Open Access Article
Open Access ArticleAn ink-jet printed dual-CD ratiometric fluorescent paper-based sensor for the visual detection of Cu2+
Ying Lia,
Fei Lua,
Qing-zhi Lia,
Yi-hua Zhou *a,
Jun Qian
*a,
Jun Qian *a,
Sheng Caob and
Chen-yu Wanga
*a,
Sheng Caob and
Chen-yu Wanga
aSchool of Printing and Packaging, Wuhan University, Wuhan 430079, Hubei, China. E-mail: yihuazhou@whu.edu.cn; qianjungreat@whu.edu.cn; Tel: +86 13317169910 Tel: +86 18971135061
bWuhan Donghu University, Wuhan 430079, Hubei, China
First published on 7th October 2021
Abstract
Copper ion (Cu2+) plays an important role in the human body because it is beneficial for metabolism. However, an excessive or slight amount of Cu2+ can cause various symptoms. Therefore, it is necessary for human health to realize the trace and visual detection of Cu2+. Referring to traditional fluorescence test papers, the qualitative and semi-quantitative detection of Cu2+ could be realized by a dual-carbon dots (CDs) ratiometric fluorescent paper-based sensor with the advantages of environmental protection, portability and low cost. In this paper, the inkjet-printed test paper with the best mixing ratio of the two CDs has been researched to maximize the spectral energy transfer of ion detection (maximum color gamut expansion). Among them, the preparation method of b-CDs has been improved, increasing the photoluminescence quantum yield (PLQY) to 88.9%. The sensitivity detection limit of the double emission ratio sensor was 0.15 nM in solution, and the human eye can distinguish at least 3 μmol L−1 Cu2+ in the paper-based sensor. Compared with the traditional single-emission sensor, the human eye was more sensitive to the color change of the emission ratio sensor. The results indicate that we not only realized the micro detection of Cu2+ with convenient methods, but also provided a promising strategy for the visual detection of Cu2+.
1. Introduction
Copper ion (Cu2+) plays an essential role in the metabolism of the human body.1 However, excessive Cu2+ intake may not only lead to various symptoms but also cause some neurological diseases, including Alzheimer's disease and Parkinson's disease.2 Simultaneously, as heavy metal pollution in the living environment has increased in recent years, people must be vigilant about this metal ion in the surrounding environment. Therefore, it is very important to explore a probe that can detect the dose of Cu2+ in domestic water with high selectivity and accuracy.Fluorescence analysis is a method of qualitative or quantitative analysis of a target based on the change of a fluorescent signal; it has high analytical sensitivity and strong selectivity, and it has been applied to medical diagnostics,3 environmental monitoring,4 explosives detection,5 and food safety testing.6 Although fluorescent sensors can be explored without complicated sample pretreatments, large equipment, and well-trained technicians, the qualitative and quantitative assays on a target species still have to rely on the aid of a fine fluorescence spectrometer. To achieve the aim of the observation of qualitative and semiquantitative assays by the naked eye, the fluorescent probes must be highly sensitive to the remarkable variations in the spectrum, including color and brightness.7 Carbon dots (CDs) are fluorescent carbon nanometer materials under 10 nm in size; they were first discovered in 2004 during the purification of single-walled carbon nanotubes by electrophoresis.8 CDs have many advantages, such as good photostability, tunable emissive color, good solubility in water and good biological compatibility, which are very suitable for the requirements of fluorescent sensors. However, the preparation of b-CDs and the improvement of photoluminous quantum yield (PLQY) are problems to be overcome in the current related research.
Inkjet printing transfers tiny droplets (picoliters, 10−9 mL) of the printing material to the designated area by a non-contact method. With the development of inkjet printing technology, inkjet inks have expanded from traditional dyes and pigments to various functional materials. At present, inkjet printing has been applied in chemistry,9 biology,10 life sciences11 and printed electronics,12 and it is widely used in other fields. Because the fluorescence response of a single CD to copper ions is not obvious enough, we preferred the ratio fluorescence analysis method to construct the detection test paper. Ratiometric fluorescent analysis is a new detection method to determine the concentration of an analyte by measuring the fluorescence intensity at two (or more) different wavelengths under the same excitation wavelength and using the ratio as a characterization parameter. Ratiometric fluorescent paper-based sensors have attracted considerable attention as useful analytical instruments due to their visual detection, low cost, portability and easy operation. To construct a ratiometric fluorescent sensor that involves two or more fluorescence materials working together, the optimization of the ratio of the involved fluorescence materials is a key step. This ratio determination can be carried out by standard assays in solution, which is straightforward but time-consuming. Ink-jet printing can precisely control the proportions and patterns of multiple components, which can help to properly design and adjust the ratio of the fluorescence intensity of the sensor. In addition, the choice of carrier is very important for the sensitivity of ion detection. Some researchers usually choose filter paper as the carrier.7 However, filter paper cannot be used for accurate semi-quantitative testing because of its blue fluorescence.
Herein, red CDs (r-CDs) and blue CDs (b-CDs) were used to construct a ratio-type fluorescence sensor, which was used to realize a test paper with color visualization caused by the change of Cu2+. Among the two fluorescent groups, b-CDs were used as internal standard probes to enhance visual contrast, and r-CDs were used as sensing probes to achieve a linear fluorescence intensity change. Citric acid and ethylenediamine were used as precursors for preparing the b-CDs, and they were dispersed in ethanol. The PLQY of the b-CDs was increased to 88.9%. At the same time, to satisfy the high selectivity and high precision of Cu2+ sensing in the dual emission ratiometric fluorescent sensor, we present a simple, fast and precise method to construct fluorescent sensors with optimized ratios by ink-jet printing. Bond paper was used as the carrier of the CDs, which improved the visual inspection effect of the test paper. The preset color model processed by the printer created significant amounts of ratiometric fluorescent sensors with different ratios in a single run. We found that when the density ratio of the r-CDs/b-CDs probe was 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, the paper-based sensor had the largest color gamut. When the concentration of Cu2+ was added from 3 to 18 μmol L−1, the fluorescence intensity ratio showed a good linear relationship from 525 to 675 nm, and the detection limit reached 0.15 nM, which was lower than the maximum level of copper in drinking water (20 μmol L−1).
3, the paper-based sensor had the largest color gamut. When the concentration of Cu2+ was added from 3 to 18 μmol L−1, the fluorescence intensity ratio showed a good linear relationship from 525 to 675 nm, and the detection limit reached 0.15 nM, which was lower than the maximum level of copper in drinking water (20 μmol L−1).
