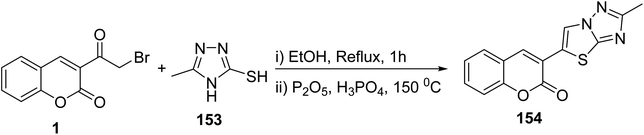Open Access Article
Open Access Article3-(Bromoacetyl)coumarins: unraveling their synthesis, chemistry, and applications
Moaz M. Abdou *a,
Ahmed Abu-Rayyanb,
Ahmed G. Bedira,
S. Abdel-Fattaha,
A. M. A. Omara,
Abdullah A. Ahmedc,
El-Sayed I. El-Desokyd and
Eslam A. Ghaithd
*a,
Ahmed Abu-Rayyanb,
Ahmed G. Bedira,
S. Abdel-Fattaha,
A. M. A. Omara,
Abdullah A. Ahmedc,
El-Sayed I. El-Desokyd and
Eslam A. Ghaithd
aEgyptian Petroleum Research Institute, Nasr City, Cairo, 11727, Egypt. E-mail: moaz.chem@gmail.com
bFaculty of Science, Applied Science Private University, P. O. BOX 166, Amman 11931, Jordan
cDepartment of Chemistry, Faculty of Science, Al-Azhar University, Cairo, 11884, Egypt
dDepartment of Chemistry, Faculty of Science, Mansoura University, Mansoura, 35516, Egypt
First published on 29th November 2021
Abstract
This review emphasizes recent developments in synthetic routes of 3-(bromoacetyl)coumarin derivatives. Also, chemical reactions of 3-(bromoacetyl)coumarins as versatile building blocks in the preparation of critical polyfunctionalized heterocyclic systems and other industrially significant scaffolds are described. Recent advances of 3-(bromoacetyl)coumarins as attractive starting points towards a wide scale of five and six-membered heterocyclic systems such as thiophenes, imidazoles, pyrazoles, thiazoles, triazoles, pyrans, pyridines, thiadiazins as well as fused heterocyclic systems have been reported. Additionally, this review covers a wide range of analytical chemistry, fluorescent sensors, and biological applications of these moieties, covering the literature till May 2021.
1. Introduction
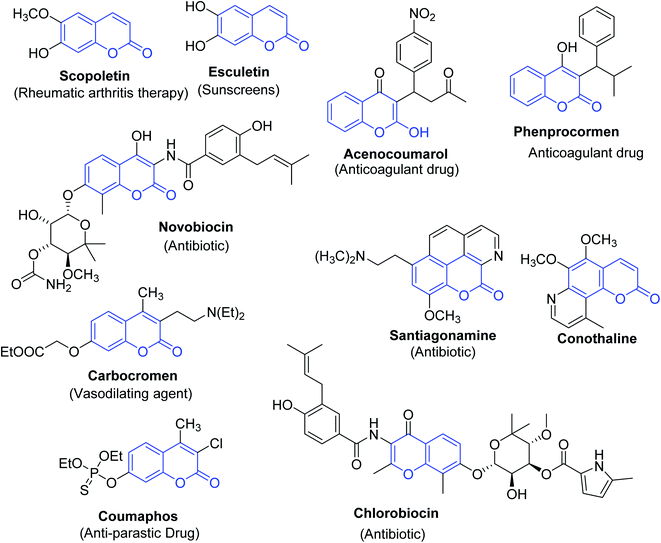
Coumarins are one of the most common host heterocyclic systems reported in the literature of organic chemistry.1,2 Furthermore, coumarins and their derivatives are seen to be the pivotal components of a plethora of many natural products and pharmaceuticals3 and synthetic dyes.4–9 The pharmacological activities discovered amongst coumarin derivatives include the treatment categories of Alzheimer's10 and haematopoietic necrosis (IHN);11 they have shown potent anticoagulant, antibiotic, antiembolic, antioxidative, and anti-ischemic activities12–16 (Fig. 1).Among these compounds, 3-(bromoacetyl)coumarin 1 and its derivatives are a prominent structural class in the synthesis of various bioactive heterocyclic scaffolds,17,18 they also are important components in drug discovery on account of their biological activities such as antiproliferative, antimicrobial activities,19 and are promising inhibitors of type 2 diabetes mellitus.20 In addition, numerous chemosensors are based on polyfunctional coumarin platforms used to detect multianalyte detection, such as different bioactive elements and various environmental pollutants.21,22 There is no survey available on the biological and chemical applications achieved since the discovery of 3-(bromoacetyl)coumarins. The articles on this type of coumarin are scattered in scientific journals.
In continuation of our investigations on the chemistry of coumarins and their azo/thio isosteric analogs23–28 and based on the above mentioned interesting biological and chemical aspects, this survey mainly highlights the advances in the synthesis of 3-(bromoacetyl)coumarin and its derivatives, besides, their transformations for the construction of different fused heterocyclic systems in detail. Additionally, a wide range of analytical chemistry, fluorescent sensors, and biological applications of these moieties are summarized.
2. Spectral data
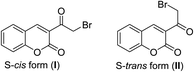
Many papers have reported the spectroscopic measurements (IR, 1H NMR, 13C NMR, and Mass) of 3-(bromoacetyl)coumarin.29,30 As IR spectrum of 3-(bromoacetyl)coumarin showed the characteristic ketonic group band at 1674, while C–H stretching vibrations at the aromatic region 3100–3000 cm−1 (ref. 29) and two carbonyl characteristic peaks at ν 1674 and 1729 cm−1 related to α,β-unsaturated ketonic and lactonic, respectively.31 1H NMR spectrum of parent 3-(bromoacetyl)coumarin 1 shows singlet signal of H-4 at δ = 8.63 ppm, while the CH2 group appears as singlet signal at δ = 4.74 ppm. Also, 13C NMR spectrum of 3-(bromoacetyl)coumarin exhibits characteristic signals at δ = 188.9, 158.9, and 35.6 ppm corresponding to α,β-unsaturated ketonic, lactonic and methylene carbons, respectively.30 In the same context, HRMS/MS is mentioned as characteristic spectrometric data for 3-(bromoacetyl)coumarin 1 shows that m/z 266.9665 (calcd. for C11H879BrO3 [M + H]+ 266.9657).30In 1991, Vasudevan et al.32 elucidated the structure 3-(bromoacetyl)coumarin 1 through its single-crystal X-ray, which showed that there are two conformers of the structure 1, S-cis (I) or S-trans (II) (Fig. 2).
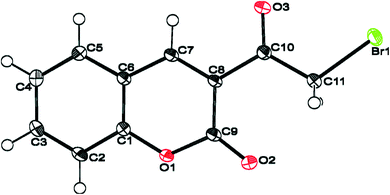
Moreover, Sparkes and coworkers33 reported a polymorph of 3-(bromoacetyl)coumarin (Fig. 3). Whereas, Chennuru et al.34 reported a single-crystal X-ray of 6-chloro-3-(bromoacetyl)coumarin (Fig. 4).
 | ||
| Fig. 3 ORTEP diagram of 3-(bromoacetyl)coumarin 1 [reprinted from ref. 33]. | ||
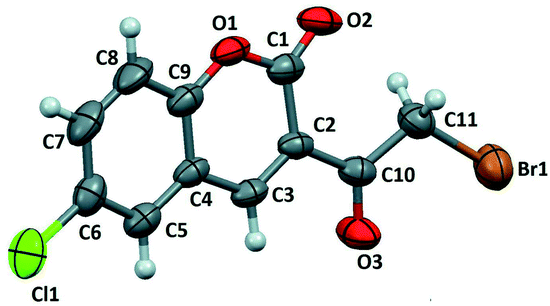 | ||
| Fig. 4 ORTEP diagram of 6-chloro-3-(bromoacetyl)coumarin [reprinted from ref. 34]. | ||
3. Synthesis
3.1. Using 3-acetylcoumarins
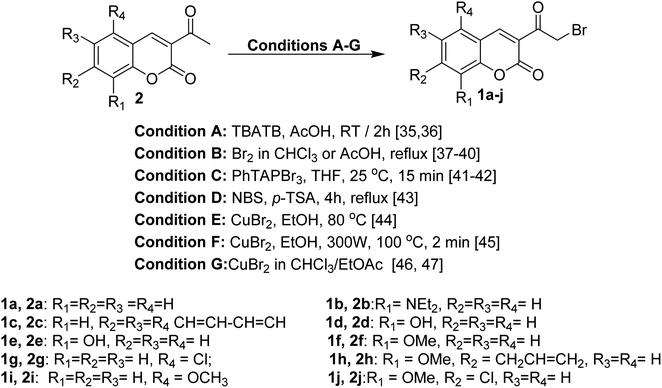
The reaction of 3-acetylcoumarins 2 with numerous reagents represents a general approach to preparing 3-bromoacetyl coumarin derivatives 1. Several brominating agents have been reported in the last two decades such as tetrabutylammonium tribromide (TBATB), bromine, phenyltrimethylammonium tribromide (PhTAPBr3), N-bromosuccinimide (NBS), and copper(II) bromide (CuBr2) (Scheme 1).35–474. Reactivity
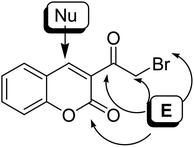
On the treatment of 3-(bromoacetyl)coumarin 1 with various nucleophiles, four possible electrophilic positions are susceptible to attack: the exo-carbonyl group (position 1), bromomethanide group (CH2Br) (position 2), lactonic carbonyl group (position 3) and the bromo atom (position 4) susceptible to attack (Fig. 5). Besides, the typically nucleophilic position for attacking is carbon 4. The reactivity of α-bromoacetylcoumarin towards oxygen, nitrogen, and sulphur nucleophiles is discussed in this review.5. Reactions
5.1. Amination
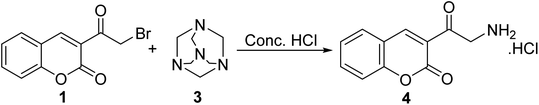
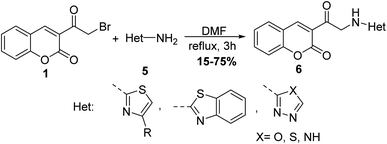
Sinnur et al.48 reported a short and efficient synthesis for aminomethyl-3-coumarinyl ketone hydrochloride 4 via refluxing 3-(bromoacetyl)coumarin 1 with hexamethylenetetramine 3 in drops of concentrated hydrochloric acid (Scheme 2).Moreover, 3-(bromoacetyl)coumarin 1 was condensed with an amino group of various heterocyclic derivatives 5 such as 2-aminothiazole, 2-aminobenzothiazole, 2-amino-1,3,4-oxadiazole, 2-amino-1,3,4-thiadiazole, and 3-amino-4H-1,2,4-triazole derivatives in DMF to give the corresponding 2H-chromen-2-ones 6 (Scheme 3).49
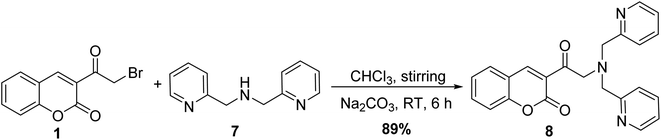
Treatment of 3-(bromoacetyl)coumarin 1 with di(2-picolyl)amine 7 in chloroform under basic condition at room temperature afforded the corresponding 3-(bis(pyridin-2-ylmethyl)glycyl)-2H-chromen-2-one 8 (Scheme 4).50,51
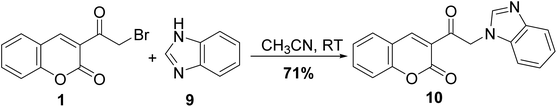
Selective nucleophilic substitution of 3-(bromoacetyl)coumarin 1 was accomplished through stirring with benzimidazole 9 in acetonitrile at ambient temperature afforded corresponding imidazole-1-carbonyl-chromenone 10 (Scheme 5).52
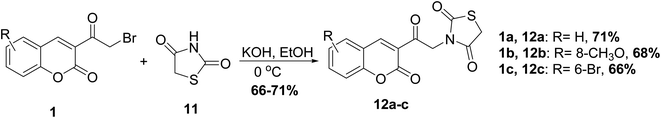
Valadbeigi et al.53 reported the synthesis of thiazolidinedione derivatives 12 through heating of 3-(bromoacetyl)coumarin 1 with thiazolidine-2,4-dione 11 in alcoholic potassium hydroxide (Scheme 6).
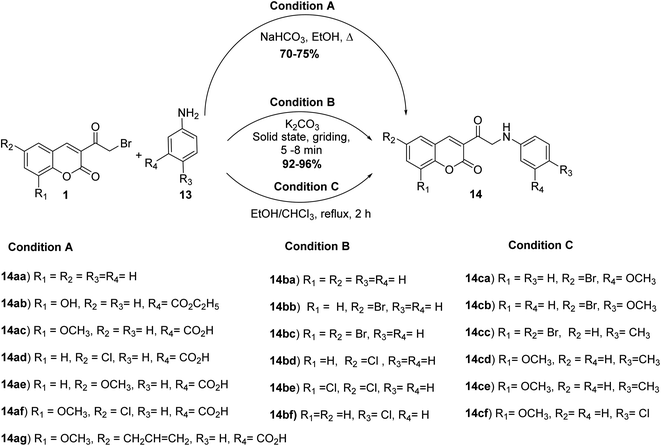
The reaction of the 3-(bromoacetyl)coumarin derivatives 1 with substituted arylamine 13 in ethanol in the absence54 or the presence of sodium bicarbonate41,55,56 or under solvent-free condition using K2CO3 (ref. 57) yielded the corresponding 3-(2-(phenylanliino)acetyl)-2H-chromen-2-ones 14 (Scheme 7).
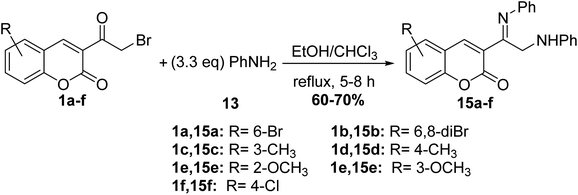
Whereas, refluxing of 3-(bromoacetyl)coumarin derivatives 1 with arylamines 13 in a mixture of ethanol and chloroform afforded the corresponding imino derivatives 15a-f (Scheme 8).54
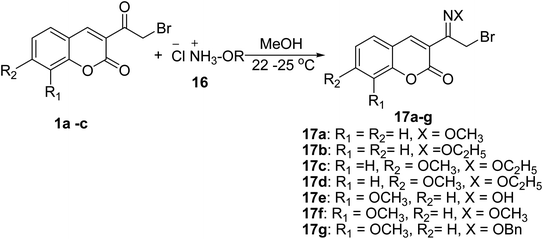
Coupling of 3-(bromoacetyl)coumarin derivatives 1 with amine hydrochlorides 16 such as hydroxylamine hydrochloride, methoxyamine hydrochloride, o-benzylhydroxylamine hydrochloride, and ethoxyamine hydrochloride in methyl alcohol to afford 3-(bromoacetyl) coumarin oximes 17 (Scheme 9).53,58–62
5.2. Azidation
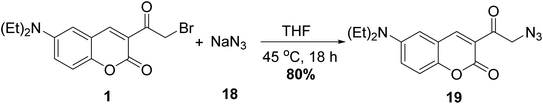
Evans and coworkers58 reported the synthesis of coumarin fluorophore bearing an azidoacyl group 19 via the treatment of 3-(bromoacetyl)coumarin 1 with sodium azide (NaN3) 18 at tetrahydrofuran (Scheme 10).5.3. Thiocyanation reaction
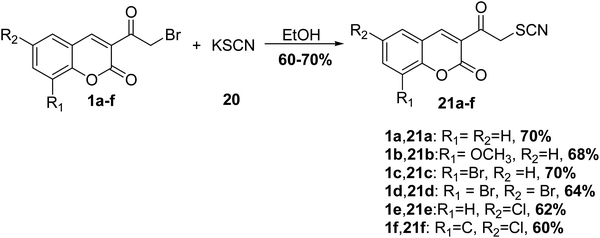
Ramanna et al.63 reported the treatment of 3-(bromoacetyl)coumarin derivatives 1 with potassium thiocyanate (KSCN) 20 in ethanol furnished 3-thiocyanatoacetyl coumarin derivatives 21 in good yields (Scheme 11).5.4. Sulfonation reaction
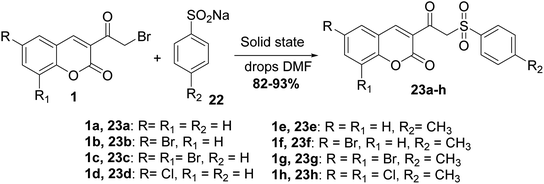
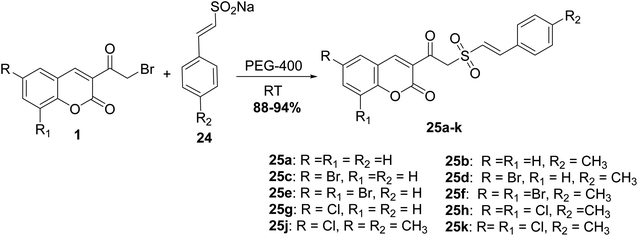
Mixing of 3-(bromoacetyl)coumarins 1 with sodium arene sulfinates 22 in solid state in the presence of few drops of DMF furnished 3-(2-(phenylsulfonyl)acetyl)coumarin derivatives 23 (Scheme 12).64,65 Furthermore, the reactions of this type were promoted under solvent-free conditions, as reported in literature.66,67A facile synthesis (E)-styryl sulfones 25a-k was accomplished via the reaction of 3-(bromoacetyl)coumarin derivatives 1 with sodium sulfinates 24 in the presence of polyethylene glycol (PEG-400) for promoting the reaction at ambient temperature (Scheme 13).68
5.5. Coupling reactions
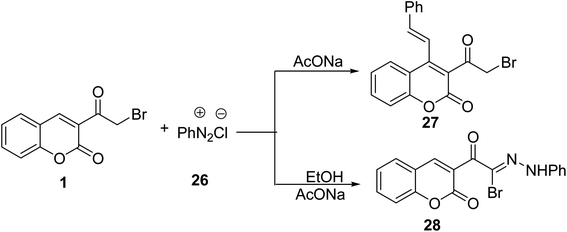
Coupling buffered solution of 3-(bromoacetyl)coumarin 1 with benzendiazonium chloride 26 yielded the corresponding 3-(2-bromoacetyl)-4-styryl-2H-chromen-2-one 27 (Scheme 14).69 While the reaction of 3-(bromoacetyl)coumarin 1 with benzenediazonium chloride 26 under the influence of sodium acetate afforded N-phenylacetohydrazonoyl bromide bearing coumarin moiety 28 (Scheme 14).705.6. Trifluoromethylation reaction
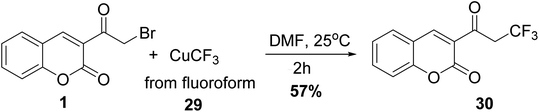
Novak and co-workers showed that trifluoromethylation of 3-(bromoacetyl)coumarin 1 with CHF3 29 derived CuCF3 at room temperature to give 2-trifluoromethylcoumarin 30 in yield 57% (Scheme 15).715.7. Phosphorylation reaction
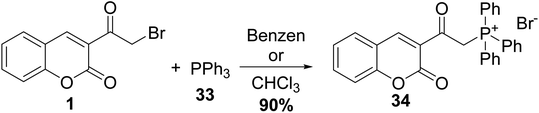
3-(Bromoacetyl)coumarin 1 was transformed to 2-oxophosphonates 32 in xylene via Arbuzov reaction conditions with triphenyl phosphite 31 (Scheme 16).72–75Wang et al. synthesized triphenylphosphonium 34 via the treatment of 3-(bromoacetyl)coumarin 1 with triphenylphosphine 33 in benzene or chloroform (Scheme 17).76
5.8. Cyanation reaction
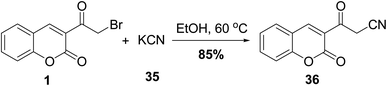
3-(Cyanoacetyl)coumarin 36 was prepared based on cyanation of 3-(bromoacetyl)coumarin 1 by treatment with potassium cyanide (KCN) 35 under ethanolic condition (Scheme 18).705.9. Reaction with active methylene compound
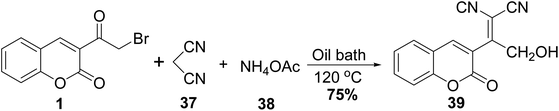
2-Hydroxy-1-(2-oxo-2H-chromen-3-yl-ethylidene)malononitrile 39 was obtained through Knoevenagel condensation of 3-(bromoacetyl)coumarin 1 with cyanoacetonitrile, 37 in the presence of ammonium acetate 38 (Scheme 19).705.10. Synthetic approach toward heterocyclic hybrids
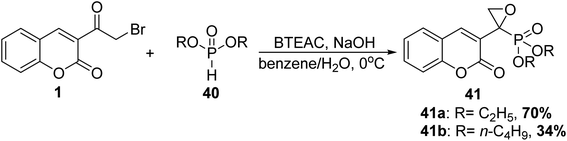
5.10.1.1. Oxirane. Oxirane phosphonates 41 were obtained via Michaelis–Becker reaction of 3-(bromoacetyl)coumarin 1 and dialkyl phosphites 40 using N-benzyl-N,N,N-triethylammonium chloride (BTEAC) as a phase-transfer catalyst (Scheme 20).77
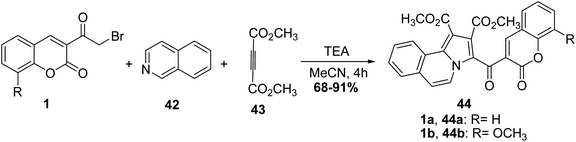
5.10.2.1. Pyrroles. An efficient synthesis of poly functionalized coumarin bearing pyrrolo[2,1-a]isoquinoline derivatives 44 was achieved via a multi-reaction of 3-(bromoacetyl)coumarin derivatives 1, isoquinoline 42, and dimethyl acetylenedicarboxylate 43 under the influence of triethylamine as catalyst (Scheme 21).78
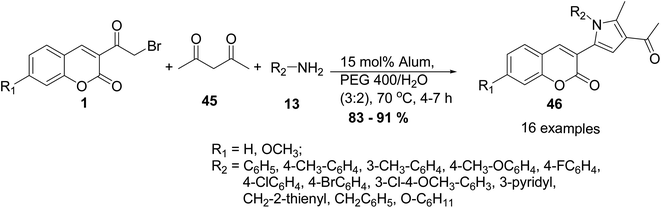
Pal et al.79 reported an eco-benign methodology for the preparation of coumarin-pyrrol hybrids 46 via three-component reactions of 3-(bromoacetyl)coumarin derivatives 1, an alkyl/arylamine 13, and acetylacetone 45 in the presence of optimized molarity of alum catalyst in water–PEG 400 (Scheme 22).
