol blue
Scavenging experiments were undertaken separately to detect the reactive radical species that are significantly responsible for decomposing the thymol blue contaminant (Fig. 12a). It was observed that after the addition of benzoic acid and EDTA, the photocatalytic performance of the nanocomposite sample does not alter significantly compared to the scavenger-free system. This signifies that the hole and the hydroxyl radicals cannot play a meaningful role in the photocatalytic degradation of thymol blue contaminant in the presence of the nanocomposite sample. However, the introduction of benzoquinone can dramatically suppress the photocatalytic efficiency of Lu2Cu2O5–Lu2O3 nanobundles, indicating that O2−˙ is the leading active species for thymol blue removal in the presence of the nanocomposite sample. The catalytic reactions for the removal of thymol blue contaminant by Lu2Cu2O5–Lu2O3 nanobundles through hydroxyl radicals, which can be generated by the leading active species, O2− radicals, can be summarized as follows.41–44
| Lu2Cu2O5–Lu2O3 nanobundles + hν → Lu2Cu2O5–Lu2O3 nanobundles* (eCB− + hVB+) |
| OH˙ + thymol blue → degradation products (e.g., H2O, CO2) |
| eCB− + thymol blue → reduction products |
| hVB+ + thymol blue → oxidation products |
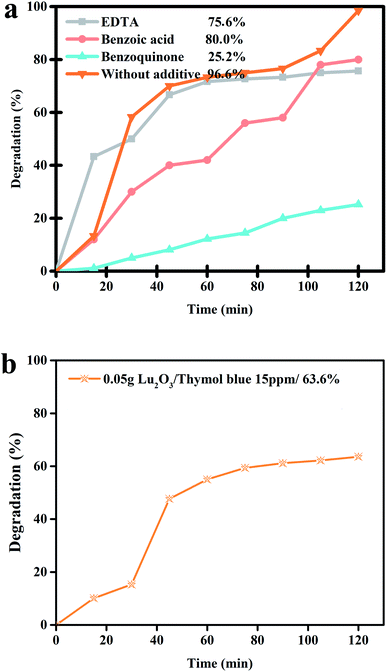 |
| | Fig. 12 (a) Radical trapping experiment of active species in the photocatalytic degradation of thymol blue over Lu2Cu2O5–Lu2O3 nanobundles and (b) photocatalytic activity of Lu2O3 over thymol blue. | |
Fig. 12b reveals the photocatalytic activity of Lu2O3 over thymol blue, indicating it degraded this colorant about 63.0%, which is much less than the degradation by Lu2Cu2O5–Lu2O3 nanocomposites. This result showed that Lu2Cu2O5–Lu2O3 nanocomposite has better activity in the photodegradation of organic dyes.
3.7. Stability and recyclability of Lu2Cu2O5–Lu2O3
The stability of Lu2Cu2O5–Lu2O3 was studied by XRD and SEM analysis after photodegradation. Fig. 13a shows the XRD pattern of the as-prepared catalyst after 5 runs. This pattern shows that Lu2Cu2O5 (reference code 83-1255) and Lu2O3 (reference code 086-2475) were present, indicating the high stability of this composite. On the other hand, the FRSEM image shows that the particles are attached to each other and agglomeration has occurred (Fig. 13b). To check the recyclability of Lu2Cu2O5–Lu2O3, the catalyst was centrifuged, washed with ethanol and water, dried at 65 °C for 24 h, and reused five times under the same conditions. As shown in Fig. 13c, Lu2Cu2O5–Lu2O3 is very stable and maintains its high photocatalytic performance across five reaction cycles. Indeed, during the fifth cycle, the reduction in photocatalytic activity is only 12.1%.
