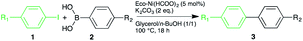Open Access Article
Open Access ArticleNew biomaterials for Ni biosorption turned into catalysts for Suzuki–Miyaura cross coupling of aryl iodides in green conditions†
Lucie Casesa,
Pauline Adlera,
Franck Pelissiera,
Sébastien Diliberto b,
Clotilde Boulangerb and
Claude Grison
b,
Clotilde Boulangerb and
Claude Grison *a
*a
aLaboratory of Bioinspired Chemistry and Ecological Innovations, UMR CNRS-University of Montpellier 5021, Cap Delta, 1682 Rue de la Valsière, 34790 Grabels, France. E-mail: claude.grison@cnrs.fr
bInstitut Jean Lamour, UMR 7198, Centre National de la Recherche Scientifique – Université de Lorraine, 57078 Metz, France
First published on 19th August 2021
Abstract
In parallel with increasing Ni production and utilisation, Ni pollution in the soil–water continuum has become an alarming and global problem. Solutions for removing Ni from industrial effluents have been widely investigated and biosorption has emerged as an efficient, cost-effective, scalable and sustainable alternative for water treatment. However, the biosorption capacity is limited by the chemical composition of the biomaterial and the Ni-enriched biomaterials are rarely valorised. In this work, the biosorption capacity of three abundant biomaterials with different chemical properties – water hyacinth, coffee grounds and pinecones – was studied before and after functionalization, and reached a maximum biosorption capacity of 51 mg g−1 of Ni(II). A bioinspired functionalization approach was investigated introducing carboxylate moieties and was conducted in green conditions. The Ni-enriched biomaterials were valorised by transformation into catalysts, which were characterised by MP-AES and XRPD. Their characterisation revealed a structure similar to nickel formate, and hence the Eco-Ni(HCOO)2 catalysts were tested in Suzuki–Miyaura reactions. Several aryl iodides were successfully cross-coupled to phenylboronic acids using Eco-Ni(HCOO)2 without any ligand, a mild and green base in a mixture of green solvents.
Introduction
Nickel is a transition metal, which is used in metallurgic processes, in electroplating, in the production of batteries and as a catalyst in fine chemistry. Within the last decade, its global mine production has almost doubled and reached 2.5 billion tons of nickel in 2020. The anthropogenic activities using Ni have led to the release of significant amounts of Ni and derivatives into the environment. Ni pollution of the soil–water continuum is serious since long-term exposure to high concentrations of Ni can cause dermatitis,1 asthma,2 cardiovascular and kidney diseases, lung fibrosis,3 lung and nasal cancers.4 Exposure to Ni pollution mainly concerns oral ingestion through water and food.5 Removal of Ni from industrial effluents before their discharge in the environment has become a priority. However, nickel is hardly extractable due to its strong affinity for aqueous media. Several processes have been reported in the literature including chemical treatments, as chemical precipitation6 and electrocoagulation,7 membrane separation processes as ultrafiltration,8 reverse osmosis, electrodialysis9 and ion exchange methods.10 Although efficient, the industrialisation of the aforementioned processes is still limited, mostly due to the high cost, the secondary pollution and the generation of problematic sludge.Biosorption has been emerging as an alternative and promising approach to water treatment. Biosorption relies on the adsorption of pollutants from wastewater using a biomaterial. This technique presents several advantages as the abundant availability in nature of its biomaterial, hence its low cost and the absence of a secondary pollution. Several types of biomaterials have been developed for the biosorption of Ni, from bacterial biomass,11,12 microalgae,13 tree bark,14 plant species15 to fruit peels.16,17 However, their biosorption capacity is limited by the chemical properties of the biomaterial. Moreover, as in every depollution process, the main drawback is the absence of valorisation of the depolluting membrane or biomaterial. In this paper, we propose to combine two strategies to overcome these limitations: the biosorption of Ni from wastewater using biomaterials modified in a bioinspired process and the utilisation of the Ni-enriched biomaterial as a catalyst in organic chemistry.
