Open Access Article
Open Access ArticleA rapid analysis of antioxidants in Sanghuangporus baumii by online extraction-HPLC-ABTS†
Qian-Hui Shenab,
Qi Huangb,
Ju-Ying Xiec,
Kun Wangd,
Zheng-Ming Qian *bc and
De-Qiang Li
*bc and
De-Qiang Li *e
*e
aGuangdong Institute for Drug Control, NMPA Key Laboratory for Rapid Testing Technology of Drugs, Guangzhou 510663, Guangdong Province, China
bKey Laboratory of State Administration of Traditional Chinese Medicine, Dongguan HEC Cordyceps R&D Co., Ltd, No. 368, Zhen'an Middle Road, Chang'an Town, Dongguan 523850, Guangdong Province, China. E-mail: qianzhengming1982@126.com
cSchool of Rehabilitation, Xiangnan University, Chenzhou 423000, Hunan Province, China
dJinzhai Shangzhen Biotechnology Co., Ltd., Liuan 237300, Anhui Province, China
eDepartment of Pharmacy, The Second Hospital of Hebei Medical University, No. 215, Heping West Road, Shijiazhuang 050000, Hebei Province, China. E-mail: deqli@163.com
First published on 27th July 2021
Abstract
In the present study, a simple and efficient approach based on the online extraction-high performance liquid chromatography coupled with ABTS antioxidant assay (OLE-HPLC-ABTS) was established to quickly and directly analyze the antioxidants in S. baumii. Through this system, the HPLC mobile phase via a guard column packed with a S. baumii sample was used for online extraction (OLE). The separation was performed on an Agilent Poroshell EC-C18 column with a gradient elution using 0.1% formic acid (A) and 0.1% formic acid–acetonitrile (B) as mobile phase systems and detected at a wavelength of 254 nm. Then, the separated compounds were reacted with the antioxidant solution (ABTS), and the response was recorded at a wavelength of 400 nm. The developed analytical method was successfully applied to S. baumii samples, and eight antioxidants were identified. The established system integrated the online extraction, separation and online antioxidant detection, which is rapid, efficient, and suitable for the rapid screening of antioxidant compounds from solid sample mixtures.
1. Introduction
Sanghuangporus baumii is the dry fruit body of Sanghuangporus baumii (Pilat) L. W. Zhou and Y.C. Dai (Basidiomycota, Agaricomycetes, Hymenochaetales, Hymenochaetaceae, and Sanghuangporus), a kind of precious and functional mushroom historically used for food and medicine.1 S. baumii was first recorded in Shennong's Classic of Material Medical and mainly distributed in oriental countries (China, Korea, and Japan). It is commonly used in the treatment of amenorrhea, dysentery and diarrhoeal diseases.2,3 Modern researches have confirmed that S. baumii mainly contains polysaccharides, flavonoids, triterpenoids and pyranone compounds with various biological activities, such as anti-tumor, anti-oxidation, anti-inflammatory, immunomodulatory and hypoglycemic.4–7 In the human body, reactive oxygen free radicals participate in numerous biological activities (immunity and signal transduction processes, etc.). The overproduction of active oxygen free radicals has a destructive effect, leading to a variety of diseases, such as heart disease, Alzheimer's disease, and tumors, by causing an oxidative damage to the normal cells and tissues.4,9 Therefore, the exploitation of natural and efficient antioxidants not only has far-reaching significance for the treatment of diseases caused by the excessive free radicals but also promotes anti-tumor, immune regulation or other pharmacological effects. At present, it has been reported that S. baumii possesses antioxidant activity.8 However, up to date, the specific antioxidant active ingredients of S. baumii are still unclear. The quantitative analysis of active ingredients in Chinese medicine is an important part for the evaluation of its quality.10,11 Therefore, screening the antioxidants from S. baumii is helpful in improving the quality evaluation of its products.Traditionally, the commonly used method for the discovery of active compounds from complex mixtures basically contains the procedure of separation–purification–activity verification or activity-guided isolation, with the disadvantages of complicated steps, long time-consumption, high energy consumption and low efficiency.12 Recently, some online antioxidant screening methods such as HPLC-ABTS/DPPH/FRAP has been widely used in the rapid screening of antioxidants from Chinese herbal medicines, such as Fallopia sachalinensis, Morchella angusticeps, and Du-zhong brick tea.13–15 However, the above-mentioned methods with extracted sample solutions for the HPLC injection analysis still require offline sample preparation programs, such as ultrasonic extraction and heating reflux extraction, often causing the large consumption of organic reagents and insufficient extraction. Fortunately, the online extraction high performance liquid chromatography (OLE-HPLC) analysis technology was successfully developed and applied to the discovery of antioxidants from traditional Chinese medicine.16,17 Through this technology, the sample was extracted by a liquid phase eluent, and the extracted compounds were separated in a single run, avoiding the complex sample processing.
