Open Access Article
Open Access ArticleEcological and cleaner process for dyeing bicomponent polyester filaments (PET/PTT) using ecological carriers: analysis of dyeing performance
Marwa Souissi *ab,
Ramzi Khiariac,
Mounir Zaagd,
Nizar Meksiab and
Hatem Dhaouadia
*ab,
Ramzi Khiariac,
Mounir Zaagd,
Nizar Meksiab and
Hatem Dhaouadia
aUniversity of Monastir, Laboratory of Environmental Chemistry and Cleaner Process (LCE2P – LR21ES04), Faculté des Sciences de Monastir, 5019 Monastir, Tunisia. E-mail: souissi.marwa20@yahoo.com
bUniversity of Monastir, National Engineering School of Monastir (ENIM), 5019 Monastir, Tunisia
cHigher Institute of Technological Studies (ISET) of Ksar-Hellal, 5070 Ksar-Hellal, Tunisia
dSociété Industrielle des Textiles (SITEX), 5070 Ksar-Hellal, Tunisia
First published on 27th July 2021
Abstract
Thanks to their excellent properties, bicomponent filaments, in particular, polyethylene terephthalate (PET)/polytrimethylene terephthalate (PTT) are more and more used in stretchable clothing. Despite the researchers' efforts, the dyeing of these filaments still presents several problems which should be resolved. Manufacturers must choose between dyeing polyester under pressure at high temperatures (close to 130 °C) to have less toxic and cheaper textile effluents and/or dyeing at low temperatures (not exceeding 100 °C) which needs the use of toxic carriers. This paper presents a new opportunity and the feasibility of dyeing bicomponent polyester filaments using an economic and clean process at a temperature equal to 100 °C and by replacing toxic carriers by ecological ones. Three kinds of ecological carriers, namely o-Vanillin, p-Vanillin and Coumarin, are used to improve the dyeing performance of bicomponent filaments with three disperse dyes having different molecular weights. They were compared to three conventional ones largely used in industry. The effect of each carrier on dyeing performance (dye bath exhaustion, color strength and CIELab coordinates) was then investigated. The obtained results prove that ecofriendly carriers constitute a good solution to replace the toxic ones and allow to obtain the same, or even better dyeing performance and fastness properties.
1. Introduction
In the last few years, thanks to awareness and strict legislations as well as ecofriendly concerns, researchers in textile industries are working hard to avoid the use of harmful products to human health and the environment.1–4 There has been a growing interest in improving the performance of textile products in terms of thermal comfort,5 resistance,6 dyeing affinity,7–10 elasticity and recovery elasticity.11–17In this context, polyester fibers are widely used while conventional filaments no longer meet the demands and progress in the textile field which targets technical textile articles offering consumers ultimate comfort,5 resistance6 and excellent elastic recovery.11–17 So great efforts are devoted to meet the expectations of the textile market and to obtain these desired characteristics such as the introduction of bicomponent filaments marked by the DuPont Company.21 These innovative filaments are designed using the same special spinneret adjacent and side by side with appropriate spinning speed and temperature (Fig. 1).18,19 To dye these filaments with an economic dyeing process, the temperature should not pass 100 °C. However, the diffusion rate of the disperse dyes used in this case does not give the desired dyeing performance. To solve this problem, many researchers have proposed to use some auxiliary products which are named “carriers”. These auxiliaries are organic and aromatic compounds with low solubility in water. They are present in the dye bath as an emulsion and increase the rate of dyeing of hydrophobic polyester fibers with disperse dyestuffs.20 The most popular carriers are o-phenylphenol, butyl benzoate, dichlorobenzene, methylnaphthalene and diphenyl.21 However, these carriers possess inherent disadvantages and hazards such as their toxicities, malodor and volatility in steam.22–24 For these reasons, many manufacturers prefer dyeing polyester filaments under pressure and at higher temperatures (close to 130 °C). This allows to have at the end of the dyeing fewer toxic effluents, and to obtain textile products not dangerous for human health, especially in sportswear and underwear where bicomponent polyester filaments are largely used. Indeed, this kind of article is generally adjusted and in contact with the human body; consequently, toxic products should never be used which can cause, in contact with sweat, very serious diseases. Therefore, the dyeing process of these textiles made with polyester filaments should be without toxic products.
In the literature, several studies have focused on the development of more ecological and economical dyeing processes by using non-harmful products: ecofriendly carriers,25–27 dispersants,26 solvents,28 saving energy consumption29 and respecting nature and human health in textile processing.7–9 The functionalization and the surface modification techniques29,30 are increasingly used in order to avoid textile discharges very loaded with toxic and non-biodegradable products.
This paper explores the possibility to dye bicomponent polyester filaments (PET/PTT) with a ecological and cleaner dyeing process. Chemical carriers which are toxic to humans and the environment will be substituted by ecological ones that respect nature, namely: o-Vanillin, p-Vanillin and Coumarin. Three different disperse dyes having different molecular weights were used. Different concentrations of the used carriers were tested and evaluated. The comparison between ecological carriers and chemical ones was established and elaborated from the point of view of dye bath exhaustion, color yield, color coordinates and colorfastness.