We have recently studied the ability of several aquatic plants to remove palladium from wastewater.18 The derived root powders were able to adsorb high concentrations of Pd, following a passive process of biosorption. However, the affinity of a biomaterial to a transition metal depends on its chemical properties. Indeed, a few natural biomaterials were chemically modified to improve their biosorption capacity. Grafted co-polymers introduced on orange peels and polyethylenimine crosslinked to glutaraldehyde itself crosslinked to biomass of Penicillium chrysogenum largely improved the maximum biosorption capacity of Ni from solution.11,17 However, these chemical methods of functionalization are far from being environmental-friendly processes.
In this paper, we first investigated a functionalization methodology to improve the affinity of three biomaterials, water hyacinth, pinecone and coffee ground, towards Ni salts. The functionalization methodology was designed and conducted in green conditions. We then studied their biosorption capacity to remove Ni from wastewater. And we finally studied the recovery of the Ni-enriched biomaterials and developed its utilisation as a catalyst in green and sustainable chemistry. The example of Ni-catalysed Suzuki–Miyaura reaction was chosen to illustrate the valorisation of the biomaterial.
Results and discussion
Biosorption of Ni using functionalized biomass feedstock
Biosorption of nickel from aqueous solution was studied in batch using three biomaterials with different chemical properties: roots of an aquatic plant, Echhornia crassipes (water hyacinth) rich in carboxylate functions, coffee grounds rich in tannins and pinecones rich in lignin. The three biomaterials were ground, dried and put into solution containing increasing concentrations of nickel sulfate (12, 40 mg L−1). Although presenting high biosorption efficiency,13–16,19 the three biomaterials rapidly showed a limited biosorption efficiency of around 36% for a solution of 40 mg L−1 of NiSO4 (Table 1 entries 1–6). Functionalization of the biomaterials was hence envisaged in order to increase their Ni biosorption performances.| Entry | Starting biomaterial | Functionalisation reagent | Name of biomaterial | Carboxylate (mmol g−1 of material) | Solution to biosorb (mg per L of metal per g of material) | Biosorption efficiency (%) |
|---|---|---|---|---|---|---|
| 1 | Echhornia crassipes | — | Ec | — | 12 mg L−1 of NiSO4 | 62 |
| 2 | Pinecone | Pn | 62 | |||
| 3 | Coffee ground | Cg | 30 | |||
| 4 | Echhornia crassipes | Ec | 40 mg L−1 of NiSO4 | 36 | ||
| 5 | Pinecone | Pn | 16 | |||
| 6 | Coffee ground | Cg | 17 | |||
| 7 | Echhornia crassipes | Succinic anhydride | Ec–SA | 1.4 | 99 | |
| 8 | Pinecone | Pn–SA | 1.1 | 78 | ||
| 9 | Coffee ground | Cg–SA | 1.4 | 80 | ||
| 10 | Echhornia crassipes | Glutaric anhydride | Ec–GA | 0.7 | 64 | |
| 11 | Echhornia crassipes | Citric acid | Ec–CA | 1.4 | 89 | |
| 12 | Pinecone | Pn–CA | 1.6 | 70 | ||
| 13 | Coffee ground | Cg–CA | 1.8 | 99 | ||
| Maximum of biosorption | ||||||
| 14 | Echhornia crassipes | Succinic anhydride | Ec–SA | 1.4 | 1024 mg L−1 of NiSO4 | 51 mg g−1 |
Our functionalization methodology was inspired from the natural mechanism of Ni storage in Ni-hyperaccumulating plant shoots, in which Ni is stored as nickel carboxylate (citrate, malate and malonate).20 Two strategies of functionalization, both based on the introduction of carboxylate moieties onto the hydroxyl groups of the biomaterials, were investigated (Scheme 1). Strategy A relies on the direct esterification of hydroxyl groups using carboxylic anhydride (succinic or glutaric anhydride). Ethyl acetate was the solvent chosen for this strategy since it does not decompose the anhydrides unlike a protic solvent. Strategy B relies on the auto-catalysed esterification of hydroxyl groups by citric acid. Unlike strategy A, ethyl acetate could not be used due to the poor solubility of citric acid in organic solvents, and water would deactivate the reaction, so ethanol was chosen. Both strategies were conducted in green conditions, using biosourced reagents (the biomass and the carboxylic acid or anhydrides) in a green solvent (ethyl acetate or ethanol).
