Open Access Article
Open Access ArticlePreparation, characterization of spherical 1,1-diamino-2,2-dinitroethene (FOX-7), and study of its thermal decomposition characteristics
Xinhua Zhaoa,
Dan Heb,
Xiaoping Mab,
Xueying Liub,
Zishuai Xub,
Lizhen Chen *a and
Jianlong Wanga
*a and
Jianlong Wanga
aSchool of Chemical Engineering and Technology, North University of China, Taiyuan 030051, China. E-mail: Chen17555@163.com
bResearch Institute of Gan Su Yin Guang Chemical Industry Group, Baiyin 730900, China
First published on 13th October 2021
Abstract
The sensitivity and properties of energetic materials strongly depend on their crystal morphology. In this article, spherical 1,1-diamino-2,2-dinitroethylene (FOX-7) was produced via a combination of the cooling crystallization method and repeated grinding technique. X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FT-IR), optical microscopy, scanning electron microscopy (SEM) and laser particle size analysis were used to characterize the structure, infrared characteristics, morphology, and particle size of the product. The results show that the obtained product has a smoother spherical morphology with granularity gradation and shows similar diffraction peak positions to raw FOX-7. Differential scanning calorimetry (DSC), thermal gravimetry (TG), and accelerating rate calorimetry (ARC) were used to analyse the thermal behavior of spherical FOX-7 under nonisothermal and adiabatic conditions. The thermal performance test results show that spherical FOX-7 releases energy faster and releases more energy as compared to raw FOX-7. These findings showed that the cooling crystallization method combined with the repeated grinding technique is suitable for efficient preparation of spherical FOX-7, which could greatly improve the thermal stability of FOX-7.
1. Introduction
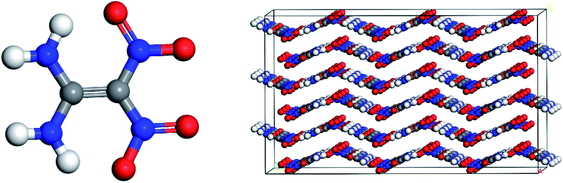
In recent years, countries all over the world have been actively developing solid propellants based on new energetic materials that have theoretically high efficacy and potentially meet the requirements of low vulnerability, which has promoted the research and development of high-energy insensitive explosives. The shortcomings of irregular crystal morphology, rough surface, relatively large aspect ratio, and agglomeration will seriously affect the performance of explosives in propellants.1 The crystal morphology of explosives not only has a direct impact on its process performance, but also has an important impact on its chemical properties and explosive performance. Studies have shown that the crystal morphology is regular and close to spherical,2 which can increase the density and dispersion of explosives, thereby improving the mechanical properties and mechanical sensitivity of the medicine column.1,1-Diamino-2,2-dinitroethene (better known as FOX-7), is a thermally stable insensitive munition (IM) high-energy material first reported by the FOA Defence Research Establishment (Sweden) in 1998.3 As shown in Fig. 1, FOX-7 has symmetrical molecular components and infinite two-dimensional wavy single-layer molecular fillers, and its special structure determines some of the physicochemical properties, in particular its low solubility, low sensitivity to friction and impact, and the absence of the melting point.4,5 The detonation speed of FOX-7 is equivalent to RDX and HMX; the sensitivity is lower than RDX and HMX, and is equivalent to TATB.6 All kinds of excellent properties make FOX-7 expected to become one of the main candidate varieties and components of insensitive explosives in the future.
In order to produce spherical FOX-7, various research activities have been carried out at home and abroad. Alok Kumar Mandal et al.2 prepared sub-micron spherical FOX-7 using a micellar nanoreactor, and the impact sensitivity was slightly improved. Ruixuan Xu et al.7 prepared nano-spherical FOX-7 by mechanical ball milling. The sensitivity of nano-spherical FOX-7 particles is reduced, and the thermal stability is improved as compared to raw FOX-7. Yuanping Zhang et al.8 used spray-drying method to prepare nanoparticle-stacked FOX-7 microspheres. The particle size of the microspheres FOX-7 formed by stacking nanoparticles (100–250 nm) is 1–5 μm, which is significantly smaller than the raw FOX-7. Particle size reduction of energetic materials has some advantages and some disadvantages.9 It was found that whilst nanosizing of explosives leads to a reduction in their sensitivity to external stimuli, it often leads to reduced shelf life. Therefore, the storage and handling of propellants containing nano energetic materials will be accompanied by hazards.10 However, in contrast, the cooling crystallization method by soaking the sample in a mixed solvent with stirring is simpler and more economical for large-scale preparation.11 The explosive crystal obtained by the recrystallization method has the characteristics of high purity and high quality. Repeated grinding technique is a completely physical process that can achieve low-cost and mass production, and it will not cause environmental pollution.
