Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Domino C–C/C–O bond formation: palladium-catalyzed regioselective synthesis of 7-iodobenzo[b]furans using 1,2,3-triiodobenzenes and benzylketones†
Raed M. Al-Zoubi *a,
Walid K. Al-Jammal
*a,
Walid K. Al-Jammal a,
Michael J. Ferguson
a,
Michael J. Ferguson b and
Graham K. Murphy
b and
Graham K. Murphy c
c
aDepartment of Chemistry, Jordan University of Science and Technology, P.O. Box 3030, Irbid, 22110, Jordan. E-mail: rmzoubi@just.edu.jo; Fax: +962-2-7201071; Tel: +962-2-7201000 ext. 23651
bDepartment of Chemistry, Gunning-Lemieux Chemistry Centre, University of Alberta, Edmonton, Alberta T6G2G2, Canada
cDepartment of Chemistry, University of Waterloo, Waterloo, Ontario N2L3G1, Canada. E-mail: graham.murphy@uwaterloo.ca
First published on 8th September 2021
Abstract
A facile and efficient synthesis of 7-iodobenzo[b]furan derivatives via a highly regioselective tandem α-arylation/intramolecular O-arylation of 5-substituted-1,2,3-triiodobenzenes and benzylketones is described. Remarkably, the α-arylation coupling reactions initiate exclusively at the least sterically-hindered position of the triiodoarene, which results in a highly chemoselective transformation. The highest yields were observed in reactions between electron-poor 1,2,3-triiodoarenes and electron-rich benzylketones, yet the optimized reaction conditions were found to be tolerant to a wide range of different functional groups. This unprecedent synthesis of 7-iodobenzo[b]furans from 1,2,3-triiodobenzenes is scalable, general in scope, and provides easy access to valuable precursors for other chemical transformations.
Introduction
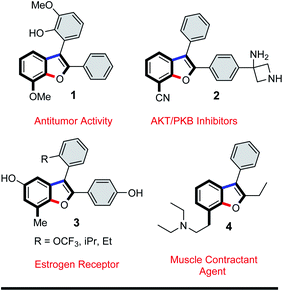
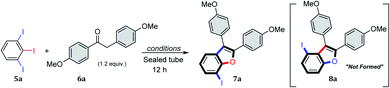
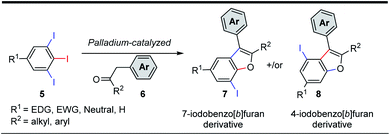
Efficient annulation protocols that quickly access highly functionalized and valuable intermediates by means of site-selective functionalization of simple precursors is a powerful tool in synthetic chemistry and biology. In addition to the bond forming efficiency and atom-economy of these domino protocols, the potential for discovering new routes to access important functionalization patterns on privileged scaffolds is of great interest in organic materials and medicinal chemistry. Indeed, benzo[b]furans are ubiquitous structural motifs found as core components in organic materials such as organic transistors1 or organic solar cells,2 and in natural products such as anigopreissin A and amurensins L,3 and in pharmaceuticals such as 6-APB® (informally called benzo-Fury),4 antitumor agents,5 antimicrobials,6 5-lipoxygenase inhibitors,7 and angiotensin II inhibitors.8 Bioactive examples of the specific 7-substituted 2,3-diarylbenzo[b]furan motif include 7-methoxy-2,3-diaryl-benzo[b]furan derivative 1 (Fig. 1), which is reported to inhibit proliferation of HeLa cells by apoptosis induction.9 The 7-cyano-2,3-diarylbenzofuran derivative 2 has inhibitory activity towards serine threonine kinase AKT for treating cancer,10 whereas the 7-methyl-2,3-diarylbenzofuran derivative 3 exhibits binding affinity to α- or β-subtype estrogen receptors.11 Lastly, the 7-aminoethyl-2,3-diarylbenzofuran derivative 4 is described as a muscle contractant with good urethral action for treatment of urinary incontinence.12 Among the numerous synthetic protocols that have been developed to make substituted benzo[b]furans,6b,13 the 2,3-diarylbenzo[b]furan scaffold has received considerable attention.14 Few of these methods employed tandem α-arylation/intramolecular O-arylations between haloarenes and ketones, even though transition metal-catalyzed domino reactions are efficient and possibly ideal methodologies.13i,k,15 In one example, Arisawa et al. reported a regioselective rhodium-catalyzed tandem C–C/C–O arylation between ortho-difluorobenzenes and ketones. In this case, the C–C coupling reaction took place at the least sterically hindered (and most electron deficient) C–F bond giving, as the state of the art, 6-substituted 2,3-diarylbenzo[b]furans in good yields.15a In another example, Willis et al. reported a two-step process for the synthesis of benzo[b]furan derivatives via two independent palladium catalyzed C–C/C–O arylation reactions between polyhaloarenes and ketones. In this instance, the first step involved an excellent chemo- and regioselective C–C coupling at the iodo substituent, even in the presence of bromine and/or fluorine substituents, giving the products in good yields.15d,e Given the importance of 2,3-diarylbenzo[b]furan derivatives in literature,16 and building on our interest in transformations of 5-substituted-1,2,3-triiodobenzenes,17 we too were encouraged to develop a new protocol to quickly access this motif. Our goal was to generate densely functionalized benzo[b]furan motifs, all with iodine at C-7 to serve as a precursor for further synthetic manipulation. Herein, we report our efforts to develop the first domino C–C/C–O arylation reaction between 1,2,3-triiodobenzenes and benzylketones to make 7-iodo-2,3-diarylbenzo[b]furan derivatives.The 5-substituted-1,2,3-triiodobenzene starting materials (5) were synthesized from anilines or benzoic acid derivatives.18 Their C–I bonds are regiochemically differentiated (Scheme 1, red vs. blue), and as such, two regioisomeric C–C/C–O arylation products are possible: the 7-iodo-2,3-disubstituted benzo[b]furan (7) and the 4-iodo-2,3-disubstituted benzo[b]furan (8). It was expected using an equimolar loading of ketone 6 would be sufficient for achieving the tandem C–C/C–O arylations across two of the aryliodide groups, and that the configuration of the benzo[b]furan products would depend solely on the site of initial C–I bond activation.
