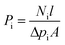Open Access Article
Open Access ArticleEffects of mesoporous silica particle size and pore structure on the performance of polymer-mesoporous silica mixed matrix membranes†
Junhui Wang a,
Gang Wangb,
Zhongshen Zhang*c,
Gangfeng Ouyang
a,
Gang Wangb,
Zhongshen Zhang*c,
Gangfeng Ouyang ade and
Zhengping Hao
ade and
Zhengping Hao *c
*c
aMOE Key Laboratory of Bioinorganic and Synthetic Chemistry/KLGHEI of Environment and Energy Chemistry, School of Chemistry, Sun Yat-Sen University, No. 135, Xingang Xi Road, Guangzhou, Guangdong 510275, China
bSchool of Materials Design & Engineering, Beijing Institute of Fashion Technology, Beijing, 100029, China
cNational Engineering Laboratory for VOCs Pollution Control Material & Technology, Research Center for Environmental Material and Pollution Control Technology, University of Chinese Academy of Sciences, Beijing, 101408, China. E-mail: zszhang@ucas.ac.cn; zphao@ucas.ac.cn
dChemistry College, Center of Advanced Analysis and Gene Sequencing, Zhengzhou University, Kexue Avenue 100, Zhengzhou 450001, China
eProvincial Key Laboratory of Emergency Test for Dangerous Chemicals, Guangdong Provincial Engineering Research Center for Ambient Mass Spectrometry, Institute of Analysis, Guangdong Academy of Sciences (China National Analytical Center Guangzhou), 100 Xianlie Middle Road, Guangzhou 510070, China
First published on 11th November 2021
Abstract
The fabrication of mixed matrix membranes (MMMs) has been regarded as an effective and economic approach to enhance the gas permeability and selectivity properties of conventional polymeric membranes for gas separation applications. However, the poor compatibility between polymeric matrix and inorganic filler in MMMs could lead to the generation of interfacial defects resulting in reduced gas selectivity. In this work, with the aim of studying the effect of particle size and pore structure of the filler on the performance of the resultant MMMs, nano/micro sized spherical mesoporous silicas with 2D/3D pore structure (MCM-41 and MCM-48) were synthesized and selected as fillers for the preparation of polydimethylsiloxane (PDMS)-based MMMs. The separation properties of the membranes prepared were characterized by permeability measurements for nitrogen and organic vapors (C3H6 and n-C4H10). Compared with microsized particles, nanosized fillers have better dispersion in the polymer matrix which could minimize the formation of non-selective microvoids around the particles, leading to higher vapor/N2 ideal selectivities of the MMMs, even at the high loading (15 wt%). Moreover, due to the conventional random packing orientation of the particles in the polymer, vapor permeation was severely hindered in the MMMs fabricated from mesoporous silica with 2D pore channels. The interface morphologies and gas diffusion paths in the MMMs have also been proposed. With an optimum loading of nanosized MCM-48 (3D pore structure), the vapor permeabilities and vapor/N2 ideal selectivities of the MMMs were shown to increase simultaneously, compared with the neat polymer membrane.