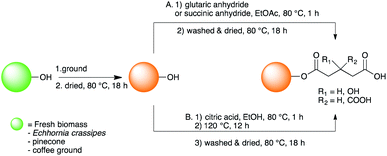 | ||
| Scheme 1 Functionalisation of biomass using (A). glutaric anhydride or succinic anhydride or using (B). citric acid for biosorption of Ni. | ||
Following the functionalization, the functionalised biomaterials were purified to remove the excess of carboxylic acids and were characterised by infrared spectroscopy (Table 2 and Fig.S1†). Infrared analyses showed new bands of higher percentage of transmission in the 1720–1740 cm−1 region corresponding to the stretching vibration of the ester group, which was formed during the functionalization process.
| Entry | Biomaterial | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ester (cm−1) O ester (cm−1) |
|---|---|---|
| a s: strong; m: moderate; w: weak; vw: very weak. | ||
| 1 | Ec | None |
| 2 | Ec–CA | 1722 (s) |
| 3 | Pn | 1738 (w) |
| 4 | Pn–CA | 1726 (s) |
| 5 | Cg | 1730 (vw) |
| 6 | Cg–CA | 1718 (m) |
The efficacy of the functionalization strategies was compared by titrating the introduced carboxylate moieties using a solution of sodium hydroxide (Table 1, entries 7–13). Both strategies A and B led to similar molar quantities of carboxylates per gram of biomaterial, except when glutaric anhydride was used, the functionalization was clearly less efficient and was therefore not pursued (Table 1, entry 10).
The functionalised biomaterials were then used to test whether functionalization had improved their biosorption capacity of nickel in batch (Table 1, entries 7–13). They were put into solutions of 40 mg L−1 of nickel sulfate, corresponding to the highest concentration at which the non-functionalised biomass was saturated with. In general, the functionalised biomass showed one-third to twice more biosorption capacity than the non-modified biomass (Table 1, entries 7–9 & 11–13), except for Ec–GA (Table 1, entry 10). Indeed the biosorption capacity of Ec–GA is similar to the one of Ec, which can be related to the poor efficiency of functionalization using glutaric acid and can be seen as a negative control of this experiment. It is noteworthy that Ec–SA and Cg–CA removed Ni in totality from the concentrated aqueous solution. The maximum capacity of biosorption of Ec–SA was hence tested using a saturating solution of 1024 mg L−1 of nickel sulfate. Ec–SA was able to adsorb up to 51 mg g−1 of Ni, which is higher than non-modified natural materials12,14–16,19 and in the same range as materials that have been modified in eco-unfriendly conditions (Table 3).11,17 These new biomaterials constitute novel, efficient and sustainable solutions to treat heavy metal polluted effluents.
