Open Access Article
Open Access ArticleRecent progress in cadmium fluorescent and colorimetric probes
Chun-tian Shi
 ab,
Zhi-yu Huangc,
Ai-bin Wu
*ab,
Yan-xiong Hua,
Ning-chen Wanga,
Ying Zhanga,
Wen-ming Shu
ab,
Zhi-yu Huangc,
Ai-bin Wu
*ab,
Yan-xiong Hua,
Ning-chen Wanga,
Ying Zhanga,
Wen-ming Shu ab and
Wei-chu Yu
*ab
ab and
Wei-chu Yu
*ab
aSchool of Chemistry and Environmental Engineering, Yangtze University, Jingzhou, Hubei, People's Republic of China. E-mail: abwu@yangtzeu.edu.cn; yuweichu@126.com
bUnconventional Oil and Gas Collaborative Innovation Center, Yangtze University, Jingzhou, Hubei, People's Republic of China
cKey Laboratory of Textile Fibers and Products, Ministry of Education, College of Materials Science and Engineering, Wuhan Textile University, Wuhan, Hubei, People's Republic of China
First published on 3rd September 2021
Abstract
Cadmium is a heavy metal which exists widely in industrial and agricultural production and can induce a variety of diseases in organisms. Therefore, its detection is of great significance in the fields of biology, environment and medicine. Fluorescent probe has been a powerful tool for cadmium detection because of its convenience, sensitivity, and bioimaging capability. In this paper, we reviewed 98 literatures on cadmium fluorescent sensors reported from 2017 to 2021, classified them according to different fluorophores, elaborated the probe design, application characteristics and recognition mode, summarized and prospected the development of cadmium fluorescent and colorimetric probes. We hope to provide some help for researchers to design cadmium fluorescent probes with higher selectivity, sensitivity and practicability.
1. Introduction
Cadmium, as an essential resource in the earth, is widely used in chemical industry, electronics industry, nuclear industry, semiconducting, quantum dots, phosphate fertilizers, rechargeable batteries, pigments, electroplating, stabilizer, metallurgy, ceramic enamels.1–6 Consequently, there is a widespread Cd2+ contamination in air, water, and soil.7 Cadmium is a non-essential substance for the organism, and is even classified as a human carcinogen.8–10 Chronic exposure to Cd2+ may cause renal dysfunction, cardiovascular diseases, lung disease, calcium metabolism disorders, eosinophilia, neurodegenerative diseases.3,11–14 According to World Health Organization (WHO), the permissible concentration of Cd2+ in drinking water is 3 μg L−1.15,16 Therefore, the detection of Cd2+ content in organisms, food, and the environment is very important and urgent for life and health.17,18Up to now, there are some traditional detection methods of Cd2+ include atomic absorption spectroscopy, ultraviolet-visible spectroscopy, ion selective electrode, inductively coupled plasma mass spectrometry, stripping voltammetry.19–23 Nevertheless, most of these detection methods require expensive equipment, tedious procedures and skilled operators, and have the characteristics of low detection sensitivity, high economic costs and time-consuming.18,23–25 By contrast, fluorescent probe detection has the characteristics of strong specificity, high sensitivity, low detection limit, high accuracy, low cost, and visualization, which can replace traditional analysis methods to detect Cd2+.15,16,26–28 Therefore, the research and application of Cd2+ fluorescent sensors is of great interest to many scientific fields, ranging from supramolecular chemistry to life sciences.
Fluorescent sensors consist of fluorophore (signalling) and receptor (guest binding) moieties, either separated by a spacer or integrated into one unit.29,30 Common fluorophores are rhodamine, naphthalimide, coumarin, quinolone, BODIPY, benzothiazole, fluorescein, diarylethylene.4,16,17,19,21,22,31–34 Common receptors are pyridine and its analogues, and other structures containing O, N, S and other heteroatoms.9 According to the generation of fluorescence signals, there are mainly the following mechanisms: photoinduced electron transfer (PET), intramolecular charge transfer (ICT), fluorescence resonance energy transfer (FRET), aggregation-induced emission (AIE), excimer/exciplex formation/extinction, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization, excited-state intramolecular proton transfer (ESIPT). In addition, Cd2+ fluorescent and colorimetric nanosensors, including metal organic framework (MOF), quantum dots (QDs), nanoclusters (NCs) and nanoparticles (NPs), have also achieved good development in recent years, which makes it more possibilities for the source of raw materials, structural design, recognition methods and application fields of Cd2+ probes.
N isomerization, excited-state intramolecular proton transfer (ESIPT). In addition, Cd2+ fluorescent and colorimetric nanosensors, including metal organic framework (MOF), quantum dots (QDs), nanoclusters (NCs) and nanoparticles (NPs), have also achieved good development in recent years, which makes it more possibilities for the source of raw materials, structural design, recognition methods and application fields of Cd2+ probes.
Trace Cd2+ is harmful to environment and organism, and the detection of Cd2+ ions by fluorescent probes is easily interfered by other transition metals, especially Zn2+ ions in the same group.3,21 Therefore, the development of Cd2+ fluorescent probes has great challenges and significance for the detection of Cd2+ in environment and living organisms. At present, a plethora of fluorescent probes for Cd2+ have been reported and this research field has become very active. A closer scrutiny of the review literatures on the research progress of Cd2+ fluorescent probes in the web of science core collection shows that J. Jia et al. reviewed Zn2+/Cd2+ fluorescent probes based on small organic molecules in 2012, and S. Y. Chen et al. reviewed Pb2+/Cd2+/Hg2+ fluorescent probes based on small organic molecules in 2021.35,36 Apart from these, there is no relevant or targeted review article that has covered research advances of Cd2+ fluorescent probes. Therefore, as a review article dedicated to the research progress of Cd2+ fluorescent probes, this article adopts a classification method different from the above reviews. Since the mechanism of some fluorescence response is inferred by the authors and has not been demonstrated absolutely, and there may be two or more possible mechanisms might simultaneously exist in the sensing process, we described the probes based on the category of fluorophore species. It should be noted that this article elaborated and summarized the Cd2+ ion fluorescent probes reported in recent years in terms of the probe design ideas, application characteristics, and binding methods, and discussed the reality and future challenges of Cd2+ ion fluorescent probes used in environmental monitoring and biological imaging.
2. Quinolone-based Cd2+ fluorescent sensor
Quinoline is a kind of fluorescent chromophore with coordination function, recognition function, strong photostability and metal ion chelating ability.9 The conjugated system of quinoline is large and prone to π–π* electronic transition. Its own N atom easily forms hydrogen bonds in polar solvents and exhibits weak fluorescence. After complexing with metal ions, the fluorescence recovers.2.1 Quinolone as a binding site for Cd2+
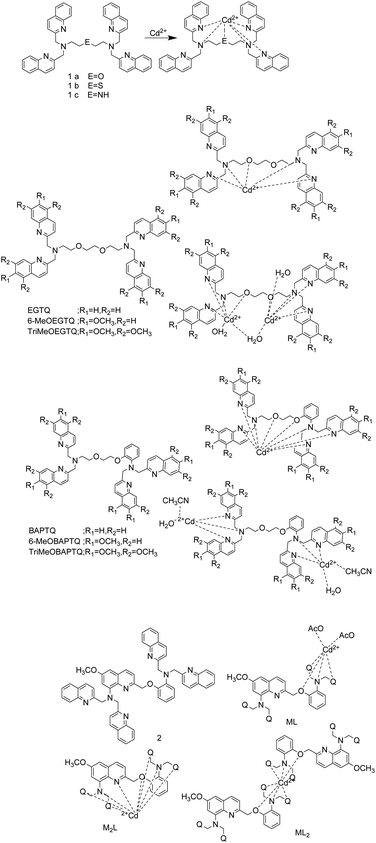
Y. Mikata's team reported on a series of fluorescent probes (Fig. 1) for detecting Cd2+, and quinoline chromophores in these probes can also be used as binding sites.1,37,38 In 2017, they synthesized probe 1a and its thia (1b) and aza (1c) derivatives.1 In DMF–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution and an excitation at 317 nm, the probes 1a, 1b and 1c exhibit weak fluorescence. The binding of the probes to Cd2+ inhibits their PET process and make the adjacent quinoline rings in the probes formed an intramolecular excimer, so these probes exhibit enhanced fluorescence emission. The detection limit of probe 1a for Cd2+ is 22 nM. The dissociation constant of the complexes of probe 1a, 1b, 1c and Cd2+ were (4.2 ± 0.7) × 10−7 M, (6.5 ± 0.2) × 10−6 M and <2 × 10−7 M, respectively. The O in probe 1a is replaced by S and N can increase the selectivity of Cd2+. For the detection of Cd2+ by these probes, although the fluorescence response interference caused by Zn2+ can be neglected, the presence of Cu2+, Ag+, Hg2+, Cr3+ and Fe3+ in the detection system can interfere with the fluorescence signal obviously.
1) solution and an excitation at 317 nm, the probes 1a, 1b and 1c exhibit weak fluorescence. The binding of the probes to Cd2+ inhibits their PET process and make the adjacent quinoline rings in the probes formed an intramolecular excimer, so these probes exhibit enhanced fluorescence emission. The detection limit of probe 1a for Cd2+ is 22 nM. The dissociation constant of the complexes of probe 1a, 1b, 1c and Cd2+ were (4.2 ± 0.7) × 10−7 M, (6.5 ± 0.2) × 10−6 M and <2 × 10−7 M, respectively. The O in probe 1a is replaced by S and N can increase the selectivity of Cd2+. For the detection of Cd2+ by these probes, although the fluorescence response interference caused by Zn2+ can be neglected, the presence of Cu2+, Ag+, Hg2+, Cr3+ and Fe3+ in the detection system can interfere with the fluorescence signal obviously.
In 2019, they designed methoxy-substituted tetrakisquinoline EGTA and BAPTA analogs probes that can recognize Cd2+ based on PET mechanism.37 All EGTQ derivatives bind to Zn2+, Cd2+, Fe3+, Co2+, Hg2+ and Ag+ as indicated by the UV-vis spectral changes. However, only Zn2+ and Cd2+ can illuminate the ligand. Although they selectivity is poor, methoxy substitution can enhance the selectivity to Cd2+ to some extent. TriMeOBAPTQ probe was synthesized by introducing three methoxy groups at positions 5, 6, and 7 of each quinoline moiety in BAPTQ. In methanol–HEPES buffer (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 50 mM HEPES, 0.1 M KCl, pH = 7.5), upon excitation at 347 nm, TriMeOBAPTQ can combine with Cd2+ in a stoichiometric ratio of 1
1, 50 mM HEPES, 0.1 M KCl, pH = 7.5), upon excitation at 347 nm, TriMeOBAPTQ can combine with Cd2+ in a stoichiometric ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 1
1 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to increase the fluorescence of the system, and detect Cd2+ as low as 9.9 nM. TriMeOBAPTQ also has affinity with Cu2+, Ag+, Hg2+, and Fe3+, even the presence of a large number of these metal ions except Cu2+ does not affect the detection of Cd2+ by TriMeOBAPTQ probe.
2 to increase the fluorescence of the system, and detect Cd2+ as low as 9.9 nM. TriMeOBAPTQ also has affinity with Cu2+, Ag+, Hg2+, and Fe3+, even the presence of a large number of these metal ions except Cu2+ does not affect the detection of Cd2+ by TriMeOBAPTQ probe.
To improve the metal ion specificity and sensitivity, they changed core structure from BAPTA to Ca2+-specific probe (quin2(8-amino-2-((2-amino-5-methylphenoxy)methyl)-6-methoxyquinoline-N,N,N′,N′-tetraacetic acid)) to synthesize probe 2, and reported it in 2020.38 Probe 2 has two chromophores, methoxy-substituted and unsubstituted quinolines. Because the aqueous solvent prevents the probe 2 from coordinating with metal ions, the test was carried out in methanol solution. The combination of probe 2 and Cd2+ can inhibit the PET process and produce CHEF (chelation-enhanced fluorescence) and excimer effects when excited by 317 nm light, which increased the fluorescence intensity of the probe 2 by 170 times at 408 nm. The detection limit of probe 2 for Cd2+ is 182 nM. The equimolar amounts of Co2+ and Ag+ shut down the Cd2+-induced fluorescence, and the excessive Zn2+ and Ni2+ can replace Cd2+ to bind to probe 2. Although the probe has enhanced fluorescence and specific selection for Cd2+ by introducing 6-methoxy-8-aminoquioline moiety, the sensor still has weak water solubility, low selectivity, and short excitation and emission wavelengths. Moreover, the detection of low concentrations of Cd2+ should prevent weak fluorescent ML2 is generated. Y. Mikata et al. proposed to introduce a suitable electron-donating substituents on the side-arm quinolones, which act as a metal binding site and chromophore to improve sensor performance.38
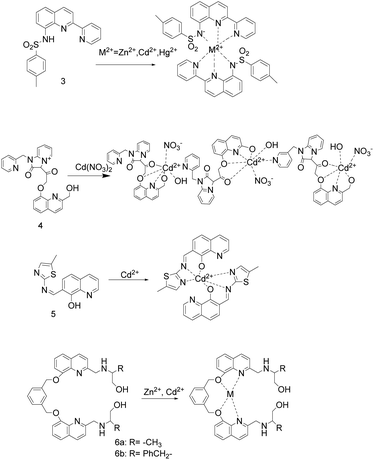
In 2017, the sulfonamidoquinoline-based derivatives sensor 3 (Fig. 2) was reported by Y. Zhang et al.39 In DMSO–water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (v/v), 0.01 M Tris–HCl, pH = 7.24) solution, sensor 3 and Zn2+/Cd2+/Hg2+ are combined in a mole ratio of 2
2 (v/v), 0.01 M Tris–HCl, pH = 7.24) solution, sensor 3 and Zn2+/Cd2+/Hg2+ are combined in a mole ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to red-shift the absorption maximum from 323 nm to 425/422/424 nm. When excited at 422 nm, the detection system including sensor 3 and Zn2+/Cd2+/Hg2+ emits red fluorescence at 634/618/630 nm, with an intensity increase of 38/96/21 times. In addition, the fluorescence intensity is linearly related to the concentration of Zn2+/Cd2+/Hg2+. The detection limit of sensor 3 to Zn2+ is 3.61 nM, to Cd2+ is 1.55 nM, and to Hg2+ is 7.02 nM, which is lower than the permissible concentrations of Cd2+ (43.8 nM) and Hg2+ (9.9 nM) in drinking water regulated by Environmental Protection Agency (EPA). The binding constants (log
1 to red-shift the absorption maximum from 323 nm to 425/422/424 nm. When excited at 422 nm, the detection system including sensor 3 and Zn2+/Cd2+/Hg2+ emits red fluorescence at 634/618/630 nm, with an intensity increase of 38/96/21 times. In addition, the fluorescence intensity is linearly related to the concentration of Zn2+/Cd2+/Hg2+. The detection limit of sensor 3 to Zn2+ is 3.61 nM, to Cd2+ is 1.55 nM, and to Hg2+ is 7.02 nM, which is lower than the permissible concentrations of Cd2+ (43.8 nM) and Hg2+ (9.9 nM) in drinking water regulated by Environmental Protection Agency (EPA). The binding constants (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ks) decreased with the increase of ion radius in group IIB, and the binding constants of Zn2+, Cd2+ and Hg2+ and sensor 3 were 9.45, 9.36 and 8.96, respectively. The sensor 3 can detect Zn2+, Cd2+ and Hg2+ in the aqueous solution under the excitation of visible light. In addition, the sensor 3 was used for Cd2+ competition experiments and fluorescence imaging in yeast cells, indicating that the sensor 3 is biocompatible and not affected by metal ions except Zn2+ and Cu2+ in the fluorescence detection of Cd2+.
Ks) decreased with the increase of ion radius in group IIB, and the binding constants of Zn2+, Cd2+ and Hg2+ and sensor 3 were 9.45, 9.36 and 8.96, respectively. The sensor 3 can detect Zn2+, Cd2+ and Hg2+ in the aqueous solution under the excitation of visible light. In addition, the sensor 3 was used for Cd2+ competition experiments and fluorescence imaging in yeast cells, indicating that the sensor 3 is biocompatible and not affected by metal ions except Zn2+ and Cu2+ in the fluorescence detection of Cd2+.
Y. P. Dai and coworkers described probe 4 (Fig. 2).9 In Tris–HCl buffer (10 mM, pH = 7.4), the detection limit to Cd2+ is 1.18 × 10−6 M and the association constants is 9.00 × 104 M−1. Upon excitation at 312 nm, probe 4 has weak fluorescence due to the double PET process from the oxygen atom of hydroxy to hydroxyquinoline group and the hydroxyquinoline to carbonyl unit, respectively. After the chelation of probe 4 with Cd2+, the PET process was inhibited, causing the fluorescence of probe 4 emission peak to redshift from 400 nm to 410 nm and the fluorescence to increase by 2.5 times. Although probe 4 is almost 100% soluble in water, reversibility, non-toxic and cell-membrane-permeable, the presence of Fe3+, Fe2+, Cu2+,Cr3+, Al3+ or Ag+ ions can quench part of the fluorescence enhancement caused by Cd2+ to some extent. At 410 nm, the fluorescence intensity of the Cd2+ complex probe 4 is very little affected by other metal ions, but Fe3+ can partially quench the fluorescence.
X. H. Ding's research group reported sensor 5 (Fig. 2).40 In CH3CN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) systems, sensor 5 combined with Cd2+ made the solution turn dark yellow immediately, and turn blue fluorescence under 365 nm UV light. The association constant of sensor 5 and Cd2+ is estimated to be 8.48 × 104 M−1. In the pH range of 6–10, sensor 5 has high selectivity, anti-interference ability and bioimaging ability. It can detect Cd2+ as low as 4 × 10−6 mol L−1 and not be interfered by other cations. In addition, the complex of sensor 5 and Cd2+ can be used as a highly selective and sensitive probe for PO43− without interference from anions.
1) systems, sensor 5 combined with Cd2+ made the solution turn dark yellow immediately, and turn blue fluorescence under 365 nm UV light. The association constant of sensor 5 and Cd2+ is estimated to be 8.48 × 104 M−1. In the pH range of 6–10, sensor 5 has high selectivity, anti-interference ability and bioimaging ability. It can detect Cd2+ as low as 4 × 10−6 mol L−1 and not be interfered by other cations. In addition, the complex of sensor 5 and Cd2+ can be used as a highly selective and sensitive probe for PO43− without interference from anions.
In 2017, X. Y. Liu's team reported two turn-on fluorescent sensors 6a and 6b (Fig. 2) for Zn2+/Cd2+, and their complexes can act as highly selective sensors to detect phosphate anion through turn-off the fluorescence.11 In CH3OH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, Tris 10 mol L−1, pH = 7.4) solution, upon the excitation at 310 nm, sensor 6a/6b has weak fluorescence. Adding Zn2+/Cd2+ into the sensor solution, an obvious fluorescence turn-on response at 430 nm, and a working curve can be established between the fluorescence intensity and the concentration. The binding constants of sensors 6a and 6b to Zn2+ or Cd2+ are (1.517 ± 0.31) × 107, (6.40 ± 0.15) × 106, (9.16 ± 0.42) × 105, and (1.03 ± 0.19) × 106 L mol−1, respectively. Adding anion into the complexes of sensor 6a/6b and Zn2+/Cd2+, and it is found that H2PO4− and HPO42− can almost quench all fluorescence. However, the article does not specifically describe the distinction between Zn2+ and Cd2+, H2PO4− and HPO42−.
1, v/v, Tris 10 mol L−1, pH = 7.4) solution, upon the excitation at 310 nm, sensor 6a/6b has weak fluorescence. Adding Zn2+/Cd2+ into the sensor solution, an obvious fluorescence turn-on response at 430 nm, and a working curve can be established between the fluorescence intensity and the concentration. The binding constants of sensors 6a and 6b to Zn2+ or Cd2+ are (1.517 ± 0.31) × 107, (6.40 ± 0.15) × 106, (9.16 ± 0.42) × 105, and (1.03 ± 0.19) × 106 L mol−1, respectively. Adding anion into the complexes of sensor 6a/6b and Zn2+/Cd2+, and it is found that H2PO4− and HPO42− can almost quench all fluorescence. However, the article does not specifically describe the distinction between Zn2+ and Cd2+, H2PO4− and HPO42−.
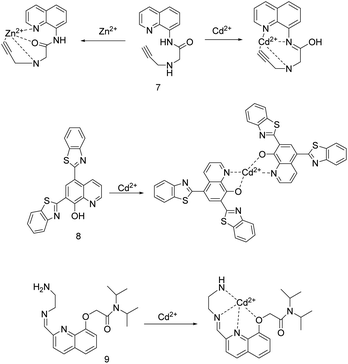
In 2019, H. H. Song's research group synthesized probe 7 (Fig. 3) by inserting an amide group into the 8-aminoquinoline fluorophore and a propargylamine chelating site.41 Probe 7 displayed selective and distinct ratiometric fluorescence response to Zn2+ and Cd2+, Zn2+ was bound as an amide tautomer in almost totally water solution, and Cd2+ was bound as an imidic acid tautomer in CH3CN aqueous medium, respectively. Under 365 nm light, in the high/low acetonitrile content aqueous solution, probe 7 and Cd2+/Zn2+ combined 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, making probe 7 emitted bright-green/green fluorescence and the emission peak is red-shifted 95/82 nm. The fluorescence intensity ratios of I500 nm/I405 nm and I498 nm/I416 nm were linearly proportional to the concentration of Cd2+ and Zn2+, respectively. The association constants of probe 7 with Cd2+ and Zn2+ are is 3.7 × 104 M−1 and 1.4 × 104 M−1, respectively. The detection limits for Cd2+ is 0.055 μM and for Zn2+ is 0.063 μM. The response of probe 7 to Zn2+ and Cd2+ is selective, reversible, and rapid. The coexistence Cu2+, Ni2+, Co2+ with Cd2+ quenched the Cd2+-induced fluorescence, while the coexistence Cr3+ with Zn2+ slightly quenched the Zn2+-induced fluorescence. Probe 7 was applied for detecting analysis of all the target ions in the tap water sample and on test paper strips, and bioimaging of Zn2+ in mung bean sprouts as well. In addition, the complex of probe 7 and Zn2+ can be used as a secondary sensor for PPi and ATP.
1, making probe 7 emitted bright-green/green fluorescence and the emission peak is red-shifted 95/82 nm. The fluorescence intensity ratios of I500 nm/I405 nm and I498 nm/I416 nm were linearly proportional to the concentration of Cd2+ and Zn2+, respectively. The association constants of probe 7 with Cd2+ and Zn2+ are is 3.7 × 104 M−1 and 1.4 × 104 M−1, respectively. The detection limits for Cd2+ is 0.055 μM and for Zn2+ is 0.063 μM. The response of probe 7 to Zn2+ and Cd2+ is selective, reversible, and rapid. The coexistence Cu2+, Ni2+, Co2+ with Cd2+ quenched the Cd2+-induced fluorescence, while the coexistence Cr3+ with Zn2+ slightly quenched the Zn2+-induced fluorescence. Probe 7 was applied for detecting analysis of all the target ions in the tap water sample and on test paper strips, and bioimaging of Zn2+ in mung bean sprouts as well. In addition, the complex of probe 7 and Zn2+ can be used as a secondary sensor for PPi and ATP.
The same year, Z. N. Lu et al. synthesized 8-hydroxyquinoline-benzothiazole conjugated probe 8 (Fig. 3) in two steps.42 This probe can greatly enhance the fluorescence by coordinating with various metal ions such as Al3+, Cd2+, Zn2+, Mg2+ in methanol containing 1% water. However, the selectivity to Cd2+ can be achieved by increasing the water content to 30% aqueous methanol solution. The aqueous methanol solution (pH = 7.4, 30% Tris–HCl buffer) and the excitation at 313 nm are selected to carry out. Probe 8 and Cd2+ combined at a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry between probe 8 and Cd2+ to emit green fluorescence, and the fluorescence intensity at 525 nm had a good linear relationship with the concentration of Cd2+ over a range of 0–5 μM. The detection limit for Cd2+ is 0.1 μM. Adding EDTA as a competing chelator can make the binding of probe 8 and Cd2+ reversible. Probe 8 can detect Cd2+ in an aqueous solution with a pH of 4–12, and be used for fluorescence imaging of Cd2+ in living cells.
1 stoichiometry between probe 8 and Cd2+ to emit green fluorescence, and the fluorescence intensity at 525 nm had a good linear relationship with the concentration of Cd2+ over a range of 0–5 μM. The detection limit for Cd2+ is 0.1 μM. Adding EDTA as a competing chelator can make the binding of probe 8 and Cd2+ reversible. Probe 8 can detect Cd2+ in an aqueous solution with a pH of 4–12, and be used for fluorescence imaging of Cd2+ in living cells.
The group headed by X. J. Wan described a quinoline Schiff base-containing sensor 9 (Fig. 3).33 After optimizing the detection environment, the researchers found that the better selectivity and sensitivity of the probe to Cd2+ under the conditions of pH = 4 methanol solution (10%) and excitation wavelength of 246 ± 2 nm. The combination of non-fluorescent sensor 9 and Cd2+ caused sensor 9 to turn on the fluorescence response at 425 nm. At this time, sensor 9 displayed excellent selectivity, sensitivity, and reversibility for detecting Cd2+ in an acidic environment, and it can detect Cd2+ as low as 2.4 nM.