1. Introduction
Organic compounds such as ethylene, propylene, and butane are important industrial raw materials. In petrochemical processes, the excess monomers would remain in the off-gas stream. If these monomers are emitted to the atmosphere, it would cause serious environmental problems, such as the formation of fine particles, photochemical smog and global warming. Therefore, it is necessary and important to recover the unreacted organic materials from the off-gas for reuse.1,2The membrane separation method has a good application prospect in the field of gas separation, owing to its low price, desirable physical robustness and good processability.3–5 However, polymeric membranes are suffered from the trade-off relationship between gas permeability and selectivity, and still do not meet the requirements of the current advanced membrane technology due to their low separation performance (moderate selectivity) both at lab and commercial scale.3,6 An economic alternative approach to solve this problem is to combine the advantages of both polymers and inorganic fillers, fabricating the mixed matrix membranes (MMMs).7–9 In recent years, different kinds of inorganic materials were employed as fillers to fabricate MMMs, such as zeolites, silicas, clays, activated carbons, carbon nanotubes, carbon molecular sieves, metal–organic frameworks (MOFs), etc.10–16
The most important factor influencing the MMM success is to obtain a good compatibility between the continuous (polymer) and disperse (filler) phase.3 Apart from adjusting the surface chemical composition of the filler to minimize filler–polymer gaps, proper regulation of the particle size is also proved to be an effective approach to avoid non-selective defects formation. Tantekin-Ersolmaz et al. investigated the effect of particle size on the performance of silicalite-PDMS mixed matrix membranes, and found that the gas permeability values decreased when relatively smaller particle sizes were employed, probably due to the enhanced area and number of zeolite–polymer interfaces that the gas molecules have to cross in these cases.17 Suen et al. found that compared with lamellar Na-montmorillonite clay (mean length = 500 nm), the incorporation of 70 nm spherical TiO2 particles into polyethersulfone (PES) could form an ideal interface morphology leaded to an increase of gas selectivity compared to the pure polymer membrane.10 It has also been reported that nanosizing the particle size of MOF could enhance its dispersion within the polymer matrix to minimize non-selective microvoid formation around the particles.11 However, on the contrary, Coronas et al. stated that compared with the nanosized particles (MCM-41), 2–4 μm particles (MCM-48) could provide a lower external surface area and minimize the agglomeration, hence improving dispersibility and interaction with the polymer.4
These inconsistent conclusions made by the above literatures could be attributed to the application of different kinds of inorganic particles for comparison, since the pore size and pore channel orientation of the inorganic fillers are of great importance for the performance of MMMs. According to the Maxwell equation, an increase in the amount of less permeable dispersed phase in the polymer matrix decreases the permeability.18 Therefore, the addition of microporous fillers (such as zeolites) usually decreases the gas permeability of MMMs, and conversely, the addition of large pore fillers would increase the gas permeability.4,12,17,18 Moreover, it has been demonstrated that the gas permeability can be significantly improved by aligning the pore orientation parallel to the gas diffusion path, by creating more available nanoporosity in the polymers having good permeation potential.12,16 Therefore, textural properties (pore size and structure) and particle size distributions should be taken into account as the critical variables to obtain a high-performance MMM.
Since the discovery of the M41S family in 1992 by Kresge et al., a variety of ordered mesoporous materials have been synthesized with various framework compositions, morphologies and pore structures.19–22 The particle shape, size and pore structure could be easily manipulated by adjusting surfactants and structure-directing agents, which makes these materials (e.g., MCM-41 and MCM-48) promising for the development of a new generation of MMMs and suitable for investigating the effects of factors that influence the performance of MMMs.4,14,23 Moreover, mesoporous materials possess pores with sizes ranging from 2 nm to 50 nm, which allow the penetration of the polymer chains (most of the cross-section areas per chain are around 1 nm).24,25 Such good contact could lead to better wetting and dispersion of particles, thus suppressing the formation of voids at the interface and improving the separation performance of the MCM-48/PS MMMs.4
In this study, in order to investigate the effects of particle size and pore structure on the performance of mesoporous silica-PDMS mixed matrix membranes, three kinds of mesoporous silicas (nm MCM-48, nm MCM-41 and μm MCM-48) with the same pore diameter, but different particle size (nano and micro) and pore structure (2D and 3D) were elaborately synthesized and employed as fillers. The MCM mesoporous silicas were characterized by X-ray diffraction (XRD), pore size analysis, and high resolution-transmission electron microscope (HR-TEM). A series of MMMs with different type and various dosage of MCM were fabricated. Characterization and gas permeation measurements show that the particle size and pore structure of fillers significantly influenced the performance of matrix membranes. Based on these research results, the interface morphologies and possible gas diffusion paths in different MCM/PDMS MMMs were proposed.
2. Experimental section
2.1 Preparation of mesoporous silica particles
2.2 Preparation of pure PDMS and mesoporous silica-PDMS MMMs
The membranes were prepared by a solvent casting method. Dense, unfilled films of PDMS were made from Momentive RTV 615 A/B (Wenhao Chip Technology, China) composed of two components: component A contains platinum catalyst while component B contains the cross-linker. After optimization, components A and B were mixed at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) ratio in heptane to make a final PDMS concentration of 60 wt%. After stirring for 1 h and ultrasonic treatment for 30 min, the polymer solution was cast onto the PVDF membrane to form the composite membrane. The prepared membranes were placed in oven at 45 °C for 48 h and then cured for 3 h at 100 °C. The basement PVDF membranes were immersed in deionized water at room temperature for 3 days and then thermally treated at 120 °C for 12 min before use.
