Open Access Article
Open Access ArticleFerrocene-based conjugated microporous polymer and its multiwalled carbon nanotube composite for direct photocatalytic benzene hydroxylation to phenol
Zhongpeng Zhu†
 a,
Long Pan†b,
Zilu Liub,
Jie Zhao*a,
Zhiping Tao*a and
Yujian Heb
a,
Long Pan†b,
Zilu Liub,
Jie Zhao*a,
Zhiping Tao*a and
Yujian Heb
aResearch Institute of Petroleum Processing, Sinopec, Beijing 100083, P. R. China. E-mail: taozp.ripp@sinopec.com; zhaojie.ripp@sinopec.com
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 12th October 2021
Abstract
Ferrocene is used as a catalytically active site and building block to construct a new conjugated microporous polymer (CMP), named Fc-POP. A corresponding carbon nanotube composite (CNTs@Fc-POP) with tubular structure was obtained through the π–π interaction between multi-walled carbon nanotubes (MWCNTs) and reactive molecules. This innovative modification method of carbon nanotubes provides a way to construct functionalized carbon materials. The two materials can achieve high conversion and selectivity of benzene hydroxylation to phenol under light irradiation using hydrogen peroxide (H2O2) as an oxidant. Due to the synergistic effect between the carbon nanotubes and the ferrocene group, the incorporation of MWCNTs can improve the yield of phenol significantly. This work explores a new photocatalystic system and expands the related photocatalytic application of CNTs.
Introduction
Phenol is an important chemical in industrial manufacturing, and has been applied in the production of plastics, fibers, medicines, rubber, dyes, etc.1 Industrially, phenol is produced through the well-known three-step cumene process,2 in which hazardous cumene peroxide and an almost equimolar amount of acetone are formed. Due to the low atom economy of the process, harsh experimental conditions and expensive equipment, direct oxidative hydroxylation of benzene to phenol using environmentally benign oxidants such as O2,3,4 NO2 (ref. 5 and 6) and H2O2 (ref. 7) has received extensive research attention. The employment of H2O2 has achieved a green process, with water as the only byproduct. However, it is challenging to introduce the hydroxyl group directly onto the benzene ring. Due to the low reactivity of the sp2 C–H bond, although almost all of the developed catalytic systems require elevated temperatures, the conversion efficiencies are still low. Another existing problem is that it is difficult to selectively oxidize benzene instead of phenol after generating more active phenol.8 One promising way to overcome the challenges is to utilize sunlight.9 Recently, several photocatalytic systems have been developed, including homogeneous,10 and metal-doped heterogeneous catalysis for the selective hydroxylation of benzene to phenol.11–13 However, the performance of the current developed photocatalytic systems are unsatisfactory. For the homogeneous catalysts, they cannot be separated from the system themselves. For the metal-doped heterogeneous catalysis, the active species can be leached from the catalysts. The most critical issue is low benzene conversion and poor phenol selectivity. Therefore, there is urgent need to develop new photocatalysts with high efficiency and selectivity.Inorganic transition-metal compounds, such as Fe, Cu, and V are the most actively studied transition metals for benzene hydroxylation.14,15 As an organometallic compound, ferrocene has good optical, electric and magnetic properties and redox reversal. Thus, it is widely used in the fields of catalysis, conductors and biology.16 Compared with inorganic iron ions, ferrocene has rarely been explored in the research of catalyzing direct oxidative hydroxylation reactions.
It was previously reported that the composites of carbon nanotubes (CNTs) and TiO2 exhibited higher photocatalytic performance than TiO2 alone.17,18 Similar to the metals mentioned above, CNTs can also exhibit metal conductivity as one of potential electronic structures. The large electron-storage capacity of CNTs has the ability to accept photon-excited electrons in mixture with titania dioxide or nanocomposites, delaying or hindering recombination.19 According to this research, CNTs enhance the photoactivity in three different ways (1) act as a sink for electrons to enhance the lifetime of separated charges; (2) serve as a surface impurity incorporation, creating band gap energy states and (3) formation of Ti–O–C or Ti–C defect sites that can absorb visible light and photocatalysis.20 Hitherto, researches has mainly focused on the CNTs and TiO2 systems. It is necessary to explore new photocatalysis system containing CNTs and expand relevant photocatalytic applications of CNTs. Another unprecedented property of CNTs produces by the fully-sp2-hybridization and conjugated π-electron system is chemical inertness.21 It should be noted that chemical inertness does not always contribute to the preparation of these CNTs materials. Due to processability and dispersibility, there are still challenges in various applications, particularly energy-related applications, catalyst supports, and reinforcement of composites.22 The covalent modification of the graphitic materials is undoubtedly a key to addressing these issues. Many approaches have been utilized for the covalent functionalization of graphitic materials.23–25 These approaches include the use of radical species, such as aryl intermediates, or oxidization, to react with the surface of CNT or graphene. In almost all instances, the reaction sites can become rehybridized from sp2 carbon to sp3 carbon atoms. This alters the exceptional physical properties of the graphitic materials.26,27 Therefore, a new method is expected to be discovered to disperse the CNT well, but with little or no structural damage to the CNT.
