Open Access Article
Open Access ArticleComposition-tuned MAPbBr3 nanoparticles with addition of Cs+ cations for improved photoluminescence†
Sai S. H. Dintakurti *abc,
Parth Vashishtha
*abc,
Parth Vashishtha b,
David Giovanni
b,
David Giovanni d,
Yanan Fang
d,
Yanan Fang b,
Norton Foo
b,
Norton Foo b,
Zexiang Shen
b,
Zexiang Shen ade,
Claude Guet
ade,
Claude Guet ab,
Tze Chien Sum
ab,
Tze Chien Sum d and
Tim White*b
d and
Tim White*b
aERI@N, Interdisciplinary Graduate School, Nanyang Technological University, Singapore 639798. E-mail: sriharsh001@e.ntu.edu.sg
bSchool of Materials Science and Engineering, Nanyang Technological University, Singapore 639798. E-mail: tjwhite@ntu.edu.sg
cDepartment of Physics, University of Warwick, Coventry, West Midlands CV4 7AL, UK
dSchool of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 639798
eCentre for Disruptive Photonic Technologies, CNRS International NTU Thales Research Alliance, Nanyang Technological University, Singapore 639798
First published on 8th July 2021
Abstract
Hybrid organic–inorganic lead halide perovskite nanoparticles are promising candidates for optoelectronic applications. This investigation describes the structural and optical properties of MAxCs1−xPbBr3 mixed cation colloidal nanoparticles spanning the complete compositional range of Cs substitution. A monotonic progression in the cubic lattice parameter (a) with changes in the Cs+ content confirmed the formation of mixed cation materials. More importantly, time-resolved photoluminescence (TRPL) revealed the optimized 13 mol% Cs nanoparticle composition exhibits the longest charge carrier lifetime and enhancement in radiative pathways. This sample also showed the highest photoluminescence quantum yield (PLQY) of ∼88% and displays ∼100% improvement in the PLQY of pure MAPbBr3 and CsPbBr3. Prototype LEDs fabricated from MA0.87Cs0.13PbBr3 were demonstrated.
Introduction
Hybrid organic–inorganic lead halide perovskites (LHPs) have been widely explored for optoelectronic applications resulting in solar cells with a record power conversion efficiency (PCE) of 25.5% and light emitting diodes with an external quantum efficiency (EQE) exceeding 20%.1–7 The key advantages of this class of materials are solution processability, inexpensive starting materials, composition tunable band gap, high hole and electron mobility, and high defect tolerance.8,9 Despite showing high efficiency, prototype perovskite materials such as MAPbX3 and FAPbX3 (where MA = methylammonium; FA = formamidinium; X = Br, I) suffer from phase instability, poor reproducibility, and thermal instability.10–12 Saidaminov et al.13 found lattice strain in perovskite induces point defects leading to structural degradation and PLQY quenching. Lattice perfection can be improved by the addition of inorganic Cs+ that improves phase stability by strain relaxation leading to more reproducible optoelectronic devices with improved thermal stability.14,15 A similar strategy of strain relaxation in FAPbI3 produced solar cells with PCE of 24.4%.16 In contrast, compressive strain in CsPbBr3 has been also found to be detrimental towards stability.17 On the other hand, Zhang et al.18 reported significant improvement in thin-film LEDs using mixed cation Cs0.87MA0.13PbBr3 material when compared to pure CsPbBr3. In general, it is likely that strain relaxation can be achieved by incorporating additional cations in the ABX3 LHPs, to improve structural stability and optoelectronic properties.Following the synthesis of mixed-cation perovskite powders and appreciable crystals, research focused on analogous nanoparticles to exploit their high PLQY, quantum confinement, narrow emission linewidth, and size-tuneable optical properties.19–21 These studies were motivated, in part, because perovskite nanoparticle LEDs show comparable performance to state-of-the-art CdSe based QDLEDs.5,22,23 Song et al.24 synthesised 15 mol% FA doped CsPbBr3 (Cs0.85FA0.15PbBr3) demonstrating 61% PLQY. Later, Vashishtha et al.13,19 demonstrated the first triple cation synthesis of Csx(MA0.17FA0.83)1−xPbBr3 (x = 0–0.15) for LED applications. These nanoparticles exhibit high phase stability and reproducibility confirming the expected advantages of mixed-cation perovskites. Premkumar et al. were the first to synthesize cesium–methylammonium mixed-cation lead bromide nanoparticles exhibiting twin PL emission peaks for Cs 20% and 40% compositions (Cs1−xMAxPbBr3, x = 0.2, 0.4).25 The twin emission was attributed to the bi-phase nature of the samples; although Cs-MA should show a continuous solid solution and be completely miscible according to perovskite tolerance factor considerations.25,26 It was also observed that these Cs-MA based LHP nanoparticles were larger than ∼12 nm with micaceous morphology that inhibits colloidal solubility/stability and reduces the advantages of strong quantum confinement.25,27 As colloidal stability is essential to fabricate thin-film optoelectronic devices, the present work was prompted by the desire to synthesise single phase MAxCs1−xPbBr3 colloidally stable nanoparticles.