2. Materials and methods
2.1. Textile fabric
A jersey knit fabric made using bicomponent multifilament (PET/PTT) yarns with average count equal to 16.5 tex is used for dyeing. The obtained knitted fabric presents a weight equal to 215 g m−2 (EN 12127) and a thickness equal to 0.92 mm (ISO 5084).2.2. Used dyes and carriers
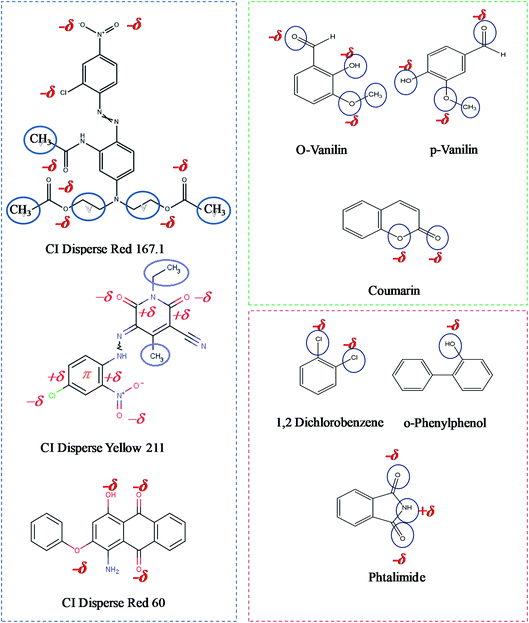
Three disperse dyestuffs, namely CI Disperse Red 60, CI Disperse Yellow 211 and CI Disperse Red 167.1 having, respectively, low, medium and high molecular weights, were used for dyeing our textile fabric.In addition, in order to improve the dyeing performance at low temperature, three ecological carriers, namely o-Vanillin, p-Vanillin and Coumarin are investigated. They are compared to three conventional toxic carriers namely: 1,2-dichlorobenzene, o-phenylphenol, and phtalimide. All carriers' molecules are illustrated in Table 1.
2.3. Dyeing procedure
The process of dyeing was carried out as following: dyebaths containing 100 mL of buffer solution of pH equal to 7 (ref. 21) and a dye concentration of 1.2% are prepared. After 15 min from the process start, carrier is introduced into the dye bath. All the mixture undergoes an agitation of 10 min in order to dissolve the carriers well before inserting the fabric to be dyed for 50 min under 100 °C.2.4. Measurement of dyebath exhaustion
After each dyeing, the dyebath exhaustion E (%) was determined by measuring the absorbance of solution at maximum wavelengths for each disperse dyes, using a spectrophotometer Hach Lange DR3900 (HACH, USA). The dyebath exhaustion E (%) is obtained by applying the formula below (eqn (1)):7,8
 | (1) |
2.5. Measurement of color and colorfastness
A spectrophotometer Spectraflash 600 Plus (Datacolor, NJ, USA) was used in order to evaluate the color of dyed samples. Light, crock and wash colorfastness of dyed fabrics were evaluated by measurements using ISO standards 105-B02, 105-X12 and 105-C06, respectively.2.6. Techniques of characterization
In order to characterize the morphology of the bicomponent filaments object of this study, a scanning electron microscope (SEM) type Hitachi S-2360 (Japan) was used. The differential scanning calorimetry (DSC) analysis was also established using Mettler machine Stare SW 9.20 software. All analysis conditions are mentioned in our previous studies.18,193. Results and discussions
3.1. Characterization of bicomponent (PET/PTT) filaments
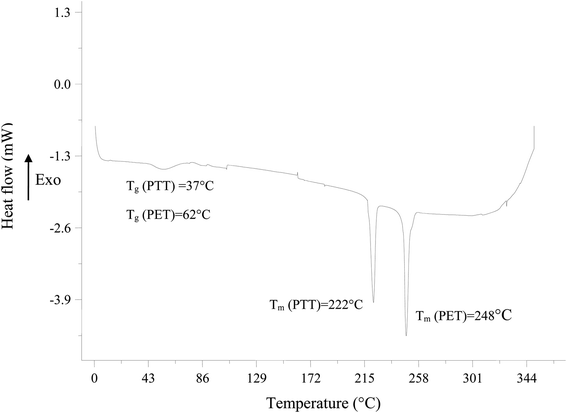
The glass transition was observed at 37 °C and 62 °C correspond to PTT and PET, respectively. From this data and according the Fox equation (eqn (2)),18,19 the Tg of bicomponent filament is equal to 51.53 °C.
 | (2) |
Furthermore, the lower the glass transition temperature, the more easily the dye fits into the amorphous zones of the filament. Indeed, below the glass transition temperature, the molecules of the polymer have practically no movement so the diffusion of the dye is impossible. However, when the glass transition temperature is reached, the fiber has sufficient energy to allow the segments of the polymer chains to rotate. Once these segments move, space will be freed up to allow the other segments to move in their turns. The onset of movement of the segments takes place over a narrow temperature interval that includes the glass transition temperature.21 So bicomponent filaments present potentially an excellent dyeing performance at a low temperature and better than that of conventional polyester filaments (100% PET).
