Open Access Article
Open Access ArticleA review on non-edible oil as a potential feedstock for biodiesel: physicochemical properties and production technologies
Marwan Abdul Hakim Shaah
a,
Md. Sohrab Hossain
 *a,
Faisal Aboelksim Salem Allafi
a,
Alyaa Alsaedi
a,
Norli Ismail
a,
Mohd Omar Ab Kadir
b and
Mardiana Idayu Ahmad
*a
*a,
Faisal Aboelksim Salem Allafi
a,
Alyaa Alsaedi
a,
Norli Ismail
a,
Mohd Omar Ab Kadir
b and
Mardiana Idayu Ahmad
*a
aSchool of Industrial Technology, Universiti Sains Malaysia, 11800 USM, Penang, Malaysia. E-mail: sohrab@usm.my; mardianaidayu@usm.my; Fax: +6046533678; Tel: +6046532216 Tel: +6046532214
bPultex Sdn Bhd, Jalan Kampung Jawa, Bayan Baru, 11950 Bayan Lepas, Penang, Malaysia
First published on 19th July 2021
Abstract
There is increasing concern regarding alleviating world energy demand by determining an alternative to petroleum-derived fuels due to the rapid depletion of fossil fuels, rapid population growth, and urbanization. Biodiesel can be utilized as an alternative fuel to petroleum-derived diesel for the combustion engine. At present, edible crops are the primary source of biodiesel production. However, the excessive utilization of these edible crops for large-scale biodiesel production might cause food supply depletion and economic imbalance. Moreover, the utilization of edible oil as a biodiesel feedstock increases biodiesel production costs due to the high price of edible oils. A possible solution to overcome the existing limitations of biodiesel production is to utilize non-edible crops oil as a feedstock. The present study was conducted to determine the possibility and challenges of utilizing non-edible oil as a potential feedstock for biodiesel production. Several aspects related to non-edible oil as a biodiesel feedstock such as overview of biodiesel feedstocks, non-edible oil resources, non-edible oil extraction technology, its physicochemical and fatty acid properties, biodiesel production technologies, advantages and limitation of using non-edible oil as a feedstock for biodiesel production have been reviewed in various recent publications. The finding of the present study reveals that there is a huge opportunity to utilize non-edible oil as a feedstock for biodiesel production.
Introduction
The fossil fuel energy is non-renewable energy, and the stock of this energy is projected to be exhausted soon. Besides, fossil fuel combustion increases CO2 concentration in the atmosphere, which is precisely related to global warming.1 It is being reported that about 15 billion tons of CO2 is dispersed into the environment annually due to the utilization of petroleum oil in diesel engines.1,2 Globally, renewable energy demand is expanding progressively to balance global energy demand with population and industrial growth. Biodiesel is a promising renewable energy source that could be utilized as the alternative to fossil fuel energy to comply with global energy demands. The rapid depletion of the petroleum-derived fuel reserves has accelerated the search for alternative energy sources. Among the renewable energy sources, biodiesel is considered a well-known alternative to petroleum-derived fuel because it is environment-friendly, economically competitive, and technically viable, and it does not require any mechanical modification of the diesel-based engines.2,3Biodiesel is a liquid biofuel that can be synthesized from industrial crops, agricultural by-products, and municipal wastes.3 Studies have been performed on biodiesel production from various matrices and develop its quantitative and qualitative properties to improve its reliability and sustainability as a renowned source of green energy.1,5 Biodiesel has been produced by utilizing various agricultural crops (i.e., corn, sugarcane, and wheat) and edible oil (rapeseed, sunflower, soybean and palm) as feedstock for the first-generation biofuel.5,6 However, the utilization of agricultural crops and edible oil to produce biodiesel have raised a potential conflict of “food vs. fuel”. Besides, this increased the biodiesel production cost due to increasing feedstock price with growing market demand along with competition for food.6 Consequently, studies have been carried out on biodiesel production from non-food crops, animal fats, fungi, bacteria, and microalgae.5–8
Various technologies have been employed to produce biodiesel from non-edible oils.3,8–10 The potential advantages of producing biodiesel from non-edible oils are available in abundance, low production cost, high oil yield and above all it does not conflict with food products. Therefore, the present study was conducted to review the potential and challenges of utilizing non-edible oils as a reliable feedstock for biodiesel production. Wherein, the feasibility of non-edible oil as a feedstock was determined by reviewing various aspects of non-edible oils such as oil composition, cultivation, oil yield, land and resources availability for cultivation. Besides, biodiesel conversion technologies and the properties of produced biodiesel from various non-edible oils were also reviewed in this study. Finally, it was pointed out the best non-edible crop, conversation technology and author views to overcome limitations of using non-edible oil as a potential feedstock for biodiesel production.
Global biodiesel production
Diesel is the most used fossil fuel for various transportations and machinery due to its intense heating power and combustion properties.11 Diesel as an energy fuel for vehicles is undeniable as Europe and the United States alone make up to 33% of diesel-powered vehicles, which was forecast to increase by 7% between 2004 and 2012.12 However, The International Energy Agency (IEA) reported that global energy consumption in transportation would increase by over 75% in 2050, resulting in a doubling of associated CO2 emissions.13 The transport sector is the ultimate source of CO2 emissions in the Nordic region, accounting for almost 40% of total CO2 emissions. CO2 is the primary greenhouse gas, and it is highly responsible for global warming. It was estimated the global energy consumption reached almost double from 6630 million tons of oil equivalents (Mtoe) in 1980 to 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 276 Mtoe in 2016. The energy consumption was mainly petroleum-based fuels, accounting for 85.52%, wherein renewable energy contributes to 3.16%.13
276 Mtoe in 2016. The energy consumption was mainly petroleum-based fuels, accounting for 85.52%, wherein renewable energy contributes to 3.16%.13
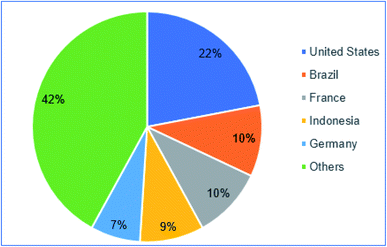
Global energy consumption has increased hastily with increasing population, lifestyle, and rural development. However, the utilization of fossil fuels-based energy is significantly increasing the environmental pollution concern and threatening our ecosystem.3,5,8 Due to the rapid depletion of primary energy sources and the ecological pollution concern of using petroleum-based fuel, the world energy commission is looking for alternative energy, wherein biodiesel is highly promising. The name ‘Biodiesel’ was promoted by the National Soy Diesel Development Board, USA in 1992.14 Generally, biodiesel is a non-petroleum-based fuel consisting of mono-alkyl esters of long-chain fatty acids, derived from vegetable oils or animal fats with an added requisite of minimizing greenhouse gas emissions. Global biodiesel production was 9.2 Mtoe in 2000, which increased to 95 Mtoe in 2018.15 Global biodiesel production and consumption are currently leading by the USA and Europe. The annual consumption of biodiesel in 2005 was 3.02 MT in Europe mad 3.32 MT in the USA. These are about 0.5–1% and about 2% of total biofuels consumption in the transportation section of USA and Europe, respectively.15 Many countries in the world are encouraged to use biodiesel in the transportation sectors, which tremendously enhanced global biodiesel consumption. It was reported that the total biodiesel consumption was 26.8 MT in 2016 by 56 countries, where 58% of the total biodiesel consumed by the five countries, as shown in Fig. 1.
Many countries encourage to increase biodiesel production and utilization in the transportation sectors. Like Europe and the USA, the biodiesel production and utilization has also started experiencing in Asian countries, including China, India and Malaysia. For instance, China and India government have targeted to utilize 15% biodiesel blended with petro-diesel in 2020. The government of Malaysia has stated lunching B10 (10% biodiesel blended with petro-diesel) in 2019, which would be increased to B20 in 2020.16 The global biodiesel production is increasing rapidly due to its potential advantage in replacing petro-diesel, such as:2,3,9,17
(i) Biodiesel is derived from renewable energy sources.
(ii) Biodiesel is highly biodegradable.
(iii) Biodiesel is noncorrosive.
(iv) Minimize the dependency on fossil fuels.
(v) Reduce greenhouses gases emission and global warming.
(vi) It can be utilized as an alternative fuel for diesel in boilers or internal combustion engines with minor mechanical modifications.
(vii) Biodiesel promotes complete combustion.
(viii) Suitable to utilize in modern diesel engines with equally engine performance of petro-diesel.
(ix) Biodiesel has a higher lubricity than petroleum-derived diesel.
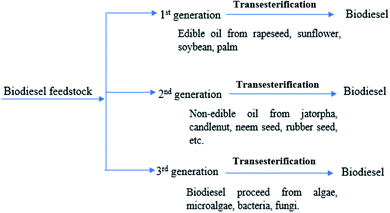
Biodiesel feedstock
Biodiesel can be derived from renewable biomass of both plant and animal matters. Several biomasses such as edible oils,18,19 non-edible oil,3,8 waste cooking oils20 and algae6 are the feedstock for the biodiesel production. It is being reported that there are 350 oil-producing crops (edible and non-edible) worldwide would utilize for biodiesel production.20 This high number of feedstock sources make biodiesel production more attractive. The feedstocks of biodiesel can be categorized based on the biomass sources as 1st generation biofuel, 2nd generation biofuel and 3rd generation biofuel. The potential feedstocks for biodiesel productions are shown in Fig. 2.Biodiesel is derived from plant oils, and animal fats have similar characteristics to petroleum-derived diesel oil.21 The edible oil utilized for biodiesel production is including sunflower oil,19 soybean oil,22 rapeseed oil,23 palm oil24 and coconut oil.25 Over 95% of biodiesel are currently obtained from edible oils.15,21 Several issues have raised with the utilization of edible oils for the biodiesel production, mainly its consequences to the world food market. Large-scale biodiesel production from edible oil crops may bring global imbalance of food supply, which might enhance the global food insecurity.7,11 The argument of whether the production of feedstock is for food or fuel continues, albeit with the overstated consequences as this may result in a price hike of biodiesel and edible oils. Recently, the environmentalist has also raised concern on biodiesel production from edible oil. They argued that the large-scale production of biodiesel requires the expansion plantation of edible oil crops, which causing deforestation and destroying the ecosystem.4,6 Thus, biodiesel production from edible oil enforces the competition between food vs. fuel economy. Wherein, the production of biodiesel from edible oil is competing with the limited land area available for the food crops production. This trend is already being observed in many countries globally, where a significant amount of land has been utilized for oil crops production to mitigate the increasing demand for biodiesel production. Eventually, the implementation of edible oil biodiesel as a substitute for petroleum diesel oil may reduce the worldwide edible oil supply.