2. Experimental
2.1 Reagents and instruments
ρ-Phenylenediamine (ρ-PDA), petroleum ether (PE), silica gel (xSiO2·yH2O), ethyl acetate (EA), citric acid (CA), ethylenediamine (EDA), disodium hydrogen phosphate (Na2HPO4·12H2O), sodium dihydrogen phosphate (NaH2PO4·2H2O), sodium chloride (NaCl), potassium chloride (KCl), ethanol, quartz sand, deionized water, and securities paper were purchased from Aladdin Ltd. All chemicals were used as received and did not require further purification. Deionized water (18.2 MΩ cm) was produced by a Millipore water purification system.A DZF-6020 vacuum dryer (Shanghai Xinmiao Co., Ltd.), AL-204-IC electronic analysis balance (METTLER-TOLEDO), HP Printer 2050 (China Hewlett–Packard Co., Ltd.), KQ-300DE ultrasound cleaner (Kunshan Instrument Co., Ltd.), H1650-W centrifuge (Hunan Instrument Co., Ltd.), FP-6500 fluorescence spectrometer (Jusco (Shanghai) Trading Co., Ltd), UV-3600 ultraviolet-visible spectrometer (Shimadzu Instruments and Equipment Co., Ltd.), TEM-2100 transmission electron microscope (Japan Electronics Co., Ltd.), FT-IR-5700 (Thermo Co., USA), X-ray photoelectron spectroscope (Thermo Fisher, UK), H-G lamp, Canon EOS 350D digital camera, and USB portable UV lamp (365 nm) were used.
2.2 Synthesis of r-CDs
According to the reported solvothermal method, r-CDs were prepared and slightly modified.13 ρ-PDA (0.5 g) was dissolved in 50 mL ethanol, and the solution was transferred to a polytetrafluoroethylene reactor. Then, it was heated in a vacuum drying chamber at 200 °C for 12 hours and cooled naturally to room temperature. The mixture was purified on a silica gel column and dried in a rotary evaporator using ethyl acetate as the eluent. The final product was obtained and the purified r-CDs were dispersed in 50 mL ethanol for further use.2.3 Synthesis of b-CDs
0.21 g (1 mmol) CA and 0.18 g (3 mmol) EDA were dissolved in 5 mL deionized water by magnetic stirring to obtain a clarified transparent solution.14 The solution was transferred to a 20 mL reactor and placed in an oven to heat to 160 °C for 4 h. The product was washed with a large amount of ethanol and centrifuged at 5000 rpm for 5 min. b-CDs were prepared by freeze-drying 24 hours after separation and purification, and the solid was ground into powder for use.2.4 Detection of Cu2+ by the ratiometric sensor
The double emission rate fluorescent probe was prepared by mixing r-CDs and b-CDs. They were mixed in 2 mL PBS buffer with different proportions and compared with each other. Because pH can affect the fluorescence performance of CDs, the pH of the solution was controlled at 7.4.15 Different concentrations of copper ions were added to the solution of a specific fluorescence probe, and the fluorescence spectrum was recorded by a fluorescence spectrometer after reaction for 3 minutes.2.5 Selectivity and interference tests
For the selectivity study of metal ions, other metal ions (including Ag+, Co2+, Ba2+, Al3+, Mn2+, Fe2+, K+, Na+, Zn2+, Ni+, and Cu2+) were added to 1 mL PBS buffer solution (with the fluorescence probe, pH 7.4). The final concentration was 10 μmol L−1, respectively.To observe the interference of the fluorescence probe with Cu2+, the metal ions (20 μmol L−1) above (1 mL) were added to 1 mL PBS buffer solution (pH 7.4) to achieve the final concentration (10 μmol L−1). Then, 1 mL Cu2+ (in PBS buffer solution) with a concentration of 10 μmol L−1 was added to the mixture, and the fluorescence spectrum was recorded again to detect the anti-interference ability of the probe.
2.6 Fabrication of paper-based sensors by ink-jet printing
In this study, a thermal ink-jet printer (HP 8020) was applied to print the CDs and metal ions. In general, the surface tension of the ink controlled the generation of tiny droplets (typically around several to tens of picoliters) and determined the spreading behavior of the ink on the substrate as well. The viscosity of the ink strongly affected the pinch-off of the droplet at the nozzle and the permeation of ink on the printing substrate. First, we developed two formulations of the CD inks (2 mg mL−1 of r-CDs, 10 mM PBS buffer solution, pH 7.4; 2 mg mL−1 of b-CDs, 10 mmol L−1 PBS buffer solution, pH 7.4) to obtain an appropriate surface tension and viscosity (in the range of 40 × 10−3 N m−1 and 4 mPa).16 The ink solutions retained their original properties after 24 h at room temperature.The ink cartridge was cleaned with deionized water until the ink residue was completely removed. The cartridge was dried in an oven at 60 °C for 3 hours and then taken out. The R-CDs and b-CDs fluorescent inks were injected into magenta (M) and cyan (C) ink cartridges, respectively. The red and blue color blocks with a density of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–10
1–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 were stacked respectively on the securities paper. The ink was printed on the stock paper by an ink-jet printer connected to a computer; the printing times were repeated 10 times (300 dpi was used). Finally, the stock paper showed strong color fluorescence under UV light of 365 nm.
10 were stacked respectively on the securities paper. The ink was printed on the stock paper by an ink-jet printer connected to a computer; the printing times were repeated 10 times (300 dpi was used). Finally, the stock paper showed strong color fluorescence under UV light of 365 nm.
To prove the selective response of the paper-based sensor, we directly ink-jet printed the ten metal ion solutions described in Section 2.5 on the prepared paper-based sensor, and we observed the fluorescence changes of the sensor's response to the ions.
Anti-interference of test paper detection: 0.1 mL of 10 μmol L−1 of the other ten metal ions were mixed, and then 1 mL Cu2+ (in PBS buffer solution) with different concentrations (0 μmol L−1, 6 μmol L−1, 12 μmol L−1, 18 μmol L−1) was added. The mixed solutions of the four concentrations were printed on the test paper, respectively, and the color changes were observed.