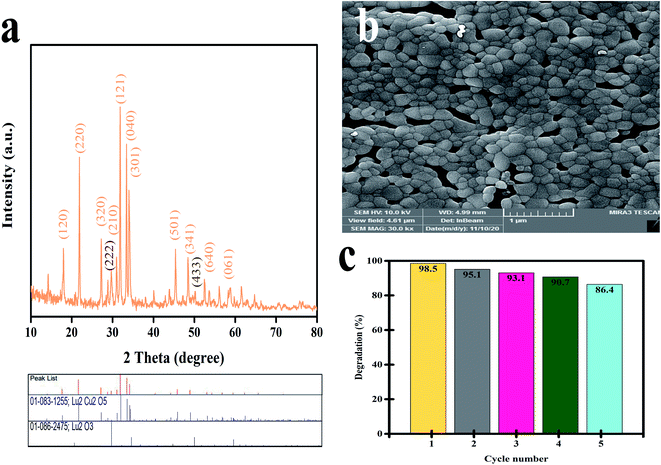 |
| | Fig. 13 (a) XRD pattern and (b) FESEM image of Lu2Cu2O5–Lu2O3 after photocatalytic reaction after 5 runs, and (c) cycling runs in the photocatalytic degradation of 10 ppm thymol blue under visible irradiation. | |
4. Conclusions
In this work, a new type of nanostructure, Lu2Cu2O5–Lu2O3 nanocomposite, was successfully designed and fabricated through a quick and eco-friendly approach. Morphological studies demonstrated that the use of 5 mmol of amylum, an appropriate green reactant, was the best option for the efficient synthesis of Lu2Cu2O5–Lu2O3 nanobundles with the most organized morphology. The features of Lu2Cu2O5-based nanostructures were carefully investigated utilizing multiple characterization methods. With an energy gap of 3.2 eV, the catalytic role of the fabricated nanobundles was evaluated for the removal of various kinds of toxic contaminants. The influences of the quantity of photocatalytic nanostructure, the concentration of the contaminant compound and the type of light source in the catalytic degradation process were evaluated. The highest photocatalytic performance was observed for 0.05 g of Lu2Cu2O5–Lu2O3 nanobundles, with an efficiency of 98.5% in 2 hours for degradation of the contaminant thymol blue at a concentration of 10 ppm under ultraviolet light irradiation. Experimental results demonstrated that superoxide radicals were largely responsible for the elimination of the toxic pollutant through the photocatalytic pathway. To our knowledge, this is the first investigation of the fabrication of Lu2Cu2O5–Lu2O3 nanocomposite through a facile and eco-friendly auto-combustion approach and of its photocatalytic efficiency. Exploitation of environmentally friendly and available reactants as well as the implementation of an efficacious fabrication method are the highlights of this research and make Lu2Cu2O5–Lu2O3 nanocomposite a superior candidate for environmental remediation.
Conflicts of interest
The authors declare that there are no conflicts of interest regarding the publication of this manuscript.
Acknowledgements
Authors are grateful to University of Bonab, the council of Iran National Science Foundation (97017837) and University of Kashan for supporting this work by Grant No. (159271/MT1).
References
- N. H. Mohamad Idris, J. Rajakumar, K. Y. Cheong, B. J. Kennedy, T. Ohno, A. Yamakata and H. L. Lee, ACS Omega, 2021, 6, 14493–14503 CrossRef CAS PubMed.
- C.-C. Wang, J.-R. Li, X.-L. Lv, Y.-Q. Zhang and G. Guo, Energy Environ. Sci., 2014, 7, 2831–2867 RSC.
- F. Guo, M. Li, H. Ren, X. Huang, W. Hou, C. Wang, W. Shi and C. Lu, Appl. Surf. Sci., 2019, 491, 88–94 CrossRef CAS.
- M. Jothibas, C. Manoharan, S. Johnson Jeyakumar, P. Praveen, I. Kartharinal Punithavathy and J. Prince Richard, Solar Energy, 2018, 159, 434–443 CrossRef CAS.
- W.-h. Zhao, Z.-q. Wei, X.-j. Wu, X.-d. Zhang, L. Zhang and X. Wang, Trans. Nonferrous Met. Soc. China, 2019, 29, 157–164 CrossRef CAS.
- M. Avais and S. Chattopadhyay, ACS Appl. Polym. Mater., 2021, 3, 789–800 CrossRef CAS.