3.2. Evaluation of dyeing performance
In order to verify the effectiveness of the three new carriers in dyeing our bicomponent polyester filaments with disperse dyestuffs (low, medium and high molecular weights), the effects of ecological carriers were evaluated and compared to conventional ones. For each carrier, a variable concentration from 0 to 0.16 mol L−1 was added to the dyebath. After each dyeing, the dyebath exhaustion (E (%)) and the color strength (K/S) of dyed fabrics were measured and analyzed. | ||
| Fig. 4 Effect of toxic carriers on dyeing performance: (a) dyebath exhaustion (%) and (b) (K/S) of dyed fabrics. | ||
In the case of the medium molecular size dye, CI Disperse Yellow 211, a carrier concentration of 0.04 mol L−1 is sufficient to improve the exhaustion of the dyebath by 24.17%, 19.46% and 26.78% using 1,2-dichlorobenzene, o-phenylphenol and phtalimide carriers, respectively. However, for dyeing bicomponent filaments using CI Disperse Red 60 (low molecular size), the use of the three carriers could not really improve the dyeing performance; exhaustion of the dyebath and (K/S) values of dyed samples remain constant or increase slightly. More importantly, a decrease in the exhaustion percentage and the color strength (K/S) is observed from 0.12 mol L−1. This is probably due to a chemical degradation of the bicomponent polyester filaments.
 | ||
| Fig. 5 Effect of ecological carriers on dyeing performance: (a) dye bath exhaustion (%) and (b) (K/S) of dyed fabrics. | ||
In addition, it is noted that the dyeing performance of bicomponent filaments depends on many parameters, in particular on the molecular size of used dyes; dyebath exhaustion and (K/S) values are higher in the cases of the disperse dyes having low and medium energies while the high energy dye shows a fairly modest dyeing performance. The large molecular size of CI Disperse Red 167.1 (Fig. 6) does not facilitate its insertion and diffusion inside the amorphous areas of the filaments. Furthermore, in the case of the dyeing of bicomponent filaments using ecological carriers, the high energy dye presents excellent dyeing performance so that the dyebath exhaustion percentage reaches a value of 90%. Likewise, it is obvious that an optimum quantity of 0.04 mol L−1 of the three ecological carriers is sufficient to maximize the color yield values in the case of the dyeing of the bicomponent filaments with all types of disperse dyes.
Furthermore, the results of dyeing performance (K/S, L*, a*, b*, and color fastnesses) of each studied carrier are different. This is due to their different chemical structures, the size of their hydrophilic (polar groups) and hydrophobic (aromatic chain) parts, and their solubility in water. As shown in Fig. 6, the carrier molecules do not have the same number of polar groups capable of interacting with those of the dye molecule. Regarding the ecological carriers, it can be seen that depending on their polar groups, p-Vanillin is more effective and can easily create interactions with the dye molecule than the other two ecological carriers (o-Vanillin, Coumarin). In addition, o-Vanillin has the same polar groups as p-Vanillin, but it has a larger steric hindrance that it makes it less effective. By comparing the ecological carriers compared to the toxic ones, it is obvious that they have a greater number of polar groups. The hydrophilic part of the carrier attracting water molecules. Thus, when the carrier is absorbed by the fiber, it carries with it a certain quantity of water (thanks to –O, –OH or –NH2 type groups) which makes it possible to fix a greater quantity of dye. The carrier is adsorbed by the fiber in a manner comparable to disperse dyes. Indeed, several types of interactions between bicomponent filaments and carriers are possible: (i) universal dispersive Londonian interaction