3. Results and conclusion
3.1 Characterization and optical properties of r-CDs/b-CDs
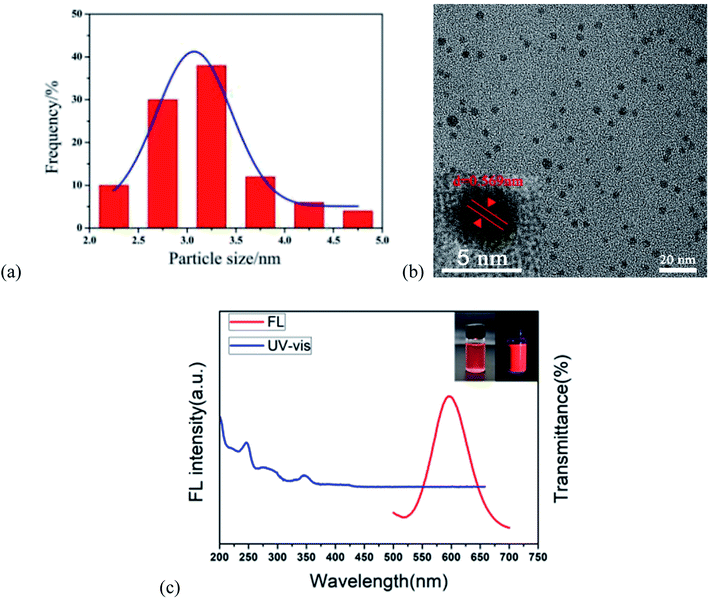
The properties of the r-CDs were characterized by transmission electron microscopy (TEM), UV-visible spectrophotometry (UV-Vis) and fluorescence spectroscopy (FL). As shown in Fig. 1a, the maximum particle size of the r-CDs reached 8.13 nm and the average particle size was 2.96 nm, with good monodispersity in ethanol and water. The crystal grains were uniformly dispersed in the solvent, and clear lattice fringes could be observed (0.569 nm, as shown in Fig. 1b). The FL spectra of the r-CDs showed an emission peak at 600 nm which was excited by 360 nm UV light (the red curve in Fig. 1c). The UV-vis spectra showed two characteristic absorption peaks centered at 246 and 345 nm, as well as the shoulder of the absorption curve at 276 nm (blue curve in Fig. 1c). These two peaks belonged to the π–π* transition of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N in the aromatic rings, which was the same as that of ρ-PDA.17–19 The weak band at 276 nm was attributed to the transition of the unshared electrons from the π–π conjugation of –NH2 and benzene rings.20 Light pink was observed in daylight, while bright orange-red fluorescence was observed in a 365 nm UV lamp (inset in Fig. 1c).
N in the aromatic rings, which was the same as that of ρ-PDA.17–19 The weak band at 276 nm was attributed to the transition of the unshared electrons from the π–π conjugation of –NH2 and benzene rings.20 Light pink was observed in daylight, while bright orange-red fluorescence was observed in a 365 nm UV lamp (inset in Fig. 1c).
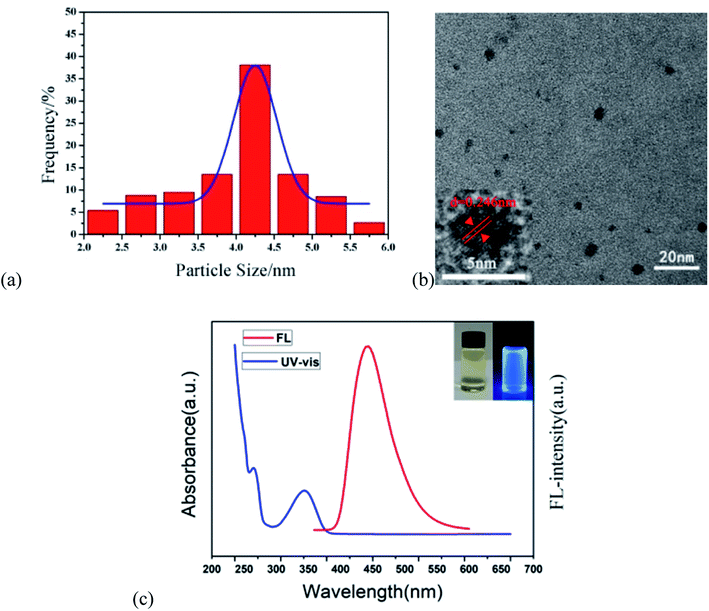
The b-CDs were prepared by the bottom-up hydrothermal method. Fig. 2a shows the good monodispersity of b-CDs in ethanol and water with an average particle size of 4.05 nm. It can be seen that the b-CDs were spherical in shape and dispersed uniformly in an aqueous solution without agglomeration (Fig. 2b). The lattice spacing of 0.246 nm indicated that the b-CDs had a graphite-like structure, as shown in the inset of Fig. 2a. The FL spectrum of the b-CDs showed blue emission centered at 443 nm with an excitation of 360 nm (the red line in Fig. 2c), and the PLQY was 88.9%. They were almost colorless and transparent under daylight and showed bright blue fluorescence under a 365 nm UV lamp (inset in Fig. 2c). The UV-vis spectra showed two characteristic absorption peaks at 260 and 352 nm (the blue curve in Fig. 2c); the absorption peaks at 260 nm were mainly due to the π–π* transition of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond, and the absorption peaks at 352 nm were due to the transition of n–π* of the C
C double bond, and the absorption peaks at 352 nm were due to the transition of n–π* of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bond.
O double bond.
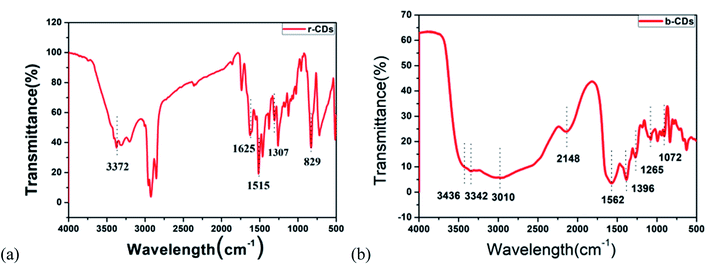
In addition, FTIR and XPS were used to study the possible chemical bonds and functional groups in the r-CDs. The FTIR spectrum in Fig. 3a shows a band at 3406 cm−1, which could be classified as the N–H stretching vibration. The other three peaks at 1625, 1515 and 1307 cm−1 are the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, C
N, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C–N
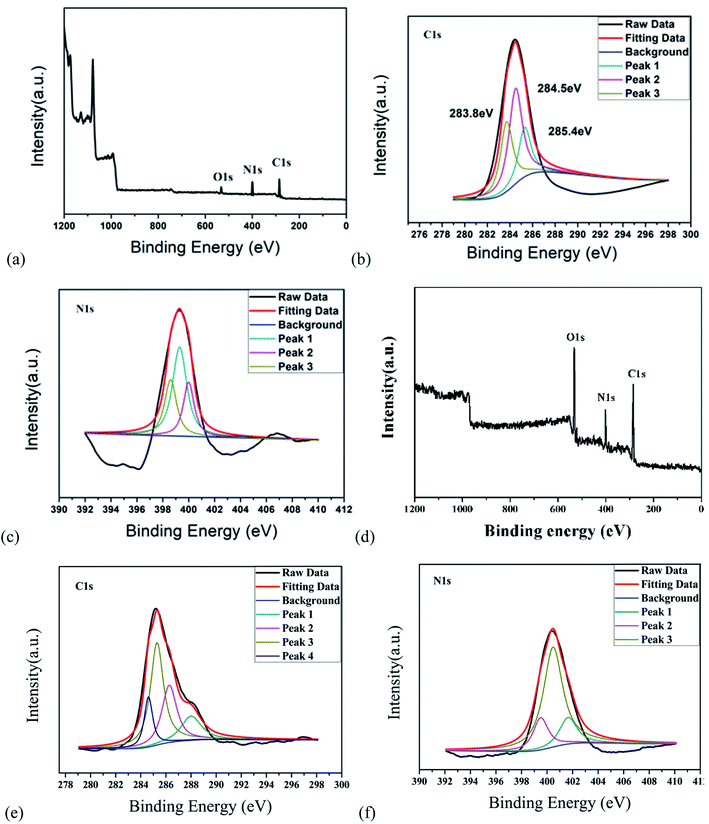
C and C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) tensile vibrations, respectively. The peak value at 829 cm−1 corresponds to the outer plane bending of the benzene ring.21–23 Furthermore, the deconvolution peaks at 398.6, 399.3 and 400 eV of the N1s band are associated with pyridine N, amino N and pyrrole N, respectively,24,25 as shown in Fig. 4a–c. Together with the UV-vis spectra, these measurements confirmed that incomplete carbonization resulted in a large number of ρ-PDA ligands remaining on the surface of the r-CDs.