| Biomaterial | Cmax Ni (mg g−1) | References |
|---|---|---|
| Algae | 13–50 | 14 |
| Lichen (Cladonia furcata) | 7.9 | 19 |
| Biomass of Penicillium chrysogenum modified with polyethylenimine (PEI) and then crosslinked with glutaraldehyde | 55 | 11 |
| Aloe barbadensis Miller modified by Na2CO3 | 29 | 15 |
| Lemon peel modified by NaOH | 36 | 16 |
| Ec–SA | 51 | This work |
Preparation and analyses of the Eco-Ni(HCOO)2 catalyst
One functionalised biomaterial, Ec–SA, was transformed into a catalyst for Suzuki–Miyaura coupling reactions (Scheme 2). Ec–SA was heated at 500 °C for 4 h to give an inorganic powder of nickel oxides that was treated with formic acid, a biosourced reducing reagent, leading to the Eco-Ni(HCOO)2 catalyst.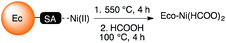 | ||
| Scheme 2 Preparation of Ec–SA biomaterial into the Eco-Ni(HCOO)2 catalyst. Ec–SA stands for Echhornia crassipes biomaterial functionalised with succinic anhydride. | ||
The composition of Eco-Ni(HCOO)2 catalyst was determined by Microwave Plasma Atomic Emission Spectroscopy (MP-AES). As expected from the biosorption experiments, the Eco-Ni(HCOO)2 catalyst presented a high mass fraction of Ni, about 30 wt% (Table 4). The high concentration in Ni was promising to promote organic catalysis.
| Catalyst | Mineral composition (wt% ± rsd) | |||
|---|---|---|---|---|
| Ni | Na | Mg | Ca | |
| Eco-Ni(HCOO)2 | 31.2 ± 2.05 | 0.88 ± 0.29 | 0.10 ± 0.31 | 2.47 ± 1.06 |
The Eco-Ni(HCOO)2 catalyst was characterised by XRPD experiments (black curve, Fig. 1). In parallel, XRPD experiment was also conducted on synthetic and non-biosourced Ni(HCOO)2 for comparison (blue curve, Fig. 1). Although more complex, the diffraction pattern of Eco-Ni(HCOO)2 shows similar peaks, as the diffraction pattern of the non-biosourced Ni(HCOO)2, that is highlighted by the green bars corresponding to the theoretical family of peaks of Ni(HCOO)2. A second family of peaks of Eco-Ni(HCOO)2 was also identified to CaSO4, as shown by the red bars in comparison to database. This salt in biosourced Ni(HCOO)2 was of plant origin.
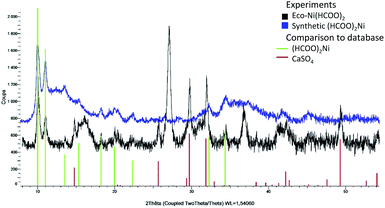 | ||
| Fig. 1 XPRD analyses of Eco-Ni(HCOO)2 (black curve) and of synthetic non-biosourced Ni(HCOO)2 (blue curve) and comparison to database (red and green bars). | ||
FT-IR and XPS analyses were conducted on the ecocatalyst (Fig. S2 and S17†). Eco-Ni(HCOO)2. The spectra were added to the ESI.† The IR spectrum shows absorption bands at 1580 cm−1 and 1325 cm−1 that are characteristic of the carboxylate group of Eco-Ni(HCOO)2.
In XPS, The Ni 2p3/2 binding energy (BE) of 857.8 eV, indicated presence of Ni(II), In conclusion, MP-AES, XRDP, XPS and FT-IR confirmed the generation of Ni(HCOO)2.
Suzuki–Miyaura reactions of aryl iodides
The Suzuki–Miyaura reaction (SMR) has arguably become one of the most utilised tools for the construction of C–C bonds and has found academic use and industrial applications for the production of polymers, fine chemicals, materials and pharmaceuticals. SMR is generally defined as the transition-metal-catalysed cross coupling between an organoboron reagent and an organic halide in the presence of a catalyst and a base. Diverse catalytic systems were developed for SMR, but Pd-based catalysts remained the most efficient and hence compatible with mild conditions.21 However, the depletion of Pd resources in parallel of its geo-economic context have risen the price of Pd to its highest records. Nickel-catalysed SMR has been recently emerging as an interesting and cost-effective alternative.22 Indeed Ni is more abundant and on average ten times cheaper than palladium. However, nickel possesses distinctive catalytic properties than palladium and nickel-catalysed SMR reactions are usually conducted in less mild conditions. Therefore nickel-catalysed SMR deserve the attention for developing greener strategies.23The activity of Eco-Ni(HCOO)2 catalyst was tested in SMR using several aryl iodides and aryl boronic acids (Table 4). Following the principles of green chemistry, not only the catalyst was biosourced but every component of the reactions was considered. Since Eco-Ni(HCOO)2 presents a degree of oxidation of Ni(II) (Fig. S17†), it should be reduced in situ to Ni(0) for SMR. A temperature of 380 °C is necessary to induce the thermal decomposition of formate nickel into Ni(0),24 but is not compatible with SMR. A hydrogen donating solvent, glycerol, was therefore chosen.
The conditions of the coupling reaction were first determined using p-iodoacetophenone and phenylboronic acid (entry 1, Table 4). The reaction led to a quantitative conversion and an excellent yield, using 5 mol% of the bio-sourced Eco-Ni(HCOO)2 catalyst without any ligand. Moreover, the reaction was conducted in eco-friendly conditions using a mild and green base, potassium carbonate, in a mixture of green solvents, glycerol and n-butanol at a moderate temperature.
The scope of the SMR was expanded to phenylboronic acids bearing electron-withdrawing groups (entries 2 & 3, Table 5). The trifluoromethyl group led to similar conversion and yield while the nitro group gave poor conversion, probably due to the low stability of the p-nitrophenylboronic acid.
Diversely para substituted iodobenzene compounds were then tested (entries 4–8, Table 5). p-Nitroiodobenzene led to excellent conversion and yield with no trace of the reduction of the nitro group, while p-iodobenzonitrile mostly led to the reduction of the nitrile group into p-iodobenzylamine.
The presence of an electron-donating group, as a fluor or a methyl, led to reduced yields since the C–I bond was reduced instead. The use of iodobenzene with a heterocyclic compound, as 3-pyridineboronic acid, gave an excellent conversion and yield. It is noteworthy that the presence of pyridine derivative did not deactivate the Eco-Ni(HCOO)2 catalyst, especially since the reaction was conducted without any ligand.
Although a few investigations have been made for Pd-catalysed SMR carried out in green conditions, a few examples are found for Ni-catalysed SMR, illustrating needs in this area.
The coupling conditions between iodobenzene and 3-pyridineboronic acid were compared to Ni-catalysed SMR from literature (Table 5). For this specific reaction, the Ni catalyst is always coordinated to complex ligands, a triazine-based Ni(II) PNP pincer complex25 or graphene oxide crosslinked nickel 5,10,15,20-tetrakis(aminophenyl)porphyrin complex.26 However, the Eco-Ni(HCOO)2 catalyst exhibits similar efficiency and is ligand-free. Moreover, hazardous solvents, dioxane and toluene, were used in the two cited reactions, while a mixture of green solvents was used here. In general, mild conditions should be preferred if applicable as using a mild and inorganic base, and a moderate temperature (Table 6, entries 2 & 3).
Conclusions
In this work, new eco-friendly modified biomaterials were found for removing efficiently Ni from aqueous solutions. A functionalization method for introducing carboxylate moieties was developed using biosourced reagent in green conditions. Water hyacinth and coffee ground functionalised with succinic anhydride led to the best biosorption capacity and could remove Ni totally from a 40 mg L−1 solution of nickel sulfate. The maximum biosorption capacity reached 51 mg g−1 of Ni(II) for water hyacinth functionalized with succinic anhydride.The Ni-enriched biomaterials post biosorption were valorised as an efficient catalyst of Suzuki–Miyaura reactions, which were conducted in green conditions. Several biaryl compounds were obtained in good yields using a biosourced ecocatalyst, no ligand, a mild base, in a mixture of green solvents at a moderate temperature.