To overcome from this distressing phenomenon, numerous researches have been conducted worldwide to determine alternative and renewable feedstocks for biodiesel production.3,8,9,18 Biodiesel derived from non-edible oil is considered 2nd generation biofuel. Jatropha, rubber seed, jojoba, tobacco seed, sea mango, neem, candlenut, mahua, karanja, yellow oleander are examples of non-edible plant sources which make up the 2nd generation feedstock.18 Moreover, animal fats sources like poultry fat, pork lard, and beef tallow can be utilized as a source for producing biodiesel.18,26 In recent years, waste edible oils such as yellow grease and waste cooking oils have also been utilized as possible sources of biodiesel production.16,20 Biodiesel can also derive from algal biomass.17 Algae can be divided into two major groups, such as unicellular (microalgae) and multicellular (macroalgae or seaweeds).27 Microalgae as a source of liquid fuel was initiated during the 1980s,17 which has sparked the study of various kinds of microalgae species to produce biodiesel in different growth environments. Generally, the high lipids content of algae (about 70%) makes it considerable source for biodiesel production. Therefore, a large amount of biodiesel can be produced a relatively small amount of algal biomass. Besides, its high photosynthetic efficiency impacts in controlling global warming. Thus, algal biomass could be applied as a leading feedstock for the production of biodiesel. However, the conversion of biodiesel from algal biomass requires advanced technology for the algal oil extraction and removal of the fermentable sugar from algal biomass.28 Besides, the algal biomass has potential applications in food, pharmaceuticals, and cosmetics industries.27 Table 1 represents the comparisons among first, second and third-generation feedstock for biodiesel production.3,6,18,29
| Biofuels | Feedstock source | Advantages | Disadvantages |
|---|---|---|---|
| 1st generation | Edible oil | - Simple conversation process | - Relative low oil yield |
| - Food vs. biofuel debate | |||
| - Causes deforestation and destroying ecosystem | |||
| 2nd generation | Non-edible oil | - Abundance availability number of non-edible crops worldwide | - Intractable structure of the feedstock |
| - No debate between food vs. fuel economy | |||
| 3rd generation | Algal biomass | - High lipids content | - It requires advance technology for biodiesel conversion |
| - High growth rate | - It has other application in food, pharmaceutical and cosmetics industries | ||
| - Its cultivation reduces global warming |
Non-edible oil as a potential feedstock for biodiesel production
Although oil compositions such as fatty acids, saturated fat, and unsaturated fat in both non-edible and edible oil oils are almost similar, the edible oil contains valuable nutrient and antioxidants. Conversely, non-edible oil derived from Jatropha, sea mango, rubber seed, and candlenut are not suitable for human consumption because it contains toxic substances in the oil.29–31 For instance, the Jatropha seed oil contains purgative and curcas.30 The rubber seed oil contains cyanogenic glucoside.31 Thus, oil extracted from the non-edible crops can utilize as an alternative feedstock for biodiesel production to overcome food versus fuel obstacles by tapping into non-edible oils for manufacturing biodiesel. The non-edible oil plants can be grown mainly in wastelands worldwide and lessen the need for further deforestation and food supply issues.29 Two main factors that make a feedstock a consideration for producing biofuel are the percentage of oil that can be derived and agricultural harvest from the farmed land. Non-edible oils could be considered as good sources of biodiesel production mainly because they are easily transferable in liquid form, renewable, efficiently combustible, low sulfur and fragrance content and biodegradable.3,29,32Table 2 shows the list of non-edible oil crops, their annual production per hector land per year (kg per ht per year) and percentage oil yield (wt%). The raw materials price is the main obstacle to produce biodiesel. It is being reported that the raw material price for biodiesel production is an account of 70–90% of the total biodiesel production.29,32 Non-edible oil crops such as such as Jatropha (2500 kg per ht per year),33 candlenut (1600 kg per ht per year),35 neem (2670 kg per ht per year),40 karanja (900–9000 kg per ht per year),41 yellow oleander (5200 kg per ht per year),46 sea mango (1900–2500 kg per ht per year)47 grow in plenty. These plants can grow almost anywhere with minimal cultivation efforts, even in sandy and saline soils, which are not suitable for food crops production.33,39,44 Thus, the utilization of the non-edible oil as a feedstock would minimize the biodiesel production cost due to the cheaper raw materials source. Generally, the plantation cost for non-edible oil crops is much cheaper than edible crops. This is because the cultivation of the edible crop requires high soil nutrition, a good irrigation system, and incentive care to maintain soil nutrients and moisture.33 Another important fact for determining the suitability of using non-edible oil as an alternative feedstock for biodiesel production is the percentage of oil content (wt%). The percentage oil content in Jatropha seed (40–60 wt%),33 rubber seed (40–50 wt%),36 see mango seed (40–50 wt%),47 candlenut (60–65 wt%),35 polanga (60–70 wt%)43 and yellow oleander (60–65 wt%)46 are much higher than edible oil crops such as rapeseed (37–50 wt%),23 soybean (20 wt%)22 and palm (20 wt%).24
| Non-edible oil crops | Scientific name | Plant type | Major crop | Yield (kg per ha per year) | Oil content (wt%) | References |
|---|---|---|---|---|---|---|
| Jatropha | Jatropha curcas | Tree | Seed | 2500 | 40–60 | 33 |
| Mahua | Madhuca longifolia | Tree | Seed | 20–200 | 35–50 | 34 |
| Candlenut | Aleurites moluccanus | Tree | Seed | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
60–65 | 35 |
| Rubber | Hevea brasiliensis | Tree | Seed | 100–150 | 40–50 | 36 |
| Soapnut | Sapindus mukorossi | Tree | Seed | — | 23–30 | 33 and 37 |
| Jojoba | Simmondsia chinensis | Shrub | Seed | 500–5000 | 40–50 | 38 |
| Tobacco | Nicotiana tabacum | Herb | Seed | 1170 | 35–49 | 39 |
| Neem | Azadirachta indica | Tree | Seed | 2670 | 25–45 | 40 |
| Karanja | Millettia pinnata | Tree | Seed | 900–9000 | 30–50 | 41 |
| Castor | Ricinus communis | Tree/shrub | Seed | 450 | 45–50 | 42 |
| Polanga | Calophyllum inophyllum L. | Tree | Seed | 3700 | 65–75 | 43 |
| Cotton | Gossypium | Tree | Seed | 649 | 17–23 | 44 |
| Kusum | Carthamus tinctorius | Tree | Seed | — | 51–62 | 45 |
| Yellow oleander | Cascabela thevetia | Tree | Seed | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
60–65 | 46 |
| Sea mango | Cerbera odollam | Tree | Seed | 1900–2500 | 40–50 | 47 |
| Tung | Vernicia fordii | Tree | Seed | 450–600 | 30–40 | 33 |
| Bottle tree | Brachychiton rupestris | Tree | Seed | 250–300 | 50–60 | 48 |
Non-edible oil crops cultivation
With rapid population growth, urbanization and industrialization, the land area available from food production decreases. It urges proper distribution of the available land for agriculture, urbanization, commercial application, and forest reservation. If the edible oil crops are utilized as feedstocks for biodiesel production, it will burden the land area available for food production. However, the non-edible crops have unique botanical features and can grow in non-fertile land like sandy, saline, and gravely soils that are not suitable for food production.18,29 For instance, Jatropha is a small tree or tall bush 5–7 m high. It is considered a multipurpose drought resistance tree. It can grow and survive in abandoned agricultural areas.29 It is a tropical tree capable of growing in different climate zones with 250–1200 mm rainfall. Jatropha tree is native to Mexico, United States, Argentina, Paraguay, Peru, Brazil, Bolivia, and throughout the tropics, including Asia and Africa.33,49 It also has been cultivated in many harsh regions, and its yields reach about 0.5 ton per hectare in these areas.49Mahua (Madhuca indica) tree is a medium-sized tree with a height of up to 20 m. It generally grows mostly in India, Pakistan, Bangladesh, and Malaysia.34,50 It is a fast-growing evergreen or semi-green tree, and it can be planted in hot and wet regions. The potential production of mahua seed is about 60 MT per annum in India.34 The oilseeds and oil yield in the mahua crops vary with the maturity of the mahua tree. Generally, total oilseed yield per annum from the mature mahua tree ranges from 20 to 200 kg ha−1, wherein the total oil yield per annum is about 2.7 tonnes per ha. The calorific value of mahua seed oil is reported to be 38.5 kJ kg−1, which is lower by 14% than the mineral diesel's calorific value (42 kJ kg−1).50 Candlenut (Aleurites moluccana L.) is a flowering tree and the height of a mature candlenut tree about 20 m. It is also known as kemiri in Bahasa Indonesia and kukui nut in Hawaii.51 The candlenut tree is a domesticated multipurpose tree, mainly growing in the Indo-Malaysia region.35 The annual production of the candlenut seed about 16 ton per ha and the yearly oil yield is around 3200 kg ha−1.51 Candlenut seed contains approximately 30–60% of candlenut oil, and the oil can be obtained from the candlenut seeds using several extraction techniques. Candlenut oil has a high iodine number (≥125) and a lower pour point.
The rubber tree is also known as the para rubber, and it derives from the amazon rain forest in Brazil. The rubber tree is distributed in Malaysia, Indonesia, India, Thailand, Sri Lanka, and Liberia. The height of a rubber tree up to 34 m and rubber seeds' weight ranging from 2–4 g.33 A typical plantation has about 350–500 trees per ha and yields about 100 to 150 kg ha−1 rubber seeds annually.52 The rubber seeds oil is brown, and the oil content in rubber seeds and kernel accounts for 40–60 wt%.33 The fatty acid content in rubber seed oil is about 17%, higher than vegetable oils. The major fatty acid compositions in rubber seeds oil are stearic acid, oleic acid, palmitic acid, linoleic acid, and linolenic acid.53
Soapnut tree is generally growing in tropical and subtropical climates.54 The soapnut tree can grow in leached and deep loamy soils; therefore, the cultivation of the soapnut tree in leached and deep loamy soils would minimize soil erosion.33 The seeds contain 23–51.8 wt% oil, the oil contains about 92% is triglycerides.37 Jojoba tree grows in Mexico, Mojave, and the Sonoran Deserts.38 Jojoba tree is 0.7–1.0 m high, and the jojoba fruits look like dark brown nutlike fruit. The seeds of the jojoba contain about 45 to 55 wt% lipid.55 The characteristics of jojoba oil differ fundamentally from edible oil. The chemical structure of the jojoba seeds oil contains long straight-chain ester, and the oil contains about 97% of waxed ester and 3% of FFAs.56 Jojoba oil is non-toxic, biodegradable, high viscosity, low volatility, high flash points, and relatively stable with a high dielectric constant.55 The high oil content and the wild nature of jojoba plants make it one of the best non-edible crops to be used as a potential feedstock for biodiesel production.
Tobacco is one of the most common non-edible crops in the world, with enormous social and economic importance. It is an annually grown herbaceous plant widespread in South and North America, Russia, India, and Macedonia.39 Tobacco seed contains 35–49 wt% of tobacco oil, and the oil does not contain nicotine.33 The primary fatty acids in tobacco seed oil are palmitic acid, linoleic acid, stearic acid, and oleic acid.57 Neem is a fast-growing tree with a height of 25 m.40 Generally, the neem tree can tolerate high temperatures and grow in non-fertile and degraded soil. The tree is originated from the Indian subcontinent but becomes a very established tree in many countries around the world, including Africa, central and south America, Bangladesh, Burma, Malaysia, Pakistan, and Sri Lanka.33 The fruiting of the neem tree starts at 3–5 years, but the maximum productivity of neem seeds begins after 15 years of plantation. The neem fruit has a shape that varies from oval to round, with a diameter of 1.0–1.5 cm and length 1.4–2.8 cm.58 Neem seeds have 45 wt% oil, and it mainly contains oleic, palmitic, and stearic acids.40
Karanja is a fast-growing and medium-sized leguminous tree. It can grow in various agro-climatic conditions, including clayey soil, stony soil, and sandy soil.59 The height of the karanja is about 25 m. The harvesting of the karanja seeds can carry out after 4–6 years of plantation. The yield of karanja seeds of 0.9 to 9.0 tonnes per ha.41 The fresh seeds contain approximately 30–35 wt% thick yellow-orange to brown oil.59 The karanja oil is considered a non-edible oil due to having karanjin and toxic di-ketone pongamol.41 The major fatty acids in karanja oil are palmitic, linoleic, oleic acid, and stearic acids.59 Castor plant grows in tropical regions worldwide, and it grows well in dry subtropical areas to wet tropics within the temperature range from 20–25 °C.60 The plant is drought and pest-resistant and can be grown practically anywhere land is available. It grows well from the. Castor seeds are poisonous to humans and animals due to the presence of ricin and other toxic compounds. The oil content in castor seeds is 46–55 wt%.42 Polanga is a medium to large evergreen and non-edible oilseed tree with an average length 8–20 m. It grows on exposed sea sands or in deep soil with a 750–5000 mm per year rainfall requirement. The tree begins yield after 4–5 years of plantation. The fruit bears a seed inside a corky shell covering and size of the seeds is 10–20 mm. The oil yield from polanga plantation is about 2000 kg per ha per annum. Polanga seeds have a high oil content (65–75%) that contains various saturated and unsaturated fatty acid.43 The polanga seeds oil is greenish with thick, woodsy or nutty-smelling.61
Kusum tree is a medium to large-sized tree with 35 to 45 feet in height. The oil content in kusum seeds is 51–62%.45 The oil contains toxic cyanogenic compounds and therefore, the kusum oil is not considered as edible oil. The fatty acid profile in kusum oil shows about 40% unsaturated fatty acid, and 53% saturated fatty acid.62 Yellow oleander is a drought-resistant, and non-edible shrub.46 The Yellow oleander plant is native to tropics and subtropics countries and is inherent to Central and South America. The height of the yellow oleander tree is about 10–18 feet. The annual production of yellow oleander seeds is about 52 tonnes per ha, and the seeds contain 60–65 wt% oil.29 Tung tree grows in native China and other countries below an altitude of 1600 m. The average height of the tung trees is about 20 m.63 The fruit's oil content is between 14–20%, seed's oil content between 30–40% and the kernel oil content between 53–60%. The average oil yield is 450–600 kg per ha per annum.33,64 Tung oil contains unsaturated fatty acids, α-eleostearic acid, β-eleostearic acid, and high conjugated triene fatty acid.63 Moringa oleifera is a fast-growing and widely cultivated plant. Generally, the moringa plants grow in tropical and subtropical areas with a required rainfall between 250 and 2000 mm. It can grow in tolerates poor soil and dry sandy soil.65,66 The moringa seed contains 38–40% of oil, and the oil contains high-quality fatty acid (oleic acid > 70%).66
Although non-edible oil crops oil is considered as an alternative feedstock for biodiesel production, the low oil yield of some non-edible crops, high FFAs, high polyunsaturated fatty acids, and low unsaturated fatty acids content in oil is the major barrier for the commercial-scale biodiesel production.18,29,63 Better quality biodiesel contains a higher amount of mono-unsaturated fatty acids, the lesser amount of saturated and polyunsaturated fatty acids. To overcome these limitations, scientists and plant biotechnologists have implemented genetic engineering technology.67 The purpose of genetically modified plants is to enhance seed oil yield in crops and improve oil quality by changing the fatty acids compositions in plant seed oil. For instance, Roesler et al.68 obtained an increase 5% rapeseed seed oil by modifying rapeseed chloroplast using a cytosolic version of acetyl-CoA carboxylase enzymes. Vigeolas and Geigenberger69 increased about 40% seed oil content in rapeseed with increasing glycerol-3-phosphate levels in seeds by overexpression of a yeast cytosolic glycerol-3-phosphate dehydrogenase. Pollard et al.70 found that the accumulation of 90% short and medium-chain fatty acids in cuphea (Cuphea hookeriana) seeds by inserting of chain length specific acyl-ACP thioesterases in seeds.
Non-edible oil extraction technology
Many technologies have been utilized for the extraction of non-edible oil from various plant seeds, such as (i) mechanical screw press, (ii) Soxhlet extraction, (iii) enzymatic extraction and (iv) microwave extraction. However, mechanical screw press and Soxhlet extraction are most used in oil extraction from non-edible plant seeds. In recent years, the scCO2 extraction technology has been extensively utilized in oil extraction due it offers various distinct advantages over others oil extraction technology. The advantages and limitations of different oil extraction technologies are presented in Table 3.| Technology | Advantages | Limitation | References |
|---|---|---|---|
| Mechanical press | - Higher yield | - High maintain cost | 72–74 |
| - Easy to operate | - Requires moisture reduction in oil seeds | ||
| - Requires further oil refining and degumming processes | |||
| - Not suitable for non-edible seed oil extraction | |||
| Soxhlet extraction | - Low cost | - Utilize volatile organic solvent | 75–79 |
| - Easy to operate | - Long operating time | ||
| - Higher yield | - High operating temperature | ||
| - Requires solvent separation process | |||
| - Requires refining process | |||
| Microwave extraction | - Enhance oil extraction yield | - Operating temperature vary with solvent boiling temperature | 80–84 |
| - Minimize solvent uses | - Generally utilized as a pretreatment for solvent extraction | ||
| - Shorter extraction time than solvent extraction | |||
| Enzymatic oil extraction | - Organic solvent free technology | - Requires prolonged extraction time | 85 and 86 |
| - Environmentally friendly | |||
| scCO2 extraction | - Green technology | - High cost of the equipment | 87–89 |
| - Does not require toxic organic solvent | |||
| - Does not require any refining and oil separation technology | |||
| - Low temperature technology | |||
| - Higher selectivity and diffusivity to fatty acids |
Mechanical press, also known as screw press or hydraulic press, is the most common method used in extracting oils from natural materials. This technique is the most conventional oil extraction method. This method is widely used on seeds with high oil content, such as olive, Jatropha, and candlenut.71–73 The mechanical pressing extraction technique operates using a rotating worm shaft to increase the pressure up 174 to 100 MPa by reducing the space and volume on the extraction chamber to squeeze out the oil from the materials.51,72 The mechanical press has a relatively simple operation process, giving a high yield of oil extraction. However, tedious pre-treatments are required on the material before the press method, such as drying, dehulling, particle size reduction, and cooking, to increase the extraction method.51,74 Uquiche et al.74 utilized microwave radiation as a substrate pre-treatment to extract hazelnut oil using mechanical extraction. The study reported that the hazelnut nut oil yield increased with the pre-treatment. Rodrigues et al.8 reported that moisture content plays an influential role in the extraction of Jatropha seed oil subjected to mechanical extraction. The disadvantage of applying mechanical extraction technology in non-edible extraction is that the extracted oil requires further oil purification of filtration and degumming processes.69 The mechanical extraction of non-edible seed oil depends on moisture content, percentage of oil containing in the seeds, and pre-treatment process of seeds.51,71 However, the mechanical extractor designs have been made for some oilseeds. Therefore, the extraction yields influence when the extractor design is utilized in other seed oil extraction.
Soxhlet extraction is commonly used for the extraction of oil using an organic solvent, where hexane is the most used solvent.74,75 Other solvents used in Soxhlet extraction for non-edible oil extraction are methanol, ethanol, diethyl ether, acetone, and chloroform.74,77 Several parameters control the performance of Soxhlet extraction, which are the type of solvent, operating temperature, and particle size of a sample. A mixture of solvents can also apply during the extraction of lipids, which have different types of polarities.77 Particle size is one of the most influential parameters in Soxhlet extraction, as the smaller particle size has a greater interfacial area between the solvent and solid matrices.76 Temperature potentially influences the extraction rate because an increase of temperate increases the solubility of the solid matrices.75 Karthikeyan et al.76 extracted non-edible oil from Catharanthus roseus seed oil using the Soxhlet extraction method with several organic solvents, including methanol, diethyl ether, acetone, and chloroform. The study reported that the highest oil yield of 31.50% was obtained using methanol as solvent at 65 °C for 3 h. Jamil et al.78 determined the influence of Soxhlet extraction process parameters such as time, temperature and solvent to seed ratio for the extraction of date pit oil. The highest yield of date pits oil was gained of 16.5 wt% at a solvent to seed ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, temperature 65 °C and time 7 h.78 Mueanmas et al.79 extracted non-edible oil from waste coffee grounds for being a feedstock for biodiesel production.
1, temperature 65 °C and time 7 h.78 Mueanmas et al.79 extracted non-edible oil from waste coffee grounds for being a feedstock for biodiesel production.
The implementation of microwave heating as a pre-treatment process in solvent extraction has recently attracted interest to the researcher as it increases the extraction efficiency of trace organic pollutants from foods and non-food materials. Studies have been conducted the determine the efficiency of the combination of microwave heating and solvent extraction method to extract several compounds from various matrices, including pectin,80 essential oils,81 pesticides,82 and polychlorobiphenyls.83 It was found that the combination of microwave heating and solvent extraction method offering many advantages, such as less solvent usage, shorter extraction time, higher extraction rate, and high quality of extract.80,82 Ibrahim et al.84 utilized microwave heating as pre-treatment during the extraction of non-edible sandbox seed oil using hexane as a solvent. The study reported that the application of microwave heating enhances the oil yield and minimizes the FFA content in solvent-extracted sandbox seed oil. The enzymatic technique has been viewed as a promising extraction method for non-edible oil extraction as an organic solvent-free extraction technology.85 The main advantage of the enzymatic oil extraction technology is that it is environmentally friendly and does not require any volatile organic solvent.86 However, the enzymatic oil extraction method requires prolonged extraction time compared to the other available technology for edible and non-edible oil extraction.
In recent years, there is an increasing interest in utilizing supercritical carbon dioxide (scCO2) technology to extract separation edible and non-edible oil due to the environmental pollution concern with the organic solvents used in conventional solvent extraction.87–89 The organic solvents used in solvent extraction are toxic and volatile, those can pose various environmental pollution concerns including atmospheric and soil toxicity.89 The scCO2 is viewed as an attractive alternative solvent to organic solvents because it is neither toxic nor volatile. Although CO2 is a greenhouse gas, the CO2 utilized in the supercritical extraction system is withdrawn from the environment and return to the environment after extraction. Therefore, the scCO2 does not contribute to greenhouse effects.90 The scCO2 extraction is viewed as the most promising technology in extraction separation of edible and non-edible oil due to the several distinct advantages over other existing technology, including higher solubility, higher selectivity, mass transfer rates, and does not require further refining process to separate the extracted oil.84,89 The component selectivity depends on the density of the supercritical fluid, which could be altered by varying the process pressure and temperature.87 Furthermore, the scCO2 extraction method can efficiently extract several compounds such as essential oil, caffeine, pesticide, lipids, and fatty acids, as reported elsewhere.87,89,91,92 The extraction is carried out by the scCO2 in the extraction vessel. Subsequently, the extracted oil is collected from the separation vessel, while the fiber retains in the extraction chamber.
A supercritical condition can be defined as a state that has a pressure and temperature greater than its critical point where vapour and liquid reach equilibrium in this phase.87,92 In the supercritical condition, the fluid has a liquid-like density and gas-like viscosities, where its diffusivity is higher than liquid solvents, making it capable of performing better mass transfer (such as extraction) and reaction rates.91 Carbon dioxide reaches supercritical conditions at a critical temperature above 31.1 °C and critical pressure above 7.39 MPa.86 Cheng et al.93 extracted lipids from microalgae using solvent extraction and scCO2 extraction methods for 241 biodiesel synthesis. The study reported that the scCO2 technology effectively produces higher selectivity lipid extraction for biodiesel synthesis. Chen et al.94 produced biodiesel from scCO2-extracted Jatropha oil using a supercritical methylation process. The study reported that the scCO2 is a superior technology for the extraction of lipids for biodiesel syntheses, since this technology does degrade triglycerides in the extracted lipids. Subroto et al.87 reported that the candlenut oil is susceptible to oxidation with temperature due to containing a high amount of unsaturated fatty acids, including the linoleic acids, accounting for 65% of the total oil. Thus, it urges a low-temperature extraction method to maintain the fatty acids content and avoid FFA production, wherein the scCO2 extraction method is highly promising.
Non-edible oil properties towards biodiesel production
Table 4 shows the physicochemical properties of non-edible oil derived from conventional extraction technology. The physicochemical properties of non-edible oil greatly influence with the conversion of biodiesel. For instance, moisture content and FFA are the main parameters for determining the transesterification process for biodiesel production.33 In conventional transesterification process, moisture present in the non-edible oil causes soap formation, and the produced soap can induce gel formation and increase the viscosity of biodiesel.98 Besides, the presence of FFA in edible oil can cause low conversion of the biodiesel production due to consuming catalyst, reduce the conventional catalytic activity and induce soap formation.33,98 It was noticed that the non-edible oil contains minimal moisture content (Table 4), which reveals that the biodiesel conversion of non-edible oil can be carried out using the conventional catalytic biodiesel conversion technology. Conversely, the non-edible oil contains FFA, and therefore the traditional alkaline catalyst biodiesel conversion technology might not be suitable for biodiesel production from non-edible oil.33| Plant seed oil | Viscosity at 40 °C | Flash point (°C) | Cloud point (°C) | Moisture content (%) | Iodine number (mg g−1) | Acid value (mg KOH per g oil) | FFA (%) | Color | References |
|---|---|---|---|---|---|---|---|---|---|
| Jatropha | 36 | 292 | 2 | 0.02 | 135 | 1.50 | 1.05 | Golden yellow | 95 |
| Castor | 222 | 294 | 14 | 0.30 | 84 | 2.41 | 1.41 | Yellow | 96 |
| Jojoba | 24 | 295 | 8 | 0.02 | 86 | 0.71 | NA | Golden yellow | 56 |
| Candlenut | 26 | NA | NA | 0.26 | 137 | 1.59 | 7 | Golden yellow | 51 |
| Karanja | 40 | 225 | 3.5 | 0.2 | 87 | 5.70 | 5 | Yellowish red | 97 |
| Mahua | 25 | 232 | 15 | NA | 71 | 36 | 18 | Yellow | 34 |
| Kusum | 25 | 268 | 12 | 0.25 | 215 | 21 | 11 | Yellowish | 98 |
| Cotton | 29 | 255 | −3.5 | 0.02 | 69 | 0.24 | 1.07 | Yellow | 99 |
| Neem | 44 | 167 | 19 | 0.25 | 85 | 18 | 17 | Reddish brown | 100 |
| Polanga | 58 | 239 | 8 | NA | 94 | 0.34 | 22 | Dark brown | 43 |
| Rubber | 76 | 198 | −9 | 0.1 | 135 | 0.52 | 17 | Dark | 101 |
The presence of viscosity in non-edible oil reveals the presence of FFAs in the oil. Acid value is another factor to decide the suitability of alkaline transesterification of non-edible oil for biodiesel synthesis. The non-edible oil contains below 2 mgKOH g−1 acid value can proceed with the conventional alkaline transesterification for biodiesel conversion.43 However, the acid value over 2 mgKOH g−1 in non-edible requires a pretreatment to reduce the acid value in non-edible oil prior to biodiesel conversion. As can see in Table 4, non-edible oil derived from castor (2.41 mgKOH g−1), karanja (5.70 mgKOH g−1), mahua (36 mgKOH g−1) and kusum (21 mgKOH g−1) contains acid value over 2 mgKOH g−1, which reveals that alkaline transesterification is not a suitable technology for biodiesel conversion from these non-edible oils. The iodine number (mg g−1) refers to the presence of unsaturated fatty acids in the oil. The higher the iodine number, the higher the unsaturated fatty acids content and thus, the lower the oxidative stability of the biodiesel.57 It is found that non-edible oil contains a high iodine number (Table 4), which reveals the oxidative stability of the non-edible oil.