associated with non-polar aliphatic groups in the two polyesters (PET) and (PTT) (purple colored sequences in the Fig. 7); (ii) the dipole–dipole forces between polarized carboxyl groups of (PTT) and (PET) and those present in the carrier molecule (−δ and +δ indicated in red color in the Fig. 6); (iv) the possible fiber-carrier interaction through the formation of hydrogen bonds between NH groups of the carrier (in the case of phthalimide as carrier) and the electron negative sites of the fibers; and finally, (v) the π–π interactions between the aromatic rings present in the carriers as well as in the fibers. As the carriers have smaller molecular sizes than the disperse dyes, they penetrate more easily into the amorphous areas of the fiber by pushing the dyes molecules away from the polymer chains. The molecules dye are then diffused into the new spaces then created.
3.3. Evaluation of color coordinates of dyed samples
Color of dyed fabrics using different conventional and ecological carriers were also evaluated and analyzed by evaluation of color coordinates. They were compared to results obtained by dyeing without carriers at high temperature (at 130 °C using the same dyeing process for 50 min and at pH value of 7). Obtained results are shown in tables (Tables 2–4). From theses tables, one can also draw the following concluding remarks:| With/without carriers | Carriers | Conc. (mol L−1) | K/S | Color coordinates | Color differencesb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | C* | H | ΔL* | Δa* | Δb* | ΔC* | ΔH | ΔECMC(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
||||
| a Mean values of three different samples dyed separately.b Mean values of color differences between samples dyed using carriers (at 100 °C) and samples dyed without carriers (at 130 °C). | ||||||||||||||
| Without carrier (dyeing temp. = 130 °C) | — | — | 17.98 | 35.96 | 58.57 | 4.41 | 58.32 | 4.31 | — | — | — | — | — | — |
| With ecological carriers (dyeing temp. = 100 °C) | o-Vanillin | 0.04 | 16.62 | 36.82 | 57.60 | 4.5 | 57.78 | 4.47 | 0.86 | −0.97 | 0.07 | −0.95 | 1.23 | 0.60 |
| 0.08 | 18.70 | 34.39 | 58.67 | 5.32 | 58.91 | 5.18 | −1.57 | 0.1 | 0.89 | 0.18 | 2.39 | 1.19 | ||
| 0.12 | 19.26 | 34.07 | 59.12 | 5.13 | 59.34 | 4.96 | −1.89 | 0.55 | 0.7 | 0.61 | 2.75 | 1.22 | ||
| 0.16 | 19.42 | 33.96 | 59.68 | 5.74 | 59.96 | 5.49 | −2.00 | 1.11 | 1.31 | 1.23 | 3.07 | 1.63 | ||
| p-Vanillin | 0.04 | 20.48 | 33.96 | 58.63 | 4.24 | 58.78 | 4.14 | −2.00 | 0.06 | −0.19 | 0.05 | 2.83 | 1.12 | |
| 0.08 | 18.93 | 34.26 | 58.91 | 3.79 | 59.03 | 3.68 | −1.70 | 0.34 | −0.64 | 0.30 | 2.49 | 1.12 | ||
| 0.12 | 17.47 | 36.08 | 58.97 | 3.55 | 59.08 | 3.45 | 0.12 | 0.40 | −0.88 | 0.35 | 0.92 | 0.84 | ||
| 0.16 | 17.78 | 35.83 | 58.62 | 3.52 | 58.73 | 3.44 | −0.13 | 0.05 | −0.91 | 0.00 | 0.93 | 0.84 | ||
| Coumarin | 0.04 | 20.65 | 35.17 | 58.44 | 4.98 | 58.65 | 4.87 | −0.79 | −0.13 | 0.55 | −0.08 | 1.25 | 0.67 | |
| 0.08 | 19.00 | 34.9 | 58.329 | 4.42 | 58.50 | 4.33 | −1.06 | −0.241 | −0.01 | −0.23 | 1.50 | 0.59 | ||
| 0.12 | 18.85 | 35.23 | 59.08 | 4.49 | 59.25 | 4.35 | −0.73 | 0.51 | 0.06 | 0.52 | 1.03 | 0.44 | ||
| 0.16 | 12.20 | 36.8 | 58.36 | 3.12 | 58.44 | 3.06 | 0.84 | −0.21 | −1.31 | −0.29 | 1.76 | 1.29 | ||
| With toxic carriers (dyeing temp. = 100 °C) | 1,2-Dichlorobenzene | 0.04 | 16.17 | 37.22 | 58.21 | 3.76 | 58.33 | 3.70 | 1.26 | −0.36 | −0.67 | −0.40 | 1.90 | 0.93 |
| 0.08 | 15.86 | 39.98 | 59.44 | 3.95 | 59.57 | 3.80 | 4.02 | 0.87 | −0.48 | 0.84 | 5.71 | 2.34 | ||
| 0.12 | 15.14 | 40.29 | 57.79 | 3.74 | 57.91 | 3.70 | 4.33 | −0.78 | −0.69 | −0.82 | 6.16 | 2.49 | ||
| 0.16 | 12.87 | 41.8 | 58.04 | 4.03 | 58.18 | 3.97 | 5.84 | −0.53 | −0.4 | −0.55 | 8.27 | 3.26 | ||
| o-Phenylphenol | 0.04 | 16.82 | 36.94 | 59.72 | 2.63 | 59.78 | 2.52 | 0.98 | 1.15 | −1.8 | 1.05 | 2.32 | 1.83 | |