After the necessary characterization of the explosive obtained by the crystallization, it is necessary to study its kinetics of thermal decomposition reaction to provide supporting data for future applications in weapon systems.12 Differential scanning calorimetry (DSC), thermal gravimetric (TG), and accelerating rate calorimeter (ARC) are commonly used methods to study the thermal decomposition behavior of energetic materials. In this study, spherical FOX-7 was successfully fabricated from the raw FOX-7 via a combination of cooling crystallization method and repeated grinding technique. The spherical FOX-7 crystal morphology, size, structure, and thermal behavior were systematically investigated in detail. It was quite useful to the evaluation of chemical properties of prepared spherical FOX-7 and to the study of its thermal changes at high temperatures.
2. Experiment
2.1 Materials
Raw FOX-7 has a purity of greater than 99.5% by HPLC and was provided by Xi'an Modern Chemistry Research Institute.13 Dimethyl sulfoxide (C2H6OS, AR grade, abbreviated as DMSO) and acetone (C3H6O, AR grade, abbreviated as ACE) were purchased from Shanghai Titan Scientific Co., Ltd. and Tianjin Shentai Chemical Reagent Co., Ltd., respectively. All reagents were used without further purification.2.2 Preparation of spherical of FOX-7
The core content is the two-step preparation technology of raw FOX-7 through cooling crystallization and repeated grinding, using the principle of preferential dissolution of particle edges and the friction caused by the collision of particles in the solvent to obtain spherical FOX-7 crystals.FOX-7 is hardly soluble in water and common organic solvents, but easily soluble in strong polar solvents such as DMSO.14 We used a mixture of DMSO and ACE to recrystallized FOX-7. It specifically includes the following steps: (1) add FOX-7 and mixed solvent (VDMSO/VACE = 2/1) into the crystallization kettle to dissolve it fully, and the initial temperature of the crystallization kettle is 60 °C; (2) lower the temperature of the crystallization kettle while stirring to form a supersaturated solution and then crystal nucleus generate and grow, culture crystal 3 hours; (3) cool down to room temperature while stirring; (4) stir for 3 days at room temperature, so that the produced crystals are repeatedly ground in the crystallization mother liquor; (5) filter, wash with ACE, and dry to obtain spherical FOX-7 crystals. The crystallization process conditions involved in the preparation method are simple and easy to control. The crystallization mother liquor can be recycled, which is beneficial to reduce the environmental pollution of organic solvents. Low cost, safe and reliable, suitable for industrialized mass production.
2.3 Characterization
X-ray diffraction (XRD) of FOX-7 was collected on a Bruker D8-Advanced eco diffractometer in the 2θ range of 5–60° with Cu Kα radiation (λ = 1.5418 Å) operated at 40 kV and 25 mA. Fourier-transform infrared spectroscopy (FT-IR) of FOX-7 was collected on a VERTEX 80 produced by Bruker (Germany).The morphology of FOX-7 was characterized by optical microscopy (Shanghai Optical Instrument Factory, XSP-10A type, China) and scanning electron microscope (Phenom, Pure, Netherlands). The particle size distribution of FOX-7 was characterized by laser particle size analyzer (Dandong Baite Instrument Co., Ltd., BT-2002 type, China).
2.4 Thermal behavior
DSC is a common equipment used to measure various thermodynamic and kinetic parameters of energetic materials.15 The DSC measurements were carried out with a METTLER DSC 3 instrument (Switzerland). The conditions of DSC were as follows: each sample was 0.9 mg and placed in an aluminum crucible with a pinhole cover from 100 to 300 °C at the heating rate of 5, 10, 15 and 20 K min−1 under N2 flow rate of 50 mL min−1.
3. Results and discussion
3.1 XRD and FT-IR
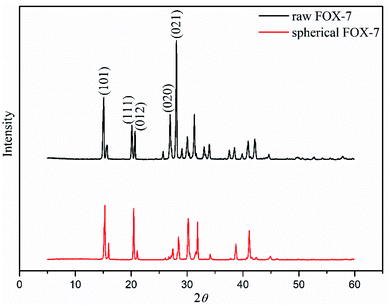
XRD and FT-IR were adopted to identify the crystalline phase of raw FOX-7 and spherical FOX-7. And the results are as follows.Fig. 2 shows the XRD patterns of raw FOX-7 and spherical FOX-7. Raw FOX-7 displays five typical peaks which are assigned to the (1 0 1), (1 1 1), (0 1 2), (0 2 0), (0 2 1) growth planes, and crystal form of FOX-7 is α-phase.18 It is noticed that the typical peaks of spherical FOX-7 are consistent with the peaks of raw FOX-7, indicating that the crystal structure of spherical FOX-7 does not change during the two processes of cooling crystallization and repeated grinding.