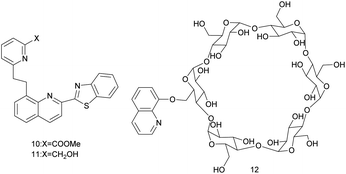
In 2019, K. Aich and colleagues reported on quinoline–benzothiazole-based fluorescent turn-on probes 10 and 11 (Fig. 4).43 In MeOH/H2O (3/7, v/v, 10 mM HEPES buffer, pH = 7.2) solution, upon the excitation at 360 nm, the fluorescence emission intensity of probes 10 and 11 are linearly related to the concentration of Cd2+. Their detection limits for Cd2+ are 3.52 × 10−10 M and 4.83 × 10−9 M, respectively, and the association constants are 3.17 × 104 M−1 and 1.60 × 104 M−1, respectively. Because the nitrogen atoms of quinoline and pyridine are strongly coordinated with Cd2+, the ICT effect of the probes is enhanced. The fluorescence emission of probe 10 is red-shifted from 488 nm to 507 nm and the fluorescence is changed from weak cyan to green. The fluorescence emission of probe 11 is increased by 20 times, the fluorescence emission is red-shifted from 490 nm to 510 nm and the fluorescence is changed from cyan to bright green. Probe 10 and probe 11 have high selectivity and reversibility (Na2EDTA) for Cd2+ detection, and are not interfered by other metal ions, and can act as a potential portable kit for detection of Cd2+ in solid state as well as in solution.
The next year, S. Nazerdeylami et al. described probe 12 (Fig. 4), which is effective for detecting Cd2+ and tetracycline.44 The 8-hydroxyquinoline dehydrogenation is connected to β-cyclodextrin, which increases the rigidity of the probe 12. In an aqueous medium, upon excitation at 350 nm, probe 12 has a strong fluorescence emission intensity at 525 nm. The N in the pyridine ring and the O in the chelator interacted with Cd2+, causing the fluorescence of the probe 12 to be quenched. The fluorescence intensity is linearly related to the Cd2+ content of 0.1–1.5 nM. At pH < 4, the solution hydrates H+ ions due to the protonation of nitrogen and oxygen, causing H+ and other metal ions to compete chelating sites. At pH > 4, cadmium hydroxide precipitates is formed. Therefore, the probe 12 is suitable for detecting Cd2+ in an aqueous medium with pH = 4, and the detection has selectivity, sensitivity, and anti-interference. The detection limit of probe 12 for Cd2+ is 0.05 nM. Further, the complex of probe 12 and Cd2+ can be used as a fluorescence-on probe of tetracycline, the fluorescence intensity is linearly related to the content of tetracycline, and can detect tetracycline as low as 0.9 μM.
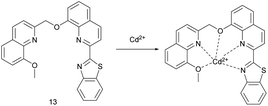
S. L. Li et al. Reported the probe 13 (Fig. 5) responding to Ag+ (PET) and Cd2+ (ICT) based on different mechanisms.45 In the CH3OH/HEPES (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH = 7.30) buffer system and the excitation wavelength of 344 nm, the fluorescence of the probe was quenched by 88% with the addition of Ag+ and showed an “on–off” behavior. With the addition of Cd2+, the maximum fluorescence emission of the probe was red shifted from 465 nm to 490 nm, the fluorescence intensity was quenched by 33%, and the fluorescence changed from blue to green. The recognition of Cd2+ is not affected by metal ions including Ag+, while the recognition of Ag+ ions is interfered by other metal ions. The selectivity and sensitivity of the probe for Cd2+ ion are stronger than that for Ag+. The complexation constant of the probe for Cd2+ is 2.23 × 104 M−1, and the detection limit for Cd2+ is 0.26 mM. The complex of probe 13 and Cd2+ could be used for sequential recognition of S2−. When Cd2+ and S2− were added alternately for 3 times, the probe still had a high level of recognition ability. Under strong acidic or alkaline conditions, probe 13 may change its own structure or lose the ability of ion coordination and cannot recognize ions.
1, v/v, pH = 7.30) buffer system and the excitation wavelength of 344 nm, the fluorescence of the probe was quenched by 88% with the addition of Ag+ and showed an “on–off” behavior. With the addition of Cd2+, the maximum fluorescence emission of the probe was red shifted from 465 nm to 490 nm, the fluorescence intensity was quenched by 33%, and the fluorescence changed from blue to green. The recognition of Cd2+ is not affected by metal ions including Ag+, while the recognition of Ag+ ions is interfered by other metal ions. The selectivity and sensitivity of the probe for Cd2+ ion are stronger than that for Ag+. The complexation constant of the probe for Cd2+ is 2.23 × 104 M−1, and the detection limit for Cd2+ is 0.26 mM. The complex of probe 13 and Cd2+ could be used for sequential recognition of S2−. When Cd2+ and S2− were added alternately for 3 times, the probe still had a high level of recognition ability. Under strong acidic or alkaline conditions, probe 13 may change its own structure or lose the ability of ion coordination and cannot recognize ions.
2.2 Quinolone does not as a binding site for Cd2+
In 2017, the first metal ion sensor 14 (Fig. 6) of 4,5-quinolinimide derivatives was synthesized by Y. Zhang et al.7 To increase the selectivity of 2,2-dipicolylamine (DPA) for Cd2+ over the same group element Zn2+, an amide group was introduced into the DPA unit. In a methanol–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
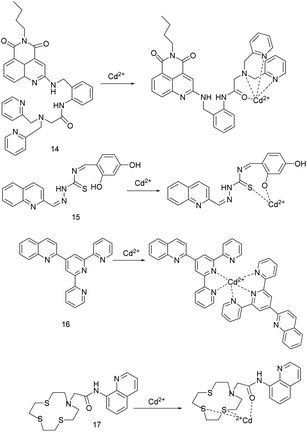
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution with a pH of 7.20, upon excitation at 430 nm, adding Cd2+ increased the fluorescence of sensor 14, and the fluorescence intensity was linearly related to the concentration of Cd2+. The complex's association constant is 9.06 × 107 M−1. The detection limit of sensor 14 for Cd2+ is 11 nM, which is lower than the permissible concentration of Cd2+ in drinking water regulated by the World Health Organization as 26 nM. The fluorescence emission of sensor 14 binding to Zn2+ is weaker and the fluorescent lifetime is shorter, which makes it possible to distinguish between Zn2+ and Cd2+. Under physiological conditions, the fluorescence of sensor 14 binding to Cd2+ can be significantly quenched by Cu2+. In addition, sensor 14 has low cytotoxicity and emits bright yellow-green fluorescence when used to fluorescence imaging of Cd2+ in yeast cells.
1) solution with a pH of 7.20, upon excitation at 430 nm, adding Cd2+ increased the fluorescence of sensor 14, and the fluorescence intensity was linearly related to the concentration of Cd2+. The complex's association constant is 9.06 × 107 M−1. The detection limit of sensor 14 for Cd2+ is 11 nM, which is lower than the permissible concentration of Cd2+ in drinking water regulated by the World Health Organization as 26 nM. The fluorescence emission of sensor 14 binding to Zn2+ is weaker and the fluorescent lifetime is shorter, which makes it possible to distinguish between Zn2+ and Cd2+. Under physiological conditions, the fluorescence of sensor 14 binding to Cd2+ can be significantly quenched by Cu2+. In addition, sensor 14 has low cytotoxicity and emits bright yellow-green fluorescence when used to fluorescence imaging of Cd2+ in yeast cells.
In 2019, P. G. Mahajan's team reported the Schiff base probe 15 (Fig. 6).6 The probe can quickly detect Cd2+, Co2+, Ni2+, Cu2+ in nano-molar level through absorption or fluorescence spectroscopy. Adding Cd2+, Ni2+, Cu2+, Co2+ to the systems of methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (7
water (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) and probe 15 for 3–5 minutes causes the probe solution to change from colorless to pale yellow, yellow, yellow, and dark yellow, and the absorption peaks red-shifts from 338 nm to 399 nm, 422 nm, 424 nm, 396 nm, respectively. The detection limits of probe 15 for Cd2+ is 1.043 nM, for Ni2+ is 0.656 nM, for Cu2+ is 0.224 nM, for Co2+ is 1.047 nM. Upon excitation at 340 nm, the complex of probe 15 with Cd2+, Ni2+, Co2+, Cu2+ absorb the energy from light followed by immediately return to ground state without photon emission, resulting in fluorescence quenching by 8.1, 9.2, 8.6, 10.5 times, respectively. The detection limits are 1.070 nM for Cd2+, 0.637 nM for Ni2+, 1.053 nM for Co2+, and 0.184 nM for Cu2+. In the aqueous solution with a pH of 6–8, and the affinity of probe 15 for metal ions is Cd2+ < Co2+ < Ni2+ < Cu2+, and the metal ions with strong affinity can replace metal ions with weak affinity to bind the probe 15.
3, v/v) and probe 15 for 3–5 minutes causes the probe solution to change from colorless to pale yellow, yellow, yellow, and dark yellow, and the absorption peaks red-shifts from 338 nm to 399 nm, 422 nm, 424 nm, 396 nm, respectively. The detection limits of probe 15 for Cd2+ is 1.043 nM, for Ni2+ is 0.656 nM, for Cu2+ is 0.224 nM, for Co2+ is 1.047 nM. Upon excitation at 340 nm, the complex of probe 15 with Cd2+, Ni2+, Co2+, Cu2+ absorb the energy from light followed by immediately return to ground state without photon emission, resulting in fluorescence quenching by 8.1, 9.2, 8.6, 10.5 times, respectively. The detection limits are 1.070 nM for Cd2+, 0.637 nM for Ni2+, 1.053 nM for Co2+, and 0.184 nM for Cu2+. In the aqueous solution with a pH of 6–8, and the affinity of probe 15 for metal ions is Cd2+ < Co2+ < Ni2+ < Cu2+, and the metal ions with strong affinity can replace metal ions with weak affinity to bind the probe 15.
Y. Xiao et al. designed the probe 16 (Fig. 6) by connecting quinoline fluorophore with tripyridine recognition group.46 Due to the strong absorption at 470 nm and the elimination of the interference of the original fluorescence in DMF–H2O (FW 40% v/v) solution, they were selected as the conditions for the study of the probe recognition performance. At 520 nm, there was a good linear relationship between the fluorescence emission intensity and the concentration of Cd2+ (0–5 μmol L−1) and Zn2+ (6–10 μmol L−1). The LOD of this fluorescent probe 16 for Cd2+ is 3.5 × 10−8 mol L−1. However, the presence of Zn2+ and Cu2+ affects the recognition of Cd2+ by the probe. Because the fluorescence of this probe induced by Zn2+ is stronger than that induced by Cd2+, the binding of Cu2+ to the probe is more stable and can quench the fluorescence induced by Cd2+.
A. Garau et al. synthesized probe 17 (Fig. 6) by inserting amide group spacer between quinoline fluorophore and 1-aza-4,7,10-trithiacyclododecane ([12]aneNS3) receptor unit.47 In MeCN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v) solution, the probe showed fluorescence enhancement in response to Cd2+ and Zn2+ due to CHEF effect, and Cd2+ induced fluorescence enhancement was stronger than that caused by Zn2+.
4, v/v) solution, the probe showed fluorescence enhancement in response to Cd2+ and Zn2+ due to CHEF effect, and Cd2+ induced fluorescence enhancement was stronger than that caused by Zn2+.
2.3 Binding mode remains unknown
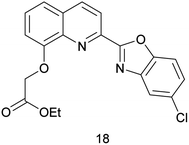
In 2018, an “on–off–on” sensor 18 (Fig. 7) for sequential recognition of Cu2+ and Cd2+ reported by J. Han et al.48 In EtOH/H2O (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) solution, sensor 18 emits strong blue fluorescence at 471 nm (the excitation at 340 nm), adding Cd2+ decreased the fluorescence intensity and red-shifted 40 nm, and the fluorescence changed from blue to green. Cu2+ binds to sensor 18 at a ratio of 1
9) solution, sensor 18 emits strong blue fluorescence at 471 nm (the excitation at 340 nm), adding Cd2+ decreased the fluorescence intensity and red-shifted 40 nm, and the fluorescence changed from blue to green. Cu2+ binds to sensor 18 at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, quenching the blue fluorescence of sensor 18. The detection limit for Cu2+ is 2.7 × 10−8 M, and the association constant is 5.643 × 104 M−1. The complex of sensor 18 and Cu2+ can be replaced by Cd2+ and emerge a strong emission peak at 510 nm to become a more sensitive and accurate fluorescent probe of Cd2+, which significantly increases the fluorescence intensity of the system. The complex of sensor 18-Cu can detect Cd2+ as low as 1.7 × 10−8 M, and the association constant is 1.374 × 104 M−1. Sensor 18 is stable in the pH range from 4 to 10, and can successively quantitatively detect Cu2+ and Cd2+ in real water samples and deproteinized milk. Test strips containing sensor 18 also has a good qualitative recognition performance for target ions.
1, quenching the blue fluorescence of sensor 18. The detection limit for Cu2+ is 2.7 × 10−8 M, and the association constant is 5.643 × 104 M−1. The complex of sensor 18 and Cu2+ can be replaced by Cd2+ and emerge a strong emission peak at 510 nm to become a more sensitive and accurate fluorescent probe of Cd2+, which significantly increases the fluorescence intensity of the system. The complex of sensor 18-Cu can detect Cd2+ as low as 1.7 × 10−8 M, and the association constant is 1.374 × 104 M−1. Sensor 18 is stable in the pH range from 4 to 10, and can successively quantitatively detect Cu2+ and Cd2+ in real water samples and deproteinized milk. Test strips containing sensor 18 also has a good qualitative recognition performance for target ions.
Among the Cd2+ fluorescent probes reported in recent five years, the research quinolone-based sensors is the most, and the spectroscopic and analytical parameters of these probes are shown in Table 1. Quinoline is widely used in the design of Cd2+ fluorescent probes because it can not only be used as fluorescent chromophore, but also as Cd2+ binding site. However, most of these sensors have some problems, such as poor water solubility, poor selectivity, poor anti-interference, short excitation and emission wavelength, which greatly limits their application in the fields of environment and biology. Compared with other sensors, sensors 9 and 18-Cu have higher selectivity, sensitivity and anti-interference ability. However, the optimum test condition of sensor 9 is pH = 4, which is not suitable for the application in real environment. The sensor 18-Cu responds to Cd2+ by replacing Cu2+ with Cd2+, which makes the detection of Cd2+ more accurate. It is not be limited to the small molecule probe directly used for Cd2+ detection, therefore, it can also be used for Cd2+ detection after the probe is complexed with other analytes in the future.
| Probe | Solvent | Excitation wavelength (nm) | Emission wavelength (nm) λem0–λem | Detection limit (nM) | Association constant (M−1) | Interfering ion(s) | Ref. |
|---|---|---|---|---|---|---|---|
| 1a | DMF–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
317 | 428 | 22 | Kd =(4.2 ± 0.7) × 10−7 M | Cu2+, Ag+, Hg2+, Cr3+ | 1 |
| TriMeO-BAPTQ | CH3OH–H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
347 | 460 | 9.9 | — | Cu2+ | 37 |
| 2 | CH3OH | 317 | 408 | 182 | — | Co2+, Ag+, Zn2+, Ni2+ | 38 |
| 3 | DMSO–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
422 | 618 | 1.55 | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ks = 9.36 Ks = 9.36 |
Zn2+, Cu2+ | 39 |
| 4 | H2O | 312 | 410 | 1180 | 9.00 × 104 | Fe3+ | 9 |
| 5 | CH3CN–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
375 | 500 | 4000 | 8.48 × 104 | PO43− | 40 |
| 6a/6b | CH3OH–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
310 | 430 | — | (1.517 ± 0.31) × 107/(1.03 ± 0.19) × 106 | Zn2+, H2PO4−, HPO42− | 11 |
| 7 | CH3CN–H2O | 321 | 405–500 | 55 | 3.7 × 104 | Cu2+, Ni2+, Co2+ | 41 |
| 8 | CH3OH–H2O (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
313 | 525 | 100 | — | — | 42 |
| 9 | CH3OH–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) 9) |
246 | 425 | 2.4 | — | — | 33 |
| 10 | CH3OH–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) 7) |
360 | 488–507 | 0.352 | 3.17 × 104 | — | 43 |
| 11 | CH3OH–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) 7) |
360 | 490–510 | 4.83 | 1.60 × 104 | — | 43 |
| 12 | H2O | 350 | 525 | 0.05 | — | Tetracycline | 44 |
| 13 | CH3OH–H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
344 | 465–490 | 260![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.23 × 104 | — | 45 |
| 14 | CH3OH–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
430 | 543 | 11 | 9.06 × 107 | Cu2+, Zn2+ | 7 |
| 15 | CH3OH–H2O (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
340 | About 420 | 1.070 | 6.73 × 105 | Co2+, Ni2+, Cu2+ | 6 |
| 16 | DMF-H2O (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
470 | 520 | 35 | — | Zn2+ and Cu2+ | 46 |
| 17 | MeCN–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
330 | 505 | — | — | Zn2+ | 12 |
| 18-Cu | EtOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) 9) |
340 | 510 | 17 | 1.374 × 104 | — | 48 |
3. Coumarin-based Cd2+ fluorescent sensor
Coumarins are characterized by high molar extinction coefficient, high fluorescence quantum yield, large stokes shift, low toxicity, good water solubility and many reaction sites. In addition, coumarin is easy to connect to different groups, and can not only act as a fluorescent chromophore, but also as recognition sites of analytes, so as to achieve fluorescence response to different analytes.34,493.1 Coumarin as a binding site for Cd2+
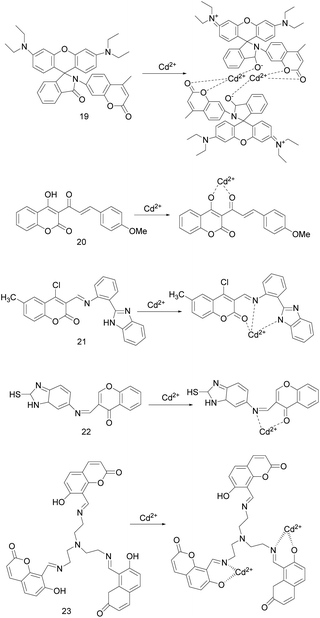
In 2017, C. Kumari's research team synthesized probe 19 (Fig. 8), which recognizes Cd2+ and turns on fluorescence.2 There is a spectral overlap between the energy donor coumarin part and the energy acceptor rhodamine part, and Cd2+ is recognized based on the FRET mechanism. In methanol/water (2/1, v/v, 1 mM HEPES buffer, pH = 7.2), the free probe has no characteristic absorption peak in the visible light region, but the absorption peak intensity at 561 nm is increased after gradually adding Cd2+. Upon the addition of Cd2+, the probe 19 and Cd2+ are combined in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to open the spirolactam ring. The solution immediately changed from colorless to pink, turned non-fluorescent to bright red fluorescent under long wavelength UV light, and shifted the fluorescence emission peak from 430 nm to 589 nm (the excitation wavelength at 340 nm). The binding constant value of the probe to Cd2+ was 7.8 × 105 M−3 and the detection level was 10.1 nM. Probe 19 can detect Cd2+ in a semi-aqueous environment with a pH of 1.0–11.0, with excellent selectivity and sensitivity, and is not interfered by other metal ions. Further, bio-imaging study and cytotoxicity test confirm that it can be used for detecting Cd2+ in living HeLa S3 cells.
1 to open the spirolactam ring. The solution immediately changed from colorless to pink, turned non-fluorescent to bright red fluorescent under long wavelength UV light, and shifted the fluorescence emission peak from 430 nm to 589 nm (the excitation wavelength at 340 nm). The binding constant value of the probe to Cd2+ was 7.8 × 105 M−3 and the detection level was 10.1 nM. Probe 19 can detect Cd2+ in a semi-aqueous environment with a pH of 1.0–11.0, with excellent selectivity and sensitivity, and is not interfered by other metal ions. Further, bio-imaging study and cytotoxicity test confirm that it can be used for detecting Cd2+ in living HeLa S3 cells.
In 2017, Shaily et al. synthesized the coumarin-chalcone conjugated probe 20 (Fig. 8).50 In HEPES-buffered solution (20 mM, CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, 3
H2O, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, v/v, pH = 7.0), probe 20 and Cd2+ follows a 1
7, v/v, pH = 7.0), probe 20 and Cd2+ follows a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding ratio, making the solution immediately change from yellow to colorless and the absorption maximum blue-shift from 387 nm to 370 nm, and the absorbance has a good linear relationship with concentration of Cd2+. When excited at 387 ± 3 nm, Cd2+ made the almost non-fluorescent probe 20 emit light blue fluorescence at 495 nm, and the fluorescence lifetime increase from 0.217 ns to 1.97 ns. According to Benesi–Hildebrand plot and nonlinear least squar fitting, the association constant for Cd2+ towards probe 20 were 9.56 × 105 M−1 and (1.34 ± 0.87) × 106 M−1, respectively. Probe 20 does not respond to metal ions except Cd2+, it can be used as a Cd2+ colorimetric and turn-on fluorescence probe. In mixed aqueous-organic media with the pH range of 7.0–9.0, it can detect Cd2+ as low as 5.84 × 10−8 M. Moreover, the test strip containing probe 20 can sense Cd2+.
1 binding ratio, making the solution immediately change from yellow to colorless and the absorption maximum blue-shift from 387 nm to 370 nm, and the absorbance has a good linear relationship with concentration of Cd2+. When excited at 387 ± 3 nm, Cd2+ made the almost non-fluorescent probe 20 emit light blue fluorescence at 495 nm, and the fluorescence lifetime increase from 0.217 ns to 1.97 ns. According to Benesi–Hildebrand plot and nonlinear least squar fitting, the association constant for Cd2+ towards probe 20 were 9.56 × 105 M−1 and (1.34 ± 0.87) × 106 M−1, respectively. Probe 20 does not respond to metal ions except Cd2+, it can be used as a Cd2+ colorimetric and turn-on fluorescence probe. In mixed aqueous-organic media with the pH range of 7.0–9.0, it can detect Cd2+ as low as 5.84 × 10−8 M. Moreover, the test strip containing probe 20 can sense Cd2+.
In 2018, K. Krishnaveni et al. reported a ratiometric probe 21 (Fig. 8) that based on ICT mechanism to recognize Cd2+/F−.3 In CH3CN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, v/v) buffered with HEPES (pH = 7.52), the probe 21 is combined with Cd2+/F− at a ratio of 1
9, v/v) buffered with HEPES (pH = 7.52), the probe 21 is combined with Cd2+/F− at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Upon the excitation wavelength at 340 ± 5 nm, the fluorescence of probe 21 was changed by Cd2+ from pale yellow to dark yellow, and the fluorescence intensity ratio of I418 nm/I530 nm was linearly related to the concentration of Cd2+. The fluorescence of probe 21 can also be changed by F−, and the fluorescence intensity ratio I415 nm/I530 nm can quantitatively detect F−. The binding constants of probe 21 for Cd2+ and F− ions were 3.51 × 10−3 M and 5.34 × 10−3 M, respectively. Probe 21 can be used as a high-selectivity and high-sensitivity proportional fluorescence sensor for Cd2+ and F− with detection limits of 1.5 × 10−10 mol L−1 and 1.2 × 10−10 mol L−1, respectively. In addition, probe 21 has reversibility (EDTA) and low cytotoxicity, and can be used for fluorescence imaging of Cd2+ in H9c2 cancer cells.
1. Upon the excitation wavelength at 340 ± 5 nm, the fluorescence of probe 21 was changed by Cd2+ from pale yellow to dark yellow, and the fluorescence intensity ratio of I418 nm/I530 nm was linearly related to the concentration of Cd2+. The fluorescence of probe 21 can also be changed by F−, and the fluorescence intensity ratio I415 nm/I530 nm can quantitatively detect F−. The binding constants of probe 21 for Cd2+ and F− ions were 3.51 × 10−3 M and 5.34 × 10−3 M, respectively. Probe 21 can be used as a high-selectivity and high-sensitivity proportional fluorescence sensor for Cd2+ and F− with detection limits of 1.5 × 10−10 mol L−1 and 1.2 × 10−10 mol L−1, respectively. In addition, probe 21 has reversibility (EDTA) and low cytotoxicity, and can be used for fluorescence imaging of Cd2+ in H9c2 cancer cells.
S. Zehra's team described coumarin derived turn-on probe 22 (Fig. 8) in 2019.49 The spectral studies were carried out in a THF/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution and an excitation at 300 ± 5 nm. Probe 22 chelating Cd2+ inhibited C
1) solution and an excitation at 300 ± 5 nm. Probe 22 chelating Cd2+ inhibited C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization of probe, so the fluorescence of probe 22 increased and the fluorescence emission intensity is linearly related to the Cd2+ of 1–15 μM. The probe 22 was combined with Cd2+ at 1
N isomerization of probe, so the fluorescence of probe 22 increased and the fluorescence emission intensity is linearly related to the Cd2+ of 1–15 μM. The probe 22 was combined with Cd2+ at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry, the Ka was 3.3 × 105 M−1, and the detection limit for Cd2+ was 0.114 μM. The response of probe 22 to Cd2+ is rapid and reversible (EDTA), without obvious interference from other metal ions, and can quantitatively detect the micro molar concentration of Cd2+ in the environmental and biological samples as well.