| 0.08 | 17.65 | 35.94 | 59.96 | 3.47 | 60.06 | 3.31 | −0.02 | 1.39 | −0.96 | 1.33 | 1.04 | 1.07 | ||
| 0.12 | 16.76 | 36.92 | 59.3 | 3.23 | 59.39 | 3.12 | 0.96 | 0.73 | −1.2 | 0.66 | 1.84 | 1.28 | ||
| 0.16 | 16.93 | 37.05 | 59.06 | 3.33 | 59.15 | 3.23 | 1.09 | 0.49 | −1.1 | 0.42 | 1.91 | 1.21 | ||
| Phtalimide | 0.04 | 17.78 | 35.8 | 58.98 | 4.38 | 59.14 | 4.25 | −0.16 | 0.41 | −0.05 | 0.41 | 0.23 | 0.19 | |
| 0.08 | 17.59 | 36.1 | 58.63 | 4.73 | 58.82 | 4.61 | 0.14 | 0.06 | 0.3 | 0.09 | 0.35 | 0.28 | ||
| 0.12 | 17.53 | 36.13 | 58.54 | 4.72 | 58.73 | 4.61 | 0.17 | −0.03 | 0.29 | 0.00 | 0.38 | 0.28 | ||
| 0.16 | 16.76 | 36.72 | 56.53 | 4.44 | 56.70 | 4.49 | 1.15 | −2.04 | 0.01 | −2.03 | 1.64 | 0.86 | ||
| With/without carriers | Carriers | Conc. (mol L−1) | K/S | Color coordinates | Color differencesb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | C* | h | ΔL* | Δa* | Δb* | ΔC* | ΔH | ΔECMC(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
||||
| a Mean values of three different samples dyed separately.b Mean values of color differences between samples dyed using carriers (at 100 °C) and samples dyed without carriers (at 130 °C). | ||||||||||||||
| Without carrier (dyeing temp. = 130 °C) | — | — | 23.80 | 76.81 | 8.45 | 48.57 | 49.29 | 80.13 | — | — | — | — | — | — |
| With ecological carriers (dyeing temp. = 100 °C) | o-Vanillin | 0.04 | 21.84 | 78.32 | 11.36 | 46.93 | 48.29 | 76.39 | 1.51 | 2.91 | −1.64 | −1.00 | 3.84 | 2.80 |
| 0.08 | 18.70 | 79.32 | 11.38 | 46.96 | 48.32 | 76.38 | 2.51 | 2.93 | −1.61 | −0.97 | 4.78 | 2.91 | ||
| 0.12 | 16.94 | 80.75 | 15.04 | 44.36 | 46.84 | 71.27 | 3.94 | 6.59 | −4.21 | −2.45 | 9.28 | 6.68 | ||
| 0.16 | 16.08 | 80.93 | 16.58 | 43.89 | 46.92 | 69.31 | 4.12 | 8.13 | −4.68 | −2.37 | 10.79 | 8.08 | ||
| p-Vanillin | 0.04 | 24.74 | 75.33 | 8.76 | 48.92 | 49.70 | 79.85 | −1.48 | 0.31 | 0.35 | 0.41 | 2.11 | 0.61 | |
| 0.08 | 23.46 | 76.89 | 8.75 | 48.82 | 49.60 | 79.84 | 0.08 | 0.3 | 0.25 | 0.31 | 0.27 | 0.24 | ||
| 0.12 | 23.35 | 76.93 | 8.72 | 48.86 | 49.63 | 79.88 | 0.12 | 0.27 | 0.29 | 0.34 | 0.26 | 0.23 | ||
| 0.16 | 22.72 | 77.85 | 8.62 | 47.91 | 48.68 | 79.80 | 1.04 | 0.17 | −0.66 | −0.61 | 1.50 | 0.51 | ||
| Coumarin | 0.04 | 24.38 | 76.32 | 8.92 | 48.04 | 48.86 | 79.48 | −0.49 | 0.47 | −0.53 | −0.43 | 0.89 | 0.53 | |
| 0.08 | 24.26 | 76.45 | 8.68 | 48.05 | 48.83 | 79.76 | −0.36 | 0.23 | −0.52 | −0.46 | 0.61 | 0.35 | ||
| 0.12 | 21.54 | 78.39 | 8.83 | 47.86 | 48.67 | 79.55 | 1.58 | 0.38 | −0.71 | −0.62 | 2.29 | 0.76 | ||
| 0.16 | 19.52 | 79.84 | 9.65 | 47.26 | 48.24 | 78.46 | 3.03 | 1.2 | −1.31 | −1.05 | 4.52 | 1.71 | ||
| With toxic carriers (dyeing temp. = 100 °C) | 1,2-Dichlorobenzene | 0.04 | 23.14 | 77.28 | 8.46 | 47.67 | 48.41 | 79.94 | 0.47 | 0.01 | −0.9 | −0.88 | 0.70 | 0.41 |
| 0.08 | 23.03 | 77.34 | 8.5 | 47.69 | 48.44 | 79.89 | 0.53 | 0.05 | −0.88 | −0.85 | 0.79 | 0.42 | ||
| 0.12 | 21.73 | 78.27 | 8.32 | 46.46 | 47.20 | 79.85 | 1.46 | −0.13 | −2.11 | −2.09 | 2.09 | 1.00 | ||
| 0.16 | 20.65 | 79.19 | 8.23 | 45.2 | 45.94 | 79.68 | 2.38 | −0.22 | −3.37 | −3.35 | 3.40 | 1.62 | ||
| o-Phenylphenol | 0.04 | 19.07 | 79.98 | 8.26 | 48.6 | 49.30 | 80.35 | 3.17 | −0.19 | 0.03 | 0.01 | 4.49 | 1.19 | |
| 0.08 | 23.80 | 77.87 | 8.38 | 48.62 | 49.34 | 80.22 | 1.06 | −0.07 | 0.05 | 0.05 | 1.50 | 0.40 | ||
| 0.12 | 22.72 | 77.84 | 8.32 | 48.9 | 49.60 | 80.34 | 1.03 | −0.13 | 0.33 | 0.31 | 1.47 | 0.43 | ||
| 0.16 | 22.41 | 77.99 | 8.31 | 49.59 | 50.28 | 80.49 | 1.18 | −0.14 | 1.02 | 0.99 | 1.69 | 0.64 | ||
| Phtalimide | 0.04 | 20.82 | 79.01 | 8.13 | 47.87 | 48.56 | 80.36 | 2.2 | −0.32 | −0.7 | −0.73 | 3.12 | 0.88 | |
| 0.08 | 23.35 | 77.02 | 8.56 | 48.59 | 49.34 | 80.01 | 0.21 | 0.11 | 0.02 | 0.05 | 0.31 | 0.12 | ||
| 0.12 | 23.03 | 77.33 | 8.54 | 48.39 | 49.14 | 79.99 | 0.52 | 0.09 | −0.18 | −0.15 | 0.75 | 0.22 | ||
| 0.16 | 23.57 | 76.9 | 8.58 | 48.7 | 49.45 | 80.01 | 0.09 | 0.13 | 0.13 | 0.16 | 0.16 | 0.11 | ||