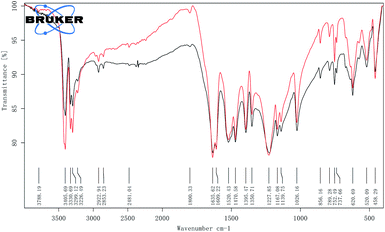
In Fig. 3, FT-IR spectra of raw FOX-7 and spherical FOX-7 showed absorbance in the ∼3220–3416 cm−1 and ∼1332–1620 cm−1 wavenumber ranges characteristic of the –NH2 and –NO2 functional groups and numerous peaks in the fingerprint region.19 FT-IR spectra of spherical FOX-7 were consistent with those of raw materials, indicating that FOX-7 did not change during the two processes of cooling crystallization and repeated grinding.
3.2 Morphology and size
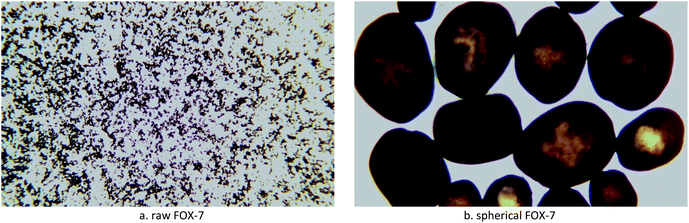
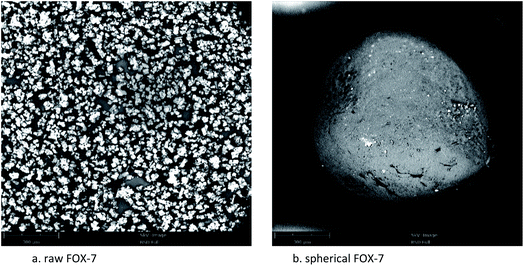
Explosive particle grading refers to the proportion of solids with different particle sizes that match each other. Relevant literature reports indicate that particle size grading can improve the formability of explosives,20,21 increase the density and compressive strength of explosives, and improve the mechanical properties of explosives. Within a certain range, the output energy, detonation pressure, and compressive strength of the explosives increase with the increase of the charge density, and the particle size grading is a very effective way to improve the packing density. Therefore, it is of great significance to develop low-sensitivity high-energy explosives and study the particle size grading technology of explosives.22The optical microscope images, scanning electron microscope images, and particle size distribution curves were shown in Fig. 4–6, respectively. Raw FOX-7 appeared as irregular fines with conspicuous edges and corners. The average particle size (d50) of raw FOX-7 is 59.26 μm with a narrow particle size distribution (d3 represents the diameter corresponding to 3% of the cumulative particle size distribution, and its value is 21.80 μm; d98 represents the diameter corresponding to 98% of the cumulative particle size distribution, and its value is 148.55 μm). After the cooling crystallization and repeated grinding process, the spherical FOX-7 with a uniform texture, few crystal deficiencies, and good dispersion, was obtained. The average particle size (d50) of spherical FOX-7 is 322.91 μm with a rather broad size distribution (d3 = 20.18 μm, d98 = 981.65 μm). The spherical FOX-7 particle size distribution curve has three peaks, showing a particle size gradation. The gaps between particles of different particle sizes are filled with each other, which can increase the density of the explosive, thereby increasing the detonation speed.