Here, MAxCs1−xPbBr3 (x = 0–1) homogeneous nanoparticles were synthesized using a facile room-temperature ligand-assisted reprecipitation (LARP) method facilitating precise linking of Cs content with the structural and optical properties.19,28 Rietveld refinement of powder X-ray diffraction (XRD) patterns and X-ray photoelectron spectroscopy (XPS) monitored crystallochemical evolution were correlated with optical properties, including PL emission wavelength, charge carrier lifetime, and PLQY. Lastly, the optimized MA0.87Cs0.13PbBr3 nanocrystals were fabricated as electrically-driven LEDs.
Synthesis
A homogenous substitution of Cs+ ions in MAPbBr3 requires overcoming the solubility differences between Cs and MA in the dimethylformamide (DMF) solvent. Following the strategy reported by Veldhuis et al.,19,28 the cesium solubility in DMF was enhanced by using a complexing agent, dibenzo 21-crown-7 ether. A continuous solid solution series with the general composition MAxCs1−xPbBr3 (where x = 0, 0.13, 0.25, 0.37, 0.5, 0.63, 0.75, 0.87, 1) was prepared with slight modification of the LARP method reported earlier where 0.4 mmol of the salts were dissolved in 2 mL of DMF to prepare the perovskite precursor solution (see Table S4†). 150 μL of this solution was added dropwise into a solution containing toluene and ligand. The resulting nanoparticles were purified and dissolved in hexane for further processing (refer ESI† for details). This procedure was used across the entire compositional range.Results and discussion
Structural properties
CsPbBr3 exhibits temperature-dependent polymorphism, and appreciable crystals are orthorhombic (Pnma) at room temperature.29 Unambiguous characterisation of CsPbBr3 nanoparticles by laboratory powder X-ray diffraction can be challenging as the changes in lattice parameters, for example orthorhombic to cubic are small, and easily obscured by Scherrer broadening and preferred orientation, but can sometimes be discerned using transmission synchrotron methods.30,31 MAPbBr3 is cubic (Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) with dynamic methyl ammonium ions. It is reasonable then to adopt a cubic model to monitor lattice changes and the compositional progression from MAPbBr3 to CsPbBr3.
m) with dynamic methyl ammonium ions. It is reasonable then to adopt a cubic model to monitor lattice changes and the compositional progression from MAPbBr3 to CsPbBr3.
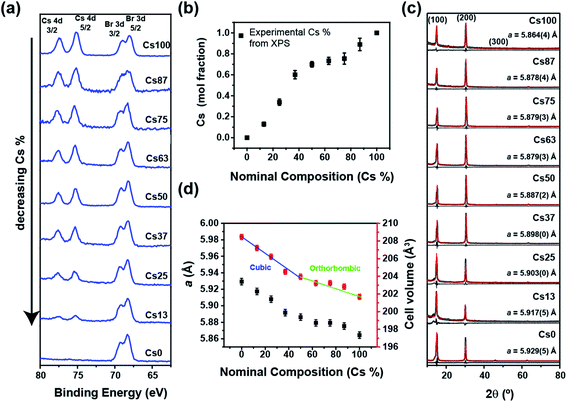
X-ray photoelectron spectroscopy (XPS) provided an independent verification of composition, and while XPS is surface sensitive, photoelectrons can escape from the centre of quantum dots/nanoparticles and the extracted compositions are representative of the perovskite chemistry.32,33 XPS examined the binding energies of the Cs 4d (74–78 eV) and Br 3d (64–71 eV) emissions (Fig. 1a and S3†), and as the intensity of Cs peaks reduces monotonically with increasing Br content, the integrated doublet peak areas were corrected for sensitivity to obtain atomic ratios. As Pb2+ stoichiometrically occupies the B-site, the Pb 4f and Cs 4d peak ratio were used to confirm the putative Cs content (Fig. 1a, b, S3 and Table S3†) and shows a slight enrichment between the nominal compositions 25 to 63% Cs. This likely arises from a systematic error in the synthesis or composition measurement rather than a miscibility gap.