- R. Sun, J. Wang, T. Yang, R. He, K. Xue, L. Wang, X. Yu, J. Wang, T. Yang and W. Wang, New J. Chem., 2019, 43, 13764–13774 RSC.
- R. Salazar, E. Brillas and I. Sirés, Appl. Catal., B, 2012, 115–116, 107–116 CrossRef CAS.
- M. Salavati-Niasari, N. Mir and F. Davar, Synthesis and characterization of NiO nanoclusters via thermal decomposition, Polyhedron, 2009, 28(6), 1111–1114 CrossRef CAS.
- E. Vasilaki, D. Vernardou, G. Kenanakis, M. Vamvakaki and N. Katsarakis, Appl. Phys. A: Mater. Sci. Process., 2017, 123, 231 CrossRef.
- M. Pirhashemi and A. Habibi-Yangjeh, J. Colloid Interface Sci., 2017, 491, 216–229 CrossRef CAS PubMed.
- M. Salavati-Niasari, F. Davar and M. R. Loghman-Estarki, Long chain polymer assisted synthesis of flower-like cadmium sulfide nanorods via hydrothermal process, J. Alloys Compd., 2009, 481(1–2), 776–780 CrossRef CAS.
- Y. Gao and T. Wang, J. Mol. Struct., 2021, 1224, 129049 CrossRef CAS.
- W. Li, Y. Zuo, L. Jiang, D. Yao, Z. Chen, G. He and H. Chen, Mater. Chem. Phys., 2020, 256, 123650 CrossRef CAS.
- M. J. Sampaio, M. J. Lima, D. L. Baptista, A. M. T. Silva, C. G. Silva and J. L. Faria, Chem. Eng. J., 2017, 318, 95–102 CrossRef CAS.
- J. Guo, C.-H. Shen, J. Sun, X.-J. Xu, X.-Y. Li, Z.-H. Fei, Z.-T. Liu and X.-J. Wen, Sep. Purif. Technol., 2021, 259, 118109 CrossRef CAS.
- M. A. Mahadadalkar, S. W. Gosavi and B. B. Kale, J. Mater. Chem. A, 2018, 6, 16064–16073 RSC.
- M. Salavati-Niasari, Nanoscale microreactor-encapsulation 14-membered nickel (II) hexamethyl tetraaza: synthesis, characterization and catalytic activity, J. Mol. Catal. A: Chem., 2005, 229(1–2), 159–164 CrossRef CAS.
- L. Liang, L. Shi, F. Wang, H. Wang, P. Yan, Y. Cong, L. Yao, Z. Yang and W. Qi, Appl. Catal., A, 2020, 599, 117618 CrossRef CAS.
- L. Liang, L. Shi, F. Wang, H. Wang and W. Qi, Sustainable Energy Fuels, 2020, 4, 5179–5187 RSC.
- K. Fang, L. Shi, L. Cui, C. Shi and W. Si, CrystEngComm, 2021, 23, 4475–4485 RSC.
- L. Liang, L. Shi, F. Wang, L. Yao, Y. Zhang and W. Qi, Int. J. Hydrogen Energy, 2019, 44, 16315–16326 CrossRef CAS.
- K. Fang, L. Shi, L. Yao and L. Cui, Mater. Res. Bull., 2020, 129, 110888 CrossRef CAS.
- A. Zaleska-Medynska, M. Marchelek, M. Diak and E. Grabowska, Adv. Colloid Interface Sci., 2016, 229, 80–107 CrossRef CAS PubMed.
- P. Naresh Kumar Reddy, D. P. M. D. Shaik, V. Ganesh, D. Nagamalleswari, K. Thyagarajan and P. Vishnu Prasanth, Appl. Surf. Sci., 2021, 561, 150092 CrossRef CAS.
- M. Ghanbari and M. Salavati-Niasari, Tl4CdI6 Nanostructures: Facile Sonochemical Synthesis and Photocatalytic Activity for Removal of Organic Dyes, Inorg. Chem., 2018, 57(18), 11443–11455 CrossRef CAS PubMed.