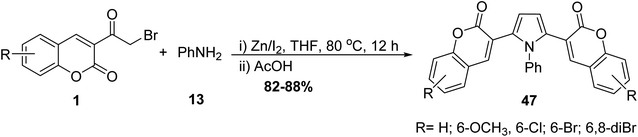
Pyrrole bis-coumarins 47 as fluorescent probes have been synthesized from the treatment of corresponding 3-(bromoacetyl)coumarin derivatives 1 with aniline 13 under catalytic condition (Zn–I2) (Scheme 23).80
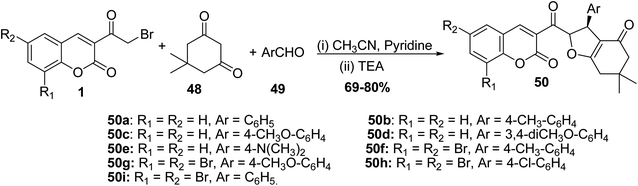
5.10.2.2. Dihydrofurans. The synthesis of coumarin substituted dihydrofurans 50a-i in good yields was performed via refluxing 3-(bromoacetyl)coumarins 1, dimedone 48, and aromatic aldehydes 49 in a mixture of acetonitrile and pyridine as a solvent containing a catalytic amount of triethylamine (Scheme 24).81
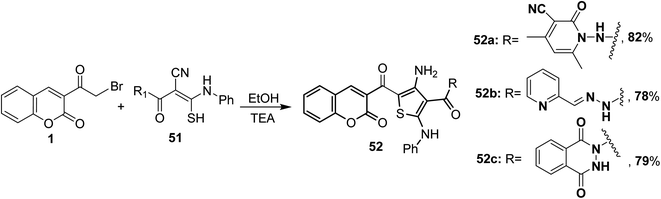
5.10.2.3. Thiophenes. Triethylamine-catalyzed heterocyclization of the ketene N,S-acetals 51 with 3-(bromoacetyl)coumarin 1 in ethanol has been employed to synthesize the corresponding 4-amino-2-phenylamino thiophenes 52a-c (Scheme 25).82
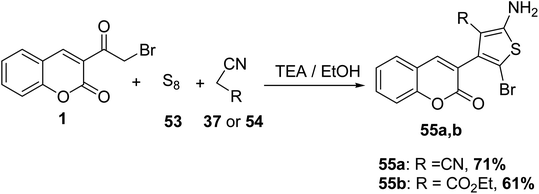
Treatment of 3-(bromoacetyl)coumarin 1 with sulfur 53 and either malononitrile 37 or ethyl cyanoacetate 54 in the presence of triethylamine furnished the corresponding 2-amino thiophene derivatives 55a and 55b, respectively (Scheme 26).70
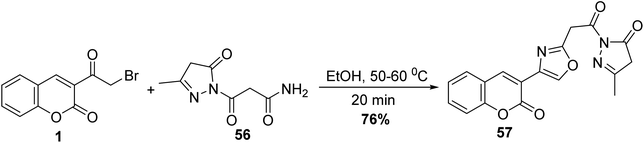
5.10.3.1. Oxazoles. Eco-friendly approach to accesses 3-methyl-1-(2-(4-(2-oxo-2H-chromen-3-yl)oxazol-2-yl)acetyl)-1H-pyrazol-5(4H)-one 57 was carried out without using any catalyst through the reaction of 3-(bromoacetyl)coumarin 1 with 3-oxopropanamide 56 in ethanol under heating (Scheme 27).83
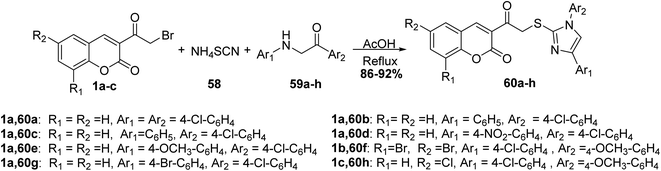
5.10.3.2. Imidazole derivatives. A simple one-pot synthesis of novel substituted imidazoles 60 has been accomplished by three-component reaction of 3-(bromoacetyl)coumarin 1, ammonium thiocyanate 58, and phenacyl aniline 59 (Scheme 28).84
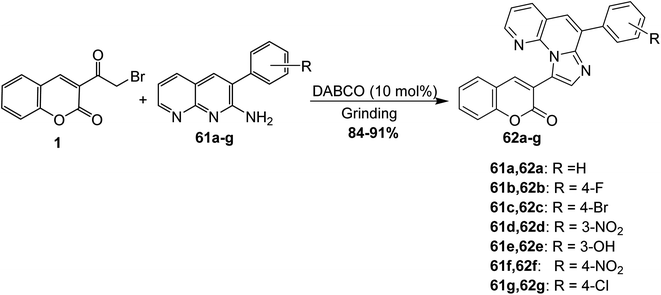
Boda et al. reported the preparation of fused imidazo[1,2-a][1,8]naphthyridines 62a-g through the solvent-free reaction of 3-(bromoacetyl)coumarin 1 and 2-amino-1,8-naphthyridines 61a-g using 1,4-diazabicyclo[2.2.2]octane (DABCO) as a catalyst (Scheme 29).85
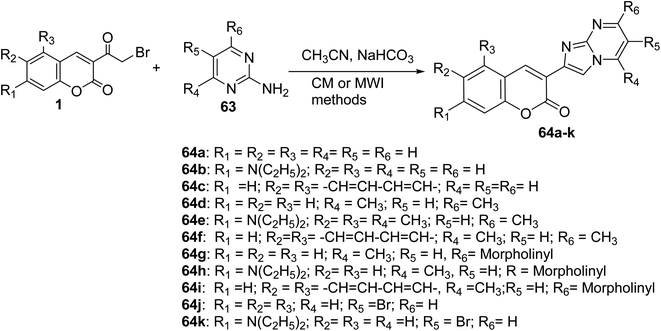
The coumarin-imidazo[1,2-a]pyrimidine derivatives 64 as pH-sensitive fluorescent compounds were carried out through thermal conventional (CM) or microwave irradiation (MWI) methods. Heating a mixture of 3-(bromoacetyl)coumarin 1 and 2-aminopyrimidine derivatives 63 in the microwave at 200 W at 100 °C afforded corresponding products in yields 5–90% compared by conventional thermal method (5–80%) (Scheme 30).37
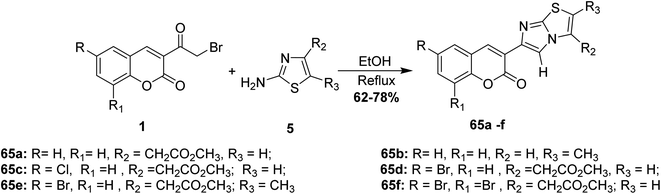
Rao and Reddy have repeated the cyclocondensation of 3-(bromoacetyl)coumarins 1 with 2-aminothiazoles 5 in refluxing ethanol yielded the corresponding imidazo[2,1-b]thiazol-5-2H-chromen-2-ones 65 (Scheme 31).86
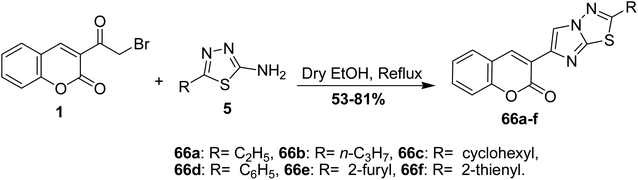
3-(2-Cyclohexylimidazo[2,1-b]-[1,3,4]thiadiazol-6-yl)-2H-chromen-2-ones 66a-f was obtained as hydrobromide salt through the reaction of 3-(bromoacetyl)coumarin 1 with 2-amino-5-cyclohexyl-1,3,4-thiadiazole 5 in refluxing ethanol (Scheme 32).87,88
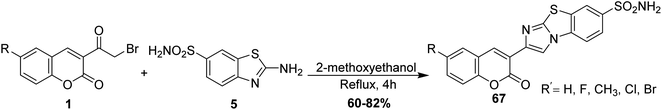
In refluxing 2-methoxyethanol, the reaction of 6-substituted-3-(bromoacetyl)coumarins 1 with 2-aminobenzo[d]thiazole-6-sulfonamide 5 was achieved, followed by neutralization using ammonia solution afforded corresponding imidazobenzothiazoles 67 (Scheme 33).89
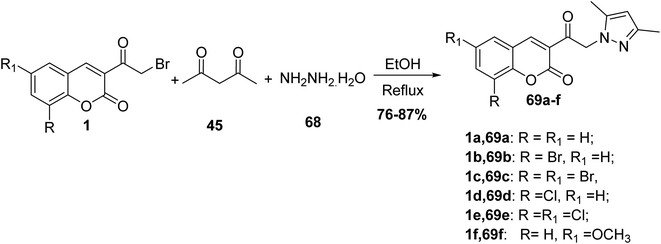
5.10.3.3. Pyrazoles. 3,5-Dimethylpyrazole derivatives 69 have been prepared through a one-pot multi-component reaction of 3-(bromoacetyl)coumarin derivatives 1, acetylacetone 45, and hydrazine hydrate 68 in refluxing ethanol (Scheme 34).90
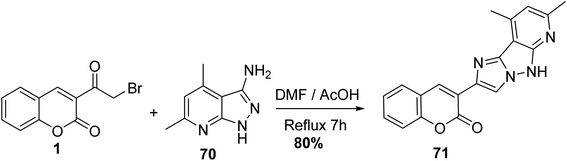
Condensation of 3-(bromoacetyl)coumarin 1 with 3-aminopyrazole 70 within DMF/AcOH yielded the corresponding imidazo[1,2-b]pyrazole 71 (Scheme 35).91
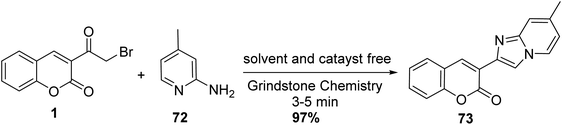
Using grindstone chemistry, the synthesis of 3-(7-methylimidazo[1,2-a]pyridin-2-yl)-2H-chromen-2-one 73 was achieved through the reaction of 3-(bromoacetyl)coumarin 1 with 2-amino-4-methylpyridine 72 under neat condition and catalyst-free (Scheme 36).92
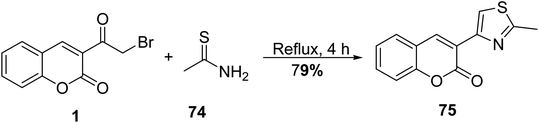
5.10.3.4. Thiazole derivatives. Gouda disclosed the reaction of 3-(bromoacetyl)coumarin 1 with thioacetamide 74 in methanol under reflux furnished 3-(2-methylthiazol-4-yl)-2H-chromen-2-one 75 (Scheme 37).93
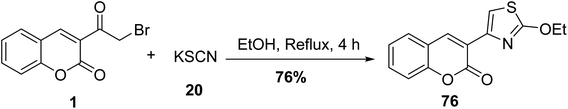
One of the most successful methods for the synthesis of 3-(2-ethoxythiazol-4-yl)-2H-chromen-2-one 76 is the refluxing 3-(bromoacetyl)coumarin 1 with potassium thiocyanate 20 in ethanol (Scheme 38).78
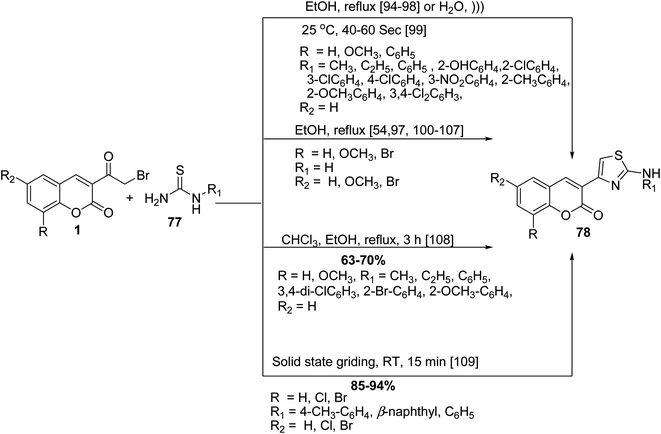
The Hantzsch thiazole synthesis of numerous 2-amino thiazolylcoumarins 78 was accomplished by cyclocondensation of 3-(bromoacetyl)coumarin derivatives 1 with various N-substituted thiourea 77 under various conditions (Scheme 39).54,94–109
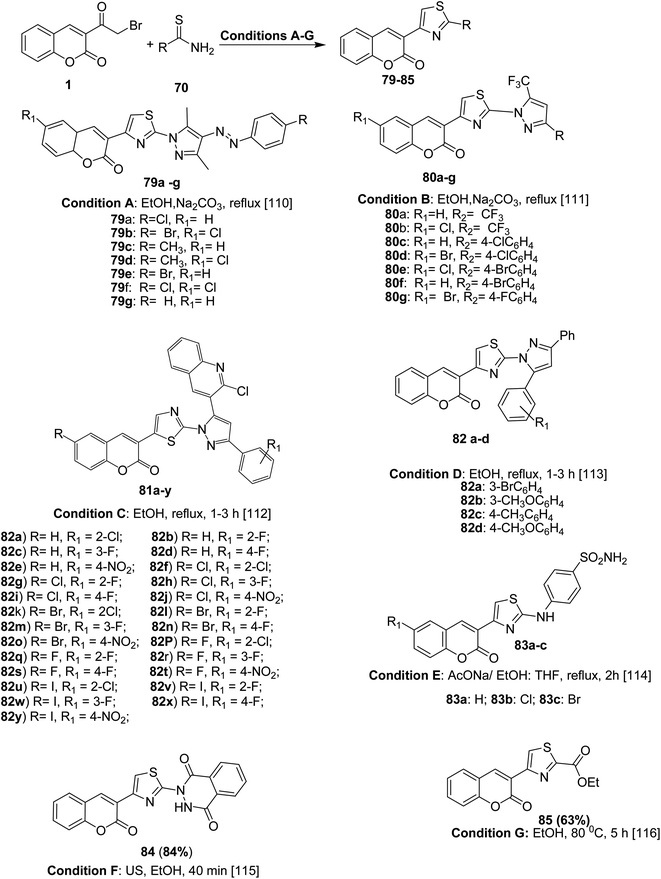
Analogously, 4-coumarinylthiazole derivatives 79–85 were efficiently prepared under conventional method or ultrasound irradiation in short reaction and high yields via the condensation of various 3-(bromoacetyl)coumarin derivatives 1 with N-substituted thioamide 74 (e.g. 2,4-thioureido benzenesulfonamide, ethyl thiooxamate, dihydrophthalazine carbothioamide, and pyrazole carbothiamides) in refluxing ethanol or tetrahydrofuran under alkaline condition (sodium acetate and sodium carbonate) (Scheme 40).110–116