1 (w/w) ratio in heptane to make a final PDMS concentration of 60 wt%. After stirring for 1 h and ultrasonic treatment for 30 min, the polymer solution was cast onto the PVDF membrane to form the composite membrane. The prepared membranes were placed in oven at 45 °C for 48 h and then cured for 3 h at 100 °C. The basement PVDF membranes were immersed in deionized water at room temperature for 3 days and then thermally treated at 120 °C for 12 min before use.
Before MMM preparation, the mesoporous silica powders were dried at 80 °C overnight. Certain amount (2–15 wt%, with respect to PDMS content) of silica powders were added to heptane and then dispersed through stirring for 30 min. After then, the PDMS components were added to the dispersion to obtain a 60 wt% polymer solution. The mixture was further stirred for 1 h and sonicated for 30 min, and then prepared similarly to that of the pure PDMS membranes.
2.3 Physico-chemical characterization
The textual properties of the mesoporous silica particles were investigated by nitrogen adsorption and desorption at 77 K using a volumetric adsorption analyzer (ASAP2020, Micromeritics Instrument Corporation, USA). The BET (Brunauer–Emmett–Teller) surface areas were calculated from N2 adsorption isotherms within the relative pressure ranging from 0.05 to 0.25. The pore size distributions were obtained from the N2 desorption isotherms using the BJH method. The ordered mesopore regularity of the samples was characterized by X-ray diffractometer (X'Pert PRO MPD, Panalytical, the Netherlands) with Cu Kα radiation (λ = 0.1541 nm) at 40 kV and 40 mA. The morphologies of membranes and mesoporous silica particles were inspected using scanning electron microscopy (SEM, S-3000N, Hitachi, Japan) and transmission electron microscopy (TEM, H-7500, Hitachi, Japan). The pore structures of the mesoporous silica were inspected using the high resolution-TEM on a JEM 2010 at 200 kV. The glass transition temperature (Tg) of the membranes was measured on a SEIKO DSC 6220. About 10 mg of each sample was placed in an aluminum pan and the measurements were carried out from −160 °C to −50 °C at a 10 °C min−1 ramping rate under a N2 atmosphere. Sorption studies were investigated using an Intelligent Gravimetric Analyzer (IGA-002, Hiden Isochema Ltd, UK). The apparatus has an ultrahigh vacuum system comprising a microbalance, temperature regulation and pressure control system, all of which are fully computerized. The adsorption and desorption isotherms were determined by the approach of monitoring equilibrium in real time using a computer algorithm.29 For each measurement, the samples were outgassed to a constant weight, at <10−6 Pa, at 100 °C.2.4 Gas separation measurements
Pure gas separation (N2, C3H6, n-C4H10) of the membranes were measured at 35 °C using a custom-built membrane module as reported in our previous work.29 The gas permeability was calculated using the following equation in the steady state:30where N is the permeate flow (cm3 s−1), l is the membrane thickness (cm), A is the membrane area (cm2), pf and pp are the feed and permeate pressure in 1.333224 × 103 Pa (cmHg), respectively. The permeability is expressed in Barrer (1 Barrer = 10−10 cm3 (STP) cm cm−2 s−1 cmHg−1).