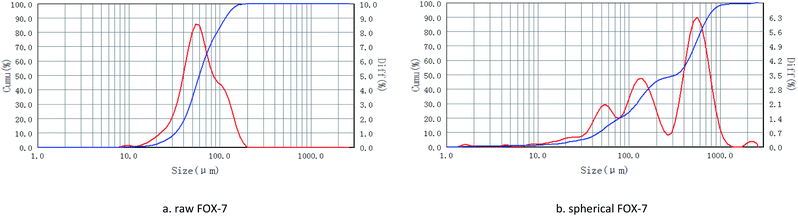 | ||
| Fig. 6 Particle size distribution curves of raw FOX-7 and spherical FOX-7 (red line: particle size; blue line: content). | ||
The concept of sphericity is introduced to quantitatively describe the sphericity of particle shape, as shown in eqn (1).23
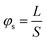 | (1) |
3.3 Thermal decomposition characteristics
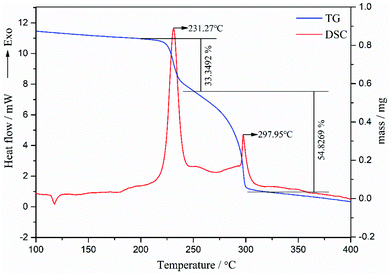
As we can see in Fig. 7, the TG curve displays two-stage mass loss in which the first stage began around 219.49 °C and completed at 242.20 °C accompanied with 33.3% mass loss. The second stage completed at 306.50 °C accompanied with 54.8% mass loss. DSC curve of raw FOX-7 exhibits one endothermic peak and two exothermic peaks, identical to the TG results. The temperature of the endothermic peak at 116.51 °C corresponds to the phase transition from α to β. The first decomposition peak temperature was 231.27 °C is mainly due to the breakage of the intra- and intermolecular hydrogen bonds of FOX-7 under heating. The adjacent FOX-7 molecules remove two amino hydrogen and one nitro oxygen to formation of water molecules, with the condensation reaction of carbon–carbon double bonds.24 The second decomposition peak temperature was 297.95 °C, which is mainly owing to the fracture of the carbon skeleton in FOX-7.19,25
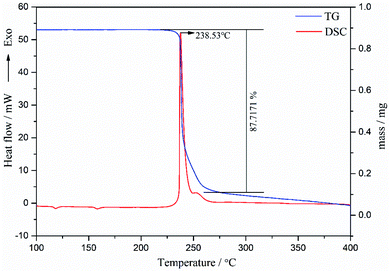
As can be seen from Fig. 8, the TG curve displays one-stage mass loss in which the stage began around 229.52 °C and completed at 248.78 °C accompanied with 87.7% mass loss. DSC curve of spherical FOX-7 exhibits two endothermic peaks and one exothermic peak, identical to the TG results. The first endothermic peak (117.29 °C) for the α → β phase transition and the second endothermic peak (157.61 °C) for the β → γ transition,9,26 which confirmed that the DSC crystal form transition peak of FOX-7 is related to the particle size of the sample.27 It should be noted that, compared with raw FOX-7, the two steps of spherical FOX-7 almost merging into a single peak (238.53 °C).28 Ostmark et al.29 explained the differences between the individual DSC curves of FOX-7 by proposing the relationship between particle size and decomposition temperature.
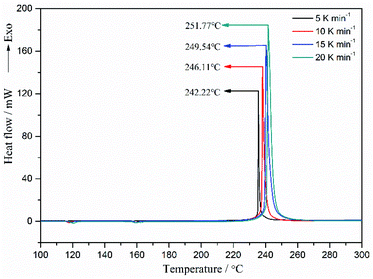
The DSC curves of spherical FOX-7 carried out at different heating rates are shown in Fig. 9. For different heating rates 5, 10, 15, and 20 K min−1 of spherical FOX-7, four different values of the peak temperature of 242.22, 246.11, 249.54, and 251.77 °C were obtained, respectively. As can be seen, both the decomposition onset temperature and exothermic peak temperature increase with the increase of the heating rate. This is because the temperature rises too fast, the reaction has not been carried out, and the higher temperature is entered, causing the reaction to lag.