tensile vibrations, respectively. The peak value at 829 cm−1 corresponds to the outer plane bending of the benzene ring.21–23 Furthermore, the deconvolution peaks at 398.6, 399.3 and 400 eV of the N1s band are associated with pyridine N, amino N and pyrrole N, respectively,24,25 as shown in Fig. 4a–c. Together with the UV-vis spectra, these measurements confirmed that incomplete carbonization resulted in a large number of ρ-PDA ligands remaining on the surface of the r-CDs.
As shown in Fig. 3b, the peak at 3436 cm−1 could be seen as the stretching vibration of the –C–H bond, which indicated that the b-CDs contained saturated alkyl groups. Moreover, the peak at 3342 cm−1 could be seen as the stretching vibration of the –OH bond. The strong peak at 3010 cm−1 could be seen as the vibration of the –NH2 bond. At the same time, the stretching vibration of the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O–NH (peptide) bond could be deduced at 1562 cm−1, and the stretching vibration of the –C–O bond could be deduced at 1396/1072 cm−1.26 The peak at 1265 cm−1 was due to the stretching vibration of the C–O bond.27 Compared with the characteristic peak position of the standard infrared spectrum, the characteristic peaks of the N–H bond and the C
O–NH (peptide) bond could be deduced at 1562 cm−1, and the stretching vibration of the –C–O bond could be deduced at 1396/1072 cm−1.26 The peak at 1265 cm−1 was due to the stretching vibration of the C–O bond.27 Compared with the characteristic peak position of the standard infrared spectrum, the characteristic peaks of the N–H bond and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond in the FT-IR spectrum belonged to the characteristic peak of the amide bond, which indicated that the b-CDs prepared in the research contain amide bonds.28 Fig. 4d, e and f show that the peaks at 284.6, 285.3, 286.28 and 288 eV (deconvolution of the C 1s bands of b-CDs) are associated with the bonds of –C−/−C
O bond in the FT-IR spectrum belonged to the characteristic peak of the amide bond, which indicated that the b-CDs prepared in the research contain amide bonds.28 Fig. 4d, e and f show that the peaks at 284.6, 285.3, 286.28 and 288 eV (deconvolution of the C 1s bands of b-CDs) are associated with the bonds of –C−/−C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–, –C–O–, –N–C
C–, –C–O–, –N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–, and –O–C
N–, and –O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O–, respectively.
O–, respectively.
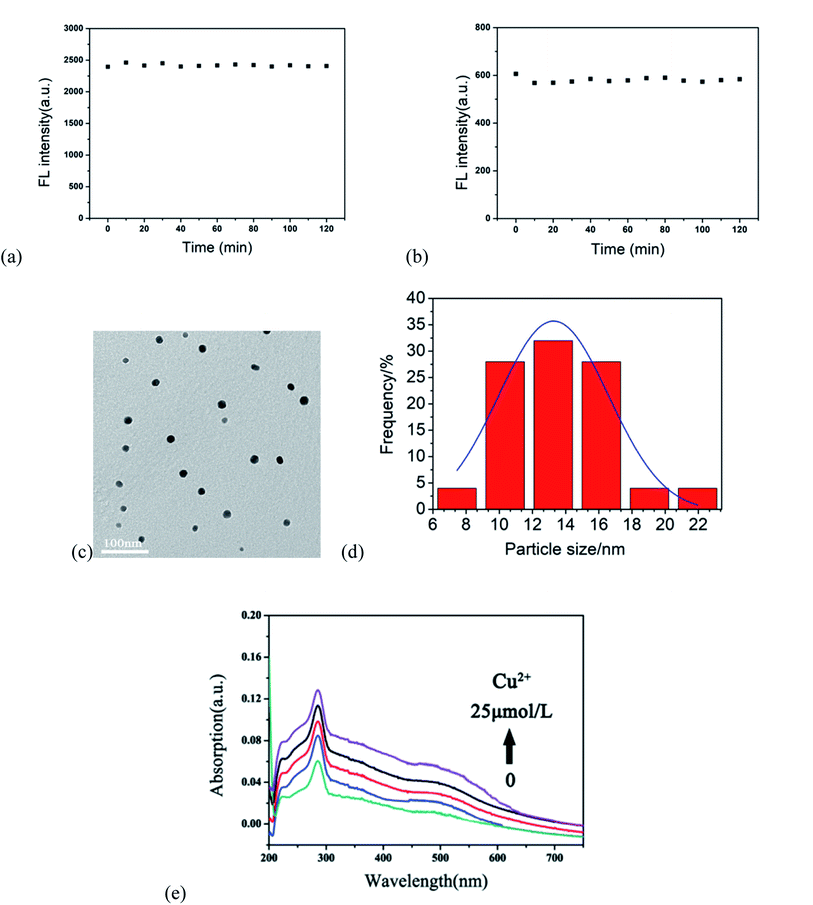
To explore the mechanism of Cu2+-induced fluorescence, the response of the r-CDs and b-CDs to Cu2+ was researched. Fig. 5a and b show the fluorescence intensity changes of the b-CDs and r-CDs in the presence of Cu2+ within 2 hours, respectively. The relative standard deviations of the linear fitting curves in Fig. 5a and b were 0.036 and 0.005, indicating the good optical stability of the two CDs. When Cu2+ was added to a mixture of b-CDs and r-CDs, the particle sizes of the r-CDs/b-CDs increased significantly. The average particle sizes of the r-CDs and b-CDs were 2.96 nm and 4.05 nm, respectively, while the particle size increased to 13.01 nm after adding Cu2+ (Fig. 5c and d). According to our conclusion, the increase in the ion size of the r-CDs/b-CDs was due to the coordination of Cu2+. The smaller r-CDs adsorbed on the surfaces of the larger b-CDs through the Cu2+, and the larger π-PDA ligand conjugation system led to the transfer of a π electron into the vacant d orbital of Cu2+.29,30 As shown in the UV-Vis spectrum in Fig. 5e, a new absorption band of 400–600 nm appeared in the r-CDs solution with increasing concentration of Cu2+. On the other hand, the UV-Vis spectrum of the b-CDs remained unchanged after Cu2+ was added. These results confirmed that when Cu2+ was added to the ratio probe, the charge was transferred from the ρ-PDA ligand to the surface of the r-CDs, resulting in the fluorescence quenching of the r-CDs.31–35
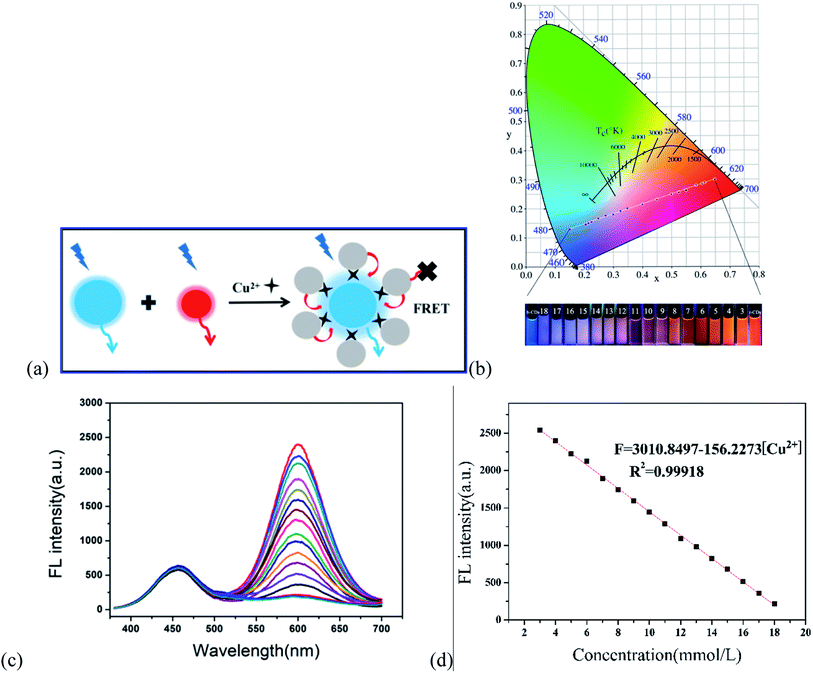