The ideal selectivity of a membrane is the ratio of the permeabilities of pure gas components A and B:
Mixed-gas permeation properties of membranes were determined with feed mixture a feed mixture containing 25 vol% C3H6 and 75 vol% N2. The pressure difference over the membrane was 0.5 bar, the permeation side was kept at atmospheric pressure. The composition of the permeate stream was determined with a gas chromatograph (7890A, Agilent). The total permeate flow was measured with a digital flowmeter. The permeability of each component can be calculated with the following expression:
3. Results and discussion
3.1 Characterization of mesoporous silica particles
3.2 Characterization of the mixed matrix membranes
 | ||
| Fig. 5 N2 permeability of different MCM/PDMS MMMs at 0.5 bar at 35 °C (the permeability of pure PDMS membrane is indicated by the red dashed line). | ||
For low nm MCM-48 additions, there is an increase for selectivity meanwhile there is no loss in overall permeability. Generally, rigidification of the polymer leads to increased selectivity, but also always leads to low permeability.11 This anomaly is due to that the MCM-48 itself is a large-pore system that compensates for the loss in PDMS permeability in the composite. According to the solution-diffusion model,40 the transport of gases in polymer membranes are governed by two factors: sorption and diffusion. While the diffusion step favors small molecules, the sorption step favors large and condensable molecules. Gas permeability and selectivity in rubbery membrane (PDMS) is more strongly influenced by the solubility factor than diffusivity factor. Vapors with condensability could cause increased polymer chain mobility and plasticization,41 therefore dissolving the rigidified polymer chains and diffusing into the pores of nm MCM-48, in which the diffusion rate could be greatly enhanced. This is why MMM with 2 wt% nanosized MCM-48 can increase the permeabilities of vapors. It should be mentioned that there is a decrease in the membrane permeability as more nm MCM-48 is added to the polymer. This is attributed to the formation of more rigidified layers and the collection of the nanosized fillers into an essentially non-porous aggregate.11 Even for the inclusion of 15 wt% filler, the use of nm MCM-48 particles created less voids and defects on the MMM (as shown in Fig. 4f) such that its vapors/N2 selectivities are still satisfactory. In sharp contrast, with the increased loading of μm MCM-48, the MMMs exhibited enhanced N2 permeability and reduction of ideal selectivities (Fig. S3†). This could be attributed to the non-selective diffusion at a defective interface (voids and defects) between micro-particles and polymer, as observed in the SEM images (Fig. S4†). Therefore, it can be revealed that compared with micro-sized particles, nanosized fillers could improve the interaction between polymer and filler and avoid the formation of non-selective defects.
The effect of pore structure of fillers on the performance of MMMs was explored by employing fillers with 3D and 2D pore structure, respectively. Both nm MCM-48 and nm MCM-41 are spherical particles with uniform diameter of ∼100 nm (Fig. 3), but with different pore structures: the former possesses three-dimensional (3D) bicontinuous cubic arrangement of mesopores, while the latter has two-dimensional (2D) hexagonal array of cylindrical mesopores which is not interconnected (Fig. 2 and 3). As shown in Fig. 6a, the incorporation of nm MCM-41 also induces the decrease of N2 permeability, which further proves the better wetting and dispersion of nanosized particles in the polymer matrix.11 However, in sharp contrast to nm MCM-48, drastic declines of C3H6/n-C4H10 permeabilities (decrease by 95%/98% to the neat polymer membrane), and C3H6/N2 and n-C4H10/N2 ideal selectivities (decrease by 88% and 94%) were observed, when the same loading of nm MCM-41 was added. As proposed in our previous report,29 the large mesopores of the fillers could provide channels with much faster diffusion rates for organic vapors, when they dissolve the rigidified polymer chains at the pore entrance and enter into the pores of the mesoporous silicas. This is why the incorporation of nm MCM-48 could increase the vapor permeabilities and vapor/N2 selectivities simultaneously. The poor performance of nm MCM-41 might be due to its 2D pore structure. As we know, the fillers disperse in the polymer matrix randomly, and most of the pore orientations cannot be parallel to the gas diffusion path, which will severely hinder the diffusion of the gases.
In order to prove the above assumptions, gas diffusions in different MMMs were investigated by measuring the gravimetric sorption isotherms. It should be noted that the isotherms for N2 cannot be obtained because there is no interaction between the membranes and N2. The adsorption amounts of C3H6 in MCM/PDMS MMMs and mesoporous silicas at 0.5 bar at 35 °C are listed in Table 1. It can be observed that C3H6 adsorption amounts of all the MCM/PDMS MMMs are higher than that of the pure polymer membrane, indicating that the vapor could diffuse into the pores of the silicas. The experimental adsorption amount of 2 wt% nm MCM-48 MMM is nearly the same as the calculated value, which means the vapor could diffuse to all the channels of the filler and no voids formed on the filler–polymer interface. However, for the 2 wt% μm MCM-48 MMM, the experimental value is larger than the theoretical one, implying the formation of extra nano/micro sized voids in which vapors adsorb and condense. Conversely, compared to the calculated value, the experimental one decreases, for 2 wt% nm MCM-41 MMM, revealing that vapors could only penetrate to partial portion of the pores in nm MCM-41, consistent with the gas separation performance.