 | ||
| Scheme 1 Possible iodinated benzofuran regioisomers from C–C/C–O arylations of 5-substituted-1,2,3-triiodobenzenes. | ||
To explore this hypothesis, 1,2,3-triiodobenzene 5a and 1,2-bis(4-methoxyphenyl)ethan-1-one (6a) were used as model substrates for reaction optimization, which is summarized in Table 1. The initial reaction of 5a with 1.0 equiv. of 6a under Miura conditions15f (0.1 equiv. Pd(OAc)2, 0.2 equiv. PPh3 in anhydrous DMF at 120 °C for 12 h) provided 18% yield of benzo[b]furan product 7a (Table 1, entry 1). The reaction was highly regioselective for initiation at the least sterically hindered of C–I bonds and gave the 7-iodobenzo[b]furan product 7a as the sole isomer, with none of regioisomer 8a being observed. Changing the solvent to toluene improved the yield of the reaction to 27% (entry 2), whereas increasing the loading of both the ligand and catalyst only slightly increased the yields, to 29% and 32%, respectively (entries 3 and 4). The optimal reaction concentration was found to be 0.1 M (entries 5 and 6), and the addition of basic additives (e.g. Cs2CO3) proved detrimental (entries 7 and 8). Changing to the more reactive catalyst tetrakis(triphenylphosphine)palladium(0) provided 37% yield of benzo[b]furan product 7a (entry 9), and under these conditions, the addition of Cs2CO3 proved beneficial, providing 7a in 64% yield (entries 10–12). Raising the reaction temperature to 130 °C increased the yield to 75% (entry 13), and any further attempts to improve the reaction (solvent, base, reaction apparatus) were found to be unproductive (entries 14–17). A final reaction using the optimized conditions (entry 13) showed the tandem α-arylation/intramolecular O-arylation reaction also performs on gram scale, giving 7a in 67% yield (entry 18).
| Entry | Catalyst (mol%) | Ligand (mol%) | Base (equiv.) | Solvent [M] | T (°C) | Yield of 7ab (%) |
|---|---|---|---|---|---|---|
| a Conditions: All reactions were carried out using 0.65 mmol (1.0 equiv., 0.1 M) of 1,2,3-triiodobenzene 5 in 6.5 solvent.b Isolated yields.c Reflux was used.d 1.0 gram scale (2.19 mmol). | ||||||
| 1 | Pd(OAc)2 (10) | PPh3 (20) | NA | DMF, [0.2] | 120 | 18% |
| 2 | Pd(OAc)2 (10) | PPh3 (20) | NA | Toluene, [0.2] | 120 | 27% |
| 3 | Pd(OAc)2 (10) | PPh3 (30) | NA | Toluene, [0.2] | 120 | 29% |
| 4 | Pd(OAc)2 (20) | PPh3 (30) | NA | Toluene, [0.2] | 120 | 32% |
| 5 | Pd(OAc)2 (20) | PPh3 (30) | NA | Toluene, [0.15] | 120 | 34% |
| 6 | Pd(OAc)2 (20) | PPh3 (30) | NA | Toluene, [0.1] | 120 | 39% |
| 7 | Pd(OAc)2 (20) | PPh3 (30) | Cs2CO3 (2) | Toluene, [0.1] | 120 | 37% |
| 8 | Pd(OAc)2 (20) | PPh3 (30) | Cs2CO3 (3) | Toluene, [0.1] | 120 | 35% |
| 9 | Pd(PPh3)4 (20) | NA | NA | Toluene, [0.1] | 120 | 37% |
