Open Access Article
Open Access ArticleSILP materials based on TiO2–SiO2 and TiO2–SiO2/lignin supports as new catalytic materials for hydrosilylation reaction – synthesis, physicochemical characterization and catalysis†
Olga Bartlewicz *ab,
Mariusz Pietrowski
*ab,
Mariusz Pietrowski a,
Marta Kaczmareka and
Hieronim Maciejewski
a,
Marta Kaczmareka and
Hieronim Maciejewski ab
ab
aFaculty of Chemistry, Adam Mickiewicz University in Poznań, Uniwersytetu Poznańskiego 8, Poznań, 61-614, Poland. E-mail: olga.bartlewicz@amu.edu.pl
bAdam Mickiewicz University Foundation, Poznań Science and Technology Park, Rubież 46, Poznań, 61-612, Poland
First published on 1st July 2021
Abstract
The oxide system TiO2–SiO2 as well as a TiO2–SiO2/lignin system have been obtained by the sol–gel synthesis method and applied as supports in Supported Ionic Liquid Phase (SILP) materials. In total 24 SILP systems were obtained with ionic liquids containing imidazolium, pyridinium, phosphonium or sulfonic cations and bis(trifluoromethylsulfonyl)imide or methylsulfate anions, and homogeneous complexes of rhodium or platinum as the active phase. The supports and catalytic materials were subjected to thorough characterization by elemental analysis, XRD, SEM-EDX, IR, and TGA, and their particle size distribution and porous properties were assessed. The new SILP materials were used in hydrosilylation of 1-octene with 1,1,1,3,5,5,5-heptamethyltrisiloxane. The effectiveness of hydrosilylation reaction catalyzed by the obtained SILP materials for the polar and nonpolar reagents was assessed. All the catalytically active materials were proved to be easy to isolate and reuse, and the best SILP systems have been shown to be active in 10 or more subsequent catalytic cycles.
Introduction
The reaction of hydrosilylation, permitting the addition of compounds containing the![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si–H group to compounds having multiple bonds, is an easy and effective method for obtaining organosilicon compounds. This reaction is commonly used not only in laboratory syntheses but also in large-scale industrial processes, including additive cross-linking of silicones, production of silane adhesion promotors and production of hybrid materials.1–4 Recently, the hydrosilylation processes have been used for functionalization (introduction of functional groups) of different materials, for instance for production of organofunctional silicones (polydimethylsiloxanes), showing chemical reactivity, in contrast to the standard, chemically inert, silicones. Thanks to these properties it is possible to produce new hybrid materials showing unique properties depending on the type of functional group attached to the siloxane chain and maintaining the characteristic features of silicones, i.e. high elasticity and thermal stability.2,5 The reaction of hydrosilylation is a catalytic process, usually taking place in the presence of homogeneous complexes of transition metals.2,4 This fact generates problems related to the effective isolation of the catalyst from the post-reaction mixture. Sometimes it is even impossible to isolate the catalyst from the product, which is highly undesirable for economical (high cost of catalysts) and ecological (high toxicity of the metals used, even in trace amounts) reasons. That is why much attention is paid to heterogeneous catalytic systems for hydrosilylation reaction, that would permit easy isolation of the catalytically active substance from the post-reaction mixture and its use in subsequent catalytic cycles.6–9 One of the recent solutions proposed for heterogenization of the homogeneous catalysts is based on the use of SILP (Supported Ionic Liquid Phase) materials. The SILP systems are obtained by physical impregnation of the support with an ionic liquid that immobilizes a given metal or metal complex. The stability and potential catalytic application of the SILP system depend on all its components.10,11 The cost of SILP system production is much lower than that of liquid–liquid systems, mainly because of small amounts of the ionic liquid (10–20% wt relative to the support mass) and the active substance needed. Moreover, the SILP system can be easily isolated from the post-reaction mixture and used again in subsequent catalytic cycles, which also contributes to cost reduction. The use of a small amount of ionic liquid is also important because of the decreased risk of adsorption of contaminants and side products that can be formed in the process, which extends the time of the catalyst use.11 SILP systems have been successfully used in many chemical reactions, e.g. in the reactions of hydroformylation or the Heck and Suzuki processes.12–14 The SILP systems based on silica support belong to the most often used catalytic materials of this type.15 Recently, other supports have been used for production of SILP materials, e.g. zeolites, carbon nanotubes, resins or oxides.16–18 From the group of the latter, particularly attractive seem to be the supports being combinations of at least two different oxides. Besides combining the unique properties of individual oxides, this solution permits enlargement of the surface area of the support. Such materials are most often obtained by the wasteless sol–gel method, which not only needs mild conditions, but also permits control of the morphology of the obtained support. Thanks to the processes of hydrolysis, condensation and removal of the solvent from the structure of the obtained alcogel, it is possible to get the oxide systems of high purity.19,20 In our studies we applied this method for the synthesis of a hybrid oxide support composed of titanium dioxide and silica. The choice of these components was based on the idea of combining their particular properties. Titanium dioxide shows high chemical and thermal resistance, is non-toxic, biocompatible, has a high oxidizing potential and unique optical properties.21–23 Silicon dioxide shows high mechanical strength and thermal resistance as well as large surface area.24,25 The hitherto studies of TiO2–SiO2 have shown that this system is an excellent support in the processes of dye adsorption26 and in catalytic processes.27,28 Another interesting idea is a combination of inorganic–organic hybrids as catalysts supports. Attachment of lignin to the oxide strengthens the system and improves its sorption properties.29 The structure of this biopolymer includes a number of functional groups, e.g. –OH, –OCH3, C
Si–H group to compounds having multiple bonds, is an easy and effective method for obtaining organosilicon compounds. This reaction is commonly used not only in laboratory syntheses but also in large-scale industrial processes, including additive cross-linking of silicones, production of silane adhesion promotors and production of hybrid materials.1–4 Recently, the hydrosilylation processes have been used for functionalization (introduction of functional groups) of different materials, for instance for production of organofunctional silicones (polydimethylsiloxanes), showing chemical reactivity, in contrast to the standard, chemically inert, silicones. Thanks to these properties it is possible to produce new hybrid materials showing unique properties depending on the type of functional group attached to the siloxane chain and maintaining the characteristic features of silicones, i.e. high elasticity and thermal stability.2,5 The reaction of hydrosilylation is a catalytic process, usually taking place in the presence of homogeneous complexes of transition metals.2,4 This fact generates problems related to the effective isolation of the catalyst from the post-reaction mixture. Sometimes it is even impossible to isolate the catalyst from the product, which is highly undesirable for economical (high cost of catalysts) and ecological (high toxicity of the metals used, even in trace amounts) reasons. That is why much attention is paid to heterogeneous catalytic systems for hydrosilylation reaction, that would permit easy isolation of the catalytically active substance from the post-reaction mixture and its use in subsequent catalytic cycles.6–9 One of the recent solutions proposed for heterogenization of the homogeneous catalysts is based on the use of SILP (Supported Ionic Liquid Phase) materials. The