Therefore, in the present study, the approach of online extraction-HPLC-ABTS (OLE-HPLC-ABTS) was established by the combination of the online extraction and HPLC-ABTS, and it was successfully applied to analyze the antioxidants in S. baumii. As a result, eight compounds with antioxidant activities were identified via Q-TOF-MS/MS. This method provides a basis for the improvement of the basic research of the medicinal substances of S. baumii and their related product development.
2. Materials and method
2.1 Fungi materials, chemicals and reagents
The sample collected from Jinzhai (Anhui, China) was identified as S. baumii by Dr. Z. M. Qian (Sunshine Lake Pharma Co., Ltd). Voucher specimens were deposited at the Sunshine Lake Pharma Co., Ltd (Dongguan, China).Protocatechuic acid (99.20%) and protocatechualdehyde (94.30%) were purchased from the Nature Standard (Shanghai, China). 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) was purchased from Aladdin (Shanghai, China). Acid-washed diatomaceous earth was purchased from Sigma-Aldrich (Shanghai, China). The deionized water used for the experiments was purified using a Milli-Q purification system (Millipore, USA).
2.2 Preparation of standard stock solutions and working solutions
The mixed standard stock solution at a concentration of 1 mg mL−1 was prepared with 50% methanol, and the working solution was further diluted to the intended concentration with 50% methanol.The stock ABTS solution was prepared by mixing ABTS (7 mM dissolved in deionized water) and potassium persulfate (5 mM dissolved in deionized water) in equal volume (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and stored in a flask protected from light using an aluminum foil at 4 °C for at least 12 h. The working ABTS radical solution was prepared by diluting with absolute ethanol to an absorbance of 0.95–1.05 AU. A UV detector was set at 400 nm according to the UV scanning result of the ABTS working reagent showing a maximum absorption at this value. This liquor was protected from light with an aluminum foil and drawn out by pump to react with the separated sample through a buffer coil.
1) and stored in a flask protected from light using an aluminum foil at 4 °C for at least 12 h. The working ABTS radical solution was prepared by diluting with absolute ethanol to an absorbance of 0.95–1.05 AU. A UV detector was set at 400 nm according to the UV scanning result of the ABTS working reagent showing a maximum absorption at this value. This liquor was protected from light with an aluminum foil and drawn out by pump to react with the separated sample through a buffer coil.
The working FRAP radical solution was prepared by mixing TPTZ (10 mM in 40 mM HCl), FeCl3 (20 mM in water) and acetate buffer (300 mM, adjusted pH to 3.6 with 1 mM NaOH solution) in the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50. Then, the reagent solution flask was covered with an aluminum foil until further use. The FRAP working solution was prepared freshly for each run.
50. Then, the reagent solution flask was covered with an aluminum foil until further use. The FRAP working solution was prepared freshly for each run.
2.3 Sample preparation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm), and the supernatant was filtered through a 0.22 μm microporous membrane (JinTeng, China) prior to HPLC analysis.
000 rpm), and the supernatant was filtered through a 0.22 μm microporous membrane (JinTeng, China) prior to HPLC analysis.For investigating the dispersion and extraction effects of different dispersants, dispersants such as silica gel, Florisil, neutral alumina and polyamide were mixed with the S. baumii sample for online extraction.