| 10 | Pd(PPh3)4 (20) | NA | Cs2CO3 (2) | Toluene, [0.1] | 120 | 54% |
| 11 | Pd(PPh3)4 (20) | NA | Cs2CO3 (3) | Toluene, [0.1] | 120 | 64% |
| 12 | Pd(PPh3)4 (20) | NA | Cs2CO3 (4) | Toluene, [0.1] | 120 | 65% |
| 13 | Pd(PPh3)4 (20) | NA | Cs2CO3 (3) | Toluene, [0.1] | 130 | 75% |
| 14 | Pd(PPh3)4 (20) | NA | Cs2CO3 (3) | Toluene, [0.2] | 130 | 61% |
| 15 | Pd(PPh3)4 (20) | NA | K2CO3 (3) | Toluene, [0.1] | 130 | 59% |
| 16 | Pd(PPh3)4 (20) | NA | Cs2CO3 (3) | O-Xylene, [0.1] | 130 | 51% |
| 17c | Pd(PPh3)4 (20) | NA | Cs2CO3 (3) | Toluene, [0.1] | 130 | 29% |
| 18d | Pd(PPh3)4 (20) | NA | Cs2CO3 (3) | Toluene, [0.1] | 130 | 67% |
Having identified optimal reaction conditions, we then investigated the scope of the α-arylation/intramolecular O-arylation reaction. 5-Substituted-1,2,3-triiodo-benzenes (5) were reacted with a series of acetophenone (6a–6c) and phenylacetone (6d, 6e) derivatives, and in each case they gave benzo[b]furan products 7 as the sole regioisomer (see Scheme 2). While the nature of the substituent (5, R1) was found to impact the reactivity observed in the domino process, it had no impact on the site-selectivity of the initial C–C bond forming step. For instance, 1,2,3-triiodoarenes bearing electron-poor/neutral substituents (5a, 5d, 5e, 5g) afforded higher isolated yields of the benzo[b]furan products (Scheme 2, 7a–7c, 7k, 7l and 7q), whereas substrates bearing electron-rich substituents (e.g. 5c) afforded moderate isolated yields of products 7h–7j. Switching from acetophenone to phenylacetone derivatives (e.g. 6d, 6e) was feasible, although this resulted in a general decrease in isolated yields of products 7d, 7j, 7n and 7o. Acetophenone derivatives possessing electron-rich substituents were found equivalent, if not slightly better than neutral derivatives, providing equivalent or higher isolated yields of the products (e.g. 7a vs. 7b, 7e vs. 7f, 7l vs. 7m). The highest isolated yield of the α-arylation/intramolecular O-arylation product was observed from electron-poor 1,2,3-triiodoarene (5d, 5g) reacting with electron-rich acetophenone 6a, giving products 7k and 7q. We also tested the reaction compatibility when one of the iodides was replaced with either bromide or chloride. The aryl chloride 5h reacted with acetophenone derivatives 6a and 6b to give products 7r and 7s in 77 and 71% yield, respectively. A similar test between aryl bromide 5i and acetophenones 6a and 6b also gave products 7t and 7u in 73 and 72% yield, respectively. In all these trials, no coupling was observed with either the bromo or chloro substituents, and the yields remained consistent with the analogous iodine-containing derivatives 7a or 7b.
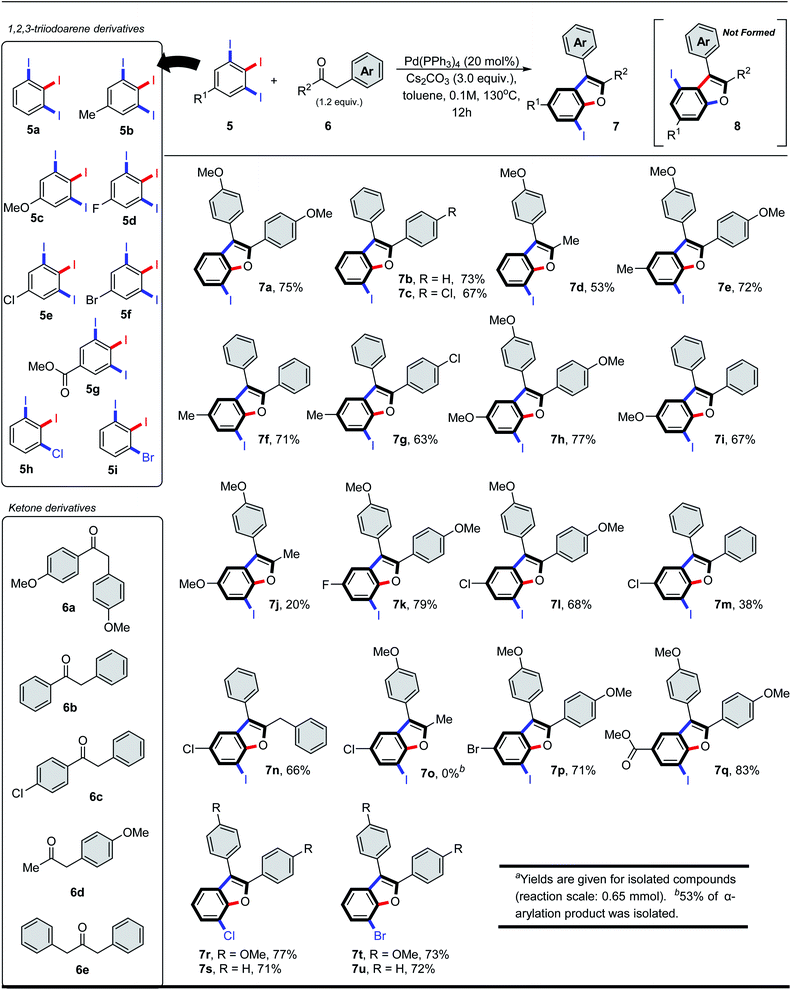 | ||