1 stoichiometry, the Ka was 3.3 × 105 M−1, and the detection limit for Cd2+ was 0.114 μM. The response of probe 22 to Cd2+ is rapid and reversible (EDTA), without obvious interference from other metal ions, and can quantitatively detect the micro molar concentration of Cd2+ in the environmental and biological samples as well.
In 2019, Y. F. Tang's study group reported probe 23 (Fig. 8), in which coumarin is a fluorophore and tri-(2-aminoethyl)-amine is a selective recognition unit for Cd2+.34 In CH3CN–HEPES (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, v/v, pH = 7.4) solution, the probe 23 binding to Cd2+ inhibited its C
10, v/v, pH = 7.4) solution, the probe 23 binding to Cd2+ inhibited its C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization and PET process. When excited by 361 nm, probe 23 showed that the blue fluorescence was turned on, the fluorescence quantum yield increased, and the fluorescence emission peak shifted from 505 nm to 457 nm. Further, the fluorescence intensity was linearly related to the amount of Cd2+. The probe 23 bound to Cd2+ in a 1
N isomerization and PET process. When excited by 361 nm, probe 23 showed that the blue fluorescence was turned on, the fluorescence quantum yield increased, and the fluorescence emission peak shifted from 505 nm to 457 nm. Further, the fluorescence intensity was linearly related to the amount of Cd2+. The probe 23 bound to Cd2+ in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio, the association constants and detection limits were 1.37 × 1011 M−2 and 1.16 × 10−7 M, respectively. The probe 23 recognizes Cd2+ well and is only slightly interfered by Zn2+. However, since EDTA makes probe 23 reversibly bind to Zn2+ and irreversibly bind to Cd2+, the effect of Zn2+ can be eliminated. Therefore, it can be used as a high selectivity and sensitivity probe to detect Cd2+. And it also can be as an imaging agent for fluorescence imaging of Cd2+ in HepG-2 cells.
2 ratio, the association constants and detection limits were 1.37 × 1011 M−2 and 1.16 × 10−7 M, respectively. The probe 23 recognizes Cd2+ well and is only slightly interfered by Zn2+. However, since EDTA makes probe 23 reversibly bind to Zn2+ and irreversibly bind to Cd2+, the effect of Zn2+ can be eliminated. Therefore, it can be used as a high selectivity and sensitivity probe to detect Cd2+. And it also can be as an imaging agent for fluorescence imaging of Cd2+ in HepG-2 cells.
The spectroscopic and analytical parameters of the coumarin-based Cd2+ fluorescent probes are compiled in Table 2. These probes recognizes Cd2+ according to different response mechanisms, and its value in practical application has been proved by test paper test, reversibility, pH application range and biological imaging. However, optimizing its water solubility, sensitivity, selectivity and anti-interference is still a problem to be solved in future research.
| Probe | Solvent | Excitation wavelength (nm) | Emission wavelength (nm) λem0–λem | Detection limit (nM) | Association constant | Interfering ion(s) | Ref. |
|---|---|---|---|---|---|---|---|
| 19 | CH3OH–H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
340 | 430–589 | 10.1 | 7.8 × 105 M−3 | — | 2 |
| 20 | CH3CN–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) 7) |
387 | 495 | 58.4 | (1.34 ± 0.87) × 106 M−1 | — | 50 |
| 21 | CH3CN–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) 9) |
340 | 418 | 0.15 | 3.51 × 10−3 M | F− | 3 |
| 22 | THF–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
300 | About 360 | 114 | 3.3 × 105 M−1 | — | 49 |
| 23 | CH3CN–H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
361 | 505–457 | 116 | 1.37 × 1011 M−2 | Zn2+ | 34 |
4. Benzothiazole based Cd2+ fluorescent sensor
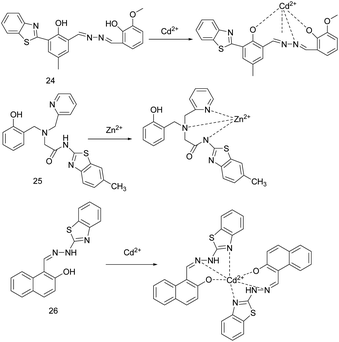
Benzothiazole is an important type of heterocyclic ring containing N and S, which can be used as a fluorophore and is widely used in the design and construction of ion recognition probes.21,24 In some cases, the N and S contained in it can also be used as a coordination site for ions.In 2018, J. Li et al. described sensor 24 (Fig. 9).4 The DMF/H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) solution and the excitation at 411 nm were selected as the exploration conditions. Due to the combined action of the ESIPT effect from the 2-(2-hydroxyphenyl)-benzothiazole moiety and AIE effect, sensor 24 exhibited weak orange fluorescence at 573 nm. The combination of sensor 24 with Zn2+ or Cd2+ inhibits the ESIPT effect and the PET process, thereby increasing the fluorescence emission, changing color from orange to yellow, and blue-shifting the fluorescence emission peak from 573 nm to 520 nm or 540 nm, respectively. The fluorescence intensity of the sensor 24 combined with Zn2+ or Cd2+ has a linear relationship with the concentration of Zn2+ or Cd2+. The detection limits of sensor 24 for Zn2+ and Cd2+ are 0.036 μM, 1.16 μM, respectively. The binding constants are 3.27 × 104 M−1 and 2.93 × 103 M−1, respectively. The addition of cysteine makes the complex of sensor 24 and Cd2+ restore the fluorescence signal of sensor 24, while the complex of sensor 24 and Zn2+ only reduces the fluorescence intensity. Thus, cysteine can be used as an auxiliary agent in the probe detection of Zn2+ and Cd2+ to distinguish them. Moreover, sensor 24 can be used for optical cell imaging of Zn2+ or Cd2+ and on-site analysis of test paper.
1, v/v) solution and the excitation at 411 nm were selected as the exploration conditions. Due to the combined action of the ESIPT effect from the 2-(2-hydroxyphenyl)-benzothiazole moiety and AIE effect, sensor 24 exhibited weak orange fluorescence at 573 nm. The combination of sensor 24 with Zn2+ or Cd2+ inhibits the ESIPT effect and the PET process, thereby increasing the fluorescence emission, changing color from orange to yellow, and blue-shifting the fluorescence emission peak from 573 nm to 520 nm or 540 nm, respectively. The fluorescence intensity of the sensor 24 combined with Zn2+ or Cd2+ has a linear relationship with the concentration of Zn2+ or Cd2+. The detection limits of sensor 24 for Zn2+ and Cd2+ are 0.036 μM, 1.16 μM, respectively. The binding constants are 3.27 × 104 M−1 and 2.93 × 103 M−1, respectively. The addition of cysteine makes the complex of sensor 24 and Cd2+ restore the fluorescence signal of sensor 24, while the complex of sensor 24 and Zn2+ only reduces the fluorescence intensity. Thus, cysteine can be used as an auxiliary agent in the probe detection of Zn2+ and Cd2+ to distinguish them. Moreover, sensor 24 can be used for optical cell imaging of Zn2+ or Cd2+ and on-site analysis of test paper.
The same year, R. Diana's research group designed probe 25 (Fig. 9) based on the ICT mechanism to respond to Cd2+.24 In ethanol–water solution, the probe 25 can quantitatively detect Fe3+ and Fe2+ through naked eye. Adding Zn2+/Cd2+, the fluorescence intensity of probe 25 increased by 3.25/4.35 times and the emission peak red-shifted from 348 nm to 370/375 nm. The detection limits for Zn2+ and Cd2+ were 205 nM and 642 nM, respectively. The binding constant for Zn2+ and Cd2+ were log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka = 4.10 and 4.25, respectively. To understand the mode of binding to the sensor, the complex of Zn2+ and the sensor is studied by X-ray crystallography, and the combination mode is shown in Fig. 10. However, due to the low selectivity, sensitivity, and anti-interference (such as: Fe3+, Fe2+, Co2+), the probe 25 is difficult to meet the detection requirements.
Ka = 4.10 and 4.25, respectively. To understand the mode of binding to the sensor, the complex of Zn2+ and the sensor is studied by X-ray crystallography, and the combination mode is shown in Fig. 10. However, due to the low selectivity, sensitivity, and anti-interference (such as: Fe3+, Fe2+, Co2+), the probe 25 is difficult to meet the detection requirements.
In 2021, S. Paul et al. reported a multifunctional naphthalene-benzothiazole based sensor 26 (Fig. 9).51 The donor part such as –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N enhances the reliability of the sensor, and the active functional group such as –OH improves the color detection efficiency of the sensor for the target analyte. Sensor 26 (1 × 10−5 M, CH3CN) can recognize Cd2+ in pure water by turning on fluorescence. When excited at 390 nm, due to the –C
N enhances the reliability of the sensor, and the active functional group such as –OH improves the color detection efficiency of the sensor for the target analyte. Sensor 26 (1 × 10−5 M, CH3CN) can recognize Cd2+ in pure water by turning on fluorescence. When excited at 390 nm, due to the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization, PET, and ESIPT mechanism, the sensor exhibits weak green fluorescence emission at 505 nm. The combination of Cd2+ with sensor 26 increases the molecular rigidity, inhibits the effects of PET and ESIPT, and promotes the CHEF process, which leads to a significant increase in the fluorescence intensity of this sensor. The association constant (Ka) of the sensor with Cd2+ is 0.91 × 106 M−1/2, and the detection limit for Cd2+ is 16.7 nM. This sensor can also detect Zn2+ or CN− in pure water medium. In addition, sensor 26 has cell membrane permeability and biocompatibility, and can detect the content of Cd2+ and CN− in bitter almonds and yeast cells.
N isomerization, PET, and ESIPT mechanism, the sensor exhibits weak green fluorescence emission at 505 nm. The combination of Cd2+ with sensor 26 increases the molecular rigidity, inhibits the effects of PET and ESIPT, and promotes the CHEF process, which leads to a significant increase in the fluorescence intensity of this sensor. The association constant (Ka) of the sensor with Cd2+ is 0.91 × 106 M−1/2, and the detection limit for Cd2+ is 16.7 nM. This sensor can also detect Zn2+ or CN− in pure water medium. In addition, sensor 26 has cell membrane permeability and biocompatibility, and can detect the content of Cd2+ and CN− in bitter almonds and yeast cells.
Like quinoline and coumarin, benzothiazole can be used not only as fluorophore, but also as Cd2+ chelating site. We provide the spectroscopic and analytical parameters of these probes benzothiazole-based fluorophore in Table 3. These probes are responsive to multiple analytes and are also disturbed by a variety of ions. Among them, the probe 24 can recognize Zn2+ and Cd2+ at the same time. The research team uses cysteine as an auxiliary agent to distinguish Zn2+ and Cd2+, which is also a way to eliminate interference and roughly identify Cd2+ in the environment. However, the quantitative detection of Cd2+ still needs a probe with high selectivity and anti-interference.
| Probe | Solvent | Excitation wavelength (nm) | Emission wavelength (nm) λem0–λem | Detection limit (nM) | Association constant (Ka) | Interfering ion(s) | Ref. |
|---|---|---|---|---|---|---|---|
| 24 | DMF–H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
411 | 573–540 | 1160 | 2.93 × 103 M−1 | Cysteine, Zn2+ | 4 |
| 25 | EtOH–H2O | 309 | 348–375 | 642 | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka = 4.25 Ka = 4.25 |
Fe3+, Fe2+, Co2+, Zn2+ | 24 |
| 26 | CH3CN | 390 | 505 | 16.7 | 0.91 × 106 M−1/2 | Zn2+, CN− | 51 |
5. Rhodamine-based Cd2+ fluorescent sensor
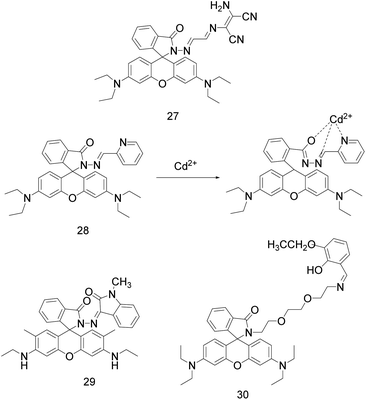
Rhodamine derivatives possess high fluorescence quantum yield, photostability, large molar extinction coefficient, bioavailability, and its excitation wavelengths and emission wavelengths in the visible light region.23,31 Furthermore, it exist an equilibrium between the nonfluorescent “spirolactam ring” and fluorescent “ring-open” forms to allow analyte sensing through “off–on” switching.23,31,52 Therefore, it is widely used in the design of fluorescent sensors.In 2017, P. Sakthivel's research group synthesized a rhodamine based senor 27 (Fig. 10) by linking to diaminomaleonitrile moiety.52 This sensor can not only identify Cd2+ by naked eyes, but also can detect Cd2+ quantitatively by UV-vis absorption and fluorescence spectra. Upon addition of Cd2+, an absorption peak emerged at 530 nm and the intensity had a linear relationship with Cd2+ concentration. Upon complexation, colourless spirolactam form is converted into colored ring opened amide form, which displays a noticeable naked-eye detection of the magenta. In HEPES buffer (acetonitrile–water = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 10 μM, pH = 7.54), sensor 27 was combined with Cd2+ in a ratio of 1
3, 10 μM, pH = 7.54), sensor 27 was combined with Cd2+ in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which increased its weak fluorescence emission at 553 nm by 200 times (excitation at 530 nm). When the Cd2+ concentration was 1.0 × 10−7 to 1.0 × 10−5 mol L−1, the fluorescence intensity [1/(F − F0)] at 553 nm had a linear relationship with 1/[Cd2+]. The association constant between sensor 27 and Cd2+ was 2.33 × 105 M−1, and the detection limit of sensor 27 for Cd2+ was 18.5 nM. It can be used for the determination of Cd2+ in river and tap water samples and as an imaging agent for Cd2+ in living cells.
1, which increased its weak fluorescence emission at 553 nm by 200 times (excitation at 530 nm). When the Cd2+ concentration was 1.0 × 10−7 to 1.0 × 10−5 mol L−1, the fluorescence intensity [1/(F − F0)] at 553 nm had a linear relationship with 1/[Cd2+]. The association constant between sensor 27 and Cd2+ was 2.33 × 105 M−1, and the detection limit of sensor 27 for Cd2+ was 18.5 nM. It can be used for the determination of Cd2+ in river and tap water samples and as an imaging agent for Cd2+ in living cells.
M. Maniyazagan et al. designed a rhodamine pyridine conjugated probe 28 (Fig. 10) based on FRET mechanism, which can recognize Cd2+ by colorimetry and fluorescence.32 In ACN/HEPES buffer (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, V/V, pH = 7.2, 10 mM), the probe was excited at 308 nm. With the addition of Cd2+, probe 28 showed orange yellow fluorescence, the maximum emission wavelength red-shifted from 480 nm to 590 nm, and the solution changed from colorless to magenta. The recognition of Cd2+ by this probe is reversible (S2−) and not interfered by other metal ions. In addition, probe 28 can also be used for imaging in HeLa cells at physiological pH. The binding constant of probe 28 to Cd2+ is 4.2524 × 104 M−1, and the detection limit is 1.025 × 10−8 M. Probe 28 should be used in the pH range of 3–9, because the fluorescence induced by Cd2+ is stronger in high acidic environment, but it cannot be induced in alkaline environment due to the formation of Cd(OH)2.
8, V/V, pH = 7.2, 10 mM), the probe was excited at 308 nm. With the addition of Cd2+, probe 28 showed orange yellow fluorescence, the maximum emission wavelength red-shifted from 480 nm to 590 nm, and the solution changed from colorless to magenta. The recognition of Cd2+ by this probe is reversible (S2−) and not interfered by other metal ions. In addition, probe 28 can also be used for imaging in HeLa cells at physiological pH. The binding constant of probe 28 to Cd2+ is 4.2524 × 104 M−1, and the detection limit is 1.025 × 10−8 M. Probe 28 should be used in the pH range of 3–9, because the fluorescence induced by Cd2+ is stronger in high acidic environment, but it cannot be induced in alkaline environment due to the formation of Cd(OH)2.
In the same year, W. Su et al. designed probe 29 (Fig. 10) by linking rhodamine 6G hydrazide with N-methylisatin via an imine linkage.8 In EtOH/H2O solution (9/1, 10 mmol HEPES buffer, pH = 7.2), probe 29 alone is non-fluorescent when excited at 500 nm. Upon the addition of Pb2+, Hg2+, Cd2+, the probe 29 generated a yellowish-green fluorescence response to Cd2+, while an orange fluorescence responses to Pb2+ and Hg2+. And the fluorescence intensity at 560/560/552 nm had a linear relationship with the concentration of Pb2+/Hg2+/Cd2+. The detection limits of probe 29 for Pb2+, Hg2+ and Cd2+ were 1.6 × 10−8, 1.2 × 10−8 and 4.7 × 10−8 mol L−1, respectively. When EDTA was added to these complexes, the fluorescence response induced by Hg2+ and Cd2+ was reversible, while the fluorescence response induced by Pb2+ was irreversible. Therefore, probe 29 can be used as a multifunctional probe for detecting Pb2+, Hg2+ and Cd2+. Because the coexistence of Cd2+ with Pb2+ or Hg2+ can enhance the fluorescence of Cd2+-induced, and the coexistence of Cd2+ with Cu2+ or Ni2+ can partially quench the fluorescence intensity of Cd2+-induced, the selectivity and practicability of the probes 29 for Cd2+ are not enough.
In 2018, M. Ghosh and colleagues reported the probe 30 (Fig. 10) for trace-level detection and discrimination of Al3+, Zn2+, Cd2+, Hg2+ in a ratiometric sensing mechanism involving PET–CHEF–FRET processes.23 In 20 mM HEPES-buffered MeOH/H2O (4/1, v/v, pH = 7.4) solution, probe 30 interacts with Cd2+ through phenolic hydroxyl group and “N” in C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, “O” in alkane proton field to form 1
N, “O” in alkane proton field to form 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with Cd2+ at a low Cd2+ concentration and 1
1 complex with Cd2+ at a low Cd2+ concentration and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 at a high Cd2+ concentration. When excited at 306 nm, the fluorescence emission peak of probe 30 blue-shifted from 397 nm to 395 nm, the fluorescence intensity increased by 36 times, and the detection limit of probe 30 for Cd2+ was 6.7 × 10−9 M. The association constant of the probe for Cd2+ is 1.35 × 105 M−1, and its affinity for Cd2+ is higher than that for Zn2+. It allows easy replacement of Zn2+ from the 30-Zn adduct to form a more stable 30-Cd adduct. Further, the simultaneous presence of Al3+ and Hg2+ can use KI to mask Hg2+, while KI does not interfere with the emission profile of probe 30. Probe 30 can identify low concentrations of Al3+ (pink), Zn2+ (green), Cd2+ (sky blue), Hg2+ (intense bloodred) according to different fluorescent signals without interference from other common ions, and can be used for imaging Zn2+, Cd2+, Hg2+ in squamous epithelial cells under a fluorescence microscope.
2 at a high Cd2+ concentration. When excited at 306 nm, the fluorescence emission peak of probe 30 blue-shifted from 397 nm to 395 nm, the fluorescence intensity increased by 36 times, and the detection limit of probe 30 for Cd2+ was 6.7 × 10−9 M. The association constant of the probe for Cd2+ is 1.35 × 105 M−1, and its affinity for Cd2+ is higher than that for Zn2+. It allows easy replacement of Zn2+ from the 30-Zn adduct to form a more stable 30-Cd adduct. Further, the simultaneous presence of Al3+ and Hg2+ can use KI to mask Hg2+, while KI does not interfere with the emission profile of probe 30. Probe 30 can identify low concentrations of Al3+ (pink), Zn2+ (green), Cd2+ (sky blue), Hg2+ (intense bloodred) according to different fluorescent signals without interference from other common ions, and can be used for imaging Zn2+, Cd2+, Hg2+ in squamous epithelial cells under a fluorescence microscope.
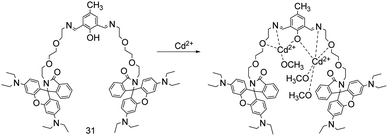
In 2019, a multi-signaling optical probe 31 (Fig. 11) reported by M. Banerjee's research group for rapid detection and discrimination of Zn2+, Cd2+ and Hg2+ at nano-molar level.31 In presence of Zn2+, Cd2+ and Hg2+, it emits deep red for Hg2+, green for Zn2+ and blue for Cd2+ upon UV light irradiation. Interestingly, Hg2+ shows intense pink in bare eye. In HEPES-buffered aqueous methanol (MeOH/H2O, 4/1, v/v, pH = 7.4), the combination of probe 31 and Cd2+ in a stoichiometric ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 makes the fluorescence emission (the excitation wavelength at 366 nm) peak blue-shift from 440 nm to 437 nm, and the blue fluorescence emission intensity is increased by 31 times. The binding constant of probe 31 for Cd2+ is 6.5 × 105 M−1, and the detection limit is 9.6 × 10−9 M. Probe 31 can be used to detect Hg2+, Cd2+, Zn2+ at the nanomolar level, although the binding ability is sequentially weakened, KI can be used to mask Hg2+ interference, Na2S can be used to mask Cd2+ interference.
2 makes the fluorescence emission (the excitation wavelength at 366 nm) peak blue-shift from 440 nm to 437 nm, and the blue fluorescence emission intensity is increased by 31 times. The binding constant of probe 31 for Cd2+ is 6.5 × 105 M−1, and the detection limit is 9.6 × 10−9 M. Probe 31 can be used to detect Hg2+, Cd2+, Zn2+ at the nanomolar level, although the binding ability is sequentially weakened, KI can be used to mask Hg2+ interference, Na2S can be used to mask Cd2+ interference.
The spectroscopic and analytical parameters rhodamine-based sensors are summarized in Table 4. Probes 27, 28 and 29 have large emission wavelengths because their molecular structures have a large degree of conjugation, which makes the fluorescence emission peak appear at a large wavelength. Based on this mechanism, we can develop sensors with larger emission wavelength for environmental detection and biological imaging. In recent five years, rhodamine-based Cd2+ fluorescent probes have good sensitivity, but their application is still disturbed by some ions. On the one hand, the probe responds to other analytes, on the other hand, the complex between the probe and Cd2+ is disturbed by other analytes. In the future development, it is necessary to optimize the ligands that recognize Cd2+, so that the probe and Cd2+ have specific recognition ability and stable binding ability. At the same time, improving the water solubility and practicability of rhodamine-based probes is still the focus of future research.
| Probe | Solvent | Excitation wavelength (nm) | Emission wavelength (nm) λem0–λem | Detection limit (nM) | Association constant (M−1) | Interfering ion(s) | Ref. |
|---|---|---|---|---|---|---|---|
| 27 | CH3CN–H2O (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
530 | 553 | 18.5 | 2.33 × 105 | — | 52 |
| 28 | CH3CN–H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) 8) |
308 | 480–590 | 10.25 | 4.2524 × 104 | S2− | 32 |
| 29 | EtOH–H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
500 | 560 | 47 | — | Pb2+, Hg2+, Cu2+, Ni2+ | 8 |
| 30 | MeOH–H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
306 | 397–395 | 6.7 | 1.35 × 105 | Al3+, Hg2+ | 23 |
| 31 | MeOH–H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
366 | 440–437 | 9.6 | 6.5 × 105 | Hg2+, Zn2+ | 31 |
6. Dansyl based Cd2+ fluorescent sensor
Dansyl group is fluorescent group with excellent performance as an electron acceptor, so it is used in the design of fluorescence sensors.53 Biological studies have shown that metallothionein is a short peptide rich in cysteine, which has high affinity for a variety of heavy metals. Cd2+ and Cu2+ ions are the main metal binding with metallothionein.54 Therefore, in the design of these probes' structure, P. Wang and co-workers introduced cysteine as the recognition group of Cd2+, connected the recognition group and dansyl fluorophore through different spacer groups, and synthesized a series of highly selective probes 32–36 (Fig. 12) for Cd2+ by solid-phase peptide synthesis (SPPS) technology.53,55–57 They described these Cd2+ fluorescent turn-on probes. These probes can be used to monitor Cd2+ in the environment and in living (HK2,53,56,57 HeLa,53–55 LNCaP57) cells. In general, they have good water solubility, selectivity, biocompatibility, and reversibility (EDTA,55 cysteine53), and can be used for Cd2+ detection or bioimaging within some specific environments (example: Cd2+ concentration and pH).In HEPES buffer (10 mM, pH = 7.4), under 365 nm UV lamp, probe 32 emits weak yellow fluorescence due to electron transfer from sulfhydryl group in Cys to dansyl group.55 Binding to Cd2+ causes the PET process of probe 32 to be blocked, to enhance fluorescence intensity and to change from yellow to green fluorescence. Upon excitation at 330 ± 10 nm, the fluorescence intensity, quantum yield and lifetime were increased (4 times, from 0.0857 to 0.1769, from 9.32 ns to 17.36 ns). Probe 32 has large stokes shift for detecting Cd2+, the binding constant is 5.18 × 1010 M−2, and the detection limit is 45 nM. Probe 32 remains stable in 2–10 pH solution and has the strongest fluorescence signal induced by Cd2+ in 7–12 pH solution. In addition, the probe 32 can realize the reversible detection for Cd2+ by EDTA.