2.4 The OLE-HPLC and post-column antioxidant system conditions
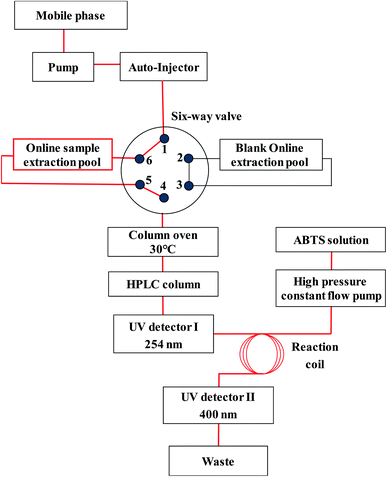
An Agilent 1260 Series high performance liquid chromatograph (HPLC) (Agilent Technologies, USA) was equipped with a quaternary pump, an online degasser, an auto-sampler, a column oven and a diode array detector (DAD). A Phenomenex hollow guard column (3 × 4 mm) with its fitted holder was used for the online extraction module.An Agilent Poroshell EC-C18 column (4.6 × 100 mm, 2.7 μm) was used for the sample separation. The column temperature was set at 30 °C. The mobile phase consisted of 0.1% formic acid–water (A) and acetonitrile contained 0.1% formic acid (B) at a flow rate of 0.8 mL min−1. The gradient elution program was as follows: 0–5 min, 15–23% B; 5–12 min, 23–25% B; 12–35 min, 25–35% B. The detection wavelength was set at 254 nm. All of the online extraction pools were connected with HPLC column through a cutting six-way valve (Fig. 1). The blank online extraction pool was connected to the liquid phase flow path for equilibrating the column, while the online sample extraction pool was connected to the flow path of the OLE-HPLC analysis. The extraction process was triggered by an auto-injector extraction of 0 μL.
Online post-column antioxidant activity was detected by combining a high pressure constant flow pump (Wufeng, China) and a 230 II UV-detector (Elite Analytical Instruments, China) to the main online extraction-HPLC system. After the online HPLC analysis, the separated sample was mixed to react with the antioxidant working solution pumped at a constant flow rate of 0.4 mL min−1 (fHPLC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) fABTS/FRAP = 2
fABTS/FRAP = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) by a high pressure constant flow pump through a reaction coil of 3 m long. Then, the antioxidant profiles of separated compounds were recorded by an UV detector (Fig. 1).
1) by a high pressure constant flow pump through a reaction coil of 3 m long. Then, the antioxidant profiles of separated compounds were recorded by an UV detector (Fig. 1).
2.5 Q-TOF-MS/MS conditions for identification analysis
HPLC-Q-TOF-MS was used to identify the compounds in the separated sample. The chromatographic conditions were the same as that of the OLE-HPLC system. The conditions of the ESI source were as follows: scanning mode, positive/negative ion mode; drying gas (N2) flow rate, 11 L min−1; drying gas (N2) temperature, 350 °C; nebulizer pressure, 40 psi; sheath gas flow rate 10 L min−1; capillary voltage, 4000 V; collision induced dissociation voltage, 120 V; ion scanning range, 100–1500 m/z. Collision energy was set at 10 V, 20 V and 40 V, respectively.2.6 Method validation
As this established approach is a qualitative analysis, only the specificity and repeatability were validated according to the guidelines of Chinese Pharmacopoeia (Ch. P).18 The specificity test was carried out via the analysis of standards and samples. In addition, three replicates of the online extraction pools prepared in parallel were analyzed via the OLE-HPLC system. Then, recording of the retention time and the area of each common peak was carried out to determine the repeatability of the developed method.3. Results and discussion
3.1 Optimization of OLE conditions
In general, the sample of the complex mixture contained macropolar and micropolar molecules. The achievement of a complete extraction efficacy in the sample pretreatment process usually required extraction through gradient organic solvents. The established system perfectly solved this problem by the inclusion of a gradient mobile phase. By comparing the results of the OLE-HPLC analysis with those obtained by the traditional ultrasound extraction under different concentrations of methanol (10%, 50%, and 100%), it was found that the developed OLE-HPLC system was able to completely and directly extract the macropolar and micropolar molecules at a higher extraction rate (Fig. S1†), with the advantages of reduced time and reduced use of organic reagents and simplified experimental procedures. Moreover, to investigate the optimum detection wavelength of the compounds contained in the sample, the different detected wavelengths (254, 330, and 360 nm) were compared. The result displayed that the detected wavelength of 254 nm had more chromatographic peaks and a smoother baseline (Fig. S2†).In addition, in order to obtain a satisfactory extraction effect, different types of dispersants mixed with the samples were investigated. Totally, 5 types of dispersants (acid-washed diatomaceous earth, silica gel, Florisil, neutral alumina, and polyamide) mixed with dried powder of S. baumii sample to make the online extraction pool were analyzed via the OLE-HPLC system. Fig. S3† indicates that the acid-washed diatomaceous earth when used as a dispersant can better disperse the compounds from the S. baumii sample and make it more completely extracted.