| Membrane | Amount adsorbed (mmol g−1) | Mesoporous silica | Amount adsorbed (mmol g−1) | |
|---|---|---|---|---|
| Experimental value | Calculated value | |||
| Pure PDMS | 0.042 | — | ||
| 2 wt% nm MCM-48 MMM | 0.057 | 0.058 | nm MCM-48 | 0.89 |
| 2 wt% μm MCM-48 MMM | 0.073 | 0.067 | μm MCM-48 | 1.34 |
| 2 wt% nm MCM-41 MMM | 0.046 | 0.051 | nm MCM-41 | 0.51 |
As shown in Fig. 7a, nm MCM-48 well disperse in the polymer matrix, and the rigidified layers form at the filler–polymer interface, possibly due to the partial pore blocking of MCM-48 by the polymer chains.4,42,43 According to the solution–diffusion model, vapors with condensability could cause increased polymer chain mobility and plasticization, therefore dissolving the rigidified polymer chains and diffusing into the pores of nm MCM-48. And once the vapors enter into the pores with large diameter, the diffusion rates could be increased dramatically. In contrast, N2 is inert and noncondensable, and as a result, it cannot pass through the rigidified polymer layer and utilize the pores of nm MCM-48. The increased tortuosity caused by the fillers leads to reduced permeability of N2. Therefore, the vapor permeabilities and vapor/N2 ideal selectivities could be increased at the same time for 2 wt% nm MCM MMMs.
 | ||
| Fig. 7 Hypotheses for the interface morphologies and gas diffusion pathways through nm MCM-48/PDMS MMM (a), μm MCM-48/PDMS MMM (b) and nm MCM-41/PDMS MMM (c). | ||
For the μm MCM-48 MMM (Fig. 7b), because of the large particle size, the fillers could not be wrapped perfectly by the polymer chains, leading to the formation of voids and defects. Both N2 and vapors prefer to diffuse through these non-selective voids, resulting in higher permeabilities and lower selectivities. In the nm MCM-41 MMM (Fig. 7c), it could not be guaranteed that the 2D pores are parallel to the gas diffusion path. As a result, even though vapors could diffuse into the pores, the increases in the diffusion path lengths experiencing when they traverse the membrane lead to decreased permeabilities. At the meantime, due to the acceleration effect of the pores of nm MCM-41, the values of the vapor/N2 ideal selectivities are still higher than one.
4. Conclusions
In this work, several kinds of MMMs fabricated with nano/micro sized mesoporous silicas with 2D/3D pore structure (MCM-41 and MCM-48) and PDMS were prepared and employed to study the effect of the particle size and pore structure of the filler on the gas separation performances of the resultant MMMs. As for the effect of particle size, nanosized fillers could have better compatibility with the polymer matrix, and no non-selective defects formed at the filler–polymer interface. As a result, the MMMs achieved enhanced vapor/N2 ideal selectivities, even at higher loading (15 wt%), compared with those of the neat polymer membrane. As regards the pore structure, due to the conventional random packing orientation of the particles in the polymer, gas permeation could be severely hindered in the MMMs fabricated from mesoporous silica with 2D pore channels. Assisted by the sorption data of mesoporous silicas, pure membrane and MMMs, the interface morphologies and gas diffusion paths in the MMMs have also been proposed. With an optimum loading of nanosized MCM-48 (3D pore structure), the vapor permeabilities and vapor/N2 ideal selectivities of the MMM were shown to increase simultaneously due to the interconnected pore channels in the filler and the good filler–polymer interaction. This work would provide guidance on the rational design of MMMs with high performance.Author contributions
J. W., Z. H. and Z. Z. conceived the project. J. W., G. W. and Z. Z. designed, analyzed, and discussed the experimental results. J. W. and G. W. performed the measurements. Z. H., Z. Z. and O. G. gave suggestions on the experiments and drafted the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge financial support from the projects of the R&D Program of Beijing Municipal Education Commission (KJZD20191443001), the National Natural Science Foundation (Nos. 21806192, 22036003, 21737006 and 22076222), the Science and Technology Program of Guangzhou (202102021203), the Natural Science Foundation of Guangdong Province (2018A030313441), Guangdong Provincial Key R&D Programme (2020B1111350002), the Fundamental Research Funds for the Central Universities (19lgpy135) and Jiangsu Key Laboratory of Vehicle Emissions Control (OVEC053).References
- R. W. Baker, J. G. Wijmans and J. H. Kaschemekat, The design of membrane vapor–gas separation systems, J. Membr. Sci., 1998, 151, 55–62 CrossRef CAS.