To achieve the effective evaluation and better functionality of spherical FOX-7, it was necessary to estimate the thermal decomposition kinetic parameters.30 The apparent activation energy (E) and pre-exponential constant (A) of spherical FOX-7 were calculated by Kissinger method (eqn (2)) and Ozawa method (eqn (3)).31,32 The critical initiation temperature (Tb) is defined as the lowest temperature at which a specific charge can be heated without thermal runaway.33–36 The initial decomposition temperature (TP0) in the exothermic decomposition process corresponding to β →0.37,38 The activation entropy (ΔS≠), activation enthalpy (ΔH≠), and activation Gibbs free energy (ΔG≠) can reflect the difference between the activated transition state and the initial reactant in the rate-determining reaction of thermal decomposition. The ΔS≠, ΔH≠, ΔG≠, and Tb at TP0 can be calculated based on eqn (4)–(8).39,40
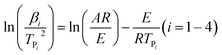 | (2) |
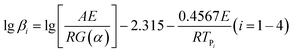 | (3) |
| TPi = TP0 + bβi + cβi2 +dβi3 (i = 1–4) | (4) |
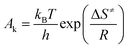 | (5) |
| ΔH≠ = Ek − RTP0 | (6) |
| ΔG≠ = ΔH≠ − TΔS≠ | (7) |
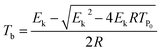 | (8) |
Decomposition kinetics basic data of spherical FOX-7 is listed in Table 1. The calculated thermal decomposition kinetics parameters are shown in Table 2. It can be found that the linear correlation coefficients of the two methods are both greater than 0.99, indicating that the measurement data is accurate and reliable. The apparent activation energy (E) is 314.03 kJ mol−1 and pre-exponential constant (A) is 5.03 × 1031 s−1, obtained by the Kissinger method. The apparent activation energy (E) is 306.82 kJ mol−1 obtained by the Ozawa method. The values of E obtained by the two methods are very close, proving that the two methods were appropriate for activation energy threshold calculations. Compared with the raw FOX-7 (Table 3),41 both apparent activation energy (E) and critical initiation temperature (Tb) of spherical FOX-7 are all increased, and the pre-exponential constant (A) also have a corresponding increase. This indicates that under thermal stimulation, spherical FOX-7 is more difficult to decompose than raw FOX-7, and the spherical outer shape effectively improves the thermal stability of FOX-7. The increased activation entropy (ΔS≠) value indicated a higher degree of disorder of spherical FOX-7 compared to that of raw FOX-7. The larger value of activation enthalpy (ΔH≠) for spherical FOX-7 results in their faster reaction rates. The positive value of activation Gibbs free energy (ΔG≠) indicates a nonspontaneous process. These data can be used as basic data for studying the physical and chemical properties of spherical FOX-7 and for theoretical calculations.42
| β/K min−1 | TP/K | 103 TP−1/K−1 | Kissinger method | Ozawa method |
|---|---|---|---|---|
| ln(β/TP2)/min−1 K−1 | lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β/K min−1 β/K min−1 |
|||
| 5 | 515.37 | 1.9404 | −10.8803 | 0.6990 |
| 10 | 519.26 | 1.9258 | −10.2022 | 1.0000 |
| 15 | 522.69 | 1.9132 | −9.8099 | 1.1761 |
| 20 | 524.92 | 1.9051 | −9.5308 | 1.3010 |
| Kissinger method | Ozawa method | TP0/K | Tb/K | Thermodynamic parameters at T0 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Ek/kJ mol−1 | A/s−1 | rk2 | Eo/kJ mol−1 | ro2 | ΔS≠/J mol−1 K−1 | ΔH≠/kJ mol−1 | ΔG≠/kJ mol−1 | ||
| 314.03 | 5.03 × 1031 | 0.9904 | 306.82 | 0.9909 | 511.76 | 518.89 | 357.50 | 309.77 | 126.82 |
| Sample | Kissinger method | TP0/K | Tb/K | Thermodynamic parameters at T0 | |||
|---|---|---|---|---|---|---|---|
| E/kJ mol−1 | A/s−1 | ΔS≠/J mol−1 K−1 | ΔH≠/kJ mol−1 | ΔG≠/kJ mol−1 | |||
| Raw FOX-7 | 249.89 | 4.50 × 1030 | 485.05 | 486.70 | 337.85 | 249.89 | 85.90 |
| Spherical FOX-7 | 314.03 | 5.03 × 1031 | 511.76 | 518.89 | 357.50 | 309.77 | 126.82 |
| Parameters | Values | Corr. |
|---|---|---|
| Onset temperature/°C | 225.50 | — |
| Onset temperature rate/K min−1 | 0.02 | 0.94 |
| Max self-heating rate/K min−1 | 2970.96 | 138![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 907.91 907.91 |
| Temperature at max rate/°C | 269.64 | — |
| Final temperature/°C | 270.67 | 2337.43 |
| Adiabatic temperature rise/°C | 45.17 | 2111.93 |
| Time to maximum rate/min | 1046.43 | 22.38 |
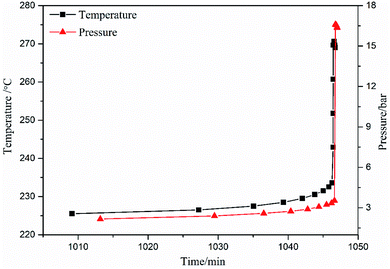
| Onset pressure/bar | 2.17 | — |
| Pressure at max rate/bar | 16.60 | — |