| Scheme 2 7-Iodinated benzofurans via regioselective tandem C–C/C–O arylations of 1,2,3-triiodobenzene and benzylketone derivative.a | ||
The regiochemical outcome for the 7-iodo-2,3-diarylbenzo[b]furan products was confirmed using X-ray diffraction methods for two C–C/C–O arylation products, 2-(4-chlorophenyl)-7-iodo-3-phenyl-1-benzo[b]furan 7c and 2-(4-chlorophenyl)-7-iodo-5-methyl-3-phenyl-1-benzo[b]furan 7g (Fig. 2).19
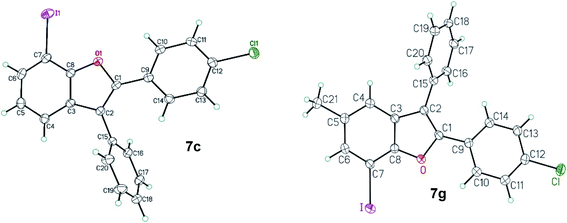 | ||
| Fig. 2 ORTEP view of 2-(4-chlorophenyl)-7-iodo-3-phenyl-1-benzo[b]furan 7c and 2-(4-chlorophenyl)-7-iodo-5-methyl-3-phenyl-1-benzo[b]furan 7g. Thermal Gaussian ellipsoids at 30% probability level. | ||
Based on our experimental results and on previously-reported mechanistic studies,15d–f,20 a reasonable catalytic cycle for the palladium-catalyzed domino C–C/C–O arylation reaction of 5-substituted-1,2,3-triiodobenzenes is proposed (Scheme 3). It begins with oxidative addition at the least sterically hindered C–I site of 5, giving PdII adduct A. Enolization of ketone 6 is followed by coordination with A and ligand exchange, giving PdII-intermediate B. Sequential reductive elimination, directed oxidative addition and deprotonation leads to PdII intermediate C. Intramolecular coordination with the enolate forms intermediate D, which can undergo reductive elimination to provide the desired benzo[b]furan 7 and regenerate the Pd0 catalyst for initiating another catalytic cycle.
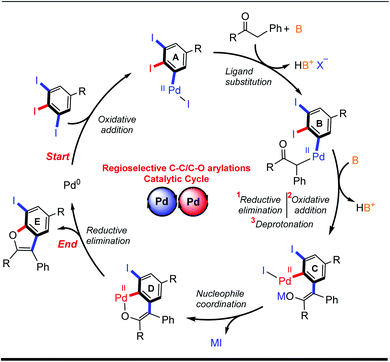 | ||
| Scheme 3 Proposed catalytic cycle for regioselective domino α-arylation/intramolecular O-arylation reaction of 5-substituted-1,2,3-triiodobenzene. | ||
In conclusion, a facile and unprecedented synthesis of 5-substituted-7-iodo-2,3-diarylbenzo[b]furan derivatives is reported. The reaction occurs via a highly regioselective, tandem α-arylation/intramolecular O-arylation between 5-substituted-1,2,3-triiodobenzenes and either acetophenone or phenylacetone derivatives, and the structures of the desired products were confirmed by X-ray diffraction. The reaction tolerated a variety of different of functional groups, and the products were isolated in yields up to 83%. The α-arylation reactions occurred solely at the terminal C–I bonds, as they were the least sterically-congested sites, facilitating the initial oxidative insertion by the catalyst.
In no instance was the regioisomeric product observed, and aryliodides possessing similarly-congested C–Br or C–Cl bonds were equally selective in their reactions. Finally, the highest isolated yields of the benzo[b]furan products were observed between electron-poor 1,2,3-triiodoarenes and electron-rich acetophenones. Various derivatives of 7 have already shown promising preliminary antimicrobial activities, and our results on this will be disclosed in due course.