- R. W. Baker and M. Jacobs, Improve monomer recovery from polyolefin resin degassing, Hydrocarbon Process., 1996, 75, 49–51 CAS.
- J. Gascon, F. Kapteijn, B. Zornoza, V. Sebastián, C. Casado and J. Coronas, Practical approach to zeolitic membranes and coatings: state of the art, opportunities, barriers, and future perspectives, Chem. Mater., 2012, 24, 2829–2844 CrossRef CAS.
- B. Zornoza, S. Irusta, C. Tellez and J. Coronas, Mesoporous silica sphere-polysulfone mixed matrix membranes for gas separation, Langmuir, 2009, 25, 5903–5909 CrossRef CAS PubMed.
- P. Bernardo, E. Drioli and G. Golemme, Membrane gas separation: a review/state of the art, Ind. Eng. Chem. Res., 2009, 48, 4638–4663 CrossRef CAS.
- L. M. Robeson, The upper bound revisited, J. Membr. Sci., 2008, 320, 390–400 CrossRef CAS.
- H. Zou, S. S. Wu and J. Shen, Polymer/silica nanocomposites: preparation, characterization, properties, and applications, Chem. Rev., 2008, 108, 3893–3957 CrossRef CAS PubMed.
- Y. Kudo, H. Mikami, M. Tanaka, T. Isaji, K. Odaka, M. Yamato and H. Kawakami, Mixed matrix membranes comprising a polymer of intrinsic microporosity loaded with surface-modified non-porous pearl-necklace nanoparticles, J. Membr. Sci., 2020, 597, 117627 CrossRef CAS.
- Y. Lu, H. Zhang, J. Y. Chan, R. Ou, H. Zhu, M. Forsyth, E. M. Marijanovic, C. M. Doherty, P. J. Marriott, M. M. B. Holl and H. Wang, Homochiral MOF–polymer mixed matrix membranes for efficient separation of chiral molecules, Angew. Chem., Int. Ed., 2019, 58, 16928–16935 CrossRef CAS PubMed.
- C.-Y. Liang, P. Uchytil, R. Petrychkovych, Y.-C. Lai, K. Friess, M. Sipek, M. Mohan Reddy and S.-Y. Suen, A comparison on gas separation between PES (polyethersulfone)/MMT (Na-montmorillonite) and PES/TiO2 mixed matrix membranes, Sep. Purif. Technol., 2012, 92, 57–63 CrossRef CAS.
- B. Ghalei, K. Sakurai, Y. Kinoshita, K. Wakimoto, A. P. Isfahani, Q. Song, K. Doitomi, S. Furukawa, H. Hirao, H. Kusuda, S. Kitagawa and E. Sivaniah, Enhanced selectivity in mixed matrix membranes for CO2 capture through efficient dispersion of amine-functionalized MOF nanoparticles, Nat. Energy, 2017, 2, 17086 CrossRef.
- G. Dong, H. Li and V. Chen, Challenges and opportunities for mixed-matrix membranes for gas separation, J. Mater. Chem. A, 2013, 1, 4610–4630 RSC.
- A. F. Bushell, M. P. Attfield, C. R. Mason, P. M. Budd, Y. Yampolskii, L. Starannikova, A. Rebrov, F. Bazzarelli, P. Bernardo, J. C. Jansen, M. Lanc, K. Friess, V. Shantarovich, V. Gustov and V. Isaeva, Gas permeation parameters of mixed matrix membranes based on the polymer of intrinsic microporosity PIM-1 and the zeolitic imidazolate framework ZIF-8, J. Membr. Sci., 2013, 427, 48–62 CrossRef CAS.
- S. Kim, E. Marand, J. Ida and V. V. Guliants, Polysulfone and mesoporous molecular sieve MCM-48 mixed matrix membranes for gas separation, Chem. Mater., 2006, 18, 1149–1155 CrossRef CAS.
- D. Vu, W. J. Koros and S. J. Miller, Effect of condensable impurity in CO2/CH4 gas feeds on performance of mixed matrix membranes using carbon molecular sieves, J. Membr. Sci., 2003, 221, 233–239 CrossRef CAS.
- A. Sharma, S. Kumar, B. Tripathi, M. Singh and Y. K. Vijay, Aligned CNT/Polymer nanocomposite membranes for hydrogen separation, Int. J. Hydrogen Energy, 2009, 34, 3977–3982 CrossRef CAS.
- Ş. B. Tantekin-Ersolmaz, Ç. Atalay-Oral, M. Tatlıer, A. Erdem-Şenatalar, B. Schoeman and J. Sterte, Effect of zeolite particle size on the performance of polymer–zeolite mixed matrix membranes, J. Membr. Sci., 2000, 175, 285–288 CrossRef.
- P. Gorgojo, S. Uriel, C. Tellez and J. Coronas, Development of mixed matrix membranes based on zeolite Nu-6(2) for gas separation, Microporous Mesoporous Mater., 2008, 115, 85–92 CrossRef CAS.
- C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, Ordered mesoporous molecular-sieves synthesized by a liquid-crystal template mechanism, Nature, 1992, 359, 710–712 CrossRef CAS.
- J. S. Beck, J. C. Vartuli, W. J. Roth, M. E. Leonowicz, C. T. Kresge, K. D. Schmitt, C. T. W. Chu, D. H. Olson and E. W. Sheppard, A new family of mesoporous molecular sieves prepared with liquid crystal templates, J. Am. Chem. Soc., 1992, 114, 10834–10843 CrossRef CAS.
- T. W. Kim, F. Kleitz, B. Paul and R. Ryoo, MCM-48-like large mesoporous silicas with tailored pore structure: facile synthesis domain in a ternary triblock copolymer–butanol–water system, J. Am. Chem. Soc., 2005, 127, 7601–7610 CrossRef CAS PubMed.
- Y. F. Lu, H. Y. Fan, A. Stump, T. L. Ward, T. Rieker and C. J. Brinker, Aerosol-assisted self-assembly of mesostructured spherical nanoparticles, Nature, 1999, 398, 223–226 CrossRef CAS.
- K. Nocon-Szmajda, A. Wolinska-Grabczyk, A. Jankowski, U. Szeluga, M. Wojtowicz, J. Konieczkowska and A. Hercog, Gas transport properties of mixed matrix membranes based on thermally rearranged poly(hydroxyimide)s filled with inorganic porous particles, Sep. Purif. Technol., 2020, 242, 116778 CrossRef CAS.
- R. W. Baker, Future directions of membrane gas separation technology, Ind. Eng. Chem. Res., 2002, 41, 1393–1411 CrossRef CAS.
- R. L. M. Raymond and F. Boyer, Polymer chain stiffness parameter, σ, and cross-sectional area per chain, Macromolecules, 1977, 10, 1167–1169 CrossRef.
- T.-W. Kim, P.-W. Chung and V. S. Y. Lin, Facile synthesis of monodisperse spherical MCM-48 mesoporous silica nanoparticles with controlled particle size, Chem. Mater., 2010, 22, 5093–5104 CrossRef CAS.
- S. S. Jinlou Gu, Y. Li, Q. He, J. Zhong and J. Shi, Surface modification-complexation strategy for cisplatin loading in mesoporous nanoparticles, J. Phys. Chem. Lett., 2010, 1, 3446–3450 CrossRef.