Since the heat released by the decomposition reaction of the sample is used not only for heating itself, but also for heating the sample ball, the test result is the entire reaction system composed of the sample and the sample ball. When all the heat released by the sample reaction is used to heat itself, the actual temperature rise and temperature rate of the sample are higher than the test value.43 Therefore, the thermal decomposition parameters measured in Table 4 need to be corrected. The corresponding equations are listed as follows:44
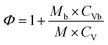 | (9) |
| ΔTS = ΦΔT | (10) |
| m0s = Φm0 | (11) |
| Tfs = T0 + ΦΔT | (12) |
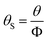 | (13) |
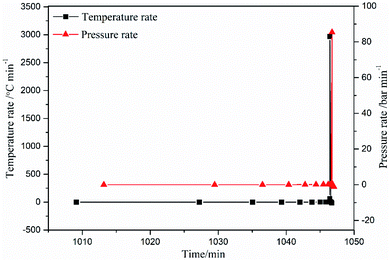
It can be seen from Fig. 10, 11 and Table 4 that the values of temperature (temperature rate) and pressure (pressure rate) were small at the beginning, but increased suddenly when the temperature exceeded 233 °C. The temperature rose from 233.57 °C to 269.64 °C within 0.162 min, the corresponding temperature rates were 2.28 K min−1 and 2970.96 K min−1, and the pressure rose from 3.53 bar to 16.60 bar. The reaction system reached the maximum temperature rate of 2970.96 K min−1 at 269.64 °C. After 0.132 minutes, the system reached the maximum temperature of 270.67 °C, and then the temperature rate became negative, and the system temperature began to drop. During the thermal decomposition of spherical FOX-7, the maximum pressure measured by the system was 16.60 bar. The high pressure and self-heating rate indicated that the energy released by the runaway reaction of spherical FOX-7 is very large. If it decomposes uncontrollably in a confined space, it may cause fire, deflagration, or even explosion.45
4. Conclusions
In conclusion, we have developed a facile approach to prepare spherical FOX-7 via a combination of cooling crystallization method and repeated grinding technique. The novel, simple and effective method was confirmed that can provide regular spherical FOX-7 with smoother surface and uniform dispersion. The average particle size of spherical FOX-7 is 322.91 μm, and its particle size distribution curve has three peaks showing a particle size gradation, which may be helpful for the potential use in military and industrial applications. The thermal decomposition of spherical FOX-7 by TG, DSC, and ARC was reported in detail. The results show that spherical FOX-7 shows more remarkable thermal stability, release energy faster and release more energy as compared to raw FOX-7. This article provides key thermodynamic, and chemical kinetic parameters for the mathematical model of the combustion process, and provides a theoretical basis for the energy prediction and safe storage performance of prepared spherical FOX-7.Author contributions
Xinhua Zhao: conceptualization, investigation, data curation, validation, writing – original draft. Dan He: conceptualization, resources. Xiaoping Ma: software, validation. Xueying Liu: supervision, funding acquisition. Zishuai Xu: methodology, supervision. Lizhen Chen: conceptualization, methodology, writing – review & editing. Jianlong Wang: resources, project administration, funding acquisition.Conflicts of interest
There are no conflicts to declare.References
- X. T. Ren, D. Y. Ye, N. Ding, J. X. He, Y. H. Lu and Q. Lei, et al., A Molecular Dynamics Simulation of Solvent Effects on the Crystal Morphology of FOX-7, Acta Armamentarii, 2015, 36(2), 272–278 Search PubMed.
- A. K. Mandal, U. Thanigaivelan, R. K. Pandey, S. Asthana, R. B. Khomane and B. D. Kulkarni, Preparation of Spherical Particles of 1,1-Diamino-2,2-dinitroethene (FOX-7) Using a Micellar Nanoreactor, Org. Process Res. Dev., 2012, 16(11), 1711–1716 CrossRef CAS.
- N. V. Latypov, J. Bergman, A. Langlet, U. Wellmar and U. Bemm, Synthesis and reactions of 1,1-diamino-2,2-dinitroethylene, Tetrahedron, 1998, 54(38), 11525–11536 CrossRef CAS.
- U. Bemm and H. Östmark, 1,1-Diamino-2,2-dinitroethylene: A Novel Energetic Material with Infinite Layers in Two Dimensions, Acta Crystallogr., 2014, 154(12), 997–1999 Search PubMed.
- H. Ostmark, A. Langlet and H. Bergman, FOX-7 - A new explosive with low sensitivity and high performance, 11th International Symposium on Detonation, Snowmass, USA, 1998 Search PubMed.
- Z. K. Long, Research development of 1,1-Diamino-2,2-dinitroethylene synthesis, Guangzhou Chem., 2013, 38(4), 71–78 CAS.
- R. X. Xu, C. W. An, H. Huang, J. Y. Wang, B. Y. Ye and B. Liu, Preparation of multi-scale FOX-7 particles and investigation of sensitivity and thermal stability, RSC Adv., 2019, 9(36), 21042–21049 RSC.