| With/without carriers | Carriers | Conc. (mol L−1) | K/S | Color coordinates | Color differencesb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | C* | h | ΔL* | Δa* | Δb* | ΔC* | ΔH | ΔECMC(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
||||
| a Mean values of three different samples dyed separately.b Mean values of color differences between samples dyed using carriers (at 100 °C) and samples dyed without carriers (at 130 °C). | ||||||||||||||
| Without carrier (dyeing temp. = 130 °C) | — | — | 20.10 | 30.09 | 45.80 | 4.26 | 46.00 | 5.31 | — | — | — | — | — | — |
| With ecological carriers (dyeing temp. = 100 °C) | o-Vanillin | 0.04 | 18.71 | 33.64 | 41.99 | 4.51 | 42.23 | 6.13 | 3.55 | −3.81 | 0.25 | 3.62 | 5.17 | 2.72 |
| 0.08 | 17.72 | 34.54 | 43.86 | 4.97 | 44.14 | 6.46 | 4.45 | −1.94 | 0.71 | 1.71 | 6.40 | 2.93 | ||
| 0.12 | 15.51 | 36.56 | 40.65 | 5.5 | 41.02 | 7.71 | 6.47 | −5.15 | 1.24 | 4.83 | 9.41 | 4.69 | ||
| 0.16 | 11.11 | 39.44 | 40.69 | 5.91 | 41.12 | 8.26 | 9.35 | −5.11 | 1.65 | 4.73 | 13.46 | 6.36 | ||
| p-Vanillin | 0.04 | 22.61 | 27.31 | 45.02 | 4.46 | 45.24 | 5.66 | −2.78 | −0.78 | 0.2 | 0.61 | 3.97 | 1.74 | |
| 0.08 | 22.51 | 27.12 | 45.16 | 4.83 | 45.42 | 6.10 | −2.97 | −0.64 | 0.57 | 0.43 | 4.26 | 1.91 | ||
| 0.12 | 21.17 | 28.06 | 45.93 | 4.27 | 46.13 | 5.31 | −2.03 | 0.13 | 0.01 | −0.28 | 2.86 | 1.26 | ||
| 0.16 | 21.63 | 27.24 | 45.24 | 4.32 | 45.45 | 5.45 | −2.85 | −0.56 | 0.06 | 0.40 | 4.05 | 1.78 | ||
| Coumarin | 0.04 | 22.82 | 26.92 | 46.05 | 5.3 | 46.35 | 6.57 | −3.17 | 0.25 | 1.04 | −0.50 | 4.58 | 2.10 | |
| 0.08 | 20.40 | 29.86 | 45.8 | 4.23 | 45.99 | 5.28 | −0.23 | 0 | −0.03 | −0.14 | 0.29 | 0.14 | ||
| 0.12 | 19.14 | 32.52 | 45.31 | 3.98 | 45.48 | 5.02 | 2.43 | −0.49 | −0.28 | 0.37 | 3.46 | 1.53 | ||
| 0.16 | 19.44 | 32.31 | 45.73 | 4.07 | 45.91 | 5.09 | 2.22 | −0.07 | −0.19 | −0.06 | 3.15 | 1.38 | ||
| With toxic carriers (dyeing temp. = 100 °C) | 1,2-Dichlorobenzene | 0.04 | 17.41 | 34.89 | 44.45 | 4.4 | 44.67 | 5.65 | 4.8 | −1.35 | 0.14 | 1.18 | 6.82 | 3.03 |
| 0.08 | 14.35 | 36.93 | 43.62 | 4.02 | 43.80 | 5.27 | 6.84 | −2.18 | −0.24 | 2.05 | 9.71 | 4.33 | ||
| 0.12 | 12.03 | 38.67 | 42.46 | 4.55 | 42.70 | 6.12 | 8.58 | −3.34 | 0.29 | 3.15 | 12.19 | 5.51 | ||
| 0.16 | 11.83 | 39.35 | 41.9 | 4.61 | 42.15 | 6.28 | 9.26 | −3.9 | 0.35 | 3.70 | 13.16 | 5.98 | ||
| o-Phenylphenol | 0.04 | 20.65 | 29.65 | 44.85 | 4.26 | 45.05 | 5.43 | −0.44 | −0.95 | 0 | 0.80 | 0.81 | 0.47 | |
| 0.08 | 21.92 | 27.03 | 45.4 | 4.58 | 45.63 | 5.76 | −3.06 | −0.4 | 0.32 | 0.22 | 4.35 | 1.92 | ||
| 0.12 | 19.67 | 32.05 | 45.15 | 4.56 | 45.38 | 5.77 | 1.96 | −0.65 | 0.3 | 0.47 | 2.82 | 1.26 | ||
| 0.16 | 18.30 | 33.78 | 42.9 | 3.89 | 43.08 | 5.18 | 3.69 | −2.9 | −0.37 | 2.77 | 5.30 | 2.54 | ||
| Phtalimide | 0.04 | 17.59 | 34.85 | 44.17 | 5.04 | 44.46 | 6.51 | 4.76 | −1.63 | 0.78 | 1.39 | 6.83 | 3.09 | |
| 0.08 | 17.78 | 34.52 | 44.14 | 5.01 | 44.42 | 6.48 | 4.43 | −1.66 | 0.75 | 1.43 | 6.37 | 2.90 | ||
| 0.12 | 17.97 | 34.33 | 44.88 | 4.32 | 45.09 | 5.50 | 4.24 | −0.92 | 0.06 | 0.76 | 6.02 | 2.66 | ||
| 0.16 | 15.00 | 36.05 | 43.01 | 2.98 | 43.11 | 3.96 | 5.96 | −2.79 | −1.28 | 2.74 | 8.54 | 3.95 | ||
– It can be shown that dyeing at 100 °C using ecological carriers allow to obtain, in the majority of cases, color yield values higher than those obtained by dyeing using conventional carriers and/or by dyeing at 130 °C (without carrier).
– For the three used dyes (with low, medium and high molecular weights), dyeing using Coumarin and p-Vanillin as carriers offer the best results in terms of (K/S).·In addition, with these two ecological carriers (Coumarin and p-Vanillin), a small quantity of 0.04 mol L−1 is sufficient to optimize the (K/S) values of dyed samples and to obtain better results than those obtained by dyeing at high temperature.