Fatty acid compositions are the crucial parameter for a biodiesel feedstock to evaluate biodiesel conversion efficiency. Generally, biodiesel conversion technology does not affect the fatty acids compositions present in the feedstocks. However, the types of fatty acids and their percentage compositions in non-edible oils vary with plant species, plant growth conditions and technology utilize to extract the oil.57,87 Table 5 shows the types of fatty acids present in the Soxhlet extracted non-edible oils. The most common fatty acids present in non-edible oil are C16 and C18 acids. The fatty acids are generally aliphatic carboxylic acids. The fatty acids is the major components of biodiesel. Generally, biodiesel contains various fatty acids, such as palmitic acid (C16:0), linoleic acid (C18:2), oleic acid (C18:1), stearic acid (C18:0), and linolenic acid (C18:3).106–108 Similar fatty acids in non-edible oil reveal that the non-edible oil could be utilized as feedstock for biodiesel production.
| Non-edible oil | Palmitic (C16:0) | Stearic (C18:0) | Oleic (C18:1) | Linoleic (C18:2) | Linolenic (C18:3) | References |
|---|---|---|---|---|---|---|
| Jatropha | 14.6 | 7.6 | 44.6 | 31.9 | 0.3 | 103 |
| Jojoba | 1.59 | 4.14 | 42.84 | 31.52 | NA | 56 |
| Candlenut | 6.23 | 2.23 | 26.26 | 39.71 | 24.86 | 87 |
| Karanja | 9.8 | 6.2 | 72.2 | 11.8 | NA | 97 |
| Mahua | 21.36 | 18.97 | 38.98 | 19.47 | 0.16 | 34 |
| Kusum | 10.35 | 11.11 | 27.08 | 6.14 | NA | 98 |
| Oleander oil | 23.28 | 7.46 | 44.23 | 21.82 | NA | 104 |
| Cotton oil | 24.15 | 2.90 | 19.32 | 50.72 | 1.45 | 105 |
| Neem oil | 14.9 | 14.4 | 61.9 | 7.5 | 0 | 106 |
| Polanga | 12.01 | 12.95 | 34.09 | 38.26 | 0.30 | 43 |
| Rubber | 10.2 | 8.7 | 24.6 | 39.6 | 16.3 | 101 |
| Rice bran | 21.76 | 2.31 | 41.86 | 30.99 | NA | 107 |
| Tobacco | 10.96 | 3.34 | 15.54 | 69.49 | 0.69 | 108 |
Biodiesel conversion technology
Biodiesel is a non-petroleum-based fuel derived from animal fats, vegetable oils, and non-vegetable oil. It is comprised of mono-alkyl esters of long-chain fatty acids. The technology to manufacture biodiesel from edible oil has been well established. However, there is a challenge on converting non-edible oil to biodiesel because of the presence of FFAs.33,102 Several technologies have been implemented to produce biodiesel from non-edible crops oil, such as micro-emulsification, pyrolysis, transesterification, and dilution. Table 6 shows the production of biodiesel from various non-edible oil subjected to various technologies.| Non-edible oil | Technology | Parameter | Yield (%) | References | ||||
|---|---|---|---|---|---|---|---|---|
| Pressure (MPa) | Time (min) | Temperature (°C) | Alcohol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oil oil |
Catalyst (%) | ||||
| Jatropha | scMeOH transesterification | 11 | 15 | 250–290 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
NA | 99 | 94 |
| Candlenut | Transesterification | 45 | 40 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1 | 99.3 | 35 | |
| Castor | Catalytic transesterification | 45 | 60 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
KOH | 97 | 60 | |
| Castor | Catalytic transesterification | 30 | 60 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
KOH | 95 | 109 | |
| Castor | Catalytic transesterification | 60 | 55 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Ni–ZnO | 95 | 110 | |
| Cotton seed oil | Catalytic transesterification | 60 | 50 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Egg shell | 92 | 111 | |
| Cotton seed oil | Catalytic transesterification | 90 | 65 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CH3ONa | 97 | 112 | |
| Cotton seed oil | Catalytic transesterification | 60 | 55 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
KOH | 96 | 99 | |
| Jojoba oil | Catalytic transesterification | 60 | 25 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
KOH | 83.5 | 113 | |
| Karanja | scMeOH transesterification | 43 | 90 | 300 | 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
NA | 81 | 59 |
| Karanja | Catalytic transesterification | NA | 65 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
H2SO4 | 97 | 114 | |
| Karanja | Transesterification | 120 | 66.8 | 10.44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
KOH | 91.05 | 115 | |
| Kusum | Catalytic transesterification | 60 | 50 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
KOH | 95 | 116 | |
| Yellow oleander | Catalytic transesterification | 30 | 60 | 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
KOH | 93 | 46 | |
| Kusum | Catalytic transesterification | 90 | 65 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
K2Al2O4 | 97 | 117 | |
| Mahua | Catalytic transesterification | 60 | 65 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
KOH | 91 | 118 | |
| Neem | Catalytic transesterification | 23 | 60 | 0.23 | Cu–ZnO | 91 | 119 | |
| Polanga | Pyrolysis | 550 | NA | NA | 46 | 120 | ||
| Sea mango | Catalytic transesterification | 180 | 150 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
SO42−/ZrO | 94 | 121 | |
| Sea mango | scMeOH transesterification | 380 | 40 | 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
NA | 78 | 122 | |
| Soap nut | Catalytic transesterification | 180 | 60 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Carbon residue | 89 | 37 | |
| Tobacco | Pyrolysis | NA | 350 | NA | NA | 67 | 123 | |
| Tobacco | Catalytic transesterification | 30 | 60 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
KOH | 91 | 124 | |
| Tung seed oil | Catalytic transesterification | 60 | 55 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
KOH | 93 | 63 | |
Pyrolysis
Pyrolysis has been utilized as an alternative method for producing renewable biofuels from biomass.116 Thus technology has also been used to synthesis biodiesel from various feedstocks including non-edible oils, animal fat and cellulosic biomass. Generally, the pyrolysis process is the thermochemical degradation of biofuel feedstock at medium temperature (300–800 °C) and high temperature (850–1300 °C) within an inert atmosphere.116,119 The pyrolysis process to produce biofuel may require a catalyst to enhance the thermal decomposition of biofuel feedstock; this process is referred to catalytic pyrolysis process. Among the various renewable fuels, pyrolytic oil, also known as bio-oil, derived from non-edible feedstock, has attracted interest to be used as an alternative biofuel. Studies have shown that the pyrolysis technology could produce a maximum 67% oil from non-edibles plant seeds.120,123 The 'pyrolysis; refers to a process of heating material at elevated temperature in the presence of the minimal amount of oxygen. In pyrolysis process, long-chain hydrocarbons breakdown to short-chain hydrocarbon, and this condensable short-chain hydrocarbon is known as bio-oil.123 Pradhan et al.125 determined the possibility of utilizing mahua pyrolysis oil as an alternative fuel, obtained from mahua seed using semi-batch type reactor with varying temperature 450 °C to 600 °C. The maximum yield obtained about 50 wt% at the optimal temperature of 525 °C. Shadangi and Mohanty126 produced niger pyrolysis oil from niger seed using conventional thermal pyrolysis. The optimal temperature was determined to be 550 °C for the maximum niger bio-oil yield of 34.5%.The quantity and quality of the extracted biofuel depend on the types of feedstocks, oil content in the plant seeds, and the types of pyrolysis use.120,125 Various types of pyrolysis reactors have been employed in the production of biofuel, including (i) circulating fluidized bed, (ii) bubbling fluidized bed, (iii) focussed solar reactor, (iv) rotating cone, and (v) ablative. The main advantages of pyrolytic bio-oil are easy to handle, store and transport. Besides, the distinct desirable properties of pyrolytic oil such as high cetane number, low viscosity, and low quantities of sulphur could be considered for being used as an alternative fuel. However, the pyrolytic bio-oil is acidic and contains different types of hydrocarbon compounds and high moisture.123 The bio-oils derived from edible and non-edible plant seeds are denser than petroleum diesel fuel, and therefore, it requires a pre-treatment process to remove moisture and neutralize prior to use as an alternative biofuel.126
Micro-emulsification
Micro-emulsification uses to formulate hybrid diesel fuels. This technology involves emulsifying oil to reduce viscosity by mixing suitable emulsifying agents (i.e., primary alcohol) in oils.33 The advantage of the micro-emulsification process is that microemulsions are thermodynamically stable and therefore it does not require any action to remain the emulsion in a single-phase and translucent at constant pressure and temperature.26 Micro-emulsification is an alternative method that produces biofuel with suitable properties with low energy consumption.Poor quality non-edible oil requires a pre-treatment technology such as cracking, blending, and hydrodeoxygenation to minimize the viscosity and FFAs content prior to producing biodiesel. However, the micro emulsification process does not require a pre-treatment process in producing biodiesel from non-edible oil. Studies reported that the utilization of the surfactant in the emulsification process enhances the yielded emulsion's stability. The most utilized surfactant in the emulsification of the non-edible is hydrophobic span 80. However, other hydrophobic and hydrophilic surfactants were also utilized in the emulsification process. Liang et al.130 utilized micro emulsification technology to stabilize bio-oil-in diesel using Span 80/Tween 80 as surfactants. Sankumgon et al.131 produce microemulsion bio-fuel from Jatropha curcas oil in the presence of the surfactant. The properties analyses of the microemulsion oil showed kinematic viscosity, moisture content, and heating values comply with the biodiesel standards and compatible to use in a diesel engine. Besides, the emission characterization showed that the microemulsions fuel combustion exhaust less smoke than diesel. The advantages of using microemulsion fuel are that it reduces toxic pollutants emission during combustion, increases combustion performance, and minimizes the ignition delay time.126–128 Microemulsion fuel properties are similar to petro diesel, but the oxidative stability is higher than petro diesel.128 The major disadvantages of utilizing micro-emulsion fuels in combustion engines are incomplete combustion and the carbon deposition in the engine.129
Transesterification
The transesterification process is the most utilized technology to produce biodiesel from non-edible oil and animal fats. Generally, non-edible contains high viscosity, FFAs and moisture. Therefore, it urges to minimize the moisture, FFAs, and viscosity before producing biodiesel. Transesterification is an effective process for reducing the moisture, FFAs and viscosity during producing biodiesel from non-edible oil.114 Besides, the transesterification process offers various advantages over the biodiesel synthesis methods, including eco-friendly, mild chemical reaction and suitable for biodiesel feedstocks.35,114 The transesterification process occurs by the chemical reaction between a triglyceride (oil/fats) and alcohol. In the transesterification process for biodiesel production, the most commonly utilized alcohols are methanol and ethanol. Methanol is being used as alcohol in the transesterification process because of its shortest carbon chain, lower cost, and lower physical properties values over other aliphatic alcohol.112 Conversely, ethanol is used in the transesterification process to produce biodiesel because it is non-toxic, renewable and environmentally friendly as it is derived from crops.26,33,111 The transesterification process has been conducted with or without catalyst; therefore, the transesterification process can be classified as catalytic transesterification and non-catalytic transesterification.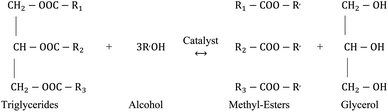 | ||
| Fig. 3 Transesterification reaction of triglycerides for the production of biodiesel from non-edible oil. | ||
An excessive amount of alcohol utilization in catalytic transesterification makes it difficult for the separation of the glycerol. Therefore, it is urged to establish an ideal alcohol/oil ratio empirically. Catalytic transesterification can be carried out by alkaline catalysts using potassium or sodium hydroxide and methoxide.59,112 Alkaline catalysts provide some distinct advantages, including low operating temperature and short reaction time.34,116 The sodium methoxide (CH3ONa) is utilized as a catalyst for biodiesel production since it offers relatively higher yields in fast reaction times with low molar concentrations.116 However, alkaline transesterification requires an absence of water and minimal FFAs in oil (≤2 wt%), making them inappropriate technology for typical industrial processes.33,94 Enzymatic transesterification is an alternative to a heterogeneous catalytic transesterification process, where the lipases isolated from different microorganisms are used as a biocatalyst.85,132 In the last decades, lipase utilization as a biocatalyst in the transesterification process has dedicated attention to its mild reaction condition, simple downstream processing, and easy separation of enzymes. Enzymatic catalytic transesterification can process low-quality biodiesel feedstock to produce biodiesel because high moisture and FFAs content in feedstocks do not affect the transesterification process biodiesel production, as the enzyme has minimal sensitivity with water and FFAs.133 Moreover, the enzymatic catalytic process, the lipase enzyme can covert FFAs and triglycerides to biodiesel in a single transesterification process.85 This process does not require subsequent purification or washing to separate enzymes. The main obstacles of the enzymatic transesterification process to produce biodiesel are the relatively slower reaction rate, cost of lipase, and the inactivation of the lipase by glycerol.133
In the conventional transesterification process in biodiesel production, the presence of FFAs and moisture in non-edible oil hinders the biodiesel conversion because of consuming more catalyst, causes soap formation, reduce catalyst effectiveness.26,94 However, these catalytic transesterification processes require relatively high reaction time and further purification technology to separate the catalyst, results in increased energy consumption and production costs.
To overcome these limitations, the transesterification of non-edible oil can be conducted using supercritical methanol (scMeOH). The critical temperature and pressure of the methanol are 512.6 K and 8.09 MPa, respectively.90 Above this critical pressure and critical temperature, methanol (MeOH) exhibits in a supercritical state, where there is no distinct phase between liquid and gas phases exist. The scMeOH can disperse through the materials like a gas and dissolve materials like a liquid. The properties of methanol in the supercritical state can be described as an intermediate between a liquid and gas phase of the methanol.26 The scMeOH is a simplified non-catalytic biodiesel conversion process since it is simultaneously conducting the transesterification of the triglycerides and esterification of fatty acids.94,133,134
Transesterification using methanol at a supercritical state has been extensively investigated in recent years to determine an effective alternative of conventional transesterification processes.26,135 The potential advantage of the scMeOH transesterification is that water and FFA content in the non-edible do not affect the biodiesel conversion process. Moreover, the water content in non-edible enhances the biodiesel conversion in scMeOH transesterification process.132 The utilization of catalyst is not essential in the scMeOH transesterification process, since methanol and oil produce homogenous phases in the supercritical state and minimize the mass transfer limitations.133 Another distinct advantage of the scMeOH transesterification process is the more straightforward separation process of glycerol from the biodiesel because biodiesel and glycerol are immiscible at ambient temperature.59,94 Román-Figueroa et al.136 synthesized biodiesel from crude castor oil using scMeOH as catalyst-free transesterification process with varying temperature (250–350 °C), pressure (10–43 MPa), treatment time (15–90 min), and methanol to oil molar ratio of 43.1. It was obtained 96.5 wt% of biodiesel conversion at scMeOH temperature 300 °C methanol to oil molar ratio of 43.1 for 90 min reaction time. García-Martínez et al.135 optimized the experimental conditions of the scMeOH transesterification process for biodiesel production from tobacco seed oil and obtained about 93 wt% biodiesel conversion at 303.4 C for 90 min with a fixed methanol and oil molar ratio of 43.1.131 Although the utilization of catalyst in scMeOH transesterification is not essential, a few studies have utilized catalyst to enhance transesterification rate and biodiesel yield with lower energy consumption.26,136 The limitations of utilizing in the scMeOH transesterification process for biodiesel conversation are the high capital cost investment required for setting up the high pressure and temperature technology. However, the high capital investment can be compensated with similar productivity by the rapid transesterification rate in a smaller reactor.
Blending of non-edible oil
Blending non-edible oil with diesel fuel is the simplest way to utilize non-edible oil as a fuel, as it does not require any chemical process. The purpose of blending non-edible oil is to minimize the viscosity and enhance the volatility since the poor volatility and high viscosity are the main obstacle to using non-edible oil as a fuel.26 Studies have determined the physicochemical properties, engine performance, and storability of non-edible oil blends with diesel fuel. It was found that the non-edible oil blends with diesel fuel increase the storability, potential improvement in physical properties, and engine performance.26,137,138 Agarwal and Rajamanoharan137 determined emission characteristics and engine performance of karanja oil blends and diesel (up to 50% v/v). The study reported that the karanja oil blends with diesel minimize the smoke emissions and enhance engine performance. Mondal et al.138 reported that blending the non-edible blends with diesel reduces poor atomization and difficulty handling by conventional fuel injection systems of compression ignition engines.Physicochemical properties of non-edible oil biodiesel
The physicochemical properties of biodiesel fuel might vary with the source of feedstocks, quality of feedstocks, the technology employed for the extraction of plant seed oil, refining process employed and the postproduction parameters. Studies reported that the biodiesel quality derived from non-edible oil is expressively depends on the chemical compositions and fatty acids compositions of the oil.26,135 Therefore, it urges the standard characterization of biodiesel fuel prior use as an alternative fuel in the diesel engine. The scientist and engineers have established standards characterization to protect biodiesel producers and consumers and support the development of biodiesel industries. The standard characterization describes the biodiesel physical and chemical properties requirements to be used as an alternative fuel. Some of these properties are viscosity (mm2 s−1), density (kg m−3), cloud points (°C), power points (°C), cetane number (MJ kg−1), acid value (mg KOH per g oil), sulphur content and oxidation stability.33,140–142 Biodiesel produces from edible and non-edible oil must mitigate the standard specification of biodiesel set by ASTM 6571, EN 14214. Table 7 presents physicochemical properties of non-edible biodiesel and standard specification of biodiesel for ASTM 6571 and EN 14214. Table 7 shows that the cetane number of non-edible biodiesel is higher than specified values by ASTM 6571 and EN 14214 standards, indicates biodiesel can utilize as alternative fuel for the combustion engine. Density indicates the ignition quality of biodiesel. The density of biodiesel can influence the biodiesel atomization efficiency in an airless combustion system.33| Non-edible oil biodiesel | Density (kg m−3) | Viscosity (mm2 s−1) | Flash point (°C) | Pour point | Cloud point (°C) | Moisture content (%) | Cetane number | Iodine number (g) (I2/100 g) | Acid value (mgKOH g−1) | Sulfated ash | Calorific value (MJ kg−1) | Oxidation stability (h) | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jatropha | 879 | 4.84 | 191 | 3 | 2.8 | 0.02 | 51 | — | 0.38 | 0.013 | 38.5 | 3.37 | 137 |
| Castor | 946 | 15.4 | 194 | −30 | −18 | 0.15 | 43.7 | 78.21 | 2.8 | NA | 38.34 | 4.4 | 109 |
| Jojoba | 871 | 5.86 | 150 | −6 | −2 | 0.053 | NA | 74.7 | 0.22 | 0.08 | 42.82 | 3.05 | 138 |
| Candlenut | 886 | 4.8 | 161 | 6 | 6.84 | 0.33 | NA | NA | 0.4 | 0.005 | 0.19 | 5.9 | 139 |
| Karanja | 870 | 5.6 | 174 | 7 | 10 | 0.04 | 57.6 | 91 | 0.21 | 0.001 | 37.8 | 3.68 | 140 |
| Mahua | 882 | 4.2 | 170 | 6 | 13 | NA | 57 | 71 | 0.47 | 0.01 | 37 | 8.2 | 34 |
| Kusum | 860 | 4.2 | 139 | −2.5 | −10.8 | NA | 47.27 | NA | 0.45 | NA | 38.33 | NA | 141 |
| Cotton | 881 | 6.81 | 173 | 5 | 7 | 0.02 | 56.06 | 125 | 0.22 | NA | 39.54 | NA | 99 |
| Neem | 867 | 5.16 | 170 | 8.5 | 15 | NA | 55 | NA | 0.61 | 0.01 | 38.25 | NA | 142 |
| Polanga | 869 | 3.99 | 140 | 4.3 | 13.2 | NA | 51 | NA | 0.34 | NA | 41.39 | 13.08 | 43 |
| Rubber | 870 | 3.7 | 110 | −2 | −6 | 0.04 | 43 | NA | 0.07 | NA | 36.5 | 8.5 | 52 |
| Tobacco | 888 | 4.22 | 165 | NA | NA | NA | 51 | 136 | 0.3 | 0.0004 | 44.6 | 8 | 143 |
| Yellow oleander | 870 | 4.2 | 175 | NA | NA | NA | NA | NA | NA | NA | 43.4 | NA | 46 |
| Sea mango | 880 | 4.5 | 138 | NA | NA | NA | NA | NA | NA | NA | 39.09 | NA | 122 |
| Soap nut | 869 | 7.7 | 151 | 4.3 | 13.2 | NA | 57.3 | 85 | 0.76 | 0.02 | 41.39 | NA | 144 |
| 870–890 | 1.9–6.0 | ≥130 | −15 to −16 | −3 to −12 | ≥0.05 | ≥47 | NA | ≤0.5 | 0.02 | NA | ≥3 | ASTM D 6751 | |
| 860–900 | 3.5–5.0 | ≥101 | NA | NA | ≥0.05 | ≥51 | 120 | ≤0.5 | 0.02 | NA | ≥6 | EN 14214 |
From Table 7, it is found that the castor biodiesel has a higher density (946 kg m−3) than standard specifications set by ASTM 6571, EN 14214. The injection and atomization properties of biodiesel far different than vegetable oil due to the presence of lower viscosity. Generally, the viscosity of the non-edible oil sharply decreases with the biodiesel transesterification processes. The lower the viscosity of biodiesel makes it easy to pump it into an engine for atomization. High viscosity of biodiesel leads to the inefficient automatization of the fuel spray, which leads to inaccurate fuel injectors operations.13,143 It was found from Table 7 that the viscosity of castor (15 mm2 s−1), cotton (6.81 mm2 s−1), and soap nut biodiesel (7.7 mm2 s−1) is higher than prescribe viscosity of biodiesel specified by ASTM 6571 and EN 14214 standards. Flashpoint is another important property of biodiesel, which indicates the lowest temperature of the fuel to spontaneously ignite in absence of a flame or spark. It is found that the non-edible biodiesel has higher flashpoint values than the recommended values reported by ASTM 6571 and EN 14214 standards, indicates that biodiesels derived from the non-edible oils are less volatile and safe to use in diesel engine.144,145 Pour point (PP) and cloud point (CP) of biodiesel indicates the low temperature application of the biofuel. From Table 7, it is found that the higher PP and CP were in neem biodiesel (8.5 and 15 °C, respectively), wherein the lowest PP and CP were found in castor biodiesel (−30 and −8 °C, respectively). Cetane number is another critical parameter to determine the biodiesel ignition quality. Generally, the cetane number provides the biodiesel readiness information for auto-ignition upon injection to the engine for combustion.146
Commercial-scale biodiesel production
In regards to the global environmental impacts of fossil fuel energy, studies have been conducted to develop sustainable liquid fuel (biodiesel) energy as an alternative to fossil fuel energy. However, biodiesel production on an industrial scale from non-edible oil faces many obstacles, including meet up the expectations of stockholders for profitability, optimal uses of capital, and other resources.147,148 The commercial-scale biodiesel producers must meet the regulatory requirements for safety and environmental standards monitored by the respective governmental agencies.149 Besides, the biodiesel producers must incorporate the recovery of chemicals and recycling/reuse the chemicals in the plant design. Currently, the commercial-scale biodiesel production from non-edible oil is being conducted either catalytic transesterification or non-catalytic transesterification.147,150 Fig. 4 shows the schematic diagram for the catalytic transesterification of biodiesel from non-edible oil. The catalytic transesterification of biodiesel production involves combining non-edible oil, alcohol, and catalyst in a reactor and agitated at a temperature of 60 °C for approximately an hour.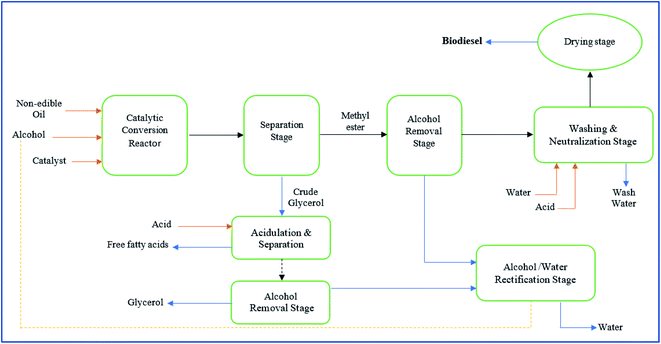 | ||
| Fig. 4 Schematic diagram for the commercial scale biodiesel production via catalytic transesterification process. | ||
In some cases, it urges to conduct two steps catalytic transesterifications to provide a complete chemical reaction with minimal uses of alcohol. In the 1st stage, 80% of alcohol and catalyst are added to the oil and then removed produced glycerol. The remaining 20% of alcohol is added to the reactor at the second stage catalytic transesterification process. Special processes are required if the non-edible oil contains excessive amounts of FFAs. The FFAs present in non-edible oil will react with the alkali catalyst to produce soap and water, as shown in Fig. 5. However, the FFAs content in non-edible oil below 5% can proceed with an alkali catalyst. The soap produced will wash out during the water washing of the biodiesel.