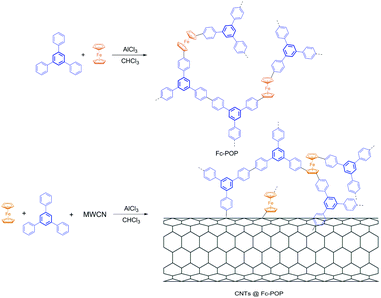
Herein, we synthesized a conjugated microporous polymer (Fc-POP) which is based on the Scholl reaction, using ferrocene and 1,3,5-triphenylbenzene as building blocks. In order to obtain higher photocatalytic efficiency and selectivity of benzene hydroxylation, the CNTs were decorated with the Fc-POP polymer to obtain the CNTs@Fc-POP composites. Additional insight into the photocatalytic benzene hydroxylation to phenol with H2O2 over the Fc-POP and CNTs@Fc-POP has been obtained to prove that the incorporation of CNTs can increase the conversion and selectivity of phenol.
Experimental
Materials and methods
Ferrocene, AlCl3 and 1,3,5-triphenylbenzene (sym-PhPh3) were commercially available and purchased from Alfa Aesar. The multi-walled carbon nanotubes (MWCNT) were purchased from Heng Qiu Science and Technology Ltd. The length of the MWCNT is 3–12 μm and the diameter is 8–15 nm. All the reagents were analytical grade and used without further purification.Synthesis of TPB
sym-PhPh3 (1.44 g, 5 mmol), chloroform (15 mL) and AlCl3 (4.82 g, 30 mmol) were sequentially added to a 50 mL round bottom flask. The resultant mixture was stirred under N2 atmosphere for 15 min and then refluxed for 48 h. After the mixture cooled to room temperature, methanol (30 mL) was added to stir for another 1 h. The resulting precipitate was collected by filtration and the solid was washed three times with water, ethanol and tetrahydrofuran (THF). The powder was abstracted with alcohol in a Soxhlet extractor for 24 h and dried in a vacuum oven at 75 °C for 12 h to get final product (pale brown powder, 99% yield).Synthesis of Fc-POP
sym-PhPh3 (0.918 g, 3 mmol), ferrocene (n(sym-PhPh3)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(Fc) = 3
n(Fc) = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, or 3
1, or 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) chloroform (15 mL) and AlCl3 (4.82 g, 30 mmol) were sequentially added to a 50 mL round bottom flask. The resultant mixture was stirred under N2 atmosphere for 15 min and then refluxed for 48 h. After the mixture cooled to room temperature, methanol (50 mL) was added to stir for another 1 h. The resulting precipitate was filtered and washed three times with HCl–H2O (v/v = 2
2) chloroform (15 mL) and AlCl3 (4.82 g, 30 mmol) were sequentially added to a 50 mL round bottom flask. The resultant mixture was stirred under N2 atmosphere for 15 min and then refluxed for 48 h. After the mixture cooled to room temperature, methanol (50 mL) was added to stir for another 1 h. The resulting precipitate was filtered and washed three times with HCl–H2O (v/v = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), ethanol and THF. The powder was further abstracted with alcohol in a Soxhlet extractor for 24 h and then dried in a vacuum oven at 75 °C for 12 h to get final product (dark black powder, 75% yield).
1), ethanol and THF. The powder was further abstracted with alcohol in a Soxhlet extractor for 24 h and then dried in a vacuum oven at 75 °C for 12 h to get final product (dark black powder, 75% yield).
Synthesis of Fc-CNT-POP
sym-PhPh3 (0.918 g, 3 mmol), multi-wall nanotubes (0.2 g), 25 mL of chloroform, and ferrocene (0.372 g, 2 mmol) were sequentially added to a 50 mL round bottom flask. The resultant mixture was stirred under N2 atmosphere for 2 min before AlCl3 (4.82 g, 30 mmol) was added to it, and then refluxed for 48 h. After cooling to room temperature, 50 mL of methanol was added into the mixture to stir for 1 h. After filtration and washing three times with HCl–H2O (v/v = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), ethanol and THF, the obtained powder was abstracted with alcohol in a Soxhlet extractor for 24 h. The final solid was dried in a vacuum oven at 75 °C for 12 h (dark black powder, 99% yield).
1), ethanol and THF, the obtained powder was abstracted with alcohol in a Soxhlet extractor for 24 h. The final solid was dried in a vacuum oven at 75 °C for 12 h (dark black powder, 99% yield).