The probe 33 was synthesized by imitating the binding site of protein, in which tryptophan (energy donor, λex = 290 nm, λem = 360 nm) and dansyl (energy acceptor, λex = 330 nm, λem = 545 nm) are fluorophores and cysteine (recognition) is ionophore.54 In the HEPES buffer (10 mM, pH = 7.4) solutions, the probe 33 can detect Cd2+ through two different excitation wavelengths. When excited at 290 ± 10 nm, probe 33 combines with Cd2+ to meet together the side chains of tryptophan and dansyl group to realize the FRET process, and the fluorescence emission increases at 510 nm and decreases at 360 nm. When excited at 330 nm, the probe 33 binds to Cd2+ to increase the fluorescence emission intensity at 545 nm by 5 times (CHEF) and the lifetime from 8.32 ns and 16.33 ns. Under the 365 nm ultraviolet lamp, the solution of the probe 33 is added with Cd2+ to emit strong green fluorescence, and other metal ions are added to emit light brown weak fluorescence. The probe 33 is combined with Cd2+ at a stoichiometric ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the binding constant is 9.12 × 1010 M−2, and the detection limit is less than 27.5 nM. Further, the emission intensity of the complex remains almost unchanged at pH 7–9.
1, the binding constant is 9.12 × 1010 M−2, and the detection limit is less than 27.5 nM. Further, the emission intensity of the complex remains almost unchanged at pH 7–9.
The dansyl-tetrapeptide fluorescent sensor 34 can produce a continuous “off–on–off” fluorescence response to Cd2+ and cysteine.53 In the HEPES buffer solution (10.0 mM, pH = 7.4), upon excitation at 330 ± 10 nm, the sensor 34 through oxygen atoms of Glu and sulfydryl of Cys binds to Cd2+ at 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the fluorescence changes from yellow to green. The fluorescence emission peak blue-shifts by 30 nm, and the fluorescence intensity increases. Adding Cys to the complex of sensor 34 and Cd2+ can restore the fluorescence signal of sensor 34. The detection limits for Cd2+ is 93 nM or for Cys is 35 nM, and lower than EPA or WHO guidelines.
1, and the fluorescence changes from yellow to green. The fluorescence emission peak blue-shifts by 30 nm, and the fluorescence intensity increases. Adding Cys to the complex of sensor 34 and Cd2+ can restore the fluorescence signal of sensor 34. The detection limits for Cd2+ is 93 nM or for Cys is 35 nM, and lower than EPA or WHO guidelines.
Sensor 35 based on the conjugated dansyl group and dipeptide.56 In HEPES buffer solutions (20.0 mM, pH = 7.4), under 365 nm ultraviolet light, the combination of sensor 35 and Cd2+ prevents the electron transfer from sulfhydryl group in Cys to the dansyl group (PET). Accordingly, the sensor 35 turns on green fluorescence. When excited at 330 nm, the fluorescence intensity at 545 nm is linearly related to the Cd2+ of 0–1.40 μM. The association constant and detection limit for Cd2+ are 1.30 × 109 M−2 and 13.8 nM, respectively. Sensor 35 can be used to detect Cd2+ with a pH range of 7.0–10.0. Due to the paramagnetism of Cu2+, fluorescence of the complex of sensor 35 and Cd2+ is partially weakened, but there is no interference from other metal ions.
In 2019, they described probe 36 based on dansyl-appended dipeptide (Gly–Cys–NH2).57 In 20.0 mM HEPES buffer solution at pH 7.4, the addition of Cd2+ led to the fluorescence enhancement of the probe, and the formation of dansulfonyl dimer shifted the fluorescence emission peak from 560 nm to 515 nm (monomer-excimer mechanism). The addition of Cu2+ led to the fluorescence quenching of the probe. Under 365 nm UV light, the fluorescence of probe 36 was bright green and the emission was significantly enhanced with the addition of Cd2+, while the fluorescence of the probe was dark brown and the emission was significantly quenched with the addition of Cu2+. The binding constants of probe 36 to Cd2+ and Cu2+ are 1.74 × 108 M−2 and 6.96 × 107 M−2, respectively. The detection limits for Cd2+ and Cu2+ are 14.5 nM and 26.3 nM, respectively. The probe with pH 7–12 can stably recognize Cd2+ (pH 7.0–12.0) and Cu2+ (pH 2.0–12.0) in 20 s, and is not interfered by other metal ions and anions. In addition, probe 36 has hypotoxicity, membrane permeability and low toxicity, which can be used to detect Cd2+ and Cu2+ in living LNCaP cells.
The spectroscopic and analytical parameters danyl-based fluorescent probes are shown in Table 5. The researchers took the sulfhydryl group in cysteine as the recognition site of Cd2+, and designed and synthesized these probes by changing the type, number and position of linkers, so as to improve the sensitivity, selectivity and anti-interference of theses probes. Moreover, the researchers use amino acids as recognition groups and linkers, which has low toxicity for biological monitoring. These sensors have good water solubility, selectivity, sensitivity and long emission wavelength. They can be used not only for environmental monitoring, but also for cell imaging.
| Probe | Solvent | Excitation wavelength (nm) | Emission wavelength (nm) λem0–λem | Detection limit (nM) | Association constant | Interfering ion(s) | Ref. |
|---|---|---|---|---|---|---|---|
| 32 | H2O | 330 | 550 | 45 | 5.18 × 1010 M−2 | — | 55 |
| 33 | H2O | 330 | 545 | 27.5 | 9.12 × 1010 M−2 | — | 54 |
| 34 | H2O | 330 | About 570–540 | 93 | — | — | 53 |
| 35 | H2O | 330 | 545 | 13.8 | 1.30 × 109 M−2 | Cu2+ | 56 |
| 36 | H2O | 365 | 560–515 | 14.5 | 1.74 × 108 M−2 | Cu2+ | 57 |
7. Diarylethylene based Cd2+ fluorescent sensor
Diarylethylene is composed of heteroaromatic ring groups connected on both sides of the vinyl group. The distance between the two aryl groups is relatively close, which can form two isomers of ring opening and ring closure. As a good photochromic dye, diarylethylene has the advantages of excellent photochromic performance, thermal stability, fatigue resistance, feasible synthesis, high photoisomerization quantum yields, and convenient functionalization.21,22,28 Therefore, it is often used as a fluorophore part in the design of probes. Diarylethene fluorescent probes have two states: ring-open and ring-closed, which can be converted under ultraviolet light (from ring-open to ring-closed) and visible light. Since the ring-open and ring-closed positions of all diarylethene probes are the same, and the state of the ring does not affect the binding site with Cd2+. Therefore, the probe 37 is taken as an example to show the ring-open and ring-closed state of diarylethylene.In 2017, D. B. Zhang's research group synthesized probe 37 (Fig. 13), which can switch between ring-open and ring-closed under the light irradiation at 297 nm and >500 nm.21 In acetonitrile solution, the addition of Cd2+/Zn2+ changed the probe 37 solution from colourless to light-yellow/dark-yellow. Upon excitation at 402 nm, the fluorescence emission red-shifted from 519 nm to 559 nm/608 nm, and the fluorescence changed from dark to bright-yellow/orange. Under 297 nm light irradiation, the ring-open complex of Cd2+ and probe 37 changed into a ring-closed complex, and the fluorescence intensity of the complex was quenched by 8.7%/19%. The fluorescence of the ring-open complex was recovered under the irradiation light >500 nm. The detection limits of probe 37 for Cd2+ is 3.2 × 10−7 M and for Zn2+ is 2.88 × 10−7 M, and the association constants (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka) for Cd2+ is 3.81 and for Zn2+ is 3.51. Probe 37 is selective and reversible (EDTA) for the detection of Zn2+ and Cd2+. The water solubility of the probe 37 is poor, and its coordination ability to Cd2+ is stronger than that to Zn2+, so Cd2+ can replace Zn2+ binding probe 37. A logic circuit was constructed with stimuli of UV/vis lights, Cd2+ and EDTA as inputs and fluorescence intensity at 559 nm as an output. In addition, because probe 37 contains a hydrazinobenzothiazole group, it can react with acids and bases, and the present of OH− is detected in the near infrared region.
Ka) for Cd2+ is 3.81 and for Zn2+ is 3.51. Probe 37 is selective and reversible (EDTA) for the detection of Zn2+ and Cd2+. The water solubility of the probe 37 is poor, and its coordination ability to Cd2+ is stronger than that to Zn2+, so Cd2+ can replace Zn2+ binding probe 37. A logic circuit was constructed with stimuli of UV/vis lights, Cd2+ and EDTA as inputs and fluorescence intensity at 559 nm as an output. In addition, because probe 37 contains a hydrazinobenzothiazole group, it can react with acids and bases, and the present of OH− is detected in the near infrared region.
In the same year, X. X. Zhang et al. reported that sensor 38 (Fig. 13).22 In THF solution, under 360 nm light, adding Cd2+, Ag+, Zn2+ increases the fluorescence of sensor 38, but only Cd2+ makes the fluorescence emission peak redshift from 439 nm to 541 nm and the fluorescence changes from dark to bright-green. 0–3.0 equiv. of Cd2+ has a linear relationship with the fluorescence intensity ratio (I541 nm/I439 nm). The ring-open and ring-closed states of sensor 38 can be converted by 313 nm and >450 nm light. Under 313 nm irradiation, the solution changes from pale yellow to magenta, and the fluorescence intensity reduces by about 87% compared with the open-ring complex. The sensor 38 detects Cd2+ with selectivity and reversibility, the detection limit for Cd2+ is 1.97 × 10−7 mol L−1, and the binding constant (Ka) to Cd2+ is 8.60 × 103 L mol−1. A logic circuit was fabricated with four inputs of the combinational stimuli of UV/vis and Cd2+/EDTA, and one output of fluorescence intensity at 541 nm.
In 2018, S. Guo and colleagues designed probe 39 (Fig. 13) and studied its recognition of Cd2+ in 392 nm excitation and acetonitrile solution.58 When Cd2+ was added, the solution of the open-ring isomer of probe 39 changed from colorless to yellow, the fluorescence emission peak red-shifted from 507 nm to 633 nm, the fluorescence changed from dark cyan to golden yellow, and the fluorescence intensity increased by 24.9 fold. After illuminating at 297 nm, a complex of the closed-ring isomer of probe 39 and Cd2+ was formed, the solution changed from yellow to plum and appeared absorption band at 547 nm, the fluorescence changed from golden yellow to fawn brown. Cd2+ was directly added to probe 39 solution of closed-ring isomer, the color changed from purple to plum, and the fluorescence changed from dark to fawn brown. Due to the incomplete cyclization and the formation of parallel conformational isomers, the compound fluorescence of the ring-closed and Cd2+ was reduced by 34%. In addition, HSO3− made the probe 39 emit bright cyan fluorescence and increase the intensity 135 times. The probe 39 can recognize Cd2+ and HSO3− with high selectivity in the acetonitrile solution, and the binding with Cd2+ is irreversible.
In 2018, Z. Wang et al. synthesized the sensors 40 and 41 (Fig. 14), the conversion between their open-ring and closed-ring isomers can be realized by irradiation at 297 nm and >500 nm.18,28 The properties of the two probes were studied in methanol solution and 350 nm light excitation. Because EDTA can make these probes bind to Cd2+ reversibly, the author used Cd2+/EDTA and UV/vis as the input stimulus and the fluorescence intensity (sensors 40 and 41 were recorded at 476 nm and 480 nm, respectively) of probes in the presence of Cd2+ as the output to construct the logic circuit.
Sensor 40 has a dark purple weak fluorescence at 435 nm.28 Adding Cd2+, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization of the sensor was inhibited, the fluorescence changed from blue to bright blue, and maximum emission peak red-shifted from 435 nm to 476 nm. Under 297 nm, the colorless open-ring isomer transforms into pink closed-ring isomer, the emission intensity at 435 nm reduces about 73% and the fluorescence changes from blue to dark, and adding Cd2+ makes the fluorescence change from dark to dark blue. Sensor 40 can detect Cd2+ as low as 2.52 × 10−7 mol L−1, and the binding constant (Ka) is 4.3 × 103 L mol−1. Sensor 40 with poor water solubility, and the fluorescence of Cd2+-induced can be completely quenched by Cu2+.
N isomerization of the sensor was inhibited, the fluorescence changed from blue to bright blue, and maximum emission peak red-shifted from 435 nm to 476 nm. Under 297 nm, the colorless open-ring isomer transforms into pink closed-ring isomer, the emission intensity at 435 nm reduces about 73% and the fluorescence changes from blue to dark, and adding Cd2+ makes the fluorescence change from dark to dark blue. Sensor 40 can detect Cd2+ as low as 2.52 × 10−7 mol L−1, and the binding constant (Ka) is 4.3 × 103 L mol−1. Sensor 40 with poor water solubility, and the fluorescence of Cd2+-induced can be completely quenched by Cu2+.
Sensor 41 chelates Cd2+/Zn2+, which inhibits the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization, increases (51.1/17.7 fold) the fluorescence, and changes from dark to light-blue/green fluorescence.18 In addition, the emission peak at 451 nm red shifts to 480 nm/500 nm, and the fluorescence intensity at 480 nm/500 nm is linearly related to the concentration of Cd2+/Zn2+. The complexing constants of open-ring isomer for Cd2+ is 5.95 × 104 L mol−1 and for Zn2+ is 4.9 × 104 L mol−1, and the detection limits for Cd2+ is 8.69 × 10−9 mol L−1 and for Zn2+ is 3.67 × 10−8 mol L−1. Under the irradiation at 297 nm, the open-ring isomer became a weaker fluorescent closed-ring isomer and the solution change from colorless to purple. Adding Cd2+/Zn2+ made it change from light dark fluorescence to dark-blue/dark-green, the fluorescence intensity increase by 28.5/14.5 times, and the emission peak red-shift 29 nm/49 nm. Probe 41 can selectively and reversibly distinguish Cd2+ and Zn2+.
N isomerization, increases (51.1/17.7 fold) the fluorescence, and changes from dark to light-blue/green fluorescence.18 In addition, the emission peak at 451 nm red shifts to 480 nm/500 nm, and the fluorescence intensity at 480 nm/500 nm is linearly related to the concentration of Cd2+/Zn2+. The complexing constants of open-ring isomer for Cd2+ is 5.95 × 104 L mol−1 and for Zn2+ is 4.9 × 104 L mol−1, and the detection limits for Cd2+ is 8.69 × 10−9 mol L−1 and for Zn2+ is 3.67 × 10−8 mol L−1. Under the irradiation at 297 nm, the open-ring isomer became a weaker fluorescent closed-ring isomer and the solution change from colorless to purple. Adding Cd2+/Zn2+ made it change from light dark fluorescence to dark-blue/dark-green, the fluorescence intensity increase by 28.5/14.5 times, and the emission peak red-shift 29 nm/49 nm. Probe 41 can selectively and reversibly distinguish Cd2+ and Zn2+.
In 2019, H. L. Liu et al. designed a fluorescence enhanced probe 42 (Fig. 15) based on C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerism suppression and CHEF mechanism to identify Cd2+.25 They tested the probe in methanol solution and at 350 nm excitation wavelength. The open-ring isomer of probe 42 combined with Cd2+ increased the fluorescence emission intensity by 106 times, red-shifted the emission peak from 452 nm to 506 nm, and changed the fluorescence from dark to cyan. The association constant between probe and Cd2+ was 3.14 × 104 L mol−1, and the detection limit for Cd2+ was 1.89 × 10−7 mol L−1. The closed-ring isomer of probe 42 changed from non-fluorescent to dark-cyan fluorescence after binding to Cd2+, and the emission peak red-shifted from 452 nm to 511 nm. Due to the formation of the closed-ring isomer and the FRET process from 2-aminoisoindole-1,3-dione to diethylene unit, its fluorescence intensity is 63% weaker than that of the complex of Cd2+ and the open-ring isomer. With 297 nm and >500 nm light irradiations, the conversion between the open-ring isomer and the closed-ring isomer of probe 42 can be realized. Adding Cu2+ made the open-ring isomer solution of probe 42 changed from colorless to yellow and the closed-ring isomer solution from purple to green, and it can be used for naked eye colorimetric detection of Cu2+ with the detection limit 4.12 × 10−8 mol L−1. As a multifunctional fluorescent probe, the probe 42 can selectively and reversibly bind and accurately detect Cd2+ and Cu2+, but Cu2+ can obviously quench the fluorescence intensity caused by Cd2+.
N isomerism suppression and CHEF mechanism to identify Cd2+.25 They tested the probe in methanol solution and at 350 nm excitation wavelength. The open-ring isomer of probe 42 combined with Cd2+ increased the fluorescence emission intensity by 106 times, red-shifted the emission peak from 452 nm to 506 nm, and changed the fluorescence from dark to cyan. The association constant between probe and Cd2+ was 3.14 × 104 L mol−1, and the detection limit for Cd2+ was 1.89 × 10−7 mol L−1. The closed-ring isomer of probe 42 changed from non-fluorescent to dark-cyan fluorescence after binding to Cd2+, and the emission peak red-shifted from 452 nm to 511 nm. Due to the formation of the closed-ring isomer and the FRET process from 2-aminoisoindole-1,3-dione to diethylene unit, its fluorescence intensity is 63% weaker than that of the complex of Cd2+ and the open-ring isomer. With 297 nm and >500 nm light irradiations, the conversion between the open-ring isomer and the closed-ring isomer of probe 42 can be realized. Adding Cu2+ made the open-ring isomer solution of probe 42 changed from colorless to yellow and the closed-ring isomer solution from purple to green, and it can be used for naked eye colorimetric detection of Cu2+ with the detection limit 4.12 × 10−8 mol L−1. As a multifunctional fluorescent probe, the probe 42 can selectively and reversibly bind and accurately detect Cd2+ and Cu2+, but Cu2+ can obviously quench the fluorescence intensity caused by Cd2+.
J. F. Lv's research group designed and synthesized a multifunctional fluorescent sensor 43 (Fig. 15) based on diarylethene containing pyrene unit.59 In acetonitrile solution, adding Cd2+ or Zn2+ makes the absorption peak of the sensor red shift and the solution color change. Under the excitation at 440 nm, the presence of Cd2+ or Zn2+ not only changes the fluorescence color, but also increases the fluorescence intensity (due to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization blocking and CHEF effect), and the fluorescence emission intensity is linearly related to a certain range of ion concentration. The binding constants of the open-loop sensor with Cd2+ or Zn2+ are 5.8 × 104 L mol−1 or 6.0 × 104 L mol−1, respectively. The detection limits for Cd2+ or Zn2+ are 1.85 × 10−9 mol L−1 or 7.68 × 10−9 mol L−1, respectively. Sensor 43 can be used for the detection of Cd2+ and Zn2+ in actual water samples, and processed into test pieces for on-site analysis and testing. However, when the sensor is used for the fluorescence detection of Cd2+ (affected by Mg2+, Zn2+, Sn2+, Co2+, Cu2+, Ni2+) and Zn2+ (affected by Cd2+, Hg2+, Cu2+, Ni2+), it is easy to be interfered by a variety of cations. Moreover, because the absorption peak, emission peak and color change caused by Cd2+ or Zn2+ are relatively close, sensor 43 can't distinguish them effectively by naked eye.
N isomerization blocking and CHEF effect), and the fluorescence emission intensity is linearly related to a certain range of ion concentration. The binding constants of the open-loop sensor with Cd2+ or Zn2+ are 5.8 × 104 L mol−1 or 6.0 × 104 L mol−1, respectively. The detection limits for Cd2+ or Zn2+ are 1.85 × 10−9 mol L−1 or 7.68 × 10−9 mol L−1, respectively. Sensor 43 can be used for the detection of Cd2+ and Zn2+ in actual water samples, and processed into test pieces for on-site analysis and testing. However, when the sensor is used for the fluorescence detection of Cd2+ (affected by Mg2+, Zn2+, Sn2+, Co2+, Cu2+, Ni2+) and Zn2+ (affected by Cd2+, Hg2+, Cu2+, Ni2+), it is easy to be interfered by a variety of cations. Moreover, because the absorption peak, emission peak and color change caused by Cd2+ or Zn2+ are relatively close, sensor 43 can't distinguish them effectively by naked eye.
The spectroscopic and analytical parameters of the diarylethene-based Cd2+ fluorescence sensor are shown in Table 6. Different from fluorophores such as quinoline, coumarin and benzothiazole, diarylethylene is only used as fluorophore (signal reporter) in probe design. The diarylethylene-based probes can realize the ring-open and ring-closed transition through ultraviolet and visible light, and its ring-open state recognition Cd2+ can produce greater fluorescence changes. Some sensors diarylethylene-based have relatively large emission wavelengths. However, many sensors either respond to ions other than Cd2+, or are disturbed by other ions when detecting Cd2+, and have low selectivity and anti-interference ability. Moreover, their water solubility and detection limit need to be improved.
| Probe | Solvent | Excitation wavelength (nm) | Emission wavelength (nm) λem0–λem | Detection limit (nM) | Association constant (Ka) | Interfering ion(s) | Ref. |
|---|---|---|---|---|---|---|---|
| 37 | CH3CN | 402 | 519–559 | 320 | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka = 3.81 Ka = 3.81 |
Zn2+ | 13 |
| 38 | THF | 360 | 439–541 | 197 | 8.60 × 103 M−1 | Ag+, Cu2+, Pb2+ | 22 |
| 39 | CH3CN | 392 | 507–633 | — | — | Cu2+ | 58 |
| 40 | MeOH | 350 | 435–476 | 252 | 4.3 × 103 M−1 | Cu2+ | 28 |
| 41 | MeOH | 350 | 451–480 | 8.69 | 5.95 × 104 M−1 | Zn2+ | 18 |
| 42 | MeOH | 350 | 452–506 | 189 | 3.14 × 104 M−1 | Cu2+ | 25 |
| 43 | CH3CN | 440 | 648 | 1.85 | 5.8 × 104 M−1 | Mg2+, Zn2+, Sn2+, Co2+, Cu2+, Ni2+ | 59 |
8. Other small organic molecules based Cd2+ sensor
In 2017, S. Chithiraikumar's research group synthesized sensor 44 (Fig. 16), which can recognize Cd2+ through absorption and fluorescence spectra.12 In acetonitrile/HEPES buffer medium (5 mM, pH = 7.3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v), the sensor 44 combines with Cd2+ to red-shift its maximum absorption from 391 nm to 418 nm, and the absorbance of probe at 418 nm to increase with the concentration of Cd2+, and the association constant for Cd2+ was 5.39 × 105 M−1 (±1.0%). Under the excitation at 400 nm, the sensor 44 binds to Cd2+ with an association constant of 3.16 × 105 M−1, which makes the fluorescence emission peak redshift from 558 nm to 561 nm, and the fluorescence emission intensity and quantum yield increase. The sensor 44 binds to Cd2+ in a pH system of 4.2 to 8.1, rapidly increases fluorescence within 0.3 min and without interference from various ions to monitor Cd2+ as low as 10.21 nM. The sensor 44 has selectivity, sensitivity, reversibility (EDTA) and bio-imaging (HeLa cells) for detecting Cd2+, the fluorescence response is enhanced when the temperature increases within 25–45 °C, and the fluorescence is quenched when the aqueous buffer is 80–100%.
5, v/v), the sensor 44 combines with Cd2+ to red-shift its maximum absorption from 391 nm to 418 nm, and the absorbance of probe at 418 nm to increase with the concentration of Cd2+, and the association constant for Cd2+ was 5.39 × 105 M−1 (±1.0%). Under the excitation at 400 nm, the sensor 44 binds to Cd2+ with an association constant of 3.16 × 105 M−1, which makes the fluorescence emission peak redshift from 558 nm to 561 nm, and the fluorescence emission intensity and quantum yield increase. The sensor 44 binds to Cd2+ in a pH system of 4.2 to 8.1, rapidly increases fluorescence within 0.3 min and without interference from various ions to monitor Cd2+ as low as 10.21 nM. The sensor 44 has selectivity, sensitivity, reversibility (EDTA) and bio-imaging (HeLa cells) for detecting Cd2+, the fluorescence response is enhanced when the temperature increases within 25–45 °C, and the fluorescence is quenched when the aqueous buffer is 80–100%.
D. D. Cheng and colleagues reported on the ICT probe 45 (Fig. 16) with tetramethyl substituted bis(difluoroboron)-1,2-bis[(1H-pyrrol-2-yl)methylene]hydrazine (Me4BOPHY) as a fluorophore and N,N-bis(pyridin-2-ylmethyl)benzenamine (BPA) as an electron donor moiety.19 Chelating Cd2+ reduces the electron-donating ability of BPA, thus quenching ICT transition and initiating π–π transition of the fluorophore, resulting in blue shift of the absorption and emission of the probe. In acetonitrile solution, Cd2+ made the absorption peak of probe 45 blue-shift from 550 nm to 475 nm. Under the excitation at 410 ± 10 nm, the fluorescence emission peak blue-shifted from 675 nm to 570 nm, and the solution changed from red to bright yellow. When the excitation wavelength at 495 nm, the fluorescence intensity ratio F570 nm/F730 nm reached a stable value within 1 min and had a functional relationship with the concentration of Cd2+, which could be used to quantitatively detect Cd2+. The probe 45 also has a fluorescence response to Zn2+, but still emits red fluorescence. Therefore, the probe 45 can detect Cd2+ as low as 6.9 nM or 0.77 ppb with selectivity, sensitivity, and anti-interference, which is far below the safety value (3 ppb) set for drinking water by WHO.
W. B. Huang et al. synthesized probe 46 (Fig. 16), in which DPA was used as the recognition group of Cd2+, and two sulfonic groups were introduced into the porphyrin fluorophore to enhance the water solubility.60 The HEPES buffer (20 mM, pH = 7.4) and the excitation at 418 nm were selected to study. Probe 46 and Cd2+ combined with a stoichiometric ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, weakened the conjugation between DPA and porphyrin (ICT), so that the fluorescence emission blue-shifted from 653 nm to 611 nm, and the fluorescence intensity ratio of F611 nm/F653 nm was linearly related to the Cd2+ concentration of 0–2.5 μM. The dissociation constant was 31.2 ± 5.2 μM, and the detection limit for Cd2+ was 3.2 × 10−8 M. Hg2+ and Cu2+ showed moderately quenching of fluorescence signal at 653 nm, but there was no enhancement of fluorescence signal at 611 nm. In an environment with no Hg2+ or Cu2+ and a pH of 6.5–10, the probe 46 can quantitatively and reversibly (EDTA) detect Cd2+. In addition, the probe has low cytotoxicity and can be used for bio-imaging in living cells.
2, weakened the conjugation between DPA and porphyrin (ICT), so that the fluorescence emission blue-shifted from 653 nm to 611 nm, and the fluorescence intensity ratio of F611 nm/F653 nm was linearly related to the Cd2+ concentration of 0–2.5 μM. The dissociation constant was 31.2 ± 5.2 μM, and the detection limit for Cd2+ was 3.2 × 10−8 M. Hg2+ and Cu2+ showed moderately quenching of fluorescence signal at 653 nm, but there was no enhancement of fluorescence signal at 611 nm. In an environment with no Hg2+ or Cu2+ and a pH of 6.5–10, the probe 46 can quantitatively and reversibly (EDTA) detect Cd2+. In addition, the probe has low cytotoxicity and can be used for bio-imaging in living cells.
J. Q. Sun's research group designed a multi-responsive squaraine-based sensor 47 (Fig. 16).17 In ethanol/H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution (10 mM HEPES buffer at pH 7.0), when excited at 470 nm, sensor 47 shows weak fluorescence at 545 nm due to ESIPT and C
1) solution (10 mM HEPES buffer at pH 7.0), when excited at 470 nm, sensor 47 shows weak fluorescence at 545 nm due to ESIPT and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization. Adding Cd2+/Al3+/Zn2+ made the sensor 47 increase the fluorescence intensity by 39/31/53 times, and shift the emission peak from 545 nm to 537 nm/550 nm/542 nm, and emit bright-green/yellow/yellow-green fluorescence under 365 nm irradiation. The stability of the combination of sensor 47 with Al3+, Zn2+, Cd2+ decreased in turn. Sensor 47 can detect Cd2+ as low as 5.76 × 10−8 M at pH 5–10, and all the other metal ions except Zn2+ can decrease the fluorescence enhancement caused by Cd2+ to a different extent. Moreover, the sensor 47 can chelate Al3+ from Aβ1-42-Al complex, indicating its potential value in the treatment of Alzheimer's disease.
N isomerization. Adding Cd2+/Al3+/Zn2+ made the sensor 47 increase the fluorescence intensity by 39/31/53 times, and shift the emission peak from 545 nm to 537 nm/550 nm/542 nm, and emit bright-green/yellow/yellow-green fluorescence under 365 nm irradiation. The stability of the combination of sensor 47 with Al3+, Zn2+, Cd2+ decreased in turn. Sensor 47 can detect Cd2+ as low as 5.76 × 10−8 M at pH 5–10, and all the other metal ions except Zn2+ can decrease the fluorescence enhancement caused by Cd2+ to a different extent. Moreover, the sensor 47 can chelate Al3+ from Aβ1-42-Al complex, indicating its potential value in the treatment of Alzheimer's disease.
N. A. Bumagina et al. described probe 48 (Fig. 17), which was excited at 495 nm and studied its ion recognition in a solution of propanol-1/cyclohexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30).61 Adding Cd2+/Hg2+ made the fluorescence change from yellow-orange to orange-green, and the maximum emission peak of probe 48 red-shift from 518 nm to 538 nm/539 nm. When the molar ratio of Cd2+ concentration to probe concentration is less than 5, the fluorescence intensity increases linearly with the increase of Cd2+ concentration. When the molar ratio of Cd2+ concentration to probe concentration is greater than 5, the ratio of I538 nm/I518 nm increases to 150 times and remains unchanged. Similarly, when the molar ratio of Hg2+ concentration to probe concentration is greater than 4, I539 nm/I518 nm increases by 40 times and remains unchanged. Probe 48 complexes Cd2+/Hg2+ with a stoichiometric ratio of 2
30).61 Adding Cd2+/Hg2+ made the fluorescence change from yellow-orange to orange-green, and the maximum emission peak of probe 48 red-shift from 518 nm to 538 nm/539 nm. When the molar ratio of Cd2+ concentration to probe concentration is less than 5, the fluorescence intensity increases linearly with the increase of Cd2+ concentration. When the molar ratio of Cd2+ concentration to probe concentration is greater than 5, the ratio of I538 nm/I518 nm increases to 150 times and remains unchanged. Similarly, when the molar ratio of Hg2+ concentration to probe concentration is greater than 4, I539 nm/I518 nm increases by 40 times and remains unchanged. Probe 48 complexes Cd2+/Hg2+ with a stoichiometric ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and the detection limit is 2 × 10−9 M/1.7 × 10−8 M, which can effectively detect Hg2+, but Co2+, Cu2+, Zn2+, Hg2+ interfere with the detection of Cd2+.
2, and the detection limit is 2 × 10−9 M/1.7 × 10−8 M, which can effectively detect Hg2+, but Co2+, Cu2+, Zn2+, Hg2+ interfere with the detection of Cd2+.
The sensor 49 (Fig. 17) synthesized by V. Tekuri et al. has good selectivity, precision and accuracy for the detection of Cd2+, and can be used for colorimetric detection of Cd2+ in environmental samples.62 When Cd2+ was added to the DMF solution of the new heterocyclic thiophene-2-carboxylic acid hydrazide based sensor 49, the solution changed from colorless to yellow, the maximum absorption red-shifted from 320 nm to 425 nm, and the absorption intensity had a linear relationship with the concentration of Cd2+ from 5.0 to 30 μM. The association constant (Ka) was 3.7 × 104 M−1, and the detection limit was 2.0 × 10−7 M.
Y. Tang's research group synthesized probe 50 (Fig. 18) by a click reaction of dialkyne and indole azide precursors.63 In ethanol–ethyl acetate (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) solution, under 283 nm, adding Cd2+/Fe3+ made the maximum fluorescence emission of probe 50 at 510 nm be quenched. But only the concentration of Cd2+ from 0 to 7.226 × 10−5 M was linearly related to the fluorescence intensity, which can be used to quantitatively detect Cd2+, and the detect limit for Cd2+ is 2.69 μM. The fluorescence quenching of probe 50 by Cd2+ reached equilibrium within 30 minutes, and it was obviously quenched at 20–50 °C, and reached the best quenching at 70 °C.
3, v/v) solution, under 283 nm, adding Cd2+/Fe3+ made the maximum fluorescence emission of probe 50 at 510 nm be quenched. But only the concentration of Cd2+ from 0 to 7.226 × 10−5 M was linearly related to the fluorescence intensity, which can be used to quantitatively detect Cd2+, and the detect limit for Cd2+ is 2.69 μM. The fluorescence quenching of probe 50 by Cd2+ reached equilibrium within 30 minutes, and it was obviously quenched at 20–50 °C, and reached the best quenching at 70 °C.
The biphenyl substituted piperidine-4-one sensor 51 (Fig. 18) in aqueous solution containing 1% CH3CN, upon irradiation at 315 nm, the fluorescence at 433 nm was increased by 3.6 times with the addition of Cd2+.64 And the maximum fluorescence intensity of sensor 51 has a linear relationship with the Cd2+ concentration of 0 to 110 equivalents, which can quantitatively detect Cd2+. The binding constant and the detection limit for Cd2+ were 3862.872 M−1 and 1 × 10−7 mol L−1, respectively. The sensor 51 reported by S. Poomalai et al. can selectively and sensitively respond to Cd2+ in the pH range of 4.5 to 8.0, and can be incorporated into the prepared polysulfone membranes to effectively detect and remove Cd2+ in real samples.64
The first report of probe 52 (Fig. 18) was reported by W. H. Hsieh et al. in 2012 as a probe for Zn2+ recognition.65 In 2017, P. Kaur et al. supplemented the ion recognition of this probe.66 In methanol solution, the solution immediately changed from yellow to colorless when probe 52 and Zn2+/Cd2+ were combined at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The probe 52 chelated Zn2+/Cd2+ to inhibit C
2. The probe 52 chelated Zn2+/Cd2+ to inhibit C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization. Upon excitation at 308 nm, adding Zn2+ and Cd2+ made probe 52 turn on fluorescence at 450 nm, the detection limits for Zn2+ was 86 nM and for Cd2+ was 50 nM, and the binding constant with Zn2+ was 4.60 and for Cd2+ was 4.87. If there is IO4− in the detection system, there is no turn-on fluorescence response caused by Zn2+/Cd2+. In addition, the probe 52 can distinguish Fe3+ (light green) and Fe2+ (purple) by naked eye.
N isomerization. Upon excitation at 308 nm, adding Zn2+ and Cd2+ made probe 52 turn on fluorescence at 450 nm, the detection limits for Zn2+ was 86 nM and for Cd2+ was 50 nM, and the binding constant with Zn2+ was 4.60 and for Cd2+ was 4.87. If there is IO4− in the detection system, there is no turn-on fluorescence response caused by Zn2+/Cd2+. In addition, the probe 52 can distinguish Fe3+ (light green) and Fe2+ (purple) by naked eye.
In 2017, the study team of B. K. Momidi designed the multi-signaling thiocarbohydrazide based colorimetric sensor 53a and 53b (Fig. 18).67 53a can specifically bind to Cd2+, 53b can multi-signal response to Hg2+, Cu2+, Cd2+, Pb2+ and the selectivity is sequentially weakened, and both sensors have good linear range 0–10−6 M, which can quantitatively detect metal ions in aqueous medium. In DMSO solution, 53a has no response to other metal ions, but when combined with Cd2+ at a stoichiometric ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in DMSO solution, the solution changes from colorless to pale pink, and the maximum absorption band red-shifts from 395 nm to 579 nm. The detection limit of 53a for Cd2+ is 3.22 μM. In DMF solution, 53b has an absorption band at 354 nm. When 53b reacts with Cd2+, Hg2+, Cu2+ and Pb2+ at a stoichiometric ratio of 1
1 in DMSO solution, the solution changes from colorless to pale pink, and the maximum absorption band red-shifts from 395 nm to 579 nm. The detection limit of 53a for Cd2+ is 3.22 μM. In DMF solution, 53b has an absorption band at 354 nm. When 53b reacts with Cd2+, Hg2+, Cu2+ and Pb2+ at a stoichiometric ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the solution changes from colorless to brownish-red, pink, yellow and orange, and the absorption peaks shows red shifted by 146 nm, 96 nm, 66 nm and 96 nm, respectively. The detection limits of 53b for Cd2+, Hg2+, Cu2+ and Pb2+ are 0.20 μM, 0.70 μM, 0.20 μM and 0.30 μM, respectively. The multi-sensing ability of 53b is due to the presence of hydroxy functionality of quinoline moiety, which has suitable binding sites (–OH, C
1, the solution changes from colorless to brownish-red, pink, yellow and orange, and the absorption peaks shows red shifted by 146 nm, 96 nm, 66 nm and 96 nm, respectively. The detection limits of 53b for Cd2+, Hg2+, Cu2+ and Pb2+ are 0.20 μM, 0.70 μM, 0.20 μM and 0.30 μM, respectively. The multi-sensing ability of 53b is due to the presence of hydroxy functionality of quinoline moiety, which has suitable binding sites (–OH, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and N in quinoline ring) to co-ordinate with the metal ions.
N and N in quinoline ring) to co-ordinate with the metal ions.
V. Kumar's team synthesized a sensor 54 (Fig. 19) with biphenyl as the fluorophore and carboxylic acid group as the binding site.68 In CH3OH solution, under excitation at 320 nm, sensor 54 emits strong fluorescence at 418 nm. Adding Cd2+ made the fluorescence emission peak blue-shift of sensor 54 by 25 nm and the fluorescence be quenched. Adding Al3+ made the fluorescence emission peak of sensor 54 red-shift 3 nm and fluorescence be increased. Adding S2− made the fluorescence emission peak of sensor 54 blue shift by 7 nm and the fluorescence be quenched. The sensor 54 combines Cd2+, Al3+, and S2− at a stoichiometric ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the detection limits for them are 1.02 μM, 0.55 μM, and 16.57 μM, respectively. When Cd2+, Al3+, and S2− exist at the same condition, the combination of sensor 54 and Al3+ is dominant, and K2HPO4 can realize the reversibility of sensor 54 to detect Cd2+/Al3+.
1, and the detection limits for them are 1.02 μM, 0.55 μM, and 16.57 μM, respectively. When Cd2+, Al3+, and S2− exist at the same condition, the combination of sensor 54 and Al3+ is dominant, and K2HPO4 can realize the reversibility of sensor 54 to detect Cd2+/Al3+.
Among the probes reported in 2017, in addition to those mentioned above, there is a ferrocenyl Schiff base sensor 55 (Fig. 19) reported by M. Findik's team.69 In an acetonitrile solution and an excitation at 378 nm, the sensor 55 bound to Zn2+/Cd2+ enhanced the weak fluorescence emission at 452 nm by 12.73/4.59 times (due to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization inhibition and CHEF), and the fluorescence intensity can reflect the concentration of Zn2+/Cd2+. The detection limits for Zn2+ was 0.68 μM and for Cd2+ was 0.94 μM, and the binding constants for Zn2+ was (1.38 ± 0.25) × 106 M−1 and for Cd2+ was (7.49 ± 0.18) × 105 M−1. In acetonitrile solution, although the sensor 55 can reversibly (EDTA) detect Zn2+ and Cd2+, the affinity for Zn2+ is stronger than that for Cd2+.
N isomerization inhibition and CHEF), and the fluorescence intensity can reflect the concentration of Zn2+/Cd2+. The detection limits for Zn2+ was 0.68 μM and for Cd2+ was 0.94 μM, and the binding constants for Zn2+ was (1.38 ± 0.25) × 106 M−1 and for Cd2+ was (7.49 ± 0.18) × 105 M−1. In acetonitrile solution, although the sensor 55 can reversibly (EDTA) detect Zn2+ and Cd2+, the affinity for Zn2+ is stronger than that for Cd2+.
In 2018, R. Purkait's research group described a multi-analyte responsive sensor 56 (Fig. 20).13 In the DMSO/water HEPES buffer (pH = 7.2; 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) and the excitation wavelength at 482 nm, adding Zn2+/Cd2+ enhanced the ICT effect of the sensor 56, due to the deprotonation of the phenolic-OH of p-cresol moiety may facilitate charge transfer transitions. Adding Zn2+/Cd2+, the sensor 56 had the maximum emission at 545/560 nm, and it emitted bright-yellow/orange fluorescence under ultraviolet light. The binding of sensor 56 to Zn2+ and Cd2+ is reversible (EDTA), the binding constants for Zn2+ is 2.7 × 104 M−1 and for Cd2+ is 0.96 × 104 M−1, and the detection limits for Zn2+ is 2.7 × 10−9 M and for Cd2+ is 6.6 × 10−9 M, which is lower than permissible levels for these ions in drinking water by EPA. In addition, sensor 56 can detect I−as low as 5 nM based on ESIPT. When the thin-layer chromatography plates stained with sensor 56 for field detection, Zn2+, Cd2+, I− change the light red fluorescence to green, orange, and red respectively.
1, v/v) and the excitation wavelength at 482 nm, adding Zn2+/Cd2+ enhanced the ICT effect of the sensor 56, due to the deprotonation of the phenolic-OH of p-cresol moiety may facilitate charge transfer transitions. Adding Zn2+/Cd2+, the sensor 56 had the maximum emission at 545/560 nm, and it emitted bright-yellow/orange fluorescence under ultraviolet light. The binding of sensor 56 to Zn2+ and Cd2+ is reversible (EDTA), the binding constants for Zn2+ is 2.7 × 104 M−1 and for Cd2+ is 0.96 × 104 M−1, and the detection limits for Zn2+ is 2.7 × 10−9 M and for Cd2+ is 6.6 × 10−9 M, which is lower than permissible levels for these ions in drinking water by EPA. In addition, sensor 56 can detect I−as low as 5 nM based on ESIPT. When the thin-layer chromatography plates stained with sensor 56 for field detection, Zn2+, Cd2+, I− change the light red fluorescence to green, orange, and red respectively.
In 2018, N. Behera et al. reported that hydrazide probe 57 (Fig. 20).10 Probe 57 can detect Al3+/Zn2+/Cd2+ as low as 5.7 × 10−9 M/1.09 × 10−6 M/1.64 × 10−6 M in physiological pH and without Cu2+, and the complexes of this probe and these metal ions can be used for detecting F− or H2PO4−. In MeOH–HEPES buffer solution (5 mM, pH = 7.3, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v), when excited at 435 nm, probe 57 emitted weak fluorescence at 495 nm due to the PET effect caused by electron transfer from nitrogen atom of imino (–CH
3 v/v), when excited at 435 nm, probe 57 emitted weak fluorescence at 495 nm due to the PET effect caused by electron transfer from nitrogen atom of imino (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) group to conjugated π system of naphthalene fluorophore. Adding Al3+/Zn2+/Cd2+ turned on the fluorescence of probe 57, the fluorescence intensity increased by 95/43/49 times, the emission peak shifted from 495 nm to 485/570/540 nm. But the presence of Cu2+ could quench the fluorescence intensity of those complexes about 20–30%. The 2
N–) group to conjugated π system of naphthalene fluorophore. Adding Al3+/Zn2+/Cd2+ turned on the fluorescence of probe 57, the fluorescence intensity increased by 95/43/49 times, the emission peak shifted from 495 nm to 485/570/540 nm. But the presence of Cu2+ could quench the fluorescence intensity of those complexes about 20–30%. The 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex formed by probe 57 and Al3+ can detect F− as low as 2.5 × 10−6 M by fluorescence quenching, and the 1
1 complex formed by probe 57 and Al3+ can detect F− as low as 2.5 × 10−6 M by fluorescence quenching, and the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex formed by probe 57 and Zn2+/Cd2+ can detect H2PO4− as low as 1.43 × 10−5 M/2.11 × 10−5 M by fluorescence quenching.
1 complex formed by probe 57 and Zn2+/Cd2+ can detect H2PO4− as low as 1.43 × 10−5 M/2.11 × 10−5 M by fluorescence quenching.
A bis(salamo)-type tetraoxime sensor 58 (Fig. 21) with two N2O2 chelating moieties as ionophore.5 In Tris–phosphate buffer solution (c = 0.2 M, DMF/H2O = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH = 7.0), the change of absorbance in UV vis test shows that sensor 58 can combine with Cd2+, Ni2+, Zn2+, and Mn2+. However, only Cd2+ changes from faint yellow to green under 365 nm light. When the excitation wavelength at 323 nm, adding Cd2+ made the emission peak of sensor 58 (due to the PET inhibition and CHEF) red-shift from 412 nm to 486 nm, the fluorescence intensity increase from 125 to 712, and the fluorescence quantum yield increase from 2.6% to 13.8%. EDTA enables the sensor 58 to detect Cd2+ reversibly and the fluorescence emission at 373 nm can be used as a signal output to establish a molecular logic gate. In an aqueous system with a pH of 3.0 to 11.0 and a temperature of 0 to 90 °C, the sensor 58 synthesized by J. Hao's team can recognize Cd2+ with naked eyes (the solution changes from colorless to yellow) and fluorescence, and detect as low as 8.61 × 10−7 M without being interfered by other metal ions. The binding constant of the sensor to Cd2+ is as 4.98 × 104 M−1. In addition, a test strip for the sensor 58 can be made to detect Cd2+ in food and the environment.
1, v/v, pH = 7.0), the change of absorbance in UV vis test shows that sensor 58 can combine with Cd2+, Ni2+, Zn2+, and Mn2+. However, only Cd2+ changes from faint yellow to green under 365 nm light. When the excitation wavelength at 323 nm, adding Cd2+ made the emission peak of sensor 58 (due to the PET inhibition and CHEF) red-shift from 412 nm to 486 nm, the fluorescence intensity increase from 125 to 712, and the fluorescence quantum yield increase from 2.6% to 13.8%. EDTA enables the sensor 58 to detect Cd2+ reversibly and the fluorescence emission at 373 nm can be used as a signal output to establish a molecular logic gate. In an aqueous system with a pH of 3.0 to 11.0 and a temperature of 0 to 90 °C, the sensor 58 synthesized by J. Hao's team can recognize Cd2+ with naked eyes (the solution changes from colorless to yellow) and fluorescence, and detect as low as 8.61 × 10−7 M without being interfered by other metal ions. The binding constant of the sensor to Cd2+ is as 4.98 × 104 M−1. In addition, a test strip for the sensor 58 can be made to detect Cd2+ in food and the environment.
A phenylacetylene based sensor 59 (Fig. 21) reported by Y. Y. Zhang et al. can be used for colorimetric detection of Fe2+ and fluorescence proportional detection of Cd2+, Zn2+.70 Adding Zn2+/Cd2+ to the ethanol/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) solution of sensor 59 enhanced the electron withdrawing ability of terpyridine and enhanced the ICT effect. Upon excitation at 340 ± 5 nm, the addition of Zn2+/Cd2+ caused the fluorescence of sensor 59 to change from blue to yellow-green/cyan, the fluorescence emission to decrease at 408 nm and to increase at 498 nm/473 nm. The fluorescence intensity ratios F498 nm/F408 nm and F473 nm/F408 nm were linearly related to the Zn2+ and Cd2+ concentrations, respectively. The detection limits of sensor 59 for Zn2+ and Cd2+ are 2.03 × 10−9 M and 2.62 × 10−8 M, respectively. However, Fe2+ can interfere the fluorescence turn on of the sensor to Zn2+ or Cd2+. Adding Fe2+, it can be observed by naked eyes that the sensor 59 solution changed from colorless to purplish red. In addition, this multifunctional sensor can be used for the detection of these ions in water samples and test strips.
1, v/v) solution of sensor 59 enhanced the electron withdrawing ability of terpyridine and enhanced the ICT effect. Upon excitation at 340 ± 5 nm, the addition of Zn2+/Cd2+ caused the fluorescence of sensor 59 to change from blue to yellow-green/cyan, the fluorescence emission to decrease at 408 nm and to increase at 498 nm/473 nm. The fluorescence intensity ratios F498 nm/F408 nm and F473 nm/F408 nm were linearly related to the Zn2+ and Cd2+ concentrations, respectively. The detection limits of sensor 59 for Zn2+ and Cd2+ are 2.03 × 10−9 M and 2.62 × 10−8 M, respectively. However, Fe2+ can interfere the fluorescence turn on of the sensor to Zn2+ or Cd2+. Adding Fe2+, it can be observed by naked eyes that the sensor 59 solution changed from colorless to purplish red. In addition, this multifunctional sensor can be used for the detection of these ions in water samples and test strips.
Also in 2018, the M. Formica's research group synthesized probes 60a and 60b (Fig. 21).71 The probes contain the 2,5-diphenyl[1,3,4]oxadiazole (PPD) fluorophore, methylene spacer and two coordinating units, namely dipicolylamine (DPA) for 60a and 1,4,7-triazacyclononane (TACN) for 60b. In 0.15 mol L−1 NMe4Cl hydroalcoholic solution (water/ethyl alcohol 50/50 v/v), upon the excitation at 277 nm, Cd2+ and 60a only forms a mononuclear complex and emits high fluorescence at 368 nm. Cd2+ and 60b forms mononuclear and dinuclear complexes, and forms excimer to emit fluorescence at 474 nm. Probes 60a and 60b complex with Cd2+ at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, with Zn2+ at 1
1, with Zn2+ at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in water or hydroalcoholic medium, but the response to Zn2+ is not as sensitive as Cd2+. Because only the mononuclear complex has a fluorescence response, so the concentration must be higher than that of the analyte when used as a sensor.
2 in water or hydroalcoholic medium, but the response to Zn2+ is not as sensitive as Cd2+. Because only the mononuclear complex has a fluorescence response, so the concentration must be higher than that of the analyte when used as a sensor.
T. H. Liu and coworkers synthesized probe 61 (Fig. 22) by covalent combination of terthiophene with 2,2′:6′,2′′-terpyridine.26 In methanol solution, probe 61 forms a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with Cd2+, which red-shifts the maximum absorption peak from 396 nm to 424 nm. Upon excitation at 395 nm, the fluorescence of the probe 61 changes from green to bright yellow, appears a new emission centering at 572 nm and decreases the original emission around 504 nm. These changes can distinguish the response of this probe to other metal ions (Co2+, Cu2+, Ni2+, Zn2+, etc.). Probe 61 can detect Cd2+ in a pH environment of 5–8 as low as 1.74 × 10−7 M, and the binding constant is 2.25 × 105 M−1. Alternating addition of metal ions and EDTA can't achieve the reversibility of the fluorescence changes induced by metal ion. Cu2+, Co2+ or Zn2+ not only blocks the probe 61 from forming a complex with Cd2+, but also ensures the quenching of the fluorescence.