The powder X-ray diffraction (XRD) patterns of drop cast samples exhibit (100) reflections characteristic of cubic MAPbBr3 (space group Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) (Fig. 1c) that was used for Rietveld refinement.34 The XRD peaks were Scherrer broadened18,35 as expected from nano-sized crystallites. Compared to Cs+ the MA+ ion scatters X-rays more weakly and has a larger effective ionic radius leading to a progressive decrease in the (200)/(100) peak intensity ratio with decreasing cesium content in MAxCs1−xPbBr3 (Fig. 1c) while the refined lattice parameter reduces monotonically with a slight deviation around 50% (Fig. 1d), in agreement with the higher cesium content observed from XPS. Furthermore, the cell volume (Fig. 1d and Table S1†) shows an inflection that may be attributed to a reduction symmetry to orthorhombic (Pbnm) as in CsPbBr3. Such compositional dimorphism could not be confirmed due to strong textural effects and crystal size broadening. Overall, XRD and XPS confirm the systematic homogenous evolution of mixed-cation structure with cesium incorporation.
m) (Fig. 1c) that was used for Rietveld refinement.34 The XRD peaks were Scherrer broadened18,35 as expected from nano-sized crystallites. Compared to Cs+ the MA+ ion scatters X-rays more weakly and has a larger effective ionic radius leading to a progressive decrease in the (200)/(100) peak intensity ratio with decreasing cesium content in MAxCs1−xPbBr3 (Fig. 1c) while the refined lattice parameter reduces monotonically with a slight deviation around 50% (Fig. 1d), in agreement with the higher cesium content observed from XPS. Furthermore, the cell volume (Fig. 1d and Table S1†) shows an inflection that may be attributed to a reduction symmetry to orthorhombic (Pbnm) as in CsPbBr3. Such compositional dimorphism could not be confirmed due to strong textural effects and crystal size broadening. Overall, XRD and XPS confirm the systematic homogenous evolution of mixed-cation structure with cesium incorporation.
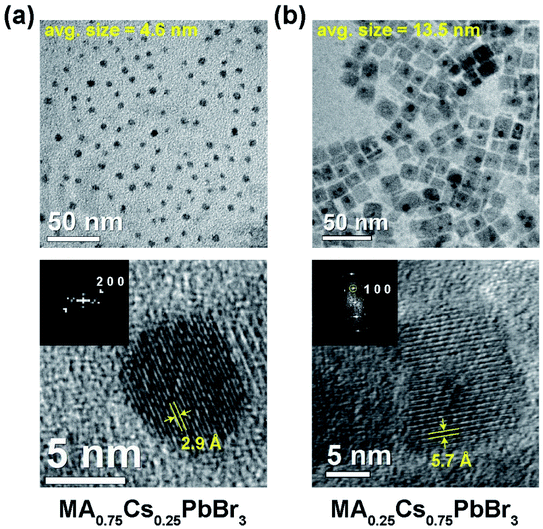
Transmission electron microscopy found the MA-rich compositions displayed spherical morphology while the Cs rich compositions show cuboid particles pointing to different surface energies of the nanoparticles, however further investigation of the surface interactions is required. An average shifted histogram was constructed to estimate particle sizes of 4.6 ± 1.3 and 13.5 ± 2.9 nm for 25 mol% Cs and 75 mol% Cs samples, respectively (Fig. 2 and S1†). A gradual increase in particle size from 3.5 nm to 18.5 nm was observed in passing from MA to Cs rich compositions. As the solubility of the Cs ions in DMF/toluene is lower than MA ions, the rate of precipitation is correspondingly faster for Cs rich precursors, and as a standard reaction time of 5 min was used for all the samples, this accounts for the increasing particle size towards the Cs endmember.