- Y. P. Zhang, C. H. Hou, X. L. Jia, J. Wang and Y. Tan, Fabrication of Nanoparticle-Stacked 1,1-Diamino-2,2-Dinitroethylene (FOX-7) Microspheres with Increased Thermal Stability, J. Nanomater., 2019, 1, 1–9 Search PubMed.
- B. Huang, M. H. Cao, F. D. Nie, H. Huang and C. W. Hu, Construction and Properties of Structure- and Size-controlled Micro/nano-Energetic Materials, Def. Technol., 2013, 9, 59–79 CrossRef.
- N. Zohari, M. H. Keshavarz and S. A. Seyedsadjadi, The Advantages and Shortcomings of Using Nano-sized Energetic Materials, Cent. Eur. J. Energ. Mater., 2013, 10(1), 135–147 CAS.
- P. Pandita, V. P. Arya and G. Kaur, Particle size reduction of RDX by sequential application of solvent-antisolvent recrystallization and mechanical methods, J. Energ. Mater., 2020, 38, 309–325 CrossRef CAS.
- Z. H. Liu, Introduction to Thermal Analysis, Chemical Industry Press, Beijing, 1st edn, 1991 Search PubMed.
- L. Hu, M. Zhang, C. Zhou and H. Y. Tian, Determination of the Purity of FOX-7 by Reversed Phase HPLC, Chin. J. Explos. Propellants, 2005, 3, 87–88 Search PubMed.
- X. H. Zhao, D. L. Cao, J. L. Wang, L. Z. Chen, Y. Y. Zhang and C. Zhou, Solubility and Crystallization of FOX-7 in DMSO-H2O, DMSO-EtOH and DMSO-ACE Binary Mixed Solvents, Chin. J. Explos. Propellants, 2019, 4(5), 473–479 Search PubMed.
- N. Unidas, Recommendations on the transport of dangerous goods, Committee of experts on the transport of dangerous goods, UN, 1995 Search PubMed.
- S. V. Pakkirisamy, S. Mahadevan, S. S. Paramashivan and A. B. Mandal, Adiabatic thermokinetics and process safety of pyrotechnic mixtures: atom bomb, Chinese, and palm leaf crackers, J. Therm. Anal. Calorim., 2012, 109, 1387–1395 CrossRef CAS.
- J. C. Tou and L. F. Whiting, The thermokinetic performance of an accelerating rate calorimeter, Thermochim. Acta, 1981, 48(1–2), 21–42 CrossRef CAS.
- H. Cai, L. Tian, B. Huang, G. Yang, D. Guan and H. Huang, 1,1-Diamino-2,2-dintroethene (FOX-7) nanocrystals embedded in mesoporous carbon FDU-15, Microporous Mesoporous Mater., 2013, 170, 20–25 CrossRef CAS.
- B. Gao, P. Wu and B. Huang, Preparation and characterization of nano-1,1-diamino-2,2-dinitroethene (FOX-7) explosive, New J. Chem., 2014, 38(6), 2334–2341 RSC.
- C. L. Lv, Experimental and theoretical research on the particle size and particle size grading of the main explosive and the shock wave sensitivity and energy output of the explosive, Master's thesis, North China Institute of Technology, Taiyuan, 2001.
- S. R. Yun, Preliminary study on the gradation law of hexogen particles, National Defense Industry Press, 1964 Search PubMed.
- Q. A. Huang, Q. Z. Cui, C. W. Zhao and L. Ning, Optimization Particle Grading of Explosive Based on Dense Packing, Sci. Technol. Eng., 2015, 15(25), 130–134 Search PubMed.
- T. Fan, Numerical simulation study on the sphericity separation process of coated nuclear fuel particles, Master's thesis, Zhejiang Univ., Hangzhou, 2021.
- A. Gindulyte, L. Massa, L. Huang and J. Karle, Proposed Mechanism of 1,1-Diamino-Dinitroethylene Decomposition: A Density Functional Theory Study, J. Phys. Chem. A, 1999, 103(50), 11045–11051 CrossRef CAS.
- D. E. Taylor, F. Rob, B. M. Rice, R. Podeszwa and K. Szalewicz, A molecular dynamics study of 1,1-diamino-2,2-dinitroethylene (FOX-7) crystal using a symmetry adapted perturbation theory-based intermolecular force field, Phys. Chem. Chem. Phys., 2011, 13(37), 16629–16636 RSC.
