Open Access Article
Open Access ArticleA new class of fluorogenic thiazolo[2,3-b]quinazolinone receptor: selective detection towards mercury and hydrogen bisulfate ions in aqueous medium†
Prajna Parimita Mohantaa,
Aparna Prabha Devia,
Bhawani Prasad Bag b,
Hari Narayan Patia and
Ajaya Kumar Behera
b,
Hari Narayan Patia and
Ajaya Kumar Behera *a
*a
aSchool of Chemistry, Sambalpur University, Jyoti Vihar, Burla-768019, Odisha, India. E-mail: ajaykumar.behera@yahoo.com; ajaykumar.behera@suniv.ac.in
bDepartment of Biotechnology and Bioinformatics, Sambalpur University, Jyoti Vihar, Burla-768019, Odisha, India
First published on 11th October 2021
Abstract
A series of fluorophoric and structurally diverse thiazoloquinazoline derivatives were synthesized in a one-pot multicomponent cascade reaction using a microwave irradiation technique. The unique structural arrangement of the synthesized compounds encouraged us to design a new type of bioactive molecular receptor. This receptor interacts with HSO4− in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and Hg2+ in 1
1 and Hg2+ in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 binding stoichiometric ratios resulting in a change in fluorescence as well as absorption spectra in aqueous medium. The ion bonded receptor complex possibly enhances the fluorescence signal of the receptor via H-bonded complex formation with HSO4− ions and co-ordinate complex formation with Hg2+ ions.
2 binding stoichiometric ratios resulting in a change in fluorescence as well as absorption spectra in aqueous medium. The ion bonded receptor complex possibly enhances the fluorescence signal of the receptor via H-bonded complex formation with HSO4− ions and co-ordinate complex formation with Hg2+ ions.
Introduction
Gradual developments in the design and synthesis of novel organic fluorophores are essential for their flawless progress in chemistry, biology, and functional-materials research.1 Extensive use of metal ions and their subsequent pollution trigger severe environmental and health problems2 in day to day life. As a consequence, construction of highly selective and sensitive fluorescence sensors based on organic frameworks that are proficient in detecting metal ions has been the subject of keen interest. In particular, thiazolo[2,3-b]quinazolinone represents a prominent framework due to its prevalence in bioactive substances.3–5 In spite of diverse applications in the field of biology and pharmacy, there are limited reports on the photophysical properties of such fused heterocycles. Since a single receptor for multiple analytes6 has drawn extensive attention amongst analytical chemists, it is quite demanding and challenging for developing such molecular sensors. Nevertheless, the approach for the construction of novel fluorophoric and structurally diverse thiazoloquinazolines towards the development of chemosensors for multiple analytes still remains a mystery.Hydrogen sulfate is one of the deleterious pollutants7 in agricultural fertilizers, industrial raw materials and nuclear fuel waste. It eventually gets into the environment and causes several problems such as skin irritation, eye damage and respiratory paralysis.8 Thus, it is necessary to detect hydrogen sulfate in real time from environmental and biological samples in the presence of other competitive ions though it is an exigent task due to its large standard Gibbs energy of hydration (−1080 kJ mol−1).9
Conversely, mercury is a highly toxic heavy transition metal and a kind of persistent toxic substances (PTS) owing to its characteristics such as environmental persistence, long-range transportation, bioaccumulation, and high toxicity.10–13 Its exposure can have numerous adverse effects on health, such as brain damage, kidney failure, various cognitive and motion disorders.14 The consequences of its toxicity has been revealed by Minamata disease and mercury poisoning in Iraq.15–17 Moreover, Hg2+ can be converted into the more toxic methylmercury (MeHg) via chemical or biological pathways, causing further harm to organisms with high trophic level after bioaccumulation and biomagnification.18–21 Therefore, developing effective and sensitive analytical methods for HSO4− and Hg2+ ions determination in water is of great significance.
In continuation of our interest in exploring the synthesis and study of fluorogenic properties of unique thiazolo[2,3-b]quinazolinones via one-pot cascade reaction under microwave irradiation technique,22 in this context, we expanded the range of accessible angular –OH functionalized thiazolo[2,3-b]quinazolinone analogues using 2-amino thiazole precursor. Accumulating evidences towards the formation of thiazolo[2,3-b]quinazolinones having unique structural arrangement and interesting fluorescence properties, we have investigated the selectivity and sensitivity of HSO4− and Hg2+ ions in aqueous (H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH, 20
MeOH, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 v/v) medium using this novel receptor.