- R. Peng, D. Zhao, N. M. Dimitrijevic, T. Rajh and R. T. Koodali, Room temperature synthesis of Ti–MCM-48 and Ti–MCM-41 mesoporous materials and their performance on photocatalytic splitting of water, J. Phys. Chem. C, 2012, 116, 1605–1613 CrossRef CAS.
- J. Wang, Y. Li, Z. Zhang and Z. Hao, Mesoporous KIT-6 silica-polydimethylsiloxane (PDMS) mixed matrix membranes for gas separation, J. Mater. Chem. A, 2015, 3, 8650–8658 RSC.
- K. De Sitter, P. Winberg, J. D'Haen, C. Dotremont, R. Leysen, J. A. Martens, S. Mullens, F. H. J. Maurer and I. F. J. Vankelecom, Silica filled poly(1-trimethylsilyl-1-propyne) nanocomposite membranes: relation between the transport of gases and structural characteristics, J. Membr. Sci., 2006, 278, 83–91 CrossRef CAS.
- S. Han, J. Xu, W. Hou, X. Yu and Y. Wang, Synthesis of high-quality MCM-48 mesoporous silica using gemini surfactant dimethylene-1,2-bis-(dodecyldimethylammonium bromide), J. Phys. Chem. B, 2004, 108, 15043–15048 CrossRef CAS.
- R. Pierotti and J. Rouquerol, Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity, Pure Appl. Chem., 1985, 57, 603–619 Search PubMed.
- Y. P. Zhai, B. Tu and D. Y. Zhao, Organosilane-assisted synthesis of ordered mesoporous poly(furfuryl alcohol) composites, J. Mater. Chem., 2009, 19, 131–140 RSC.
- B. Dou, Q. Hu, J. Li, S. Qiao and Z. Hao, Adsorption performance of VOCs in ordered mesoporous silicas with different pore structures and surface chemistry, J. Hazard. Mater., 2011, 186, 1615–1624 CrossRef CAS PubMed.
- A. L. Khan, C. Klaysom, A. Gahlaut, A. U. Khan and I. F. J. Vankelecom, Mixed matrix membranes comprising of Matrimid and -SO3H functionalized mesoporous MCM-41 for gas separation, J. Membr. Sci., 2013, 447, 73–79 CrossRef CAS.
- T. S. Chung, L. Y. Jiang, Y. Li and S. Kulprathipanja, Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation, Prog. Polym. Sci., 2007, 32, 483–507 CrossRef CAS.
- H. Ren, J. Jin, J. Hu and H. Liu, Affinity between metal–organic frameworks and polyimides in asymmetric mixed matrix membranes for gas separations, Ind. Eng. Chem. Res., 2012, 51, 10156–10164 CrossRef CAS.
- D. Q. Vu, W. J. Koros and S. J. Miller, Mixed matrix membranes using carbon molecular sieves: II. Modeling permeation behavior, J. Membr. Sci., 2003, 211, 335–348 CrossRef CAS.
- Y. Li, T.-S. Chung, C. Cao and S. Kulprathipanja, The effects of polymer chain rigidification, zeolite pore size and pore blockage on polyethersulfone (PES)-zeolite A mixed matrix membranes, J. Membr. Sci., 2005, 260, 45–55 CrossRef CAS.
- B. D. Freeman, Basis of permeability/selectivity tradeoff relations in polymeric gas separation membranes, Macromolecules, 1999, 32, 375–380 CrossRef CAS.
- S. Shahid and K. Nijmeijer, High pressure gas separation performance of mixed-matrix polymer membranes containing mesoporous Fe(BTC), J. Membr. Sci., 2014, 459, 33–44 CrossRef CAS.
- P. Duan, J. C. Moreton, S. R. Tavares, R. Semino, G. Maurin, S. M. Cohen and K. Schmidt-Rohr, Polymer infiltration into metal–organic frameworks in mixed-matrix membranes detected in situ by NMR, J. Am. Chem. Soc., 2019, 141, 7589–7595 CrossRef CAS PubMed.
- R. F. Boyer and R. L. Miller, Correlation of liquid-state compressibility and bulk mo1dulus with cross-sectional area per polymer-chain, Macromolecules, 1984, 17, 365–369 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra05125c |
| This journal is © The Royal Society of Chemistry 2021 |