According to the principle of ligand metal charge transfer (LMCT) shown in Fig. 6a, the competitive binding of r-CDs and Cu2+ ions in solution led to aggregation of the r-CDs. The fluorescence spectra of b-CDs and r-CDs showed the maximum emissions at 443 and 600 nm, respectively (at the excitation wavelength of 360 nm). The fluorescence intensity ratio of r-CDs/b-CDs was changed for further study. It was found that the color gamut evolution of the double emission ratio probe was maximized only when the ratio was 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. In this case, the corresponding coordinates of the b-CDs and r-CDs were (0.15, 0.13) and (0.65, 0.3), respectively. The chromaticity coordinates of the r-CDs/b-CDs would change along the line between the two kinds of carbon dots in the chromaticity coordinate system, which was the basis of visual detection. Both nanoparticles could be excited at the same time, and double emission peaks were observed at 443 and 600 nm. At the same time, Fig. 6b shows the corresponding fluorescence color under a 365 nm UV lamp. Compared with b-CDs and r-CDs, the ratio sensor showed orange-red fluorescence, which indicated remarkable variations in the spectrum, including color and brightness. Moreover, the fluorescence intensity at 600 nm (F) in Fig. 6c decreased proportionately ranging from 3 to 18 μmol L−1 Cu2+ ions with good linearity, based on the correlation coefficient (R2) of 0.99918 (in Fig. 6d). The detection limit was 3 times the background standard deviation divided by the linear slope (3S/k), which was calculated as 0.15 nM.
1. In this case, the corresponding coordinates of the b-CDs and r-CDs were (0.15, 0.13) and (0.65, 0.3), respectively. The chromaticity coordinates of the r-CDs/b-CDs would change along the line between the two kinds of carbon dots in the chromaticity coordinate system, which was the basis of visual detection. Both nanoparticles could be excited at the same time, and double emission peaks were observed at 443 and 600 nm. At the same time, Fig. 6b shows the corresponding fluorescence color under a 365 nm UV lamp. Compared with b-CDs and r-CDs, the ratio sensor showed orange-red fluorescence, which indicated remarkable variations in the spectrum, including color and brightness. Moreover, the fluorescence intensity at 600 nm (F) in Fig. 6c decreased proportionately ranging from 3 to 18 μmol L−1 Cu2+ ions with good linearity, based on the correlation coefficient (R2) of 0.99918 (in Fig. 6d). The detection limit was 3 times the background standard deviation divided by the linear slope (3S/k), which was calculated as 0.15 nM.
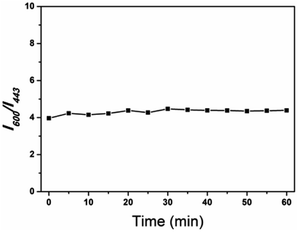
Moreover, the photobleaching tolerance of the ratio sensor was characterized by continuous irradiation of the H-G lamp with a 365 nm wavelength. As shown in Fig. 7, the ratio of the fluorescence intensity at 600 and 443 nm showed almost no change within 1 hour, and the relative standard deviation of the linear fitting curve was 0.063, indicating relatively high fluorescence stability.
3.2 Detection of Cu2+ with the R-CDs/R-CDs sensor
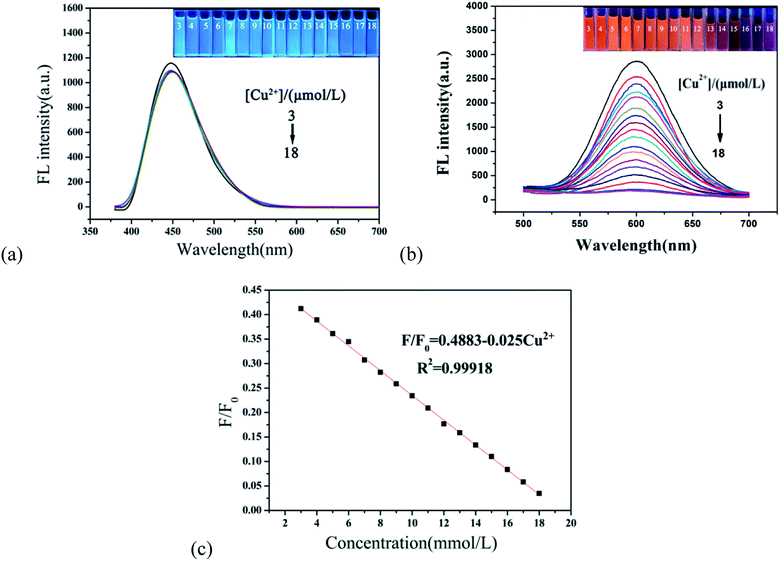
In order to evaluate the sensitivity of the r-CDs/b-CDs sensor to Cu2+, the fluorescence response of r-CDs/b-CDs solution (10 μmol L−1, pH 7.4) to Cu2+ with different concentrations from 0 to 50 μmol L−1 was measured.As shown in Fig. 8a and b, two distinct peaks at 600 nm and 443 nm can be recorded under a single excitation. The fluorescence intensity of the r-CDs decreased significantly with the addition of Cu2+, while the b-CDs were not affected. As shown in Fig. 8c, when the Cu2+ concentration changed from 3 to 18 μmol L−1, the ratio of the fluorescence intensity to the original fluorescence intensity (F/F0, F0 = 2400) of the sensor at 600 nm had a good linear relationship with the concentration of Cu2+. The linear equation was (F/F0) = 0.4883–0.025 Cu2+ (the correlation coefficient R2 = 0.99918). As shown in Fig. 8c, the detection limit was calculated to be not much different from that in Fig. 6. This test result was in full compliance with the EPA regulation that the maximum allowable content of Cu2+ in drinking water is 20 μM. This work showed that the r-CDs alone used to detect Cu2+ had no obvious fluorescence color change, so that it could not be distinguished by the naked eye, especially for low Cu2+ with a concentration range of 3 to 18 μmol L−1.