Experimental
General
All commercial reagents and chromatography solvents were used as obtained unless otherwise stated. Ethanol, toluene, ethyl acetate, hexanes, anhydrous sodium sulfate (Na2SO4, BDH), Pd(PPh3)4 (Strem Chemicals). Anhydrous solvents were distilled over appropriate drying agents prior to use. Analytical thin layer chromatography (TLC) was performed on Merck silica gel 60 F254. Merck Silica gel 60 (0.063–0.2 mm) was used for column chromatography. Visualization of TLC was accomplished with UV light (254 nm). NMR spectra were recorded on a Bruker-Avance 400 MHz spectrometer. The residual solvent protons (1H) or the solvent carbon (13C) were used as internal standards. 1H-NMR data are presented as follows: chemical shift in ppm (δ) downfield from trimethylsilane (multiplicity, integration, coupling constant). The following abbreviations are used in reporting NMR data: s, singlet; bs, broad singlet; d, doublet; t, triplet; q, quartet; dq, doublet of quartets; dd, doublet of doublets; m, multiplet. High resolution mass spectra were recorded using Chemical Ionization (CI) and electrospray ionization (ESI) techniques.General procedure for palladium-catalyzed regioselective domino α-arylation/intramolecular O-arylation of 5-substituted-1,2,3-triiodoarenes and benzylketones
A flame-dried round bottom flask equipped with a condenser was charged with 5-substituted-1,2,3-triiodobenzene (5a–5i, 0.65 mmol, 1.0 equiv.), ketone (6a–6e, 0.78 mmol, 1.2 equiv.), tetrakis(triphenylphosphine)palladium(0) (20 mol%), cesium carbonate (3.0 equiv.) and 6.5 mL toluene (0.1 M). The reaction mixture was sealed with a septum, purged with argon and then heated to 130 °C for 12 h. The reaction was cooled to room temperature, diluted with 15 mL of distilled water and extracted with ethyl acetate (2 × 50 mL). The organic layers were combined and washed with brine, dried over Na2SO4, filtered and concentrated under reduced pressure. The crude product was purified by flash chromatography (15% EtOAc/hexanes) to yield the pure desired product.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was generously funded by the Jordan University of Science and Technology (JUST) – Deanship of Research – Jordan (Sabbatical grant No. 344/2018 for R. M. A.) and by the Department of Chemistry at the University of Waterloo and NSERC of Canada (NSERC, grant No. 2019-04086). We thank the Department of Chemistry at University of Alberta for X-ray crystallographic analysis.Notes and references
-
(a) J. Gao, R. Chen and H. Fan, CN111072685A, Hangzhou Normal University, Peop. Rep. China, 2020
; (b) H. Fan, S. Zou, J. Gao, R. Chen, Q. Ma, W. Ma, H. Zhang, G. Chen, X. Huo, Z. Liu, Y. Dang and W. Hu, J. Mater. Chem. C, 2020, 8, 11477–11484 RSC
; (c) J. H. Park, J. R. Noh and D. G. Ku, KR2019075555A, Chung-Ang University, Industry-Academic Cooperation Foundation, S. Korea, 2019
; (d) G. H. Rao, M. Pandey, K. Narayanaswamy, R. Srinivasa Rao, S. S. Pandey, S. Hayase and S. P. Singh, ACS Omega, 2018, 3, 13919–13927 CrossRef CAS
; (e) W. Hao, S. Zou, J. Gao, H. Zhang, R. Chen, H. Li and W. Hu, Org. Electron., 2018, 53, 57–65 CrossRef CAS
; (f) D. Chen, J. Li, W. Ma, B. Li, Y. Zhen, X. Zhu, W. Hu, H. Tsuji and E. Nakamura, Asian J. Org. Chem., 2018, 7, 2228–2232 CrossRef CAS
.
-
(a) M. N. Shah, M. F. Shah, J. Ma, M. I. Shah, Y. Yang and X. Pan, J. Mater. Sci., 2021, 56, 2528–2538 CrossRef CAS
; (b) P. Nagarjuna, A. Bagui, R. S. Rao, V. Gupta and S. P. Singh, ACS Appl. Energy Mater., 2019, 2, 1019–1025 CrossRef CAS
; (c) B. Liu, X. Wang, G. Wang, J. Liu, M. He, X. Hu, Y. Chen and F. Wang, CN110343237A, Hunan University of Arts and Science, Peop. Rep. China, 2019
; (d) P. Heinrichova, J. Pospisil, S. Stritesky, M. Vala, M. Weiter, P. Toman, D. Rais, J. Pfleger, M. Vondracek, D. Simek, L. Fekete, P. Horakova, L. Dokladalova, L. Kubac and I. Kratochvilova, J. Phys. Chem. C, 2019, 123, 11447–11463 CrossRef CAS
; (e) Z. Tang, B. Liu, A. Melianas, J. Bergqvist, W. Tress, Q. Bao, D. Qian, O. Inganaes and F. Zhang, Adv. Mater., 2015, 27, 1900–1907 CrossRef CAS PubMed
; (f) H. Kang, S. Y. An, B. Walker, S. Song, T. Kim, J. Y. Kim and C. Yang, J. Mater. Chem. A, 2015, 3, 9899–9908 RSC
.
-
(a) C. Sundin, C. E. Zetterstroem, D. D. Vo, R. Brkljaca, S. Urban and M. Elofsson, Sci. Rep., 2020, 10, 2103 CrossRef CAS PubMed
; (b) R. Brkljaca, H.-M. Dahse, K. Voigt and S. Urban, Nat. Prod. Commun., 2019, 14, 1–6 CrossRef
; (c) R. Brkljaca, J. M. White and S. Urban, J. Nat. Prod., 2015, 78, 1600–1608 CrossRef CAS PubMed
; (d) N. Amessis-Ouchemoukh, I. M. Abu-Reidah, R. Quirantes-Pine, C. Rodriguez-Perez, K. Madani, A. Fernandez-Gutierrez and A. Segura-Carretero, Phytochem. Anal., 2014, 25, 389–398 CrossRef CAS PubMed
; (e) A. E. Fructus, AF Consulting, France, 2005, FR2867977A1
; (f) K. S. Huang, M. Lin and G. F. Cheng, Phytochemistry, 2001, 58, 357–362 CrossRef CAS PubMed
.