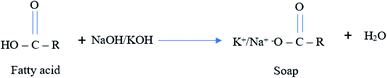 | ||
| Fig. 5 Soap formation during alkaline catalytic transesterification of non-edible oil containing excessive amount of FFAs. | ||
However, the alkali transesterification process of non-edible oil containing over 5 wt% FFAs would inhibit glycerol and methyl ester separation due to the emulsion formation during waster wash. Therefore, it requires a pretreatment process to reduce FFAs in the non-edible oil by converting FFAs to methyl ester, as shown in Fig. 6.151 The treated oil can proceed with an alkaline catalytic transesterification process to convert triglycerides to methyl ester. After transesterification, the glycerol is separated using either a centrifuge or a settling tank. The glycerol separation gently occurs due to its low solubility in the ester. The presence of excess alcohol may slow the separation process, but the excess alcohol generally not removed the reaction stream until completely separated the methyl ester and glycerol.152 The transesterification is a reversible process, and therefore the glycerol may recombine with methyl ester to form monoglycerides in the absence of alcohol. The glycerol stream remaining in the separator contains alcohol, catalyst, and soap. At this stage, the glycerol has little value due to the presence of the contaminate. However, the glycerol can refine with two steps refining processes by removing soap and excess alcohol. Almost 85% of glycerol can be recovered with the paurity up to 99.7%.148
 | ||
| Fig. 6 Pretreatment of non-edible oil containing excessive amount of FFAs before biodiesel production. | ||
The separated methyl esters pass through an alcohol stripper to remove the alcohol before neutralization and water washing of the biodiesel. The produced biodiesel is neutralized by adding acid to neutralize the residual catalyst and split any soap forming during the reaction. The acids react with soap and produce FFAs and water-soluble salt.152 The salt generated will wash out during the water washing of the biodiesel. Finally, the produced biodiesel is cooled in ambient temperature and makes it ready for storage and transportation. Fig. 7 shows the commercial-scale biodiesel production from non-edible oil via the non-catalytic transesterification process. The most preferred non-catalytic transesterification process is the esterification of non-edible oil using supercritical methanol (scMeOH).150 Transesterification is also an important process in the scMeOH transesterification process of non-edible oil for biodiesel production. There is also an esterification reaction between alcohol and FFAs, a relatively much faster rate than the conventional transesterification process. scMeOH transesterification is a catalyst-free transesterification process.10 It is viewed as an eco-friendly, less energy intensive, highly efficient and superior transesterification process for converting non-edible to methyl ester. Methanol over critical pressure (8.09 MPa) and temperature (239 °C) acts as a solvent for dissolving oil uniformly during transesterification.59,150 The potential advantages of this transesterification process is that it reduces treatment time and it processes the non-edible oil that contains high FFAs, which is not possible for the conventional catalytic transesterification process.10,59 In this process, the possibility of the hydrolysis of the biodiesel is neglected due to the production of a small amount of water during biodiesel production. Various parameters potentially influence the scMEOH transesterification process, including pressure, temperature, reaction time, mixing intensity, and MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oil molar ratio.10,59
oil molar ratio.10,59
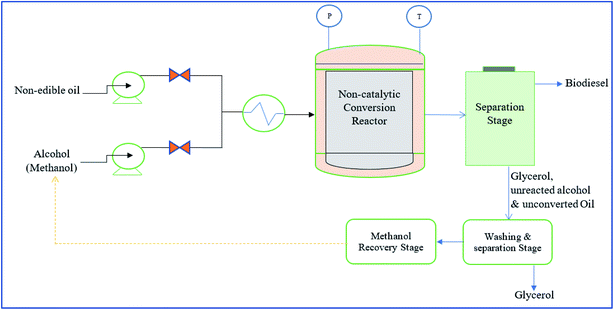 | ||
| Fig. 7 Schematic diagram non-catalytic transesterification process for the commercial scale biodiesel production from non-edible oil. | ||
Techno-economic feasibility of the biodiesel production
The techno-economic feasibility analysis is crucial for commercial-scale biodiesel production from non-edible oil feedstock. The parameters that influence the techno-economic feasibility analyses include commercially available transesterification technologies, feedstock cost, the market price of biodiesel, and other by-products producing during biodiesel production.150 Table 8 shows the techno-economic feasibility analyses for biodiesel production from non-edible oil. It is being estimated that the average cost of biodiesel production is $0.50.29,157 The factors considered for calculating the biodiesel production cost were operational costs (feedstock price, chemicals, labor, and utilities), fixed charges (tax) and general costs such as research, development, and finance.157 However, the feedstock price is the most influential factor in biodiesel production, which covers about 80% of the total biodiesel production cost. Besides, the catalyst price is also influenced biodiesel production. However, the utilization of low-cost materials, such as biomass waste-derived catalysts would minimize biodiesel production. The biodiesel conversion technology, the labor cost of harvesting non-edible crops, and transportation cost of non-edible crops from the plantation to biodiesel production plant must consider during techno-economic feasibility analyses for biodiesel production from non-edible oil.| Source | Type of reactor | Catalyst | Quantity (t per year) | Utility and labour cost ($ per L) | Oil price (t per $) | Total production cost (×106 $) | Cost of biodiesel ($ per L) | Biodiesel selling price ($ per L) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Jatropha oil | Continuous stirred tank reactor | Homogeneous base | 9900 | 0.032 | 322 | 0.394 | 0.145 | 0.75 | 150 |
| Supercritical methanol | Catalyst free | 110 | 0.37 | 322 | 0.443 | 0.210 | 0.75 | ||
| Low quality non-edible oil | Continuous reactor | Homogeneous base | 8000 | 0.047 | 478 | 0.458 | 0.162 | 0.70–0.80 | 153 |
| Tamanu seed oil | Continuous stirred tank reactor | Heterogeneous nano-catalyst | 2400 | 0.036 | 400 | 0.421 | 0.159 | 0.70 | 154 |
| Cotton oil | Continuous reactor | Homogeneous base | 5102 | 0.025 | 980 | 0.532 | 0.180 | 0.58 | 149 |
| Neem oil | Batch reactor | Heterogeneous base | 3285 | 0.052 | 840 | 1.06 | 0.230 | 1.03 | 155 |
| Waste cooking oil | Semi-industrial reactor | Homogeneous base | 1500 | 0.075 | 990 | 0.81 | 0.270 | 0.65 | 156 |
Potential and challenges of non-edible oil for biodiesel feedstock
With the emerging modern technologies, the non-edible feedstock is an efficient and low-cost resource for future biofuel development. Being a clean source of fuel generated from domestic and renewable resources as well as having the ability to be mixed with diesel to get a biodiesel blend, the demand of biodiesel production has increased immensely.29 Compared to common diesel, its non-toxic and eco-friendly characteristics allow it to utilize as alternative fuel for the combustion engine to reduce greenhouse gas emissions and global warming. Another advantage is that it prolongs engine life and performance due to its lubrication properties. Furthermore, its high flashpoint ensures the safety of operation as biodiesel is more secure than diesel and that no alterations are necessary when using it in engines.29,107 The cultivation of non-edible oil crops does not compete with existing food crops or vegetable cultivation. The adaptability of the non-edible crops' cultivation in marginal land with low moisture and fertility demand has given its potentiality to utilize as a potential feedstock for biodiesel production. Generally, non-edible plant grows in wastelands those are not suitable for food crops. Thus, the utilization of non-edible crops as a feedstock would eliminate the debate between food vs. biodiesel. Moreover, the distinct properties of biodiesel derived from non-edibles crops, such as biodegradability, renewability, ready availability, lower sulphur content and higher heat content, make it possible alternative feedstock for biodiesel production.Based on the literature survey, the development of non-edible crops as a potential feedstock for biodiesel poses challenges towards self-reliance energy security. This is because:
(i) Non-edible crops are of forest origin, and it is therefore harvesting, collection and transportation are problematic.
(ii) Lowering fuel economy, seasonal availability of non-edible crops and improper marketing channels are the major drawbacks for setting up biodiesel production industries.
(iii) The presence of high FFAs and moisture content requires a pre-treatment to minimize FFAs and water content in the oil prior to the transesterification process.
(iv) Lack of post-harvest technologies for non-edible crops affects its oil quality.
(v) Existing technologies for oil extraction and biodiesel conversion are not cost effective since these technologies require multiple purification and separation processes.
Thus, the utilization of non-edible oil as feedstock for biodiesel possess challenges and opportunity to utilize as an alternative fuel of petro-diesel for the environment and economic benefits. These urges to conduct further research on cultivation of non-edible crops and cost-effective technology for biodiesel conversion. Since non-edible crops can grow in harsh and aired lands with low moisture requirements, thus the plantation can be carried out in the most unused lands, particularly in developing countries, such as sea sore, bank of the river, desert, and other wastelands those are not suitable for edible crops. These will allow the maximum utilization of the limited land area available for crop production. The presence of FFAs and moisture content in non-edible oil could be avoided with the implementation of advance technology, particularly with the implementation waterless extraction technology like supercritical fluids technology.
Conclusions
The present study was conducted to determine the non-edible oil as a potential feedstock for biodiesel production. The utilization of edible oil as a feedstock arises from concerns on food vs. fuel debate and the production cost argument due to high feedstock price. Therefore, the demand for non-edible oil as a feedstock for biodiesel production is sharply increasing. No edible oil is not suitable for human consumption due to the presence of toxic components. Besides, non-edible oil plants can grow non-fertile and harsh lands with lower moisture requirements, and those lands are not suitable for edible crop production. The physicochemical properties and fatty acids compositions of non-edible oil are comparable to edible oil's properties and fatty acids compositions. Thus, there is ample opportunity to utilize non-edible oil as a potential feedstock for biodiesel production. However, high FFA and water content in non-edible oil are the main barrier to utilizing non-edible oil as a potential feedstock. The extraction of non-edible oil subjected to scCO2 extraction technology yielded non-edible oil with minimal FFAs content and moisture content. On the other hand, the scMeOH has been found as a promising technology for biodiesel conversion since the methanol in the supercritical state has the least interaction with the FFAs content and certain amounts of water in non-edible oil enhance the biodiesel conversation rate. The operation cost associated with the high pressure and temperature of scMeOH transesterification might affect the biodiesel production cost. However, implementing the supercritical fluid's technology for simultaneous extraction and biodiesel conversion and optimizing the experimental condition would minimize cost to produce biodiesel from non-edible oils. The determined non-edible oil yield and biodiesel properties reviewed in the present study indicate a huge opportunity to utilize non-edible oil as a potential feedstock for biodiesel production without affecting the global food economy.Author contributions
Marwan Abdul Hakim Shaah: conceptualization, methodology and writing—original draft; Md. Sohrab Hossain: conceptualization, methodology, supervision and writing-review and editing; Faisal Aboelksim Salem Allafi: methodology and formal analysis; Alyaa Alsaedi: methodology and investigation; Norli Ismail: conceptualization, resources and validation; Mohd Omar Ab Kadir: investigation and project administration; Mardiana Idayu Ahmad: conceptualization, methodology, data curation and funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thanks and acknowledge the Ministry of Higher Education (MOHE), Malaysia for financial support through the Fundamental Research Grant Scheme (203/PTEKIND/6711817).Notes and references
- A. Shahsavari and M. Akbari, Renewable Sustainable Energy Rev., 2018, 90, 275–291 CrossRef CAS.
- K. Agarwal, J. G. Gupta and A. Dhar, Prog. Energy Combust. Sci., 2017, 61, 113–149 CrossRef.
- M. Munir, M. Ahmad, M. Saeed, A. Waseem, M. Rehan, A.-S. Nizami, M. Zafar, M. Arshad and S. Sultana, Renewable Sustainable Energy Rev., 2019, 109, 321–332 CrossRef CAS.
- J. Popp, M. Harangi-Rákos, Z. Gabnai, P. Balogh, G. Antal and A. Bai, Molecules, 2016, 21, 285 CrossRef PubMed.
- A. A. Karabulut, E. Crenna, S. Sala and A. Udias, J. Cleaner Prod., 2018, 172, 3874–3889 CrossRef.
- D. F. Correa, H. L. Beyer, H. P. Possingham, S. R. Thomas-Hall and P. M. Schenk, Renewable Sustainable Energy Rev., 2017, 74, 1131–1146 CrossRef.
- A. R. Behera, K. Dutta, P. Verma, A. Daverey and D. K. Sahoo, J. Environ. Manage., 2019, 252, 109686 CrossRef CAS PubMed.
- J. Rodrigues, I. Miranda, J. Gominho, M. Vasconcelos, G. Barradas, H. Pereira, F. Bianchi-de-Aguiar and S. Ferreira-Dias, Ind. Crops Prod., 2016, 83, 614–619 CrossRef CAS.
- H. Pan, H. Li, H. Zhang, A. Wang, D. Jin and S. Yang, Energy Convers. Manage., 2018, 166, 534–544 CrossRef CAS.
- C. Román-Figueroa, P. Olivares-Carrillo, M. Paneque, F. J. Palacios-Nereo and J. Quesada-Medina, Energy, 2016, 107, 165–171 CrossRef.
- E. Trutnevyte, W. McDowall, J. Tomei and I. Keppo, Renewable Sustainable Energy Rev., 2016, 55, 326–337 CrossRef.
- I. Staffell, D. Scamman, A. V. Abad, P. Balcombe, P. E. Dodds, P. Ekins, N. Shah and K. R. Ward, Energy Environ. Sci., 2019, 12, 463–491 RSC.
- R. Salvucci, S. Petrović, K. Karlsson, M. Wråke, T. P. Uteng and O. Balyk, Energies, 2019, 12, 2232 CrossRef CAS.
- A. Karmakar and S. Mukherjee, Indian J. Agric. Res., 2017, 51, 529–535 Search PubMed.
- R. M. Czarny, The Nordic Dimension of Energy Security, Springer, 2020, pp. 67–99 Search PubMed.
- M. A. A. Farid, A. M. Roslan, M. A. Hassan, M. Y. Hasan, M. R. Othman and Y. Shirai, Sustain. Energy Technol. Assess., 2020, 39, 100700 Search PubMed.
- S. Jayakumar, M. M. Yusoff, M. H. A. Rahim, G. P. Maniam and N. Govindan, Renewable Sustainable Energy Rev., 2017, 72, 33–47 CrossRef CAS.
- U. Rajak and T. N. Verma, Energy Convers. Manage., 2018, 166, 704–718 CrossRef CAS.
- E. Mercuri, D. Manca, O. Abderazag, R. Patel and I. Mujtaba, Computer Aided Chemical Engineering, Elsevier, 2016, vol. 38, pp. 1713–1718 Search PubMed.
- A. J. Folayan, P. A. L. Anawe, A. E. Aladejare and A. O. Ayeni, Energy Rep., 2019, 5, 793–806 CrossRef.
- A. Sajjadi, A. A. A. Raman and H. Arandiyan, Renewable Sustainable Energy Rev., 2016, 63, 62–92 CrossRef.
- da Silva César, M. A. Conejero, E. C. B. Ribeiro and M. O. Batalha, Renewable Energy, 2019, 133, 1147–1157 CrossRef.
- S. V. Mazanov, A. R. Gabitova, R. A. Usmanov, F. M. Gumerov, S. Labidi, M. B. Amar, J.-P. Passarello, A. Kanaev, F. Volle and B. Le Neindre, J. Supercrit. Fluids, 2016, 118, 107–118 CrossRef CAS.
- M. C. Nongbe, T. Ekou, L. Ekou, K. B. Yao, E. Le Grognec and F.-X. Felpin, Renewable Energy, 2017, 106, 135–141 CrossRef CAS.
- P. Zareh, A. A. Zare and B. Ghobadian, Energy, 2017, 139, 883–894 CrossRef CAS.
- P. Adewale, M.-J. Dumont and M. Ngadi, Renewable Sustainable Energy Rev., 2015, 45, 574–588 CrossRef CAS.
- I. Gómez and P. Huovinen, Antarctic Seaweeds: Diversity, Adaptation and Ecosystem Services, 2020, p. 219 Search PubMed.
- T. Dong, E. P. Knoshaug, R. Davis, L. M. Laurens, S. Van Wychen, P. T. Pienkos and N. Nagle, Algal Res., 2016, 19, 316–323 CrossRef.
- A. Atabani, A. Silitonga, H. Ong, T. Mahlia, H. Masjuki, I. A. Badruddin and H. Fayaz, Renewable Sustainable Energy Rev., 2013, 18, 211–245 CrossRef CAS.
- A. Kumar and S. Sharma, Ind. Crops Prod., 2008, 28, 1–10 CrossRef CAS.
- M. Abdullah, J. Salimon, E. Yousif and N. Salih, J. Assoc. Arab Univ. Basic Appl. Sci., 2013, 14, 83–86 Search PubMed.
- G. O. Ferrero, E. M. S. Faba, A. A. Rickert and G. A. Eimer, Renewable Energy, 2020, 150, 128–135 CrossRef CAS.
- A. Demirbas, A. Bafail, W. Ahmad and M. Sheikh, Energy Explor. Exploit., 2016, 34, 290–318 CrossRef CAS.
- N. Acharya, P. Nanda, S. Panda and S. Acharya, Eng. Sci. Technol., 2017, 20, 511–517 Search PubMed.
- L. N. Pham, B. Van Luu, H. D. Phuoc, H. N. T. Le, H. T. Truong, P. D. Luu, M. Furuta, K. Imamura and Y. Maeda, J. Oleo Sci., 2018, ess17220 Search PubMed.
- H. K. G. Singh, S. Yusup, B. Abdullah, K. W. Cheah, F. N. Azmee and H. L. Lam, J. Environ. Manage., 2017, 203, 1011–1016 CrossRef PubMed.
- R. Mathiarasi and N. Partha, J. Saudi Chem. Soc., 2017, 21, 11–17 CrossRef CAS.
- A. Sandouqa and Z. Al-Hamamre, Renewable energy, 2019, 130, 831–842 CrossRef.
- I. Gravalos, N. Ziakas, S. Loutridis and T. Gialamas, Comput. Electron. Agric., 2019, 166, 104986 CrossRef.
- E. Aransiola, E. Ehinmitola, A. Adebimpe, T. Shittu and B. Solomon, Advances in Eco-Fuels for a Sustainable Environment, 2019, pp. 53–87 Search PubMed.
- R. L. Patel and C. Sankhavara, Renewable Sustainable Energy Rev., 2017, 71, 464–474 CrossRef CAS.
- E. B. Mubofu, Sustainable Chem. Processes, 2016, 4, 1–12 CrossRef.
- P. Sahoo, L. Das, M. Babu and S. Naik, Fuel, 2007, 86, 448–454 CrossRef CAS.
- R. Durairaj, A. Anderson, G. Mageshwaran, G. Britto Joseph and M. Balamurali, Int. J. Ambient Energy, 2019, 40, 396–400 CrossRef CAS.
- A. Poshetti and M. Tandale, Advances in Energy Research, Springer, 2020, vol. 2, pp. 467–478 Search PubMed.
- K. Yadav, A. Pal, U. Ghosh and S. K. Gupta, Int. J. Ambient Energy, 2019, 40, 152–157 CrossRef.
- S. P. Khurana and R. K. Gaur, Plant Biotechnology: Progress in Genomic Era, Springer, 2019 Search PubMed.
- M. T. Akhtar, M. Ahmad, A. Shaheen, M. Zafar, R. Ullah, M. Asma, S. Sultana, M. Munir, N. Rashid and K. Malik, Front. Energy Res., 2019, 7, 4 CrossRef.
- M. Balat, Energy Convers. Manage., 2011, 52, 1479–1492 CrossRef CAS.
- A. Mahalingam, Y. Devarajan, S. Radhakrishnan, S. Vellaiyan and B. Nagappan, Alexandria Eng. J., 2018, 57, 2627–2631 CrossRef.
- E. Subroto, R. Manurung, H. J. Heeres and A. A. Broekhuis, Ind. Crops Prod., 2015, 63, 294–302 CrossRef CAS.
- S. E. Onoji, S. E. Iyuke, A. I. Igbafe and D. B. Nkazi, Energy Convers. Manage., 2016, 110, 125–134 CrossRef CAS.
- M. Ameen, M. T. Azizan, A. Ramli, S. Yusup and M. S. Alnarabiji, Ultrason. Sonochem., 2019, 51, 90–102 CrossRef CAS PubMed.
- Y.-H. Chen, T.-H. Chiang and J.-H. Chen, Fuel, 2012, 92, 377–380 CrossRef CAS.
- A. Azad, M. Rasul and C. Bhatt, Energy Procedia, 2019, 156, 159–165 CrossRef CAS.
- Z. Al-Hamamre and A. Al-Salaymeh, Fuel, 2014, 123, 175–188 CrossRef CAS.
- Z.-h. Ni, F.-s. Li, H. Wang, S. Wang, S.-y. Gao and L. Zhou, Renewable Energy, 2020, 145, 93–98 CrossRef CAS.
- D. Sharma, P. Kaur, G. Singh, D. Singh, S. Verma and J. Singh, Anal. Chem. Lett., 2019, 9, 564–581 CrossRef CAS.
- V. Ortiz-Martínez, M. Salar-García, F. Palacios-Nereo, P. Olivares-Carrillo, J. Quesada-Medina, A. P. De Los Ríos and F. Hernández-Fernández, J. Supercrit. Fluids, 2016, 113, 23–30 CrossRef.
- B. Y. Makama, O. Wilson and J. H. Claver, Am. J. Environ. Prot., 2018, 6, 35–38 CAS.
- Y. Singh, A. Sharma, N. Singh and A. Singla, SN Appl. Sci., 2019, 1, 1–9 CAS.
- S. Banka and S. P. Parikh, Asia-Pac. J. Chem. Eng., 2019, 14, e2310 CrossRef.
- S. L. Gadhave and S. S. Ragit, Biofuels, 2020, 11, 49–55 CrossRef CAS.
- Z. Liu, J. Li, G. Knothe, B. K. Sharma and J. Jiang, Energy Fuels, 2019, 33, 5110–5115 CrossRef CAS.
- S. Boukandoul, S. Casal and F. Zaidi, Agriculture, 2018, 8, 150 CrossRef CAS.
- V. Karthickeyan, Fuel, 2019, 235, 538–550 CrossRef CAS.
- A. J. Folayan, P. A. L. Anawe, A. E. Aladejare and A. O. Ayeni, Energy Rep., 2019, 5, 793–806 CrossRef.
- K. Roesler, D. Shintani, L. Savage, S. Boddupalli and J. Ohlrogge, J. Plant Physiol., 1997, 113(1), 75–81 CrossRef CAS PubMed.
- H. Vigeolas and P. Geigenberger, Planta, 2004, 219, 827–835 CrossRef CAS PubMed.
- M. R. Pollard, L. Anderson, C. Fan, D. J. Hawkins and H. M. Davies, Arch. Biochem. Biophys., 1991, 284, 306–312 CrossRef CAS.
- C. Martín, A. Moure, G. Martín, E. Carrillo, H. Domínguez and J. C. Parajó, Biomass Bioenergy, 2010, 34, 533–538 CrossRef.
- A. H. Tambunan, J. Situmorang, J. Silip, A. Joelianingsih and T. Araki, Biomass Bioenergy, 2012, 43, 12–17 CrossRef CAS.
- G. Veneziani, S. Esposto, A. Taticchi, R. Selvaggini, S. Urbani, I. Di Maio, B. Sordini and M. Servili, J. Agric. Food Chem., 2015, 63, 6066–6074 CrossRef CAS PubMed.
- E. Uquiche, M. Jeréz and J. Ortíz, Innovative Food Sci. Emerging Technol., 2008, 9, 495–500 CrossRef CAS.
- S. Qin, Y. Sun, X. Meng and S. Zhang, Energy Explor. Exploit., 2010, 28, 37–46 CrossRef CAS.
- M. Karthikeyan, S. Renganathan, G. Baskar and S. Nambirajan, Energy Sources, Part A, 2017, 39, 1746–1753 CrossRef CAS.
- A. P. Ibrahim, R. O. Omilakin and E. Betiku, Renewable Energy, 2019, 141, 349–358 CrossRef CAS.
- F. Jamil, H. Ala’a, L. Al-Haj, M. A. Al-Hinai, P. Hellier and U. Rashid, Energy Convers. Manage., 2016, 117, 264–272 CrossRef CAS.
- A. Mueanmas, R. Nikhom, A. Petchkaew, J. Iewkittayakorn and K. Prasertsit, Renewable Energy, 2019, 133, 1414–1425 CrossRef.
- S. Q. Liew, G. C. Ngoh, R. Yusoff and W. H. Teoh, Int. J. Biol. Macromol., 2016, 93, 426–435 CrossRef CAS PubMed.