3.3 Optimizing experimental conditions for detecting Cu2+ with the ratio fluorescence paper-based sensor
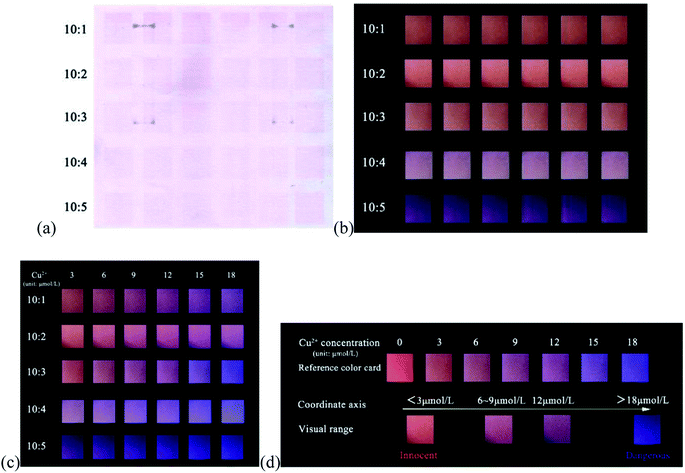
According to the above strategy, we prepared a fluorescent test paper for visual semi-quantitative detection of Cu2+ ions. However, when the dual-colored carbon dots with a fluorescence intensity ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (r-CDs/b-CDs) were transferred to the paper by ink-printing, it was found that the detection color gamut of Cu2+ only changed from purple to blue, and the visualization effect was not obvious, as shown in Fig. 9. The content of r-CDs was thus increased.
1 (r-CDs/b-CDs) were transferred to the paper by ink-printing, it was found that the detection color gamut of Cu2+ only changed from purple to blue, and the visualization effect was not obvious, as shown in Fig. 9. The content of r-CDs was thus increased.
 | ||
| Fig. 9 The range of color change reflected by Cu2+ with the same density value of r-CDs/b-CDs ink-jet printed on paper (compared with a reference color card). | ||
Therefore, two-color carbon dots with different density values were inkjet printed on paper: the neutral r-CDs and b-CDs (pH 7.4) were used as red and blue fluorescent inks, respectively, for ink-jet printing on securities paper to prepare the fluorescent test paper. The fluorescence brightness of the test paper could be enhanced by repeated inkjet printing 10 times. As shown in Fig. 10a, the test paper with fluorescent ink was light pink under sunlight and showed a highly uniform orange-red fluorescence under the irradiation of a 365 nm UV lamp. The concentration of Cu2+ in the water was tested by directly observing the color of the test paper under an ultraviolet lamp.
The fluorescence response of the ratio paper-based sensor depended on the emission intensity at 443 and 600 nm, which was closely related to the density of r-CDs and b-CDs in the inkjet printing. As shown in Fig. 10b, when the density ratio of r-CDs/b-CDs was 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 10
1 or 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (the content of r-CDs was high), the orange-red fluorescence of the detection paper was strong, which could completely shield the blue fluorescence. The Cu2+ concentration-related color change detected on the paper-based sensor was only close to purple. However, when the density ratio was 10
2 (the content of r-CDs was high), the orange-red fluorescence of the detection paper was strong, which could completely shield the blue fluorescence. The Cu2+ concentration-related color change detected on the paper-based sensor was only close to purple. However, when the density ratio was 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 or 10
4 or 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (the ratio of r-CDs became low), the red fluorescence was covered by the blue fluorescence of the b-CDs, and the Cu2+ concentration-related color change was not obvious. It can be seen that a too high or too low ratio of r-CDs/b-CDs may lead to very significant changes in the fluorescence color. In the ratio of 10
5 (the ratio of r-CDs became low), the red fluorescence was covered by the blue fluorescence of the b-CDs, and the Cu2+ concentration-related color change was not obvious. It can be seen that a too high or too low ratio of r-CDs/b-CDs may lead to very significant changes in the fluorescence color. In the ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, as shown in Fig. 10c, an obvious fluorescence color transition from orange to blue can be observed. Therefore, the r-CDs/b-CDs ratiometric probe with a pH of 7.4 and a density ratio of 10
3, as shown in Fig. 10c, an obvious fluorescence color transition from orange to blue can be observed. Therefore, the r-CDs/b-CDs ratiometric probe with a pH of 7.4 and a density ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 was regarded as the best conditions in the following experiments. When the detection concentration range of Cu2+ was in the range of 3–18 μmol L−1, there was a continuous evolution from orange-red to pink to purple to blue on the paper to realize visual detection. Fig. 10d can be used as a standard contrast color card to preliminarily determine the concentration range of Cu2+ detection by the naked eye.
3 was regarded as the best conditions in the following experiments. When the detection concentration range of Cu2+ was in the range of 3–18 μmol L−1, there was a continuous evolution from orange-red to pink to purple to blue on the paper to realize visual detection. Fig. 10d can be used as a standard contrast color card to preliminarily determine the concentration range of Cu2+ detection by the naked eye.
3.4 Sensor selectivity and interference detection of Cu2+
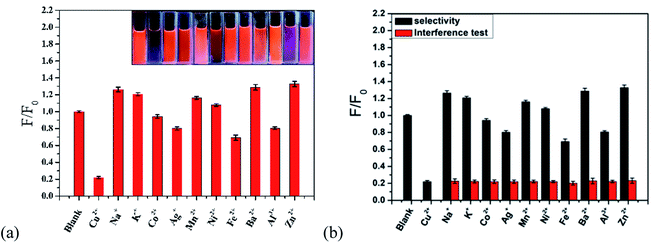
As shown in Fig. 11a, to research the selectivity of the r-CDs/b-CDs probe to Cu2+, the fluorescence intensity ratios (F/F0) under the same conditions with different metal ions were tested, including Ag+, Co2+, Ba2+, Al3+, Mn2+, Fe2+, K+, Na+, Zn2+, Ni+, and Cu2+. It can be seen that the fluorescence intensity of the ratiometric fluorescence probe was quenched by about 77.97% at 600 nm after adding 10 μmol L−1 Cu2+.The fluorescence intensity did not decrease significantly with other ions. In contrast, the fluorescence intensity ratio (F/F0) of the probe changed significantly after adding 10 μmol L−1 Cu2+. Obviously, the ratio probe can still detect Cu2+ in the presence of all possible interference ions. The excellent selectivity and excellent anti-interference can be attributed to the higher affinity of Cu2+ to ρ-PDA-r-CDs/b-CDs than of the other metal ions (Fig. 11b).