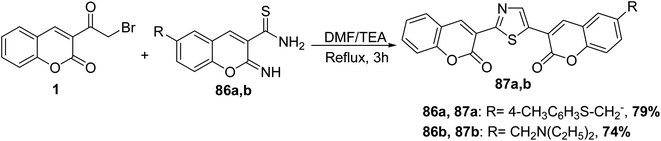
3-(Bromoacetyl)coumarin 1 was reacted with the appropriate carbothioamides 86 in DMF in the presence of triethylamine to give the corresponding 3,3′-(thiazole-2,4-diyl)bis-chromen-2-ones 87a,b (Scheme 41).117
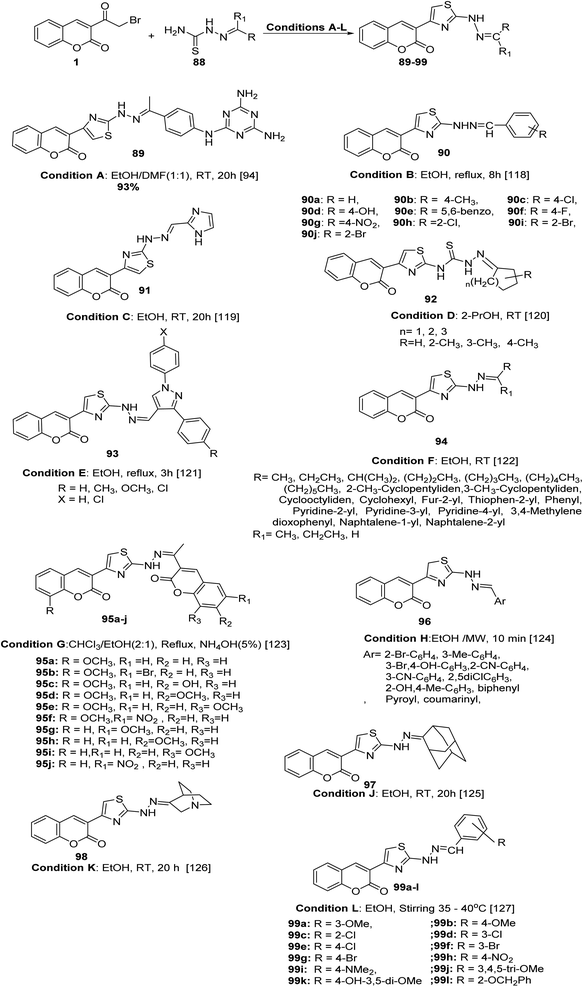
New sets of hydrazinyl thiazolyl coumarin derivatives 89–99 were accomplished in high and efficient yield from the one-pot Hantzsch reaction; the proposed mechanism of the reaction involves the cyclocondensation of the appropriate thiosemicarbazones 88 with 3-(bromoacetyl)coumarin 1 under various conditions (Scheme 42).94,118–127
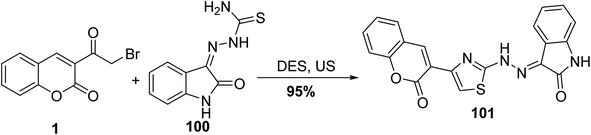
Utilizing deep eutectic solvent (DES) and ultrasound for the preparation of 2-oxochroman-3-thiazol-2-hydrazono-indolin-2-one 101 via the reaction of 1 with hydrazinecarbothioamide 100 (Scheme 43).94,128
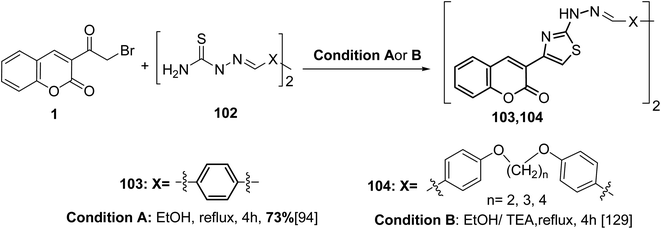
The bis(thiazole-4,2-diyl)bis(2H-chromen-2-ones) 103 and 104 were obtained via one-pot cyclisation reaction of bis(hydrazinecarbothioamides) 102 with 3-(bromoacetyl)coumarin 1 (Scheme 44).94,129
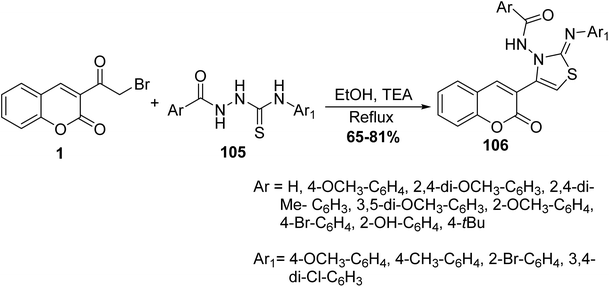
Cyclization reaction of 3-(bromoacetyl)coumarin 1 with thiosemicarbazides 105 in the presence of a catalytic amount of trimethylamine in ethanol yielded thiazolylcoumarin derivatives 106 (Scheme 45).130
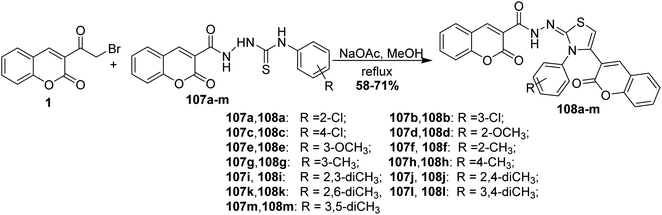
Refluxing of 3-(bromoacetyl)coumarin 1 and coumarinothiosemicarbazides 107a-m in methanol containing drops of acetic acid as catalyst gave bis-coumarin–iminothiazole hybrids 108a-m in good yields (Scheme 46).131
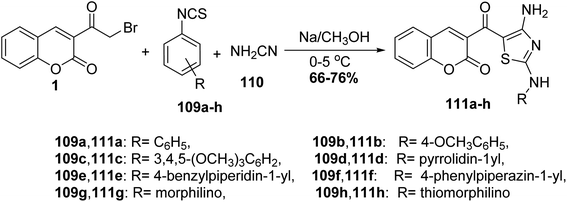
The multi-component reaction of 3-(bromoacetyl)coumarin derivatives 1, phenylisothiocyanates 109a-h with cyanamide 110 in freshly prepared sodium methoxide yielded annulated 3-(4-amino-2-(phenylamino)thiazole-5-carbonyl)-2H-chromen-2-one derivatives 111a-h in moderate yields (Scheme 47).132
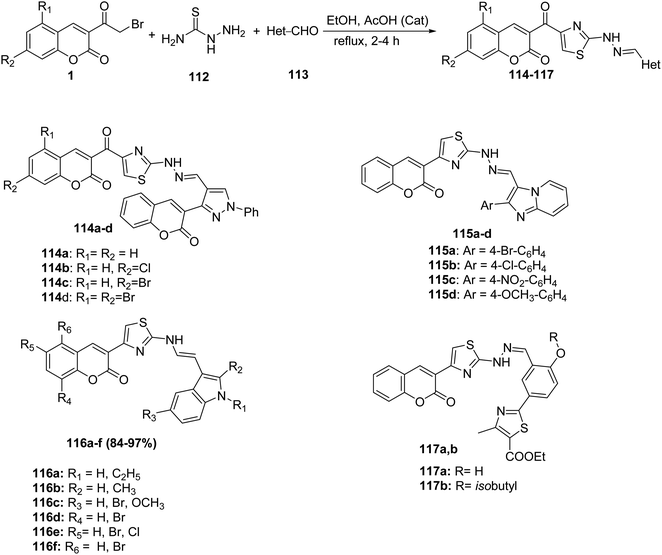
Novel series of thiazolylcoumarins 114-117 were prepared via multi-component condensation reaction of 3-(bromoacetyl)coumarin derivatives 1 thiosemicarbazide 112 and aldehydes 113 with different substitution patterns (aryl,133,134 pyrazole,134 imidazo[1,2-a]pyridine,135 indole136) in ethanol with a catalytic amount of acetic acid (Scheme 48).
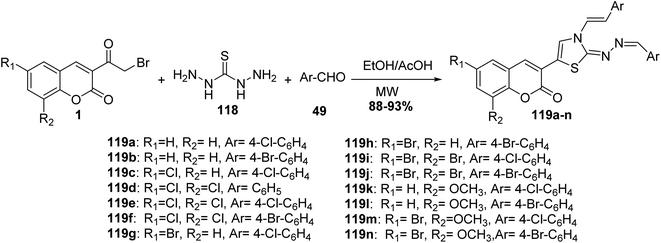
New series of coumarin based thiazoles 119a-n were accomplished via mixing of substituted 3-(bromoacetyl)coumarins 1, aldehydes 49, and thiocarbohydrazide 118 in the presence of a catalytic amount of acetic acid in the microwave for 6–8 min (Scheme 49).137
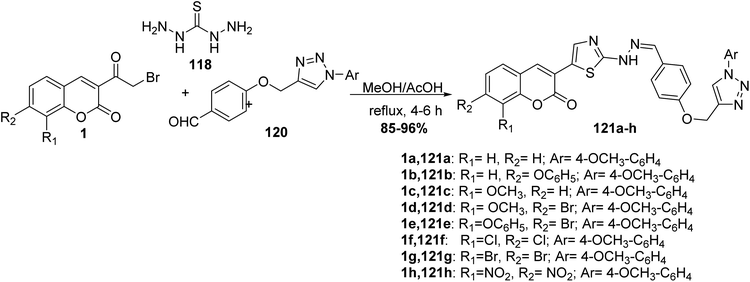
Three-component condensation of 3-(bromoacetyl)coumarin derivatives 1, thiocarbohydrazide 118 and aldehyde 120 were carried out under refluxing condition in ethanol in the presence of a catalytic amount of acetic acid to afford novel series of substituted 1,2,3-triazole-hydrazinyl-1,3-thiazole scaffolds 121a-h (Scheme 50).138
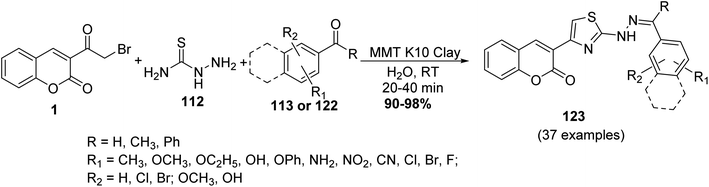
A water-mediated MCR protocol has been described for the synthesis of thiazolyl coumarins 123 from a three-component reaction of 3-(bromoacetyl)coumarin 1, aldehydes 113 or ketones 122, and thiosemicarbazide 112 catalyzed by montmorillonite K10 clay at ambient temperature (Scheme 51).139
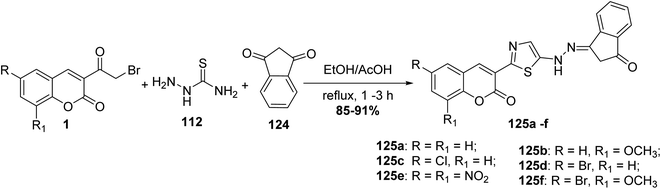
One-pot, synthesis of thiazolylhydrazone derivatives 125a-f through multi-component condensation of 3-(bromoacetyl)coumarin derivatives 1, thiosemicarbazide 112 and 1,3-indandione 124 in refluxing ethanol using a catalytic amount of acetic acid (Scheme 52).140
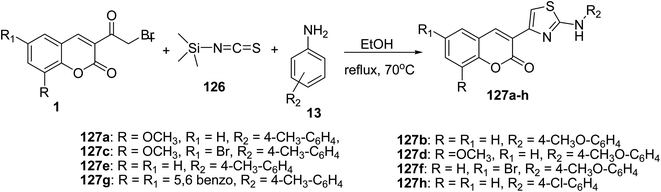
Multi-component synthesis of 3-(2-amino-4-thiazolyl)coumarins 127a-h have been obtained in good yields by refluxing of 3-(bromoacetyl)coumarin derivatives 1, trimethylsilyl isothiocyanate 126, and different primary amines 13 in ethanol (Scheme 53).141
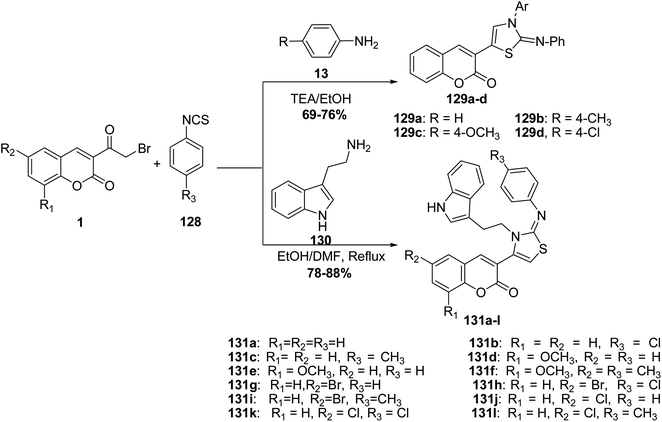
The reaction of 3-(bromoacetyl)coumarins 1 with phenylisothiocyanate 128 and aniline derivatives 13 afforded the thiazole derivatives 129a–d (Scheme 54).70 On the other hand, an efficient three-component synthesis of 2-arylimino-3-thiazolines 131 by the condensation of 3-(bromoacetyl)coumarin derivatives 1, arylisothiocyanates 128, and amine 130 (Scheme 54).142
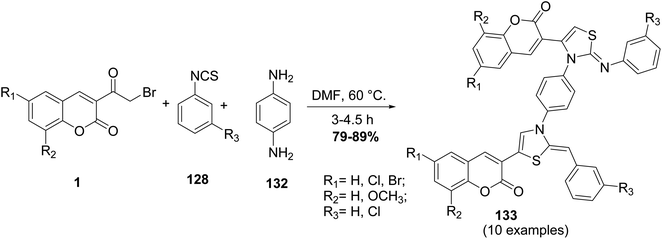
A one-pot multi-component approach involving different substituted of 3-(bromoacetyl)coumarin derivatives 1, phenyl isothiocyanates 128, and p-phenylenediamine 132 in refluxing DMF have been carried out for getting the new series of bis (phenylimino dihydro thiazolyl-2H-chromene) 133 (Scheme 55).143
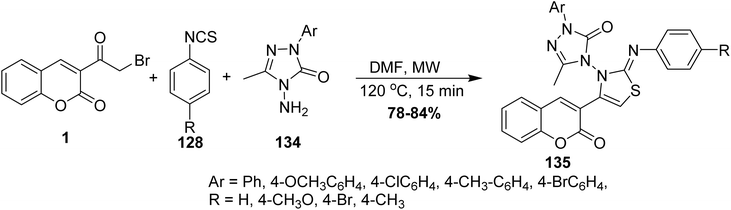
Microwave irradiation was reported as a green chemistry method for the synthesis of coumarin-3-yl-thiazol-3-yl-1,2,4-triazolin-3-ones 135 by Shaikh et al.144 via mixing of 3-(bromoacetyl)coumarin derivatives 1, 1,2,4-triazolone, 134 and aryl isothiocyanate 128 in DMF without using a catalyst (Scheme 56).