3.2 The extraction efficiency of the OLE-HPLC system
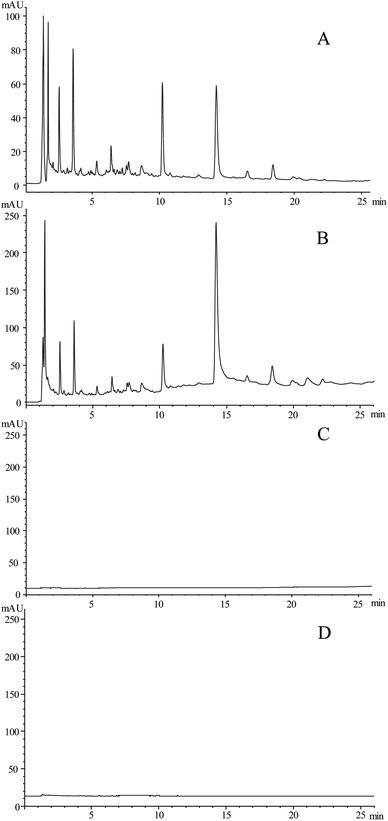
To verify the effectiveness of the developed OLE-HPLC method, the pretreated sample (ultrasound extraction) and the OLE sample were analyzed. The results showed that the chromatograms of the OLE sample were almost similar to those of the ultrasound pretreated sample (Fig. 2A and B). Moreover, the same OLE sample was analyzed for the second time to test if the online extraction was complete in one attempt (Fig. 2C). Results showed that the main compounds of S. baumii could be completely extracted in one attempt by the OLE-HPLC method, and the extraction efficiency of the OLE-HPLC method was almost identical to the traditional extraction method. The negative sample is shown in Fig. 2D.3.3 Comparison of HPLC-ABTS and HPLC-FRAP assays
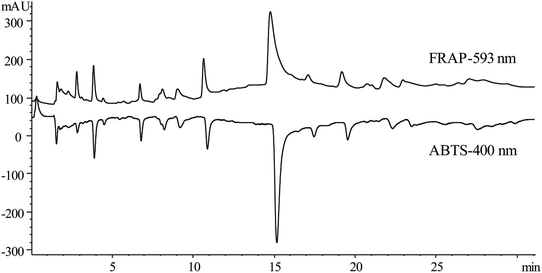
In this study, two common approaches (ABTS and FRAP) were used to evaluate the antioxidant capacities of the samples. Antioxidant effects can work through numerous mechanistic reaction pathways, such as metal chelation, quenching of singlet oxygen and radical scavenging.19The FRAP assay is based on a redox reaction of metal ion assessed through a redox-linked reduction of complex Fe3+–TPTZ to a blue-colored complex (Fe2+–TPTZ). Antioxidants increased the production of Fe2+–TPTZ, and a positive peak was observed at 593 nm. The ABTS radical scavenging assay was based on the oxidization of ABTS free radicals to green free radical cation ABTS˙+ under the action of appropriate oxidants (potassium persulfate, etc.). The antioxidants inhibited the production of ABTS+, and a negative peak was observed at 400 nm.
Two samples of the online extraction pools prepared in parallel were analyzed by the two post-column online antioxidant assays of ABTS and FRAP. The wavelength of the HPLC-ABTS assay was set at 400 nm according to the UV scanning result of the ABTS working reagent showing a maximum absorption at this value, and the chromatogram showing the negative peaks was obtained after each run. However, the wavelength of the HPLC-FRAP assay was set at 593 nm, and a chromatogram with positive peaks was recorded. The results (Fig. 3) showed that the chromatographic peak response of the compounds possessing antioxidant activity was maximum, and the baseline noise was smoother in the HPLC-ABTS system. Besides, the chromatogram with a negative peak provided a more intuitive display of antioxidant compounds. Eventually, the HPLC-ABTS post-column assay was used for the online antioxidant analysis.
3.4 Method validation
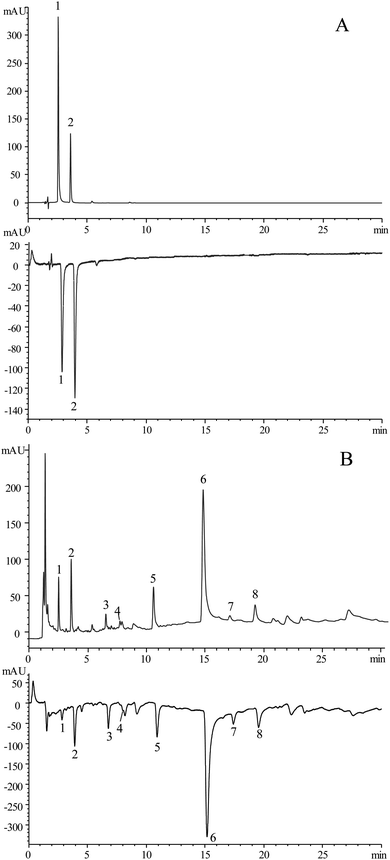
To determine the repeatability of the developed method, three samples of the online extraction pools prepared in parallel were extracted by the online extraction-HPLC-ABTS system. Then, the calculated RSD values of the retention time and peak areas from each common peak were <6%, which indicated that the method was suitable for the intended use and demonstrated great repeatability. Furthermore, the specificity test is verified via the analysis of the standards and samples shown in Fig. 4.3.5 OLE-HPLC-ABTS analysis of S. baumii
After the optimization and validation of the method, the OLE-HPLC-ABTS system was successfully applied to the mixed standard stock solution and the OLE sample. The chromatographs with the positive peaks (UV detector at 254 nm) and the antioxidant activity chromatograms with negative peaks (UV detector at 400 nm) are obtained, as shown in Fig. 4. In recent years, numerous approaches for screening of the antioxidant in other species of Sanghuangporus were reported.4,20–22 Comparison with the reported methods showed that the established approach only required 35 min to complete the whole process of antioxidant screening, while the reported methods needed at least 60 min (Table 1). In addition, the OLE-HPLC-ABTS assay only needed less than 50 mL of mobile phase, while the reported methods required above 180 mL of the total solvent. In addition, the reported methods only proved the total antioxidant capacity of S. Baumii extraction, while the current approach identified the 8 antioxidant components from S. Baumii. Thus, the developed OLE-HPLC-ABTS system for the analysis of the antioxidants in S. Baumii was simple, rapid and highly efficient.| No. | Species of Sanghuangporus | Sample preparation | Sample antioxidant analysis | Total time (min) | Total solvent volume (mL) | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| Extraction methods | Extraction solutions | Time (min) | Method | Time (min) | |||||
| 1 | S. sanghuang | Dipping extraction | 70% ethanol | 7200 | ABTS, DPPH | 20 | 7220 | Unclear | 4 |
| S. baumii | |||||||||
| S. vaninii | |||||||||
| 2 | S. sanghuang | Ultrasonic extraction | 80% ethanol | 40 | ABTS, DPPH | 20/30 | 60/70 | 180 | 20 |
| 3 | Phellinus linteus | Reflux extraction | Methanol | 300 | HPLC-DPPH | 30 | 330 | 3 × 104 | 21 |
| 4 | Phellinus igniarius | Heat-assisted extraction | Water | 420 | FRAP, DPPH | 30/20 | 450/440 | Unclear | 22 |
| Phellinus linteus | |||||||||
| This study | S. baumii | Online extraction | — | — | HPLC–ABTS | 35 | 35 | 42 | — |
3.6 Identification of antioxidants in S. baumii
The S. baumii sample was further analyzed by the HPLC-Q-TOF-MS system to identify the components with antioxidant activity. Two peaks, protocatechuic acid (1) and protocatechualdehyde (2), were identified by comparing the retention time and the mass spectrometric data of the chromatographic peaks with that of the reference substances. By verifying the mass spectrometry data of the chromatographic peak with the reported data,23–26 other 6 chromatographic peaks were preliminarily identified, namely osmundacetone (3), hispidin (4), davallialactone (5), hypholomine B (6), (4Z)-3-(3,4-dihydroxyphenyl)-6-[(E)-2-(3,4-dihydroxyphenyl)vinyl]-1-hydroxy-4-(2-oxopropylidene)-3a,4-dihydro-3H,8H-furo[3,4-c]oxepin-8-one (7), and inoscavin A (8). The detailed mass data is provided in Table 2. The results showed that the main antioxidant components in S. baumii are flavonoids and polyphenols.| Peaks no. | RT (min) | Molecular formula | Molecular ion (m/z) | Fragment ion (m/z) | Identification |
|---|---|---|---|---|---|
| 1 | 2.530 | C7H6O4 | 153.0189 | 109.0291 | Protocatechuic acid |
| 2 | 3.586 | C7H5O3 | 137.0241 | 108.0211 | Protocatechualdehyde |
| 3 | 6.403 | C10H10O3 | 177.0552 | 177.0548, 134.0368, 133.0284 | Osmundacetone |
| 4 | 7.530 | C13H10O5 | 245.0456 | 159.0456, 201.0558 | Hispidin |
| 5 | 10.207 | C25H20O9 | 463.1033 | 379.0827, 259.0612, 159.0433 | Davallialactone |
| 6 | 14.152 | C26H18O10 | 489.0840 | 445.0935, 403.0829, 241.0506 | Hypholomine B |
| 7 | 16.488 | C25H20O9 | 463.1034 | 405.0618, 379.0808, 259.0586 | (4Z)-3-(3,4-Dihydroxyphenyl)-6-[(E)-2-(3,4-dihydroxyphenyl)vinyl]-1-hydroxy-4-(2-oxopropylidene)-3a,4-dihydro-3H,8H-furo[3,4-c]oxepin-8-one |
| 8 | 18.381 | C25H18O9 | 461.0877 | 377.0661, 257.0442, 159.0432 | Inoscavin A |
4. Conclusion
In this study, the OLE-HPLC-ABTS technology was used to establish an online screening method for the rapid discovery of the antioxidants in S. baumii. By combining of the online extraction and online antioxidant assay, the advantages of extremely lower sample consumption, higher analytical efficiency and less usage of the extraction solvent were observed compared to the previous methods. The results of the antioxidant screening from S. Baumii showed that the main antioxidant substances are flavonoids and polyphenols, which could be used as the markers for the quality control of S. baumii and its products. In future, the analytical method for the determination of these antioxidants should be developed for the analysis of different S. baumii samples. Besides, by virtue of low sample consumption (mg level), the developed method is more suitable for the analysis of the active substances in valuable medicinal materials, such as Cordyceps sinensis. To conclude, as a green analytical chemistry method with little pollution, it provided a possible developmental direction for the analysis and quality control of TCM as well as the discovery of bioactive compounds from them.Funding information
This study was funded by the NMPA Key Laboratory Open Project Fund for Rapid Testing Technology of Drugs, Talent Training Project of Hebei Province (A201902012), the Natural Science Foundation of Hebei Province of China (H2020206091), and the Research and Demonstration on Three-dimensional, Standardized and Highly Efficient Cultivation Mode and Key Technology of Deep Processing of Sanghuang PV Greenhouse (1804G07020180).Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.Author contributions
Qianhui Shen: investigation, visualization, methodology, and writing (original draft preparation). Qi Huang: visualization, supervision, and writing (review and editing). Juying Xie: conceptualization and writing (review and editing). Kun Wang: resources, project administration, and funding acquisition. Luqi Peng: writing (review and editing). Zhengming Qian: conceptualization, validation, methodology, project administration, funding acquisition, and writing (review and editing). Deqiang Li: conceptualization, project administration, funding acquisition, and writing (review and editing).Conflicts of interest
All the authors declare that they have no conflicts of interests.References
- J. Huang, K. Wang, S. Zuo, L. Chen, Z. Y. Ding, M. El-shazly and B. B. Zhang, Unsaturated fatty acid promotes the production of triterpenoids in submerged fermentation of Sanghuangporus baumii, Food Biosci., 2020, 37, 100712 CrossRef CAS.
- H. M. Cao, G. P. Hu, X. P. Shi, X. M. Du, J. W. Wang, Z. H. Deng, X. P. Li, S. Y. Zheng and L. X. Wang, Research progress on medicinal fungus Sanghuang, Sci. Seric., 2019, 45, 0285–0292 Search PubMed.
- W. K. Wang, N. Lu, J. Yan, L. Z. Fu, J. L. Song, W. D. Yuan and Z. F. Zhou, Effects of growth age on nutrition, content of active components and antioxidant activities of fruiting bodies of Sanghuangporus baumii cultivated on oak segment, Mycosystema, 2020, 39, 1–12 Search PubMed.
- W. C. Lin, J. S. Deng, S. S. Huang, S. H. Wu, H. Y. Lin and G. J. Huang, Evaluation of antioxidant, anti-inflammatory and anti-proliferative activities of ethanol extracts from different varieties of Sanghuang species, RSC Adv., 2017, 7, 7780–7788 RSC.
- J. G. Han, M. W. Hyun, C. S. Kim, J. W. Jo, J. H. Cho, K. H. Lee, W. S. Kong, S. K. Han, J. Oh and G. H. Sung, Species identity of Phellinus linteus (Sanghuang) extensively used as a medicinal mushroom in Korea, J. Microbiol., 2016, 54, 290–295 CrossRef CAS PubMed.
- N. T. Thanh, N. N. Tuan, P. C. Kuo, D. M. Dung, D. L. Phuong, D. T. T Giang, T. S. Wu and T. D. Thang, Chemical constituents from the fruiting bodies of Phellinus igniarius, Nat. Prod. Res., 2018, 32, 2392–2397 CrossRef PubMed.
- H. Feng, S. J. Zhang, J. M. F Wan, L. F. Gui, M. C. Ruan, N. Li, H. Y. Zhang, Z. G. Liu and H. L. Wang, Polysaccharides extracted from Phellinus linteus ameliorate high-fat high-fructose diet induced insulin resistance in mice, Carbohydr. Polym., 2018, 200, 144–153 CrossRef CAS PubMed.
- Q. Xu, W. X. Zhou, C. Wang, Q. Li, M. Y. Sun, X. X. Jiao, J. J. Lou and L. X. Zhang, Research status on active substances of Phellinus igniarius, Edible Fungi of China, 2019, 38, 1–6 CrossRef PubMed.
- O. A. Wintola, A. A. Olajuyigbe, A. J. Afolayan, R. M. Coopoosamy and O. O. Olajuyigbe, Chemical composition, antioxidant activities and antibacterial activities of essential oil from Erythrina caffra Thunb. growing in South Africa, Heliyon, 2021, 7, e07244 CrossRef PubMed.
- H. Hoellinger, M. Re, A. Deroussent, R. P. Singh and T. Cresteil, Quantitative liquid chromatographic determination of sanguinarine in cell culture medium and in rat urine and plasma, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2004, 799, 195–200 CrossRef CAS PubMed.
- Z. M. Qian, C. H. Li, Y. L. Song, M. X. Zhou and W. J. Li, Rapid Determination of Adenosine in Cordyceps by Online Extraction HPLC, J. Chromatogr. Sci., 2019, 57, 381–384 CAS.
- Y. P. Zhang, S. Y. Shi, X. Xiong, X. Q. Chen and M. J. Peng, Comparative evaluation of three methods based on high-performance liquid chromatography analysis combined with a 2,2′-diphenyl-1-picrylhydrazyl assay for the rapid screening of antioxidants from Pueraria lobata flowers, Anal. Bioanal. Chem., 2012, 402, 2965–2976 CrossRef CAS PubMed.
- S. Lachowicz, J. Oszmiański, A. Wojdyło, T. Cebulak, L. Hirnle and M. Siewiński, UPLC-PDA-Q/TOF-MS identification of bioactive compounds and on-line UPLC-ABTS assay in Fallopia japonica Houtt and Fallopia sachalinensis (F. Schmidt) leaves and rhizomes grown in Poland, Eur. Food Res. Technol., 2019, 245, 691–706 CrossRef CAS.
- J. K. You, M. Yan, Y. Q. Zhuang, S. R. Wu and J. L. Cui, Screening and characterization of antioxidative active ingredients in Morchella angusticeps by HPLC-DAD-MS-DPPH, Edible Fungi of China, 2019, 38, 52–58 Search PubMed.
- S. Y. Shi, K. Guo, R. N. Tong, Y. G. Liu, C. Y. Tong and M. J. Peng, Online extraction-HPLC-FRAP system for direct identification of antioxidants from solid Du-zhong brick tea, Food Chem., 2019, 288, 215–220 CrossRef CAS PubMed.
- Z. M. Qian, B. W. Fang, H. M. Chen, C. H. Li, Q. Huang, L. Chen, W. J. Li and D. Q. Li, Online liquid microextraction coupled with HPLC-ABTS for rapid screening of natural antioxidants: case study of three different teas, J. Chromatogr. Sci., 2020, 58, 875–879 CAS.
- Z. M. Qian, C. H. Li, Y. L. Song, M. X. Zhou and W. J. Li, Rapid determination of adenosine in Cordyceps by online extraction HPLC, J. Chromatogr. Sci., 2019, 57, 381–384 CAS.
- Chinese Pharmacopoeia Commission, Pharmacopoeia of the People's Republic of China, Beijing, China, 1st edn, 2015 Search PubMed.
- Z. Wang, C. M. Tang, F. W. Dai, G. S. Xiao and G. Q. Luo, HPLC determination of phenolic compounds in different solvent extracts of mulberry leaves and antioxidant capacity of extracts, Int. J. Food Prop., 2021, 24, 544–552 CrossRef CAS.
- C. S. Cai, J. X. Ma, C. R. Han, Y. Jin, G. Z. Zhao and X. W. He, Extraction and antioxidant activity of total triterpenoids in the mycelium of a medicinal fungus, Sanghuangporus sanghuang, Sci. Rep., 2019, 9, 7418 CrossRef PubMed.
- Y. E. Jeon, Y. S. Lee, S. S. Lin, S. J. Kim, S. H. Jung, Y. S. Bae, J. S. Yi and I. J. Kang, Evaluation of the antioxidant activity of the fruiting body of Phellinus linteus using the on-line HPLC-DPPH method, J. Korean Soc. Appl. Biol. Chem., 2009, 52, 472–479 CrossRef CAS.
- S. S. Guo and W. D. Rausch, Compare of antioxidant and anti-inflammatory activity of different Phellinus, Chinese Journal of Pharmacovigilance, 2017, 14, 394–398 Search PubMed.
- Y. Zhang, Extraction and separation of active components from medicinal fungi Phellinus igniarius and Inonotus obliquus and its anti-gout activity, MPhil dissertation, Changchun Normal University, 2020.
- T. Y. Zhao, Z. Z. Wang, N. Q. Zhang, X. Y. Su, J. P. Liu, P. Y. Li and C. Z. Wang, Rapid identification on chemical constituents of Phellinus igniarius by UPLC-Q-TOF-MSE combined with UNIFI platform, Special Wild Economic Animal and Plant Research, 2018, 1, 20–25 Search PubMed.
- J. Y. Jung, I. K. Lee, S. J. Seok, H. J. Lee, Y. H. Kim and B. S. Yun, Antioxidant polyphenols from the mycelial culture of the medicinal fungi Inonotus xeranticus and Phellinus linteus, J. Appl. Microbiol., 2010, 104, 1824–1832 CrossRef PubMed.
- K. Kojima, Y. Ogihara, Y. Sakai, H. Mizukami and A. Nagatsu, HPLC profiling of Phellinus linteus, J. Nat. Med., 2008, 62, 441–446 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra04300e |
| This journal is © The Royal Society of Chemistry 2021 |