1 complex with Cd2+, which red-shifts the maximum absorption peak from 396 nm to 424 nm. Upon excitation at 395 nm, the fluorescence of the probe 61 changes from green to bright yellow, appears a new emission centering at 572 nm and decreases the original emission around 504 nm. These changes can distinguish the response of this probe to other metal ions (Co2+, Cu2+, Ni2+, Zn2+, etc.). Probe 61 can detect Cd2+ in a pH environment of 5–8 as low as 1.74 × 10−7 M, and the binding constant is 2.25 × 105 M−1. Alternating addition of metal ions and EDTA can't achieve the reversibility of the fluorescence changes induced by metal ion. Cu2+, Co2+ or Zn2+ not only blocks the probe 61 from forming a complex with Cd2+, but also ensures the quenching of the fluorescence.
In 2019, J. K. Xiong's team reported the sensor 62 (Fig. 22) of a perylene bisimide derivative conjugated with lactose and DPA.14 In an aqueous solution with a pH of 3.0–9.5, the sensor 62 can highly and selectively bind to Cd2+ in a stoichiometric ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the binding constant is (6.0 ± 0.3) × 104 M−1. Although this sensor can detect Cd2+ as low as 5.2 × 10−7 M, the present of Zn2+, Co2+ or Cu2+ can quench the fluorescence induced by Cd2+.
1, and the binding constant is (6.0 ± 0.3) × 104 M−1. Although this sensor can detect Cd2+ as low as 5.2 × 10−7 M, the present of Zn2+, Co2+ or Cu2+ can quench the fluorescence induced by Cd2+.
P. Ravichandiran et al. designed probe 63 (Fig. 23) by introducing a furan-2-carboxamide group to a phenoxazine fluorophore and a phenyl sulfonyl chelating site.72 In CH3CN/H2O (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) medium, when excited at 340 nm, probe 63 emitted weak fluorescence due to the C
1, v/v) medium, when excited at 340 nm, probe 63 emitted weak fluorescence due to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization and ICT effects. The combination of probe 63 with Cd2+ strengthened the charge transfer between the secondary amine and the amide group (–CONH) of the benzoxazine moiety and enhanced the ICT effect. In addition, the use of polar organic solvent acetonitrile, these result in a 130-fold increase in the fluorescence of probe 63 at 530 nm. The binding constant is 1.36 × 106 M−2, and the detection limit for Cd2+ is 0.60 μM. Probe 63 recognizes Cd2+ with selectivity and reversibility (EDTA), and can be used for detecting Cd2+ in living cells. In addition, the complex of probe 63 and Cd2+ can turn-off the fluorescence to detect CN− with a detection limit of 0.145 μM.
N isomerization and ICT effects. The combination of probe 63 with Cd2+ strengthened the charge transfer between the secondary amine and the amide group (–CONH) of the benzoxazine moiety and enhanced the ICT effect. In addition, the use of polar organic solvent acetonitrile, these result in a 130-fold increase in the fluorescence of probe 63 at 530 nm. The binding constant is 1.36 × 106 M−2, and the detection limit for Cd2+ is 0.60 μM. Probe 63 recognizes Cd2+ with selectivity and reversibility (EDTA), and can be used for detecting Cd2+ in living cells. In addition, the complex of probe 63 and Cd2+ can turn-off the fluorescence to detect CN− with a detection limit of 0.145 μM.
The research group of M. Das synthesized a pyridyl based Schiff base sensor 64 (Fig. 23).73 In methanol solution, under excitation wavelength at 290 nm, adding Cd2+ and Ni2+ quenched the fluorescence of sensor 64. The sensor 64 has maximum absorption at 234 nm and 272 nm, adding Cd2+/Ni2+ led to the maximum absorption peaks red-shift from 272 nm to 280/281 nm, and the interference of other metal ions and anions was neglected. The binding constants of sensor 64 to Cd2+ and Ni2+ were 4.29 × 106 L mol−1 and 26.88 × 106 L mol−1 calculated by Yoe Jones method, respectively. The detection limit for Cd2+ was 1.4688 μM, and for Ni2+ was 3.08 μM.
M. Vera et al. designed sensor 65 (Fig. 23).29 They inserted –CH2– between the DTPA (diethylenetriaminepentaacetate) and the naphthalene chromophore to make the complex stable and have little impact on the environment when Cd2+ was detected by sensor 65. Furthermore, sensor 65 can distinguish Zn2+ and Cd2+, and without fluorescence quenching caused by paramagnetic effect. In an aqueous solution and the excitation wavelength at 280 nm, sensor 65 has strong naphthalene-monomer emission at 355 nm and excimer emission at 410 nm. The sensor 65 is coordinated with Cd2+, so that the fluorescence emission of excimer is increased by 70–100%, while the monomer emission is hardly affected. The sensor 65 is coordinated with Zn2+, so that the fluorescence emission of excimer is decreased by 60%, while the monomer emission is increased.
P. Kaur's research group synthesized a 1,2,3-triazole linked fluorescein derivative probe 66 (Fig. 24).30 In an ethanol/water (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) solution, probe 66 has high selectivity to Cd2+ and Co2+. It binds to Cd2+ and Co2+ through the N, O, N cornered caged of probe 66. Due to the PET effect, probe 66 shows fluorescence turn off for Cd2+ and fluorescence turn on for Co2+, and the detection limits at 9–10 μM.
1, v/v) solution, probe 66 has high selectivity to Cd2+ and Co2+. It binds to Cd2+ and Co2+ through the N, O, N cornered caged of probe 66. Due to the PET effect, probe 66 shows fluorescence turn off for Cd2+ and fluorescence turn on for Co2+, and the detection limits at 9–10 μM.
Using 2,9-dimethyl-1,10-phenanthroline and o-aminophenol as raw materials, M. X. Huang et al. synthesized a simple, sensitive and practical fluorescence enhanced probe 67 (Fig. 24) for Cd2+.74 The performance of probe 67 to recognize Cd2+ was tested in DMF–water (v/v, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) solution, excitation wavelength at 275 nm, and emission wavelength at 377 nm. The addition of Cd2+ restricted the PET process (electron transfer from the N of imine to phenanthroline ring) and the C
7) solution, excitation wavelength at 275 nm, and emission wavelength at 377 nm. The addition of Cd2+ restricted the PET process (electron transfer from the N of imine to phenanthroline ring) and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization, resulting in charge rearrangement and rigidity enhancement. Therefore, Cd2+ led to fluorescence enhancement and fluorescence lifetime reduction of the probe 67. The binding constant between the probe 67 and Cd2+ is 3.15 × 105 M−1. The emission intensity of probe 67 at 377 nm has a good linear relationship with Cd2+ at 0.025–2.5 μM, and it can be quantitatively detected as low as 29.3 nM. Cd2+ can enhance the fluorescence of probe 67 in 6.6 s, but its same group elements Cu2+ and Pb2+ can quench the fluorescence induced by Cd2+ to some extent. The researchers not only proposed the binding method of the probe 67 and Cd2+ by Job plot, FT-IR and 1H NMR titration experiments, but also confirmed the accuracy of the probe in the detection of Cd2+ by the determination of Cd2+ in different water samples.
N isomerization, resulting in charge rearrangement and rigidity enhancement. Therefore, Cd2+ led to fluorescence enhancement and fluorescence lifetime reduction of the probe 67. The binding constant between the probe 67 and Cd2+ is 3.15 × 105 M−1. The emission intensity of probe 67 at 377 nm has a good linear relationship with Cd2+ at 0.025–2.5 μM, and it can be quantitatively detected as low as 29.3 nM. Cd2+ can enhance the fluorescence of probe 67 in 6.6 s, but its same group elements Cu2+ and Pb2+ can quench the fluorescence induced by Cd2+ to some extent. The researchers not only proposed the binding method of the probe 67 and Cd2+ by Job plot, FT-IR and 1H NMR titration experiments, but also confirmed the accuracy of the probe in the detection of Cd2+ by the determination of Cd2+ in different water samples.
The probe 68 (Fig. 25), which was designed by conjugation of terpydine (a receptor for Zn2+ and Cd2+) and pyridinium with the styryl group, has good selectivity and anti-interference ability for Cd2+ recognition.75 Probe 68 responds to Cd2+ based on the ICT and AIE mechanisms. In DMSO/H2O system, upon excitation at 375 nm, the energy consumed by the intramolecular rotation of multiple pyridinium rings made the probe 68 emit weak fluorescence. As the water fraction in the solution increases to more than 80%, molecules aggregation and inhibition of intramolecular rotation made the probe 68 emit bright fluorescence. Adding Cd2+ made the fluorescence emission peak of the probe 68 red shift from 480 nm to 530 nm, but adding Zn2+ did not emerge obvious fluorescence spectral changes. W. X. Lin et al. introduced pyridinium group and alkane with sixteen carbon atoms into the probe 68 to increase its water solubility and cell membrane permeability, which can be used to detect and label Cd2+ in cells.75
The next year, P. P. Namitha's study team reported sensor 69 (Fig. 25).76 In an ethanol solution and the excitation wavelength at 427 nm, the strong fluorescence of sensor 69 was quenched by Cd2+ due to the PET effect, and the fluorescence intensity ratio I0/I has a linear relationship with Cd2+ of 0.25–2 μM. Ni2+, Zn2+ and Cu2+ can interfere with Cd2+ detection when the concentration exceeds 10 times of Cd2+ concentration, and when they exceed 100 times, they can slightly quench the fluorescence caused by Cd2+. The sensor 69 can detect Cd2+ as low as 0.1499 μM with excellent selectivity, light stability and water solubility, and can be used to measure Cd2+ in the NIR region.
In 2020, P. Liu et al. reported a fluorescent probe 70 (Fig. 26) based on ICT to detect Cd2+.77 In HEPES buffer (1% DMSO as cosolvent) and the excitation wavelength at 380 nm, probe 70 has no colour and fluorescence response to cations other than Cd2+. The addition of Cd2+ turns the yellow weak fluorescence of probe 70 into red strong fluorescence, and the fluorescence intensity is linearly related to the Cd2+ concentration of 5.0 × 10−8 to 5.0 × 10−5 mol L−1. The association constant between probe and Cd2+ was 7.97 × 104 M−1, and the detection limit for Cd2+ was 6.31 nM. In addition, the probe solution changes from yellow to maroon red by adding Cd2+, so it can be used as a new visual sensor for Cd2+. The probe 70 is easy to synthesize, and has excellent selectivity, sensitivity, reversibility (EDTA), stability, and a wide pH (3–13) range for Cd2+ detection. In addition, the probe 70 can be used for fluorescence imaging of Cd2+ in living cells.
The sensor 71 (Fig. 26) designed by B. Jana's research team has high response efficiency to Cd2+ in water medium with pH of 1–10 and lower temperature.78 In aqueous medium (water/DMSO, v/v: 95/5), sensor 71 has maximum absorption at 367 nm and 293 nm, the addition of Cd2+ makes maximum absorption shift from 293 nm to 313 nm. Under 367 nm, the addition of Cd2+ increases the fluorescence emission intensity of sensor 71 at 482 nm by 3 times, the fluorescence lifetime increases from 6.49 ns to 9.78 ns, and the fluorescence changes from colorless to faint blue. The dissociation constant (Kd) of Cd2+ and probe complex is 1.7 × 10−7, and the detection limit for Cd2+ is 0.95 μM. The fluorescence intensity (F0/(F−F0)) and Cd2+ (1/[Cd2+]) with a concentration of 0.05–1 μM has a linear relationship, which can be used to quantitatively detect Cd2+. However, the presence of Hg2+ can be reduced the fluorescence intensity of the complex by 9–10%.
In 2020, E. K. Inal et al. reported the sensor 72 (Fig. 27).79 The ethanol–water solutions and the excitation wavelength at 369 nm are selected to carry out. The sensor 72 emits weak fluorescence at 464 nm, then addition of Zn2+/Cd2+/Hg2+ makes the sensor has obvious fluorescence emission at 452/474/491 nm, the fluorescence intensity and Zn2+/Cd2+/Hg2+ concentration has a linear relationship. The detection limit for Zn2+/Cd2+/Hg2+ is 2.7 × 10−7 M/6.0 × 10−7 M/7.5 × 10−7 M. The sensor 72 can detect Zn2+, Cd2+, Hg2+ in ethanol–water solutions with a pH of 8–9 (due to the protonation of nitrogen in imine bond, the fluorescence response to metal ions in acidic medium is very weak), but the sensor has a stronger affinity for Cu2+ and Fe3+. Moreover, Co2+ and Ni2+ interferes with the selectivity of the sensor 72 for Zn2+ and Cd2+.
The research group of S. G. J. Andrews reported probe 73 (Fig. 27).80 The coordination of probe 73 with Cd2+ or Pb2+ led to inhibit the PET process and produce the CHEF effect, so probe 73 emitted strong fluorescence. Probe 73 has selectivity, sensitivity, reversibility (EDTA) and visualization for the recognition of Cd2+ and Pb2+ in an aqueous buffer (<80%) medium, and is not affected by other metal ions or interferes with each other. In methanol/HEPES buffer (5 mM, v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, pH = 7.3), upon excitation at 420 nm, adding Cd2+ makes the emission peak of probe 73 redshift from 553 nm to 578 nm, and can detect Cd2+ as low as 12.96 nM in a pH system of 4.8–8.5. Upon excitation at 410 nm, adding Pb2+ makes the emission peak of probe 73 redshift from 499 nm to 505 nm, and can detect Pb2+ as low as 10.63 nM in a pH system of 5.0–8.4. The binding constants of probe for Cd2+ is 3.10 × 105 M−1 and for Pb2+ is 2.40 × 105 M−1. The probe 73 can be used as a naked-eye sensing probe for Cd2+ and Pb2+. Under visible light, the solution of the probe 73 changes from pale yellow to dark yellow and light pink, respectively. Under ultraviolet light, the probe solution changes from pale yellow to green and red, respectively. In addition, probe 73 can be used for fluorescence imaging of Cd2+ and Pb2+ in MCF 7 cells.
9, pH = 7.3), upon excitation at 420 nm, adding Cd2+ makes the emission peak of probe 73 redshift from 553 nm to 578 nm, and can detect Cd2+ as low as 12.96 nM in a pH system of 4.8–8.5. Upon excitation at 410 nm, adding Pb2+ makes the emission peak of probe 73 redshift from 499 nm to 505 nm, and can detect Pb2+ as low as 10.63 nM in a pH system of 5.0–8.4. The binding constants of probe for Cd2+ is 3.10 × 105 M−1 and for Pb2+ is 2.40 × 105 M−1. The probe 73 can be used as a naked-eye sensing probe for Cd2+ and Pb2+. Under visible light, the solution of the probe 73 changes from pale yellow to dark yellow and light pink, respectively. Under ultraviolet light, the probe solution changes from pale yellow to green and red, respectively. In addition, probe 73 can be used for fluorescence imaging of Cd2+ and Pb2+ in MCF 7 cells.
Y. F. Zhang et al. synthesized the sensor 74 (Fig. 28) by connecting 2-(2-((2-hydroxyphenyl)imino)ethylidene)amino unit (as the recognition site of Pb2+ and Cd2+) and tert-butyldiphenylsilyl (as the reaction center of F−, and improving the stability of the probe molecule and protecting the molecule from modification) to fluoresceins.81 Sensor 74 can recognize Pb2+ and Cd2+ quickly and sensitively by using F− as auxiliary reagent. In EtOH/H2O (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) sensor solution, adding Pb2+ or Cd2+ increased the absorption band at 533 nm and decreased the absorption band at 374 nm, resulting in the change of ratiometric spectral. When F− is added to the sensor solution containing Pb2+ or Cd2+, the solution containing Pb2+ fades from light purple to colorless, and the solution containing Cd2+ deepens from light purple to dark purple. Through these phenomena, this sensor can be used as an indirect sensor to identify F−. The researchers believe that these phenomena are caused by the different radius of Pb2+ and Cd2+ and the different coordination mode with F−. The binding constants (Ka) of the sensor 74 with Pb2+ or Cd2+ are 3.292 × 104 M−1 and 2.349 × 104 M−1, respectively. The detection limits of sensor 74 for Pb2+ and Cd2+ are 0.42 μM and 0.53 μM, respectively.
1, v/v) sensor solution, adding Pb2+ or Cd2+ increased the absorption band at 533 nm and decreased the absorption band at 374 nm, resulting in the change of ratiometric spectral. When F− is added to the sensor solution containing Pb2+ or Cd2+, the solution containing Pb2+ fades from light purple to colorless, and the solution containing Cd2+ deepens from light purple to dark purple. Through these phenomena, this sensor can be used as an indirect sensor to identify F−. The researchers believe that these phenomena are caused by the different radius of Pb2+ and Cd2+ and the different coordination mode with F−. The binding constants (Ka) of the sensor 74 with Pb2+ or Cd2+ are 3.292 × 104 M−1 and 2.349 × 104 M−1, respectively. The detection limits of sensor 74 for Pb2+ and Cd2+ are 0.42 μM and 0.53 μM, respectively.
F. D. Carlos' team has developed an acridine based fluorophore sensor 75 (Fig. 28).82 In EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3Cl (99
CH3Cl (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) solution, the combination of sensor 75 with Cd2+ suppressed the PET effect and enhanced the fluorescence. Under the excitation of 400 nm light, the fluorescence intensity of sensor 75 was enhanced by 746% with the addition of Cd2+, and was not affected by interference ions. The linear range of fluorescence intensity and Cd2+ concentration was 0.10–1.00 μmol L−1. The binding constant of sensor 75 to Cd2+ is 1.05 × 109 L mol−1, and the detection limit and quantitative detection limit are 9.98 and 33.31 nmol L−1, respectively. Sensor 75 has repeatability, reproducibility, robustness and reversibility (EDTA) for detecting Cd2+. In addition, sensor 75 can be used to detect Cd2+ in Brazilian sugarcane wine and tobacco smoke extract.
1 v/v) solution, the combination of sensor 75 with Cd2+ suppressed the PET effect and enhanced the fluorescence. Under the excitation of 400 nm light, the fluorescence intensity of sensor 75 was enhanced by 746% with the addition of Cd2+, and was not affected by interference ions. The linear range of fluorescence intensity and Cd2+ concentration was 0.10–1.00 μmol L−1. The binding constant of sensor 75 to Cd2+ is 1.05 × 109 L mol−1, and the detection limit and quantitative detection limit are 9.98 and 33.31 nmol L−1, respectively. Sensor 75 has repeatability, reproducibility, robustness and reversibility (EDTA) for detecting Cd2+. In addition, sensor 75 can be used to detect Cd2+ in Brazilian sugarcane wine and tobacco smoke extract.
Rhodanine and salicylidene Schiff bases can bind to metal ions strongly, and have good photophysical properties and water solubility.83 Therefore, the probe 76 (Fig. 28) was designed and synthesized by S. Park et al. When probe 76 was used to detect Zn2+ or Cd2+ in aqueous medium (λex = 329 nm), the fluorescence intensity at 519 nm increased significantly.83 However, the addition of cysteine can make the fluorescence induced by Cd2+ disappear, while the fluorescence induced by Zn2+ remain, so the distinction between Zn2+ and Cd2+ is realized. The binding constant of probe to Zn2+ or Cd2+ is 2.0 × 107 M−1 or 1.0 × 107 M−1, and the detection limit for Zn2+ or Cd2+ is 1.07 μM or 1.37 μM. The probe 76 can respond to Zn2+ (detection is affected by Co2+, Cu2+, Fe3+, Fe2+, Na+) or Cd2+ (detection is affected by Co2+, Cu2+, Fe3+, Fe2+, Na+, Zn2+) in the pH range of 7.0–9.0 for water sample analysis and cell imaging, but its anti-interference ability is poor.
Because thiosemicarbazide has many coordination modes with metal ions, P. S. Kumar et al. synthesized the probe 77 (Fig. 29) by connecting it to the fluorophore unit (4-tert-butyl-2,6-diformylphenol).84 The probe can be used for the fluorescence detection of Zn2+, Cd2+ and Hg2+ in H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO (95
DMSO (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 v/v) medium and 360 nm excitation wavelength. The fluorescence intensity ratios I488 nm/I540 nm and I470 nm/I540 nm were linearly correlated with the concentrations of Zn2+ and Cd2+, respectively. Hg2+ makes the probe emit weak reddish brown fluorescence near 578 nm. The binding constants of Zn2+, Cd2+ and Hg2+ complexes are 9.8 × 103, 1.39 × 105 and 2.03 × 1013 M−1, respectively. The binding ability of the probe to Zn2+, Cd2+ and Hg2+ increased in turn, and the metal ions with strong binding ability could interfere with the detection of metal ions with weak binding ability. Probe 77 can respond to Zn2+, Cd2+ and Hg2+ in the pH range of 3–10, with detection limits as low as 5.1, 3.4 and 0.51 μM.
5 v/v) medium and 360 nm excitation wavelength. The fluorescence intensity ratios I488 nm/I540 nm and I470 nm/I540 nm were linearly correlated with the concentrations of Zn2+ and Cd2+, respectively. Hg2+ makes the probe emit weak reddish brown fluorescence near 578 nm. The binding constants of Zn2+, Cd2+ and Hg2+ complexes are 9.8 × 103, 1.39 × 105 and 2.03 × 1013 M−1, respectively. The binding ability of the probe to Zn2+, Cd2+ and Hg2+ increased in turn, and the metal ions with strong binding ability could interfere with the detection of metal ions with weak binding ability. Probe 77 can respond to Zn2+, Cd2+ and Hg2+ in the pH range of 3–10, with detection limits as low as 5.1, 3.4 and 0.51 μM.
Z. Aydin et al. reported the Schiff base probes 78a and 78b (Fig. 29) with N-phenyl-o-phenylenediamine as Cd2+ binding part and benzaldehyde and cinnamaldehyde as chromophores.85 For the recognition of Cd2+ ions in aqueous solution, the probe 78a changes from colorless to orange, and the probe 78b changes from yellow to reddish. These probes can quantitatively detect Cd2+ based on absorbance. Although the ions has no effect on the colorimetric detection of Cd2+ by 78b, its spectrometric titration is interfered by Zn2+. The binding constants of 78a and 78b with Cd2+ were 2.65 × 1012 M−2 and 4.95 × 1012 M−2, respectively. The detection limits of 78a and 78b were 4.38 × 10−7 M and 1.02 × 10−7 M, respectively. The two probes have selectivity and reversibility for the recognition of Cd2+, and can be used as colorimetric sensors for the detection of Cd2+ in aqueous solution.
J. J. Celestina et al. rapidly synthesized the colorimetric and fluorescent sensor 79 (Fig. 29) for Cd2+ from 4- aminoantipyrine and 9-anthracene carboxaldehyde under microwave irradiation.86 The addition of Cd2+ makes the sensor produce PET-CHEF effects and inhibit the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization, resulting in the solution changes from yellow to light brown and the fluorescence changes from yellow to green. The detection limit of the sensor 79 for Cd2+ is as low as 0.02 μM, and the detection is reversible and specific. In addition, sensor 79 can be used for the detection of Cd2+ in water samples and fluorescence imaging in L929 cells.
N isomerization, resulting in the solution changes from yellow to light brown and the fluorescence changes from yellow to green. The detection limit of the sensor 79 for Cd2+ is as low as 0.02 μM, and the detection is reversible and specific. In addition, sensor 79 can be used for the detection of Cd2+ in water samples and fluorescence imaging in L929 cells.
P. Lasitha et al. synthesized a squaramine ring based probe 80 (Fig. 30), which can respond to Zn2+ and Cd2+ by turning on fluorescence.87 Under the excitation of 400 nm light, the maximum emission peak of the probe shifts from 464 nm to long wavelength with the addition of Zn(CH3COO)2 and Cd(CH3COO)2. With the addition of Zn(CH3COO)2, a new emission band appeared at 560 nm, and gradually red shifted to 595 nm. Similarly, with the addition of Cd(CH3COO)2, a new emission band appeared near 530 nm, and gradually red shifted to 540 nm. The order of stokes shift emission is the same as that of anion basicity (ClO4− < Cl− < NO3− < SO42− < CH3COO−). The detection limits for Zn(CH3COO)2 and Cd(CH3COO)2 were 5 × 10−7 M (32.6 ppb) and 7 × 10−8 M (7.8 ppb), respectively.
M. Muddassir's team prepared a complex sensor 81 (Fig. 30) of 3,5-dimethylpyrazole with Cu2+ perchlorate.88 Sensor 81 can identify Cd2+ in aqueous solution by fluorescence enhancement, and is not interfered by other metal ions. M. Muddassir et al. confirmed the binding of the complex with Cd2+ by X-ray photoelectron spectroscopy (XPS) analysis.88 They speculate that the high selectivity and sensitivity to Cd2+ may be due to the interaction between Cd2+ and 3,5-dimetylpyrazole ligand through Lewis acid–base interactions, which promotes more effective energy transfer from ligand to Cu2+. In addition, the complex probe can also respond to acetone by fluorescence quenching.
Vanillinyl-hydrazone Schiff base probe 82 (Fig. 30) is a turn-on fluorescent probe for Mg2+, Zn2+, Cd2+ and I−, which was reported by S. Dey's team in 2020.89 In DMSO/H2O medium (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and under 480 nm excitation wavelength, the different emission wavelengths and fluorescence colors of this probe can be used as the methods to distinguish Zn2+ (λem = 546 nm, yellow green), Cd2+ (λem = 576 nm, yellow) and Mg2+ (λem = 625 nm, orange). The binding constants of probe 82 to Zn2+, Cd2+ and Mg2+ are 8.07 × 104 M−1, 6.5 × 104 M−1 and 4.38 × 104 M−1, respectively. The detection limits for Zn2+, Cd2+ and Mg2+ were 14 × 10−9 M, 6.8 × 10−9 M and 11.5 × 10−9 M, respectively. In different detection environments, probe 82 can also be used as a fluorescent probe for I−, and the detection limit is as low as 5.9 nM.
1) and under 480 nm excitation wavelength, the different emission wavelengths and fluorescence colors of this probe can be used as the methods to distinguish Zn2+ (λem = 546 nm, yellow green), Cd2+ (λem = 576 nm, yellow) and Mg2+ (λem = 625 nm, orange). The binding constants of probe 82 to Zn2+, Cd2+ and Mg2+ are 8.07 × 104 M−1, 6.5 × 104 M−1 and 4.38 × 104 M−1, respectively. The detection limits for Zn2+, Cd2+ and Mg2+ were 14 × 10−9 M, 6.8 × 10−9 M and 11.5 × 10−9 M, respectively. In different detection environments, probe 82 can also be used as a fluorescent probe for I−, and the detection limit is as low as 5.9 nM.
Using fluorene as fluorophore, 2,2′,6′,2′′-terpyridine as ligand and benzothiazole as electron acceptor, D. Yue et al. synthesized probe 83 (Fig. 31) with high electron absorption, fluorescence quantum yield and photostability.90 They also introduced 2-(2-ethoxyethoxy)-ethyl group to enhance the solubility of the probe. Zn2+ and Cd2+ were identified by different emission colors in acetonitrile. The detection limits for Zn2+ and Cd2+ were 11.25 nM and 1.80 nM, respectively.
The spectroscopic and analytical parameters of other Cd2+ sensors based on small organic molecules are listed in Table 7. There are many problems in these probes, such as low sensitivity, poor water solubility and weak anti-interference ability. Some probes recognize Cd2+ only through colorimetric response, and they generally have high selectivity and anti-interference, but their sensitivity is not high (the minimum detection limit is 102 nM). Fluorescent sensors usually have high sensitivity, but in these reports, there are relatively a few sensors with detection limits as low as 10−7 M. Few sensors can meet the requirements of water solubility, selectivity, sensitivity, anti-interference and practicability (such as appropriate pH, temperature and response time).
| Probe | Solvent | Excitation wavelength (nm) | Emission wavelength (nm) λem0–λem | Detection limit (nM) | Association constant | Interfering ion(s) | Ref. |
|---|---|---|---|---|---|---|---|
| 44 | CH3CN–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) 5) |
400 | 558–561 | 10.21 | 3.16 × 105 M−1 | — | 12 |
| 45 | CH3CN | 410; 495 | 675–570; 730–570 | 6.9 | — | Zn2+ | 19 |
| 46 | H2O | 418 | 653–611 | 32 | Kd = 31.2 ± 5.2 μM | Hg2+, Cu2+ | 60 |
| 47 | EtOH–H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
470 | 545–537 | 57.6 | — | Many | 17 |
| 48 | NPA/CYH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) 30) |
495 | 518–538 | 2 | — | Co2+, Cu2+, Zn2+, Hg2+ | 61 |
| 49 | DMF | — | — | 200 | 3.7 × 104 M−1 | — | 62 |
| 50 | EtOH – EAC (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
283 | 510 | 2690 | — | — | 63 |
| 51 | CH3CN–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99) 99) |
315 | 433 | 100 | 3862.872 M−1 | — | 64 |
| 52 | MeOH | 308 | 450 | 50 | Kb = 4.87 | IO4−, Zn2+ | 66 |
| 53a/b | DMSO; DMF | — | — | 3220; 200 | — | —; Hg2+, Cu2+, Pb2+ | 67 |
| 54 | MeOH | 320 | 418–393 | 1020 | — | Al3+ | 68 |
| 55 | CH3CN | 378 | 452 | 940 | (7.49 ± 0.18) × 105 M−1 | Zn2+ | 69 |
| 56 | DMSO–H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
482 | 560 | 6.6 | 0.96 × 104 M−1 | Zn2+, I− | 13 |
| 57 | MeOH–H2O (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
435 | 495–540 | 1640 | — | Cu2+ | 10 |
| 58 | DMF–H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
323 | 412–486 | 861 | 4.98 × 104 M−1 | — | 5 |
| 59 | EtOH–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
340 | 473 | 26.2 | — | Fe2+ | 70 |
| 60a/b | EtOH–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
277 | 368/474 | — | — | — | 71 |
| 61 | MeOH | 395 | 572 | 174 | — | Cu2+, Co2+, Zn2+ | 26 |
| 62 | H2O | 528 | 600 | 520 | (6.0 ± 0.3) × 104 M−1 | Zn2+, Co2+, Cu2+ | 14 |
| 63 | CH3CN–H2O (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
340 | 530 | 600 | 1.36 × 106 M−2 | CN− | 72 |
| 64 | MeOH | — | — | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 688 688 |
4.29 × 106 M−1 | 73 | |
| 65 | H2O | 280 | 355/410 | — | — | Zn2+ | 29 |
| 66 | EtOH–H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
— | — | 9000–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | Co2+ | 30 |
| 67 | DMF–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) 7) |
275 | 377 | 29.3 | 3.15 × 105 M−1 | Cu2+, Pb2+ | 74 |
| 68 | DMSO–H2O | 375 | 480–530 | — | — | Fe3+, Co2+, Cu2+, Pb2+ | 75 |
| 69 | EtOH | 427 | 656 | 149.9 | — | Ni2+, Zn2+, Cu2+ | 76 |
| 70 | DMSO–H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
380 | 513–515 | 6.31 | 7.97 × 104 M−1 | — | 77 |
| 71 | DMSO–H2O (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99) 99) |
367 | 482 | 950 | Kd = 1.7 × 10−7 | Hg2+ | 78 |
| 72 | EtOH–H2O | 369 | 464–474 | 600 | — | Cu2+, Fe3+, Co2+, Ni2+ | 79 |
| 73 | MeOH–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) 9) |
420 | 553–578 | 12.96 | 3.10 × 105 M−1 | Pb2+ | 80 |
| 74 | EtOH–H2O (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
— | — | 530 | 2.349 × 104 M−1 | Pb2+ | 81 |
| 75 | EtOH–CH3Cl (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
400 | 416 | 9.98 | 1.05 × 109 L mol−1 | — | 82 |
| 76 | H2O | 329 | 519 | 1370 | 1.0 × 107 M−1 | Co2+, Cu2+, Fe3+, Fe2+, Na+, Zn2+ | 83 |
| 77 | DMSO–H2O (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99) 99) |
360 | 578 | 3400 | 1.39 × 105 M−1 | Zn2+, Hg2+ | 84 |
| 78a/b | H2O | — | — | 438/102 | 2.65 × 1012 M−2/4.95 × 1012 M−2 | — | 85 |
| 79 | EtOH | 398 | 345–404 and 465 | 20 | — | Many | 86 |
| 80 | DMSO | 400 | 530–540 | 70 | — | Zn2+ | 87 |
| 81 | CH3CN–H2O | 267 | 650 | — | — | — | 88 |
| 82 | DMSO–H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
480 | 576 | 6.8 | 6.5 × 104 M−1 | Zn2+, Mg2+, I− | 89 |
| 83 | CH3CN | 410 | 460 | 1.8 | — | Zn2+ | 90 |
9. Nanosensor-based Cd2+ fluorescent sensor
Compared with small organic molecules fluorescent probes, the nanosensor-based fluorescent and colorimetric probes on avoid complex synthesis and purification processes.91 In addition, nanosensors have the characteristics of wide excitation wavelength, adjustable size, high quantum yield and good photochemical stability.91,92 Therefore, the development of nanosensors in Cd2+ fluorescent probes is also worthy of our attention.In 2017, Q. Wu's team first reported a probe 84 that enhances the fluorescence of the second near-infrared (NIR-II) region based on quantum dots identification Zn2+ and Cd2+.93 The research group prepared oil-soluble Ag2S QDs by capping Ag2S nanocrystals with n-dodecyl mercaptan groups. However, due to the limited application of the oil-soluble probe, they replaced dodecyl mercaptan as a surface ligand with thioglycollic acid containing carboxyl group to obtain water-soluble probe 84. The UV-vis absorption spectrum of probe 84 has no obvious peak in the range of 250–910 nm, and the fluorescence emission intensity gradually decreases with the increase of excitation wavelength. After adding Zn2+ or Cd2+, thioglycollic acid on the surface of probe 84 reacts with ions to form Zn–thiol or Cd–thiol complex passivation shell around the surface of Ag2S quantum dots. Due to the passivation shell repairing surface defects and inhibiting non-radiative recombination pathway, the fluorescence intensity of probe 84 at 1100 nm increased by 7 or 8.5 times under 480 nm light excitation, and the fluorescence lifetime increased from 3.25 ns to 7.35 or 10.42 ns, respectively. The fluorescence intensity ratio (I/I0) of probe 84 has a linear relationship with the concentration (1–40 μM) of Zn2+ or Cd2+, and can be used for quantitative detection of Zn2+ or Cd2+. The detection limits of probe 84 for Zn2+ and Cd2+ are 760 nM and 546 nM, respectively. In addition, the probe 84 has strong practicability. It can not only detect Zn2+ or Cd2+ in lake water and tap water samples, but also be used for cell imaging of Zn2+ in NIR-II region.
According to the interaction between C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N group and specific metal ions, J. T. Wang et al. obtained 3D porous MOF-UiO-66-N
N group and specific metal ions, J. T. Wang et al. obtained 3D porous MOF-UiO-66-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 with perfect octahedral morphology and particle size of about 100 nm through the interaction of –NH2 of UiO-66-NH2 with formaldehyde.94 MOF-UiO-66-N
CH2 with perfect octahedral morphology and particle size of about 100 nm through the interaction of –NH2 of UiO-66-NH2 with formaldehyde.94 MOF-UiO-66-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 was used as the fluorescent probe 85 of Cd2+. In aqueous solution and light excitation at 342 nm, probe 85 has an emission peak at 468 nm. When metal ions were added to probe 85, only Fe3+ quenched its fluorescence intensity, Cd2+ and Zn2+ increased its fluorescence intensity, and the effects of other metal ions could be neglected. However, the increase of luminescence intensity of probe 85 caused by Cd2+ is the strongest, and the fluorescence intensity ratio (I/I0) has a good linear relationship with Cd2+ concentration (0–500 μM). Therefore, probe 85 can be used as a fluorescence on probe for quantitative detection of Cd2+, the detection limit is as low as 0.336 μM (37.8 ppb), and the KSV value is 3.81 × 103 M−1. In addition, probe 85 has low-toxicity and pH (4–10) independence, and is promising for Cd2+ detection in water and intracellular environments.
CH2 was used as the fluorescent probe 85 of Cd2+. In aqueous solution and light excitation at 342 nm, probe 85 has an emission peak at 468 nm. When metal ions were added to probe 85, only Fe3+ quenched its fluorescence intensity, Cd2+ and Zn2+ increased its fluorescence intensity, and the effects of other metal ions could be neglected. However, the increase of luminescence intensity of probe 85 caused by Cd2+ is the strongest, and the fluorescence intensity ratio (I/I0) has a good linear relationship with Cd2+ concentration (0–500 μM). Therefore, probe 85 can be used as a fluorescence on probe for quantitative detection of Cd2+, the detection limit is as low as 0.336 μM (37.8 ppb), and the KSV value is 3.81 × 103 M−1. In addition, probe 85 has low-toxicity and pH (4–10) independence, and is promising for Cd2+ detection in water and intracellular environments.
In 2017, L. Li's research team reported a fluorescent probe 86 based on dithizone (DZ) etched cadmium telluride nanoparticles (CdTe NPs) for the detection of Cd2+.92 After DZ was added to L-cysteine-capped CdTe NPs, the Cd–thiol complex surface layers was destroyed and the surface passivation was reduced due to the chemical etching effect of DZ, resulting in the reduction of fluorescence intensity and fluorescence lifetime. After adding Cd2+ to the weakly fluorescent CdTe NPs–DZ mixture (probe 86), the recognition site of Cd2+ on the surface of NPs can selectively bind Cd2+ to re-passivate the surface of NPs, resulting in the increase of fluorescence intensity and fluorescence lifetime. Under ultrapure water medium and excitation wavelength of 328 ± 5 nm, DZ or Cd2+ can be quantitatively detected within 40 min according to the fluorescence intensity (468 nm) ratio before and after adding DZ or Cd2+. The detection limit for DZ is 2.7 μM, and the detection limit for Cd2+ is 0.13 μM. In addition, Ag+ and Cu2+ almost completely quench the fluorescence of non-etched and DZ etched NPs. However, thiourea can be selected as a masking agent to eliminate the interference of Ag+ and Cu2+.
In 2017, Y. Yang et al. reported a super stable Eu-MOF luminescent probe 87. The probe 87 has extremely high thermal stability, air stability and chemical stability, and has fluorescence response to multiple analytes.95 The luminescence sensing of metal ions by probe 87 was studied in aqueous solution and 324 nm excitation wavelength. It was found that the fluorescence intensity of probe 87 at 613 nm gradually increased (2.18 times)/decreased (about 85%) with the addition of Cd2+/Mn2+. According to Stern–Volmer (SV) equation: I0/I = 1 + KSV[M], the quenching effect coefficients (KSV) of probe 87 for Cd2+ and Mn2+ are −54 or 443 L mol−1 respectively, indicating that Mn2+ has high fluorescence quenching efficiency for probe 87. Probe 87 can detect Cd2+ and Mn2+ as low as 10−6 M. The research team speculated that the increase or quenching of fluorescence of probe 87 caused by Cd2+/Mn2+ may be due to the cation exchange between free [Me2NH2]+ and target ions and the interaction between metal ions and organic ligands. In addition, methanol or ether can significantly enhance or weaken the emission of probe 87 at 613 nm, which is the first time to use Ln-MOFs as a luminescent probe to identify ether molecules.
Because biomass contains a lot of carbohydrates, it can be used as raw material for preparing carbon dots (CDs). In 2018, D. Gu's team synthesized a nitrogen and sulfur co-doped sensor 88 with scallion as carbon source, which can detect Cd2+ by fluorescence quenching.96 The response of sensor 88 to Cd2+ was studied in HAC–NaAc buffer solution (pH = 5.0) and 390 nm excitation wavelength. The linear relationship can be established between the emission intensity ratio of sensor 88 at 455 nm and Cd2+ concentration of 0.1–3.0 μM or 5.0–30.0 μM respectively, and the corresponding binding constants were 2.816 × 105 ± 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 670 L mol−1 or 3.636 × 104 ± 2120 L mol−1 respectively. The sensor 88 has no obvious fluorescence response to other metal ions except Cd2+, and the fluorescence quenched by Cd2+ is hardly disturbed by other metal ions. It can detect Cd2+ as low as 15.0 nM. In addition, the sensor 88 has extremely low cytotoxicity and detection limit, and has a good application prospect for Cd2+ ion sensing and biological imaging in vivo or in vitro.
670 L mol−1 or 3.636 × 104 ± 2120 L mol−1 respectively. The sensor 88 has no obvious fluorescence response to other metal ions except Cd2+, and the fluorescence quenched by Cd2+ is hardly disturbed by other metal ions. It can detect Cd2+ as low as 15.0 nM. In addition, the sensor 88 has extremely low cytotoxicity and detection limit, and has a good application prospect for Cd2+ ion sensing and biological imaging in vivo or in vitro.
Y. A. Pan et al. selected a semi-rigid ligand with carboxyl group (2,5-bis(phenylamino)-1,4-benzenedicar boxylic acid, L) as an aromatic organic linker and selected Zn2+ ions with outer shell electronic configuration similar to Cd2+ as ion exchange interaction sites to construct a new three-dimensional Zn-MOF sensor 89.97 Since Cd2+ replaces Zn2+ and reacts with the carboxyl group of L, a more stable Zn–Cd-MOF structure is obtained, which limits the rotation of L in sensor 89 and improves the rigidity of the whole system, the fluorescence of probe 89 increases when identifying Cd2+. Under 365 nm UV irradiation, it can be seen that the probe 89 turns on yellow fluorescence induced by Cd2+. When excited at 333 ± 5 nm, probe 89 has a fluorescence emission peak at 529 nm. After adding Al3+ and Cd2+ ions, the fluorescence emission of probe 89 is enhanced, but Al3+ causes the emission peak of probe 89 to red-shift to 555 nm without interfering with the quantitative detection of Cd2+ at 529 nm according to the fluorescence intensity ratio I/I0. The fluorescence of probe 89 is stable within 20 min after adding Cd2+, and the presence of Cu2+, Mn2+, Ni2+ and Co2+ in the detection system will have a slight impact on the determination of Cd2+. In addition, the probe 89 can recognize nitrobenzene by fluorescence quenching, and the detection limit is 1.19 μg mL−1.
Z. Y. Zhang's research team reported a graphite-like nitride doped carbon quantum dots-capped gold nanoparticle (Au@g-CNQDs, probe 90).98 Because the surface of probe 90 has numerous heptazine, carboxyl and hydroxyl groups, a trace amount of Cd2+ ions can be adsorbed on the probe 90 surface through “cooperative effect”. In Tris–HCl buffer solution, the color of probe 90 changes from wine-red to blue-violet within 30 s after the addition of Cd2+, and the absorption peak of probe 90 decreases at 520 nm and appears at 650 nm. The absorption ratio (A650 nm/A520 nm) of probe 90 has a linear relationship with Cd2+ concentration (0.01–3.0 μM), and Cd2+ can be detected quantitatively with a detection limit of 10 nM. In addition, the probe 90 can accurately and quickly detect the distribution of Cd2+ ions in mouse organs and tissues, indicating that it has considerable application potential in practical application and clinical detection.
Through the reaction of chelidamic acid-picolylamine (CP) substituted diacetylene monomer and the reaction between 10,12-pentacosadiynoic acid and CP chelating part, P. Thanh Chung et al. synthesized probe 91 based on conjugated polyacetylene.99 In HEPES buffer (10 mM, pH = 7.4) and excitation wavelength of 530 ± 5 nm, the addition of Cd2+ rapidly turned on the fluorescence of probe 91, and the fluorescence emission intensity increased with the concentration of Cd2+ ion from 0 to 300 μM. In addition, since two pyridine and chelidamic acid moieties of probe 91 chelating Cd2+ shortens the effective conjugate chain length of probe 91, different amounts of Cd2+ caused the colorimetric change of probe 91 from blue to red(violet). Probe 91 binds to Cd2+ in a stoichiometric ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the LOD for Cd2+ is as low as 1.85 × 10−5 M.
1, and the LOD for Cd2+ is as low as 1.85 × 10−5 M.
M. Y. Tang et al. reported probe 92 of nitrogen sulfur doped carbon dots (N&S CDs) with high water solubility, selectivity, sensitivity and stability to Cd2+.100 Using triethylenetetramine (TETA) and 2-mercaptothiazoline (2-MT) as raw materials can significantly improve the quantum yield of CDs, and they have rich sulfhydryl and amino groups that can provide more opportunities for application implementation. When excited at 340 nm, the probe 92 can quickly (2 min) recognize Cd2+ in ultrapure water and real water samples and quench the bright blue fluorescence at 459 nm. Because the fluorescence quenching of the probe caused by the potential aggregation of Cd2+ and sulfhydryl and amide groups on the surface of N&S CDs. Probe 92 has no obvious reaction with metal ions, biomolecules and anions other than Cd2+, and the presence of other metal ions in the detection system also has no obvious interference with the detection of Cd2+. The probe 92 can quantitatively detect the Cd2+ concentration of 0.02–1 μM, and the detection limit is 0.018 μM. In addition, the probe 92 not only has low toxicity and good cell permeability, but also remains stable in the pH range of 4–12 (the best detection pH is 9).
C. Wei's team reported the first quaternary QDs (Zn–Ag–In–S QDs) probe 93 based on aggregation-induced emission enhancement (AIEE) mechanism.91 Under the phosphate buffer solution (pH = 7.4) and the excitation wavelength of 360 nm, the probe 93 reacts with metal ions for 20 min before fluorescence detection. Since L-cysteine is used as a stabilizer and ligand of Zn–Ag–In–S QDs, it can coordinate with Cd2+ through thiol groups, resulting in enhanced fluorescence emission and blue-shifted the emission peak of probe 93, and formed macroscopic floc in the presence of high concentration of Cd2+. Because the coordination of thiol groups with Cd2+ weakens the electrostatic repulsion between QDs, forms microheterojunctions and passivates surface defects, so the probe 93 shows enhanced fluorescence. In addition, Cd2+ may diffuse into Zn–Ag–In–S QDs to widen the band gap, and the emission peak of probe 93 is blue shifted at 520 nm. Probe 93 can quantitatively detect Cd2+ (25 μM to 2 mM), and the detection limit is 1.56 μM. In addition, the presence of Pb2+, Hg2+ and Cu2+ can also cause the fluorescence change (quenching the fluorescence) of probe 93. The effects of Pb2+ and Hg2+ are eliminated by adding ammonium fluoride and thiosemicarbazide as masking agents, and the effects of Cu2+ are decreased by chelating Cu2+ with L-cysteine in advance.
In 2020, according to the hard-soft acid base (HSAB) principle, the soft donor site can react with soft toxic metals. M. Arnab's team constructed a three-dimensional columnar layered Zn-MOF sensor 94 using bis(pyridin-4-ylmethylene)benzene-1,4-diamine (as the Cd2+ ligand), thiophene-2,5-dicarboxylic acid and Zn(NO3)2.101 When Cd2+ was added to the CH3CN solution of sensor 94, it can be observed by the naked eye that the color immediately changes from a very faint green colour to a deep orange. Upon the excitation at 330 nm, the addition of Cd2+ combined the emission peaks of sensor 94 at 390 nm and 468 nm into one emission peak at 446 nm, and the blue emission peak moved to 408 nm with the increase of the amount of Cd2+. In addition, the addition of Cd2+ enhanced the fluorescence lifetime and fluorescence intensity (408 nm) of sensor 94. The detection limit of sensor 94 for Cd2+ is 0.132 μM. Moreover, by repeatedly cleaning the sensor 94 that has been used for Cd2+ detection, the reversibility of the sensor can be realized.
Z. Q. Zhou's team reported a fluorescent probe 95, which turns on fluorescence to identify Cd2+ by replacing Mn2+ near the surface of Mn:ZnSe QDs with Cd2+.102 The doping of Mn2+ near the QD surface reduces the fluorescence intensity of probe 95. Due to the similar ion radius between Cd2+ and Zn2+, the external Mn2+ is replaced by Cd2+, resulting in the probe 95 emitting strong orange fluorescence and the fluorescence intensity increases by 20 times. The researchers optimized the detection conditions of probe 95 for the detection of Cd2+ in aqueous medium, such as the optimal concentration of probe 95 is 0.01 mg mL−1, the optimal pH is 7 (higher pH is conducive to the deprotonation and electronegativity of MPA, so as to enhance the electrostatic attraction with Cd2+) and the optimal reaction time is 15 min. Strong orange fluorescence of probe 95 was observed only when incubated with Cd2+. Under the excitation of 367 nm light, the fluorescence intensity at 600 nm is linear with Cd2+ concentration (0.02–60 μM), and Cd2+ as low as 18 nM can be detected.
P. Subhash Chandra et al. synthesized carbon dots sensor 96 using curry leaves as carbon source for the first time.103 Since the amine group in probe 96 provides donate an electron pair to excited state vacant orbital of the Cd2+ to form ligand-to-metal charge transfer (LMCT) process, the sensor recognizes Cd2+ by fluorescence off. The addition of Cd2+ not only reduced the fluorescence intensity and fluorescence lifetime of probe 96, but also shifted its absorption peak and emission peak to lower wavelengths by about 10 nm. At the excitation wavelength of 390 nm, Cd2+ was added to the aqueous solution of probe 96 for 15 min, and the fluorescence emission of probe 96 at 450 nm was detected. The fluorescence intensity ratio at 450 nm before and after adding Cd2+ to probe 96 has a good linear relationship with the concentration of Cd2+ (0.01–8 M), and Cd2+ as low as 0.29 nM can be detected quantitatively. Other metal ions do not affect the fluorescence emission of probe 96 or the fluorescence response of probe 96 to Cd2+. However, due to the presence of phenol (–OH) and carbonyl groups (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, positive charge carrier) on the surface of the probe 96, the fluorescence properties of the probe are pH dependent.
O, positive charge carrier) on the surface of the probe 96, the fluorescence properties of the probe are pH dependent.
T. Alizadeh's team synthesized a fluorescent imprinted polymer probe 97 by a combination of hydrophilic functional monomers and hydrophobic ligands.104 The luminescent complexing agent 5-((3hydroxynaphthalen-2-yl)methylene)pyrimidine-2,4,6(1,3,5)-trione (HMPT) forms a stable complex with Cd2+, then the mixture of acryl amide, vinyl benzene and ethylene glycol dimethacrylate are added for copolymerization, and finally Cd2+ is extracted from the polymer, resulting in a cavity, which can be selectively recombined with Cd2+. At the excitation wavelength of 365 nm, Cd2+ was added to turn on the high-intensity red fluorescence of probe 97, and the emission peak blue-shifted from 440 nm to 502 nm. After the probe 97 reacted with Cd2+ for 20 min, the emission intensity at 502 nm had a good correlation with the concentration of Cd2+ (10 to 0.05 mM). The limit of detection (LOD) and limit of quantification (LOQ) of probe 97 for Cd2+ were 12.3 and 41 nM, respectively. Probe 97 not only has high selectivity, but also has low cytotoxicity. It also can be used as a fluorescent probe for the detection of Cd2+ in living cells.
In 2021, W. T. Li et al. reported a dual-emission ratiometric fluorescence probe 98, in which silicon oxide-coated copper nanoclusters (CuNCs@SiO2) as signal reference and cadmium telluride quantum dots (CdTe QDs) as signal response.105 Since SiO2 coating prevents the reaction of CuNCs with other substances, it is ensured that CuNCs@SiO2 can provide a stable and reliable reference signal. In addition, based on the strong affinity of the two nitrogen atoms of 1,10-phenanthroline (Phen) for Cd in CdTe QDs, the researchers prepared CdTe QDs–Phen complex probe. Due to the photo-induced hole transfer (PHT) between CdTe QDs and Phen, the fluorescence of CdTe QDs–Phen complex probe is weak. After adding Cd2+, Phen separated from CdTe QDs–Phen complex and formed [Cd(Phen)2(H2O)2]2+ and CdTe QDs, so the fluorescence intensity of CdTe QDs recovered. Under the excitation of 365 nm light, the characteristic fluorescence emission peaks of CuNCs@SiO2 and CdTe QDs are well separated at 440 nm and 580 nm respectively, and the addition of Cd2+ only increases the fluorescence of CdTe QDs at 580 nm. Therefore, the Cd2+ content can be quantitatively analyzed according to the fluorescence intensity ratio (I580/I436) of probe 98 between 580 nm and 436 nm. The ratio fluorescent probe 98 can be sensitive with a detection limit of 1.1 μg L−1 (2.75 μg kg−1) in a detection time of 6 min, and the detection can eliminate background interference, with high sensitivity and accuracy. In addition, the fluorescent color captured by the smartphone camera can be converted into digital values representing red, green and blue (RGB) color modes through the Color Picker app, and Cd2+ can be quantitatively analyzed through the ratio (G/R) of green (G) and red channels (R).
Table 8 shows the spectroscopic and analytical parameters of Cd2+ recognition based on nanosensors. Sensors based on MOF, QDs, nanoclusters and nanoparticles have achieved good development in recent years. For example, probe with enhanced near-infrared fluorescence emission are reported for the first time, AIE sensor based on quaternary quantum dots are reported for the first time, and quantum dot probe are synthesized using biomass as carbon source, etc. These nanosensors have good water solubility, but their selectivity, sensitivity, anti-interference ability and practicability (such as pH and response time) still need to be improved. These nanosensors can identify Cd2+ ions by chemical etching reaction, displacement reaction, cavity-imprinting, chelation passivation on the surface of nanoparticles and other methods. Researchers can design and improve the performances of nanosensors based on these mechanisms. In addition, the MOF sensor 85 based on C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N recognition of Cd2+, the quaternary quantum dot sensor 93 synthesized with cysteine as the ligand of Cd2+, and the ratiometric sensor 98 with 1,10-phenanthroline chelating Cd2+ to restore CdTe fluorescence. These reaction sites are similar to the design of small organic molecules sensors. Therefore, the idea of constructing and improving nanosensors can also be obtained based on the research results of small organic molecules probes in Cd2+ recognition.
N recognition of Cd2+, the quaternary quantum dot sensor 93 synthesized with cysteine as the ligand of Cd2+, and the ratiometric sensor 98 with 1,10-phenanthroline chelating Cd2+ to restore CdTe fluorescence. These reaction sites are similar to the design of small organic molecules sensors. Therefore, the idea of constructing and improving nanosensors can also be obtained based on the research results of small organic molecules probes in Cd2+ recognition.
| Probe | Luminous structure | Solvent | Excitation wavelength (nm) | Emission wavelength (nm) λem0–λem | Detection limit (nM) | Interfering ion(s) | Ref. |
|---|---|---|---|---|---|---|---|
| 84 | Ag2S QDs | H2O | 480 (250–910) | 1100 | 546 | Zn2+ | 93 |
| 85 | Zr-MOF | H2O | 342 | 486 | 336 | Fe3+, Zn2+ | 94 |
| 86 | CdTe NPs | H2O | 328 | 468 | 130 | Ag+, Cu2+ | 92 |
| 87 | Eu-MOF | H2O | 324 | 613 | 1000 | Mn2+ | 95 |
| 88 | CDs | H2O | 390 | 455 | 15 | — | 96 |
| 89 | Zn-MOF | H2O | 333 | 529 | 1.19 μg mL−1 | Cu2+, Mn2+, Ni2+, Co2+ | 97 |
| 90 | Au@g-CNQDs | H2O | — | — | 10 | — | 98 |
| 91 | Polydiacetylene | H2O | 530 | About 625 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
— | 99 |
| 92 | N&S CDs | H2O | 340 | 459 | 18 | — | 100 |
| 93 | Zn–Ag–In–S QDs | H2O | 360 | 520 | 1560 | Pb2+, Hg2+, Cu2+ | 91 |
| 94 | Zn-MOF | CH3CN | 330 | 390 and 468–446 -408 | 132 | — | 101 |
| 95 | Mn:ZnSe QDs | H2O | 367 | 600 | 18 | — | 102 |
| 96 | CDs | H2O | 390 | 450 | 0.29 | 103 | |
| 97 | Imprinted polymer | CH3CN | 365 | 440–502 | 12.3 (LOQ = 41) | — | 104 |
| 98 | CuNCs@SiO2; CdTe QDs | H2O | 365 | 580 and 436 | 1.1 μg L−1 | — | 105 |
10. Conclusions and outlooks
In this paper, we summarized the reports of Cd2+ fluorescent and colorimetric probes with different fluorophores since 2017, and found that there are still some problems to be solved in the practical application of Cd2+ fluorescent sensors. For example, the specific selectivity and anti-interference ability of most probes remain poor, which greatly affect the qualitative and quantitative detection of Cd2+ in complex environment. Secondly, the water solubility of many probes are poor, which inevitably result in addition of organic solvent into the detection medium and then pollution formation. Thirdly, the detection sensitivity of some probes are not enough to meet the maximum allowable concentration of Cd2+ in drinking water stipulated by WHO. Fourthly, few of the probes can realize near-infrared recognition of Cd2+, and most of the probes only identify Cd2+ in the blue and green light regions, which are easily interfered by the background fluorescence of biological samples and limits their application in biological system.Therefore, the design and development of Cd2+ fluorescent probes in the future, on the one hand, need to discover and optimize the novel recognition groups with high selectivity, sensitivity and water solubility. On the other hand, overall consideration of the economy of raw materials, the simplicity of preparation, the practicability and feasibility of the probes, should be paid more attention. Finally and most importantly, the recognition pattern and mechanism of the probes should be elucidated clearly. Only in this way can we effectively perform the molecular structure design and find the lead compound with high performance.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Science and Technology Major Project of China (No. 2017ZX05049-003-007) and the National Natural Science Foundation of China (No. 21801022).Notes and references
- Y. Mikata, A. Kizu, K. Nozaki, H. Konno, H. Ono, S. Mizutani and S. Sato, Inorg. Chem., 2017, 56, 7404–7415 CrossRef CAS PubMed.
- C. Kumari, D. Sain, A. Kumar, S. Debnath, P. Saha and S. Dey, Dalton Trans., 2017, 46, 2524–2531 RSC.
- K. Krishnaveni, M. Iniya, D. Jeyanthi, A. Siva and D. Chellappa, Spectrochim. Acta, Part A, 2018, 205, 557–567 CrossRef CAS PubMed.
- J. Li, Y. H. Chen, T. T. Chen, J. Qiang, Z. J. Zhang, T. W. Wei, W. Zhang, F. Wang and X. Q. Chen, Sens. Actuators, B, 2018, 268, 446–455 CrossRef CAS.
- J. Hao, X.-Y. Li, Y. Zhang and W.-K. Dong, Materials, 2018, 11, 523 CrossRef PubMed.
- P. G. Mahajan, N. C. Dige, B. D. Vanjare, E. Kamaraj, S. Y. Seo and K. H. Lee, J. Photochem. Photobiol., A, 2019, 385, 112089 CrossRef CAS.
- Y. Zhang, X. F. Guo, M. M. Zheng, R. Yang, H. M. Yang, L. H. Jia and M. M. Yang, Org. Biomol. Chem., 2017, 15, 2211–2216 RSC.
- W. Su, S. Z. Yuan and E. J. Wang, J. Fluoresc., 2017, 27, 1871–1875 CrossRef CAS PubMed.
- Y. P. Dai, K. Yao, J. X. Fu, K. Xue, L. Yang and K. X. Xu, Sens. Actuators, B, 2017, 251, 877–884 CrossRef CAS.
- N. Behera and V. Manivannan, J. Photochem. Photobiol., A, 2018, 353, 77–85 CrossRef CAS.
- X. Y. Liu, P. Wang, J. X. Fu, K. Yao, K. Xue and K. X. Xu, J. Lumin., 2017, 186, 16–22 CrossRef CAS.
- S. Chithiraikumar, C. Balakrishnan and M. A. Neelakantan, Sens. Actuators, B, 2017, 249, 235–245 CrossRef CAS.
- R. Purkait, S. Dey and C. Sinhaa, New J. Chem., 2018, 42, 16653–16665 RSC.
- J. K. Xiong, K. R. Wang, K. X. Wang, T. L. Han, H. Y. Zhu, R. X. Rong, Z. R. Cao and X. L. Li, Sens. Actuators, B, 2019, 297, 126802 CrossRef CAS.
- L. Lvova, F. Caroleo, A. Garau, V. Lippolis, L. Giorgi, V. Fusi, N. Zaccheroni, M. Lombardo, L. Prodi, C. Di Natale and R. Paolesse, Front. Chem., 2018, 6, 258 CrossRef PubMed.
- A. Maity, U. Ghosh, D. Giri, D. Mukherjee, T. K. Maiti and S. K. Patra, Dalton Trans., 2019, 48, 2108–2117 RSC.
- J. Q. Sun, B. F. Ye, G. M. Xia and H. M. Wang, Sens. Actuators, B, 2017, 249, 386–394 CrossRef CAS.
- Z. Wang, S. Q. Cui, S. Y. Qiu and S. Z. Pu, J. Photochem. Photobiol., A, 2018, 367, 212–218 CrossRef CAS.
- D. D. Cheng, X. L. Liu, Y. D. Xie, H. T. Lv, Z. Q. Wang, H. Z. Yang, A. X. Han, X. M. Yang and L. Zang, Sensors, 2017, 17, 2517 CrossRef PubMed.
- T. Nemeth, T. Toth, G. T. Balogh and P. Huszthy, Period. Polytech., Chem. Eng., 2017, 61, 249–257 CrossRef CAS.
- D. B. Zhang, S. Y. Li, R. M. Lu, G. Liu and S. Z. Pu, Dyes Pigments, 2017, 146, 305–315 CrossRef CAS.
- X. X. Zhang, R. J. Wang, C. B. Fan, G. Liu and S. Z. Pu, Dyes Pigments, 2017, 139, 208–217 CrossRef CAS.
- M. Ghosh, S. Ta, M. Banerjee, M. Mahiuddin and D. Das, ACS Omega, 2018, 3, 4262–4275 CrossRef CAS PubMed.
- R. Diana, U. Caruso, S. Concilio, S. Piotto, A. Tuzi and B. Panunzi, Dyes Pigments, 2018, 155, 249–257 CrossRef CAS.
- H. L. Liu, S. Q. Cui, F. Shi and S. Z. Pu, Dyes Pigments, 2019, 161, 34–43 CrossRef CAS.
- T. H. Liu, K. Liu, J. L. Zhang and Z. L. Wang, ChemistrySelect, 2018, 3, 5559–5565 CrossRef CAS.
- Y. Y. Zhang, X. Z. Chen, X. Y. Liu, M. Wang, J. J. Liu, G. Gao, X. Y. Zhang, R. Z. Sun, S. C. Hou and H. M. Wang, Sens. Actuators, B, 2018, 273, 1077–1084 CrossRef CAS.
- Z. Wang, S. Q. Cui, S. Y. Qiu and S. Z. Pu, Tetrahedron, 2018, 74, 7431–7437 CrossRef CAS.
- M. Vera, H. S. Ortega, M. Inoue and L. Machi, Supramol. Chem., 2019, 31, 336–348 CrossRef CAS.
- P. Kaur, B. Lal, N. Kaur, G. Singh, A. Singh, G. Kaur and J. Singh, J. Photochem. Photobiol., A, 2019, 382, 111847 CrossRef CAS.
- M. Banerjee, M. Ghosh, S. Ta, J. Das and D. Das, J. Photochem. Photobiol., A, 2019, 377, 286–297 CrossRef CAS.
- M. Maniyazagan, R. Mariadasse, J. Jeyakanthan, N. K. Lokanath, S. Naveen, K. Premkumar, P. Muthuraja, P. Manisankar and T. Stalin, Sens. Actuators, B, 2017, 238, 565–577 CrossRef CAS.
- X. J. Wan, H. Q. Ke, J. N. Tang and G. H. Yang, Talanta, 2019, 199, 8–13 CrossRef CAS PubMed.
- Y. F. Tang, Y. Huang, Y. H. Chen, L. X. Lu, C. Wang, T. M. Sun, M. Wang, G. H. Zhu, Y. Yang, L. Zhang and J. L. Zhu, Spectrochim. Acta, Part A, 2019, 218, 359–365 CrossRef CAS PubMed.
- J. Jia, X. Tang, Y. He, M. Zhang and G. Xing, Chin. J. Org. Chem., 2012, 32, 1803–1811 CrossRef CAS.
- S. Y. Chen, Z. Li, K. Li and X. Q. Yu, Coord. Chem. Rev., 2021, 429, 20 CrossRef.
- Y. Mikat, M. Kaneda, H. Konno, A. Matsumoto, S. Sato, M. Kawamura and S. Iwatsuki, Dalton Trans., 2019, 48, 3840–3852 RSC.
- Y. Mikata, M. Kaneda, M. Tanaka, S. Iwatsuki, H. Konno and A. Matsumoto, Eur. J. Inorg. Chem., 2020, 2020, 757–763 CrossRef CAS.
- Y. Zhang, X. F. Guo, L. B. Zheng and L. H. Jia, J. Lumin., 2017, 188, 283–288 CrossRef CAS.
- X. H. Ding, F. F. Zhang, Y. J. Bai, J. X. Zhao, X. Chen, M. Ge and W. Sun, Tetrahedron Lett., 2017, 58, 3868–3874 CrossRef CAS.
- H. H. Song and Z. Zhang, Dyes Pigments, 2019, 165, 172–181 CrossRef CAS.
- Z. N. Lu, L. Wang, X. Zhang and Z. J. Zhu, Spectrochim. Acta, Part A, 2019, 213, 57–63 CrossRef CAS PubMed.
- K. Aich, S. Das, S. Gharami, L. Patra and T. K. Mondal, ChemistrySelect, 2019, 4, 8068–8073 CrossRef CAS.
- S. Nazerdeylami, J. B. Ghasemi, A. Amiri, G. M. Ziarani and A. Badiei, Methods Appl. Fluoresc., 2020, 8, 025009 CrossRef CAS PubMed.
- S. L. Li, D. L. Cao, W. B. Ma, Z. Y. Hu, X. J. Meng, Z. C. Li, C. C. Yuan, T. Zhou and X. H. Han, RSC Adv., 2020, 10, 18434–18439 RSC.
- Y. Xiao, J. Ma, D. H. Li, L. Liu and H. L. Wang, J. Photochem. Photobiol., A, 2020, 399, 112613 CrossRef CAS.
- A. Garau, M. C. Aragoni, M. Arca, A. Bencini, A. J. Blake, C. Caltagirone, C. Giorgi, V. Lippolis and M. A. Scorciapino, Chempluschem, 2020, 85, 1789–1799 CrossRef CAS PubMed.
- J. Han, X. Tang, Y. Wang, R. J. Liu, L. Wang and L. Ni, Spectrochim. Acta, Part A, 2018, 205, 597–602 CrossRef CAS PubMed.
- S. Zehra, R. A. Khan, A. Alsalme and S. Tabassum, J. Fluoresc., 2019, 29, 1029–1037 CrossRef CAS PubMed.
- A. K. Shaily and N. Ahmed, New J. Chem., 2017, 41, 14746–14753 RSC.
- S. Paul and P. Banerjee, Sens. Actuators, B, 2021, 329, 129172 CrossRef CAS.
- P. Sakthivel, K. Sekar, G. Sivaraman and S. Singaravadivel, J. Fluoresc., 2017, 27, 1109–1115 CrossRef CAS PubMed.
- P. Wang, L. P. Duan and Y. W. Liao, Microchem. J., 2019, 146, 818–827 CrossRef CAS.
- P. Wang, K. Chen and Y. S. Ge, J. Lumin., 2019, 208, 495–501 CrossRef CAS.
- P. Wang, D. G. Zhou and B. Chen, Spectrochim. Acta, Part A, 2019, 207, 276–283 CrossRef CAS PubMed.
- P. Wang, Y. An and Y. W. Liao, Spectrochim. Acta, Part A, 2019, 216, 61–68 CrossRef CAS PubMed.
- P. Wang, J. Wu and C. H. Zhao, Spectrochim. Acta, Part A, 2020, 226, 117600 CrossRef CAS PubMed.
- S. Guo, G. Liu, C. Fan and S. Pu, RSC Adv., 2018, 8, 22786–22798 RSC.
- J. F. Lv, G. Liu, C. B. Fan and S. Z. Pu, Spectrochim. Acta, Part A, 2020, 227, 117581 CrossRef CAS PubMed.
- W. B. Huang, W. Gu, H. X. Huang, J. B. Wang, W. X. Shen, Y. Y. Lv and J. Shen, Dyes Pigments, 2017, 143, 427–435 CrossRef CAS.
- N. A. Bumagina, E. V. Antina and D. I. Sozonov, J. Lumin., 2017, 183, 315–321 CrossRef CAS.
- V. Tekuri and D. R. Trivedi, Anal. Chim. Acta, 2017, 972, 81–93 CrossRef CAS PubMed.
- Y. Tang, H. Liu, G. Jiang and Z. Gu, J. Appl. Spectrosc., 2017, 84, 911–914 CrossRef CAS.
- S. Poomalai, A. K. Padinjareveetil, S. Ramasamy, T. S. Govindaraj, M. S. Paulraj and I. Enoch, Luminescence, 2017, 32, 1405–1410 CrossRef CAS PubMed.
- W. H. Hsieh, C.-F. Wan, D.-J. Liao and A.-T. Wu, Tetrahedron Lett., 2012, 53, 5848–5851 CrossRef CAS.
- P. Kaur, J. Singh, R. Singh, V. Kaur and D. Talwar, Polyhedron, 2017, 125, 230–237 CrossRef CAS.
- B. K. Momidi, V. Tekuri and D. R. Trivedi, Spectrochim. Acta, Part A, 2017, 180, 175–182 CrossRef CAS PubMed.
- V. Kumar, P. Kumar and R. Gupta, RSC Adv., 2017, 7, 23127–23135 RSC.
- M. Findik, A. Ucar, H. Bingol, E. Guler and E. Ozcan, Res. Chem. Intermed., 2017, 43, 401–412 CrossRef CAS.
- Y. Y. Zhang, X. Z. Chen, J. J. Liu, G. Gao, X. Y. Zhang, S. C. Hou and H. M. Wang, New J. Chem., 2018, 42, 19245–19251 RSC.
- M. Formica, G. Ambrosi, V. Fusi, L. Giorgi, M. Arca, A. Garau, A. Pintus and V. Lippolis, New J. Chem., 2018, 42, 7869–7883 RSC.
- P. Ravichandiran, A. Boguszewska-Czubara, M. Maslyk, A. P. Bella, P. M. Johnson, S. A. Subramaniyan, K. S. Shim and D. J. Yoo, Dyes Pigments, 2020, 172, 107828 CrossRef CAS.
- M. Das and M. Sarkar, ChemistrySelect, 2019, 4, 681–692 CrossRef CAS.
- M.-X. Huang, C.-H. Lv, Q.-D. Huang, J.-P. Lai and H. Sun, RSC Adv., 2019, 9, 36011–36019 RSC.
- W. X. Lin, X. C. Xie, Y. J. Wang and J. J. Chen, Z. Anorg. Allg. Chem., 2019, 645, 645–648 CrossRef CAS.
- P. P. Namitha, A. Saji, S. Francis and L. Rajith, J. Fluoresc., 2020, 30, 527–535 CrossRef CAS PubMed.
- P. Liu, J. Liu, F. Yao, X. M. Zhan and X. P. Qi, J. Photochem. Photobiol., B, 2020, 202, 111717 CrossRef CAS PubMed.
- B. Jana, S. Maity, A. Mondal and J. Ganguly, J. Lumin., 2020, 222, 117128 CrossRef CAS.
- E. K. Inal, J. Fluoresc., 2020, 30, 891–900 CrossRef CAS PubMed.
- S. G. J. Andrews, S. B. J. Silviya, D. Jeyanthi, E. S. Devi, J. W. Jebaraj and C. Balakrishnan, Analyst, 2020, 145, 4576–4586 RSC.
- Y. F. Zhang, L. Chen, J. Yang, Y. R. Zhang and M. S. Yuan, Spectrochim. Acta, Part A, 2020, 232, 118163 CrossRef CAS PubMed.
- F. D. Carlos, L. A. da Silva, C. Zanlorenzi and F. S. Nunes, Inorg. Chim. Acta, 2020, 508, 119634 CrossRef CAS.
- S. Park, H. Lee, Y. Yi, M. H. Lim and C. Kim, Inorg. Chim. Acta, 2020, 513, 119936 CrossRef CAS.
- P. S. Kumar and K. P. Elango, Spectrochim. Acta, Part A, 2020, 241, 118610 CrossRef CAS PubMed.
- Z. Aydin and M. Keles, Turk. J. Chem., 2020, 44, 791–804 CrossRef CAS PubMed.
- J. J. Celestina, P. Tharmaraj and C. D. Sheela, Opt. Mater., 2020, 109, 110176 CrossRef CAS.
- P. Lasitha, S. Dasgupta and G. N. Patwari, ChemPhysChem, 2020, 21, 1564–1570 CrossRef CAS PubMed.
- M. Muddassir, A. Alarifi, M. Afzal, K. A. Alshali, N. A. Y. Abduh and A. Beagan, RSC Adv., 2020, 10, 42137–42146 RSC.
- S. Dey, R. Purkait, D. Mallick and C. Sinha, ChemistrySelect, 2020, 5, 8274–8283 CrossRef CAS.
- D. Yue, X. Zhang, Y. Tan, Z. Wang and Y. Zhang, J. Lumin., 2020, 228, 117618 CrossRef CAS.
- C. Wei, X. Wei, Z. Hu, D. Yang, S. Mei, G. Zhang, D. Su, W. Zhang and R. Guo, Anal. Methods, 2019, 11, 2559–2564 RSC.
- L. Li, L. Liao, Y. Ding and H. Zeng, RSC Adv., 2017, 7, 10361–10368 RSC.
- Q. Wu, M. Zhou, J. Shi, Q. Li, M. Yang and Z. Zhang, Anal. Chem., 2017, 89, 6616–6623 CrossRef CAS PubMed.
- J. Wang, T. Xia, X. Zhang, Q. Zhang, Y. Cui, Y. Yang and G. Qian, RSC Adv., 2017, 7, 54892–54897 RSC.
- Y. Yang, L. Chen, F. Jiang, X. Wan, M. Yu, Z. Cao, T. Jing and M. Hong, J. Mater. Chem. C, 2017, 5, 4511–4519 RSC.
- D. Gu, L. Hong, L. Zhang, H. Liu and S. Shang, J. Photochem. Photobiol., B, 2018, 186, 144–151 CrossRef CAS PubMed.
- Y. Pan, J. Wang, X. Guo, X. Liu, X. Tang and H. Zhang, J. Colloid Interface Sci., 2018, 513, 418–426 CrossRef CAS PubMed.
- Z. Zhang, Z. Zhang, H. Liu, X. Mao, W. Liu, S. Zhang, Z. Nie and X. Lu, Biosens. Bioelectron., 2018, 103, 87–93 CrossRef CAS PubMed.
- P. Thanh Chung, Y. K. Kim, J. B. Park, S. Jeon, J. Ahn, Y. Yim, J. Yoon and S. Lee, ChemPhotoChem, 2019, 3, 1133–1137 CrossRef.
- M. Tang, X. Liu, N. Zhang, J. Pang, Y. Zou, F. Chai, H. Wu and L. Chen, Anal. Methods, 2019, 11, 5214–5221 RSC.
- A. Mandal, A. Adhikary, A. Sarkar and D. Das, Inorg. Chem., 2020, 59, 17758–17765 CrossRef CAS PubMed.
- Z.-Q. Zhou, L.-Y. Yang, Y.-P. Liao, H.-Y. Wu, X.-H. Zhou, S. Huang, Y. Liu and Q. Xiao, Anal. Methods, 2020, 12, 552–556 RSC.
- S. C. Pandey, A. Kumar and S. K. Sahu, J. Photochem. Photobiol., A, 2020, 400, 112620 CrossRef CAS.
- T. Alizadeh, A. R. Sharifi and M. R. Ganjali, RSC Adv., 2020, 10, 4110–4117 RSC.
- W. Li, X. Zhang, X. Hu, Y. Shi, Z. Li, X. Huang, W. Zhang, D. Zhang, X. Zou and J. Shi, J. Hazard. Mater., 2021, 408, 124872 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2021 |