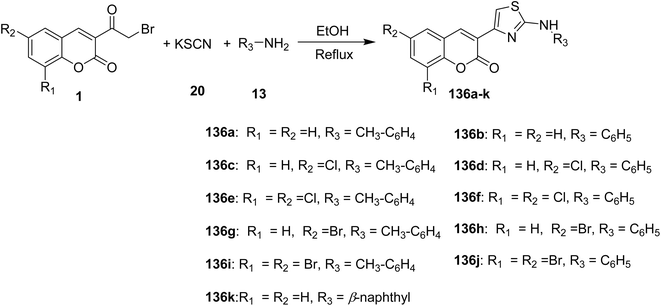
An efficient synthesis of 3-[2-(arylamino)thiazol-4-yl]coumarins 136a-k via grinding of 3-(bromoacetyl)coumarin derivatives 1, arylamines, 13 and potassium thiocyanate 20 in the least amount of ethanol as solvent under free catalyst and neat condition (Scheme 57).109
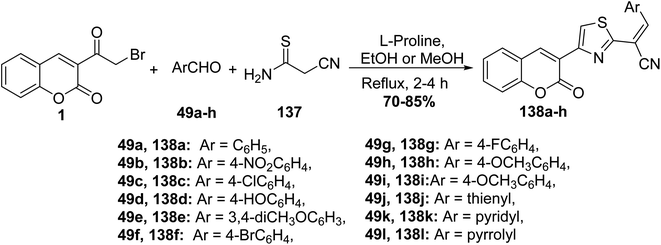
L-Proline catalyzed efficient one-pot three-component route for the synthesis of (2-oxo-2H-chromen-3-yl-thiazol-2-yl)-3-arylacrylonitriles 138a-h via treating 3-(bromoacetyl)coumarin 1 with numerous aryl/heteryl aldehydes 49 and 2-cyanothioacetamide 137 (Scheme 58).145
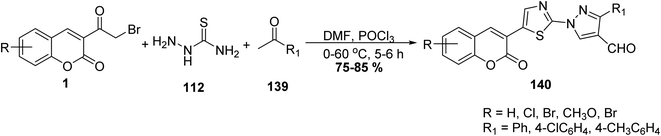
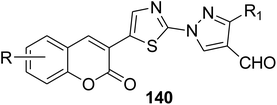
5.10.3.5. Thiazolopyrazolones. A mixture of 3-(bromoacetyl)coumarin derivatives 1, acetophenones 139, and thiosemicarbazide 112 were subjected to a one-pot multi-component Vilsmeier–Haack reaction condition afforded series of substituted thiazolyl-3-aryl-pyrazole-4-carbaldehydes bearing coumarin moiety 140 in moderate yields (Scheme 59).146
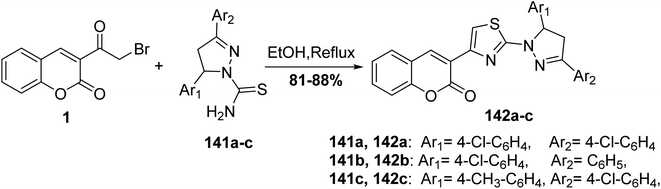
4,5-Dihydropyrazolyl–thiazole–coumarin systems 142 were obtained via the reaction of 3-(bromoacetyl)coumarin 1 and 3,5-disubstituted phenyl-4,5-dihydropyrazole-1-carbothioamide 141 in ethanol (Scheme 60).147
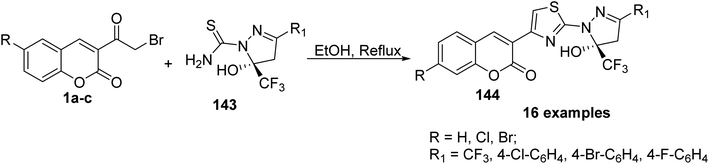
5-Hydroxy-5-trifluoromethyl-4,5-dihydropyrazol-1-4-(coumarin-3-yl)thiazoles 144 were obtained by refluxing of 3-(bromoacetyl)coumarin derivatives 1 with 5-hydroxy-5-trifluoromethyl-4,5-dihydropyrazol-1-thiocarboxamides 143 in ethanol (Scheme 61).148
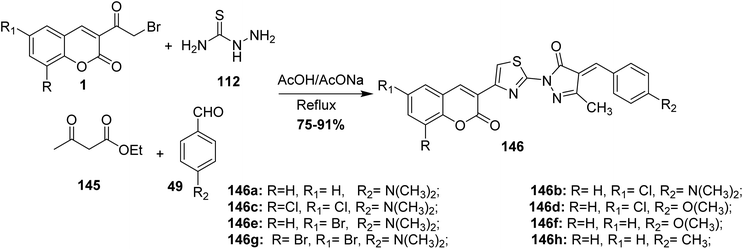
Synthesis of coumarin-substituted thiazolyl-pyrazolone derivatives 146 was reported by Pavurala et al. via a one-pot reaction of 3-(bromoacetyl)coumarin derivatives 1, thiosemicarbazide 89, aryl aldehyde 49, and ethyl acetoacetate 145 in boiling acetic acid (Scheme 62).149
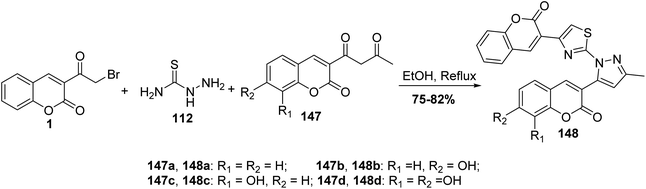
Series of pyrazoles bearing coumarin moieties 148 were prepared underwent Hantzsch cyclocondensation of 3-(bromoacetyl)coumarin 1, thiosemicarbazide 112 and various 3-(acetoacetyl) coumarins 147 in refluxing ethanol (Scheme 63).150
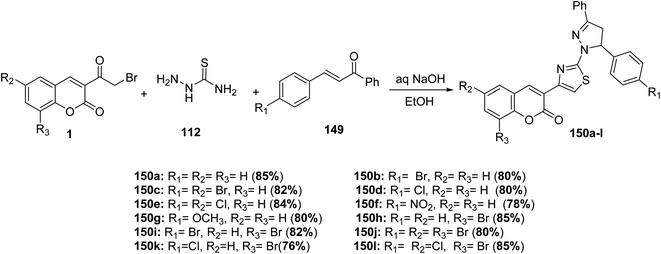
One pot, three-component reaction of chalcones 149, thiosemicarbazide 112, and different substituted 3-(bromoacetyl)coumarin derivatives 1 in refluxing ethanol containing catalytic amount of aqueous sodium hydroxide was achieved as an effective route for the synthesis of 4,5-dihydro-3,5-diphenylpyrazol-1-thiazol-4-2H-chromen-2-one derivatives 150a-l in one step (Scheme 64).149
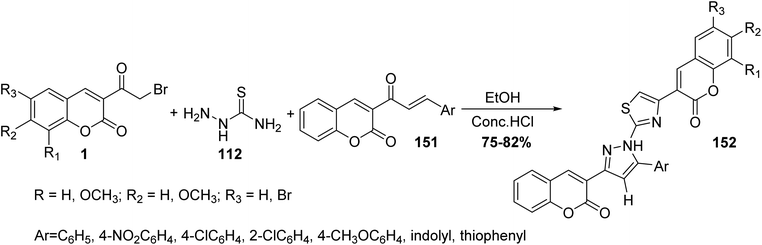
In the same fashion, Ghodsi et al. have been reported the synthesis of fused substituted thiazolyl-pyrazole-biscoumarin 152 through cyclocondensation of different coumarin chalcones 151, thiosemicarbazide 112, and 3-(bromoacetyl)coumarin derivatives 1 in ethanol in the presence of hydrochloric acid (Scheme 65).151
5.10.3.6. Thiazolotriazoles. On the other hand, the reaction of 3-(bromoacetyl)coumarin 1 with 5-phenyl-4H-1,2,4-triazole-3-thiol 153 gave fused thiazolo[3,2-b][1,2,4]triazol-5-chromenone 154 (Scheme 66).152
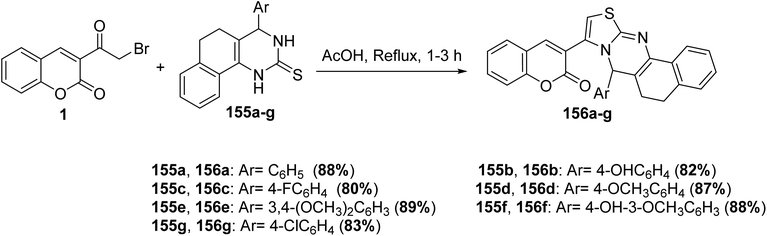
5.10.3.7. Thiazolopyrimidines. Novel fused thiazolo[3,2-a]pyrimidines 156a-g have been obtained in good yields by treatment of 3-(bromoacetyl)coumarin 1 with aryl-3,4-dihydropyrimidin-2(1H)-thiones 155a-g under conventional heating in acetic acid as solvent (Scheme 67).153,154
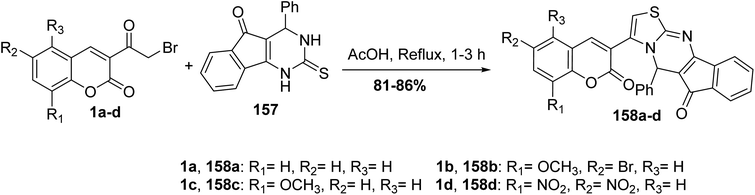
The cyclocondensation reaction of 3-(bromoacetyl)coumarin derivatives 1 with 4-phenyl-2-thioxo-indeno[1,2-d]pyrimidinone 157 in boiling acetic acid furnished phenylindeno[1,2-d]thiazolo[3,2-a]pyrimidin-6(5H)-ones 158 in high yields (Scheme 68).155
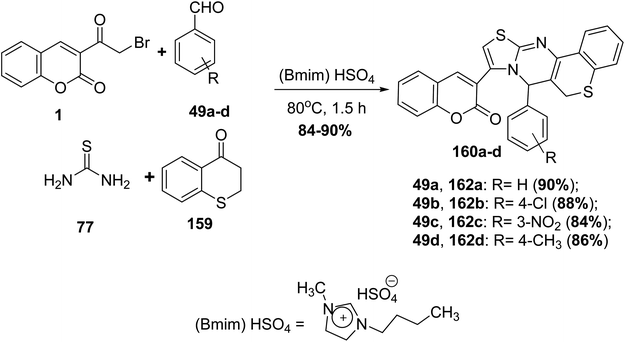
A new version of the Biginelli reaction using new variants was applied for the synthesis of substituted thiazolo[3,2-a]thiochromeno[4,3-d]pyrimidine 160a-d through mixing an equimolar ratio of 3-(bromoacetyl)coumarin 1, thiochromanone 159, substituted benzaldehyde 49a-d and thiourea 77 in one-pot reaction in the presence of [Bmim]HSO4 as a mediated ionic liquid catalyst, leading to the formation of a double electrophilic pyrimidine-2(5H)-thione as an intermediate which cyclized directly to furnish the targeting products 160a-d (Scheme 69).157
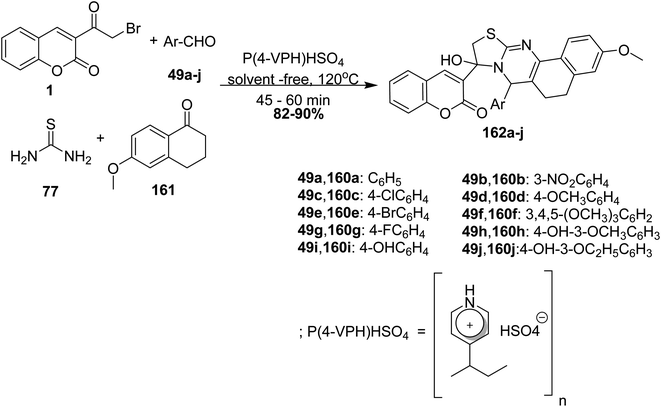
5.10.3.8. Thiazoloquinazolines. Biginelli reaction of 3-(bromoacetyl)coumarin 1, aryl aldehyde 49a-j, thiourea 77 and 6-methoxy-1-tetralone 161 in the presence of in poly(4-vinylpyridinium)hydrogen sulfate [P(4-VPH)HSO4] as Brønsted acid catalyst under neat condition afforded aryl-thiazolo[2,3-b]quinazoline derivatives 162a-j (Scheme 70).156
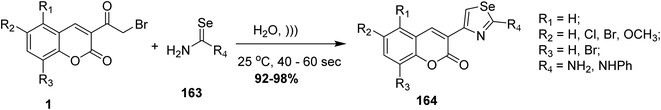
5.10.3.9. Selenazoles. An efficient synthesis of functionalized selenazoles 164 was achieved via ultrasonic irradiation of 3-(bromoacetyl)coumarin 1 with selenourea 163 at ambient temperature an aqueous medium under ultrasonic irradiation (Scheme 71).99
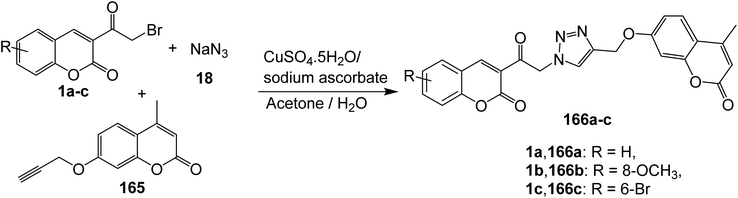
5.10.4.1. Triazoles. Cu(I)-catalyzed Huisgen 1,3-dipolar cycloaddition reaction of 3-(bromoacetyl)coumarin derivatives 1, sodium azide 18, and coumarin propargyl ethers 165 has been employed for the construction of bis-coumarinyl triazoles 166 (Scheme 72).158
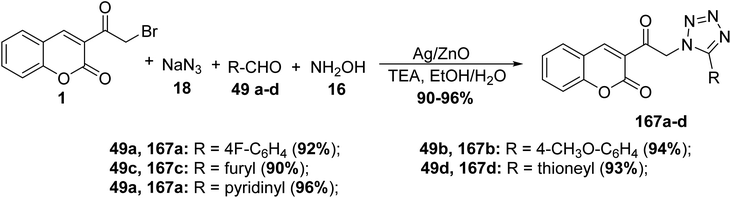
5.10.5.1. Tetrazoles. 1,5-Disubstituted tetrazole based chromone derivatives 167a-d were synthesized employing four-component condensation of 3-(bromoacetyl)coumarin 1, aldehyde derivatives 49a-d, sodium azide 18, and hydroxylamine 16 in ethanol containing catalytic drops of trimethylamine, the reaction was supported by nanorods of zinc oxide (NRs) and Ag-doped ZnO nanocomposites (NCs) as photocatalysts (Scheme 73).159
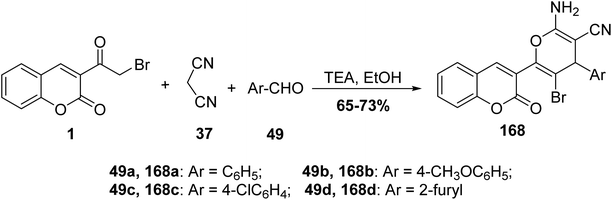
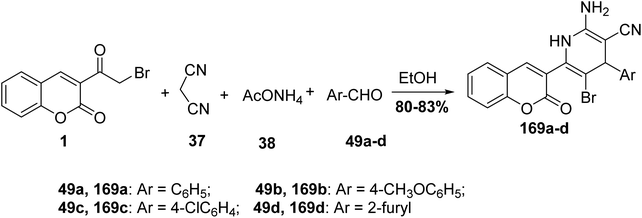
5.10.6.1. Pyran derivatives. Mohareb and MegallyAbdo70 described the preparation of 2-amino-3-cyano-pyran derivatives 168 using three-component reactions of 3-(bromoacetyl)coumarin 1 with malononitrile 37 and aromatic aldehydes 49 in boiling ethanol containing catalytic drops of trimethylamine (Scheme 74).