80 v/v) medium using this novel receptor.
Results and discussion
Taking our previously reported results into consideration,22 we attempted analogous one pot three component cascade reaction of equimolar mixture of 2-amino thiazole 1, aryl aldehydes 2a-h and cyclic-1,3-dicarbonyls 3a-b under acid mediated microwave heating at 150 °C in a microwave synthesizer. Here also, unexpected formation of a series of compound 5 was achieved in spite of compound 4 (Scheme 1). The isolated target molecules are elucidated unambiguously using NMR and mass spectroscopy. Two different series of thiazoloquinazolinone derivatives 5aa-5ah and 5ba-5bh were synthesized in good yields on heating the mixture of 1, 2a-h separately with dimedone 3a and 1,3-cyclohexanedione 3b respectively (Scheme 1).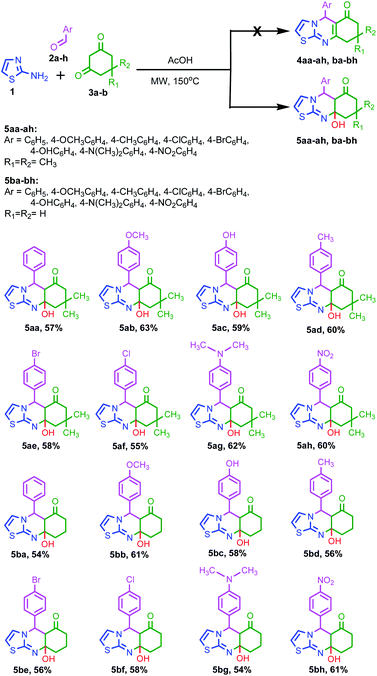 | ||
| Scheme 1 Synthesis of 9a-hydroxy-5-phenyl-5,5a,7,8,9,9a-hexahydrothiazolo[2,3-b]quinazolin-6-ones 5aa-5ah, ba-5bh from 2-amino thiazole 1, cyclic 1,3-diketones 2a-h and aromatic aldehydes 3a-b. | ||
Interestingly, an additional experiment was done to know the presence of –OH group leading a crucial role for the distinct photophysical property of the thiazoloquinazolinone (I) (Fig. 1). Benzothiazoloquinazolinone (II) (Fig. 1) was also synthesized by previously reported procedure.22 Fluorescence spectra of benzothiazoloquinazolinone (II) were then measured upon addition of 5 equivalents various metal ions. From the fluorescence spectra, it was observed that no such significant change in emission intensities at 395 nm was occurred after addition of the metal ions (see Fig. S49 in the ESI†).
Perceiving the significance of –OH bond formation, we have investigated the sensing mechanism of the synthesized compounds possessing different substituents in compounds 5aa, 5ac, 5ag, 5ah and 5ba. All the derivatives of the thiazolo[2,3-b]quinazolinones besides, compounds 5ag, 5ah, 5bg and 5bh displayed cyan colour fluorescence under UV lamp. The initial solvatochromism studies of compound 5aa (R) was performed in acetonitrile, chloroform, DMSO, 1,4-dioxan, ethanol, hexane and methanol–water. In the UV-visible spectra, two strong absorption band appeared at 286 and 404 nm in H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH (20
MeOH (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 v/v) solvent medium may be attributed to π → π* and n → π* transition. The fluorescence emission spectrum of receptor R was measured in the same solvent medium with an excitation wavelength 404 nm and maximum fluorescence emission intensity was observed at 460 nm. Further, the receptor R possessing sulfur, nitrogen atoms and one free –OH group appended to one side of the molecule may act as suitable binding site for cation in accordance with soft acid soft base (SASB) concept and for anions by non-covalent interaction such as hydrogen bonding.
80 v/v) solvent medium may be attributed to π → π* and n → π* transition. The fluorescence emission spectrum of receptor R was measured in the same solvent medium with an excitation wavelength 404 nm and maximum fluorescence emission intensity was observed at 460 nm. Further, the receptor R possessing sulfur, nitrogen atoms and one free –OH group appended to one side of the molecule may act as suitable binding site for cation in accordance with soft acid soft base (SASB) concept and for anions by non-covalent interaction such as hydrogen bonding.
Consequently, we have investigated the binding affinity of receptors 5aa, 5ac, 5ag, 5ah and 5ba towards various anions and cations. The anion and cation binding affinity of different receptors was primarily examined by fluorescence and UV-vis spectroscopic techniques. Fluorescence and UV-visible spectra were measured upon addition of 5 equivalents various anions such F−, CI−, Br−, I−, AcO−, H2PO4−, HSO4− in the form of their tetrabutylammonium (TBA) salts and CN− ion as potassium salt to a 10 μM receptor solutions in H2O/MeOH (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
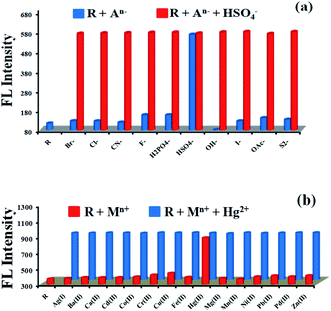
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 v/v) solvent medium. Similarly, the spectroscopic responses were also monitored by adding 5 equivalents of different metal ions such as Na+, K+, Ag+, Al3+, Ba2+, Ca2+, Cd2+, Co2+, Cr2+, Cu2+, Fe2+, Hg2+, Mg2+, Mn2+, Ni2+, Pb2+, Pd2+, Zn2+ in the form of their perchlorate/chloride//nitrate salts (10−3 to 10−4 M) dissolved in Milli-Q water. From the fluorescence spectra, it was observed that the emission intensities at 460 nm were increased by about 10 fold in presence of 5 equivalents of HSO4− or Hg2+ ions (Fig. 2) when added separately to the receptor solutions, however other ions could not produce any significant changes in the fluorescence spectra of the receptor solution under an identical condition. On the other hand in the UV-vis studies, it was observed that the absorption band at 404 nm was decreased to a smaller extent while the absorption band at 286 nm shows a significant decrease with a hypsochromic shift of 21 nm from 286 nm to 265 nm only in presence of HSO4− ion (see Fig. S50a in the ESI†). Moreover, in case of 5 equivalents of Hg2+ ions a hypsochromically shifted (59 nm) new and hence a distinct absorption band was appeared at 345 nm (see Fig. S50b in the ESI†) with simultaneous disappearance of the absorption band at 404 nm. These findings clearly indicated that the receptor has a very high selectivity and significant affinity towards HSO4− and Hg2+ ions.
80 v/v) solvent medium. Similarly, the spectroscopic responses were also monitored by adding 5 equivalents of different metal ions such as Na+, K+, Ag+, Al3+, Ba2+, Ca2+, Cd2+, Co2+, Cr2+, Cu2+, Fe2+, Hg2+, Mg2+, Mn2+, Ni2+, Pb2+, Pd2+, Zn2+ in the form of their perchlorate/chloride//nitrate salts (10−3 to 10−4 M) dissolved in Milli-Q water. From the fluorescence spectra, it was observed that the emission intensities at 460 nm were increased by about 10 fold in presence of 5 equivalents of HSO4− or Hg2+ ions (Fig. 2) when added separately to the receptor solutions, however other ions could not produce any significant changes in the fluorescence spectra of the receptor solution under an identical condition. On the other hand in the UV-vis studies, it was observed that the absorption band at 404 nm was decreased to a smaller extent while the absorption band at 286 nm shows a significant decrease with a hypsochromic shift of 21 nm from 286 nm to 265 nm only in presence of HSO4− ion (see Fig. S50a in the ESI†). Moreover, in case of 5 equivalents of Hg2+ ions a hypsochromically shifted (59 nm) new and hence a distinct absorption band was appeared at 345 nm (see Fig. S50b in the ESI†) with simultaneous disappearance of the absorption band at 404 nm. These findings clearly indicated that the receptor has a very high selectivity and significant affinity towards HSO4− and Hg2+ ions.
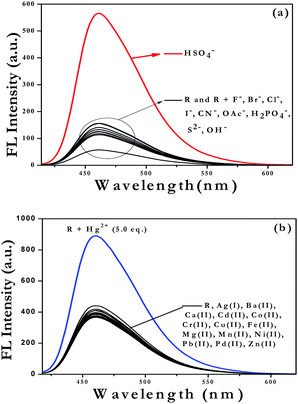 | ||
Fig. 2 Fluorescence spectra of receptor R (10 μM) in presence of 5.0 equivalents of various anions (a) and cations (b) in MeOH/H2O (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80) as solvent medium (λex = 404 nm). 80) as solvent medium (λex = 404 nm). | ||
Furthermore, in order to check the interference of other ions, competitive binding studies were carried out by monitoring the fluorescence spectra of receptor in presence of 5 equivalents various anions having 5 equivalents of HSO4− ions and 5 equivalents various cations including 5 equivalents Hg2+ ions separately in MeOH/H2O (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 v/v) solvent medium upon excitation at 404 nm. From the histograms (Fig. 3), it was observed that the fluorescence intensity at 460 nm was enhanced only by 5 equivalents of HSO4− or Hg2+ ions (Fig. 3a and b) indicating a high selectivity of the receptors towards these ions. Besides, the receptor R (compound 5aa), the sensing properties of thiazoloquinazolinone derivatives possessing electron rich (compound 5ac and 5ag) and electron deficient (compound 5ah) phenyl substituents have been investigated for sensing. From the results, it was concluded that the compound 5ac showed similar type of optical responses as the compound 5aa. No satisfactory results were obtained in case of 5ag and 5ah as the compounds are poorly emissive/non-emissive in nature. In addition, the anion and cation selectivities of the compound 5ba also checked whether this compound offers steric hindrance towards binding (ESI, Fig. S60†). The findings suggest that the compounds 5aa and 5ba show similar fluorescence responses. Thus, the steric hindrance does not interfere the binding. As a result, only the compound 5aa was taken for further investigation towards sensing.
80 v/v) solvent medium upon excitation at 404 nm. From the histograms (Fig. 3), it was observed that the fluorescence intensity at 460 nm was enhanced only by 5 equivalents of HSO4− or Hg2+ ions (Fig. 3a and b) indicating a high selectivity of the receptors towards these ions. Besides, the receptor R (compound 5aa), the sensing properties of thiazoloquinazolinone derivatives possessing electron rich (compound 5ac and 5ag) and electron deficient (compound 5ah) phenyl substituents have been investigated for sensing. From the results, it was concluded that the compound 5ac showed similar type of optical responses as the compound 5aa. No satisfactory results were obtained in case of 5ag and 5ah as the compounds are poorly emissive/non-emissive in nature. In addition, the anion and cation selectivities of the compound 5ba also checked whether this compound offers steric hindrance towards binding (ESI, Fig. S60†). The findings suggest that the compounds 5aa and 5ba show similar fluorescence responses. Thus, the steric hindrance does not interfere the binding. As a result, only the compound 5aa was taken for further investigation towards sensing.
The fluorescence intensity at 460 nm is nearly the same as receptor R in presence of 5 equivalents HSO4− or Hg2+ ion even in the presence of 5 equivalents other interfering ions (Fig. 3a and b). These findings further supported that, presence of other ions in the analytical sample could not produce any interference in the detection of HSO4− or Hg2+ ion and hence the receptor is found to be highly selective towards HSO4− and Hg2+ ion when analyzed separately.
Additionally, the interference of cations for HSO4− and anions for Hg2+ ion were also checked by measuring fluorescence spectra in presence of 5 equivalents various cations with receptor solution having 5 equivalents of HSO4− ions and 5 equivalents various anions with receptor solution having 5 equivalents of Hg2+ ions separately in MeOH/H2O (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 v/v) solvent medium upon excitation at 404 nm. From the histograms (see Fig. S51 in the ESI†), the fluorescence intensity at 460 nm is nearly the same as receptor R in presence of 5 equivalents HSO4− ions and 5 equivalents of other cations having 5 equivalents of Hg2+ ions.
80 v/v) solvent medium upon excitation at 404 nm. From the histograms (see Fig. S51 in the ESI†), the fluorescence intensity at 460 nm is nearly the same as receptor R in presence of 5 equivalents HSO4− ions and 5 equivalents of other cations having 5 equivalents of Hg2+ ions.
Similarly, the fluorescence intensity at 460 nm is nearly the same as receptor R in presence of 5 equivalents Hg2+ and 5 equivalents of other anions having 5 equivalents of HSO4− ion. These findings further supported that presence of other anions in the analytical sample could not produce any interference in the detection of Hg2+ ion except HSO4− ion and presence of other cations could not produce any interference in the detection of HSO4− ion except Hg2+ ion. Thus, we can envisage that the receptor can be utilized for selective and sensitive detection of HSO4− or Hg2+ with only exception when both are present.
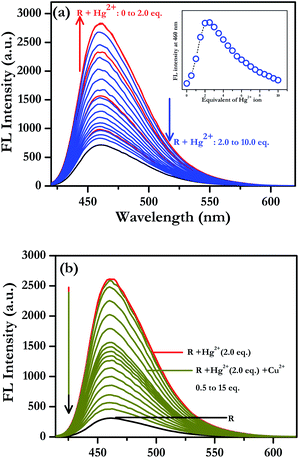
In order to understand the binding characteristics of the receptors and HSO4− or Hg2+ ions, fluorescence titration experiments were conducted upon gradual addition of a standard solution of HSO4− or Hg2+ ions to the receptor solution separately. On gradual addition of a standard solution of HSO4− ion to a 10 μM receptor R solution indicated a progressive increase in intensity of the receptor emission band at 460 nm (see Fig. S52 in the ESI†). A fluorescence change from weakly emissive to bright cyan was observed with increasing concentration of HSO4− ion upon UV-illumination. From the fluorescence titration spectra, the binding constant of receptor-HSO4− was estimated to be 2.79 × 106 with a nonlinear regression fit of R2 = 0.998 (see Fig. S52 in the ESI†). Conversely, an initial increase in intensity of the receptor emission band at 460 nm was observed for gradual addition of two equivalents of Hg2+ ions to a 10 μM receptor R solution (Fig. 4a). On further addition of Hg2+ ions beyond two equivalents to the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 receptor–Hg2+ complex exhibited a subsequent decrease in intensity at 460 nm (Fig. 4a). Here, we can suggest that after binding of two equivalents of mercury ions, additional Hg2+ ions remain in the vicinity of the solution of the complex that quenches the intensity possibly due to heavy metal ion effect acting as a self-quencher. In order to understand the quenching behavior, an additional experiment was carried out taking copper as quencher. Upon gradual addition of Cu2+ ions to a 1
2 receptor–Hg2+ complex exhibited a subsequent decrease in intensity at 460 nm (Fig. 4a). Here, we can suggest that after binding of two equivalents of mercury ions, additional Hg2+ ions remain in the vicinity of the solution of the complex that quenches the intensity possibly due to heavy metal ion effect acting as a self-quencher. In order to understand the quenching behavior, an additional experiment was carried out taking copper as quencher. Upon gradual addition of Cu2+ ions to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 receptor–Hg2+ complex solution, it was observed that the fluorescence intensity at 460 nm get quenched as was observed in case of Hg2+ ion addition beyond 2 equivalents (Fig. 4b). These findings suggest that the fluorescence quenching is due to the presence of heavy metal ions in the vicinity of the 1
2 receptor–Hg2+ complex solution, it was observed that the fluorescence intensity at 460 nm get quenched as was observed in case of Hg2+ ion addition beyond 2 equivalents (Fig. 4b). These findings suggest that the fluorescence quenching is due to the presence of heavy metal ions in the vicinity of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 receptor–Hg2+ complex in the solution. From the Benesi–Hildebrand plot, the binding constant was estimated to be 2.29 × 104 M−1 with linear regression fit of R2 = 0.985 for Hg2+ ion as measure of fluorescence intensity at 460 nm (see Fig. S53 in the ESI†). Such a very high binding constant indicated a very strong affinity of the receptor R towards HSO4− or Hg2+ ion.
2 receptor–Hg2+ complex in the solution. From the Benesi–Hildebrand plot, the binding constant was estimated to be 2.29 × 104 M−1 with linear regression fit of R2 = 0.985 for Hg2+ ion as measure of fluorescence intensity at 460 nm (see Fig. S53 in the ESI†). Such a very high binding constant indicated a very strong affinity of the receptor R towards HSO4− or Hg2+ ion.
In the UV-visible titration of receptor R, it was observed that the receptor absorption band at 404 nm gradually decreases along with the simultaneous increase of a hypsochromically shifted new absorption band at 345 nm with incremental concentration of Hg2+ ion by the gradual addition of Hg2+ ions (see Fig. S54 in the ESI†). The UV-visible titration profile is in accordance with the fluorescence titration profile that further supported a certain interaction of the receptor R with Hg2+ ions. Thus, we can propose that the sulfur (S), nitrogen (N) and hydroxyl oxygen (O) binding sites of the receptor R bind with the Hg2+ ions through co-ordinate interaction due to soft acid soft base concept.
The binding stoichiometry of receptor R with HSO4− or Hg2+ ions was quantitatively analyzed by Job's continuous variation plot. It revealed that maximum fluorescence intensity at 460 nm for 0.5 mole fractions of HSO4− or Hg2+ ions indicating the binding ratios between R with HSO4− ion and R with Hg2+ ion in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 respectively (see Fig. S55 in the ESI†). Further, the sensitivity of the receptor R has been evaluated by determining the detection limit. From the fluorescence measurement the detection limit for HSO4− and Hg2+ ion by the receptor R were found to be 3.6 μM and 17.1 μM respectively (see Fig. S56 in the ESI†). This limit of detection HSO4− is far below the permissible concentration of HSO4− ion (1000–1200 mg L−1 = 0.01 M) in drinking water as recommended by WHO.23
2 respectively (see Fig. S55 in the ESI†). Further, the sensitivity of the receptor R has been evaluated by determining the detection limit. From the fluorescence measurement the detection limit for HSO4− and Hg2+ ion by the receptor R were found to be 3.6 μM and 17.1 μM respectively (see Fig. S56 in the ESI†). This limit of detection HSO4− is far below the permissible concentration of HSO4− ion (1000–1200 mg L−1 = 0.01 M) in drinking water as recommended by WHO.23
To further elucidate the binding mode of the receptor R with HSO4−, 1H NMR-titration experiment has been performed which illustrated that the chemical shift of the –OH group shifted towards downfield region. Moreover, after the continuous addition of HSO4− anion of 0.5, 1.0 and 2.0 equiv. to the receptor solution, the resonance at δ11.18 ppm shifted towards downfield to δ11.21, 11.23 and 11.26 ppm respectively.
In order to gain insight into the structural basis of photophysical properties of receptor R, theoretical calculations of R, R + Hg2+ and R + HSO4− were performed using Gaussian 09 program. The structures of R, R + Hg2+ and R + HSO4− were optimized using B3LYP and B3LYP/LANL2DZ basis sets respectively. The frontier molecular orbital analysis reveals that in receptor R, HOMO is spread over thiazole and pyridine scaffolds where as the LUMO is located only over pyridine ring (Fig. 5d). Moreover, in R + Hg2+ (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) HOMO appears over thiazole and pyridine moieties, where as LUMO is located over mercury (Fig. 5f). Moreover, the calculated HOMO–LUMO energy gap difference for R + Hg2+ (1
2) HOMO appears over thiazole and pyridine moieties, where as LUMO is located over mercury (Fig. 5f). Moreover, the calculated HOMO–LUMO energy gap difference for R + Hg2+ (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (3.15 eV) is lesser than that of the receptor R (4.48 eV) which indicates favorable coordination between the receptor R and Hg2+ in 1
2) (3.15 eV) is lesser than that of the receptor R (4.48 eV) which indicates favorable coordination between the receptor R and Hg2+ in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio. However, in R + HSO4− HOMO is found over the thiazole and pyridine moieties and LUMO spreads all over the molecules (Fig. 5e). The calculated energy difference between HOMO and LUMO for R + HSO4− (8.19 eV) is higher than that of the receptor R (4.48 eV). The larger HOMO–LUMO gap refers to higher kinetic stability and lower chemical reactivity. The molecule binding with HSO4− is definitely stable.
2 ratio. However, in R + HSO4− HOMO is found over the thiazole and pyridine moieties and LUMO spreads all over the molecules (Fig. 5e). The calculated energy difference between HOMO and LUMO for R + HSO4− (8.19 eV) is higher than that of the receptor R (4.48 eV). The larger HOMO–LUMO gap refers to higher kinetic stability and lower chemical reactivity. The molecule binding with HSO4− is definitely stable.
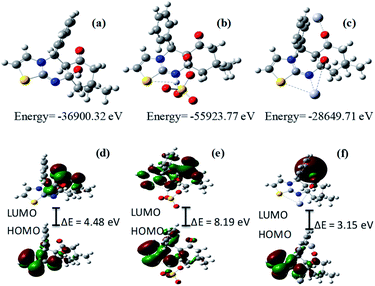 | ||
| Fig. 5 Optimized structure of (a) R, (b) R + HSO4− and (c) R + Hg2+. Frontier molecular orbital of (d) receptor R (e) R + HSO4−and (f) R + Hg2+. | ||
Conclusions
In summary, we were able to extend our methodology for the synthesis of –OH functionalized thiazoloquinazolinone derivatives under one-pot multicomponent cascade reaction using 2-amino thiazole precursor. The unique structural arrangement of the synthesized compounds stimulated us to design a new type of bioactive novel molecular receptor. All the experimental findings clearly suggested that this receptor interact with HSO4− in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and with Hg2+ in 1
1 and with Hg2+ in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 binding stoichiometric ratio resulting in a change in fluorescence as well as absorption spectra in aqueous medium. The ion bonded receptor complex possibly enhances the fluorescence signal of the receptor at 460 nm via H-bonded complex formation with HSO4− ions and co-ordinate complex formation with Hg2+ ions.
2 binding stoichiometric ratio resulting in a change in fluorescence as well as absorption spectra in aqueous medium. The ion bonded receptor complex possibly enhances the fluorescence signal of the receptor at 460 nm via H-bonded complex formation with HSO4− ions and co-ordinate complex formation with Hg2+ ions.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support by UGC (DRS-SAP) and infrastructural research facility from FIST-DST, New Delhi, India are highly appreciated. We are grateful to NIT-Rourkela, IIT-Chennai and IISC-Bangalore for providing HPLC, NMR and mass spectra. We thank to Dr S. N. Sahu and Dr H. Chakraborty of our department for their valuable suggestion during photophysical studies. P. P. M. thanks UGC, New Delhi, India for providing BSR fellowship.Notes and references
- (a) H. Li and J. C. Vaughan, Chem. Rev., 2018, 118, 9412–9454 CrossRef CAS PubMed; (b) G. Feng and B. Liu, Acc. Chem. Res., 2018, 51, 1404–1414 CrossRef CAS PubMed; (c) S. He, J. Song, J. Qu and Z. Cheng, Chem. Soc. Rev., 2018, 47, 4258–4278 RSC; (d) Z. Yang, A. Sharma, J. Qi, X. Peng, D. Y. Lee, R. Hu, D. Lin, J. Qu and J. S. Kim, Chem. Soc. Rev., 2016, 45, 4651–4667 RSC; (e) J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS PubMed; (f) K. M. Dean and A. E. Palmer, Nat. Chem. Biol., 2014, 10, 512–523 CrossRef CAS PubMed; (g) D. Ding, K. Li, B. Liu and B. Z. Tang, Acc. Chem. Res., 2013, 46, 2441–2453 CrossRef CAS PubMed; (h) J. Chan, S. C. Dodani and C. J. Chang, Nat. Chem., 2012, 4, 973–984 CrossRef CAS PubMed; (i) T. M. Figueira-Duarte and K. Müllen, Chem. Rev., 2011, 111, 7260–7314 CrossRef CAS PubMed.
- (a) X. R. He, H. B. Liu, Y. L. Li, S. Wang, Y. J. Li, N. Wang, J. C. Xiao, X. H. Xu and D. B. Zhu, Adv. Mater., 2005, 17, 2811–2815 CrossRef CAS; (b) E. M. Nolanand and S. J. Lippard, Chem. Rev., 2008, 108, 3443–3480 CrossRef PubMed; (c) G. Aragay, J. Ponsand and A. Merkoci, Chem. Rev., 2011, 111, 3433–3458 CrossRef CAS PubMed; (d) J. Y. Jung, M. Kang, J. Chun, J. Lee, J. Kim, J. Kim, Y. Kim, S. J. Kim, C. Lee and J. Yoon, Chem. Commun., 2013, 49, 176–178 RSC.
- (a) C. C. Cheng, D. F. Liua and T. C. Chou, Heterocycles, 1993, 35, 775–789 CrossRef CAS; (b) G. Shukla, A. K. Tiwari, V. K. Singh, A. Bajpai, H. Chandra and A. K. Mishra, Chem. Biol. Drug Des., 2008, 72, 533–539 CrossRef CAS PubMed; (c) M. A. El-Sherbeny, Drug Res., 2000, 50, 848–853 CAS.
- (a) M. A. Khalilzadeh, H. Kamiri-Maleh and V. K. Gupta, Electroanalysis, 2015, 27, 1766–1773 CrossRef CAS; (b) H. Karimi-Maleh, F. Tahernejad-Javazmi, V. K. Gupta, H. Ahmar and M. H. Asadi, J. Mol. Liq., 2014, 196, 258–263 CrossRef CAS.
- K. Arya, R. Tomar and D. S. Rawat, Med. Chem. Res., 2014, 23, 896–904 CrossRef CAS.
- J.-T. Hou, B.-Y. Liu, K. Li, K.-K. Yu, M.-B. Wu and X.-Q. Yu, Talanta, 2013, 116, 434–440 CrossRef CAS PubMed.
- B. A. Moyer, L. H. Delmau, C. J. Fowler, A. Ruas, D. A. Bostick, J. L. Sessler, E. Katayeu, G. D. Pantos, J. M. Llinares, M. A. Hossain, S. O. Kang, K. Bowman-James, R. V. Eldik and K. Bowman-James, Advances in Inorganic Chemistry, Academic Press, New York, 2006, pp. 175–204 Search PubMed.
- S. M. Hezaveh, H. Khanmohammadi and M. Zendehdel, Spectrochemica Acta, 2018, 199, 21–31 CrossRef CAS PubMed.
- F. P. Schmidtchen, Top. Curr. Chem., 1986, 132, 101–133 CrossRef CAS.
- M. T. Tsui and W.-X. Wang, Environ. Sci. Technol., 2006, 40, 4025–4030 CrossRef CAS PubMed.
- M. Loewen, S. Kang, D. Armstrong, Q. Zhang, G. Tomy and F. Wang, Environ. Sci. Technol., 2007, 41, 7632–7638 CrossRef CAS PubMed.
- N. E. Selin, Annu. Rev. Environ. Resour., 2009, 34, 43–63 CrossRef.
- C. B. Liu, X. B. Hua, H. W. Liu, B. Yu, Y. X. Mao, D. Y. Wang, Y. G. Yin, L. G. Hu, J. B. Shi and G. B. Jiang, Ecotoxicol. Environ. Saf., 2018, 150, 327–334 CrossRef CAS PubMed.
- P. B. Tchounwou, W. K. Ayensu, N. Ninashvili and D. Sutton, Environ. Toxicol., 2003, 18, 149–175 CrossRef CAS PubMed.
- H. H. Harris, I. J. Pickering and G. N. George, Science, 2003, 301, 1203 CrossRef CAS PubMed.
- B. Weiss, Toxicol. Sci., 2007, 97, 223–225 CrossRef CAS PubMed.
- L. Amin-Zaki, S. Elhassani, M. A. Majeed, T. W. Clarkson, R. A. Doherty and M. Greenwood, Pediatrics, 1974, 54, 587–595 CAS.
- Y. Yin, Y. Li, C. Tai, Y. Cai and G. Jiang, Nat. Commun., 2014, 5, 4633 CrossRef CAS PubMed.
- J. M. Parks, A. Johs, M. Podar, R. Bridou, R. A. Hurt, S. D. Smith, S. J. Tomanicek, Y. Qian, S. D. Brown, C. C. Brandt, A. V. Palumbo, J. C. Smith, J. D. Wall, D. A. Elias and L. Liang, Science, 2013, 339, 1332–1335 CrossRef CAS PubMed.
- M. Meng, J.-b. Shi, C.-b. Liu, N.-l. Zhu, J.-j. Shao, B. He, Y. Cai and G.-b. Jiang, RSC Adv., 2015, 5, 40036–40045 RSC.
- C. R. Hammerschmidt, M. B. Finiguerra, R. L. Weller and W. F. Fitzgerald, Environ. Sci. Technol., 2013, 47, 3671–3677 CrossRef CAS PubMed.
- P. P. Mohanta, H. N. Pati and A. K. Behera, RSC Adv., 2020, 10, 15354–15359 RSC.
- WHO/SDE/WSH/03.04/114, Sulfate in Drinking-water Background document for development of WHO Guidelines for Drinking-water Quality Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, NMR, mass spectra, UV and fluorescence spectra of the synthesized compounds. See DOI: 10.1039/d1ra05824j |
| This journal is © The Royal Society of Chemistry 2021 |