Cesium-rich were less susceptible to degradation during electron beam irradiation enabling the collection of higher magnification bright-field images with lattices where 2.9 Å and 5.7 Å fringes correspond to the (200) and (100) planes of cubic perovskite (Fig. 2).
Photophysical properties
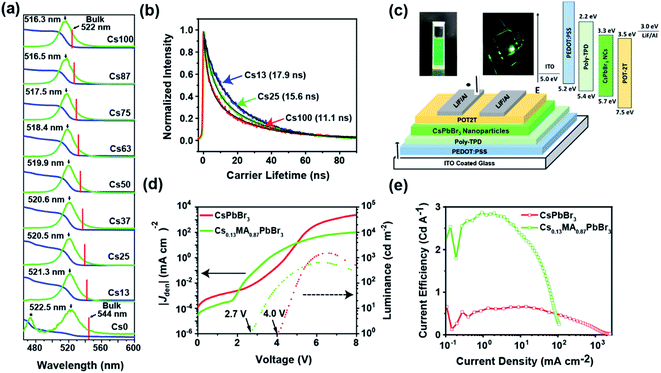
Cs+ incorporation in MAPbBr3 results in a blue shift of absorption edge as well as the PL emission peak moving from 522 nm for 0 mol% Cs sample to 516 nm for 100 mol% Cs sample (Fig. 3a), consistent with previous reports of pure MAPbBr3 and CsPbBr3 nanoparticles.19,36,37 The bulk PL emission was estimated by linearly interpolating the PL emission peak from reported CsPbBr3 and MAPbBr3 data. This enables a deconvolution of size and composition effects. This blue shift in favour with contraction of lattice parameter by Cs+ incorporation, further verifies a homogenous distribution of Cs+ on the A site.25,26 Moreover, all of these PL peaks exhibit FWHM smaller than 22 nm ensuring the monodispersity in the nanoparticles (Fig. 3a) and suitability for utilization in display devices. Pure MAPbBr3 also shows the presence of a blue emission peak at 475 nm that has been previously observed in pure MAPbBr3 and FAPbBr3 nanoparticles and assigned to a 2D impurity phase forming with the octylamine ligand38,39 whose formation could be attributed to the low growth rate of nanoparticles during LARP synthesis. Reducing the ligand concentration may remove this fraction but results in larger particles. Moreover, the synthesis procedure used consistent experimental conditions across all samples to maintain a comparable size range, so further optimization on MAPbBr3 nanoparticles was not performed.To further investigate the optical properties, time resolved photoluminescence spectroscopy (TRPL) was conducted on the colloidal solution of as synthesized samples and analyzed using a bi-exponential decay fit (Tables 1, S2† and Fig. 3b). The 100 mol% Cs sample showed an exciton lifetime of 11 ns, which is consistent with a previous report of CsPbBr3 nanoparticles synthesized using a similar method.40 By comparison, MAPbBr3 nanoparticles exhibit an extremely long exciton lifetime of 25 ns due to the additional presence of blue-emitting species favoured by the formation of nanoplatelets, resulting in a downhill energy transfer process to the nanoparticles bandgap and the prolongation of population lifetime.41 In contrast, mixed cation perovskites show a reverse trend in exciton lifetime such that increasing Cs+ content reduces carrier lifetime from 17.9 ns for 13 mol% Cs to 9.4 ns for 87 mol% Cs sample (Table 1). In the current study, the major variables affecting the PLQY are particle size, defect number and composition. Orthorhombic (Pbnm) CsPbBr3 nanoparticles reportedly have residual strain accommodated through octahedral tilting of the PbBr6 octahedra17,42,43 leading to higher defect concentrations and the degradation of photoluminescence properties.13,17 As MAPbBr3 is cubic (Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) without octahedral tilting, addition of methylammonium ions is expected to relax the lattice strain. The change in particle size is progressive, hence the increase in PLQY and subsequent decrease could be due to anomalous changes in strain affecting the number of structural defects. A future study will seek to quantitatively correlate quantifying strain and defect population.
m) without octahedral tilting, addition of methylammonium ions is expected to relax the lattice strain. The change in particle size is progressive, hence the increase in PLQY and subsequent decrease could be due to anomalous changes in strain affecting the number of structural defects. A future study will seek to quantitatively correlate quantifying strain and defect population.
| Sample composition | Bulk PL emission (nm) | Nanoparticle PL emission (nm) | Nanoparticle PL fwhm (nm) | Crystallite size (TEM) (nm) | PLQY (%) | Carrier lifetime (±0.5 ns) | Bandgap (eV) (±0.1 eV) |
|---|---|---|---|---|---|---|---|
| Cs100 | 522.0 (ref. 27 and 37) | 516.3 | 19.5 | 18.5 ± 3.5 | 48 | 11.1 | 2.37 |
| Cs87 | 524.8 | 516.5 | 18.9 | — | 60 | 9.4 | 2.36 |
| Cs75 | 527.5 | 517.5 | 18.8 | 13.5 ± 2.9 | 67 | 9.9 | 2.35 |
| Cs63 | 530.3 | 518.4 | 19.7 | — | 76 | 9.7 | 2.34 |
| Cs50 | 533.0 | 519.9 | 20.2 | 11.2 ± 2.6 | 74 | 15.6 | 2.33 |
| Cs37 | 535.8 | 520.6 | 20.3 | — | 74 | 15.7 | 2.32 |
| Cs25 | 538.5 | 520.5 | 21.2 | 4.6 ± 1.3 | 78 | 15.6 | 2.33 |
| Cs13 | 541.8 | 521.3 | 22.1 | — | 88 | 17.9 | 2.31 |
| Cs0 | 544.0 (ref. 37) | 522.5 | 25.1 | 3.5 ± 0.9 | 42 | 25.5* | 2.30 |
The reduction in the exciton lifetimes and PLQY indicates the enhancement of a non-radiative channel that is undesirable for PL efficiency.44 To investigate this effect, PLQY was measured in the colloidal solutions and found the 13 mol% Cs (MA0.87Cs0.13PbBr3) shows the highest PLQY (∼88%) with increased radiative recombination pathways (Table 1) compared to CsPbBr3 (48%) and MAPbBr3 (∼42%). MA0.75Cs0.25PbBr3 (78%) and MA0.63Cs0.37PbBr3 (74%) also show good PLQY performance attributable to lattice strain relaxation.13
The excellent properties of MA0.87Cs0.13PbBr3 such as high PLQY with colloidal stability in non-polar solvents and narrow emission linewidth (22 nm) are highly suitable for LED applications. Therefore, prototype LEDs using purified nanoparticle samples of MA0.87Cs0.13PbBr3 and CsPbBr3 were fabricated. The LED structure is comprised of indium tin oxide (ITO)/poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT:PSS, 45 nm)/poly(4-butylphenyl-diphenylamine) (poly-TPD, 30 nm)/nanoparticles (20 nm)/2,4,6-tris[3-(diphenylphosphinyl)phenyl]-1,3,5-triazine (POT2T, 45 nm)/LiF (0.8 nm)/Al (100 nm) respectively (Fig. 3c). As presented in the energy diagram (Fig. 3c), PEDOT:PSS and poly-TPD act as the hole injecting layer, whereas POT2T serves as an electron injecting layer. The energy level data of the transport layers and CsPbBr3 was reported earlier by Brown et al. illustrate (Fig. 3c) the compatibility of transport layers and photoactive CsPbBr3 layer.45 The inset of Fig. 3c shows a clear and stable colloidal solution and a photograph of LED at 4.0 V. As evident from JV curve, both LEDs exhibit low leakage current in the order of 10−4 mA cm−2 (Fig. 3d). However, the MA0.87Cs0.13PbBr3 shows a low turn-on voltage of 2.0 V, whereas the CsPbBr3 LED the turn-on voltage is 4.0 V. The LED electroluminescence (EL) spectra exhibit a slight red-shift compared to their original PL in suspension that is ascribed to the transition of nanoparticles from colloidal to thin-film form due to Förster resonance energy transfer (FRET) between neighbouring nanoparticles (Fig. 3e).46 Fig. 1e, S2 and Table S2† show the current efficiency, power efficiency, and EQE vs. current density curve of both LEDs; the MA0.87Cs0.13PbBr3 LED shows a current efficiency of 2.8 cd A−1 with EQE of 0.83% and power efficiency of 2.34 lm W−1, whereas, the CsPbBr3 LED only exhibits a current efficiency of 0.64 cd A−1 with EQE of 0.20% and power efficiency of 0.41% lm W−1. Future work will likely improve the performance of these LED prototypes. While the efficiency values in both devices are low, the MA0.87Cs0.13PbBr3 LED shows a remarkable 4.3 times current efficiency compared to the standard CsPbBr3 LEDs.
Conclusion
The development of Cs-MA mixed cation colloidal perovskites using a room-temperature LARP synthesis method has been investigated. Structural and compositional studies using powder XRD and XPS confirmed the incorporation of Cs+ on the “A” site of MAPbBr3 nanoparticles with high PLQY achieved ranging from 60–88%. TRPL demonstrated increased charge carrier lifetime for optimized MA0.87Cs0.13PbBr3 which in turn results in a PLQY of ∼88%. The increment in PLQY for mixed-cation nanoparticles was attributed to the relaxation in structural strain. However, investigation at the atomic scale is needed to establish the origin of local strain in these hybrid perovskites. Lastly, the colloidally stable ink of 13 mol% Cs and CsPbBr3 were used to fabricate into functioning electrically-driven LED prototypes.Conflicts of interest
There are no conflicts to declare.Acknowledgements
SSHD and TJW acknowledge financial support from the Singapore National Research Foundation, Prime Minister's Office, through the Competitive Research Program (CRP Award No. NRF-CRP14-2014-03). PV acknowledges NTU, Singapore Presidential Postdoctoral Fellowship via grant 04INS000581C150 and Mr Alasdair A. M. Brown for his assistance. ZXS gratefully acknowledges the following funding AcRF Tier 1 (Reference No: RG156/19) and AcRF Tier 3 (MOE2016-T3-1-006 (S)). TCS and DG acknowledge the financial support from the Ministry of Education Tier 2 grants (MOE2017-T2-2-002 and MOE2019-T2-1-006); and the NRF Investigatorship (NRF-NRFI-2018-04). We would like to acknowledge the Energy Research Institute @ NTU, Singapore for access to their facilities. The authors would also like to acknowledge the Facility for Analysis, Characterization, Testing and Simulation (FACTS) at NTU, Singapore, for use of their electron microscopy and X-ray diffraction facilities.References
- Best Research Cell Efficiency, https://www.nrel.gov/pv/assets/pdfs/pv-efficiency-chart.201812171.pdf, 2019 Search PubMed.
- B. Zhao, S. Bai, V. Kim, R. Lamboll, R. Shivanna, F. Auras, J. M. Richter, L. Yang, L. Dai, M. Alsari, X.-J. She, L. Liang, J. Zhang, S. Lilliu, P. Gao, H. J. Snaith, J. Wang, N. C. Greenham, R. H. Friend and D. Di, High-efficiency perovskite–polymer bulk heterostructure light-emitting diodes, 2018 Search PubMed
.
- P. Vashishtha, M. Ng, S. B. Shivarudraiah and J. E. Halpert, Chem. Mater., 2018, 31, 83–89 CrossRef
.
- L. Xie, P. Vashishtha, T. M. Koh, P. C. Harikesh, N. F. Jamaludin, A. Bruno, T. J. Hooper, J. Li, Y. F. Ng and S. G. Mhaisalkar, Adv. Mater., 2020, 32, 2003296 CrossRef CAS PubMed
.
- Y. Dong, Y.-K. Wang, F. Yuan, A. Johnston, Y. Liu, D. Ma, M.-J. Choi, B. Chen, M. Chekini and S.-W. Baek, Nat. Nanotechnol., 2020, 15, 668–674 CrossRef CAS
.
- M. Jeong, I. W. Choi, E. M. Go, Y. Cho, M. Kim, B. Lee, S. Jeong, Y. Jo, H. W. Choi and J. Lee, Science, 2020, 369, 1615–1620 CrossRef CAS
.
- R. R. Tamming, J. Butkus, M. B. Price, P. Vashishtha, S. K. K. Prasad, J. E. Halpert, K. Chen and J. M. Hodgkiss, ACS Photonics, 2019, 6, 345–350 CrossRef CAS
.
- M. M. Lee, J. Teuscher, T. Miyasaka, T. N. Murakami and H. J. Snaith, Science, 2012, 338, 643–647 CrossRef CAS
.
- S. D. Stranks and H. J. Snaith, Nat. Nanotechnol., 2015, 10, 391–402 CrossRef CAS
.
- V. Kumar, J. Barbé, W. L. Schmidt, K. Tsevas, B. Ozkan, C. M. Handley, C. L. Freeman, D. C. Sinclair, I. M. Reaney and W. C. Tsoi, J. Mater. Chem. A, 2018, 6, 23578–23586 RSC
.
- B. Slimi, M. Mollar, I. Ben Assaker, A. Kriaa, R. Chtourou and B. Mari, Monatshefte für Chemie, 2017, 148, 835–844 CrossRef CAS
.
- P. Vashishtha, S. Bishnoi, C.-H. Angus Li, M. Jagadeeswararao, T. J. N. Hooper, N. Lohia, S. B. Shivarudraiah, M. S. Ansari, S. N. Sharma and J. E. Halpert, ACS Appl. Electron. Mater., 2020, 2(11), 3470–3490 CrossRef CAS
.
- P. Vashishtha, S. A. Veldhuis, S. S. Dintakurti, N. L. Kelly, B. E. Griffith, A. A. Brown, M. S. Ansari, A. Bruno, N. Mathews and Y. Fang, J. Mater. Chem. C, 2020, 8, 11805–11821 RSC
.
- M. Saliba, T. Matsui, J.-Y. Seo, K. Domanski, J.-P. Correa-Baena, M. K. Nazeeruddin, S. M. Zakeeruddin, W. Tress, A. Abate and A. Hagfeldt, Energy Environ. Sci., 2016, 9, 1989–1997 RSC
.
- H. P. Kim, J. Kim, B. S. Kim, H. M. Kim, J. Kim, A. R. b. M. Yusoff, J. Jang and M. K. Nazeeruddin, Adv. Opt. Mater., 2017, 5, 1600920 CrossRef
.
- G. Kim, H. Min, K. S. Lee, D. Y. Lee, S. M. Yoon and S. I. Seok, Science, 2020, 370, 108 CrossRef CAS
.
- X. Li, Y. Luo, M. V. Holt, Z. Cai and D. P. Fenning, Chem. Mater., 2019, 31, 2778–2785 CrossRef CAS
.
- L. Zhang, X. Yang, Q. Jiang, P. Wang, Z. Yin, X. Zhang, H. Tan, Y. M. Yang, M. Wei and B. R. Sutherland, Nat. Commun., 2017, 8, 15640 CrossRef CAS PubMed
.
- M. I. Saidaminov, J. Kim, A. Jain, R. Quintero-Bermudez, H. Tan, G. Long, F. Tan, A. Johnston, Y. Zhao, O. Voznyy and E. H. Sargent, Nat. Energy, 2018, 3, 648–654 CrossRef CAS
.
- X. Zhang, H. Liu, W. Wang, J. Zhang, B. Xu, K. L. Karen, Y. Zheng, S. Liu, S. Chen and K. Wang, Adv. Mater., 2017, 29, 1606405 CrossRef PubMed
.
- C. Zhang, S. Wang, X. Li, M. Yuan, L. Turyanska and X. Yang, Adv. Funct. Mater., 2020, 1910582 CrossRef CAS
.
- X. Dai, Z. Zhang, Y. Jin, Y. Niu, H. Cao, X. Liang, L. Chen, J. Wang and X. Peng, Nature, 2014, 515, 96–99 CrossRef CAS
.
- P. O. Anikeeva, J. E. Halpert, M. G. Bawendi and V. Bulovic, Nano Lett., 2009, 9, 2532–2536 CrossRef CAS PubMed
.
- J. Song, J. Li, L. Xu, J. Li, F. Zhang, B. Han, Q. Shan and H. Zeng, Adv. Mater., 2018, 30, 1800764 CrossRef PubMed
.
- S. Premkumar, K. Kundu and S. Umapathy, Nanoscale, 2019, 11, 10292–10305 RSC
.
- C. J. Bartel, C. Sutton, B. R. Goldsmith, R. Ouyang, C. B. Musgrave, L. M. Ghiringhelli and M. Scheffler, Sci. Adv., 2019, 5, eaav0693 CrossRef CAS PubMed
.
- J. Butkus, P. Vashishtha, K. Chen, J. K. Gallaher, S. K. Prasad, D. Z. Metin, G. Laufersky, N. Gaston, J. E. Halpert and J. M. Hodgkiss, Chem. Mater., 2017, 29, 3644–3652 CrossRef CAS
.
- S. A. Veldhuis, Y. F. Ng, R. Ahmad, A. Bruno, N. F. Jamaludin, B. Damodaran, N. Mathews and S. G. Mhaisalkar, ACS Energy Lett., 2018, 3, 526–531 CrossRef CAS
.
- S. Hirotsu, J. Harada, M. Iizumi and K. Gesi, J. Phys. Soc. Jpn., 1974, 37, 1393–1398 CrossRef CAS
.
- P. Cottingham and R. L. Brutchey, Chem. Commun., 2016, 52, 5246–5249 RSC
.
- C. Tenailleau, S. Aharon, B.-E. Cohen and L. Etgar, Nanoscale Adv., 2019, 1, 147–153 RSC
.
- F. Weigert, A. Müller, I. Häusler, D. Geißler, D. Skroblin, M. Krumrey, W. Unger, J. Radnik and U. Resch-Genger, Sci. Rep., 2020, 10, 1–15 CrossRef PubMed
.
- C. Ferraris, M. Srinivasan and T. J. White, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, C536–C537 Search PubMed
.
- M. R. Linaburg, E. T. McClure, J. D. Majher and P. M. Woodward, Chem. Mater., 2017, 29, 3507–3514 CrossRef CAS
.
- U. Holzwarth and N. Gibson, Nat. Nanotechnol., 2011, 6, 534 CrossRef CAS
.
- J. Huang, Y.-H. Wu, Z.-G. Zhu, W. Y. Shih and W.-H. Shih, Chem. Phys. Lett., 2018, 702, 21–25 CrossRef CAS
.
- T. Yin, Y. Fang, X. Fan, B. Zhang, J.-L. Kuo, T. J. White, G. M. Chow, J. Yan and Z. X. Shen, Chem. Mater., 2017, 29, 5974–5981 CrossRef CAS
.
- X. Y. Chin, A. Perumal, A. Bruno, N. Yantara, S. A. Veldhuis, L. Martínez-Sarti, B. Chandran, V. Chirvony, A. S.-Z. Lo and J. So, Energy Environ. Sci., 2018, 11, 1770–1778 RSC
.
- S. Bhaumik, S. A. Veldhuis, Y. F. Ng, M. Li, S. K. Muduli, T. C. Sum, B. Damodaran, S. Mhaisalkar and N. Mathews, Chem. Commun., 2016, 52, 7118–7121 RSC
.
- P. Vashishtha, B. E. Griffith, A. A. M. Brown, T. J. N. Hooper, Y. Fang, M. S. Ansari, A. Bruno, S. H. Pu, S. G. Mhaisalkar, T. White and J. V. Hanna, ACS Appl. Electron. Mater., 2020, 2, 4002–4011 CrossRef CAS
.
- S. Zhou, R. Tang, H. Li, L. Fu, B. Li and L. Yin, J. Power Sources, 2019, 439, 227065 CrossRef CAS
.
- Y. Chen, Y. Lei, Y. Li, Y. Yu, J. Cai, M.-H. Chiu, R. Rao, Y. Gu, C. Wang, W. Choi, H. Hu, C. Wang, Y. Li, J. Song, J. Zhang, B. Qi, M. Lin, Z. Zhang, A. E. Islam, B. Maruyama, S. Dayeh, L.-J. Li, K. Yang, Y.-H. Lo and S. Xu, Nature, 2020, 577, 209–215 CrossRef CAS PubMed
.
- L. Leppert, S. E. Reyes-Lillo and J. B. Neaton, J. Phys. Chem. Lett., 2016, 7, 3683–3689 CrossRef CAS
.
- J. Shi, F. Li, J. Yuan, X. Ling, S. Zhou, Y. Qian and W. Ma, J. Mater. Chem. A, 2019, 7, 20936–20944 RSC
.
- A. A. M. Brown, T. J. N. Hooper, S. A. Veldhuis, X. Y. Chin, A. Bruno, P. Vashishtha, J. N. Tey, L. Jiang, B. Damodaran and S. H. Pu, Nanoscale, 2019, 11, 12370–12380 RSC
.
- P. Vashishtha and J. E. Halpert, Chem. Mater., 2017, 29, 5965–5973 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra03965b |
| This journal is © The Royal Society of Chemistry 2021 |