Experimental
Materials and methods
All chemicals and solvents were purchased from commercial sources. Plants of Eichhornia crassipes were purchased from a specialized grower (Nymphea company, France). Bark pine and pinecone were collected from black pine trees, Pinus nigra, in the region of Montpellier in autumn 2018. Coffee grounds were obtained directly from our laboratory.The samples for MP-AES analyses were digested in 10 mL of reversed aqua regia (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 hydrochloric acid (37%): nitric acid (65%)) under a microwave-assisted digestion (Multiwave-Go Anton Paar) with the following program: 20–165 °C in 20 min and then 10 min isothermal at 165 °C. Samples were filtered and then diluted to 0.4 mg L−1 in 1% aqueous nitric acid. Mineral compositions were determined by using a microwave plasma-atomic emission spectroscopy (MP-AES) 4200 (Agilent Technologies) equipped with a concentric nebulizer and a double-pass cyclonic spray chamber. The pump speed during analysis was kept at 10 rpm and the sample introduction tube diameter was 0.89 mm. The analytical cycle consisted of 30 s rinsing with aq. 1% nitric acid followed by 25 s of sample uptake (pump speed 40 rpm) and then 20 s of equilibration before the reading at preselected integration times (pump speed 10 rpm). The integration time was set to 3 s for all elements. Unless otherwise stated, the automatic background correction mode available in the software was used. An Agilent SPS3 autosampler was used throughout the study.
2 hydrochloric acid (37%): nitric acid (65%)) under a microwave-assisted digestion (Multiwave-Go Anton Paar) with the following program: 20–165 °C in 20 min and then 10 min isothermal at 165 °C. Samples were filtered and then diluted to 0.4 mg L−1 in 1% aqueous nitric acid. Mineral compositions were determined by using a microwave plasma-atomic emission spectroscopy (MP-AES) 4200 (Agilent Technologies) equipped with a concentric nebulizer and a double-pass cyclonic spray chamber. The pump speed during analysis was kept at 10 rpm and the sample introduction tube diameter was 0.89 mm. The analytical cycle consisted of 30 s rinsing with aq. 1% nitric acid followed by 25 s of sample uptake (pump speed 40 rpm) and then 20 s of equilibration before the reading at preselected integration times (pump speed 10 rpm). The integration time was set to 3 s for all elements. Unless otherwise stated, the automatic background correction mode available in the software was used. An Agilent SPS3 autosampler was used throughout the study.
Gas chromatography analyses were performed using a Thermo Scientific Trace 1300 device equipped with an EI ionization source and an ISQ-QD detector. The reactions were monitored by using para-cymene as internal standard and FID methods for the calibration.
IR spectra were recorded on a PerkinElmer Spectrum 100 FTIR® spectrometer in ATR (Attenuated Total Reflexion) mode. The number of scans was 32, the resolution was 1 point per cm. The acquisition was done from 650 to 4000 cm−1. The detector used was a DTGS (Deuterated-TriGlycine Sulfate). The background was done in air.
NMR spectra were recorded on a Brücker Avance 400 spectrometer at room temperature. 1H frequency was at 400 MHz and 13C frequency was at 100 MHz. 1H and 13C NMR data match that reported in the literature.
Procedure for preparation of the biomaterial
Eichhornia crassipes roots, bark pines and pinecones were dried in an oven at 80 °C for 8 hours. They were then ground, sifted through a 1.25 mesh sieves, and washed with water (3 × 100 mL g−1). They were then dried for 18 hours at 80 °C before functionalization. Coffee grounds were washed several times with hot water until the filtrate became colourless and then were dried for 18 hours at 80 °C before functionalization.General procedure for functionalization
General procedure for biosorption
1 g of functionalised biomaterial was added to a solution of nickel sulfate (1 L of 12, 40 and 1024 mg L−1). The suspension was stirred for 2 hours at room temperature. The solid was filtered and dried at 85 °C for 24 hours. The concentrations of nickel in solution were determined by MP-AES before and after biosorption.Preparation of the catalyst Eco-Ni(HCOO)2
The Ni-enriched biomaterial (Ec–CA–Ni) was heated at 550 °C under air for 4 hours. The resulting powder (243 mg) was heated in formic acid (7 mL) under reflux for 4 hours. The resulting blue–green suspension was cooled to room temperature, then filtrated and washed with 15 mL of formic acid. The resulting solid was rinsed with distilled water in order to dissolve the ecocatalyst. The resulting bright green solution was collected by filtration, then concentrated under reduced pressure, affording Eco-Ni(II) formate as a light green solid (160 mg).General procedure for Suzuki–Miyaura coupling reaction
To a solution of aryl iodide (0.5 mmol, 1 eq.), boronic acid (0.6 mmol, 1.2 eq.) and potassium carbonate (1 mmol, 2 eq.) in a mixture of degassed n-butanol and glycerol (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1 mL) was added the catalyst Eco-Ni(HCOO)2 (5% mol). The suspension mixture was then stirred at 100 °C for 18 hours. The reaction was quenched by adding distilled water (5 mL) and ethyl acetate (10 mL). After separation of the layers, the aqueous layer was extracted 3 times with 10 mL of ethyl acetate. The combined organic layers were washed with a saturated solution of Eco-CaO (thermal treatment of Crepidula fornicata)27 and then dried on anhydrous magnesium sulfate and filtered. The solvent was removed under reduced pressure conversions and yields were established by GC MS/FID, using p-cymene as intern standard, and then confirmed by 1H NMR and 13C NMR.
1, 1 mL) was added the catalyst Eco-Ni(HCOO)2 (5% mol). The suspension mixture was then stirred at 100 °C for 18 hours. The reaction was quenched by adding distilled water (5 mL) and ethyl acetate (10 mL). After separation of the layers, the aqueous layer was extracted 3 times with 10 mL of ethyl acetate. The combined organic layers were washed with a saturated solution of Eco-CaO (thermal treatment of Crepidula fornicata)27 and then dried on anhydrous magnesium sulfate and filtered. The solvent was removed under reduced pressure conversions and yields were established by GC MS/FID, using p-cymene as intern standard, and then confirmed by 1H NMR and 13C NMR.
Author contributions
C. G. conceived and designed the study. F. P. carried out the MP AES assessments and L. C. and P. A. carried out the experimental works of biosorption and organic synthesis. S. D. and C. B. performed XRDP analysis. C. G. wrote the paper.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank the French National Center for Scientific Research (CNRS) and the University of Montpellier for financial support. Sci-GuidEdit is acknowledged for writing assistance and proof reading the article.Notes and references
- H. Hill, A. Goldenberg, M. P. Sheehan, A. Patel and S. E. Jacob, Dermatitis, 2015, 26, 245–253 CrossRef CAS PubMed.
- M. Saito, R. Arakaki, A. Yamada, T. Tsunematsu, Y. Kudo and N. Ishimaru, Int. J. Mol. Sci., 2016, 17, 202 CrossRef PubMed.
- Y.-H. Chiou, R.-H. Wong, M.-R. Chao, C.-Y. Chen, S.-H. Liou and H. Lee, Environ. Mol. Mutagen., 2014, 55, 624–632 CrossRef CAS PubMed.
- D. J. Sivulka, Regul. Toxicol. Pharmacol., 2005, 43, 117–133 CrossRef CAS.
- G. Genchi, A. Carocci, G. Lauria, M. S. Sinicropi and A. Catalano, Int. J. Environ. Res. Public Health, 2020, 17, 679 CrossRef CAS.
- G. Escudero, E. Espinoza and F. Rao, Int. Res. J. Pure Appl. Chem., 2017, 15, 1–7 CrossRef CAS.
- C.-H. Huang, S.-Y. Shen, C.-W. Chen, C.-D. Dong, M. Kumar, B. Dakshinamoorthy and J.-H. Chang, Sustainability, 2020, 12, 7693 CrossRef CAS.
- G. Borbély and E. Nagy, Desalination, 2009, 240, 218–226 CrossRef.
- T. Benvenuti, M. A. S. Rodrigues, A. Moura Bernardes and J. Zoppas Ferreira, in Electrodialysis and Water Reuse: Novel Approaches, ed. A. Moura Bernardes, M. A. Siqueira Rodrigues and J. Zoppas Ferreira, Springer, Berlin, Heidelberg, 2014, pp. 133–144 Search PubMed.
- A. Kilislioglu, Ion Exchange Technologies, BoD – Books on Demand, 2012 Search PubMed.
- S. Deng and Y.-P. Ting, Water Res., 2005, 39, 2167–2177 CrossRef CAS PubMed.
- R. S. A. Husain, A. J. Thatheyus and D. Ramya, Am. J. Microbiol. Res., 2013, 1(3), 48–52 Search PubMed.
- P. S. Lau, H. Y. Lee, C. C. K. Tsang, N. F. Y. Tam and Y. S. Wong, Environ. Technol., 1999, 20, 953–961 CrossRef CAS.
- D. H. K. Reddy, D. K. V. Ramana, K. Seshaiah and A. V. R. Reddy, Desalination, 2011, 268, 150–157 CrossRef CAS.
- S. Gupta, S. K. Sharma and A. Kumar, Water Sci. Eng., 2019, 12, 27–36 CrossRef.
- M. Villen-Guzman, D. Gutierrez-Pinilla, C. Gomez-Lahoz, C. Vereda-Alonso, J. M. Rodriguez-Maroto and B. Arhoun, Environ. Res., 2019, 179, 108849 CrossRef CAS.
- N. Feng, X. Guo, S. Liang, Y. Zhu and J. Liu, J. Hazard. Mater., 2011, 185, 49–54 CrossRef CAS.
- A. Garcia, P.-A. Deyris, P. Adler, F. Pelissier, T. Dumas, Y.-M. Legrand and C. Grison, J. Cleaner Prod., 2021, 299, 126895 CrossRef CAS.
- A. Sarı, M. Tuzen, Ö. D. Uluözlü and M. Soylak, Biochem. Eng. J., 2007, 37, 151–158 CrossRef.
- C. Grison, V. Escande, E. Petit, L. Garoux, C. Boulanger and C. Grison, RSC Adv., 2013, 3, 22340–22345 RSC.
- I. Maluenda and O. Navarro, Molecules, 2015, 20, 7528–7557 CrossRef CAS PubMed.
- F.-S. Han, Chem. Soc. Rev., 2013, 42, 5270 RSC.
- S. E. Hooshmand, B. Heidari, R. Sedghi and R. S. Varma, Green Chem., 2019, 21, 381–405 RSC.
- V. Escande, C. Poullain, G. Clavé, E. Petit, N. Masquelez, P. Hesemann and C. Grison, Appl. Catal., B, 2017, 210, 495–503 CrossRef CAS.
- M. Mastalir, B. Stöger, E. Pittenauer, G. Allmaier and K. Kirchner, Org. Lett., 2016, 18, 3186–3189 CrossRef CAS.
- M. Keyhaniyan, A. Shiri, H. Eshghi and A. Khojastehnezhad, New J. Chem., 2018, 42, 19433–19441 RSC.
- C. Grison, PCT Int. Appl. WO2015036714 (A1), 2015.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods, IR spectra of biomaterials and Eco-Ni(HCOO)2, description of products, XPS of Eco-Ni(HCOO)2. See DOI: 10.1039/d1ra04478h |
| This journal is © The Royal Society of Chemistry 2021 |