- S. Adeel, F.-u.-. Rehman, A. Hameed, N. Habib, S. Kiran, K. M. Zia and M. Zuber, J. Nat. Fibers, 2020, 17, 745–758 CrossRef CAS.
- L. Wu, M. Hu, Z. Li, Y. Song, C. Yu, H. Zhang, A. Yu, Q. Ma and Z. Wang, Food Chem., 2016, 192, 596–602 CrossRef CAS PubMed.
- H. Rostami and S. M. T. Gharibzahedi, Carbohydr. Polym., 2016, 143, 100–107 CrossRef CAS PubMed.
- A. P. Ibrahim, R. O. Omilakin and E. Betiku, Renewable Energy, 2019, 141, 349–358 CrossRef CAS.
- X. Li, X.-Y. He, Z.-L. Li, Y.-D. Wang, C.-Y. Wang, H. Shi and F. Wang, Fuel, 2012, 92, 89–93 CrossRef CAS.
- C.-H. Su, H. C. Nguyen, T. L. Bui and D.-L. Huang, J. Cleaner Prod., 2019, 223, 436–444 CrossRef CAS.
- E. Subroto, E. Widjojokusumo, B. Veriansyah and R. R. Tjandrawinata, J. Food Sci. Technol., 2017, 54, 1286 CrossRef CAS PubMed.
- W. B. Kueh, S. Yusup and N. Osman, J. CO2 Util., 2018, 24, 220–227 CrossRef.
- S. Hossain, N. N. Norulaini, A. A. Naim, A. M. Zulkhairi, M. M. Bennama and A. M. Omar, J. CO2 Util., 2016, 16, 121–129 CrossRef.
- S. Hossain, V. Balakrishnan, N. N. N. Ab Rahman, Z. A. Rajion and M. O. Ab Kadir, J. Supercrit. Fluids, 2013, 83, 47–56 CrossRef.
- S. D. Manjare and K. Dhingra, Mater. Sci. Energy Technol., 2019, 2, 463–484 Search PubMed.
- K. Tyśkiewicz, M. Konkol and E. Rój, Molecules, 2018, 23, 2625 CrossRef PubMed.
- C.-H. Cheng, T.-B. Du, H.-C. Pi, S.-M. Jang, Y.-H. Lin and H.-T. Lee, Bioresour. Technol., 2011, 102, 10151–10153 CrossRef CAS PubMed.
- C.-H. Chen, W.-H. Chen, C.-M. J. Chang, S.-M. Lai and C.-H. Tu, J. Supercrit. Fluids, 2010, 52, 228–234 CrossRef CAS.
- J. Salimon and R. Abdullah, Sains Malays., 2008, 37, 379–382 CAS.
- A. Yusuf, P. Mamza, A. Ahmed and U. Agunwa, Int. J. Sci. Environ. Technol., 2015, 4, 1392–1404 Search PubMed.
- W. S. Eipeson, J. Manjunatha, P. Srinivas and T. S. Kanya, Ind. Crops Prod., 2010, 32, 118–122 CrossRef.
- R. Murmu, H. Sutar and S. Patra, Adv. Chem. Eng. Sci., 2017, 7, 464 CrossRef CAS.
- O. Onukwuli, L. N. Emembolu, C. N. Ude, S. O. Aliozo and M. C. Menkiti, Egypt. J. Pet., 2017, 26, 103–110 CrossRef.
- K. Viswanathan, Environ. Sci. Pollut. Res., 2018, 25, 13548–13559 CrossRef CAS PubMed.
- M. Abdullah, J. Salimon, E. Yousif and N. Salih, J. Assoc. Arab Univ. Basic Appl. Sci., 2013, 14, 83–86 Search PubMed.
- H. Mardhiah, H. C. Ong, H. Masjuki, S. Lim and H. Lee, Renewable Sustainable Energy Rev., 2017, 67, 1225–1236 CrossRef CAS.
- N. Acharya, P. Nanda, S. Panda and S. Acharya, J. King Saud Univ., Eng. Sci., 2019, 31, 184–190 CrossRef.
- A. K. Yadav, M. E. Khan and A. Pal, Korean J. Chem. Eng., 2017, 34, 340–345 CrossRef CAS.
- M. Talavera-Prieto, A. G. Ferreira, A. n. T. Portugal and A. P. Egas, J. Chem. Eng. Data, 2019, 64, 1166–1176 CrossRef.
- R. Gangadhara and N. Prasad, Chem. Int., 2016, 2, 59 CAS.
- S. Rani, M. Joy and K. P. Nair, Ind. Crops Prod., 2015, 65, 328–333 CrossRef CAS.
- P. N. Giannelos, F. Zannikos, S. Stournas, E. Lois and G. Anastopoulos, Ind. Crops Prod., 2002, 16, 1–9 CrossRef CAS.
- S. Keera, S. El Sabagh and A. Taman, Egypt. J. Pet., 2018, 27, 979–984 CrossRef.
- G. Baskar, I. A. E. Selvakumari and R. Aiswarya, Bioresour. Technol., 2018, 250, 793–798 CrossRef CAS PubMed.
- D. Sinha and S. Murugavelh, Perspect. Sci., 2016, 8, 237–240 CrossRef.
- U. Rashid, F. Anwar and G. Knothe, Fuel Process. Technol., 2009, 90, 1157–1163 CrossRef CAS.
- A. Bouaid, L. Bajo, M. Martinez and J. Aracil, Process Saf. Environ. Prot., 2007, 85, 378–382 CrossRef CAS.
- M. Naik, L. Meher, S. Naik and L. Das, Biomass Bioenergy, 2008, 32, 354–357 CrossRef CAS.
- P. Verma and M. Sharma, Fuel, 2016, 180, 164–174 CrossRef CAS.
- Y. Sharma and B. Singh, Fuel, 2010, 89, 1470–1474 CrossRef CAS.
- M. Yadav and Y. C. Sharma, J. Cleaner Prod., 2018, 199, 593–602 CrossRef CAS.
- Y. Devarajan, D. B. Munuswamy and A. Mahalingam, Process Saf. Environ. Prot., 2017, 111, 283–291 CrossRef CAS.
- B. Gurunathan and A. Ravi, Bioresour. Technol., 2015, 190, 424–428 CrossRef CAS PubMed.
- P. Shadangi and R. K. Singh, Fuel, 2012, 97, 450–456 CrossRef.
- J. Kansedo and K. T. Lee, Chem. Eng. J., 2013, 214, 157–164 CrossRef CAS.
- G. T. Ang, K. T. Tan, K. T. Lee and A. R. Mohamed, Energy Convers. Manage., 2015, 99, 242–251 CrossRef CAS.
- B. Yan, S. Zhang, W. Chen and Q. Cai, J. Anal. Appl. Pyrolysis, 2018, 136, 248–254 CrossRef CAS.
- V. Veljković, S. Lakićević, O. Stamenković, Z. Todorović and M. Lazić, Fuel, 2006, 85, 2671–2675 CrossRef.
- D. Pradhan, H. Bendu, R. Singh and S. Murugan, Energy, 2017, 118, 600–612 CrossRef CAS.
- P. Shadangi and K. Mohanty, Fuel, 2014, 126, 109–115 CrossRef.
- S. Fernando and M. Hanna, Energy Fuels, 2004, 18, 1695–1703 CrossRef CAS.
- A. de Jesus, M. M. Silva and M. G. R. Vale, Talanta, 2008, 74, 1378–1384 CrossRef CAS PubMed.
- T. Nguyen, L. Do and D. A. Sabatini, Fuel, 2010, 89, 2285–2291 CrossRef CAS.
- J. Liang, Y. Qian, X. Yuan, L. Leng, G. Zeng, L. Jiang, J. Shao, Y. Luo, X. Ding and Z. Yang, Renewable Energy, 2018, 126, 774–782 CrossRef CAS.
- A. Sankumgon, M. Assawadithalerd, N. Phasukarratchai, N. Chollacoop and C. Tongcumpou, Renew. Energy Focus, 2018, 24, 28–32 CrossRef.
- A. Gog, M. Roman, M. Toşa, C. Paizs and F. D. Irimie, Renewable energy, 2012, 39, 10–16 CrossRef CAS.
- F. Moazeni, Y.-C. Chen and G. Zhang, J. Cleaner Prod., 2019, 216, 117–128 CrossRef CAS.
- R. Sawangkeaw, K. Bunyakiat and S. Ngamprasertsith, Green Chem., 2007, 9, 679–685 RSC.
- N. García-Martínez, P. Andreo-Martínez, J. Quesada-Medina, A. P. de los Ríos, A. Chica, R. Beneito-Ruiz and J. Carratalá-Abril, Energy Convers. Manage., 2017, 131, 99–108 CrossRef.
- C. Román-Figueroa, P. Olivares-Carrillo, M. Paneque, F. J. Palacios-Nereo and J. Quesada-Medina, Energy, 2016, 107, 165–171 CrossRef.
- A. K. Agarwal and K. Rajamanoharan, Appl. Energy, 2009, 86, 106–112 CrossRef CAS.
- N. A. Mondal, M. Jakaria and M. M. Hasan, Sci. Educ., 2018, 6, 1–10 Search PubMed.
- Y. Rao, R. Voleti, V. Hariharan, P. Reddy and A. Raju, Energy Environ., 2009, 21, 1343 CrossRef.
- M. Sánchez, M. R. Avhad, J. M. Marchetti, M. Martínez and J. Aracil, Energy Convers. Manage., 2016, 129, 293–304 CrossRef.
- H. Imdadul, N. Zulkifli, H. Masjuki, M. Kalam, M. Kamruzzaman, M. Rashed, H. Rashedul and A. Alwi, Environ. Sci. Pollut. Res., 2017, 24, 2350–2363 CrossRef CAS PubMed.
- D. Kumar, B. Singh, A. Banerjee and S. Chatterjee, J. Cleaner Prod., 2018, 183, 26–34 CrossRef CAS.
- N. Kumar and M. Tomar, Int. J. Energy Res., 2019, 43, 3223–3236 CrossRef.
- A. Karmakar and S. Mukherjee, Indian J. Agric. Res., 2017, 51, 529–535 Search PubMed.
- N. Usta, B. Aydoğan, A. Çon, E. Uğuzdoğan and S. Özkal, Energy Convers. Manage., 2011, 52, 2031–2039 CrossRef CAS.
- A. Azad, M. Rasul, M. Khan, S. C. Sharma, M. Mofijur and M. Bhuiya, Renewable Sustainable Energy Rev., 2016, 61, 302–318 CrossRef CAS.
- O. Fasanya, A. A. Osigbesan and O. P. Avbenake, Biodiesel Technology and Applications, 2021, pp. 389–427 Search PubMed.
- V. Ávila Vázquez, R. A. Díaz Estrada, M. M. Aguilera Flores, C. Escamilla Alvarado and H. C. Correa Aguado, Biofuels, 2020, 11, 753–762 Search PubMed.
- N. A. M. Jamaluddin, T. M. I. Riayatsyah, A. S. Silitonga, M. Mofijur, A. H. Shamsuddin, H. C. Ong, T. M. I. Mahlia and S. M. A. Rahman, Processes, 2019, 7, 636 CrossRef CAS.
- L. E. Rincón, J. J. Jaramillo and C. A. Cardona, Renewable Energy, 2014, 69, 479–487 CrossRef.
- I. Chanakaewsomboon, C. Tongurai, S. Photaworn, S. Kungsanant and R. Nikhom, J. Environ. Chem. Eng., 2020, 8, 103538 CrossRef CAS.
- L. Tran-Nguyen, L. K. Ong, A. W. Go, Y.-H. Ju and A. E. Angkawijaya, Asia-Pac. J. Chem. Eng., 2020, 15, e2490 Search PubMed.
- N. Gebremariam and J. M. Marchetti, Biomass Bioenergy, 2021, 149, 106102 CrossRef.
- R. Naveenkumar and G. Baskar, Bioresour. Technol., 2020, 315, 123852 CrossRef CAS PubMed.
- O. Oke, O. Adeyi, B. I. Okolo, C. J. Ude, J. A. Adeyi, K. K. Salam, U. Nwokie and I. Nzeribe, Bioresour. Technol., 2021, 332, 125141 CrossRef PubMed.
- M. A. A. Farid, A. M. Roslan, M. A. Hassan, M. Y. Hasan, M. R. Othman and Y. Shirai, Sustain. Energy Technol. Assess., 2020, 39, 100700 Search PubMed.
- S. Rezania, B. Oryani, J. Park, B. Hashemi, K. K. Yadav, E. E. Kwon, J. Hur and J. Cho, Energy Convers. Manage., 2019, 201, 112155 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |