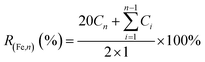Open Access Article
Open Access ArticleH2O2-independent chemodynamic therapy initiated from magnetic iron carbide nanoparticle-assisted artemisinin synergy†
Fan Zhao‡
ab,
Jing Yu‡ *ab,
Weiliang Gaoab,
Xue Yangc,
Liying Liangab,
Xiaolian Sund,
Dan Sue,
Yao Ying
*ab,
Weiliang Gaoab,
Xue Yangc,
Liying Liangab,
Xiaolian Sund,
Dan Sue,
Yao Ying ab,
Wangchang Liab,
Juan Liab,
Jingwu Zhengab,
Liang Qiaoab,
Wei Caiab,
Shenglei Che
ab,
Wangchang Liab,
Juan Liab,
Jingwu Zhengab,
Liang Qiaoab,
Wei Caiab,
Shenglei Che *ab and
Xiaozhou Mou*c
*ab and
Xiaozhou Mou*c
aCollege of Materials Science and Engineering, Zhejiang University of Technology, Hangzhou 310014, China. E-mail: yujing@zjut.edu.cn; cheshenglei@zjut.edu.cn
bResearch Center of Magnetic and Electronic Materials, Zhejiang University of Technology, Hangzhou 310014, China
cClinical Research Institute, Zhejiang Provincial People's Hospital, Hangzhou 310014, China. E-mail: mouxz@zju.edu.cn
dKey Laboratory of Drug Quality Control and Pharmacovigilance (China Pharmaceutical University), Ministry of Education, Nanjing 210009, China
eDepartment of Oncology, Zhejiang Provincial People's Hospital, Hangzhou 310014, China
First published on 22nd November 2021
Abstract
Chemodynamic therapy (CDT) is a booming technology that utilizes Fenton reagents to kill tumor cells by transforming intracellular H2O2 into reactive oxygen species (ROS), but insufficient endogenous H2O2 makes it difficult to attain satisfactory antitumor results. In this article, a H2O2-free CDT technique with tumor-specificity is developed by using pH-sensitive magnetic iron carbide nanoparticles (PEG/Fe2C@Fe3O4 NPs) to trigger artemisinin (ART) to in situ form ROS. ART-loaded PEG/Fe2C@Fe3O4 NPs are fabricated for the enormous release of Fe2+ ions induced by the acidic conditions of the tumor microenvironment after magnetic-assisted tumor enrichment, which results in the rapid degradation of the PEG/Fe2C@Fe3O4 NPs and release of ART once endocytosed into tumor cells. In situ catalysis reaction between the co-released Fe2+ ions and ART generates toxic ROS and then induces apoptosis of tumor cells. Both in vitro and in vivo experiments demonstrate that the efficient Fe-enhanced and tumor-specific CDT efficacy for effective tumor inhibition based on ROS generation. This work provides a new direction to improve CDT efficacy based on H2O2-independent ROS generation.
Introduction
Chemodynamic therapy (CDT), that employs highly cytotoxic reactive oxygen species (ROS) generated from endogenous hydrogen peroxide (H2O2) via the Fenton-type reactions to induce tumor cell apoptosis, has been intensively investigated for tumor therapy nowadays.1,2 Benefiting from the overproduced endogenous H2O2 which is the characteristic feature of the tumor microenvironment (TME), CDT has been recognized as an emerging therapeutic strategy with high therapeutic specificity and low invasiveness.3,4 However, the concentration of intratumoral H2O2 (50–100 μM), although at a higher level compared with normal cells, is still insufficient to maximize the Fenton catalytic efficacy, which restricts the clinical application of CDT.5–7 To address this limitation, substantial efforts have been devoted to increasing the level of intratumoral H2O2, such as applying natural enzymes like glucose oxidase (GOx), nicotinamide adenine dinucleotide phosphate oxidase (NOX), and superoxide dismutase (SOD) as H2O2 self-supplying systems to in situ generate H2O2 inside tumor.8–10 Nevertheless, the rigorous reaction condition further confines the therapeutic efficiency of CDT utilizing these strategies. Recently, Wang and coauthors applied ferrous ion (Fe2+) to catalyze linoleic acid hydroperoxide (LAOOH) to produce abundant ROS through the Russell mechanism, overcoming the dependence on H2O2 concentration by CDT.11 This suggests a potential alternative to H2O2-dependent CDT.Artemisinin (ART), a class of sesquiterpene lactone extracted from Artemisia annua, has been broadly used to treat malaria.12,13 Over the last 10 years, studies have suggested that ART containing endoperoxide bridge also has a therapeutic effect on various tumor cells.14,15 The critical mechanism for antitumor ability of ART is that peroxide bridge structure (R–OO–R′) inside of ART could be catalysis-cleaved by Fe2+ within tumor cells to produce toxic carbon-centered free radicals (a kind of ROS).16–19 Thus, a H2O2-independent CDT agent can be constructed based on ART. Noteworthy that Fe2+ plays an irreplaceable role in tumor cell killing for ART-based drugs and sufficient Fe2+ may make a remarkable contribution to maximize the therapy efficiency of ART.20–24 Whereas, the concentration of Fe2+ at the tumor site is generally much lower than the optimum concentration of Fe2+ which can exhaust the antitumor ability of ART, leading to an unsatisfactory therapeutic efficiency.25 Therefore, designing a nanocarrier for co-delivery of ART and Fe2+ to tumor cells can be an ideal strategy for the enhancement of CDT that independent from endogenous H2O2.
Iron carbide nanoparticles (ICNPs), a kind of nano-intermetallic iron-based compounds, have gained enormous attention for their extraordinary magnetic property which renders them to be T2 contrast agents in magnetic resonance imaging (MRI).26–29 Besides, ICNPs with appropriate surface modification could be excellent drug carriers, and the high saturation magnetization of ICNPs endows them magnetic targeting property.30–32 Interestingly, our previous works suggested that ICNPs in which the valence state of iron tends to be zero valence can easily release Fe2+ under acidic condition and chronically remain stable in the neutral environment.33 Consequently, taking advantage of their pH-sensitivity, ICNPs can be designed as ‘iron storage pool’ to specifically release Fe2+ at acidic microenvironment of the tumor to trigger ART.
Herein, core–shell structured ICNPs (Fe2C@Fe3O4 nanoparticles), which were capable of pH-triggered Fe2+-release, were designed as the nanocarriers for ART. Benefiting from the high saturation magnetization, an enrichment of Fe2C@Fe3O4 NPs together with ART in tumor sites was feasible by magnetic targeting. Combined with the acidic microenvironment of tumor, the designed Fe2C@Fe3O4 nanocarrier was able to resolve at tumor, release both Fe2+ and ART synchronously for yielding quantity of toxic ROS in situ to damage proteins and nucleic acid, and consequently induce cell death which significantly enhance the CDT efficiency (Scheme 1). This study paves a new direction to improve CDT efficacy based on H2O2-free ROS generation.
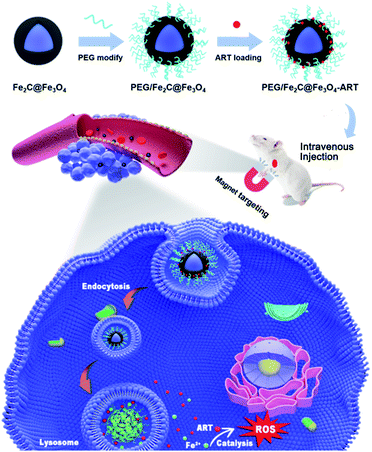 | ||
| Scheme 1 Schematic illustration of ART loaded PEG/Fe2C@Fe3O4 NPs to tumor cells assisted by an externally applied magnetic field and the antitumor mechanism of the ART delivery system. | ||
Results and discussion
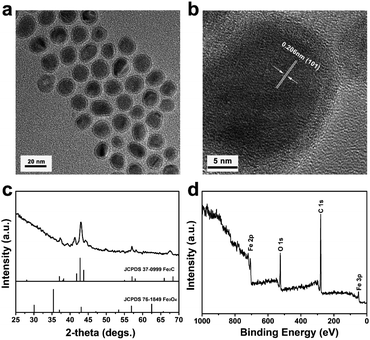
Fe2C@Fe3O4 NPs were synthesized by a two-step synthetic method.34 Based on transmission electron microscopy (TEM) image (Fig. 1a), the as-synthesized NPs showed monodisperse core–shell structure with uniform size at approximately 18 nm, which was consistent with the data of dynamic light scattering (DLS) (Fig. S1†). The core size of Fe2C@Fe3O4 NPs was measured to be 14 ± 2 nm, while the shell thickness was approximately 1 ± 0.5 nm. The crystallization of the NPs could be identified in the high-resolution transmission electron microscopy (HRTEM) image (Fig. 1b). The lattice spacing in the core was 0.206 nm, corresponding to the (101) plane of highly crystallized Fe2C, while the shell structure showed an amorphous feature of Fe3O4.34 The crystal structure of the NPs was further determined by X-ray diffraction (XRD) (Fig. 1c). All the diffraction peaks could be well-indexed to the Fe2C core (JCPDS no. 37-0999), while no obvious peak of Fe3O4 was observed, due to its low crystallization. To verify the composition of the Fe2C@Fe3O4 NPs, X-ray photoelectron spectroscopy (XPS) was applied, and the result indicated the coexistence of carbon, oxygen, and iron (Fig. 1d). The energy-dispersive spectroscopy (EDS) further confirmed that obtained Fe2C@Fe3O4 NPs were composed of Fe, C, and O elements (Fig. S2†). Besides, the magnetic property of Fe2C@Fe3O4 NPs was tested by using a vibrating sample magnetometer (VSM) at room temperature. The saturation magnetization (Ms) value of Fe2C@Fe3O4 NPs was 65.483 emu g−1 (Fig. S3†), suggesting their potential as T2 contrast agents as well as nanocarriers with magnetic targeting.To endow a hydrophilic nature as well as a long-circulation property to Fe2C@Fe3O4 NPs, DSPE-PEG was applied to form PEG/Fe2C@Fe3O4 NPs. The data of FT-IR and macrophotograph showed the successful modification of PEG on Fe2C@Fe3O4 NPs (Fig. S4 and S5†). TEM image of PEG/Fe2C@Fe3O4 NPs obtained from their aqueous dispersions, indicating that there was no obvious morphology change after the ligand addition process (Fig. S6†). In addition, hydrodynamic diameter of PEG/Fe2C@Fe3O4 NPs was increased to 96.1 nm and zeta potential was measured to be −21.5 mV (Fig. S7†), which was suitable for enhanced permeability and retention (EPR) effect to achieve practical applications. Furthermore, Fe2C@Fe3O4 NPs after PEGylation could keep long-term stability in the aqueous phase (Fig. S8†).
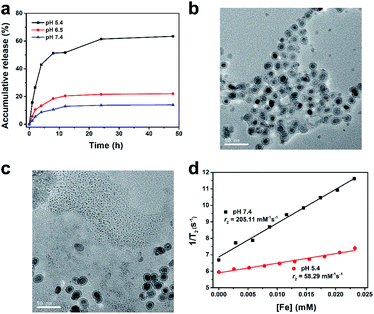
The antitumor activity of ART was related to the concentration of Fe2+. Hence, Fe2+ release characteristic of PEG/Fe2C@Fe3O4 NPs was firstly explored. Three solutions with pH value of 7.4, 6.5, and 5.4 were applied that corresponding to the environment of normal tissue, tumor site, and lysosomes. As shown in Fig. 2a, the degradation of PEG/Fe2C@Fe3O4 NPs in different solutions severed an obvious pH-sensitivity. Iron ions released in pH 5.4 solution increased significantly in the first 4 h, with the amount reached to 63.42% after 24 h, while only 14.02% and 22.04% of iron ions could be released under pH 7.4 and 6.5, respectively. TEM images of PEG/Fe2C@Fe3O4 NPs after being dispersed in pH 5.4 and 7.4 media for 24 h, further confirming that the NPs could be degraded only in pH 5.4 solution (Fig. 2b and c). In addition, strong hypo-intensities in T2-weighted magnetic resonance imaging (T2-MRI) of PEG/Fe2C@Fe3O4 NPs, which induced from their high magnetization, was reduced by altering the incubation solution from physiological condition (pH 7.4) to acidic solution (pH 5.4), with the transverse relaxivity (r2) decreased from 205.11 mM−1 s−1 to 58.29 mM−1 s−1 (Fig. 2d). This result further proved the dissolution of the NPs in acidic environment. Color of the solution turned to bright yellow at pH of 5.4, and after adding potassium ferricyanide (a Fe2+ indicator), the color became blue, suggesting an efficient release of Fe2+ at acidic environment (Fig. S9†). The excellent pH-sensitive Fe2+ release ability of PEG/Fe2C@Fe3O4 NPs was attributed to successful introduction of amorphous Fe3O4 shells which performed stronger pH sensitivity after amorphization.33,35,36 The above results demonstrated that PEG/Fe2C@Fe3O4 NPs were good Fe2+ donors in low pH environment.
PEG was modified onto hydrophobic Fe2C@Fe3O4 NPs through ligand addition method, endowing the interspace for loading ART by hydrophobic–hydrophobic interaction. The appearance of the characteristic peaks at the wavelength of 1737 cm−1, between 800 cm−1 and 1200 cm−1 confirming the loading of ART onto PEG/Fe2C@Fe3O4 NPs, which were ascribed to the C–O stretching vibrations, and the O–O and C–O modes of O–O–C (peroxide), respectively (Fig. S10†).37 The ART loading efficiency was calculated to be 21.9 wt% by using ultraviolet and visible (UV-vis) spectra. Result from TEM image showed the ART loaded PEG/Fe2C@Fe3O4 (PEG/Fe2C@Fe3O4-ART) NPs could still keep their morphology with good dispersity in aqueous solution (Fig. S11†).
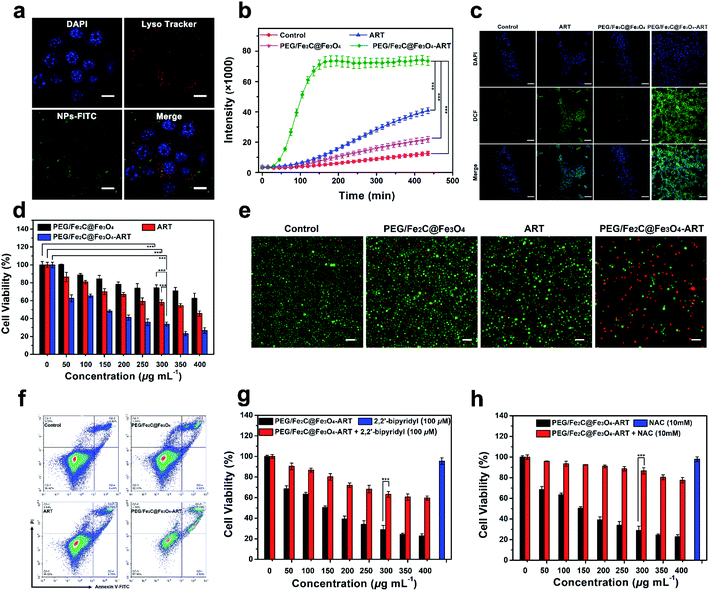
The result from EPR spectrometry showed ART was able to generate carbon-centered free radicals (a kind of ROS) in Fe2+-riched environment (Fig. S12†).21,23 Considering the acidic environment of lysosome and the efficient release of Fe2+ under low pH solution, locating PEG/Fe2C@Fe3O4 NPs as an ART activator in lysosomes after being cellular uptake was important. After labelling PEG/Fe2C@Fe3O4 NPs with fluorescein isothiocyanate (FITC), cellular uptake assay was carried out by staining nuclei with 4,6-diamidino-2-phenylindole (DAPI) and lysosomes with Lyso-Tracker red. As shown Fig. 3a, green fluorescence from the FITC-labeled NPs was well overlapped with the red fluorescence of Lyso-Tracker, indicating that PEG/Fe2C@Fe3O4 NPs were internalized and located in the acidic lysosomal compartment, and should produce ROS consequently.
To verify the capability of PEG/Fe2C@Fe3O4-ART NPs in generating ROS in tumor cells, 2,7-dichlorofluorescein diacetate (DCFH-DA), a fluorescent ROS-detection probe was used. Fluorescence emitted from PEG/Fe2C@Fe3O4-ART NPs increased with the incubation time, and the fluorescence intensity after 9 h of incubation was 1.8 and 3.6-fold higher compared to the ART alone and PEG/Fe2C@Fe3O4 NPs treated group respectively (Fig. 3b). Similarly, observed under a confocal laser scanning microscope (CLSM), a bright green fluorescence could be clearly observed in cells incubated with PEG/Fe2C@Fe3O4-ART NPs within 4 h, while in contrast, fluorescence from the cells incubated with ART alone or PEG/Fe2C@Fe3O4 NPs was much weaker (Fig. 3c). These results demonstrated that intracellular PEG/Fe2C@Fe3O4-ART NPs could boost the generation of ROS in situ for tumor therapy.
Cell growth inhibition was then evaluated through MTT assay. As shown in Fig. 3d and S13,† ART alone and PEG/Fe2C@Fe3O4 NPs were not obviously toxic to 4T1, MDA-MB-231, and HeLa cells. Whereas, the cytotoxicity of PEG/Fe2C@Fe3O4-ART NPs was significantly enhanced, demonstrating Fe2C@Fe3O4 NPs could improve the antitumor efficiency of ART. Results from live/dead cell staining assay further confirmed this conclusion. By staining live cells with calcein-AM and dead cells with propidium iodide (PI), red fluorescence from PEG/Fe2C@Fe3O4-ART NPs 24 h-treated 4T1 cells indicated the induction of cell death (Fig. 3e). It has been reported that cell killing through ROS-mediated pathway could mainly induce cell apoptosis. Flow cytometry was further applied to identify the type of cell death by using the Annexin V-FITC/PI apoptosis detection kit. Obviously, the PEG/Fe2C@Fe3O4-ART-treated groups had higher ratios of apoptotic tumor cells compared with other groups (Fig. 3f).
From the phenomenon above, the reason for the enhanced apoptosis of PEG/Fe2C@Fe3O4-ART NPs could be ascribed to the efficient in situ generation of ROS by released Fe2+ and ART. Consequently, Fe2+ is sufficient for ART-based tumor therapy. Therefore, 2,2-bipyridyl, a widely used Fe2+ chelator was applied to confirm the dependance of Fe2+ in the cell killing.38 Cells incubated with PEG/Fe2C@Fe3O4-ART in the presence of 2,2-bipyridyl showed suppressed intracellular ROS production (Fig. S14†), and as a result, significantly increased the cell viability compared with that without 2,2-bipyridyl (Fig. 3g). It proved the involvement of the released Fe2+ in the cytotoxicity of PEG/Fe2C@Fe3O4-ART NPs. To verify the significance of ROS, N-acetyl-cysteine (NAC),39,40 a ROS scavenger, was applied. As shown in Fig. 3h, the addition of NAC significantly reversed the cytotoxicity induced by PEG/Fe2C@Fe3O4-ART NPs, further confirmed the cell apoptosis was primarily origined from ROS. From the above, we can conclude that PEG/Fe2C@Fe3O4 NPs can provide Fe2+ in tumor cells and in situ activate ART to generate abundant ROS, which result in the cytotoxicity for cell killing.
Inspired by the excellent in vitro cell growth inhibition based on PEG/Fe2C@Fe3O4-ART NPs, in vivo biological performances were then evaluated on 4T1 tumor-bearing mice. Benefiting from the magnetic property of PEG/Fe2C@Fe3O4 NPs, biodistribution of NPs was initially evaluated by measuring Fe concentration in major organs and tumors 24 h post-injection. Compared with magnetic field-free group, in which PEG/Fe2C@Fe3O4 NPs were primarily distributed in liver, and a few NPs accumulated in tumor tissue via EPR effect, the magnetic targeting group could effectively accumulate NPs in tumor tissue by locating a magnant at tumor (Fig. S15†). Furthermore, in vivo NIR images further verified PEG/Fe2C@Fe3O4 NPs could be effectively accumulated in tumor site by magnetic targeting (Fig. S16†). Together with the acid environment of tumor, the effective enrichment of NPs in tumors by magnetic targeting makes PEG/Fe2C@Fe3O4 NPs as good carriers to effective deliver ART and donate Fe2+ for the in situ activation of ART by ROS generation.
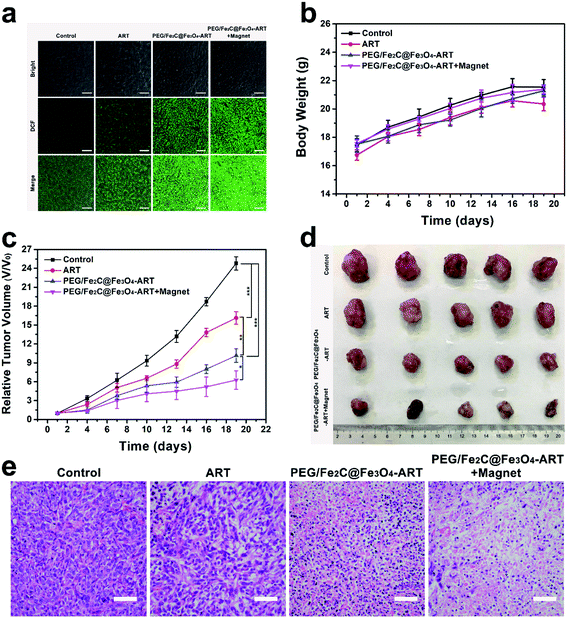
Thus, we next investigated the in vivo tumor ROS concentration. As shown in Fig. 4a, bright green fluorescence was emitted only in the group that had been subjected to magnetic-assisted intravenous (i.v.) injection of PEG/Fe2C@Fe3O4-ART NPs after staining with DCFH-DA, illustrating amounts of ROS could be generated by specifically accumulating PEG/Fe2C@Fe3O4-ART NPs in tumor with Fe2+-ART catalysis. The high ROS intensity led to tumor cell apoptosis and even necrosis. Therefore, tumor inhibition assay was then carried out. 4T1 tumor-bearing female Balb/c mice were established and divided into four groups, which were i.v. injected with either saline, ART only, PEG/Fe2C@Fe3O4-ART NPs or PEG/Fe2C@Fe3O4-ART NPs with magnetic targeting. Tumor volume and body weight were monitored one-day post-injection. No obvious body-weight changes were observed during the overall therapeutic process (Fig. 4b). More importantly, tumor growth in the group treated with magnetic-assisted i.v. injection of PEG/Fe2C@Fe3O4-ART NPs was significantly suppressed (Fig. 4c and d), compared to the control group, the ART-only group, and i.v. injection of PEG/Fe2C@Fe3O4-ART NPs group (Fig. S17†). Besides, tumor sections from mice dissected at the end of the treatment period conducted hematoxylin–eosin (H&E) and TdT-mediated dUTP nick-end labeling (TUNEL) staining, which showed that the multitude of cell necrosis and apoptosis occurred in the tumor sections from mice treated with PEG/Fe2C@Fe3O4-ART NPs (Fig. 4e and S18†). By contrast, no obvious cell necrosis was observed in the control group or the ART-only group, indicating the improved chemotherapeutic efficacy of PEG/Fe2C@Fe3O4-ART NPs for ART-based chemotherapy.
Eventually, systemic biotoxicity valuation is crucial for nanomaterials to clinical research. To explore long-term biosafety, the normal hematology parameters and standard blood biochemical indexes were also measured in healthy mice. As shown in the blood biochemistry and hematology analysis, no obvious changes were induced by PEG/Fe2C@Fe3O4-ART NPs (Fig. S19†). H&E staining also showed no obvious pathological toxicity in the major organs, indicating high biocompatibility of PEG/Fe2C@Fe3O4-ART NPs (Fig. S20†).
Conclusions
In summary, a H2O2-free and tumor-selective chemodynamic therapy agent was constructed by ART loaded magnetic iron carbide NP, which in situ generated ROS by releasing Fe2+ and ART under tumor specificity environment after magnetic targeting. The core–shell structured PEG/Fe2C@Fe3O4 magnetic nanoparticles were synthesized to deliver ART into the tumors, where they could rapidly co-release Fe2+ and ART in a tumor-specific manner. The released ART molecules then transformed into toxic ROS by in situ catalysis of Fe2+, resulting in apoptosis of tumor cells. These features synchronously endow the nanocarrier with enhanced efficacy and biosafety for tumor-specific therapy. Both in vitro and in vivo experiments confirmed the high chemotherapeutic efficacy and revealed the related therapeutic mechanism. This work sets an example of iron-based magnetic nanocarrier with excellent Fe2+ and drug release behavior in the tumor microenvironment after magnetic-assisted tumor enrichment for in situ enhanced Fe2+-dependent chemodynamic.Experimental section
Materials
Iron carbonyl (Fe(CO)5, 99%), oleylamine (OAm), oleic acid (OAc, 90%), 1-octadecene (ODE, 90%) and ammonium bromide (NH4Br, 99%) were purchased from Alfa Aesar. Hexane (C6H14), and ethanol (C2H6OH) of analytic grade were from the Juhua Group Factory, China. Artemisinin (ART, 98%) was purchased from J&K. 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-2000 (DSPE-PEG) was purchased from Toyongbio corporation, china. All chemicals were used as received without further purification.Characterization
X-ray diffraction (XRD) patterns were recorded on a X'Pert PRO X-ray diffractometer equipped with Cu Kα (λ = 1.54178 Å) radiation. Transmission electron microscopy (TEM) was carried out on a FEI Tecnai G2 F30 microscope. High-resolution TEM (HRTEM) was carried out on a FEI Tecnai G2 F30 microscope. Dynamic light scattering was measured using a particle size analyzer (ZetaPALS, Brookhaven Instruments). X-ray photoelectron spectroscopy (XPS) measurements were carried out on an Axis Ultra imaging photoelectron spectrometer (Kratos Analytical Ltd). Magnetization was measured by a superconducting quantum interference device (SQUID). The concentrations of Fe were quantified using an inductively coupled plasma-atomic emission spectrometer (ICP-AES, Profile, Leeman, USA). Fourier transform infrared radiation (FT-IR) spectrum was determined using a Nicolet 6700 spectrometer. Ultraviolet visible (UV-vis) absorption spectra were measured on a HACH DR6000 ultraviolet visible absorption spectrometer.Synthesis of Fe2C@Fe3O4 NPs
In the typical synthesis, bcc-Fe NPs were firstly synthesized by the following steps. ODE (15 mL), NH4Br (0.05–0.1 mmol) and OAm (0.4 mL) were mixed magnetically and degassed under a gentle Ar flow for 1 h in a four-neck flask. The solution was then heated to 100 °C and kept at this temperature for 1 h before it was heated further to 180 °C. After that, Fe(CO)5 (0.5–0.7 mL) was injected to the reaction mixture and kept there for 30 min. OAc (0.1 mL) was added via a syringe and the resultant solution was aged at 140 °C for another 30 min before it was cooled down to room temperature. The complex was collected through magnetic separation and rinsed three times with ethanol. After centrifugation (5 min, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm), the product was collected and re-dispersed in hexane. ODE (3.0 g), OAm (10 mL) was magnetically blended in the four-neck flask and degassed under a gentle Ar flow for 1 h at 120 °C. Then, the resulted bcc-Fe NPs (5 mmol, in 10 mL hexane) was added via a syringe and the reaction solution was heated at 280–340 °C for 15–45 min. The black-brown colored solution was cooled down to room temperature. Ethanol was added and the mixture was centrifuged (5 min, 10
000 rpm), the product was collected and re-dispersed in hexane. ODE (3.0 g), OAm (10 mL) was magnetically blended in the four-neck flask and degassed under a gentle Ar flow for 1 h at 120 °C. Then, the resulted bcc-Fe NPs (5 mmol, in 10 mL hexane) was added via a syringe and the reaction solution was heated at 280–340 °C for 15–45 min. The black-brown colored solution was cooled down to room temperature. Ethanol was added and the mixture was centrifuged (5 min, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm) three times. The precipitates were re-dispersed in hexane.
000 rpm) three times. The precipitates were re-dispersed in hexane.
Preparation of ART-loaded Fe2C@Fe3O4 NPs
Fe2C@Fe3O4 (5 mg) was added to chloroform (10 mL) and then sonicated at room temperature. Then artemisinin (10 mg) and DSPE-PEG (20 mg) dissolving in 10 mL chloroform was slowly added to the above Fe2C@Fe3O4 chloroform mixture solution and sonicated for 30 min before the solvent was evaporated by a rotary evaporator. The final products were washed with ethanol to remove the free drug. The loading efficiency of ART was measured by a UV-vis spectrophotometer. Unloaded ART in the supernatant was converted into a UV-absorbing compound through treatment with sodium hydroxide aqueous solution at 50 °C for 30 min and the detection wavelength was 292 nm.Fe2+ release from PEG/Fe2C@Fe3O4 NPs
PEG/Fe2C@Fe3O4 NPs solution (1 mL) with Fe concentration of 2 mg mL−1 was sealed in a dialysis bag (MCWO: 1000 Da), and immersed in 20 mL buffer media (PBS) at different pH values (7.4, 6.5 and 5.4) in a centrifuge tube. Centrifuge tubes were then shaken with a speed of 200 rpm at 37 °C. At given intervals, 1 mL of buffer was collected and analyzed the Fe concentration by ICP-AES. 1 mL fresh buffer medium was returned. Fe released (R(Fe,n)) was calculated by the following equation:where Cn is the iron concentration tested in nth collection, Ci is the iron concentration in the ith collection. Unit of Cn and Ci are mg mL−1.
In vitro MRI test
The in vitro MRI tests were conducted on a 3.0 T clinical MRI instrument (Philips). To test the in vitro longitudinal relaxation rate r2, PEG/Fe2C@Fe3O4 NPs at the iron concentration from 0.003 mM to 0.021 mM with the interval of 0.003 mM were dispersed in 1 mL of solution with pH 5.4 and 7.4 containing 1 wt% agarose gel respectively and placed in centrifuge tubes (1.5 mL). MR images were acquired using a T2-weighted sequence with the following parameters: repetition time (TR) = 500 ms; echo time (TE) from 12.25 ms to 196 ms, with the interval of 12.25 ms; field of view (FOV) = 180 mm; slice thickness = 2 mm; NEX = 1.Cell culture
4T1 cell line was obtained from the Zhejiang Provincial People's Hospital. All cell-culture-related reagents were purchased from Invitrogen. Cells were cultured in DMEM culture medium supplemented with 10% FBS and 1% penicillin/streptomycin at 37 °C under 5% CO2 with 100% humidity.The intracellular location of PEG/Fe2C@Fe3O4 NPs in 4T1 cells
The as-prepared of PEG/Fe2C@Fe3O4 NPs was labeled with fluorescein isothiocyanate (FITC) (λex = 488 nm, λem = 517 nm) defined as PEG/Fe2C@Fe3O4-FITC. 4T1 cells (5 × 104 cells per well) were seeded into a 24-well plate. When the cell confluence reached 80%, 300 μg mL−1 of PEG/Fe2C@Fe3O4-FITC NPs were added into the culture medium and co-cultured with cells at 37 °C for 4 h. After that, 4T1 cells were counterstained with Lyso-Tracker red (λex = 577 nm, λem = 590 nm) for 30 min and DAPI (λex = 359 nm, λem = 461 nm) for 15 min. Finally, the cells were washed three times with PBS to remove the NPs that have not been endocytic. The images were taken by using a confocal microscope (Leica SP8).Intracellular ROS burst detection
4T1 cells were plated at a density of 5 × 104 cells per well into a 24-well plate and were incubated for 12 h. Cells were loaded with 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA, 10 μM) for 30 min and then washed with PBS for three times. Then, cells were treated by ART or PEG/Fe2C@Fe3O4 NPs ([Fe] = 300 μg mL−1) or PEG/Fe2C@Fe3O4-ART NPs ([Fe] = 300 μg mL−1). PEG/Fe2C@Fe3O4-ART group has an equivalent ART dosage to the free ART group. The intensity of fluorescence was recorded every 15 min over a period of 9 h via excitation of 488 nm and emission at 525 nm by BioTek ELx800.For the fluorescent microscopic examination, 4T1 cells with the cell density of 1 × 105 cells per well were plated onto coverslips in 12-well plates overnight. Then, cells were pre-stained with 10 μM DCFH-DA for 30 min and washed with PBS to remove the free DCFH-DA. Later, the cells were incubated with ART or PEG/Fe2C@Fe3O4 NPs ([Fe] = 300 μg mL−1) or PEG/Fe2C@Fe3O4-ART NPs ([Fe] = 300 μg mL−1) for 4 h before they were washed with PBS for three times. The images were acquired by a Leica SP8 confocal microscope with excitation at 488 nm and emission from 525 nm.
In vitro cytotoxicity assays
The in vitro cytotoxicity was evaluated by standard MTT assay. 4T1 cells were seeded into 96-well cell culture plates at 5 × 103 cells per well and incubated overnight at 37 °C under 5% CO2. After that, cells were treated with various formulations of free ART, PEG/Fe2C@Fe3O4, and PEG/Fe2C@Fe3O4-ART at the same Fe2C@Fe3O4 and ART concentrations. After further incubation for 24 h, the culture media were replaced by medium containing 0.5 mg mL−1 3-[4,5-dimethylthiazol-2-yl-]-2,5-diphenyltetrazolium bromide (MTT). Finally, the MTT solution was replaced by 100 μL of dimethyl sulfoxide (DMSO) after co-incubation for 4 h. By using an ELISA reader (Tecan m200), cell proliferation was measured. Furthermore, after 4T1 cells were treated under the aforementioned conditions, cells were stained with calcein acetoxymethyl ester (calcein-AM, 2 μM, Yeason Biotech, China) and propidium iodide (PI, 4.5 μM, Yeason Biotech, China) to distinguish the live cells from dead cells through a fluorescence microscope (Nikon ECLIPSE Ti).Assessment of apoptosis
Apoptotic status was determined by FITC-conjugated Annexin-V/PI assay kit (Sony) using flow cytometry following the manufacturer's instructions. Briefly, 5 × 104 per well 4T1 cells were seeded in 6-well plates for 12 h and treated with drugs for a further 24 h. The cells were rinsed with PBS and detached using EDTA-free trypsin. The detached cells were resuspended in 100 μL binding buffer containing FITC-conjugated Annexin-V/PI and incubated at RT for 15 min. The cells were diluted in 400 μL of buffer and analyzed by flow cytometry (NovoCyte 3130, USA). Apoptosis and necrosis were evaluated using PI vs. Annexin V plots. The cells stained with Annexin V only were classified as early apoptosis and the Annexin V and PI double-stained cells were classified as late apoptosis or necrosis.Animal modal
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Zhejiang Chinese Medical University and experiments were approved by the Animal Ethics Committee of Zhejiang Chinese Medical University. 6 week female Balb/c mice, with an average weight of 17 g, were provided by the Shanghai Laboratory Animal Center, Shanghai, China. Mice were injected with 1 × 106 4T1 cells (0.1 mL cells in PBS) subcutaneously at the right axillary region.Biodistribution studies
To qualitatively investigate the biodistribution of PEG/Fe2C@Fe3O4 NPs, 6 mice were assigned into two groups randomly. When the tumor volume reached to about 150 mm3, mice were intravenously injected of PEG/Fe2C@Fe3O4 NPs (10 mgFe kg−1). In the magnetic targeting group, a magnet was located at the tumor site. Mice were sacrificed after heart perfusion at time point of 24 h with major organs (heart, liver, spleen, lung and kidneys) and tumors dissected, rinsed and weighted. The in vivo biodistribution of Fe element was then measured by ICP-AES and calculated as Fe percentage over administrated dose per gram of tissues.In vivo tumor chemotherapy of PEG/Fe2C@Fe3O4-ART
When the tumor volume reached to about 100 mm3, 20 mice were randomly divided into 4 groups: (1) mice were intravenously injected of saline only as control; (2) mice were intravenously injected of saline with free ART; (3) mice were intravenously injected of saline with PEG/Fe2C@Fe3O4-ART NPs; (4) mice were intravenously injected of saline with PEG/Fe2C@Fe3O4-ART NPs as well as a magnet located at the tumor site. Both PEG/Fe2C@Fe3O4-ART group and PEG/Fe2C@Fe3O4-ART + magnet group have an equivalent ART dosage to the free ART group and have an equivalent particles dosage. All groups were injected once daily every three days in the period of 18 days with the dosage of 50 mgFe kg−1 and tumor volume was monitored 1 day post injection. Tumor volumes and body weight were monitored 1 day post injection during the treatment (n = 5). Tumor volume was calculated according to the formula of (a × b2)/2, where a and b are the long and short diameters of the tumor, respectively.At given intervals of 19 days, mice from each group were euthanized and major visceral organs (heart, liver, spleen, lung, and kidney) and tumor were recovered, followed by fixing with 10% neutral buffered formalin. After the organs were embedded in paraffin and sectioned at 5 μm, hematoxylin and eosin (H&E) and TdT-mediated dUTP nick-end labeling (TUNEL) staining was performed. The slides were observed under optical microscope (Nikon ECLIPSE Ti).
Quantitative analysis of the ROS generation in tumors
At the 3rd day post injection, one mouse from each group was euthanized, and tumors were harvested from the necropsy. The tumor tissues were firstly embedded and frozen by an OCT (Jung, Tissue freezing medium, Leica). Cross sections of 10 μm thickness were cut using a cryomicrotome (Leica), staining with DCFH-DA, 10 μM for 30 min and mounted on the glass slides. The slides were then observed under a confocal microscope (Leica SP8, λex = 488 nm; λem = 525 nm).Statistical analysis
Quantitative data were expressed as mean ± s.d. One-way ANOVA statistical analysis was used to analyze differences between datum. Values with p < 0.05 were considered statistically significant (* means p < 0.05, ** means p < 0.01, *** means p < 0.001).Author contributions
Fan Zhao: contributed equally to this work, carried out all experiments and performed the statistical analysis, contributed to discussion, writing – original draft. Jing Yu: contributed equally to this work, carried out all experiments and performed the statistical analysis, conceptualization, writing – review & editing. Weiliang Gao: participated in the animal studies and molecular biology experiments. Xue Yang: participated in the animal studies and molecular biology experiments. Liying Liang: participated in molecular biology experiments. Xiaolian Sun: supervision. Dan Su: supervision. Juan Li: supervision. Yao Ying: supervision. Wangchang Li: supervision. Liang Qiao: supervision. Jingwu Zheng: supervision. Wei Cai: supervision. Shenglei Che: conceptualization, supervision, writing – reviewing and editing. Xiaozhou Mou: conceptualization, supervision, writing – reviewing and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported in part by the National Natural Science Foundation of China (NSFC) (No. 52073258, 51602285, 81701821), Natural Science Foundation of Zhejiang Province (No. LY20E020017, LQ19H160016), Young Elite Scientist Sponsorship Program by CAST (No. 2017QNRC001), the Fundamental Research Funds for the Provincial Universities of Zhejiang (No. RF-A2019004), the Open Project Program of MOE Key Laboratory of Drug Quality Control and Pharmacovigilance (No. DQCP20/21MS02), and Foundation of Health Department of Zhejiang Province (No. 2018KY239, 2019RC010), and the Technological Program of Zhejiang Traditional Medicine (No. 2019ZB014).References
- Z. Shen, J. Song, B. C. Yung, Z. Zhou, A. Wu and X. Chen, Emerging strategies of cancer therapy based on ferroptosis, Adv. Mater., 2018, 30, 1704007 CrossRef PubMed.
- Z. Tang, Y. Liu, M. He and W. Bu, Chemodynamic therapy: tumour microenvironment-mediated fenton and fenton-like reactions, Angew. Chem., Int. Ed., 2019, 58, 946–956 CrossRef CAS PubMed.
- C. Wu, S. Wang, J. Zhao, Y. Liu, Y. Zheng, Y. Luo, C. Ye, M. Huang and H. Chen, Biodegradable Fe(III)@WS2-PVP nanocapsules for redox reaction and tme-enhanced nanocatalytic, photothermal, and chemotherapy, Adv. Funct. Mater., 2019, 29, 1901722 CrossRef.
- L. Wang, M. Huo, Y. Chen and J. Shi, Iron-engineered mesoporous silica nanocatalyst with biodegradable and catalytic framework for tumor-specific therapy, Biomaterials, 2018, 163, 1–13 CrossRef CAS PubMed.
- M. Wu, Y. Ding and L. Li, Recent progress in the augmentation of reactive species with nanoplatforms for cancer therapy, Nanoscale, 2019, 11, 19658–19683 RSC.
- L.-S. Lin, J. Song, L. Song, K. Ke, Y. Liu, Z. Zhou, Z. Shen, J. Li, Z. Yang, W. Tang, G. Niu, H.-H. Yang and X. Chen, Simultaneous fenton-like ion delivery and glutathione depletion by MnO2-based nanoagent to enhance chemodynamic therapy, Angew. Chem., Int. Ed., 2018, 57, 4902–4906 CrossRef CAS PubMed.
- Q. Chen, C. Liang, X. Sun, J. Chen, Z. Yang, H. Zhao, L. Feng and Z. Liu, H2O2-responsive liposomal nanoprobe for photoacoustic inflammation imaging and tumor theranostics via in vivo chromogenic assay, Proc. Natl. Acad. Sci., 2017, 114, 5343 CrossRef CAS PubMed.
- P. a. Ma, H. Xiao, C. Yu, J. Liu, Z. Cheng, H. Song, X. Zhang, C. Li, J. Wang, Z. Gu and J. Lin, Enhanced cisplatin chemotherapy by iron oxide nanocarrier-mediated generation of highly toxic reactive oxygen species, Nano Lett., 2017, 17, 928–937 CrossRef CAS PubMed.
- M. Huo, L. Wang, Y. Chen and J. Shi, Tumor-selective catalytic nanomedicine by nanocatalyst delivery, Nat. Commun., 2017, 8, 357 CrossRef PubMed.
- L.-H. Fu, C. Qi, Y.-R. Hu, J. Lin and P. Huang, Glucose oxidase-instructed multimodal synergistic cancer therapy, Adv. Mater., 2019, 31, 1808325 CrossRef PubMed.
- B. Wang, H. Zhang, J. An, Y. Zhang, L. Sun, Y. Jin, J. Shi, M. Li, H. Zhang and Z. Zhang, Sequential intercellular delivery nanosystem for enhancing ROS-Induced antitumor therapy, Nano Lett., 2019, 19, 3505–3518 CrossRef CAS PubMed.
- D. L. Klayman, Qinghaosu (Artemisinin): An antimalarial drug from china, Science, 1985, 228, 1049–1055 CrossRef CAS PubMed.
- Y. Tu, Artemisinin—a gift from traditional chinese medicine to the world (Nobel Lecture), Angew. Chem., Int. Ed., 2016, 55, 10210–10226 CrossRef CAS PubMed.
- A. K. Das, Anticancer effect of antimalarial artemisinin compounds, Ann. Med. Health Sci. Res., 2015, 5, 93–102 CrossRef CAS PubMed.
- A. Bhaw-Luximon and D. Jhurry, Artemisinin and its derivatives in cancer therapy: status of progress, mechanism of action, and future perspectives, Cancer Chemother. Pharmacol., 2017, 79, 451–466 CrossRef CAS PubMed.
- Y. Chen, X. Lin, H. Park and R. Greever, Study of artemisinin nanocapsules as anticancer drug delivery systems, Nanomedicine, 2009, 5, 316–322 CrossRef CAS PubMed.
- T. Efferth, A. Benakis, M. R. Romero, M. Tomicic, R. Rauh, D. Steinbach, R. Häfer, T. Stamminger, F. Oesch, B. Kaina and M. Marschall, Enhancement of cytotoxicity of artemisinins toward cancer cells by ferrous iron, Free Radicals Biol. Med., 2004, 37, 998–1009 CrossRef CAS PubMed.
- Y. K. Wong, C. Xu, K. A. Kalesh, Y. He, Q. Lin, W. S. F. Wong, H.-M. Shen and J. Wang, Artemisinin as an anticancer drug: recent advances in target profiling and mechanisms of action, Med. Res. Rev., 2017, 37, 1492–1517 CrossRef CAS PubMed.
- A. Shahbazfar, P. Zare, M. Ranjbaran, H. Tayefi-Nasrabadi, O. Fakhri, Y. Farshi, S. Shadi and A. Khoshkerdar, A survey on anticancer effects of artemisinin, iron, miconazole, and butyric acid on 5637 (bladder cancer) and 4T1 (Breast cancer) cell lines, J. Cancer Res. Ther., 2014, 10, 1057–1062 CrossRef PubMed.
- J. Fu and Y. Zhu, Lysosomes activating chain reactions against cancer cells with a pH-switched prodrug/procatalyst co-delivery nanosystem, J. Mater. Chem. B, 2017, 5, 996–1004 RSC.
- H. Zhang, Q. Chen, X. Zhang, X. Zhu, J. Chen, H. Zhang, L. Hou and Z. Zhang, An intelligent and tumor-responsive Fe2+ donor and Fe2+-dependent drugs cotransport system, ACS Appl. Mater. Interfaces, 2016, 8, 33484–33498 CrossRef CAS PubMed.