Tables 2–4 also summarize the colorimetric coordinates (L*, a*, b*, C* and h) of samples dyed at 100 °C using various ecological and toxic carriers. The color differences (ΔL*, Δa*, Δb*, ΔC*, ΔH and ΔECMC(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) between these samples and standard fabric dyed at 130 °C without carriers were also calculated and evaluated. This allowed us to compare the variation of shades using the two different dyeing processes (at 130 °C without carrier and at 100 °C with carriers). The obtained results show that the colorimetric coordinates undergo a more and less important change after the addition of ecological and toxic carriers. The o-Vanillin for example allows to obtain lighter shades than those obtained at 130 °C and then colors differences greater than 1.
1)) between these samples and standard fabric dyed at 130 °C without carriers were also calculated and evaluated. This allowed us to compare the variation of shades using the two different dyeing processes (at 130 °C without carrier and at 100 °C with carriers). The obtained results show that the colorimetric coordinates undergo a more and less important change after the addition of ecological and toxic carriers. The o-Vanillin for example allows to obtain lighter shades than those obtained at 130 °C and then colors differences greater than 1.
Moreover, as the use of p-Vanillin and Coumarin allowed to obtain much greater color yield values than those obtained using the dyeing process at high temperature(for the three studied dyes), color differences (ΔECMC(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) obtained in these cases are relatively greater than 1. It is due to the fact that colors of dyed samples have become darker (ΔL* < 0).
1)) obtained in these cases are relatively greater than 1. It is due to the fact that colors of dyed samples have become darker (ΔL* < 0).
Regarding toxic carriers, they allow to obtain similar shades to those obtained at high temperature process in particular in the case of CI Disperse Yellow 211 dye with color differences values (ΔECMC(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) less than 1. However, they have not been performing in improving the dyeing performance (exhaustion dye bath and color yield) of bicomponent polyester filaments in the case of dyeing bicomponent filaments with high molecular weight dye (CI Disperse Red 167.1). These results confirm the effectiveness of ecological carriers which showed an improvement in the diffusion of all types of dyes into the polyester filaments.
1)) less than 1. However, they have not been performing in improving the dyeing performance (exhaustion dye bath and color yield) of bicomponent polyester filaments in the case of dyeing bicomponent filaments with high molecular weight dye (CI Disperse Red 167.1). These results confirm the effectiveness of ecological carriers which showed an improvement in the diffusion of all types of dyes into the polyester filaments.
3.4. Evaluation of color fastnesses
The color fastnesses values (wash, crock and light fastnesses) of samples dyed using ecological and toxic carriers were established and the results were recapitulated in Table 5. The obtained results show that the use of ecological carriers allows to obtain the same values of color fastnesses obtained using dyeing process at 130 °C and this for the three disperse dyes of high, medium and low energies. However, the use of the toxic carriers caused a decrease in the fastness of the polyester samples, especially the cases of 1,2-dichlorobenzene and o-phenylphenol.| Dyes | With/without carriers | Wash fastness (ISO 105-C06) | Crock fastness (ISO 105-X12) | Light fastness (ISO 105-B02) | |||
|---|---|---|---|---|---|---|---|
| Staining | Color change | Dry | Wet | ||||
| CI Disperse Red 60 | Without carrier | — | 3–4 | 4 | 4 | 3–4 | 6–7 |
| With ecological carriers | o-Vanillin | 4 | 3–4 | 4 | 3–4 | 6–7 | |
| p-Vanillin | 4 | 4 | 4 | 3–4 | 7 | ||
| Coumarin | 4 | 4 | 4 | 3–4 | 6–7 | ||
| With toxic carriers | 1,2-Dichlorobenzene | 3–4 | 3 | 3–4 | 3 | 5 | |
| o-Phenylphenol | 3–4 | 3–4 | 3–4 | 3–4 | 6 | ||
| Phtalimide | 3–4 | 4 | 4 | 4 | 6 | ||
| CI Disperse Yellow 211 | Without carrier | — | 4 | 4 | 4–5 | 4–5 | 7 |
| With ecological carriers | o-Vanillin | 4 | 3–4 | 4 | 4 | 7 | |
| p-Vanillin | 4 | 4–5 | 4–5 | 4–5 | 7 | ||
| Coumarin | 4 | 3–4 | 4 | 3–4 | 7 | ||
| With toxic carriers | 1,2-Dichlorobenzene | 3–4 | 3 | 3–4 | 3 | 6 | |
| o-Phenylphenol | 3–4 | 3–4 | 3–4 | 3–4 | 6 | ||
| Phtalimide | 4 | 4 | 4–5 | 4–5 | 7 | ||
| CI Disperse Red 167.1 | Without carrier | — | 5 | 4–5 | 5 | 4–5 | 7–8 |
| With ecological carriers | o-Vanillin | 4 | 4 | 4 | 4 | 7–8 | |
| p-Vanillin | 4–5 | 4 | 4–5 | 4–5 | 7–8 | ||
| Coumarin | 4 | 4 | 4 | 4 | 7–8 | ||
| With toxic carriers | 1,2-Dichlorobenzene | 3–4 | 3–4 | 3–4 | 3–4 | 6 | |
| o-Phenylphenol | 3–4 | 3–4 | 3–4 | 3–4 | 6 | ||
| Phtalimide | 4 | 4–5 | 4 | 4 | 7 | ||
4. Conclusion
Bicomponent polyester filaments (PET/PTT) are dyed using an ecological dyeing process which allowed to reduce the dyeing temperature by 30 °C. The use of three environmentally friendly carriers named o-Vanillin, p-Vanillin and Coumarin has highly improved the dyeing performance of all classes of disperse dyes with excellent dyeing fastnesses. In order to remain faithful to any process that respects man and environment, p-Vanillin seems the most suitable choice to be used as an ecological carrier replacing toxic carriers; it has allowed to obtain the best performance of dyeing with excellent fastnesses. It is also much less expensive and very abandoning, unlike o-Vanillin and Coumarin, as their synthesis is more complicated and they are much more expensive. As perspectives to this work, our upcoming publication will be devoted to study the phenomenon of adsorption of disperse dyes after the addition of ecological carriers. The kinetics dyeing of bicomponent filament with three disperse dyes and their interaction with adding ecological carriers will studied. In addition, different models such as pseudo first order, pseudo second order, Elovich and intra-particles models will be exploited to identify the adequate dyeing mechanism.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project is carried out under the MOBIDOC scheme, funded by the EU through the EMORI program and managed by the ANPR.References
- G. C. De Oliveira Neto, J. M. Ferreira Correia, P. C. Silva, A. G. de Oliveira Sanches and W. C. Lucato, J. Cleaner Prod., 2019, 228, 1514–1525 CrossRef.