Instrumental characterization and structural analysis
Solid-state cross-polarization magic angle spinning (CP/MAS) NMR spectra of the polymers and composite were obtained on a Bruker Avance III 400 Nuclear Magnetic Resonance (NMR) spectrometer (Bruker Daltonics Inc., Switzerland). The infrared (IR) spectra were obtained from a Fourier transform infrared (FTIR) spectrometer (PerkinElmer, USA). Transmission Electron Microscope (TEM) observations were obtained using a Tecnai G2 20 S-TWIN microscope (FEI, USA) at the accelerating voltage of 200 kV. Nitrogen sorption–desorption isotherm was measured at 77 K using a Micromeritics 2020M + C system after the sample was degassed at 120 °C overnight and the surface areas were determined by Brunauer–Emmett–Teller(BET) method. (Micromeritics Instrument Corporation, USA). The Pore Size Distribution (PSD) was calculated by Non-local density functional theory (NLDFT). Electron paramagnetic resonance (EPR) measurements were carried out on a Bruker model A300 spectrometer with a 300 W Xenon lamp. All Gas Chromatography (GC) experiments were carried out and recorded with a SHIMADZU GC-2010 with flame ionization detector (FID) detector.Photocatalytic reaction
The general experimental procedure for the hydroxylation of benzene was carried out as following and detailed conditions are presented in Table 2. Benzene (0.2 mL) and photocatalyst (50 mg) were added in a mixture of acetonitrile (4 mL) and water (4 mL). After ultrasonic treatment for 15 min, the catalyst was dispersed evenly. Then hydrogen peroxide (30 wt%, 0.8 mL, 8 mmol) was slowly added into the stirring mixture, following by irradiation for 4 h with a 300 W Xenon light. The reaction was quenched with 9.6 mL of ethanol and the heterogenous catalyst was separated by filter membrane. GC was employed to qualitatively analyze the filtrate and standard chemicals were used to determine the conversion and selectivity of phenols.Results and discussion
Referring to the previous research from Tan et al.,28 the conjugated microporous polymers (Fc-POP) were synthesized though the Scholl reaction which involved the coupling reaction between two aromatic molecules and accompanied by the formation of a new aryl–aryl (as shown in Scheme 1). Acting as light backbones, 1,3,5-triphenylbenzene guarantees the porosity and specific surface area whilst its fluorescence enhances the photo-catalytic properties of the polymers. Ferrocene acts as active site in the reaction of benzene hydroxylation to phenol. Different from the physical incorporation heterogeneous catalysis, the active species were uniformly distributed and connected to the skeleton by covalent bond that can avoid leaching and losing themselves.In order to synthesize Fc-POP with different ferrocene contents, different molar ratio of sym-PhPh3 and ferrocene: n(sym-PhPh3)/n(ferrocene) = 3/1, 2/1 or 3/2 was added before the reaction started and the final polymers were named after FC-POP1, FC-POP2 and FC-POP3 respectively. All obtained polymers are chemically stable, even when exposed to HCl or NaOH solution.
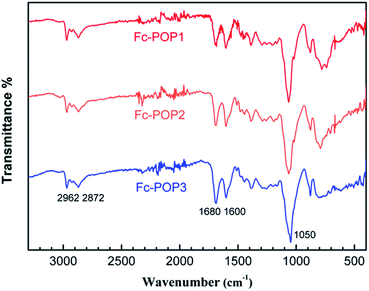
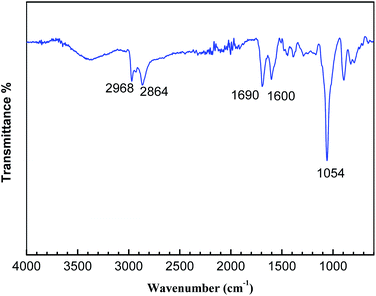
The structures of these polymers were confirmed at molecular level by a FTIR spectrometer and 13C CP/MAS NMR spectrometer. A comparison of FTIR spectra of the polymers is shown in Fig. 1. The weak peaks near 3000 cm−1 are attributed to C–H stretching in the aromatic rings; peaks near 1650 cm−1, 1600 cm−1 and 1450 cm−1 are attributed to the benzene ring skeleton vibrations; the peak at 1050 cm−1 is attributed to the cyclopentadiene rings skeleton vibrations. It can be inferred from the spectra that the conjugated microporous polymers (Fc-POP) were successfully synthesized and they are consistent with the proposed structure. However, the same wave numbers and transmittance of characteristic peaks can be found in the three FT-IR spectra of Fc-POP1–3. It indicates that the structure and ferrocene content of these three polymers are almost same. If ferrocene was incorporated with various ratios, the peak intensity of benzene rings and cyclopentadienyl rings should differ from each other.
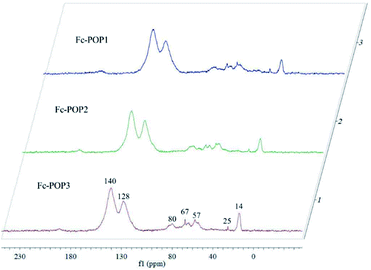
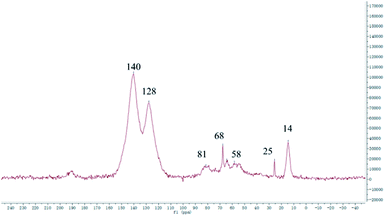
The 13C CP/MAS NMR spectra of the three polymers are shown in Fig. 2. These polymers show the almost the same spectra. The peaks at 140 and 128 ppm are ascribed to the coupling of benzene carbons and noncoupling benzene carbons respectively. While the peaks at 80 and 67 ppm are corresponded to the coupling of ferrocene carbons and noncoupling ferrocene carbons respectively. The peaks around 14 and 57 ppm of all samples are assigned to the carbons of remaining ethanol adsorbed in the micropores or ultra-micropores, which is consistent with published reports.28 As the micropores or ultra-micropores are very small, there is usually some solvent molecules left in the materials, which is hard to completely remove even after drying in a vacuum oven.29 In all the three 13C CP/MAS NMR spectra, the peaks intensities at 80 and 67 ppm are low and almost the same. The result are consistent with the FTIR spectra, indicating that the three polymers contain the same amount of incorporated ferrocene. Our initial goal to obtain different ferrocene-incorporated conjugated microporous polymers was not realized, which might be due to the lower reactivity of ferrocene in the Scholl reaction. Regardless of the amount of ferrocene added, only a small certain portion was incorporated into the polymers.