- Y. Wang, Y. Han, Y. Yang, J. Yang, X. Guo, J. Zhang, L. Pan, G. Xia and B. Chen, Effect of interaction of magnetic nanoparticles of Fe3O4 and artesunate on apoptosis of K562 cells, Int. J. Nanomed., 2011, 6, 1185–1192 CAS.
- Y. Ding, J. Wan, Z. Zhang, F. Wang, J. Guo and C. Wang, Localized Fe(II)-induced cytotoxic reactive oxygen species generating nanosystem for enhanced anticancer therapy, ACS Appl. Mater. Interfaces, 2018, 10, 4439–4449 CrossRef CAS PubMed.
- H. Zhang, H. Zhang, X. Zhu, X. Zhang, Q. Chen, J. Chen, L. Hou and Z. Zhang, Visible-light-sensitive titanium dioxide nanoplatform for tumor-responsive Fe2+ liberating and artemisinin delivery, Oncotarget, 2017, 8, 58738–58753 CrossRef PubMed.
- N. Shterman, B. Kupfer and C. Moroz, Comparison of transferrin receptors, iron content and isoferritin profile in normal and malignant human breast cell lines, Pathobiology, 1991, 59, 19–25 CrossRef CAS PubMed.
- J. Yu, F. Chen, W. Gao, Y. Ju, X. Chu, S. Che, F. Sheng and Y. Hou, Iron carbide nanoparticles: an innovative nanoplatform for biomedical applications, Nanoscale Horiz., 2017, 2, 81–88 RSC.
- V. Davydov, A. Rakhmanina, I. Kireev, I. Alieva, O. Zhironkina, O. Strelkova, V. Dianova, T. D. Samani, K. Mireles, L. H. Yahia, R. Uzbekov, V. Agafonov and V. Khabashesku, Solid state synthesis of carbon-encapsulated iron carbide nanoparticles and their interaction with living cells, J. Mater. Chem. B, 2014, 2, 4250–4261 RSC.
- C. Giordano, A. Kraupner, S. C. Wimbush and M. Antonietti, Iron carbide: an ancient advanced material, Small, 2010, 6, 1859–1862 CrossRef CAS PubMed.
- J. Yu, X. Chu and Y. Hou, Stimuli-responsive cancer therapy based on nanoparticles, Chem. Commun., 2014, 50, 11614–11630 RSC.
- J. Yu, C. Yang, J. Li, Y. Ding, L. Zhang, M. Z. Yousaf, J. Lin, R. Pang, L. Wei, L. Xu, F. Sheng, C. Li, G. Li, L. Zhao and Y. Hou, Multifunctional Fe5C2 nanoparticles: a targeted theranostic platform for magnetic resonance imaging and photoacoustic tomography-guided photothermal therapy, Adv. Mater., 2014, 26, 4114–4120 CrossRef CAS PubMed.
- J. Yu, Y. Ju, L. Zhao, X. Chu, W. Yang, Y. Tian, F. Sheng, J. Lin, F. Liu, Y. Dong and Y. Hou, Multistimuli-regulated photochemothermal cancer therapy remotely controlled via Fe5C2 nanoparticles, ACS Nano, 2016, 10, 159–169 CrossRef CAS PubMed.
- Y. Ju, H. Zhang, J. Yu, S. Tong, N. Tian, Z. Wang, X. Wang, X. Su, X. Chu, J. Lin, Y. Ding, G. Li, F. Sheng and Y. Hou, Monodisperse Au–Fe2C janus nanoparticles: an attractive multifunctional material for triple-modal imaging-guided tumor photothermal therapy, ACS Nano, 2017, 11, 9239–9248 CrossRef CAS PubMed.
- J. Yu, F. Zhao, W. Gao, X. Yang, Y. Ju, L. Zhao, W. Guo, J. Xie, X.-j. Liang, X. Tao, J. Li, Y. Ying, W. Li, J. Zheng, L. Qiao, S. Xiong, X. Mou, S. Che and Y. Hou, Magnetic Reactive oxygen species nanoreactor for switchable magnetic resonance imaging guided cancer therapy based on pH-sensitive Fe5C2@Fe3O4 nanoparticles, ACS Nano, 2019, 13, 10002–10014 CrossRef CAS PubMed.
- Z. Yang, T. Zhao, X. Huang, X. Chu, T. Tang, Y. Ju, Q. Wang, Y. Hou and S. Gao, Modulating the phases of iron carbide nanoparticles: from a perspective of interfering with the carbon penetration of Fe@Fe3O4 by selectively adsorbed halide ions, Chem. Sci., 2017, 8, 473–481 RSC.
- C. Zhang, W. Bu, D. Ni, S. Zhang, Q. Li, Z. Yao, J. Zhang, H. Yao, Z. Wang and J. Shi, Synthesis of iron nanometallic glasses and their application in cancer therapy by a localized fenton reaction, Angew. Chem., Int. Ed., 2016, 55, 2101–2106 CrossRef CAS PubMed.
- X. Chen, H. Zhang, M. Zhang, P. Zhao, R. Song, T. Gong, Y. Liu, X. He, K. Zhao and W. Bu, Amorphous Fe-based nanoagents for self-enhanced chemodynamic therapy by re-establishing tumor acidosis, Adv. Funct. Mater., 2019, 30, 1908365 CrossRef.
- A. Lawal, R. A. Umar, M. G. Abubakar, U. Z. Faruk and U. Wali, FTIR and UV-Visible spectrophotometeric analyses of artemisinin and its derivatives, J. Pharm. Biomed. Sci., 2012, 24, 6–14 Search PubMed.
- M. A. Kohanski, D. J. Dwyer, B. Hayete, C. A. Lawrence and J. J. Collins, A common mechanism of cellular death induced by bactericidal antibiotics, Cell, 2007, 130, 797–810 CrossRef CAS PubMed.
- N. C. Yip, I. S. Fombon, P. Liu, S. Brown, V. Kannappan, A. L. Armesilla, B. Xu, J. Cassidy, J. L. Darling and W. Wang, Disulfiram modulated ROS-MAPK and NFκB pathways and targeted breast cancer cells with cancer stem cell-like properties, Br. J. Cancer, 2011, 104, 1564 CrossRef CAS PubMed.
- P. Liu, S. Brown, P. Channathodiyil, V. Kannappan, A. L. Armesilla, J. L. Darling and W. Wang, Cytotoxic effect of disulfiram/copper on human glioblastoma cell lines and ALDH-positive cancer-stem-like cells, Br. J. Cancer, 2013, 108, 9940–9942 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Figures and discussions. See DOI: 10.1039/d1ra04975e |
| ‡ These authors contributed equally to this manuscript. |
| This journal is © The Royal Society of Chemistry 2021 |