5.10.6.2. Pyridines. On the other hand, repeating the previous reaction using a catalytic amount of ammonium acetate 38 in lieu of triethylamine afforded the pyridine systems 169a-d (Scheme 75).70
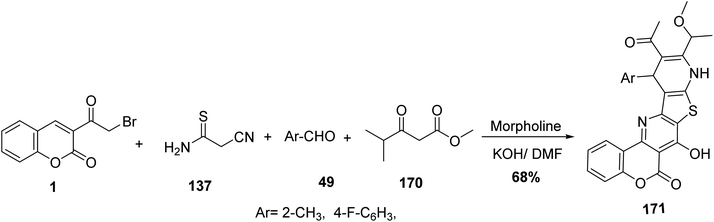
Multicomponent condensation of 3-(bromoacetyl)coumarin 1, cyanothioacetamide 137, benzaldehyde derivatives 49 and methyl 4-methyl-3-oxopentanoate 170 led to formation of fused chromeno[3′′,4′′:5′,6′]pyrido[2′,3′:4,5]thieno[3,2-e]pyridine derivatives 171 (Scheme 76).160
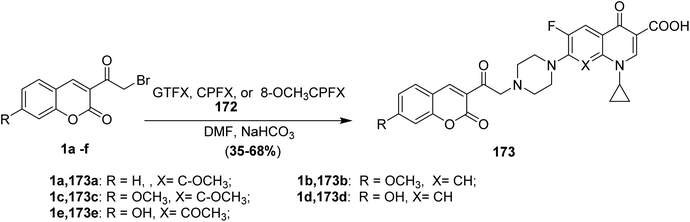
5.10.7.1. Fluoroquinolone derivatives. Nucleophilic substitution reactions of fluoroquinolones 172 (GTFX, CPFX, and 8-OCH3CPFX) with 3-(bromoacetyl)coumarin derivatives 1 in dimethylformamide, in the presence of NaHCO3, provide fluoroquinolone derivatives 173 (Scheme 77).62
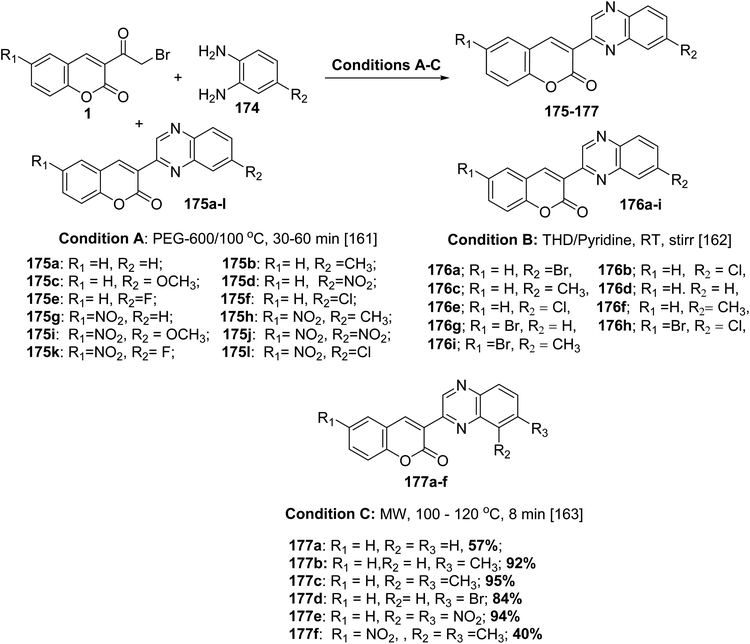
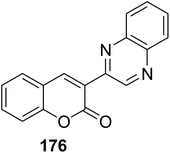
5.10.7.2. 3-(Quinoxalin-2-yl)-2H-chromen-2-ones. 3-(Quinoxalin-2-yl)-2H-chromen-2-ones 175-177 have been synthesized via substituted 3-(bromoacetyl)coumarins 1 and substituted o-phenylenediamines 174 in the presence of a catalyst such as PEG-600 or pyridine or without catalyst through microwave irradiation (Scheme 78).161–163
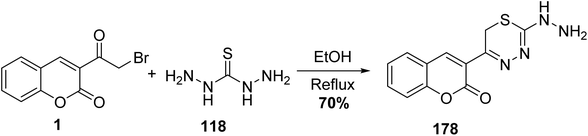
5.10.8.1. Thiadiazin derivatives. One-pot condensation reaction between 3-(bromoacetyl)coumarin 1 and thiocarbohydrazide 118 as bishydrazide in ethanol and in the presence a catalytic amount of acetic acid afforded 2-hydrazino[1,3,4]thiadiazin-5-chromenone 178 (Scheme 79).164
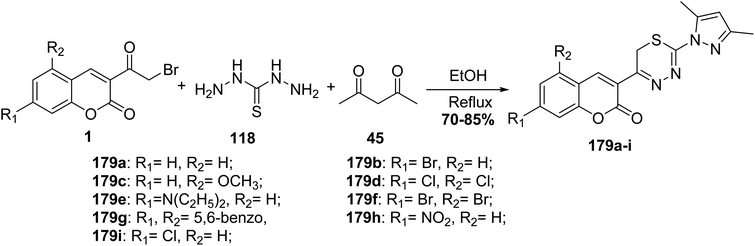
5.10.8.2. Pyrazolyl-thiadiazine derivatives. Refluxing of an equimolar mixture of substituted 3-(bromoacetyl)coumarins 1, acetylacetone 45, and thiocarbohydrazide 118 in ethanol furnished pyrazolyl-thiadiazinyl-2H-chromenones 179a–i (Scheme 80).165
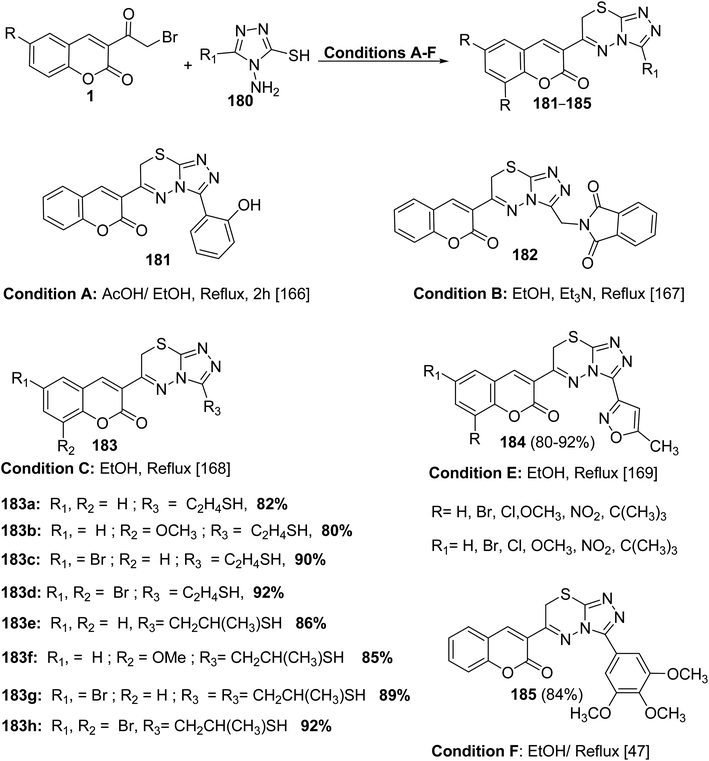
5.10.8.3. Triazolo[3,4-b]thiadiazines. Series of functionalized 4-amino-4H-1,2,4-triazole-3-thiols 180 on reaction with substituted 3-(bromoacetyl)coumarins 1 under simple reaction conditions formed the title products coumarin-substituted [1,2,4]triazolo[3,4-b][1,3,4]thiadiazine hybrids 181–185 in good to excellent yields (Scheme 81).47,166–169
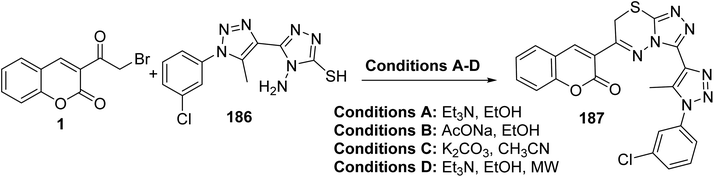
Triazolo[3,4-b]thiadiazine 187 was produced from the treatment of 3-(bromoacetyl)coumarin 1 with 4-aminotriazole-3-thiol 186 under both conventional and microwave conditions (Scheme 82).170
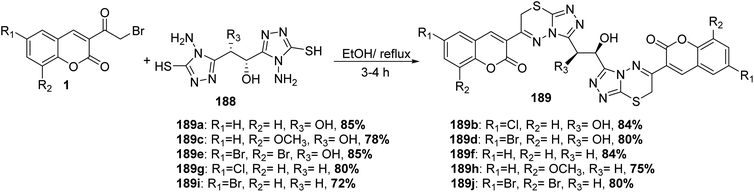
Bis coumarinyl bis triazolothiadiazinyl ethane derivatives 189 were synthesized through the reaction of ethane-1,2-diyl bis-4-amino-4H-1,2,4-triazole-3-thiols 188 with different substituted 3-(bromoacetyl)coumarin derivatives 1 in the presence of ethanol solvent (Scheme 83).165
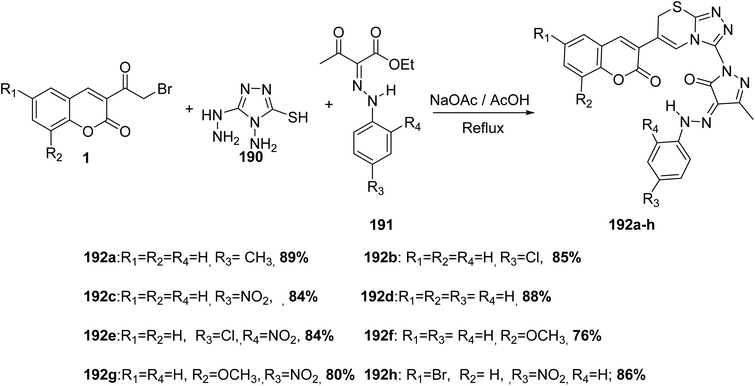
A one-pot, multi-component reaction of 3-(bromoacetyl)coumarins 1, 4-amino-5-hydrazino-4H-[1,2,4]triazole-3-thiol 190 and various ethyl 2-(2-(aryl)hydrazono)-3-oxobutanoate dervatives 191 in acetic acid in the presence of sodium acetate provide a direct route for the synthesis of corresponding triazolothiadiazinyl-pyrazolone 192a-h (Scheme 84).171
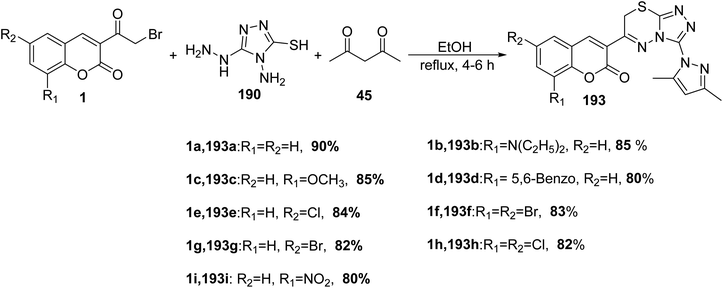
Pavurala and Vedula172 disclosed multi-component one-pot synthesis of pyrazolyl triazolo thiadiazinyl chromen-2–ones 193 was achieved via the multi-component reaction of 3-(bromoacetyl)coumarins 1, 4-amino-5-hydrazino-4H-[1,2,4]triazole-3-thiol 190 and acetylacetone 45 in absolute ethanol (Scheme 85).
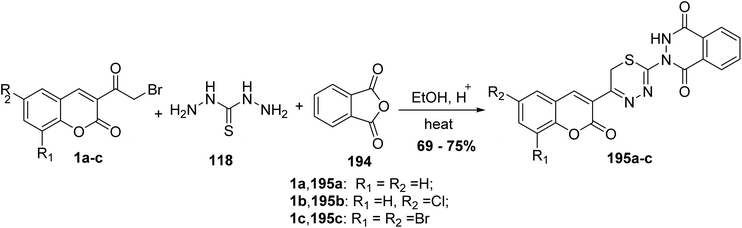
5.10.8.4. Thiadiazinyl-phthalazine-1,4-diones. Rao Chunduru and Rao173 reported the synthesis of thiadiazinyl-phthalazine-1,4-dione derivatives 195 via one-pot condensation reaction of 3-(bromoacetyl)coumarins 1, thiocarbohydrazide 118, and phthalic anhydride 194 in ethanol containing a catalytic amount of acetic acid (Scheme 86).
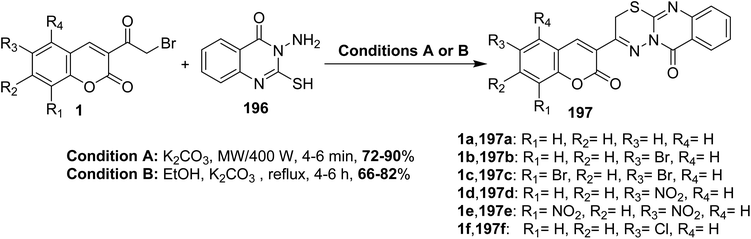
5.10.8.5. Thiadiazino[2,3-b]quinazolin-6(2H)-ones. An efficient one-pot synthesis of chromenyl[1,3,4]thiadiazino[2,3-b]quinazolin-6(2H)-ones 197 in high yields through cyclocondensation of 3-(bromoacetyl)coumarins 1 with 3-amino-2mercapto-3H-quinazolin-4-one 196 under the conventional and microwave conditions in the presence of potassium carbonate (Scheme 87).174
6. Applications
6.1. Biological activities
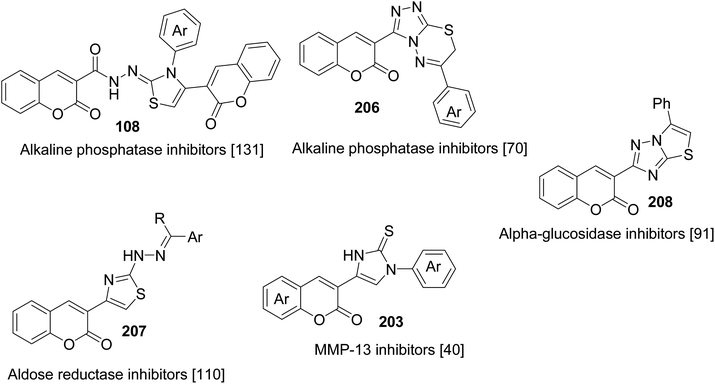
3-(Bromoacetyl)coumarins are being employed as privileged building blocks for the production of several bioactive heterocyclic compounds with a broad spectrum of medicinal agents including antibacterial, antifungal, antioxidant, anticancer, anti-inflammatory, anti-hepatocarcinoma, and antiproliferative agents (Table 1). Moreover, many approaches have also been explored for the construction and synthesis of a diverse range of inhibitors of metalloproteinase with significant inhibitory effects. These as a versatile scaffold include, for example, alkaline phosphatase,131 aldose reductase,110 alpha-glucosidase,91 and MMP-13 (ref. 40) inhibitors (Fig. 6).| Structures | Activities | Ref. |
|---|---|---|
 |
Antibacterial activity against: (E. coli, S. aureus and P. aeruginosa) | 175 |
| Antifungal activity against: (A. flavus, C. keratinophilum, and C. albicans) | ||
| Antioxidant activity: (moderate potency in scavenging DPPH radical (approximately 65%) | ||
 |
Antibacterial activity (zone of inhibition, ZI) against: S. aureus, ZI = 36.8 ± 0.6 mm | 176 |
| • S. mutans, ZI = 25.4 ± 0.5 mm | ||
| • K. pneumoniae, ZI = 27.2 ± 0.5 mm | ||
| • E. coli, ZI = 26.3 ± 0.5 mm | ||
 |
Anti-influenza A virus H1N1: IC50 = 4.84 μg mL−1 in MDCK cells | 108 |
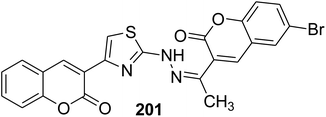 |
Antimicrobial agents: M. tuberculosis (MIC = 15 μM) | 177 |
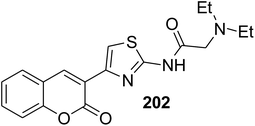 |
Anti-Alzheimeractivity: anti-cholinesterases (IC50 = 43 nM) | 178 |
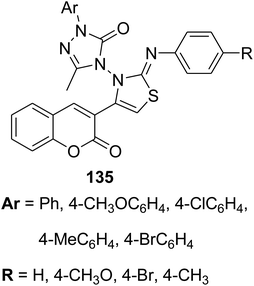 |
Anticancer activity against | 144 |
| • Breast cancer | ||
| • Lung cancer | ||
| • Leukemia | ||
| • Human cervical cancer | ||
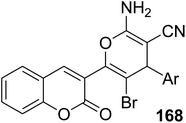 |
Anticancer activity against human gastric cancer NUGC | 70 |
| • 168a: Ar = 2-furyl, IC50 = 29 nM (against human gastric cancer NUGC) | ||
| • 168b: Ar = 4-Cl-C6H5, IC50 = 89 nM (against MCF) | ||
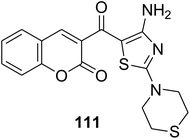 |
Anticancer activity (against MCF-7, HepG2 and SW480 cells): IC50 = 7.5–16.9 μg mL−1 | 132 |
 |
Anticancer activity (against Hela cell line) | 146 |
| • R = 6,8-diCl, R1 = 4-MeC6H4, IC50 = 5.75 μM | ||
| • R = 6,8-diBr, R1 = 4-MeC6H4, IC50 = 6.25 μM | ||
 |
Anticancer activity (against Melanoma tumor cell line): 55.75% GI | 99 |
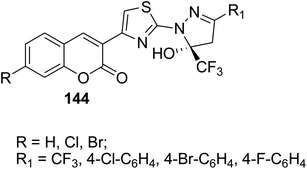 |
Anti-inflammatory agents: 73–86% of inhibition after 1 h | 148 |
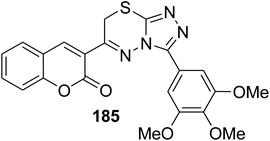 |
Antiproliferative activity: IC50 = 10.364 ± 0.270 μM | 170 |
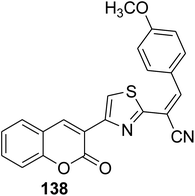 |
Anti-hepatocarcinoma activity: IC50 = 2.33 ± 0.004 μM | 145 |
6.2. Analytical applications
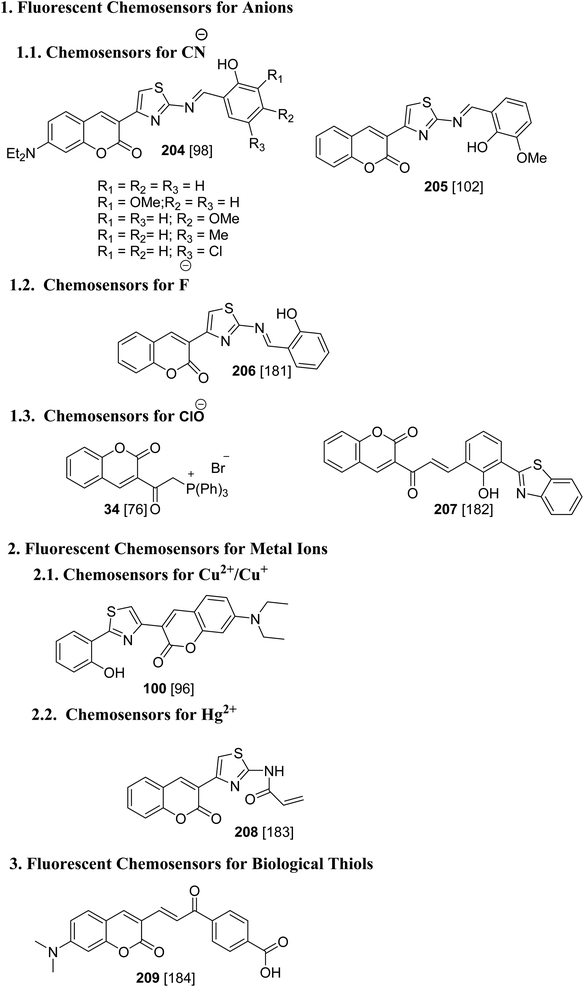
3-(Bromoacetyl)coumarin and 3-bromoacetyl-7-methoxycoumarin were used for the analysis of an emerging contaminant, perfluorinated substances.179,180 3-(Bromoacetyl)coumarins are versatile scaffolds with pivotal templates which have a vast array of applications in the field of fluorescent chemosensors towards metal cations, anions, and biomolecules181–184 (Fig. 7).7. Conclusion
This review has illuminated different aspects of 3-bromoacetylcoumarin 1 and its derivatives chemistry up to the beginning of 2021. It implies many sections on the synthesis of bromoacetylcoumarin derivatives. Besides different chemical reactions of bromoacetylcoumarins with various reagents, their biological evaluations and analytical application have been presented. Eventually, we hope that showcasing information accumulated over the years in developing 3-(bromoacetyl)coumarins core ranging from chemistry to applications will supplement the ongoing and forthcoming efforts towards the advancement of new functional molecular materials in the industry, biochemistry, and the environment.Conflicts of interest
There are no conflicts to declare.References
- M. M. Abdou, R. A. El-Saeed and S. Bondock, Recent advances in 4-hydroxycoumarin chemistry. Part 1: Synthesis and reactions, Arabian J. Chem., 2019, 12(1), 88–121 CrossRef CAS.
- M. M. Abdou, R. A. El-Saeed and S. Bondock, Recent advances in 4-hydroxycoumarin chemistry. Part 2: Scaffolds for heterocycle molecular diversity, Arabian J. Chem., 2019, 12(7), 974–1003 CrossRef CAS.
- M. M. Abdou, 3-Acetyl-4-hydroxycoumarin: Synthesis, reactions and applications, Arabian J. Chem., 2017, 10, S3664–S3675 CrossRef CAS.
- M. A. Metwally, S. Bondock, E. El-Desouky and M. M. Abdou, Synthesis, structure elucidation and application of some new azo disperse dyes derived from 4-hydroxycoumarin for dyeing polyester fabrics, Am. J. Chem., 2012, 2, 347–354 CrossRef CAS.
- M. A. Metwally, S. Bondock, E. S. I. El-Desouky and M. M. Abdou, A facile synthesis and tautomeric structure of novel 4-arylhydrazono-3-(2-hydroxyphenyl)-2-pyrazolin-5-ones and their application as disperse dyes, Color. Technol., 2013, 129(6), 418–424 CAS.
- M. M. Abdou, S. Bondock, S. El-Desouky and M. Metwally, Synthesis, spectroscopic studies and technical evaluation of novel disazo disperse dyes derived from 3-(2-hydroxyphenyl)-2-pyrazolin-5-ones for dyeing polyester fabrics, Am. J. Chem., 2013, 3, 59–67 Search PubMed.
- M. Metwally, S. Bondock, S. El-Desouky and M. Abdou, Synthesis, Structure Investigation and Dyeing Assessment of Novel Bisazo Disperse Dyes Derived from 3-(2'-Hydroxyphenyl)-1-phenyl-2-pyrazolin-5-ones, J. Korean Chem. Soc., 2012, 56(3), 348–356 CrossRef CAS.
- M. Metwally, S. Bondock, S. El-Desouky and M. Abdou, Synthesis, tautomeric structure, dyeing characteristics, and antimicrobial activity of novel 4-(2-arylazophenyl)-3-(2-hydroxyphenyl)-1-phenyl-2-pyrazolin-5-ones, J. Korean Chem. Soc., 2012, 56(1), 82–91 CrossRef CAS.
- M. A. Abbas, A. M. Eid, M. M. Abdou, A. Elgendy, R. A. El-Saeed and E. G. Zaki, Multifunctional Aspects of the Synthesized Pyrazoline Derivatives for AP1 5L X60 Steel Protection Against MIC and Acidization: Electrochemical, In Silico, and SRB Insights, ACS Omega, 2021, 6(13), 8894–8907 CrossRef CAS PubMed.
- S. F. Razavi, M. Khoobi, H. Nadri, A. Sakhteman, A. Moradi, S. Emami, A. Foroumadi and A. Shafiee, Synthesis and evaluation of 4-substituted coumarins as novel acetylcholinesterase inhibitors, Eur. J. Med. Chem., 2013, 64, 252–259 CrossRef CAS PubMed.
- Y. Hu, W. Chen, Y. Shen, B. Zhu and G.-X. Wang, Synthesis and antiviral activity of coumarin derivatives against infectious hematopoietic necrosis virus, Bioorg. Med. Chem. Lett., 2019, 29(14), 1749–1755 CrossRef CAS PubMed.
- S. Sandhu, Y. Bansal, O. Silakari and G. Bansal, Coumarin hybrids as novel therapeutic agents, Bioorg. Med. Chem., 2014, 22(15), 3806–3814 CrossRef CAS PubMed.
- A. Carneiro, M. J. Matos, E. Uriarte and L. Santana, Trending Topics on Coumarin and Its Derivatives in 2020, Molecules, 2021, 26(2), 501 CrossRef CAS PubMed.
- C. Ranjan Sahoo, J. Sahoo, M. Mahapatra, D. Lenka, P. Kumar Sahu, B. Dehury, R. Nath Padhy and S. Kumar Paidesetty, Coumarin derivatives as promising antibacterial agent(s), Arabian J. Chem., 2021, 14(2), 102922 CrossRef CAS.
- F. Salehian, H. Nadri, L. Jalili-Baleh, L. Youseftabar-Miri, S. N. A. Bukhari, A. Foroumadi, T. T. Küçükkilinç, M. Sharifzadeh and M. Khoobi, A Review: Biologically active 3, 4-heterocycle-fused coumarins, Eur. J. Med. Chem., 2020, 113034 Search PubMed.
- G. A. Gonçalves, A. R. Spillere, G. M. das Neves, L. P. Kagami, G. L. von Poser, R. F. S. Canto and V. L. Eifler-Lima, Natural and synthetic coumarins as antileishmanial agents: A review, Eur. J. Med. Chem., 2020, 112514 CrossRef PubMed.
- S. Mamidala, V. Vangala, S. R. Peddi, R. Chedupaka, V. Manga and R. R. Vedula, A facile one-pot, three component synthesis of a new series of 1, 3, 4-thiadiazines: Anticancer evaluation and molecular docking studies, J. Mol. Struct., 2021, 1233, 130111 CrossRef CAS.
- H. M. Alshibl, E. S. Al-Abdullah and H. M. Alkahtani, Coumarin: a promising scaffold for design and development of bioactive agents, Curr. Bioact. Compd., 2020, 16(6), 837–852 CrossRef CAS.
- H.-L. Qin, Z.-W. Zhang, L. Ravindar and K. Rakesh, Antibacterial activities with the structure-activity relationship of coumarin derivatives, Eur. J. Med. Chem., 2020, 112832 CrossRef CAS PubMed.
- E. Elahabaadi, A. A. Salarian and E. Nassireslami, Design, Synthesis, and Molecular Docking of Novel Hybrids of Coumarin-Dithiocarbamate Alpha-Glucosidase Inhibitors Targeting Type 2 Diabetes Mellitus, Polycyclic Aromat. Compd., 2021, 1–11 Search PubMed.
- D. Cao, Z. Liu, P. Verwilst, S. Koo, P. Jangjili, J. S. Kim and W. Lin, Coumarin-based small-molecule fluorescent chemosensors, Chem. Rev., 2019, 119(18), 10403–10519 CrossRef CAS PubMed.
- D. Srikrishna, C. Godugu and P. K. Dubey, A review on pharmacological properties of coumarins, Mini-Rev. Med. Chem., 2018, 18(2), 113–141 CrossRef CAS PubMed.
- M. M. Abdou, R. A. El-Saeed, M. A. Abozeid, M. G. Sadek, E. Zaki, Y. Barakat, H. Ibrahim, M. Fathy, S. Shabana and M. Amine, Advancements in tetronic acid chemistry. Part 1: Synthesis and reactions, Arabian J. Chem., 2019, 12(4), 464–475 CrossRef CAS.
- M. M. Abdou, Z. Seferoğlu, M. Fathy, T. Akitsu, M. Koketsu, R. Kellow and E. Amigues, Synthesis and chemical transformations of 3-acetyl-4-hydroxyquinolin-2 (1 H)-one and its N-substituted derivatives: bird's eye view, Res. Chem. Intermed., 2019, 45(3), 919–934 CrossRef CAS.
- M. M. Abdou, Chemistry of 4-hydroxy-2 (1H)-quinolone. Part 2. As synthons in heterocyclic synthesis, Arabian J. Chem., 2018, 11(7), 1061–1071 CrossRef CAS.
- M. M. Abdou, Chemistry of 4-Hydroxy-2 (1H)-quinolone. Part 1: Synthesis and reactions, Arabian J. Chem., 2017, 10, S3324–S3337 CrossRef CAS.
- M. M. Abdou, Utility of 4-hydroxythiocoumarin in organic synthesis, Arabian J. Chem., 2017, 10, S3955–S3961 CrossRef CAS.
- M. M. Abdou, R. A. El-Saeed, K. M. Elattar, Z. Seferoğlu and J. Boukouvalas, Advancements in tetronic acid chemistry. Part 2: use as a simple precursor to privileged heterocyclic motifs, Mol. Diversity, 2016, 20(4), 989–999 CrossRef CAS PubMed.
- D. Sajan, Y. Erdogdu, R. Reshmy, Ö. Dereli, K. K. Thomas and I. H. Joe, DFT-based molecular modeling, NBO analysis and vibrational spectroscopic study of 3-(bromoacetyl) coumarin, Spectrochim. Acta, Part A, 2011, 82(1), 118–125 CrossRef CAS PubMed.
- F. N. Magwenzi, S. D. Khanye and C. G. Veale, Unexpected transformations of 3-(bromoacetyl) coumarin provides new evidence for the mechanism of thiol mediated dehalogenation of α-halocarbonyls, Tetrahedron Lett., 2017, 58(10), 968–972 CrossRef CAS.
- W. H. El-Shwiniy, M. A. Gamil, S. A. Sadeek, W. A. Zordok and A. F. El-farargy, Ligational, DFT modeling and biological properties of some new metal complexes with 3-(bromoacetyl) coumarin and 1, 10-phenanthroline, Appl. Organomet. Chem., 2020, 34(8), e5696 CrossRef CAS.
- K. Vasudevan and M. Kulkarni, Structure of 3-(bromoacetyl) coumarin, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1991, 47(4), 775–777 CrossRef.
- H. A. Sparkes and J. A. Howard, 3-(Bromoacetyl) coumarin: a second P21/c modification, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2007, 63(9), o3895 CrossRef CAS.
- R. Chennuru, B. Maddimsetti, S. Gundlapalli, R. R. C. Babu and S. Mahapatra, Crystal structure of 3-bromoacetyl-6-chloro-2H-1-benzopyran-2-one, Acta Crystallogr., Sect. E: Crystallogr. Commun., 2015, 71(8), o615–o616 CrossRef CAS PubMed.
- K. Kavitha, D. Srikrishna, P. K. Dubey and P. Aparna, An efficient one-pot four-component Gewald reaction: Synthesis of substituted 2-aminothiophenes with coumarin–thiazole scaffolds under environmentally benign conditions, J. Sulfur Chem., 2019, 40(2), 195–208 CrossRef CAS.
- D. Srikrishna and P. K. Dubey, A facile and expedient microwave-assisted solvent-free method for the synthesis of 2-amino-4-(2-oxo-2 H-chromen-3-yl) nicotinonitriles, J. Iran. Chem. Soc., 2018, 15(7), 1647–1654 CrossRef CAS.
- V. Rajeswar Rao and T. Padmanabha Rao, Studies of thiazolyl, imidazolyl-2H-1-benzopyran-2-ones, Indian Journal of Chemistry-Section B, 1986, 25, 413–415 Search PubMed.
- C. Koelsch, Bromination of 3-acetocoumarin, J. Am. Chem. Soc., 1950, 72(7), 2993–2995 CrossRef CAS.
- T. M. A.-D. Musa, M. N. A. Aljibouri, B. F. Abbas and N. Hasani, Synthesis, Spectroscopic and Computational Studies of Some Metals Chelates with Chromene-2-one and Pyrazine-Based Ligands, Indones. J. Chem., 20(1), 160–174 CrossRef CAS.
- C. Bandyopadhyay, K. R. Sur, R. Patra and S. Banerjee, One-Pot Synthesis of 2-Alkyl/Arylamino-4-oxo-4 H-1-Benzopyran-3-Carboxaldehyde from 4-oxo-4 H-1-Benzopyran-3-Carboxaldehyde, J. Chem. Res., 2003, 2003(8), 459–460 CrossRef.
- A. Olyaei, E. Feizy and A. Aghajanzadeh, One-pot synthesis of novel (E)-3-(3, 8a-dihydro-2 H-oxazolo [3, 2-a] pyridin-2-ylidene) chroman-2-one derivatives, J. Heterocycl. Chem., 2020, 58(3), 757–765 CrossRef.
- C. Bandyopadhyay, K. R. Sur, R. Patra and A. Sen, Synthesis of Coumarin Derivatives from 4-Oxo-4H-1-benzopyran-3-carboxaldehyde via 3-(Arylaminomethylene) chroman-2, 4-dione, Tetrahedron, 2000, 56(22), 3583–3587 CrossRef CAS.
- A. Alizadeh and R. Ghanbaripour, An efficient synthesis of pyrrolo [2, 1-a] isoquinoline derivatives containing coumarin skeletons via a one-pot, three-component reaction, Res. Chem. Intermed., 2015, 41(11), 8785–8796 CrossRef CAS.
- S. K. Dwivedi, S. S. Razi and A. Misra, Sensitive colorimetric detection of CN− and AcO− anions in a semi-aqueous environment through a coumarin–naphthalene conjugate azo dye, New J. Chem., 2019, 43(13), 5126–5132 RSC.
- V. La Pietra, L. Marinelli, S. Cosconati, F. S. Di Leva, E. Nuti, S. Santamaria, I. Pugliesi, M. Morelli, F. Casalini and A. Rossello, Identification of novel molecular scaffolds for the design of MMP-13 inhibitors: a first round of lead optimization, Eur. J. Med. Chem., 2012, 47, 143–152 CrossRef CAS PubMed.
- N. Siddiqui, M. F. Arshad and S. A. Khan, Synthesis of some new coumarin incorporated thiazolyl semicarbazones as anticonvulsants, Acta Pol. Pharm., 2009, 66, 161–167 CAS.
- W. Ma, P. Chen, X. Huo, Y. Ma, Y. Li, P. Diao, F. Yang, S. Zheng, M. Hu and W. You, Development of triazolothiadiazine derivatives as highly potent tubulin polymerization inhibitors: Structure-activity relationship, in vitro and in vivo study, Eur. J. Med. Chem., 2020, 208, 112847 CrossRef CAS PubMed.
- K. Sinnur, S. Siddappa, S. P. Hiremath and M. Purohit, Synthesis of Substituted 2-(2-Oxobenzopyran-3-yl) indoles, ChemInform, 1987, 18(12), no CrossRef.
- S. K. Sahu, A. Mishra and R. K. Behera, Synthesis of thiazole, benzothiazole, oxadiazole, thiadiazole, triazole and thiazolidinone incorporated coumarins, Indian J. Heterocycl. Chem., 1996, 91–94 CAS.
- S. Khan, A. Zafar and I. Naseem, Copper-redox cycling by coumarin-di (2-picolyl) amine hybrid molecule leads to ROS-mediated DNA damage and apoptosis: a mechanism for cancer chemoprevention, Chem.-Biol. Interact., 2018, 290, 64–76 CrossRef CAS PubMed.
- S. Khan, A. M. Malla, A. Zafar and I. Naseem, Synthesis of novel coumarin nucleus-based DPA drug-like molecular entity: In vitro DNA/Cu (II) binding, DNA cleavage and pro-oxidant mechanism for anticancer action, PLoS One, 2017, 12(8), e0181783 CrossRef PubMed.
- L. R. Singh, S. R. Avula, S. Raj, A. Srivastava, G. R. Palnati, C. Tripathi, M. Pasupuleti and K. V. Sashidhara, Coumarin–benzimidazole hybrids as a potent antimicrobial agent: synthesis and biological elevation, J. Antibiot., 2017, 70(9), 954–961 CrossRef CAS PubMed.
- E. Valadbeigi and S. Ghodsi, Synthesis and characterization of some new thiazolidinedione derivatives containing a coumarin moiety for their antibacterial and antifungal activities, Med. Chem., 2017, 7, 178–185 CAS.
- O. Hishmat, J. Miky, A. Farrag and E. Fadl-Allah, Synthesis of some coumarin derivatives and their antimicrobial activity, Arch. Pharmacal Res., 1989, 12(3), 181–185 CrossRef CAS.
- P. Yagodinets, O. Skripskaya, I. Chernyuk, V. Bezverkhnii, L. Vlasik and V. Sinchenko, Synthesis and antimicrobial activity of coumarin-containing imidazoles, Pharm. Chem. J., 1996, 30(5), 335–336 CrossRef.
- V. R. Kumar and V. R. Rao, Synthesis of 3-(1-aryl-2-mercaptoimidazolyl)-2H-1-benzopyran-2-one derivatives, Indian Journal of Chemistry-Section B, 2002, 41, 415–418 Search PubMed.
- N. Guravaiah and V. R. Rao, Efficient, Stereoselective Approach to the Synthesis of 3-(1-Phenyl-2-(Z-styrylsulfonyl)-1 H-imidazol-4-yl)-2 H-chromen-2-ones, Synth. Commun., 2011, 41(8), 1167–1174 CrossRef CAS.
- V. Evans, P. Duncanson, M. Motevalli and M. Watkinson, An investigation into the synthesis of azido-functionalised coumarins for application in 1, 3-dipolar “click” cycloaddition reactions, Dyes Pigm., 2016, 135, 36–40 CrossRef CAS.
- E. Valadbeigi and S. Ghodsi, Synthesis and Study of Some New Quinolone Derivatives Containing a 3-acetyl Coumarin for Their Antibacterial and Antifungal Activities, Iran. J. Pharm. Res., 2017, 16(2), 554 CAS.
- R. Kalkhambkar, G. Kulkarni, H. Shivkumar and R. N. Rao, Synthesis of novel triheterocyclic thiazoles as anti-inflammatory and analgesic agents, Eur. J. Med. Chem., 2007, 42(10), 1272–1276 CrossRef CAS PubMed.
- A. Foroumadi, M. Oboudiat, S. Emami, A. Karimollah, L. Saghaee, M. H. Moshafi and A. Shafiee, Synthesis and antibacterial activity of N-[2-[5-(methylthio) thiophen-2-yl]-2-oxoethyl] and N-[2-[5-(methylthio) thiophen-2-yl]-2-(oxyimino) ethyl] piperazinylquinolone derivatives, Bioorg. Med. Chem., 2006, 14(10), 3421–3427 CrossRef CAS PubMed.
- L.-S. Feng, M.-L. Liu, S. Zhang, Y. Chai, B. Wang, Y.-B. Zhang, K. Lv, Y. Guan, H.-Y. Guo and C.-L. Xiao, Synthesis and in vitro antimycobacterial activity of 8-OCH3 ciprofloxacin methylene and ethylene isatin derivatives, Eur. J. Med. Chem., 2011, 46(1), 341–348 CrossRef CAS PubMed.
- S. Ramanna, V. R. Rao, T. S. Kumari and T. Padmanabha Rao, Synthesis of N-(4-2H-1-benzopyran-2-one-2-thiazolyl) phthalimides, Phosphorus, Sulfur Silicon Relat. Elem., 1995, 107(1–4), 197–204 CrossRef CAS.
- N. Guravaiah and V. R. Rao, Simple strategy for sulfonyl ethynylogs of coumarins, Synth. Commun., 2011, 41(18), 2693–2700 CrossRef CAS.
- M. A. Abdelrahman, H. S. Ibrahim, A. Nocentini, W. M. Eldehna, A. Bonardi, H. A. Abdel-Aziz, P. Gratteri, S. M. Abou-Seri and C. T. Supuran, Novel 3-substituted coumarins as selective human carbonic anhydrase IX and XII inhibitors: Synthesis, biological and molecular dynamics analysis, Eur. J. Med. Chem., 2021, 209, 112897 CrossRef CAS PubMed.
- N. Guravaiah and V. R. Rao, Solvent-free synthesis of new heteryl β-ketosulfones, J. Chem. Res., 2009, 2009(2), 87–89 CrossRef.
- R. S. Lamani, N. S. Shetty, R. R. Kamble and I. A. M. Khazi, Synthesis and antimicrobial studies of novel methylene bridged benzisoxazolyl imidazo [2, 1-b][1, 3, 4] thiadiazole derivatives, Eur. J. Med. Chem., 2009, 44(7), 2828–2833 CrossRef CAS PubMed.
- N. Guravaiah and V. R. Rao, Facile polyethylene glycol (PEG-400) promoted synthesis of some new heteryl (E)-styryl sulfones, J. Chem. Res., 2009, 2009(4), 237–239 CrossRef.
- M. A. Khalil, S. M. Sayed and M. A. Raslan, Synthesis of New Pyrazolo [5, 1-c] triazine, Triazolo [5, 1-c] triazine, Triazino [4, 3-b] indazole and Benzimidazo [2, 1-c] triazine Derivatives Incorporating Chromen-2-one Moiety, J. Korean Chem. Soc., 2013, 57(5), 612–617 CrossRef CAS.
- R. M. Mohareb and N. Y. MegallyAbdo, Uses of 3-(2-bromoacetyl)-2H-chromen-2-one in the synthesis of heterocyclic compounds incorporating coumarin: synthesis, characterization and cytotoxicity, Molecules, 2015, 20(6), 11535–11553 CrossRef CAS PubMed.
- P. Novak, A. Lishchynskyi and V. V. Grushin, Trifluoromethylation of α-Haloketones, J. Am. Chem. Soc., 2012, 134(39), 16167–16170 CrossRef CAS PubMed.
- B. Arbuzov, Michaelis-Arbusow-und perkow-reaktionen, Pure Appl. Chem., 1964, 9(2), 307–336 CAS.
- D. Marquarding, F. Ramirez, I. Ugi and P. Gillespie, Austausch-Reaktionen von Phosphor (v)-Verbindungen und ihre pentakoordinierten Zwischenstufen, Angew. Chem., 1973, 85(3), 99–127 CrossRef CAS.
- B. A. Arbuzov, Über die “nichtklassische” Arbuzov-Umlagerung, Zeitschrift für Chemie, 1974, 14(2), 41–49 CrossRef CAS.
- C. Pardin, J. N. Pelletier, W. D. Lubell and J. W. Keillor, Cinnamoyl inhibitors of tissue transglutaminase, J. Org. Chem., 2008, 73(15), 5766–5775 CrossRef CAS PubMed.
- Q.-M. Wang, L. Jin, Z.-Y. Shen, J.-H. Xu, L.-Q. Sheng and H. Bai, Mitochondria-targeting turn-on fluorescent probe for HClO detection and imaging in living cells, Spectrochim. Acta, Part A, 2020, 228, 117825 CrossRef CAS PubMed.
- R. Nikolova, A. Bojilova and N. A. Rodios, Reaction of 3-bromobenzyl and 3-bromoacetyl coumarin with phosphites. Synthesis of some new phosphonates and phosphates in the coumarin series, Tetrahedron, 1998, 54(47), 14407–14420 CrossRef CAS.
- A. Alizadeh and R. Ghanbaripour, An efficient synthesis of pyrrolo[2,1-a]isoquinoline derivatives containing coumarin skeletons via a one-pot, three-component reaction, Res. Chem. Intermed., 2015, 41(11), 8785–8796 CrossRef CAS.
- G. Pal, S. Paul and A. R. Das, Alum-Catalyzed Synthesis of 3-(1H-Pyrrol-2-yl)-2H-chromen-2-ones: A Water–PEG 400 Binary Solvent Mediated, One-Pot, Three-Component Protocol, Synthesis, 2013, 45(09), 1191–1200 CrossRef CAS.
- L. Shastri, S. Kalegowda and M. Kulkarni, The synthesis of pyrrole bis-coumarins, new structures for fluorescent probes, Tetrahedron Lett., 2007, 48(40), 7215–7217 CrossRef CAS.
- V. S. R. Chunduru and R. R. Vedula, One-pot synthesis of coumarin substituted dihydrofurans, Indian Journal of Chemistry-Section B, 2012, 51, 1417–1420 Search PubMed.
- R. E. Khidre, I. A. M. Radini and D. A. Ibrahim, Design and synthesis of some new thiophene and 1, 3, 4-thiadiazole based heterocycles, Phosphorus, Sulfur Silicon Relat. Elem., 2019, 194(11), 1040–1047 CrossRef CAS.
- R. B. Toche, V. M. Patil, S. A. Chaudhari, S. M. Chavan and R. W. Sabnis, Green Synthesis of Pyrazole and Oxazole Derivatives, J. Heterocycl. Chem., 2019, 56(1), 38–43 CrossRef CAS.
- R. K. Ramagiri, M. K. Thupurani and R. R. Vedula, Synthesis, characterization, and antibacterial activity of some novel substituted imidazole derivatives via one-pot three-component, J. Heterocycl. Chem., 2015, 52(6), 1713–1717 CrossRef CAS.
- S. Banoth, S. Perugu and S. Boda, Green Synthesis of Fused Imidazo [1, 2-a][1, 8] naphthyridine Derivatives Catalyzed by DABCO under Solvent-Free Solid-State Conditions and Their Biological Evaluation, J. Heterocycl. Chem., 2018, 55(3), 709–715 CrossRef CAS.
- V. R. Rao and V. R. Reddy, Synthesis of some new types of 3-coumarinyl-substituted pyrazolopyrimidines and imidazothiazoles, Chem. Heterocycl. Compd., 2008, 44(3), 360 CrossRef CAS.
- N. S. Begum, D. Vasundhara, C. Girija, G. Kolavi, V. Hegde and I. Khazi, Synthesis, spectroscopic and crystal structure analysis of a compound with pharmocophoric substituent: 2-cyclohexyl-6-(2-oxo-2H-chromen-3-yl)-imidazo [2, 1-b][1, 3, 4] thiadiazole-5-carbaldehyde, J. Chem. Crystallogr., 2007, 37(3), 193–198 CrossRef CAS.
- G. Kolavi, V. Hegde and I. A. Khazi, Intramolecular amidation: Synthesis of novel imidazo [2, 1-b][1, 3, 4] thiadiazole and imidazo [2, 1-b][1, 3] thiazole fused diazepinones, Tetrahedron Lett., 2006, 47(16), 2811–2814 CrossRef CAS.
- N. Chandak, J. K. Bhardwaj, R. K. Sharma and P. K. Sharma, Inhibitors of apoptosis in testicular germ cells: synthesis and biological evaluation of some novel IBTs bearing sulfonamide moiety, Eur. J. Med. Chem., 2013, 59, 203–208 CrossRef CAS PubMed.
- P. Santhosh, V. R. Chunduru and V. R. Rao, One-pot synthesis of trisubstituted pyrazoles via multicomponent approach, Chem. Heterocycl. Compd., 2011, 47(4), 448–451 CrossRef CAS.
- M. A. Gouda, Synthesis and antioxidant evaluation of some new pyrazolopyridine derivatives, Arch. Pharm., 2012, 345(2), 155–162 CrossRef CAS PubMed.
- K. Godugu and C. G. R. Nallagondu, Solvent and catalyst-free synthesis of imidazo [1, 2-a] pyridines by grindstone chemistry, J. Heterocycl. Chem., 2021, 58(1), 250–259 CrossRef CAS.
- A. Saeed, M. Arif, M. F. Erben, U. Flörke and J. Simpson, One-pot synthesis, quantum chemical calculations and X-ray diffraction studies of thiazolyl-coumarin hybrid compounds, Spectrochim. Acta, Part A, 2018, 198, 290–296 CrossRef CAS PubMed.
- S. S. Razi, P. Srivastava, R. Ali, R. C. Gupta, S. K. Dwivedi and A. Misra, A coumarin-derived useful scaffold exhibiting Cu2+ induced fluorescence quenching and fluoride sensing (On–Off–On) via copper displacement approach, Sens. Actuators, B, 2015, 209, 162–171 CrossRef CAS.
- R. S. Koti, G. D. Kolavi, V. S. Hegde and I. Khazi, Intramolecular Amidation: Synthesis of Novel Thiazole-Fused Diazepinones, Synth. Commun., 2007, 37(1), 99–105 CrossRef CAS.
- A. Helal, M. H. O. Rashid, C.-H. Choi and H.-S. Kim, Chromogenic and fluorogenic sensing of Cu2+ based on coumarin, Tetrahedron, 2011, 67(15), 2794–2802 CrossRef CAS.
- S. Bondock, T. Naser and Y. A. Ammar, Synthesis of some new 2-(3-pyridyl)-4, 5-disubstituted thiazoles as potent antimicrobial agents, Eur. J. Med. Chem., 2013, 62, 270–279 CrossRef CAS PubMed.
- B. Aydıner, Ö. Şahin, D. Çakmaz, G. Kaplan, K. Kaya, Ü. Ö. Özdemir, N. Seferoğlu and Z. Seferoğlu, A highly sensitive and selective fluorescent turn-on chemosensor bearing a 7-diethylaminocoumarin moiety for the detection of cyanide in organic and aqueous solutions, New J. Chem., 2020, 44(44), 19155–19165 RSC.
- G. Ramesh, B. Janardhan and B. Rajitha, Green approach: an efficient synthesis of 2, 4-disubstituted-1, 3-thiazoles and selenazoles in aqueous medium under ultrasonic irradiation, Res. Chem. Intermed., 2015, 41(11), 8099–8109 CrossRef CAS.
- W. S. Hamama, M. A. Berghot, E. A. Baz and M. A. Gouda, Synthesis and Antioxidant Evaluation of Some New 3-Substituted Coumarins, Arch. Pharm., 2011, 344(11), 710–718 CrossRef CAS PubMed.
- B. Z. Kurt, I. Gazioglu, F. Sonmez and M. Kucukislamoglu, Synthesis, antioxidant and anticholinesterase activities of novel coumarylthiazole derivatives, Bioorg. Chem., 2015, 59, 80–90 CrossRef CAS PubMed.
- Ö. Şahin, Ü. Ö. Özdemir, N. Seferoğlu, Z. K. Genc, K. Kaya, B. Aydıner, S. Tekin and Z. Seferoğlu, New platinum (II) and palladium (II) complexes of coumarin-thiazole Schiff base with a fluorescent chemosensor properties: Synthesis, spectroscopic characterization, X-ray structure determination, in vitro anticancer activity on various human carcinoma cell lines and computational studies, J. Photochem. Photobiol., B, 2018, 178, 428–439 CrossRef PubMed.
- A. Saeed, S. A. Ejaz, M. Shehzad, S. Hassan, M. al-Rashida, J. Lecka, J. Sévigny and J. Iqbal, 3-(5-(Benzylideneamino) thiazol-3-yl)-2 H-chromen-2-ones: a new class of alkaline phosphatase and ecto-5′-nucleotidase inhibitors, RSC Adv., 2016, 6(25), 21026–21036 RSC.
- M. A. Gouda, M. A. Berghot, E. A. Baz and W. S. Hamama, Synthesis, antitumor and antioxidant evaluation of some new thiazole and thiophene derivatives incorporated coumarin moiety, Med. Chem. Res., 2012, 21(7), 1062–1070 CrossRef CAS.
- A. Helal, M. H. O. Rashid, C.-H. Choi and H.-S. Kim, New regioisomeric naphthol-substituted thiazole based ratiometric fluorescence sensor for Zn2+ with a remarkable red shift in emission spectra, Tetrahedron, 2012, 68(2), 647–653 CrossRef CAS.
- I. Zhuravel, S. Kovalenko, S. Vlasov and V. Chernykh, Solution-phase Synthesis of a Combinatorial Library of 3-[4-(Coumarin-3-yl)-1, 3-thiazol-2-ylcarbamoyl] propanoic acid Amides, Molecules, 2005, 10(2), 444–456 CrossRef CAS PubMed.
- A. Abdel-Aziem, B. S. Baaiu, A. W. Elbazzar and F. Elabbar, A facile synthesis of some novel thiazoles, arylazothiazoles, and pyrazole linked to thiazolyl coumarin as antibacterial agents, Synth. Commun., 2020, 50(16), 2522–2530 CrossRef CAS.
- H. Osman, S. K. Yusufzai, M. S. Khan, B. M. Abd Razik, O. Sulaiman, S. Mohamad, J. A. Gansau, M. O. Ezzat, T. Parumasivam and M. Z. Hassan, New thiazolyl-coumarin hybrids: Design, synthesis, characterization, X-ray crystal structure, antibacterial and antiviral evaluation, J. Mol. Struct., 2018, 1166, 147–154 CrossRef CAS.
- V. S. R. Chunduru and R. V. Rao, One pot synthesis of 3-[2-(arylamino) thiazol-4-yl] coumarins in a three-component synthesis and a catalyst and solvent-free synthesis on grinding, J. Chem. Res., 2010, 34(1), 50–53 CrossRef CAS.
- A. Ibrar, Y. Tehseen, I. Khan, A. Hameed, A. Saeed, N. Furtmann, J. Bajorath and J. Iqbal, Coumarin-thiazole and-oxadiazole derivatives: synthesis, bioactivity and docking studies for aldose/aldehyde reductase inhibitors, Bioorg. Chem., 2016, 68, 177–186 CrossRef CAS PubMed.
- S. Kumar, R. Aggarwal and C. Sharma, Synthesis of Some 2-(3-Alkyl/aryl-5-trifluoromethylpyrazol-1-yl)-4-(coumarin-3-yl) thiazoles as Novel Antibacterial Agents, Synth. Commun., 2015, 45(17), 2022–2029 CrossRef CAS.
- M. I. Ansari and S. A. Khan, Synthesis and antimicrobial activity of some novel quinoline-pyrazoline-based coumarinyl thiazole derivatives, Med. Chem. Res., 2017, 26(7), 1481–1496 CrossRef CAS.
- M. Madni, M. N. Ahmed, S. Hameed, S. W. A. Shah, U. Rashid, K. Ayub, M. N. Tahir and T. Mahmood, Synthesis, quantum chemical, in vitro acetyl cholinesterase inhibition and molecular docking studies of four new coumarin based pyrazolylthiazole nuclei, J. Mol. Struct., 2018, 1168, 175–186 CrossRef CAS.
- N. Chandak, P. Kumar, P. Kaushik, P. Varshney, C. Sharma, D. Kaushik, S. Jain, K. R. Aneja and P. K. Sharma, Dual evaluation of some novel 2-amino-substituted coumarinylthiazoles as anti-inflammatory–antimicrobial agents and their docking studies with COX-1/COX-2 active sites, J. Enzyme Inhib. Med. Chem., 2014, 29(4), 476–484 CrossRef CAS PubMed.
- F. S. Elsharabasy, S. M. Gomha, T. A. Farghaly and H. S. Elzahabi, An Efficient Synthesis of Novel Bioactive Thiazolyl-Phthalazinediones under Ultrasound Irradiation, Molecules, 2017, 22(2), 319 CrossRef PubMed.
- G. Wang, D. He, X. Li, J. Li and Z. Peng, Design, synthesis and biological evaluation of novel coumarin thiazole derivatives as α-glucosidase inhibitors, Bioorg. Chem., 2016, 65, 167–174 CrossRef CAS PubMed.
- S. M. Sanad and A. E. Mekky, Efficient synthesis and characterization of novel bis (chromenes) and bis (benzo [f] chromenes) linked to thiazole units, Synth. Commun., 2021, 51(4), 611–624 CrossRef CAS.
- D. Kini and M. Ghate, Synthesis and Oral Hypoglycemic Activity of 3-[5'-Methyl-2'-aryl-3'-(thiazol-2′′-yl amino) thiazolidin-4'-one] coumarin Derivatives, Eur. J. Chem., 2011, 8(1), 386–390 CAS.
- K. Z. Łączkowski, K. Jachowicz, K. Misiura, A. Biernasiuk and A. Malm, Synthesis and biological evaluation of novel 2-(1H-imidazol-2-ylmethylidene) hydrazinyl-1, 3-thiazoles as potential antimicrobial agents, Heterocycl. Commun., 2015, 21(2), 109–114 Search PubMed.
- F. Chimenti, S. Carradori, D. Secci, A. Bolasco, P. Chimenti, A. Granese and B. Bizzarri, Synthesis and biological evaluation of novel conjugated coumarin-thiazole systems, J. Heterocycl. Chem., 2009, 46(3), 575–578 CrossRef CAS.
- N. Harikrishna, A. M. Isloor, K. Ananda, A. Obaid and H.-K. Fun, 1, 3, 4-Trisubstituted pyrazole bearing a 4-(chromen-2-one) thiazole: Synthesis, characterization and its biological studies, RSC Adv., 2015, 5(54), 43648–43659 RSC.
- F. Chimenti, B. Bizzarri, A. Bolasco, D. Secci, P. Chimenti, A. Granese, S. Carradori, M. D’Ascenzio, M. M. Scaltrito and F. Sisto, Synthesis and anti-Helicobacter pylori Activity of 4-(Coumarin-3-yl) thiazol-2-ylhydrazone Derivatives, ChemInform, 2011, 42(13), 1269–1274 CrossRef.
- S. KhanYusufzai, H. Osman, M. S. Khan, S. Mohamad, O. Sulaiman, T. Parumasivam, J. A. Gansau and N. Johansah, Design, characterization, in vitro antibacterial, antitubercular evaluation and structure–activity relationships of new hydrazinyl thiazolyl coumarin derivatives, Med. Chem. Res., 2017, 26(6), 1139–1148 CrossRef CAS.
- M. T. Gabr, N. S. El-Gohary, E. R. El-Bendary, M. M. El-Kerdawy and N. Ni, Microwave-assisted synthesis and antitumor evaluation of a new series of thiazolylcoumarin derivatives, EXCLI Journal, 2017, 16, 1114 Search PubMed.
- K. Łączkowski, K. Misiura, A. Biernasiuk, A. Malm, A. Paneth and T. Plech, Synthesis, Antimicrobial Activity and Molecular Docking Studies of 1, 3-Thiazole Derivatives Incorporating Adamantanyl Moiety, J. Heterocycl. Chem., 2016, 53(2), 441–448 CrossRef.
- K. Z. Łączkowski, K. Landowska, A. Biernasiuk, K. Sałat, A. Furgała, T. Plech and A. Malm, Synthesis, biological evaluation and molecular docking studies of novel quinuclidinone derivatives as potential antimicrobial and anticonvulsant agents, Med. Chem. Res., 2017, 26(9), 2088–2104 CrossRef.
- R. Raza, A. Saeed, M. Arif, S. Mahmood, M. Muddassar, A. Raza and J. Iqbal, Synthesis and biological evaluation of 3-thiazolocoumarinyl schiff-base derivatives as cholinesterase inhibitors, Chem. Biol. Drug Des., 2012, 80(4), 605–615 CrossRef CAS PubMed.
- M. Imran, M. Bakht, A. Khan, M. Alam, E. H. Anouar, M. B. Alshammari, N. Ajmal, A. Vimal, A. Kumar and Y. Riadi, An Improved Synthesis of Key Intermediate to the Formation of Selected Indolin-2-Ones Derivatives Incorporating Ultrasound and Deep Eutectic Solvent (DES) Blend of Techniques, for Some Biological Activities and Molecular Docking Studies, Molecules, 2020, 25(5), 1118 CrossRef CAS PubMed.
- M. Hosny, M. E. Salem, A. F. Darweesh and A. H. Elwahy, Synthesis of Novel Bis (thiazolylchromen-2-one) Derivatives Linked to Alkyl Spacer via Phenoxy Group, J. Heterocycl. Chem., 2018, 55(10), 2342–2348 CrossRef CAS.
- U. Salar, M. Taha, K. M. Khan, N. H. Ismail, S. Imran, S. Perveen, S. Gul and A. Wadood, Syntheses of new 3-thiazolyl coumarin derivatives, in vitro α-glucosidase inhibitory activity, and molecular modeling studies, Eur. J. Med. Chem., 2016, 122, 196–204 CrossRef CAS PubMed.