SILP systems are obtained by physical impregnation of the support with an ionic liquid that immobilizes a given metal or metal complex. The stability and potential catalytic application of the SILP system depend on all its components.10,11 The cost of SILP system production is much lower than that of liquid–liquid systems, mainly because of small amounts of the ionic liquid (10–20% wt relative to the support mass) and the active substance needed. Moreover, the SILP system can be easily isolated from the post-reaction mixture and used again in subsequent catalytic cycles, which also contributes to cost reduction. The use of a small amount of ionic liquid is also important because of the decreased risk of adsorption of contaminants and side products that can be formed in the process, which extends the time of the catalyst use.11 SILP systems have been successfully used in many chemical reactions, e.g. in the reactions of hydroformylation or the Heck and Suzuki processes.12–14 The SILP systems based on silica support belong to the most often used catalytic materials of this type.15 Recently, other supports have been used for production of SILP materials, e.g. zeolites, carbon nanotubes, resins or oxides.16–18 From the group of the latter, particularly attractive seem to be the supports being combinations of at least two different oxides. Besides combining the unique properties of individual oxides, this solution permits enlargement of the surface area of the support. Such materials are most often obtained by the wasteless sol–gel method, which not only needs mild conditions, but also permits control of the morphology of the obtained support. Thanks to the processes of hydrolysis, condensation and removal of the solvent from the structure of the obtained alcogel, it is possible to get the oxide systems of high purity.19,20 In our studies we applied this method for the synthesis of a hybrid oxide support composed of titanium dioxide and silica. The choice of these components was based on the idea of combining their particular properties. Titanium dioxide shows high chemical and thermal resistance, is non-toxic, biocompatible, has a high oxidizing potential and unique optical properties.21–23 Silicon dioxide shows high mechanical strength and thermal resistance as well as large surface area.24,25 The hitherto studies of TiO2–SiO2 have shown that this system is an excellent support in the processes of dye adsorption26 and in catalytic processes.27,28 Another interesting idea is a combination of inorganic–organic hybrids as catalysts supports. Attachment of lignin to the oxide strengthens the system and improves its sorption properties.29 The structure of this biopolymer includes a number of functional groups, e.g. –OH, –OCH3, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, which means that the material is able to easily link to other substances, including enzymes or metal complexes.30
O, which means that the material is able to easily link to other substances, including enzymes or metal complexes.30
In this paper we report the studies of new SILP materials obtained with the use of the oxide system TiO2–SiO2 and TiO2–SiO2/lignin as supports. These supports were subjected to impregnation with ionic liquids containing imidazolium, pyridinium, phosphonium or sulfonium cations or methylsulfate or bis(trifluoromethylsulfonyl)imide anions. Then the complexes of rhodium or platinum were immobilized on the supports surface. The obtained SILP systems were characterized as to their physicochemical and adsorption properties.
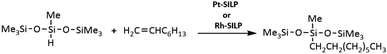
As our research group has many-year experience in catalysis of hydrosilylation processes,31–33 this very reaction was used to test the catalytic activities of the new Rh-SILP and Pt-SILP systems and the possibilities of their isolation from post-reaction mixture and reuse. The reactions of hydrosilylation were performed with the use of polar and nonpolar olefins (1-octene, allyl-glycidyl ether, octafluoropentyl ether) and 1,1,1,3,5,5,5-heptamethyltrisiloxane, triethylsilane and triethoxysilane.
Experimental
Synthesis of titanium dioxide–silica support (TiO2–SiO2)
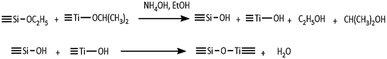
The synthesis of the inorganic oxide system TiO2–SiO2 was carried out by the sol–gel method with using tetraethoxysilane (TEOS) as silicon precursor and titanium(IV) isopropoxide (TIPP) as a titanium precursor.26 Schematic synthesis reaction of TiO2–SiO2 is presented in Scheme 1 and the detailed procedure of synthesis is described in ESI.†Preparation of TiO2–SiO2/lignin support
Preparation of Rh-SILP and Pt-SILP materials
SLIP materials were prepared using the method of physical impregnation. The earlier calcinated TiO2–SiO2 or TiO2–SiO2/lignin support (2 g) was impregnated with an ionic liquid (10% wt), which immobilized platinum (dichloro(1,5-cyclooctadiene)platinum(II)) or rhodium (Wilkinson's catalyst) catalyst (4 × 10−3%),35,37 as described in detail in ESI.†Results and discussion
Physicochemical characterization of TiO2–SiO2 oxide system
By the sol–gel synthesis a series of TiO2–SiO2 oxide systems were obtained. In order to establish the effects of the composition of the oxide systems on their adsorption properties, the precursors of silicon and titanium were used at different molar ratios of 0.75 (TEOS)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 (TIPP), 0.50 (TEOS)
0.25 (TIPP), 0.50 (TEOS)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.50 (TIPP) and 0.25 (TEOS)
0.50 (TIPP) and 0.25 (TEOS)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75 (TIPP). The porous properties of the obtained oxide systems were compared with those of the individual oxides being components of the TiO2–SiO2 system, obtained by the same method, see Table S2 (ESI).† The results confirmed the influence of different proportions of silicon and titanium precursors on the porous properties of the TiO2–SiO2 systems obtained. With increasing molar concentration of TEOS, the surface area as well as the pore volume and average pore diameter of the support increase. The adsorption parameters of all TiO2–SiO2 systems obtained were better than those of the individual components of the system (silica dioxide and titanium dioxide). The largest surface area was obtained for the TiO2–SiO2 system synthesized with the highest molar ratio of TEOS, while the smallest surface area was found for the system synthesized with the greater amount of TIPP. The molar ratio of silicon and titanium precursors also affected the thermal stability of the oxide systems obtained. As follows from the results of thermogravimetric measurements (TG), a greater amount of tetraethoxysilane used for the synthesis results in a higher thermal resistance of the oxide system, Table 1. Unfortunately, the thermal stabilities of the TiO2–SiO2 systems although satisfactory, were not as high as the stabilities of the individual oxides TiO2 and SiO2.
0.75 (TIPP). The porous properties of the obtained oxide systems were compared with those of the individual oxides being components of the TiO2–SiO2 system, obtained by the same method, see Table S2 (ESI).† The results confirmed the influence of different proportions of silicon and titanium precursors on the porous properties of the TiO2–SiO2 systems obtained. With increasing molar concentration of TEOS, the surface area as well as the pore volume and average pore diameter of the support increase. The adsorption parameters of all TiO2–SiO2 systems obtained were better than those of the individual components of the system (silica dioxide and titanium dioxide). The largest surface area was obtained for the TiO2–SiO2 system synthesized with the highest molar ratio of TEOS, while the smallest surface area was found for the system synthesized with the greater amount of TIPP. The molar ratio of silicon and titanium precursors also affected the thermal stability of the oxide systems obtained. As follows from the results of thermogravimetric measurements (TG), a greater amount of tetraethoxysilane used for the synthesis results in a higher thermal resistance of the oxide system, Table 1. Unfortunately, the thermal stabilities of the TiO2–SiO2 systems although satisfactory, were not as high as the stabilities of the individual oxides TiO2 and SiO2.
From among the obtained TiO2–SiO2 systems, the one characterized with the largest surface area of 328 m2 g−1 (Table S2 (ESI)†) was chosen for further studies. This system was synthesized using TEOS and TIPP at the molar ratio of 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25. The effectiveness of the catalytic process significantly depends on the appropriate preparation of the support surface for adsorption. At first the support was subjected to calcination to eliminate the physically adsorbed water and contaminants introduced at the stage of synthesis. The calcination of TiO2–SiO2 resulted in a decrease in its surface area (Table 2), which indicates a high contribution of micropores in the TiO2–SiO2 structure prior to this process, which may limit the effectiveness of adsorption. In the process of calcination the micropores join one another to form mesopores, which is confirmed by the increase in the pore diameter in the support after the process, Table 2.
0.25. The effectiveness of the catalytic process significantly depends on the appropriate preparation of the support surface for adsorption. At first the support was subjected to calcination to eliminate the physically adsorbed water and contaminants introduced at the stage of synthesis. The calcination of TiO2–SiO2 resulted in a decrease in its surface area (Table 2), which indicates a high contribution of micropores in the TiO2–SiO2 structure prior to this process, which may limit the effectiveness of adsorption. In the process of calcination the micropores join one another to form mesopores, which is confirmed by the increase in the pore diameter in the support after the process, Table 2.
| Inorganic support | BET surface area [m2 g−1] | Total pore volume [cm3 g−1] | Average pore diameter [nm] |
|---|---|---|---|
| Before CP | 464 | 0.93 | 7.97 |
| After CP | 328 | 0.91 | 10.95 |
The temperature of calcination determines the formation of particular crystalline forms in the support structure. When it is above 600 °C, the TiO2–SiO2 support is composed of anatase and rutile, with significant prevalence of the latter. According to literature data, rutile shows poorer adsorption properties than anatase as the latter has large surface area and high degree of the surface hydroxylation. A too high temperature of calcination leads to pore sintering and formation of agglomerates.38,39 In view of the above, the temperature of 600 °C was chosen as the optimum.
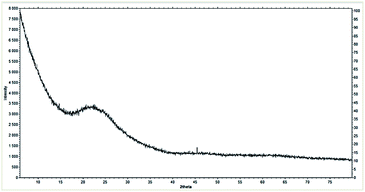
XRD diffractogram (Fig. 1) of the mixed oxide support after calcination revealed its amorphous structure, which is reflected in its large surface area. The intensive bands corresponding to amorphous silica overlap the bands assigned to the crystalline structure of titanium dioxide (Fig. 1.). The XRD patterns of SiO2 and TiO2 oxides are presented in Fig. S15 in ESI.†
Determination of the particle size distribution, shown in Fig. 2, proved that for TiO2–SiO2 (TS) system it is monomodal in the range 122–396 nm. The largest volume contribution bring the particles of 220 nm (33%) and 255 nm (32%) diameters. The support obtained in the synthesis with the use of precursors of the two oxides was found to have smaller size particles than the individual oxides SiO2 and TiO2, but poorer homogeneity. Titanium dioxide and silica have bimodal particle size distributions, while the system of TiO2–SiO2 and lignin (TS_L) has a trimodal one. The latter system was also characterized by a high polydispersity coefficient (0.868) indicating its great inhomogeneity. Moreover, the incorporation of lignin to the oxide system TiO2–SiO2 resulted in enlargement of the particle size of the system, the dominant particles in TS_L have the mean diameter of 1313 nm.
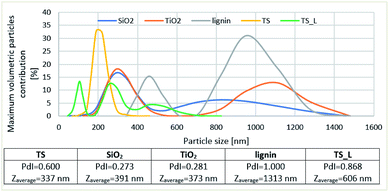 | ||
| Fig. 2 Distribution of particles size and maximum volumetric particles contribution in the selected supports. | ||
To confirm the composition of the systems studied, they were subjected to energy-dispersive X-ray microanalysis (EDX), Table 3, which confirmed the presence of titanium dioxide and silica in the structures of TS and TS_L. These two supports showed high contents of silicon, titanium and oxygen, while the TS_L apart from these elements had a high content of carbon and trace amounts of sulfur.
| Support | Element content [wt%] | |||||
|---|---|---|---|---|---|---|
| C | O | Na | Si | Ti | S | |
| TS | 2.08 | 47.51 | — | 30.89 | 19.50 | — |
| TS_L | 9.74 | 46.57 | 1.04 | 26.49 | 16.09 | 0.2 |
Preparation of SILP materials
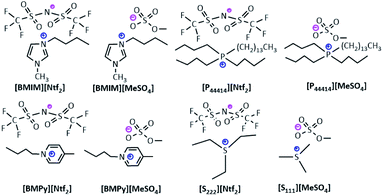
The aim of the study was to evaluate the impact of the support structure as well as the type of the ionic liquid making the SILP system, on its stability and catalytic performance. The analysis was performed for eight ionic liquids containing imidazolium, pyridinium, phosphonic or sulfonic cations and methylsulfate and bis(trifluoromethylsulfonyl)imide anions, Fig. 3. The methods of syntheses of the ionic liquids, their spectroscopic and thermogravimetric characterization are given in ESI.†Another important parameter of the Supported Ionic Liquid Phase systems is the amount of the ionic liquid, its excess may hinder the assess of products to the catalyst surface, while a too small amount of the liquid leads to poor impregnation of the support surface and faster leaching of the immobilized catalytically active phase. In order to determine the optimum amount of the liquid, a series of the SILP systems containing 10%, 15% or 20% wt of [P44414][Ntf2] were obtained and subjected to catalytic tests, Table S8.† For the systems containing the platinum complex as the active phase, the catalytic activity was at the same level for the samples with 10% and 15% content of ionic liquid, but for the ionic liquid content of 20%, the reaction efficiency was significantly lower. For the samples with the rhodium complex as the active phase, the highest yield was obtained for the ionic liquid content of 10% wt, while it was lower for the systems with 15% and 20% wt of IL. In view of the fact that the improvement in catalytic activity with increasing content of ionic liquid was rather insignificant and taking into account the economic reasons, the further studies were performed for the SILP samples with 10% wt of an ionic liquid relative to the mass of the support. As a result of physical impregnation of the supports TiO2–SiO2 and TiO2–SiO2/lignin with the above mentioned ionic liquids and immobilization of the platinum or rhodium complexes, a series of 24 new SILP materials was obtained. For the sake of comparison 4 SILP systems with sulfonic ionic liquids supported on silica were prepared, Table 4.
| Support | Ionic liquid | Catalyst | Sample name |
|---|---|---|---|
| TiO2–SiO2 | — | — | TS |
| [BMIM][Ntf2] | Rh(PPh3)3Cl | TS_1.1_Rh | |
| Pt(cod)Cl2 | TS_1.1_Pt | ||
| [BMPy][Ntf2] | Rh(PPh3)3Cl | TS_2.1_Rh | |
| Pt(cod)Cl2 | TS_2.1_Pt | ||
| [P44414][Ntf2] | Rh(PPh3)3Cl | TS_3.1_Rh | |
| Pt(cod)Cl2 | TS_3.1_Pt | ||
| [S222][Ntf2] | Rh(PPh3)3Cl | TS_4.1_Rh | |
| Pt(cod)Cl2 | TS_4.1_Pt | ||
| [BMIM][MeSO4] | Rh(PPh3)3Cl | TS_1.2_Rh | |
| Pt(cod)Cl2 | TS_1.2_Pt | ||
| [BMPy][MeSO4] | Rh(PPh3)3Cl | TS_2.2_Rh | |
| Pt(cod)Cl2 | TS_2.2_Pt | ||
| [P44414][MeSO4] | Rh(PPh3)3Cl | TS_3.2_Rh | |
| Pt(cod)Cl2 | TS_3.2_Pt | ||
| [S111][MeSO4] | Rh(PPh3)3Cl | TS_4.2_Rh | |
| Pt(cod)Cl2 | TS_4.2_Pt | ||
| TiO2–SiO2_ lignin | — | — | TS_L |
| [P44414][Ntf2] | Rh(PPh3)3Cl | TS_L_3.1_Rh | |
| Pt(cod)Cl2 | TS_L_3.1_Pt | ||
| [S222][Ntf2] | Rh(PPh3)3Cl | TS_L_4.1_Rh | |
| Pt(cod)Cl2 | TS_L_4.1_Pt | ||
| [P44414][MeSO4] | Rh(PPh3)3Cl | TS_L_3.2_Rh | |
| Pt(cod)Cl2 | TS_L_3.2_Pt | ||
| [S111][MeSO4] | Rh(PPh3)3Cl | TS_L_4.2_Rh | |
| Pt(cod)Cl2 | TS_L_4.2_Pt | ||
| SiO2 | [S222][Ntf2] | Rh(PPh3)3Cl | S_4.1_Rh |
| Pt(cod)Cl2 | S_4.1_Pt | ||
| [S111][MeSO4] | Rh(PPh3)3Cl | S_4.2_Rh | |
| Pt(cod)Cl2 | S_4.2_Pt |
Physicochemical characterization of the obtained SILP materials
| Sample | BET surface area [m2 g−1] | Total pore volume [cm3 g−1] | Average pore diameter [nm] | αa | Layer thicknessb [nm] |
|---|---|---|---|---|---|
| a Pore filling degree of support as the ratio IL volume/support pore volume.b Ratio of the IL volume used for coating and the initial surface area. | |||||
| TS | 328 | 0.91 | 10.95 | — | — |
| TS_L | 176 | 0.51 | 11.43 | — | — |
| TS_1.1_Pt | 214 | 0.65 | 11.93 | 0.28 | 0.65 |
| TS_2.1_Pt | 204 | 0.69 | 11.25 | 0.24 | 0.62 |
| TS_3.1_Pt | 189 | 0.69 | 12.36 | 0.24 | 0.57 |
| TS_4.1_Pt | 210 | 0.69 | 11.38 | 0.24 | 0.64 |
| TS_L_3.1_Pt | 82 | 0.36 | 13.71 | 0.29 | 0.46 |
| TS_L_4.1_Pt | 90 | 0.38 | 13.43 | 0.25 | 0.51 |
| TS_1.2_Pt | 224 | 0.66 | 11.21 | 0.27 | 0.68 |
| TS_2.2_Pt | 241 | 0.69 | 10.95 | 0.20 | 0.70 |
| TS_3.2_Pt | 222 | 0.67 | 10.26 | 0.23 | 0.65 |
| TS_4.2_Pt | 246 | 0.72 | 10.37 | 0.18 | 0.72 |
| TS_L_3.2_Pt | 106 | 0.41 | 13.95 | 0.19 | 0.60 |
| TS_L_4.2_Pt | 127 | 0.45 | 12.22 | 0.11 | 0.72 |
| Sample | Element content [wt%] | |||||||
|---|---|---|---|---|---|---|---|---|
| C | O | F | Si | S | Ti | P | ||
| TS | 2.1 | 47.5 | — | 30.8 | — | 19.5 | — | |
| TS_L | 9.7 | 46.5 | — | 26.4 | 0.2 | 16.1 | — | |
| TS_1.1_Rh | 2.6 | 42.8 | 1.4 | 31.4 | 0.9 | 20.5 | — | |
| TS_2.1_Rh | 3.6 | 43.6 | 1.5 | 30.4 | 0.9 | 19.6 | — | |
| TS_3.1_Rh | 6.2 | 44.8 | 1.4 | 28.9 | 0.7 | 16.9 | 0.5 | |
| TS_L_3.1_Rh | 15.3 | 43.2 | 1.4 | 22.4 | 1.2 | 14.4 | 0.6 | |
| TS_4.1_Rh | 5.2 | 42.2 | 1.8 | 28.0 | 2.2 | 20.2 | — | |
| TS_L_4.1_Rh | 11.9 | 40.3 | 4.3 | 22.5 | 3.2 | 16.5 | — | |
| TS_1.2_Rh | 4.2 | 46.0 | — | 28.9 | 1.3 | 19.5 | — | |
| TS_2.2_Rh | 4.7 | 45.9 | — | 27.3 | 1.1 | 20.7 | — | |
| TS_3.2_Rh | 5.0 | 45.9 | — | 27.9 | 1.2 | 18.9 | 0.5 | |
| TS_L_3.2_Rh | 13.1 | 43.2 | — | 26.5 | 0.7 | 14.7 | 0.5 | |
| TS_4.2_Rh | 4.2 | 46.6 | — | 27.7 | 2.2 | 19.0 | — | |
| TS_L_4.2_Rh | 12.9 | 44.7 | — | 22.7 | 3.0 | 14.8 | — | |
Results of the elemental analysis of selected SILP systems revealed increased percentage contents of N, C, H and S relative to those in pure supports. This result is an additional evidence of the presence of an ionic liquid on the surface of TiO2–SiO2 and TiO2–SiO2/lignin supports. On the basis of the percentage contents of carbon in the SILP samples studied it was possible to estimate the degree of the supports coverage with the ionic liquid, using the Berendsen formula.41 The surface coverage was 1.54 μmol m−2 for TS_4.1_Pt, 2.12 μmol m−2 for TS_4.2_Pt and 1.51 μmol m−2 for TS_L_4.1_Pt. The results of elemental analysis are displayed in Table S6 in the ESI.† Comparisons of SEM images of the support before and after the impregnation with an ionic liquid and rhodium complex impregnation revealed changes in the number of agglomerates in the systems structures, Fig. 4. After the adsorption of ionic liquid the sample structure is more homogeneous and the agglomerates are much smaller than in pure supports. It is not only a consequence of impregnation but also of the process of SILP materials preparation. As a result of vigorous stirring of the support in the solution containing the ionic liquid and a given metal complex, the TiO2–SiO2 particles were broken and divided into smaller ones, which reduced the number and size of the agglomerates. The SILP surface is also appreciably smoothened relative to that of the pure support, which is attributed to the presence of the ionic liquid inside the pores of TiO2–SiO2 support.
 | ||
| Fig. 4 The structure of TiO2–SiO2 before (a) and after (b) physical impregnation of [P44414][Ntf2] and rhodium catalyst on its surface. | ||
The mean diameter of the nanoparticles was observed to decrease after the ionic liquid adsorption. Moreover, as follows from the data presented in Table S7,† the process of adsorption resulted in deterioration of the sample homogeneity, manifested by an increase in the polydispersity index (PdI) for TS_4.1_Pt and TS_L_4.1._Pt. According to the results of thermogravimetric (TG) measurements, the SILP systems containing ionic liquids with imidazolium, pyridinium and phosphonium cations are stable above 300 °C, irrespective of the anion used. Interestingly, for the SILP systems with sulfonic ionic liquids, the type of anion had an impact on their thermal stability, the systems with [S222][Ntf2] were stable up to about 270 °C, while those with [S111][MeSO4], were stable up to 200 °C. The incorporation of lignin to TiO2–SiO2 also resulted in deterioration of thermal stability. However, all SILP materials were thermally stable at 100 °C, at which hydrosilylation was performed. Results of TG analysis are shown in Table S3 (ESI).†
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the amount of rhodium or platinum was of 10−5 mol per 1 mol Si–H. The reaction was selective, leading only to the anti-Markovnikov β-addition product, Scheme 4.
1, and the amount of rhodium or platinum was of 10−5 mol per 1 mol Si–H. The reaction was selective, leading only to the anti-Markovnikov β-addition product, Scheme 4.
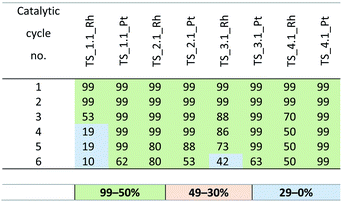
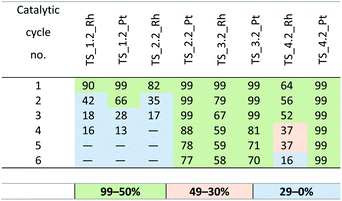
At the next step, the optimum concentrations of the rhodium and platinum complexes were chosen. The use of the complexes at 10−4 mol was found to shorten the reaction to 20 minutes with no significant effect on the reaction efficiency. When the complexes were used at the concentration of 10−6 mol, the yield was lowered to 30% and the reaction time was extended to over 1 hour. The best results were obtained for the systems with the active complexes concentration of 10−5 mol of Rh or Pt per mol Si–H. The yields of the reactions catalyzed with the SILP materials obtained in six subsequent catalytic cycles are presented in Tables 7 and 8. The best SILP systems were active much longer, which is evidenced by the TON and TOF coefficients given in Table 9. The methodology of catalytic tests is described in detail in the ESI.†
| SILP material | TON | TOF, ×106 h−1 |
|---|---|---|
| TS_1.1_Rh | 304![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.61 |
| TS_1.1_Pt | 792![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.58 |
| TS_2.1_Rh | 706![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.41 |
| TS_2.1_Pt | 682![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.36 |
| TS_3.1_Rh | 639![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.28 |
| TS_3.1_Pt | 668![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.34 |
| TS_4.1_Rh | 534![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.07 |
| TS_4.1_Pt | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 302 302![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.60 |
| TS_1.2_Rh | 166![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.33 |
| TS_1.2_Pt | 206![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.21 |
| TS_2.2_Rh | 134![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.27 |
| TS_2.2_Pt | 802![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.60 |
| TS_3.2_Rh | 616![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.23 |
| TS_3.2_Pt | 809![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.62 |
| TS_4.2_Rh | 262![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.52 |
| TS_4.2_Pt | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 140 140![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.28 |
According to the yields obtained, all the SILP systems were active in the test hydrosilylation reaction. A significant difference in yield was noted between the SILP with [Ntf2]− and that with [MeSO4]− anion, the yield of the former was much higher and they were catalytically active much longer that the systems with [MeSO4]−. The difference was particularly pronounced for the systems with the rhodium complex, as indicated by the values of TON and TOF. For the samples with [Ntf2]− anion, such as: TS_1.1_Rh and TS_2.1_Rh, the value of TON were 304![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 706
000 and 706![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and TOF were 0.61 × 106 and 1.41 × 106 h−1, respectively. The samples with [MeSO4]− anion – TS_1.2_Rh and TS_2.2 were characterized by lower values of TON and TOF coefficients of 166
000 and TOF were 0.61 × 106 and 1.41 × 106 h−1, respectively. The samples with [MeSO4]− anion – TS_1.2_Rh and TS_2.2 were characterized by lower values of TON and TOF coefficients of 166![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 134
000 and 134![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (TON) and 0.33 and 0.27 × 106 h−1 (TOF), respectively. It should be mentioned that the systems of SILP with the ionic liquids containing aromatic cations are considered less effective because of the electrostatic interactions in the ring strongly binding the cation and the anion, which hinders the access of substrates to the immobilized catalyst.42,43 This phenomenon does not take place in the branched ionic liquids, i.e. phosphonium and sulfonic ones. The SILP systems with the two latter liquids show very good catalytic performance, irrespective of the type of anion. The systems with platinum complexes show greater stability and higher catalytic activity. It may be a consequence of the stronger ionic character of Pt (II) in [Pt(cod)Cl2] than Rh(I) in the Wilkinson catalyst. Thus, the platinum catalyst enters into stronger interactions with ionic liquids, endowing the catalyst with higher stability and better catalytic performance. For the platinum catalysts, the best results were obtained for the systems with TiO2–SiO2 as a support and sulfonic ionic liquid (TS_4.1_Pt and TS_4.2_Pt), that were active even in 15 subsequent catalytic cycles. From among the SILP systems with the rhodium complex, the best catalytic performance was noted for TS_2.1_Rh.
000 (TON) and 0.33 and 0.27 × 106 h−1 (TOF), respectively. It should be mentioned that the systems of SILP with the ionic liquids containing aromatic cations are considered less effective because of the electrostatic interactions in the ring strongly binding the cation and the anion, which hinders the access of substrates to the immobilized catalyst.42,43 This phenomenon does not take place in the branched ionic liquids, i.e. phosphonium and sulfonic ones. The SILP systems with the two latter liquids show very good catalytic performance, irrespective of the type of anion. The systems with platinum complexes show greater stability and higher catalytic activity. It may be a consequence of the stronger ionic character of Pt (II) in [Pt(cod)Cl2] than Rh(I) in the Wilkinson catalyst. Thus, the platinum catalyst enters into stronger interactions with ionic liquids, endowing the catalyst with higher stability and better catalytic performance. For the platinum catalysts, the best results were obtained for the systems with TiO2–SiO2 as a support and sulfonic ionic liquid (TS_4.1_Pt and TS_4.2_Pt), that were active even in 15 subsequent catalytic cycles. From among the SILP systems with the rhodium complex, the best catalytic performance was noted for TS_2.1_Rh.
The catalytic performance of the SILP materials based on TiO2–SiO2 support were compared with the results obtained for the systems based on mesoporous silica. In the tests we used Davisil grade 62 silica of the surface area of 302 m2 g−1, pore volume of 1.14 cm3 g−1 and average pore diameter of 11.39 nm. The silica surface was impregnated with sulfonic liquid with [Ntf2]− or [MeSO4]− anions and then the platinum [Pt(cod)Cl2] or rhodium [Rh(PPh3)3Cl] complexes were immobilized on the silica. As follows from the results of catalytic tests presented in Table S9, ESI,† the yield of the hydrosilylation reaction was much lower than when using the corresponding SILP systems supported on TiO2–SiO2. For the systems Rh-SILP significant differences were observed in the system's stability in subsequent catalytic cycles. For the systems S_4.1_Rh and S_4.2_Rh, already in the second catalytic cycle the yield of the reaction decreased by more than half and in subsequent cycles – to 20–30% or to zero. For the systems with platinum and silica support, the decrease in catalytic activity and stability was also faster than for the corresponding systems supported on TiO2–SiO2. The TON and TOF values for the most active system based on SiO2 were 585![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 1.17 × 106 h−1, respectively, while for the most active system supported on TiO2–SiO2 the TON and TOF vales were 1
000 and 1.17 × 106 h−1, respectively, while for the most active system supported on TiO2–SiO2 the TON and TOF vales were 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 302
302![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 2.60 × 106 h−1, respectively. An important result was determination of the profiles of hydrosilylation reaction catalyzed by the tested catalysts. The profiles were obtained for TS_4.1_Pt and TS_2.1_Rh, as the reactions in the presence of these catalysts were characterized by high yields. The catalytic activities of the SILP systems obtained were compared with those of the precursors of the catalysts, i.e. [Pt(cod)Cl2] and [Rh(PPh3)3Cl], used for their preparation, and the results are presented in Fig. 5. In the FT-IR in situ analysis, the decay of the band assigned to the
000 and 2.60 × 106 h−1, respectively. An important result was determination of the profiles of hydrosilylation reaction catalyzed by the tested catalysts. The profiles were obtained for TS_4.1_Pt and TS_2.1_Rh, as the reactions in the presence of these catalysts were characterized by high yields. The catalytic activities of the SILP systems obtained were compared with those of the precursors of the catalysts, i.e. [Pt(cod)Cl2] and [Rh(PPh3)3Cl], used for their preparation, and the results are presented in Fig. 5. In the FT-IR in situ analysis, the decay of the band assigned to the ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si–H bond in the HMTS molecule at 913 cm−1 was monitored. The reaction profiles were determined on the basis of the
Si–H bond in the HMTS molecule at 913 cm−1 was monitored. The reaction profiles were determined on the basis of the ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si–H conversion expressed as the change in the area of the monitored band. The SILP materials studied were found to lead to full Si–H conversion in a time shorter than 30 minutes, while the system TS_2.1_Rh was characterized by a much longer time of activation, close to 27 minutes, than the homogeneous rhodium catalyst. The reaction profiles obtained for the two catalysts are much different. In the presence of the Wilkinson catalyst after a nine-minute activation, the Si–H conversion rapidly increased up to almost 100% conversion in 10 minutes. In the presence of the rhodium catalyst, the process of activation started in the second minute of the reaction and lasted till the 22nd minute and then the Si–H conversion fast increased reaching almost 100%. The reaction profiles in the presence of a platinum homogeneous catalyst and the Pt-SILP system are very similar. The Pt-SILP system needed a little longer time of activation than [Pt(cod)Cl2], however, the time needed for full Si–H conversion was by 3 minutes shorter. The process of hydrosilylation in the presence of each of the platinum catalysts was fast and with high conversion.
Si–H conversion expressed as the change in the area of the monitored band. The SILP materials studied were found to lead to full Si–H conversion in a time shorter than 30 minutes, while the system TS_2.1_Rh was characterized by a much longer time of activation, close to 27 minutes, than the homogeneous rhodium catalyst. The reaction profiles obtained for the two catalysts are much different. In the presence of the Wilkinson catalyst after a nine-minute activation, the Si–H conversion rapidly increased up to almost 100% conversion in 10 minutes. In the presence of the rhodium catalyst, the process of activation started in the second minute of the reaction and lasted till the 22nd minute and then the Si–H conversion fast increased reaching almost 100%. The reaction profiles in the presence of a platinum homogeneous catalyst and the Pt-SILP system are very similar. The Pt-SILP system needed a little longer time of activation than [Pt(cod)Cl2], however, the time needed for full Si–H conversion was by 3 minutes shorter. The process of hydrosilylation in the presence of each of the platinum catalysts was fast and with high conversion.
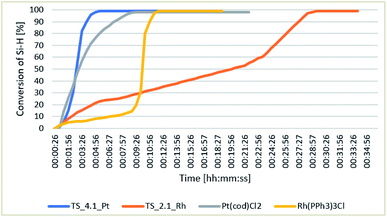 | ||
| Fig. 5 Changes in the conversion of Si–H as a function of time for the hydrosilylation of 1-octene with HMTS, catalyzed by SILP materials with rhodium and platinum complexes. | ||
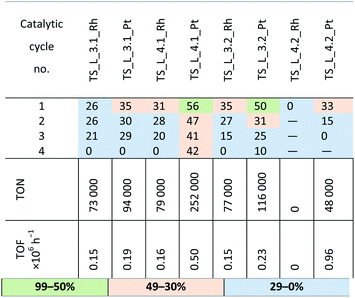
Another SILP system studied was supported on a hybrid, inorganic–organic material composed of TiO2–SiO2 and lignin and contained phosphonium and sulfonic ionic liquids and the earlier used homogeneous platinum and rhodium catalysts. The catalytic materials were used in the hydrosilylation of 1-octene with HMTS, the results are presented in Table 10. The SILP materials supported on TiO2–SiO2/lignin were characterized by low catalytic activity. Almost for all such SILP materials the reaction yield was below 30%. The extension of reaction time to one hour did not improve the yield. The best catalytic performance was obtained for the SILP system with ionic liquid containing [Ntf2]−. Low catalytic activity may be a result of the decreased surface area and pore volume in TiO2–SiO2/lignin than for the support without lignin. The changes also affected the effectiveness of the ionic liquids adsorption on the support surface and further immobilization of a metal complex, consequently, the obtained SILP systems with lignin showed poor stability and poor leaching resistance.
For the SILP systems showing the best catalytic performance: TS_4.1_Pt, TS_4.2_Pt and TS_2.1_Rh, the effects of different reagents on the product yield were evaluated. The reactions were run for polar and nonpolar olefins: 1-octene, allyl-glycidyl ether, octafluoropentyl ether, and the silicon compounds: HMTS, triethylsilane and triethoxysilane (TriEOS). The results are collected in Table 11 and imply that Pt-SILP materials show higher catalytic activity in the first cycle, in almost all hydrosilylation reactions tested, irrespective of the type of reagents used. For all SILP systems studied the attempts were made to isolate them from the post-reaction mixture and reuse in subsequent cycles, but in most cases the materials were inactive or their catalytic activity rapidly decreased after the second or the third catalytic cycle. The reactions with allyl-glycidyl ether and octafluoropentyl ether catalyzed by Pt-SILP were characterized by very high yield, but when Rh-SILP was used, their yield was very low. Irrespective of the type of SILP catalytic system, the efficiency of the reaction of olefin hydrosilylation with triethylsilane was very low. The use of triethoxysilane (TriEOS) resulted in the increase in the yield of the reactions with all olefins. The best results were obtained for the reaction of TriEOS with 1-octene in the presence of Pt-SILP materials, not only a high yield was obtained, but the Pt-SILP systems maintained activity in subsequent catalytic cycles. Slightly worse results were obtained in the reaction of hydrosilylation between TriEOS and the ethers. The catalytic activity of SILP TS_2.1_Rh was found much lower than the activities of the systems containing platinum. The best catalytic results were obtained for the systems with HMTS and TriEOS, containing electron-withdrawing substituents, and nonpolar olefin (1-octene). For these systems, in the presence of Pt-SILP catalysts, not only high yields were obtained, but also the catalytically active phase was not leached and could be reused in subsequent catalytic cycles. As the product is nonpolar, the polar liquid with the immobilized catalytically active phase is not washed out from the SILP surface. The hydrosilylation reactions with the use of triethylsilane are very slow and difficult to run, often their course is compared to that of the reaction with triphenylsilane in which the access to the Si–H bond is spatially hindered by phenyl groups. Triethylsilane contains electron-donating substituents and shows poor ability to form Si–C bonds, because of very low effectiveness of hydrogen atom transfer from Si–H bond to the carbon atom in the olefin double bond.
| Si–H | H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2-R, where -R: CHCH2-R, where -R: |
Yield of the reaction [%] | ||
|---|---|---|---|---|
| TS_4.1_Pt | TS_2.1_Rh | TS_4.2_Pt | ||
| HMTS | -C5H11 | 99 | 99 | 99 |
| TON | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 302 302![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 140 140![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
706![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
| TOF × 106 h−1 | 2.60 | 2.28 | 1.41 | |
| -OCH2CHOCH2 | 86 | 7 | 85 | |
| TON | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | 330![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
| TOF × 106 h−1 | 0.07 | — | 0.66 | |
| -OCH2CF2CF2CF2CHF2 | 99 | 37 | 98 | |
| TON | 128![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
| TOF × 106 h−1 | 0.26 | 0.07 | 0.20 | |
| Et3SiH | -C5H11 | 6 | 6 | 42 |
| TON | — | — | 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
| TOF × 106 h−1 | — | — | 0.13 | |
| -OCH2CHOCH2 | 7 | 0 | 18 | |
| TON | — | — | 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
| TOF × 106 h−1 | — | — | 0.10 | |
| -OCH2CF2CF2CF2CHF2 | 62 | 0 | 53 | |
| TON | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
| TOF × 106 h−1 | 0.19 | — | 0.11 | |
| TriEOS | -C5H11 | 91 | 24 | 99 |
| TON | 308![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
307![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
| TOF × 106 h−1 | 0.61 | 0.05 | 0.61 | |
| -OCH2CHOCH2 | 99 | — | 54 | |
| TON | 116![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
| TOF × 106 h−1 | 0.23 | — | 0.17 | |
| -OCH2CF2CF2CF2CHF2 | 99 | 0 | 93 | |
| TON | 129![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | 147![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
| TOF × 106 h−1 | 0.26 | — | 0.30 | |
In view of the observed loss of catalytic abilities of SILP materials in subsequent catalytic cycles, selected samples of the post-reaction mixture was subjected to ICP analysis. The amounts of platinum and rhodium in these samples were below the level of detection (1 ppm). Taking into account our eelier experience with catalytic systems containing ionic liquids, we also checked the leaching of ionic liquids from the SILP systems studied. The sample of Pt-SILP with phosphonic ionic liquid [P44414][MeSO4] was analyzed by 31P NMR to assess the content of the liquid in the first and fifth catalytic cycle. The presence of the ionic liquid in the SILP samples after the first and the fifth catalytic cycle was confirmed, but its content in the reaction mixture was insignificant. Of course, slow leaching of the ionic liquid may have some insignificant effect on the catalytic activity of the SILP systems, but the majority of them maintained their activity up to at least 10 cycles and the most active ones – up to 15 subsequent cycles.
Materials and methods
Detail information on the analytical methods applied and the materials used in the studies reported is given in the ESI.†Conclusions
Twenty four new SILP systems were obtained, supported on the oxide system TiO2–SiO2 and its combination with lignin (organic polymer). The structures, morphologies and physicochemical properties of the supports and the SILP systems were fully characterized. The determined adsorption parameters and thermal stability of the supports were proved to depend on the ratio of silicon and titanium precursors used in the sol–gel synthesis of the supports. The effectiveness of impregnation of the supports (TiO2–SiO2 or TiO2–SiO2/lignin) surfaces with the ionic liquids and immobilization of the catalytically active phase (homogeneous platinum or rhodium complexes) was verified by elemental analysis, SEM-EDX, IR and BET methods. According to the thermogravimetric analysis results, all obtained SILP systems were thermally stable at a temperature significantly higher than the hydrosilylation reaction temperature. All SILP systems obtained and studied in this work were catalytically active in hydrosilylation of 1-octene with 1,1,1,3,5,5,5-heptamethyltrisiloxane. The catalytically active materials were proved to be easy to isolate, so that they could be reused, and have been shown to be active in subsequent catalytic cycles. The Pt-SILP systems were found to more active than the systems with rhodium complexes, which was interpreted as a result of the more ionic character of the complex [Pt(cod)Cl2]. From among the Pt-SILP materials, the highest catalytic activity was observed for those containing phosphonium and sulfonic ionic liquids and among the Rh-SILP materials, those with pyridinium ionic liquids. The SILP systems with the [Ntf2]− anion were found to be more stable and in the reaction with their presence the Si–H conversion was higher than that in the systems with [MeSO4]−. FT-IR in situ results revealed that the activation time of the Pt-SILP systems is much more shorter than the corresponding time for the Rh-SILP materials. The reactions run with reagents of polar and nonpolar character revealed that the obtained SILP systems showed the highest catalytic activity and stability in the systems leading to products of nonpolar character. The polar reaction products lead to leaching of the ionic liquid together with the metal complex, thus to reduced activity or even inactivity in subsequent catalytic cycles. Taking into account the ease of synthesis of the SILP catalytic materials, their activity and possibility of reuse in subsequent catalytical cycles, they seem to make excellent alternatives to the homogeneous catalysts used in the reaction of hydrosilylation. The use of such materials could solve the problems with mass transport between the phases in standard two-phase systems and help reduce the cost related to the use of transition metal complexes.Author contributions
Conceptualization, methodology, investigation, writing and editing original draft, project administration – Olga Bartlewicz; formal analysis, editing of original draft – Mariusz Pietrowski; investigation – Marta Kaczmarek; supervision, editing of original draft – Hieronim Maciejewski.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by grant Preludium no. UMO-2019/35/N/ST4/00494 financed by the National Science Centre Poland.References
- H. Maciejewski, K. Szubert and B. Marciniec, Catal. Commun., 2012, 24, 1–4 CrossRef CAS.
- B. Marciniec, H. Maciejewski, C. Pietraszuk and P. Pawluć, Hydrosilylation. Comprehensive Review on Recent Advances, Springer, 2009 Search PubMed.
- Y. Nakajima and S. Shimada, RSC Adv., 2015, 5, 20603–20616 RSC.
- Z. Rappoport and Y. Apeloig, The Chemistry of Organic Silicon Compounds, Wiley, 2001 Search PubMed.
- R. G. Jones, W. Ando and J. Chojnowski, Silicon-Containing Polymers, Kluwer Acad. Press, 2000 Search PubMed.
- W. Zieliński, R. Kukawka, H. Maciejewski and M. Śmiglak, Molecules, 2016, 21, 1115–1125 CrossRef PubMed.
- M. Jankowska-Wajda, R. Kukawka, M. Śmiglak and H. Maciejewski, New J. Chem., 2018, 42, 5229–5236 RSC.
- Y. Naganawa, Y. Maegawa, H. Guo, S. S. Gholap, S. Tanaka, K. Sato, S. Inagaki and Y. Nakajima, Dalton Trans., 2019, 48, 5534–5540 RSC.
- O. Bartlewicz, I. Dąbek, A. Szymańska and H. Maciejewski, Catalysts, 2020, 10, 1227–1246 CrossRef CAS.
- C. P. Mehnert and R. A. Cook, J. Am. Chem. Soc., 2002, 124, 12932–12933 CrossRef CAS PubMed.
- R. Fehrmann, A. Riisager and M. Haumann, Supported Ionic Liquids. Fundamental and Applications, Wiley-VCH, Weinheim, 2014 Search PubMed.
- J. M. Marinkovic, A. Riisager, R. Franke, P. Wasserscheid and M. Haumann, Ind. Eng. Chem. Res., 2019, 58, 2409–2420 CrossRef CAS.
- J. Brunig, Z. Csendes, S. Weber, N. Gorgas, R. Bittner, W. Limbeck, K. Bica, H. Hoffmann and K. Kirchner, ACS Catal., 2018, 8, 1048–1051 CrossRef.
- F. Giacalone and M. Gruttadauria, ChemCatChem, 2016, 8(4), 664–684 CrossRef CAS.
- B. V. Romanovsky and I. G. Tarhanov, Russ. Chem. Rev., 2017, 86, 444–458 CrossRef CAS.
- S. More, S. Jadhav, R. Salunkhe and A. Kumbhar, Mol. Catal., 2017, 442, 126–132 CrossRef CAS.
- J. Brunig, Z. Csendes, S. Weber, N. Gorgas, R. W. Bittner, A. Limbeck, K. Bica, H. Hoffmann and K. Kirchner, ACS Catal., 2018, 8, 1048–1051 CrossRef.
- R. Castro-Amoedo, Z. Csendes, J. Brunig, M. Sauer, A. Foelske-Schmitz, N. Yigit, G. Rupprechter, T. Gupta, A. M. Martins, K. Bica, H. Hoffmann and K. Krichner, Catal. Sci. Technol., 2018, 8, 4812–4820 RSC.
- L. L. Hench and J. K. West, Chem. Rev., 1990, 90, 33–72 CrossRef CAS.
- U. G. Akpan and B. H. Hameed, Appl. Catal., A, 2010, 375, 1–11 CrossRef CAS.
- G. Wypych, Handbook of Fillers, ChemTec Publishing, Toronto, 2nd edn, 1999 Search PubMed.
- X. Chen and S. S. Mao, Chem. Rev., 2007, 107, 2891–2959 CrossRef CAS PubMed.
- O. Ola and M. M. Maroto-Valer, J. Photochem. Photobiol., C, 2015, 24, 16–42 CrossRef CAS.
- S. F. A. Taliba, W. H. Azmib, I. Zakariaa, W. A. N. W. Mohameda, A. M. I. Mamata, H. Ismaila and W. R. W. Daud, Energy Procedia, 2015, 79, 366–371 CrossRef.
- R. Q. Zhang and W. J. Fan, J. Cluster Sci., 2006, 17, 541–563 CrossRef CAS.
- K. Siwińska-Stefańska, O. Bartlewicz, P. Bartczak, A. Piasecki and T. Jesionowski, Adsorption, 2019, 25, 485–499 CrossRef.
- M. Cozzolino, M. Di Serio, R. Tesser and E. Santacesaria, Appl. Catal., A, 2007, 325(2), 256–262 CrossRef CAS.
- G. Mul, A. Zwijnenburg, B. van der Linden, M. Makkee and J. A. Moulijn, J. Catal., 2001, 201, 128–137 CrossRef CAS.
- Ł. Klapiszewski, K. Siwińska-Stefańska and D. Kołdyńska, Chem. Eng. J., 2017, 314, 169–181 CrossRef.
- Ł. Klapiszewski, J. Zdarta and T. Jesionowski, Colloids Surf., B, 2018, 162, 90–97 CrossRef PubMed.
- B. Marciniec, K. Szubert, M. J. Potrzebowski, I. Kownacki and H. Maciejewski, ChemCatChem, 2009, 1, 304–310 CrossRef CAS.
- M. Jankowska-Wajda, O. Bartlewicz, A. Walczak, A. R. Stefankiewicz and H. Maciejewski, J. Catal., 2019, 374, 266–275 CrossRef CAS.
- R. Kukawka, A. Pawłowska-Zygarowicz, R. Januszewski, J. Działkowska, M. Pietrowski, M. Zieliński, H. Maciejewski and M. Śmiglak, Catalysts, 2020, 10, 1414–1425 CrossRef CAS.
- A. Kołodziejczak-Radzimska, J. Zdarta, F. Ciesielczyk and T. Jesionowski, Korean J. Chem. Eng., 2018, 35, 2220–2231 CrossRef.
- O. Bartlewicz, M. Zieliński, M. Kaczmarek and H. Maciejewski, Mol. Catal., 2021, 509, 111615–111624 CrossRef CAS.
- T. Jesionowski, Ł. Klapiszewski and G. Milczarek, J. Mater. Sci., 2014, 49, 1376–1385 CrossRef CAS.
- R. Kukawka, A. Pawlowska-Zygarowicz, J. Dzialkowska, M. Pietrowski, H. Maciejewski, K. Bica and M. Smiglak, ACS Sustainable Chem. Eng., 2019, 7, 4699–4706 CrossRef CAS.
- A. L. Linsebigler, G. Lu and J. Yates, Chem. Rev., 1995, 95, 735–758 CrossRef CAS.
- D. Kondarides, Photocatalysis, Encyclopedia of Life Support Systems, Oxford, 2010 Search PubMed.
- M. Thommes, K. Kaneko, A. V. Neimark, J. P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol and K. S. W. Sing, Pure Appl. Chem., 2015, 87, 1051–1069 CAS.
- G. E. Berendsen and L. de Golan, J. Liq. Chromatogr. Relat. Technol., 1978, 1(5), 561–586 CrossRef CAS.
- V. Calo, A. Nacci and A. Monopoli, Eur. J. Org. Chem., 2006, 3791–3802 CrossRef CAS.
- S. Subbiah, V. Srinivasadesikan, M.-C. Tseng and Y.-H. Chu, Molecules, 2009, 14, 3780–3813 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra03966k |
| This journal is © The Royal Society of Chemistry 2021 |