- K. Chatragadda and A. A. Vargeese, A Kinetics Investigation on the Nitro-Nitrite Rearrangement Mediated Thermal Decomposition of High Temperature Monoclinic Phase of 1,1-Diamino-2,2-Dinitroethylene (γ-FOX-7), J. Chem. Sci., 2017, 129, 281–288 CrossRef CAS.
- Q. B. Fu, Y. J. Shu, Y. G. Huang, J. H. Zhou and Y. X. Zhang, Preparation and thermal properties of FOX-7 crystal, Journal of Propellants and Explosives, 2009, 32(4), 6–9 CAS.
- I. J. Lochert, FOX-7 - A new insensitive explosive, DSTO Aeronautical and Maritime Research Laboratory, Australia, 2001 Search PubMed.
- H. Ostmark, H. Bergman and U. Bemm, in 2,2-dinitro-ethene-1,1-diamine (FOX-7) - properties, analysis and scale-up, 32nd International Annual Conference of ICT on Energetic Materials - Ignition, Combustion and Detonation, Karlsruhe, Germany, 2001 Search PubMed.
- M. Zhang, F. Q. Zhao, Y. J. Yang, J. K. Zhang, N. Li and H. X. Gao, Effect of rGO–Fe2O3 nanocomposites fabricated in different solvents on the thermal decomposition properties of ammonium perchlorate, CrystEngComm, 2018, 20, 7010–7019 RSC.
- H. E. Kissinger, Reaction Kinetics in Differential Thermal Analysis, Anal. Chem., 1957, 29(11), 1702–1706 CrossRef CAS.
- T. A. Ozawa, A New Method of Analyzing Thermo-gravimatric Data, Bull. Chem. Soc. Jpn., 1965, 38, 1881–1886 CrossRef CAS.
- T. L. Zhang, R. Z. Hu, Y. Xie and F. Li, The estimation of critical temperatures of thermal explosion for energetic materials using non-isothermal DSC, Thermochim. Acta, 1994, 244, 171–176 CrossRef CAS.
- A. K. Burnham, R. K. Weese, A. P. Wemhoff and J. L. Maienschein, A historical and current perspective on predicting thermal cook-off behavior, J. Therm. Anal. Calorim., 2007, 89(2), 407–415 CrossRef CAS.
- J. M. Pickard, Critical Ignition Temperature, Thermochim. Acta, 2002, 392(2), 37–40 CrossRef.
- Q. Ma, H. C. Lu, L. Y. Liao, G. Fan and J. Huang, One-Pot Synthesis, Crystal Structure, and Thermal Decomposition Behavior of 1,1′-Diamino-4,4′,5,5′-Tetranitro-2,2′-Biimidazole, J. Energ. Mater., 2016, 35(2), 1–11 Search PubMed.
- Y. Yue, X. D. Li, Y. T. Sun, J. A. Tian, H. M. Liu and B. D. Wu, et al., Preparation and characterization of HMX/EVA/hBNNSs micro-composites with improved thermal stability and reduced sensitivity, Def. Technol., 2021, 17, 650–656 CrossRef.
- R. Z. Hu, S. L. Gao and F. Q. Zhao, Thermal analysis kinetics, Science Press, Beijing, 2nd edn, 2008 Search PubMed.
- M. T. Hosamani, N. H. Ayachit and D. K. Deshpande, Activation energy (ΔG≠), enthalpy (ΔH≠), and entropy (ΔS≠) of some indoles and certain of their binary mixtures, J. Therm. Anal. Calorim., 2012, 107, 1301–1306 CrossRef CAS.
- P. L. Houston, Chemical Kinetics and Reaction Dynamics, McGraw Hill Companies, Inc., New York, 2001 Search PubMed.
- Q. B. Fu, Synthesis and properties of 1,1-diamino-2,2-dinitroethylene, PhD thesis, Sichuan Univ., Chengdu, 2007.
- P. G. Boswell, On the calculation of activation energies using a modified Kissinger method, J. Therm. Anal., 1980, 18(2), 353–358 CrossRef CAS.
- Z. M. Fu, J. Y. Huang, X. M. Qian and C. G. Feng, The Research of Thermal Stability of Chemicals by Accelerating Rate Calorimeter, Fire Saf. Sci., 2001, 10(3), 149–153 Search PubMed.
- W. Liu, Z. X. Liu, J. L. Wang, D. L. Cao and L. Z. Chen, Adiabatic decomposition analyses of 3,4-dinitropyrazole by accelerating rate calorimeter, Sci. Technol. Eng., 2018, 18(14), 121–125 Search PubMed.
- S. J. Chu, Thermal Analyses of Explosives, Science Press, Beijing, 1994 Search PubMed.
| This journal is © The Royal Society of Chemistry 2021 |