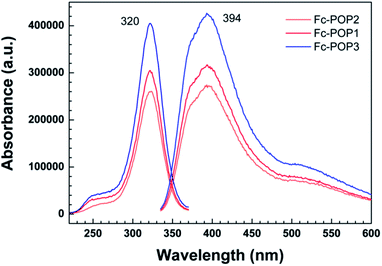
The excitation and emission spectra of the three materials (Fc-POP1, Fc-POP2 and Fc-POP3) were tested, as shown in Fig. 3. It can be seen that the three polymers have the same maximum excitation and occurrence wavelength, but the intensity is slightly different. Their maximum excitation wavelength is about 320 nm and the maximum emission wavelength is about 390 nm, proving that the material we obtained is a fluorescent solid material, which plays an important role in direct photocatalytic benzene hydroxylation to phenol.
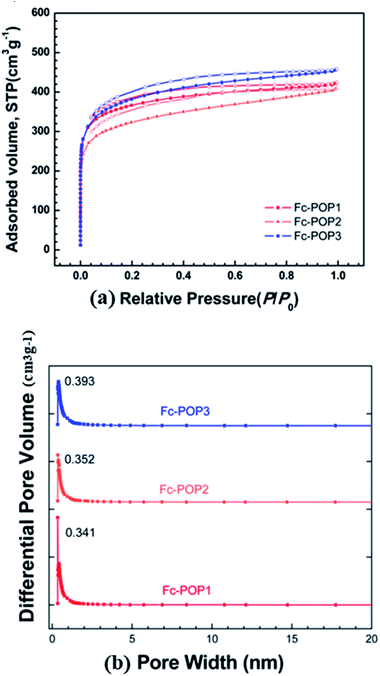
The porosity of the Fc-POPs was also analyzed by N2 sorption analysis. The nitrogen adsorption–desorption isotherms of the three polymers at 77 K are displayed in Fig. 4 and the characterization data such as the surface area and pore volume are summarized in Table 1. According to the IUPAC classification, the adsorption–desorption isotherms are all categorized into type I. In low pressure range (P/P0 < 0.001), the isotherms show an obvious rising trend, indicating an extensive micropores structure. Additionally, hysteresis loops were observed in the entire relative pressure range extending to the lowest attainable pressures, which is due to the irreversible uptake of gas molecules in the pores (or through pore entrances) with the same diameter as that of N2 molecule.30 Using the five adsorption points in 0.01–0.1 relative pressure, the BET specific surface areas of Fc-POP1–3 were measured to be 1320, 1200 and 1360 m2 g−1 respectively, showing very little difference. This further demonstrates the conclusion from IR and NMR that almost the same amount of ferrocene was incorporated in these three materials. The pore distribution (Fig. 4) of Fc-POP1–3 indicates that they possess ultra-micropores (smaller than 0.7 nm). Compared to the previously reported MOPs, Fc-POP1–3 possess smaller pores in the micropore region, with a relatively narrow pore size distribution and no obvious peaks beyond 2 nm in the pore size distribution.
| Polymers | SBETa (m2 g−1) | Smicrob (m2 g−1) | Vtotalc (cm3 g−1) | Dpored (nm) |
|---|---|---|---|---|
| a Specific surface area calculated from the nitrogen adsorption isotherm using the BET method.b Micropore surface area calculated from the adsorption branch of the nitrogen adsorption–desorption isotherm using the t-plot method.c Total pore volume at P/P0 = 0.99.d Data calculated from nitrogen adsorption isotherms with the NLDFT method. | ||||
| Fc-POP1 | 1326 | 1153 | 0.64 | 0.393 |
| Fc-POP2 | 1164 | 937 | 0.62 | 0.352 |
| Fc-POP3 | 1361 | 1130 | 0.70 | 0.341 |
| Entry | Catalyst (x mg) | Light source | Solvent | Vben (mL) | Yield (%) |
|---|---|---|---|---|---|
| 1 | Fc-POP3 (25) | Xe lamp | Acetonitrile | 0.8 | 2.7 |
| 2 | Fc-POP3 (25) | Dark | Acetonitrile | 0.8 | 0.3 |
| 3 | TPB (25) | Xe lamp | Acetonitrile | 0.8 | — |
| 4 | Fc-POP3 (25) | Xe lamp | Mixture | 0.8 | 5.2 |
| 5 | Fc-POP3 (50) | Xe lamp | Mixture | 0.1 | 9.4 |
| 6 | Fc-POP3 (50) | Xe lamp | Mixture | 0.4 | 13.1 |
| 7 | Fc-POP3 (50) | Xe lamp | Mixture | 0.8 | 18.1 |
Considering the highest specific surface area and best fluorescence, Fc-POP3 was chose to investigate the catalytic activity for the benzene hydroxylation reaction. Using H2O2 (H2O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) benzene ratio of 3
benzene ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) as cocatalyst and oxidant, xenon light as the photon source, the benzene hydroxylation reaction was initially conducted in acetonitrile (CH3CN). It was found that Fc-POP3 can effectively hydroxylate benzene to phenol under this condition. As shown in Table 2, entries 2 and 3, the control experiments without ferrocene or light irradiation achieved very low or even undetectable products, indicating the light irradiation and ferrocene are essential for the reaction and the hydroxylation of benzene to phenol over Fc-POP3.
4) as cocatalyst and oxidant, xenon light as the photon source, the benzene hydroxylation reaction was initially conducted in acetonitrile (CH3CN). It was found that Fc-POP3 can effectively hydroxylate benzene to phenol under this condition. As shown in Table 2, entries 2 and 3, the control experiments without ferrocene or light irradiation achieved very low or even undetectable products, indicating the light irradiation and ferrocene are essential for the reaction and the hydroxylation of benzene to phenol over Fc-POP3.
Solvent plays an important role in this reaction. According to the research about the solvents investigated, a CH3CN/H2O mixed solvent 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) gave the best performance by showing the highest benzene conversion ratio.31 So the mixture solvent was used in our catalytic system to find out the effect on the reaction. Comparing entries 1 and 4 in Table 2, the reaction conducted in the mixed solvent is more effective in benzene hydroxylation, and the phenol production (5.2%) is almost twice than that in bare CH3CN (2.7%). After further optimization of the benzene concentration and catalyst loading, the best yield of phenol can reach 18.1%. All the yield of phenol is calculated by using GC.
1 (v/v) gave the best performance by showing the highest benzene conversion ratio.31 So the mixture solvent was used in our catalytic system to find out the effect on the reaction. Comparing entries 1 and 4 in Table 2, the reaction conducted in the mixed solvent is more effective in benzene hydroxylation, and the phenol production (5.2%) is almost twice than that in bare CH3CN (2.7%). After further optimization of the benzene concentration and catalyst loading, the best yield of phenol can reach 18.1%. All the yield of phenol is calculated by using GC.
Recently, carbon nanotubes were introduced to TiO2 photocatalysts and the resultant composite catalyst showed very good activity on oxidation of organic compounds. It was the synergistic effect between TiO2 and carbon nanotubes that promoted the transmittance of photo-generated electrons from the former to the latter, consequently prolonging the life of photo-generated electron–hole pairs and granting the composite catalyst the ability to absorb visible light. In order to find out whether the CNTs possess the similar synergistic effect in our catalytic system and whether it can enhance the product yield, we tried to combine our porous Fc-POP with CNTs. The defect/edge-selective functionalization protocol is an attractive and versatile alternative used in various nanocarbon functionalization strategies.32 Compared with other functionalization systems, defect/edge-selective functionalization is benign enough not to damage graphitic frameworks. Thus, it allows preservation of the intrinsic properties of graphitic materials. Friedel–Crafts acylation reaction has been used for the defect/edge-selective functionalization of carbon materials.33 Considering the similar reaction mechanism and condition between the Friedel–Crafts acylation reaction and Scholl reaction, we tried to modify the Fc-POP into WMCNTs through the defect/edge-selective functionalization method.
The composite of Fc-POP and MWCNTs (CNTs@Fc-POP) was successfully synthesized just via adding some MWCNTs into the ferrocene and sym-PhPh3 solution and stirring several hours before the polymerization reaction. The FT-IR spectrum and 13C CP/MAS NMR spectrum of CNTs@Fc-POP are shown in Fig. 5 and Fig. 6. Due to the π–π interaction between the MWCNTs and reactive molecules (ferrocene and sym-PhPh3), these molecules stack on the surface of the MWCNTs and can react with the defect/edge sites (mostly sp2 C–H) of MWCNTs, the polymer shell will be fabricated and coated with the MWCNTs after the polymerization reaction.
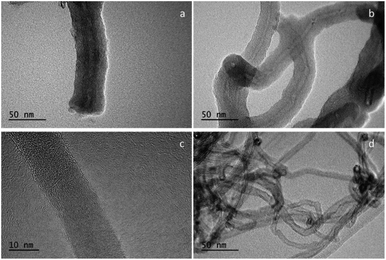
From the representative TEM images of CNTs@Fc-POP and MWCNTs shown in Fig. 7, it can be seen that the obtained composite which has one-dimension tubular structure. The MWCNTs with about 12 nm mean diameter are individually coated with the uniform gray polymer shell with a thickness of about 10 nm. This demonstrates the successful synthesis of a novel MWCNTs composite through a facile method.
The nitrogen adsorption–desorption isotherms of CNTs@Fc-POP at 77 K are displayed in Fig. 8. Compared with the isotherms of Fc-POPs with almost no change of adsorption quantity in the media and high pressure range, there is an obvious increase of CNTs@Fc-POP isotherms in this range. Fc-POPs possess a relatively narrow pore size distribution and no obvious peaks beyond 2 nm in the pore size distribution, while the micropores specific surface area of CNTs@Fc-POP is 580 m2 g−1, 60% of the total. Because of the combination of MWCNTs, the rest 40% was attributed to meso- and macro-pores. For heterogeneous catalysts, porosity is beneficial to the catalytic process: a large specific surface area can help the full mixing of reactants and catalytic sites. Apart from this, meso- and macro-pores accelerate the mass transfer rates of reactants and products.
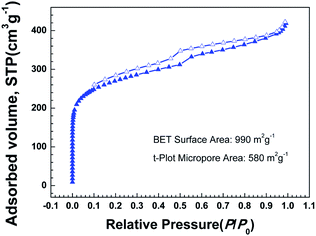 | ||
| Fig. 8 Nitrogen adsorption–desorption isotherms of CNTs@Fc-POP measured at 77 K, the adsorption and desorption branches are labeled with solid and open symbols, respectively. | ||
The catalytic activity of the CNTs@Fc-POP in hydroxylation of benzene was investigated under the same conditions as Fc-POP (Table 3). The highest yield was improved to 25.3%. We also examined the recyclability of the heterogeneous photocatalysts. The catalysts were recycled by centrifugation. The recovered catalyst was repeatedly used after being dried overnight in vacuum, and no obviously deterioration of conversion is determinated for the reaction after three times of benzene hydroxylation.
| Entry | Catalyst (x mg) | Light source | Solvent | Vben (mL) | Yield (%) |
|---|---|---|---|---|---|
| 1 | Fc-POP3 (50) | Xe lamp | Mixture | 0.8 | 18.1 |
| 2 | CNTs@Fc-POP (50) | Xe lamp | Mixture | 0.8 | 25.3 |
| 3 | Recycle-1 | Xe lamp | Mixture | 0.8 | 24.2 |
| 4 | Recycle-2 | Xe lamp | Mixture | 0.8 | 23.8 |
| 5 | Recycle-3 | Xe lamp | Mixture | 0.8 | 24.1 |
| 6 | CNTs@Fc-POP (50) | Dark | Mixture | 0.8 | 0.2 |
The chemical stability of photocatalyst was evaluated after long term test. The catalytic activity of the CNTs@Fc-POP in hydroxylation of benzene was investigated over 26 h. The best yield of phenol can reach 25.3% at 4 h, while the yield was decreased after 4 h due to the increase of byproducts. The TEM and ICP were used to characterize the chemical stability of CNTs@FC-POP after 26 h. It was observed that the MWCNTs are still coated with the uniform polymer shell with a thickness of about 10 nm after the long term reaction. The structure was not damaged, but the surface becomes a little irregular. The material CNTs@Fc-POP is also characterized via ICP-OES after reaction. It has been observed that the content of Fe is 4.8% after a long term reaction, while the content is 4.9% before reaction, meaning that the catalyst is stable enough over a long periods of time.
The excellent performance was attributed to the following reasons: (1) carbon nanotubes have a large amount of unpaired electrons on the wall which make carbon nanotubes have the properties of both metals and semi-conductors and thus promote the surface photocatalysis which is governed by electron transmission; (2) carbon nanotubes have good capacity to store electrons and can receive the photo-excited electrons from polymer shell and inhibit the combination of photogenerated electron–hole pairs; (3) the addition of carbon nanotubes introduced meso-pores and macro-pores, which accelerate the mass transfer rates of reactants and products. Compared with other reported heterogeneous catalysts using Fe as active site (as shown in Table 4), our catalysts performed the highest yield. Furthermore, they have the advantages of simple synthesis, mild reaction conditions and cheap starting materials.
| Materials | SBETa (m2 g−1) | Yield of phenol (%) | Ref. |
|---|---|---|---|
| a Specific surface area calculated from the nitrogen adsorption isotherm using the BET method. | |||
| CNTs@Fc-POP | 990 | 25 | This work |
| 5.0Fe/NACH-600N | 600 | 20 | 34 |
| AlFePO (0.02)450 | 248 | 22 | 35 |
| SBA-15-N = ferrocene | 658 | 15 | 16 |
| Fe–CN/TS-1 | 266 | 10 | 36 |
| Fe/MWCNTs | 216 | 11 | 37 |
| MIL-100 (Fe) | — | 22 | 38 |
| Fc-MCN | 51 | 14 | 39 |
| Fe-g-C3N4/SBA-15 | 451 | 12 | 40 |
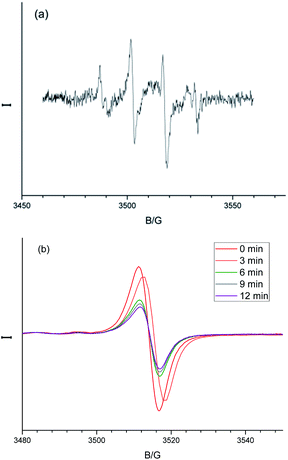
To further understand the catalysed benzene oxidation, we moved forward the mechanism investigation. The benzene hydroxylation to produce phenol by H2O2 is generally believed to proceed via an oxygenation pathway induced by the in situ-formed ·OH. When ethanol (1 mL), a ·OH radical scavenger, was added into the oxidation of benzene, no oxidation occurred anymore, indicating that the oxidation may follow a pathway through the ·OH radical-induced benzene hydroxylation. Moreover, when the photolysis of H2O2 was performed in the presence of the CNTs@Fc-POP composite, the formation of ·OH was confirmed by electron spin resonance (ESR) and DMPO (5,5-dimethyl-1-pyrrolineN-oxide) spin-trapping technique, as the signal for the DMPO-·OH additive was observed on the ESR spectra (Fig. 9a).
In most case, the ·OH radicals are generated under Fenton like conditions (Fe2+ + H2O2 → Fe3+ + ·OH + OH−) and the regeneration of Fe2+ (by reduction of Fe3+ to Fe2+) is the key step in the catalytic cycle. It is reasonable to consider that the electrons from the MWCNTs of CNTs@Fc-POP can possibly reduce the Fe3+ to Fe2+ for H2O2 activation. To test this hypothesis, we used the ESR measurements to study the reduction process. Only Fe3+ with 3d5 structure affords a single peak signal on the spectra, while Fe2+ shows no clear response in the ESR spectra. So the peak intensity of the ESR signal is proportional to the concentration of unpaired electrons (Fe3+) in the sample and the decreasing intensity can be used to value the Fe3+ concentration change. Interestingly, when testing CNTs@Fc-POP under light illumination, a gradual decrease of Fe3+ signal was observed over time, which directly proves that the photo-excited electrons can reduce the Fe3+ to Fe2+ in the system (Fig. 9b).
P. W. Cyr41,42 reported that irradiation of thin films of poly (ferrocenylmethylphenylsilane) ([Fe(η5-C5H4)2SiMePh]n) cast from chloroform solution with UV light leads to photooxidation of ferrocene centers in the polymer main chain, indicating that the ferrocene is photo-active. The control experiments were also conducted using the ferrocene as catalyst in dark and under illumination conditions respectively. The yield of phenol is only 0.3% in dark condition while the yield can be 8.2% under illumination condition, also proving the photo-active role of ferrocene.
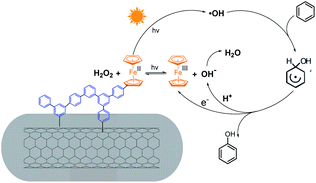
On the basis of the observations described above, the mechanism for photocatalytic benzene hydroxylation to phenol over CNTs@Fc-POP was proposed (Scheme 2). The light-trigged catalytic cycle starts with the reaction of Fc(II) and H2O2 to produce a hydroxyl radical (·OH), OH− and Fc(III) (Fe2+ + H2O2 → Fe3+ + ·OH + OH−). The ·OH radical attacks the aromatic ring, followed by dimerization of the resulting hydroxycyclohexadienyl radicals. Subsequently, the hydroxycyclohexadienyl radicals donate an electron to Fc(III) to achieve regeneration of Fc(II) and yield phenol, while releasing H+ to react with OH− to produce H2O. Then, the regenerated Fc(II) reacts with H2O2 to restart the catalytic cycle.
Conclusions
By using ferrocene and sym-Ph3Ph as building blocks, we synthesized ferrocene-incorporated conjugated micropores polymer (Fc-POP). To increase its photo-catalytic properties, we synthesized novel carbon-organic composite CNTs@Fc-POP. These two kinds of materials can be used as heterogeneous catalysts, and their catalytic activity was studied within the context of hydroxylation of benzene to phenol. Both of them show very better activities than reported catalysts, and the yields are 18.1% and 25.3%, respectively.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The financial support of National Natural Science Foundation of China (Grant No. 21272263), National key research and development plan (2016YFF0203700) is acknowledged.References
- T. Dong, J. Li, F. Huang, L. Wang, J. Tu, Y. Torimoto, M. Sadakata and Q. X. Li, Chem. Commun., 2005, 21, 2724 RSC.
- H. Hock and S. Lang, Ber. Dtsch. Chem. Ges., 1944, 77, 257 CrossRef.
- Y.-Y. Gu, X.-H. Zhao, G.-R. Zhang, H.-M. Ding and Y.-K. Shan, Appl. Catal., A, 2007, 328, 150 CrossRef CAS.
- bS. S. Acharyya, S. Ghosh, R. Tiwari, C. Pendem, T. Sasaki and R. Bal, ACS Catal., 2015, 5, 2850 CrossRef.
- I. Yuranov, D. A. Bulushev, A. Renken and L. Kiwi-Minsker, Appl. Catal., A, 2007, 319, 128 CrossRef CAS.
- Y. Li, Z. Feng, R. A. van Santen, E. J. M. Hensen and C. Li, J. Catal., 2008, 255, 190 CrossRef CAS.
- B. Guo, L. Zhu, X. Hu, Q. Zhang, D. Tong, G. Li and C. Hu, Catal. Sci. Technol., 2011, 1, 1060 RSC.
- C. I. Herrerias, X. Yao, Z. Li and C.-J. Li, Chem. Rev., 2007, 107, 2546 CrossRef CAS PubMed.
- H. Tong, S. Ouyang, Y. Bi, N. Umezawa, M. Oshikiri and J. Ye, Adv. Mater., 2012, 24, 229 CrossRef CAS PubMed.
- P. T. Tanev, M. Chibwe and T. J. Pinnavaia, Nature, 1994, 368, 321 CrossRef CAS PubMed.
- K. Ohkubo, T. Kobayashi and S. Fukuzumi, Angew. Chem., Int. Ed., 2011, 50, 8652 CrossRef CAS PubMed.