- A. Ibrar, S. Zaib, I. Khan, F. Jabeen, J. Iqbal and A. Saeed, Facile and expedient access to bis-coumarin–iminothiazole hybrids by molecular hybridization approach: synthesis, molecular modelling and assessment of alkaline phosphatase inhibition, anticancer and antileishmanial potential, RSC Adv., 2015, 5(109), 89919–89931 RSC.
- A. Ayati, T. O. Bakhshaiesh, S. Moghimi, R. Esmaeili, K. Majidzadeh-A, M. Safavi, L. Firoozpour, S. Emami and A. Foroumadi, Synthesis and biological evaluation of new coumarins bearing 2, 4-diaminothiazole-5-carbonyl moiety, Eur. J. Med. Chem., 2018, 155, 483–491 CrossRef CAS PubMed.
- R. Deshineni, R. Velpula, S. Koppu, J. Pilli and G. Chellamella, One-pot multi-component synthesis of novel ethyl-2-(3-((2-(4-(4-aryl) thiazol-2-yl) hydrazono) methyl)-4-hydroxy/isobutoxyphenyl)-4-methylthiazole-5-carboxylate derivatives and evaluation of their in vitro antimicrobial activity, J. Heterocycl. Chem., 2020, 57(3), 1361–1367 CrossRef CAS.
- R. Gondru, J. Banothu, R. K. Thatipamula and R. Bavantula, 3-(1-Phenyl-4-((2-(4-arylthiazol-2-yl) hydrazono) methyl)-1 H-pyrazol-3-yl)-2 H-chromen-2-ones: one-pot three component condensation, in vitro antimicrobial, antioxidant and molecular docking studies, RSC Adv., 2015, 5(42), 33562–33569 RSC.
- R. Gali, J. Banothu and R. Bavantula, One-Pot Multicomponent Synthesis of Novel Substituted Imidazo [1, 2-a] Pyridine Incorporated Thiazolyl Coumarins and their Antimicrobial Activity, J. Heterocycl. Chem., 2015, 52(3), 641–646 CrossRef CAS.
- R. Gali, J. Banothu, R. Gondru, R. Bavantula, Y. Velivela and P. A. Crooks, One-pot multicomponent synthesis of indole incorporated thiazolylcoumarins and their antibacterial, anticancer and DNA cleavage studies, Bioorg. Med. Chem. Lett., 2015, 25(1), 106–112 CrossRef CAS PubMed.
- S. Mamidala, S. R. Peddi, R. K. Aravilli, P. C. Jilloju, V. Manga and R. R. Vedula, Microwave irradiated one pot, three component synthesis of a new series of hybrid coumarin based thiazoles: Antibacterial evaluation and molecular docking studies, J. Mol. Struct., 2021, 1225, 129114 CrossRef CAS.
- R. Gondru, S. Kanugala, S. Raj, C. G. Kumar, M. Pasupuleti, J. Banothu and R. Bavantula, 1, 2, 3-triazole-thiazole hybrids: Synthesis, in vitro antimicrobial activity and antibiofilm studies, Bioorg. Med. Chem. Lett., 2021, 33, 127746 CrossRef CAS PubMed.
- K. Godugu, T. R. Gundala, R. Bodapati, V. D. S. Yadala, S. S. Loka and C. G. R. Nallagondu, Synthesis, photophysical and electrochemical properties of donor–acceptor type hydrazinyl thiazolyl coumarins, New J. Chem., 2020, 44(17), 7007–7016 RSC.
- G. Rajitha, V. Ravibabu, G. Ramesh, B. Rajitha, R. Jobina, B. Siddhardha and S. V. Laxmi, One-pot multicomponent synthesis of novel thiazolylhydrazone derivatives and their antimicrobial activity, Res. Chem. Intermed., 2015, 41(12), 9703–9713 CrossRef CAS.
- R. K. Ramagiri, R. R. Vedula and M. K. Thupurani, A facile one-step multi-component approach toward the synthesis of 3-(2-amino-4-thiazolyl) coumarins by using trimethylsilyl isothiocyanate and their antioxidant and anti-inflammatory activity, Phosphorus, Sulfur Silicon Relat. Elem., 2015, 190(9), 1393–1397 CrossRef CAS.
- T. B. Aychiluhim and V. R. Rao, Multi-component Synthesis of 3-{3-[2-(1 H-Indol-3-yl) ethyl]}-2, 3-dihydro-2-(aryliminothiazol-4-yl)-2 H-chromen-2-ones, Org. Prep. Proced. Int., 2014, 46(1), 66–75 CrossRef CAS.
- S. Pavurala, K. Vaarla and R. R. Vedula, One-pot synthesis of bis (phenylimino dihydro thiazolyl-2 H-chromene) derivatives, Synth. Commun., 2017, 47(4), 330–334 CrossRef CAS.
- S. K. J. Shaikh, M. S. Sannaikar, M. N. Kumbar, P. K. Bayannavar, R. R. Kamble, S. R. Inamdar and S. D. Joshi, Microwave-Expedited Green Synthesis, Photophysical, Computational Studies of Coumarin-3-yl-thiazol-3-yl-1, 2, 4-triazolin-3-ones and Their Anticancer Activity, ChemistrySelect, 2018, 3(16), 4448–4462 CrossRef CAS.
- K. Kavitha, D. Srikrishna and P. Aparna, An efficient one-pot three-component synthesis of 2-(4-(2-oxo-2H-chromen-3-yl) thiazol-2-yl)-3-arylacrylonitriles and their cytotoxic activity evaluation with molecular docking, J. Saudi Chem. Soc., 2019, 23(3), 325–337 CrossRef CAS.
- K. Vaarla, R. K. Kesharwani, K. Santosh, R. R. Vedula, S. Kotamraju and M. K. Toopurani, Synthesis, biological activity evaluation and molecular docking studies of novel coumarin substituted thiazolyl-3-aryl-pyrazole-4-carbaldehydes, Bioorg. Med. Chem. Lett., 2015, 25(24), 5797–5803 CrossRef CAS PubMed.
- M. Madni, M. N. Ahmed, M. Hafeez, M. Ashfaq, M. N. Tahir, D. M. Gil, B. Galmés, S. Hameed and A. Frontera, Recurrent π–π stacking motifs in three new 4, 5-dihydropyrazolyl–thiazole–coumarin hybrids: X-ray characterization, Hirshfeld surface analysis and DFT calculations, New J. Chem., 2020, 44(34), 14592–14603 RSC.
- R. Aggarwal, S. Kumar, P. Kaushik, D. Kaushik and G. K. Gupta, Synthesis and pharmacological evaluation of some novel 2-(5-hydroxy-5-trifluoromethyl-4, 5-dihydropyrazol-1-yl)-4-(coumarin-3-yl) thiazoles, Eur. J. Med. Chem., 2013, 62, 508–514 CrossRef CAS PubMed.
- S. Pavurala and R. R. Vedula, Synthesis of 3-(2-(4, 5-Dihydro-3, 5-diphenylpyrazol-1-yl) thiazol-4-yl)-2 H-chromen-2-one Derivatives via Multicomponent Approach, Synth. Commun., 2014, 44(5), 583–588 CrossRef CAS.
- S. Ben Mohamed, Y. Rachedi, M. Hamdi, F. Le Bideau, C. Dejean and F. Dumas, An Efficient Synthetic Access to Substituted Thiazolyl-pyrazolyl-chromene-2-ones from Dehydroacetic Acid and Coumarin Derivatives by a Multicomponent Approach, Eur. J. Org. Chem., 2016, 2016(15), 2628–2636 CrossRef CAS.
- N. O. Mahmoodi and S. Ghodsi, Thiazolyl-pyrazole-biscoumarin synthesis and evaluation of their antibacterial and antioxidant activities, Res. Chem. Intermed., 2017, 43(2), 661–678 CrossRef CAS.
- V. A. Vardhan, V. R. Kumar and V. R. Rao, Condensation of 3-methyl/ethyl-5-mercapto-s-triazole with 3-acetylcoumarin and its derivatives, Indian Journal of Chemistry-Section B, 1999, 38, 18–23 Search PubMed.
- J. Banothu, M. Khanapur, S. Basavoju, R. Bavantula, M. Narra and S. Abbagani, Synthesis, characterization and biological evaluation of fused thiazolo [3, 2-a] pyrimidine derivatives, RSC Adv., 2014, 4(44), 22866–22874 RSC.
- B. Sakram, B. Sonyanaik, K. Ashok, S. Rambabu and S. Johnmiya, Benzo [h] thiazolo [2, 3-b] quinazolines by an efficient p-toluenesulfonic acid-catalyzed one-pot two-step tandem reaction, Res. Chem. Intermed., 2016, 42(3), 1699–1705 CrossRef CAS.
- G. Rajitha, C. Arya, B. Janardhan, S. Laxmi, G. Ramesh and U. S. Kumari, 3-Aryl/Heteryl-5-Phenylindeno [1, 2-d] thiazolo [3, 2-a] pyrimidin-6 (5 H)-ones: Synthesis, Characterization, and Antimicrobial Investigation, Russ. J. Bioorg. Chem., 2020, 46(4), 612–619 CrossRef CAS.
- R. Velpula, J. Banothu, R. Gali, Y. Sargam and R. Bavantula, One-pot and solvent-free synthesis of 3-(9-hydroxy-3-methoxy-7-aryl-6, 7, 9, 10-tetrahydro-5$ H $-benzo [$ h $] thiazolo [2, 3-$ b $] quinazolin-9-yl)-2$ H $-chromen-2-ones and their antibacterial evaluation, Turk. J. Chem., 2015, 39(3), 620–629 CrossRef CAS.
- L. Suresh, P. S. V. Kumar, Y. Poornachandra, C. G. Kumar, N. J. Babu and G. Chandramouli, An expeditious four-component domino protocol for the synthesis of novel thiazolo [3, 2-a] thiochromeno [4, 3-d] pyrimidine derivatives as antibacterial and antibiofilm agents, Bioorg. Med. Chem., 2016, 24(16), 3808–3817 CrossRef CAS PubMed.
- A. Anand, R. J. Naik, H. M. Revankar, M. V. Kulkarni, S. R. Dixit and S. D. Joshi, A click chemistry approach for the synthesis of mono and bis aryloxy linked coumarinyl triazoles as anti-tubercular agents, Eur. J. Med. Chem., 2015, 105, 194–207 CrossRef CAS PubMed.
- Y. A. Attia, Y. M. Mohamed, M. M. Awad and S. Alexeree, Ag doped ZnO nanorods catalyzed photo-triggered synthesis of some novel (1H-tetrazol-5-yl)-coumarin hybrids, J. Organomet. Chem., 2020, 919, 121320 CrossRef CAS.
- I. Dyachenko, V. Dyachenko, I. Yakushev, V. Khrustalev and V. Nenaidenko, Chromeno [3 ′′, 4 ′′: 5′, 6′] pyrido [2′, 3′: 4, 5] thieno [3, 2-e] pyridine—A New Heterocyclic System. Synthesis and Molecular and Crystal Structures, Russ. J. Org. Chem., 2020, 56(9), 1669–1672 CrossRef CAS.
- K. Kavitha, D. Srikrishna, P. K. Dubey and P. Aparna, Tetrabutylammonium tribromide: an effective green reagent for the one-pot reaction of 3-acetyl-2H-chromen-2-ones withoff-phenylenediamines, Org. Chem., 2018, 172–185 CAS.
- A. A. Kamble, R. R. Kamble, M. N. Kumbar and G. Tegginamath, Pyridine-catalyzed synthesis of quinoxalines as anticancer and anti-tubercular agents, Med. Chem. Res., 2016, 25(6), 1163–1174 CrossRef CAS.
- J.-F. Zhou, G.-X. Gong, K.-B. Shi and Y.-L. Zhu, Synthesis of 3-(quinoxalin-2-yl)-2 H-chromen-2-ones under microwave irradiation, Monatsh. Chem., 2009, 140(6), 651–654 CrossRef CAS.
- V. S. R. Chunduru and R. R. Vedula, One-Pot Synthesis of 1, 3, 4-Thiadiazin-5-yl-chromen-2-one Derivatives via Three-Component Reaction, Synth. Commun., 2012, 42(10), 1454–1460 CrossRef CAS.
- S. Pavurala, K. Vaarla, R. Kesharwani, L. Naesens, S. Liekens and R. R. Vedula, Bis coumarinyl bis triazolothiadiazinyl ethane derivatives: Synthesis, antiviral activity evaluation, and molecular docking studies, Synth. Commun., 2018, 48(12), 1494–1503 CrossRef CAS.
- N. Lechani, M. Hamdi, B. Kheddis-Boutemeur, O. Talhi, Y. Laichi, K. Bachari and A. M. Silva, Synthetic Approach Toward Heterocyclic Hybrids of [1, 2, 4] Triazolo [3, 4-b][1, 3, 4] thiadiazines, Synlett, 2018, 29(11), 1502–1504 CrossRef CAS.
- R. E. Khidre, I. A. M. Radini and B. F. Abdel-Wahab, Synthesis of New Heterocycles Incorporating 3-(N-phthalimidomethyl)-1, 2, 4-triazole as Antimicrobial Agents, Egypt. J. Chem., 2016, 59(5), 731–744 CrossRef.
- P. V. Kumar and V. R. Rao, Synthesis and antitubercular, antiviral and anticancer activity of 3-(3-mercaptoalkyl-7H-[1, 2, 4] triazolo [3, 4-b][1, 3, 4]-thiadiazin-6-yl) chromen-2-one and its derivatives, Indian Journal of Chemistry-Section B, 2008, 47, 106–111 Search PubMed.
- K. Vaarla and R. R. Vedula, Synthesis of Novel 3-(3-(5-Methylisoxazol-3-yl)-7H-[1,2,4]Triazolo [3,4-b][1,3,4]Thiadiazin-6-yl)-2H-Chromen-2-Ones, J. Heterocycl. Chem., 2016, 53(1), 69–73 CrossRef CAS.
- H. A. El-Sayed, M. G. Assy and A. Mohamed, An efficient synthesis and antimicrobial activity of N-bridged triazolo [3, 4-b] thiadiazine and triazolo [3, 4-b] thiadiazole derivatives under microwave irradiation, Synth. Commun., 2020, 50(7), 997–1007 CrossRef CAS.
- T. B. Aychiluhim and V. R. Rao, Efficient, One-Pot Synthesis of Triazolothiadiazinyl-pyrazolone and Pyrazolyl-triazolothiadiazine Derivatives via Multicomponent Reaction, Synth. Commun., 2014, 44(10), 1422–1429 CrossRef CAS.
- S. Pavurala and R. R. Vedula, An Efficient, Multicomponent Synthesis of Pyrazolyl Triazolo Thiadiazinyl Chromen - 2 - Ones, J. Heterocycl. Chem., 2015, 52(1), 306–309 CrossRef CAS.
- V. S. Rao Chunduru and V. R. Rao, Synthesis of Aryl and Heteryl 1,3,4-Thiadiazinyl-phthalazine-1,4-dione Derivatives via a Multicomponent Approach, Synth. Commun., 2013, 43(7), 923–929 CrossRef CAS.
- V. N. Kumar, E. De Clercq and B. Rajitha, One-Pot Synthesis of Novel 3-(2-Oxo-2H-chromen-3-yl)-[1,3,4] Thiadiazino [2,3-b] Quinazolin-6 (2H)-ones Under Microwave Irradiation, Phosphorus, Sulfur Silicon Relat. Elem., 2007, 182(2), 273–279 CrossRef CAS.
- R. Kenchappa, Y. D. Bodke, S. K. Peethambar, S. Telkar and V. K. Bhovi, Synthesis of β-amino carbonyl derivatives of coumarin and benzofuran and evaluation of their biological activity, Med. Chem. Res., 2013, 22(10), 4787–4797 CrossRef CAS.
- S. M. H. Sanad, A. A. M. Ahmed and A. E. M. Mekky, Synthesis, in vitro and in-silico study of novel thiazoles as potent antibacterial agents and MurB inhibitors, Arch. Pharm., 2020, 353(4), 1900309 CrossRef CAS PubMed.
- A. Arshad, H. Osman, M. C. Bagley, C. K. Lam, S. Mohamad and A. S. M. Zahariluddin, Synthesis and antimicrobial properties of some new thiazolyl coumarin derivatives, Eur. J. Med. Chem., 2011, 46(9), 3788–3794 CrossRef CAS.
- F. Sonmez, B. Zengin Kurt, I. Gazioglu, L. Basile, A. Dag, V. Cappello, T. Ginex, M. Kucukislamoglu and S. Guccione, Design, synthesis and docking study of novel coumarin ligands as potential selective acetylcholinesterase inhibitors, J. Enzyme Inhib. Med. Chem., 2017, 32(1), 285–297 CrossRef CAS PubMed.
- T. Ohya, N. Kudo, E. Suzuki and Y. Kawashima, Determination of perfluorinated carboxylic acids in biological samples by high-performance liquid chromatography, J. Chromatogr. B: Biomed. Sci. Appl., 1998, 720(1), 1–7 CrossRef CAS.
- E. Poboży, E. Król, L. Wójcik, M. Wachowicz and M. Trojanowicz, HPLC determination of perfluorinated carboxylic acids with fluorescence detection, Mikrochim. Acta, 2011, 172(3–4), 409–417 CrossRef PubMed.
- S. K. Padhan, M. B. Podh, P. K. Sahu and S. N. Sahu, Optical discrimination of fluoride and cyanide ions by coumarin-salicylidene based chromofluorescent probes in organic and aqueous medium, Sens. Actuators, B, 2018, 255, 1376–1390 CrossRef CAS.
- F. Yan, X. Sun, Y. Jiang, R. Wang, Y. Zhang and Y. Cui, Dual-function fluorescent probe with red emission for sensing viscosity and OCl− and its applications in bioimaging, Dyes Pigm., 2020, 182, 108531 CrossRef CAS.
- P. E. Hande, A. B. Samui and P. S. Kulkarni, Selective nanomolar detection of mercury using coumarin based fluorescent Hg (II)—Ion imprinted polymer, Sens. Actuators, B, 2017, 246, 597–605 CrossRef CAS.
- N. Kumar, Y. Hori, M. Nishiura and K. Kikuchi, Rapid no-wash labeling of PYP-tag proteins with reactive fluorogenic ligands affords stable fluorescent protein conjugates for long-term cell imaging studies, Chem. Sci., 2020, 11(14), 3694–3701 RSC.
| This journal is © The Royal Society of Chemistry 2021 |