-
(a) S. D. Brandt, H. M. Walters, J. S. Partilla, B. E. Blough, P. V. Kavanagh and M. H. Baumann, Psychopharmacology, 2020, 237(12), 3703–3714 CrossRef CAS
; (b) R. Roque Bravo, H. Carmo, F. Carvalho, M. d. L. Bastos and D. Dias da Silva, J. Appl. Toxicol., 2019, 39, 1083–1095 CrossRef CAS PubMed
; (c) J. Welter-Luedeke and H. H. Maurer, Ther. Drug Monit., 2016, 38, 4–11 CrossRef CAS PubMed
.
-
(a) Y. Gao, C. Ma, X. Feng, Y. Liu and X. Haimiti, Chem. Biodiversity, 2020, 17, e1900622 CrossRef CAS PubMed
; (b) Y.-h. Miao, Y.-h. Hu, J. Yang, T. Liu, J. Sun and X.-j. Wang, RSC Adv., 2019, 9, 27510–27540 RSC
; (c) S. Wang, B. Li, B. Liu, M. Huang, D. Li, L. Guan, J. Zang, D. Liu and L. Zhao, Bioorg. Med. Chem., 2018, 26, 4602–4614 CrossRef CAS PubMed
; (d) T. Ou, W. Peng, Z. Sun, Q. Zhang, S. Wang and Z. Huang, CN108530453A, Sun Yat-Sen University, Peop. Rep. China, 2018
; (e) S. M. Gomha, A. O. Abdelhamid, N. A. Abdelrehem and S. M. Kandeel, J. Heterocycl. Chem., 2018, 55, 995–1001 CrossRef CAS
; (f) L. Fu, J. Liu, F. Jiang, X. Jiang, J. Liu and W. Liu, CN102351852A, Shanghai Jiao Tong University, Peop. Rep. China, 2012
.
-
(a) M. Idrees, Y. G. Bodkhe, N. J. Siddiqui and S. S. Kola, Asian J. Chem., 2020, 32, 896–900 CAS
; (b) B. Thorat, M. Mandewale, B. Nazirkar, B. Kale, A. Beldar and R. Yamgar, World J. Pharm. Res., 2015, 4, 524–550 CAS
; (c) A. Hiremathad, M. R. Patil, K. R. Chethana, K. Chand, M. A. Santos and R. S. Keri, RSC Adv., 2015, 5, 96809–96828 RSC
; (d) R. Kenchappa, Y. D. Bodke, B. Asha, S. Telkar and M. Aruna Sindhe, Med. Chem. Res., 2014, 23, 3065–3081 CrossRef CAS
.
-
(a) M. M. M. El-Miligy, A. A. Hazzaa, H. El-Messmary, R. A. Nassra and S. A. M. El-Hawash, Bioorg. Chem., 2017, 72, 102–115 CrossRef CAS PubMed
; (b) L. Baumgartner, S. Sosa, A. G. Atanasov, A. Bodensieck, N. Fakhrudin, J. Bauer, G. Del Favero, C. Ponti, E. H. Heiss, S. Schwaiger, A. Ladurner, U. Widowitz, R. Della Loggia, J. M. Rollinger, O. Werz, R. Bauer, V. M. Dirsch, A. Tubaro and H. Stuppner, J. Nat. Prod., 2011, 74, 1779–1786 CrossRef CAS PubMed
; (c) G. Grewall, R. Scannell, X. Cai, M. Young and A. Fura, WO2003011848A1, Millennium Pharmaceuticals, Inc., USA, 2003
; (d) J. M. Janusz, P. A. Young, J. M. Ridgeway, M. W. Scherz, K. Enzweiler, L. I. Wu, L. Gan, J. Chen, D. E. Kellstein, S. A. Green, J. L. Tulich, T. Rosario-Jansen, I. J. Magrisso, K. R. Wehmeyer, D. L. Kuhlenbeck, T. H. Eichhold and R. L. M. Dobson, J. Med. Chem., 1998, 41, 3515–3529 CrossRef CAS
; (e) M. R. Hellberg, G. Graff, D. A. Gamache, J. C. Nixon and W. H. Garner, US5811438A, Alcon Laboratories, Inc., USA, 1998
.
-
(a) S. E. Yoo, K. Y. Lee, H. H. Seo, S. J. Kim, N. J. Kim and S. H. Lee, KR191898B1, Korea Research Institute of Chemical Technology, S. Korea, 1999
; (b) S.-e. Yoo, S.-H. Lee and S.-K. Kim, Bioorg. Med. Chem., 1997, 5, 445–459 CrossRef CAS PubMed
; (c) D. Middlemiss, S. P. Watson, B. C. Ross, M. D. Dowle, D. I. C. Scopes, J. G. Montana, P. Shah, G. C. Hirst, T. A. Panchal, J. M. S. Paton, M. Pass, T. Hubbard, J. Hamblett, K. S. Cardwell, T. I. Jack, G. Stuart, S. Coote, J. Bradshaw, G. M. Drew, A. Hilditch, K. L. Clark, M. J. Robertson, M. K. Bayliss, M. Donnelly, E. Palmer and G. R. M. Manchee, Bioorg. Med. Chem. Lett., 1993, 3, 589–594 CrossRef CAS
; (d) D. Middlemiss, S. P. Watson, B. C. Ross, M. D. Dowle, D. I. C. Scopes, J. G. Montana, G. C. Hirst, T. A. Panchal, J. M. S. Paton, T. Hubbard, G. Stuart, G. M. Drew, A. Hilditch, A. Travers, M. J. Robertson, A. A. E. Hunt, E. Pahner and G. R. Manchee, Bioorg. Med. Chem. Lett., 1993, 3, 2043–2046 CrossRef CAS
.
- G.-X. He, J.-M. Yuan, H.-M. Zhu, K. Wei, L.-Y. Wang, S.-L. Kong, D.-L. Mo, C.-X. Pan and G.-F. Su, Bioorg. Med. Chem. Lett., 2017, 27, 1660–1664 CrossRef CAS
.
- M. P. Bell, C. R. O'Dowd, J. S. S. Rountree, G. P. Trevitt, T. Harrison and M. M. McFarland, WO2011055115A1, Almac Discovery Limited, UK, 2011
.
-
(a) D. Noeteberg, E. Kallin and M. Wennerstaal, US20110112142A1, Karo Bio AB, Sweden, 2011
; (b) D. Noeteberg, E. Kallin and M. Wennerstaal, WO2009124968A1, Karo Bio AB, Sweden, 2009
.
- C. Philippo, G. Courtemanche, E. Fett, M. C. Orts, P. Bovy, S. E. O'Connor and A. M. Galzin, WO9732870A1, Synthelabo S. A. Inc., France, 1997
.
-
(a) C. Sreenivasulu, A. Gopi Krishna Reddy and G. Satyanarayana, Org. Chem. Front., 2017, 4, 972–977 RSC
; (b) J.-t. Liu, C. J. Simmons, H. Xie, F. Yang, X.-l. Zhao, Y. Tang and W. Tang, Adv. Synth. Catal., 2017, 359, 693–697 CrossRef CAS
; (c) J. Liao, P. Guo and Q. Chen, Catal. Commun., 2016, 77, 22–25 CrossRef CAS
; (d) H. K. Potukuchi, A. P. Spork and T. J. Donohoe, Org. Biomol. Chem., 2015, 13, 4367–4373 RSC
; (e) S. Agasti, S. Maity, K. J. Szabo and D. Maiti, Adv. Synth. Catal., 2015, 357, 2331–2338 CrossRef CAS PubMed
; (f) R. Zhu, J. Wei and Z. Shi, Chem. Sci., 2013, 4, 3706–3711 RSC
; (g) W. Zeng, W. Wu, H. Jiang, L. Huang, Y. Sun, Z. Chen and X. Li, Chem. Commun., 2013, 49, 6611–6613 RSC
; (h) J. Zhao, Q. Zhang, L. Liu, Y. He, J. Li, J. Li and Q. Zhu, Org. Lett., 2012, 14, 5362–5365 CrossRef CAS PubMed
; (i) F. Churruca, R. SanMartin, I. Tellitu and E. Dominguez, Eur. J. Org. Chem., 2005, 2481–2490 CrossRef CAS
; (j) Y. Hu, K. J. Nawoschik, Y. Liao, J. Ma, R. Fathi and Z. Yang, J. Org. Chem., 2004, 69, 2235–2239 CrossRef CAS PubMed
; (k) V. R. Veeramaneni, M. Pal and K. R. Yeleswarapu, Tetrahedron, 2003, 59, 3283–3290 CrossRef CAS
; (l) W. Ma, J. Huang, X. Huang, S. Meng, Z. Yang, C. Li, Y. Wang, T. Qi and B. Li, Org. Chem. Front., 2019, 6, 493–497 RSC
; (m) Z. Zhou, G. Liu, Y. Shen and X. Lu, Org. Chem. Front., 2014, 1, 1161–1165 RSC
.
- M. D. Collini, D. H. Kaufman, E. S. Manas, H. A. Harris, R. A. Henderson, Z. B. Xu, R. J. Unwalla and C. P. Miller, Bioorg. Med. Chem. Lett., 2004, 14, 4925–4929 CrossRef CAS PubMed
.