The fluorescence changes of the paper-based sensor with the different metal ions were tested to study the specific response of the ratio-based paper-based sensor to Cu2+. As shown in Fig. 12a, the fluorescence quenching effect of Cu2+ on the sensor was higher than that of other metal ions under the naked eye. To research the applicability of the test paper, the ten metal ion solutions were mixed with a certain concentration of Cu2+ solution. The fluorescent test paper showed obvious color responses to different Cu2+ concentrations (in Fig. 12a). It turned blue completely when the concentration was 18 μmol L−1. The results showed that our fluorescent test paper could meet the requirements of semi-quantitative detection of Cu2+ under the interference of other ions. It also shows the good selectivity of the paper-based sensor for Cu2+ detection and the possibility of the sensor to detect Cu2+ in practical applications.
 | ||
| Fig. 12 Anti-interference visual detection of the ratio fluorescent test paper for (a) single metal ions and (b) a mixed solution. | ||
4. Conclusion
First, CA and EDA were used to prepare b-CDs. They were dispersed in ethanol with a higher PLQY. A dual-color fluorescence ratio ratiometric test paper was made with r-CDs/b-CDs. B-CDs and r-CDs were used in inkjet printing at a density ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 to realize the fluorescence visualization detection of Cu2+. The orange-red fluorescence of the r-CDs at 600 nm could be quenched because of the competitive binding between ρ-PDA on the surface of the r-CDs and Cu2+ in solution. Because the blue fluorescence of b-CDs at 443 nm was almost unaffected, a continuous broad-color evolution from orange to blue can be observed when Cu2+ with an increased concentration was added under a 365 nm UV lamp. Single r-CDs and r-CDs/b-CDs were used to compare the results, and the intensity reduction of the single CDs was so insignificant that the naked eye could not distinguish it well, especially for a low concentration of Cu2+. Therefore, the dual-emission fluorescence paper-based sensor would be a good choice for Cu2+ vision detection. The ink-jet printing paper prepared with dual-colored CDs ink showed a dose-sensitive color response with a distinguishable scale as low as 3 μmol L−1 Cu2+, which could be observed by the naked eye. The sensor has a large change in fluorescence color difference in the range of 3–18 μmol L−1 Cu2+, which fully shows the possibility of semi-quantitative detection of Cu2+ by the paper-based sensor. Moreover, the filter paper was replaced with non-fluorescent bond paper, which increased the accuracy of the test paper. In addition, CDs are environmentally friendly compared to other tested substances and can be tested without recycling in the natural environment. The results of the paper suggest the unique optical properties of nanomaterials to develop promising visual test papers for environmental and medical applications.
10 to realize the fluorescence visualization detection of Cu2+. The orange-red fluorescence of the r-CDs at 600 nm could be quenched because of the competitive binding between ρ-PDA on the surface of the r-CDs and Cu2+ in solution. Because the blue fluorescence of b-CDs at 443 nm was almost unaffected, a continuous broad-color evolution from orange to blue can be observed when Cu2+ with an increased concentration was added under a 365 nm UV lamp. Single r-CDs and r-CDs/b-CDs were used to compare the results, and the intensity reduction of the single CDs was so insignificant that the naked eye could not distinguish it well, especially for a low concentration of Cu2+. Therefore, the dual-emission fluorescence paper-based sensor would be a good choice for Cu2+ vision detection. The ink-jet printing paper prepared with dual-colored CDs ink showed a dose-sensitive color response with a distinguishable scale as low as 3 μmol L−1 Cu2+, which could be observed by the naked eye. The sensor has a large change in fluorescence color difference in the range of 3–18 μmol L−1 Cu2+, which fully shows the possibility of semi-quantitative detection of Cu2+ by the paper-based sensor. Moreover, the filter paper was replaced with non-fluorescent bond paper, which increased the accuracy of the test paper. In addition, CDs are environmentally friendly compared to other tested substances and can be tested without recycling in the natural environment. The results of the paper suggest the unique optical properties of nanomaterials to develop promising visual test papers for environmental and medical applications.
Data availability
The data used to support the findings of this study are included within the article.Conflicts of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.Acknowledgements
The authors gratefully recognize the financial support from the Key Laboratory of Intelligent and Green Flexographic printing (ZBKT202002; ZBKT201903).References
- Y. J. Zhou, X. Y. Huang and C. Liu, et al., Color-Multiplexing-Based Fluorescent Test Paper: Dosage-Sensitive Visualization of Arsenic (III) with Discernable Scale as Low as 5 ppb, Anal. Chem., 2016, 88, 6105–6109 CrossRef CAS PubMed.
- C. Yuan, B. H. Liu and F. Liu, et al., Fluorescence “Turn On” Detection of Mercuric Ion Based on Bis(dithiocarbamato)copper (II) Complex Functionalized Carbon Nanodots, Anal. Chem., 2014, 86, 1123–1130 CrossRef CAS PubMed.
- Y. H. Wang, C. Zhang and X. C. Chen, et al., Ratiometric fluorescent paper sensor utilizing hybrid carbon dots-quantum dots for the visual determination of copper ions, Nanoscale, 2016, 8, 5977–5984 RSC.
- X. Y. Huang, Y. J. Zhou and C. Liu, et al., A single dual-emissive nanofluorophore test paper for highly sensitive colorimetry-based quantification of blood glucose, Biosens. Bioelectron., 2016, 86, 530–535 CrossRef CAS PubMed.
- C. L. Jiang, B. H. Liu, M. Y. Han and Z. P. Zhang, et al., Fluorescent Nanomaterials for Color-Multiplexing Test Papers toward Qualitative/Quantitative Assays, Small Methods, 2019, 2, 1700379 CrossRef.
- X. Liu, N. Zhang and T. Bing, et al., Carbon Dots Based Dual-Emission Silica Nanoparticles as a Ratiometric Nanosensor for Cu2+, Anal. Chem., 2014, 85, 2289–2296 CrossRef PubMed.
- C. Liu, D. Ning and C. Zhang, et al., Dual-colored carbon dot ratiometric fluorescent test paper based on a specific spectral energy transfer for semiquantitative assay of copper ions, ACS Appl. Mater. Interfaces, 2017, 22, 18897–18903 CrossRef PubMed.
- H. J. Plaehn, Y. L. Gu, K. Kaker, L. Gearheart and R. Ray, et al., Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments, J. Am. Chem. Soc., 2004, 40, 12736–12737 Search PubMed.