- L. Chen, L. Wang, X. Wu and X. Ding, J. Cleaner Prod., 2017, 143, 1137–1143 CrossRef CAS.
- X. Ren, J. Cleaner Prod., 2000, 8, 473–481 CrossRef.
- A. K. A. Rathi, J. Cleaner Prod., 2003, 11, 583–590 CrossRef.
- H. M. Jeong, B. K. Ahn, S. M. Cho and B. K. Kim, J. Polym. Sci., Part B: Polym. Phys., 2000, 38, 3009–3017 CrossRef CAS.
- T. Kitagawa, K. Yabuki and R. Young, Polym. J., 2001, 42, 2101–2112 CAS.
- M. Souissi, A. Guesmi and A. Moussa, J. Cleaner Prod., 2018, 202, 1045–1055 CrossRef CAS.
- M. Souissi, A. Guesmi and A. Moussa, J. Cleaner Prod., 2018, 204, 1143–1153 CrossRef CAS.
- R. Campardelli, P. Trucillo, M. Iorio and E. Reverchon, Dyes Pigm., 2019, 10, 79–85 Search PubMed.
- J. Ru, X. Qian and Y. Wang, Sci. Rep., 2018, 8, 1–9 CAS.
- C. L. Moore and H. A. Bruck, Smart Mater. Struct., 2002, 11, 130–139 CrossRef CAS.
- S. V. Ahir, A. R. Tajbakhsh and E. M. Terentjev, Adv. Funct. Mater., 2006, 16, 556–560 CrossRef CAS.
- Y. Zhu, J. Hu, L. Y. Yeung, Y. Liu, F. Ji and K. Yeung, Smart Mater. Struct., 2006, 15, 1385–1394 CrossRef CAS.
- W. Fumei, G. Fei and X. Bugao, Elastic strain of PTT/PET self-crimping fibers, J. Eng. Fibers Fabr., 2013, 8, 50–55 Search PubMed.
- J. Hu, J. Lu and Y. Zhu, Polym. Rev., 2008, 48, 275–301 CrossRef CAS.
- L. Jin, W. Fumei and X. Bugao, Text. Res. J., 2011, 81, 538–544 CrossRef.
- C. Sihai and W. Shanyuan, J. Macromol. Sci., Part B: Phys., 2011, 50, 1447–1459 CrossRef.
- M. Souissi, R. Khiari, W. Haddar, M. Zaag, N. Meksi and H. Dhaouadi, Process, 2020, 8, 501 CrossRef CAS.
- M. Souissi, R. Khiari, M. Zaag, N. Meksi and H. Dhaouadi, Polym. Bull., 2021, 78, 2685–2707 CrossRef CAS.
- F. J. C. Fité, Text. Res. J., 1995, 65, 362–368 CrossRef.
- M. A. Iskender, B. Becerir and A. Koruyucu, Text. Res. J., 2005, 75, 462–465 CrossRef CAS.
- M. A. Tavanaie, A. M. Shoushtari and F. Goharpey, J. Cleaner Prod., 2010, 18, 1866–1871 CrossRef CAS.
- W. Ingamells and A. Yabani, J. Soc. Dye. Colour., 1997, 93, 417–423 CrossRef.
- V. Pasquet, A. Perwuelz, N. Behary and J. Isaad, J. Cleaner Prod., 2013, 43, 20–26 CrossRef CAS.
- A. M. Al-Etaibi and M. A. El-Apasery, Int. J. Environ. Res. Public Health, 2019, 16, 4603 CrossRef CAS PubMed.
- H. Barani and M. Montazer, J. Liposome Res., 2008, 18, 249–262 CrossRef CAS PubMed.
- S. S. Pawar, S. Maiti, S. Biranje, K. Kulkarni and R. V. Adivarekar, Heliyon, 2019, 5, 1–9 CrossRef PubMed.
- B. Gupta, N. Revagade and J. Hilborn, Prog. Polym. Sci., 2007, 32, 455–482 CrossRef CAS.
- Y. Ren, J. Gong, R. Fu, Z. Li, Z. Yu, J. Lou and J. Zhang, J. Clean. Prod., 2017, 148, 375–385 CrossRef CAS.
- S. Chen, S. Zhang, M. Galluzzi, F. Li, X. Zhang, X. Yang and P. Huang, Chem. Eng. J., 2019, 358, 634–642 CrossRef CAS.
- T. H. Oh, S. S. Han, W. S. Lyoo and H. Y. Jeon, Polym. Eng. Sci., 2010, 51, 232–236 CrossRef.
| This journal is © The Royal Society of Chemistry 2021 |