-
(a) M. Arisawa, S. Nakane, M. Kuwajima and M. Yamaguchi, Heterocycles, 2012, 86, 1103–1118 CrossRef CAS
; (b) M. Sekiguchi, Y. Saito and Y. Toya, JP2008094777A, Mitsui Chemicals Inc., Japan, 2008
; (c) M. Kawamura, T. Obikawa, S. Matsunami, I. Takada and Y. Kijima, Anthracene derivative and organic electroluminescent device using the same, WO2008143229A1, Idemitsu Kosan Co., Ltd., Japan, Sony Corporation, 2008, 95
; (d) M. C. Willis, D. Taylor and A. T. Gillmore, Tetrahedron, 2006, 62, 11513–11520 CrossRef CAS
; (e) M. C. Willis, D. Taylor and A. T. Gillmore, Org. Lett., 2004, 6, 4755–4757 CrossRef CAS PubMed
; (f) Y. Terao, T. Satoh, M. Miura and M. Nomura, Bull. Chem. Soc. Jpn., 1999, 72, 2345–2350 CrossRef CAS
; (g) C.-F. Zhu, C.-H. Gao, W.-J. Hao, Y.-L. Zhu, S.-J. Tu, D.-C. Wang and B. Jiang, Org. Chem. Front., 2020, 8, 127–132 RSC
.
-
(a) C. Yao, H. Sun, X. Wang, W. Liu, M. Li, B. Teng, F. Zhang and C. Shang, Application of amurensin H derivatives in preparing medicament for treating liver-related diseases, CN110433152A, Institute of Materia Medica, Chinese Academy of Medical Sciences, Peop. Rep. China, 2019, 31
; (b) X. Xu, J. Shi, C. Li, W. Zhang, X. Shen, X. Ma, W. Chen, L. Deng, X.-M. Li and Y. Guo, Chem. Nat. Compd., 2017, 53, 883–886 CrossRef CAS
; (c) T. S. Peat, O. Dolezal, J. Newman, D. Mobley and J. J. Deadman, J. Comput.-Aided Mol. Des., 2014, 28, 347–362 CrossRef CAS PubMed
; (d) E. Gallicchio, N. Deng, P. He, L. Wickstrom, A. L. Perryman, D. N. Santiago, S. Forli, A. J. Olson and R. M. Levy, J. Comput.-Aided Mol. Des., 2014, 28, 475–490 CrossRef CAS PubMed
.
-
(a) R. M. Al-Zoubi, K. T. Jaradat, W. K. Al-Jammal and R. McDonald, Synlett, 2020, 31, 953–958 CrossRef CAS
; (b) R. M. Al-Zoubi, M. K. Al-Omari, W. K. Al-Jammal and M. J. Ferguson, RSC Adv., 2020, 10, 16366–16376 RSC
; (c) R. M. Al-Zoubi, W. K. Al-Jammal and R. McDonald, New J. Chem., 2020, 44, 3612–3623 RSC
; (d) R. M. Al-Zoubi, A. Ibdah, W. K. Al-Jammal, M. S. Al-Zoubi, A. A. Almasalma and R. McDonald, Synthesis, 2018, 50, 384–390 CrossRef CAS
; (e) R. M. Al-Zoubi, M. S. Al-Zoubi, K. T. Jaradat and R. McDonald, Eur. J. Org. Chem., 2017, 5800–5808 CrossRef CAS
; (f) R. M. Al-Zoubi, M. S. Al-Zoubi, A. H. Abazid and R. McDonald, Asian J. Org. Chem., 2015, 4, 359–367 CrossRef CAS
; (g) R. M. Al-Zoubi, W. K. Al-Jammal, M. Y. El-Khateeb and R. McDonald, Eur. J. Org. Chem., 2015, 3374–3384 CrossRef CAS
.
-
(a) R. M. Al-Zoubi, W. K. Al-Jammal and R. McDonald, ChemistrySelect, 2020, 5, 2848–2853 CrossRef CAS
; (b) R. M. Al-Zoubi, H. Al-Mughaid and R. McDonald, Aust. J. Chem., 2015, 68, 912–918 CrossRef CAS
; (c) R. M. Al-Zoubi, H. Al-Mughaid, M. A. Al-Zoubi, K. T. Jaradat and R. McDonald, Eur. J. Org. Chem., 2015, 5501–5508 CrossRef CAS
; (d) R. M. Al-Zoubi, H. A. Futouh and R. McDonald, Aust. J. Chem., 2013, 66, 1570–1575 CrossRef CAS
.
- CCDC 2047366 and 2047367 contain the supplementary crystallographic data for compounds 7g and 7c respectively.†.
-
(a) G. Danoun, A. Tlili, F. Monnier and M. Taillefer, Angew. Chem., Int. Ed., 2012, 51, 12815–12819 CrossRef CAS PubMed
; (b) D. A. Culkin and J. F. Hartwig, Acc. Chem. Res., 2003, 36, 234–245 CrossRef CAS PubMed
; (c) J. M. Fox, X. Huang, A. Chieffi and S. L. Buchwald, J. Am. Chem. Soc., 2000, 122, 1360–1370 CrossRef CAS
; (d) M. Palucki and S. L. Buchwald, J. Am. Chem. Soc., 1997, 119, 11108–11109 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization, MS spectra, and copies of 1H and 13C NMR spectra for all new compounds. CCDC 2047366 and 2047367. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1ra05730h |
| This journal is © The Royal Society of Chemistry 2021 |