- E. B. Secor and M. C. Hersam, Emerging Carbon and Post-Carbon Nanomaterial Inks for Printed Electronics, J. Phys. Chem. Lett., 2015, 4, 620–626 CrossRef PubMed.
- J. Li, F. Rossignol and J. Macdonald, Inkjet printing for biosensor fabrication: combining chemistry and technology for advanced manufacturing, Lab Chip, 2015, 12, 2538–2558 RSC.
- A. L. Rutz, K. E. Hyland and A. E. Jakus, et al., A Multimaterial Bioink Method for 3D Printing Tunable, Cell-Compatible Hydrogels, Adv. Mater., 2015, 27, 1607–1614 CrossRef CAS PubMed.
- J. P. Lombardi, R. S. Aga and E. M. Heckman, et al., Characterization of DNA biopolymer-based UV photodetector fabricated by inkjet printing, Electron. Lett., 2015, 51, 778–780 CrossRef CAS.
- K. Jiang, S. Sun, L. Zhang, Y. Lu, A. G. Wu, C. Z. Cai and He. W. Lin, Red, Green, and Blue Luminescence by Carbon Dots: Full-Color Emission Tuning and Multicolor Cellular Imaging, Angew. Chem., Int. Ed., 2015, 54, 5360–5363 CrossRef CAS PubMed.
- H. Ding, S. B. Yu and J. S. Wei, et al., Full-Color Light-Emitting Carbon Dots with a Surface-State-Controlled Luminescence Mechanism, ACS Nano, 2015, 10, 484–491 CrossRef PubMed.
- C. Zheng, X. Q. An and J. Gong, Novel pH sensitive N-doped carbon dots with both long fluorescence lifetime and high quantum yield, RSC Adv., 2015, 5, 32319–32322 RSC.
- W. H. Zhou. Research on the coating characteristics and printing performance of inkjet printing paper based on optical recognition. South China University of Technology, 2014 Search PubMed.
- D. Qu. Preparation and application of doped graphene quantum dots. Changchun Institute of Optics, Fine Mechanics and Physics, Chinese Academy of Sciences. 2017 Search PubMed.
- Jaidev and S. Ramaprabhu, Poly(p-phenylenediamine)/graphene nanocomposites for supercapacitor applications, J. Mater. Chem., 2012, 22, 18775 RSC.
- Y. L. Min, T. Wang and Y. G. Zhang, et al., The synthesis of poly(p-phenylenediamine) microstructures without oxidant and their effective adsorption of lead ions, J. Mater. Chem., 2011, 21, 6683–6690 RSC.
- A. Karimzadeh, M. Hasanzadeh and N. Shadjou, et al., Electrochemical biosensing using b-CDs: Recent advances in analytical approach, TrAC, Trends Anal. Chem., 2018, 105, 484–491 CrossRef CAS.
- J. Soleymani, M. Hasanzadeh and M. H. Somi, et al., Targeting and sensing of some cancer cells using folate bioreceptor functionalized nitrogen-doped graphene quantum dots, Int. J. Biol. Macromol., 2018, 118, 1021–2034 CrossRef CAS PubMed.
- Z. Y. Li and T. Bian, et al., Highly luminescent nitrogen-doped carbon quantum dots as effective fluorescent probes for mercuric and iodide ions, J. Mater. Chem. C, 2015, 3, 1922–1928 RSC.
- J. M. Liu, L. P. Lin and X. X. Wang, et al., Highly Selective and Sensitive Detection of Cu2+ with Lysine Enhancing Bovine Serum Albumin Modified-Carbon Dots Fluorescent Probe, Analyst, 2012, 137, 2637–2642 RSC.
- Y. Dong, R. Wang and G. Li, et al., Polyamine-Functionalized Carbon Quantum Dots as Fluorescent Probes for Selective and Sensitive Detection of Copper Ions, Anal. Chem., 2012, 84, 6220–6224 CrossRef CAS PubMed.
- C. M. Jones, C. R. Johnson and S. A. Asher, et al., Resonance Raman Studies of the Excited Electronic States of (CN)5FeIII(imidazole)2– and (NH3)5RuIII(imidazole)3+, J. Am. Chem. Soc., 1985, 107, 3772–3780 CrossRef CAS.
- E. Bernarducci, P. K. Bharadwaj and K. Krogh-Jespersen, et al., Electronic Structure of Alkylated Imidazoles and Electronic Spectra of Tetrakis(imidazole)copper (II) Complexes, J. Am. Chem. Soc., 1983, 105, 3860–3866 CrossRef CAS.
- E. Bernarducci, W. F. Schwindinger and J. L. Hughey, et al., Electronic Spectra of Copper (II)-Imidazole and Copper (II)-Pyrazole Chromophores, J. Am. Chem. Soc., 1981, 103, 1686–1691 CrossRef CAS.
- K. Krogh-Jesperse and H. J. Schugar, Detailed Correlations between the Ligand-to-Metal Charge-Transfer (LMCT) Spectra of Copper (II) and Ruthenium (III) Imidazoles and Imidazolates. Electronic Structures of Carbon-Bound Ruthenium (III) Imidazoles and Imidazolates, Inorg. Chem., 1984, 23, 4390–4393 CrossRef.
- T. G. Fawcett, E. Bernarducci and K. Krogh-Jespersen, Charge-Transfer Absorptions of Cu (II)-Imidazole and Cu (II)-Imidazolate Chromophores, J. Am. Chem. Soc., 1980, 102, 2598–2604 CrossRef CAS.
- L. Zhang, H. Wang and W. Yu, et al., Facile and large-scale synthesis of functional poly(m-phenylenediamine) nanoparticles by Cu2+-assisted method with superior ability for dye adsorption, J. Mater. Chem., 2012, 22, 18244–18251 RSC.
- Y. Dong, H. Pang and H. B. Yang, et al., Carbon-Based Dots Co-doped with Nitrogen and Sulfur for High Quantum Yield and Excitation-Independent Emission, Angew. Chem., Int. Ed., 2013, 52, 7800–7804 CrossRef CAS PubMed.
- Y. Song, S. Zhu and S. Xiang, et al., Investigation into the fluorescence quenching behaviors and applications of carbon dots, Nanoscale, 2014, 6, 4676 RSC.
- R. Ye, C. Xiang and J. Lin, et al., Coal as an abundant source of graphene quantum dots, Nat. Commun., 2013, 4, 2943 CrossRef PubMed.
- Q. Mei, H. Jing and Y. Li, et al., Smartphone based visual and quantitative assays on upconversional paper sensor, Biosens. Bioelectron., 2016, 75, 427 CrossRef CAS PubMed.
- H. Ding, S. B. Yu and J. S. Wei, et al., Full-Color Light-Emitting Carbon Dots with a Surface-State-Controlled Luminescence Mechanism, ACS Nano, 2016, 10, 484 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2021 |