- S. Zhang and P. Cheng, CrystEngComm, 2015, 17, 4250–4271 RSC.
- V. M. Denisov, L. T. Denisova, L. G. Chumilina and S. D. Kirik, Phys. Solid State, 2013, 55, 2023–2026 CrossRef CAS.
- R. Guo, J. You, F. Han, C. Li, G. Zheng, W. Xiao and X. Liu, Appl. Surf. Sci., 2017, 396, 1076–1084 CrossRef CAS.
- J. M. S. Skakle and A. R. West, J. Mater. Chem., 1995, 5, 765–768 RSC.
- L. Li, J. Wang, K. Su, D. Huo and Y. Qi, J. Alloys Compd., 2016, 658, 500–504 CrossRef CAS.
- L. T. Denisova, V. M. Denisov, L. G. Chumilina, S. D. Kirik and S. A. Istomin, Phys. Solid State, 2013, 55, 2613–2615 CrossRef CAS.
- S. Zinatloo-Ajabshir, M. S. Morassaei and M. Salavati-Niasari, Eco-friendly synthesis of Nd2Sn2O7-based nanostructure materials using grape juice as green fuel as photocatalyst for the degradation of erythrosine, Composites, Part B, 2019, 167, 643–653 CrossRef CAS.
- M. Sukumar, L. John Kennedy, J. Judith Vijaya, B. Al-Najar and M. Bououdina, J. Magn. Magn. Mater., 2018, 465, 48–57 CrossRef CAS.
- M. Sukumar, L. J. Kennedy, J. J. Vijaya, B. Al-Najar and M. Bououdina, Mater. Sci. Semicond. Process., 2019, 100, 225–235 CrossRef CAS.
- F. Ansari, A. Sobhani and M. Salavati-Niasari, Green synthesis of magnetic chitosan nanocomposites by a new sol–gel auto-combustion method, J. Magn. Magn. Mater., 2016, 410, 27–33 CrossRef CAS.
- S. Huang, Y. Xu, M. Xie, H. Xu, M. He, J. Xia, L. Huang and H. Li, Colloids Surf., A, 2015, 478, 71–80 CrossRef CAS.
- F. Miao, Q. Wang, L.-C. Zhang and B. Shen, Appl. Surf. Sci., 2021, 540, 148401 CrossRef CAS.
- S. Ahmadian-Fard-Fini, D. Ghanbari and M. Salavati-Niasari, Photoluminescence carbon dot as a sensor for detecting of Pseudomonas aeruginosa bacteria: hydrothermal synthesis of magnetic hollow NiFe2O4-carbon dots nanocomposite material, Composites, Part B, 2019, 161, 564–577 CrossRef CAS.
- S. Sunayana, C. Nitin, R. Chaturvedi and M. Sharma, Int. J. Chem. Sci., 2010, 8, 1580–1590 Search PubMed.
- S. Zinatloo-Ajabshir, M. S. Morassaei, O. Amiri and M. Salavati-Niasari, Green synthesis of dysprosium stannate nanoparticles using Ficus carica extract as photocatalyst for the degradation of organic pollutants under visible irradiation, Ceram. Int., 2020, 46(5), 6095–6107 CrossRef CAS.
- M. Karami, M. Ghanbari, O. Amiri and M. Salavati-Niasari, Sep. Purif. Technol., 2020, 253, 117526 CrossRef CAS.
- M. Amiri, M. Salavati-Niasari, A. Pardakhty, M. Ahmadi and A. Akbari, Caffeine: a novel green precursor for synthesis of magnetic CoFe2O4 nanoparticles and pH-sensitive magnetic alginate beads for drug delivery, Mater. Sci. Eng., C, 2017, 76, 1085–1093 CrossRef CAS PubMed.
- S. M. Tabatabaeinejad, O. Amiri, M. Ghanbari and M. Salavati-Niasari, J. Mol. Liq., 2021, 117883 CrossRef.
|
| This journal is © The Royal Society of Chemistry 2021 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *a
*a