- K. Ohkubo, A. Fujimoto and S. Fukuzumi, J. Am. Chem. Soc., 2013, 135, 5368 CrossRef CAS PubMed.
- P. Devaraji, N. K. Sathu and C. S. Gopinath, ACS Catal., 2014, 4, 2844 CrossRef CAS.
- G. Tanarungsun, W. Kiatkittipong, P. Praserthdam, H. Yamada, T. Tagawa and S. Assabumrungrat, J. Ind. Eng. Chem., 2008, 14, 596 CrossRef CAS.
- J. S. Choi, T. H. Kim, K. Y. Choo, J. S. Sung, M. B. Saidutta, S. D. Song and Y. W. Rhee, J. Porous Mater., 2005, 12, 301 CrossRef CAS.
- D. R. Burri, I. R. Shaikh, K.-M. Choi and S.-E. Park, Catal. Commun., 2007, 8, 731 CrossRef CAS.
- Y. Zhang, Z. R. Tang, X. Fu and Y. J. Xu, ACS Nano, 2010, 4, 7303 CrossRef CAS PubMed.
- B. K. Vijayan, N. M. Dimitrijevic, D. Finkelstein-Shapiro, J. Wu and K. A. Gray, ACS Catal., 2012, 2, 223 CrossRef CAS.
- A. Kongkanand and P. V. Kamat, ACS Nano, 2007, 1, 13 CrossRef CAS PubMed.
- K. Woan, G. Pyrgiotakis and W. Sigmund, Adv. Mater., 2009, 21, 2233 CrossRef CAS.
- A. Hirsch, Nat. Mater., 2010, 9, 868 CrossRef CAS PubMed.
- A. Hirsch, Angew. Chem., Int. Ed., 2002, 41, 1853 CrossRef CAS PubMed.
- D. Tasis, N. Tagmatarchis, A. Bianco and M. Prato, Chem. Rev., 2006, 106, 1105 CrossRef CAS PubMed.
- V. Georgakilas, M. Otyepka, A. B. Bourlinos, V. Chandra, N. Kim, K. C. Kemp, P. Hobza, R. Zboril and K. S. Kim, Chem. Rev., 2012, 112, 6156 CrossRef CAS PubMed.
- S. Eigler and A. Hirsch, Angew. Chem., Int. Ed., 2014, 53, 7720 CrossRef CAS PubMed.
- E. Bekyarova, S. Sarkar, F. Wang, M. E. Itkis, I. Kalinina, X. Tian and R. C. Haddon, Acc. Chem. Res., 2013, 46, 65 CrossRef CAS PubMed.
- A. Ciesielski and P. Samor, Adv. Mater., 2016, 28, 6030 CrossRef CAS PubMed.
- B. Li, Z. Guan, X. Yang, W. D. Wang, W. Wang, I. Hussain, K. Song, B. Tan and T. Li, J. Mater. Chem. A, 2014, 2, 11930 RSC.
- S. Ren, M. J. Bojdys, R. Dawson, A. Laybourn, Y. Z. Khimyak, D. J. Adams and A. I. Cooper, Adv. Mater., 2012, 24, 2357 CrossRef CAS PubMed.
- K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquerol and T. Siemieniewska, Pure Appl. Chem., 1985, 57, 603 CAS.
- D. Bianchi, R. Bortolo, R. Tassinari, M. Ricci and R. Vignola, Angew. Chem., Int. Ed., 2000, 39, 4321 CrossRef CAS PubMed.
- Z. Xiang, Q. Dai, J.-F. Chen and L. Dai, Adv. Mater., 2016, 28, 6253 CrossRef CAS PubMed.
- J. M. Seo, L. S. Tan and J. B. Baek, Adv. Mater., 2017, 29, 1606317 CrossRef PubMed.
- J. S. Choi, T. H. Kim, K. Y. Choo, J. S. Sung, M. B. Saidutta, S. O. Ryu, S. D. Song, B. Ramachandra and Y. W. Rhee, Appl. Catal., A, 2005, 290, 1 CrossRef CAS.
- R. Navarro, S. Lopez-Pedrajas, D. Luna, J. M. Marinas and F. M. Bautista, Appl. Catal., A, 2014, 474, 272 CrossRef CAS.
- X. Ye, Y. Cui, X. Qiu and X. Wang, Appl. Catal., B, 2014, 383, 152 Search PubMed.
- S. Song, H. Yang, R. Rao, H. Liu and A. Zhang, Appl. Catal., A, 2010, 375, 265 CrossRef CAS.
- D. Wang, M. Wang and Z. Li, ACS Catal., 2015, 5, 6852 CrossRef CAS.
- X. Ye, Y. Cui and X. Wang, ChemSusChem, 2014, 7, 738 CrossRef CAS PubMed.
- X. Chen, J. Zhang, X. Fu, M. Antonietti and X. Wang, J. Am. Chem. Soc., 2009, 131, 11658 CrossRef CAS PubMed.
- P. W. Cyr, M. Tzolov, I. Manners and E. H. Sargent, Macromol. Chem. Phys., 2003, 204, 915 CrossRef CAS.
- P. W. Cyr, E. J. D. Klem, E. H. Sargent and I. Manners, Chem. Mater., 2005, 17, 5770 CrossRef CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |