Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
New sensing platform of poly(ester-urethane)urea doped with gold nanoparticles for rapid detection of mercury ions in fish tissue†
Hany Abd El-Raheem ac,
Rabeay Y. A. Hassan
ac,
Rabeay Y. A. Hassan *ab,
Rehab Khaledd,
Ahmed Farghalic and
Ibrahim M. El-Sherbiny*a
*ab,
Rehab Khaledd,
Ahmed Farghalic and
Ibrahim M. El-Sherbiny*a
aCenter of Materials Sciences, Zewail City of Science and Technology, October Gardens, 6th of October City, 12578, Giza, Egypt. E-mail: ielsherbiny@zewailcity.edu.eg; ryounes@zewailcity.edu.eg
bApplied Organic Chemistry Department, National Research Centre (NRC), Dokki, 12622, Giza, Egypt
cMaterials Science and Nanotechnology Department, Faculty of Postgraduate Studies for Advanced Sciences, Beni-Suef University, Beni-Suef, Egypt
dChemistry Department, Faculty of Science, Beni-Suef University, Beni-Suef, Egypt
First published on 28th September 2021
Abstract
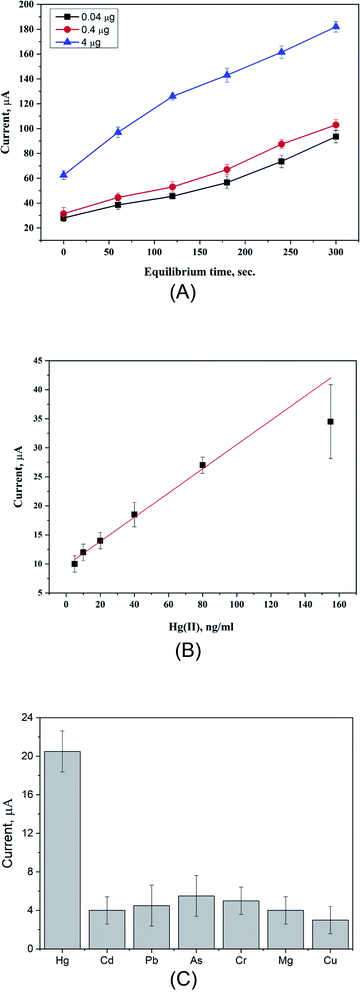
A new electrochemical sensor has been fabricated based on the in situ synthesis of poly(ester-urethane) urea (PUU) doped with gold nanoparticles (AuNPs), and the obtained composite materials (PUU/AuNPs) were used as a new sensing platform for highly sensitive and selective detection of mercury(II) ions in fish tissue. PUU was synthesized and fully characterized by XRD, TGA, DSC, and FTIR to analyze the chemical structure, thermal stability, and morphological properties. As a polymeric structure, the PUU consists of urethane and urea groups that possess pronounced binding abilities to Hg2+ ions. SEM-EDX was carried out to confirm this kind of interaction. Using ferricyanide as the redox probe, PUU alone exhibited weak electrochemical signals due to its low electrical conductivity. Therefore, a new series of nanocomposites of PUU with different nanostructured materials were applied, and their electrochemical performances were evaluated. Among these materials, the PUU/AuNP-modified electrode showed high voltammetric signals towards Hg2+. Consequently, the parameters affecting the performance of the assay, such as electrode composition, scan rate, and sensing time, as well as the effect of electrolyte and pH were studied and optimized. The sensor showed a linear range of 5 ng mL−1 to 155 ng mL−1 with the regression coefficient R2 = 0.986, while the calculated values of the limit of detection (LOD) and limit of quantification (LOQ) were 0.235 ng mL−1 and 0.710 ng mL−1, respectively. In terms of cross reactivity testing, the sensor exhibited a high selectivity against heavy metals which are commonly determined in seafood (Cd2+, Pb2+, As3+, Cr3+, Mg2+, and Cu2+). For real applications, total Hg2+ ions in fish tissue were determined with very high recovery and no prior complicated treatments.
Introduction
Liquid mercury was found historically in Egyptian tombs from 1500 BC. It was isolated by the ancient Egyptians and the Chinese from the mineral cinnabar (mercuric sulfide).1 Mercury, a heavy, silvery-white liquid metal, remains a highly toxic element and the most worrying pollutant at the global scale as it exists inclusively in the ambient atmosphere, water, soil, and numerous bioregions.2 Inhalation of mercury vapor leads to harmful effects on nervous, digestive and immune systems, and the lungs and kidneys. The inorganic salts of mercury are corrosive to the skin, eyes, and gastrointestinal tract, and may induce kidney toxicity if ingested.3–5 Even at low concentrations, mercury is not only a threat to human health, but also to animals, microorganisms, and plants.6,7 An inorganic species Hg2+, is one of the common and most stable forms of mercury toxins, and has high tendency to be bio-accumulated in living organisms throughout the food chains, which leads to high concentrations at the top of the aquatic food chain.8 Fish and other seafood products can absorb this toxic heavy metal in the gastrointestinal tract, and is then transformed to people who consume amounts of these food products.9,10 Consequently, symptoms of health problems include skin rashes and dermatitis, mood swings, memory loss, mental disturbances, muscle weakness, changes in nerve responses, performance deficits on tests of cognitive function.11 Thus, due to these health risks, it is very crucial to detect Hg2+ with simple and efficient methods to mitigate such possible risks. Mercury can be quantified by several analytical techniques, such as inductively coupled plasma atomic emission spectrometry (ICP-AES),12 inductively coupled plasma-mass spectrometry (ICP-MS),3 atomic absorption spectroscopy (AAS),13 High performance liquid chromatography-cold vapor-inductively coupled plasma mass spectrometry (HPLC/CV/ICP/MS),14 and flame atomic absorption spectrophotometry (FAAS).15 These methods deliver high sensitivity, low detection, and wide linearity range with low limit of detection. However, they do not meet demands for online monitoring, quick, portable, easy to use, small amounts of chemical reagents, and cheap analysis. Based on this, electrochemical techniques meet these expectations and are recommended as an alternative due to their high sensitivity, good selectivity, portability, low cost, simple instrumentation, fast analysis, and easy to operate.16–20In such kind of electrochemical techniques, working electrode materials are the main components that are employed as solid platforms for immobilization of recognition or sensing elements and regulate the charge as well as mass transfer.20–22 In that essence, numerous nanomaterials that provided synergic electrocatalytic properties along with expanding the working surface area, hence, loading capacity and mass transport of reactants were implemented for achieving high performance in terms of selectivity and sensitivity issues. In this regard, polyurethanes (PUs), the distinctive class of polymers which are widely used in construction materials, biomedical, actuators, and transducers, have been applied in the construction of electrochemical sensing platforms.23–29 Basically, PUs are synthesized by combining three chemical constituents (diisocyanate (aliphatic or aromatic), polyether diol (long-chain polyester), and diamine (small molecule chain-extender diol)).27,30,31 Modified poly(urethane urea) (PUU) provides high thermal and mechanical stability.32 Therefore, PUU has been implemented in sensing of gas, humidity, pressure, hormone, and glucose.33–38 In the electrochemical sensor sector, polyurethane composite decorated with gold nanoparticles has been used to detect dopamine in the cerebrospinal synthetic fluid.39 Also, polyurethane modified with gold nanoparticles has been used to determine tryptophan.40 Thus, the PUU was selected in this study as a polymeric platform for the voltammetric determination of Hg2+ ions in fish tissues. The PUU was prepared through the one-shot technique and then doped with different nanomaterials to enhance the PUU-electrochemical features. The PUU-based platforms were characterized by FTIR, TGA, DSC, X-ray diffraction, TEM, SEM-mapping, and cyclic voltammetry. Eventually, the modified electrodes with the PUU/Au NPs were finally selected as the targeted sensing platforms.
Materials and methods
Chemical reagents and substances
4,4′-Diphenylmethane-diisocyanate (MDI), castor oil (CO) and ethanolamine (EA) were purchased from Merck, Germany. Tin(II) octoate, Sn(Oct)2 was used as a catalyst and it has been provided by Sigma-Aldrich. Tetrahydrofuran (THF) and N,N-dimethylformamide (DMF) were obtained from Fisher chemicals. Phthalic acid (C8H6O4) was used as a supporting electrolyte and prepared in Milli-Q water. The synthesized nanomaterials were used in this study include, silver (Ag), gold (Au), platinum (Pt), and copper (Cu) nanoparticles in addition to metal oxides such as aluminum oxide (Al2O3), manganese oxide (MnO2) nanorods, and multi-walled carbon nanotube (MWCNTs, Sigma-Aldrich). Carbon paste electrodes were prepared by using graphite powder and paraffin oil, brought from Acros Organics and Fluka, respectively.Chemical synthesis of poly(ester-urethane) urea (PUU)
As presented in Scheme 1, the PUU, including MDI, CO, EA, is chemically synthesized by one-shot poly-condensation technique as reported in our previous work.27,41 The solutions of MDI, CO, EA with molar ratios (1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5) were placed in 250 mL polypropylene beakers containing tin(II)-2-ethylhexanoate (0.03 wt%, concerning to the reactants) and stirred vigorously. The resulting viscous polymer was then poured into a silica mold and heated at 50 °C for 100 h in an oven to attain a complete polymerization. Then, the samples were transmitted to a roll mill mixer for 15 minutes to eliminate any air bubbles through the casting process. Consequently, the sample was subjected to hot press, and compression molded into 1 mm plates at 175 °C for 45 min at a pressure of 62.05 MPa, and then cooled down to room temperature. The resulting PUU was dissolved in a mixture of tetrahydrofuran and N,N dimethylformamide (THF/DMF, 1
0.5) were placed in 250 mL polypropylene beakers containing tin(II)-2-ethylhexanoate (0.03 wt%, concerning to the reactants) and stirred vigorously. The resulting viscous polymer was then poured into a silica mold and heated at 50 °C for 100 h in an oven to attain a complete polymerization. Then, the samples were transmitted to a roll mill mixer for 15 minutes to eliminate any air bubbles through the casting process. Consequently, the sample was subjected to hot press, and compression molded into 1 mm plates at 175 °C for 45 min at a pressure of 62.05 MPa, and then cooled down to room temperature. The resulting PUU was dissolved in a mixture of tetrahydrofuran and N,N dimethylformamide (THF/DMF, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v).
1 v/v).
Preparation of gold nanoparticles (Au NPs)
Using a chemical reduction method, the synthesis of Au nanospheres was carried out as follows: a solution of chloroauric acid (HAuCl4·3H2O) with the concentration of 147 mM was prepared in 50 mL of Milli-Q water. The solution was heated until boiling in 100 mL glass flask. A volume of 2 mL of 1% (w/v) tri-sodium citrate was then added rapidly under vigorous stirring. The solution color turned from pale yellow to blue and eventually to dark red color. The suspension was then centrifuged for 15 min at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, and re-suspended in water to be used for drop-casting on the electrode surface.
000 rpm, and re-suspended in water to be used for drop-casting on the electrode surface.
Preparation of working electrode
For electrochemical characterization, the PUU was doped with different nanomaterials via forming a homogenous suspension which is then mixed with graphite powder (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 w/w) and paraffin oil to form a carbon paste. The resulting paste was then packed into a cylindrical plastic tube with 5 mm internal diameter. The surface of the modified electrodes was smoothed and polished with wet filter paper, and rinsed with Milli-Q water. Finally, prior to each electrochemical measurement, surfaces of the modified electrode were activated electrochemically by running continues voltammetric cycles from −0.4 to 1.0 V (6 voltammetric cycles) in KCl 0.1 M, using a scan rate of 50 mV s−1 and equilibrium time of 15 seconds.
10 w/w) and paraffin oil to form a carbon paste. The resulting paste was then packed into a cylindrical plastic tube with 5 mm internal diameter. The surface of the modified electrodes was smoothed and polished with wet filter paper, and rinsed with Milli-Q water. Finally, prior to each electrochemical measurement, surfaces of the modified electrode were activated electrochemically by running continues voltammetric cycles from −0.4 to 1.0 V (6 voltammetric cycles) in KCl 0.1 M, using a scan rate of 50 mV s−1 and equilibrium time of 15 seconds.
Measurements and devices
The voltammetric measurements were carried out using a computer-controlled Gamry potentiostat/galvanostat/ZRA G750 (Gamry, Pennsylvania, USA). The electrochemical system is connected with a three-electrode setup (the PUU-based carbon paste electrode as the working electrode, Ag/AgCl as a reference electrode, Pt disc as a counter electrode). Cyclic voltammetry (CV) technique was carried out in a 50 mL electrochemical cell containing phthalic acid (pH 2.5) as supporting electrolyte medium, at the potential window between −0.4 to 1.0 V and scan rate 50 mV s−1. Heavy metal ions were accumulated at the modified electrode surface for 15 seconds at the applied potential of 0.0 V).The pH measurements were performed by using a bench-top pH-meter (JENWAY, Model 3510, UK). Fourier transform infrared (FTIR) spectra were obtained using Thermo Scientific IS10 FTIR spectrometer (USA). The FTIR spectra were collected in the range of 4000–400 cm−1 at room temperature, utilizing 64 scans at 4 cm−1 resolutions. For morphological characterizations, high-resolution transmission electron microscopy (HR-TEM, JEOL JEM-2100) at an accelerating voltage of 200 kV was used for imaging and analysis. Scanning Electron Microscope (SEM, JEOL, JXA-840A) at an accelerating applied potential of 15 keV. Differential Scanning Calorimetry (DSC), Q20 V24.10 Build 122 (USA), approximately 6 mg of a sample was measured, temperature range from 0 to 400 °C at a heating rate of 10 °C min−1, under nitrogen flow of 40 mL min−1. Thermogravimetric Analysis (TGA), (DTG-60H, Shimadzu, Japan). Approximately 8 mg of sample was measured from 0 to 500 °C at a rate of 10 °C min−1 under a nitrogen flow of 40 mL min−1. X-ray diffraction (XRD) analysis was performed using Shimadzu XRD6000 Japan, operating with nickel filtered, Cu–K target, voltage 40 kV, current 30 mA, and scan speed 8 degrees per min.
Fish sample pre-treatment and mercury ions determination
Different fish samples and shrimps were brought from local markets. The muscle tissues of fishes and shrimps were removed, homogenized, and 1.0 gram of each dried tissue sample was transferred into a 25 mL digestion flask and 10 mL concentrated HNO3 (69–70%) was added. The flask was then heated at 40 °C for 1 hour and the temperature was then raised up to 150 °C for another 3 hours. Digestion was continued until all tissue samples dissolved completely in the conc. acid, i.e. continuous boiling of the acid–tissue mixture until dense white fumes appeared. Next, the digested samples were cooled and filtered through the Whatman No. 42 filter paper. The samples were diluted up to 5 mL of distilled water for analysis.42Results and discussion
Synthesis and characterization of poly(ester-urethane) urea (PUU)
The PUU was prepared by one-shot polycondensation technique, three monomers (MDI, CO, EA) are synthesized, both CO and EA carry hydroxyl and amino groups to react with isocyanates MDI group to give urethane and urea groups localized on the same chain. The final polymeric structure provides urethane and urea groups that have the ability to bind with some heavy metals. Gold nanoparticles are introduced into the PUU to provide the electrochemical activity of the materials because of their good electrochemical behavior.Before involving the PUU into electrochemical studies, a series of characterization techniques such as FTIR, TGA, DSC, X-ray diffraction, TEM, and the SEM were applied to analyze the chemical structure, thermal stability, and morphological properties of the synthesized PUU. Successful urethane-urea formation was confirmed by infrared spectra of MDI, EA, CO and PUU, and presented in Fig. 1A. In the spectrum of CO, the absorption peaks at 3411 cm−1, 2927 cm−1, 2856 cm−1, and 1744 cm−1 are ascribed to O–H stretching vibration, CH2 symmetric and asymmetric, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ester group respectively. The absorption peaks at 3377 cm−1 and 3615 cm−1 are peaks ascribed to N–H/O–H groups of EA. A strong band at 2274 cm−1 is assigned to the NCO group of MDI43,44 and the stretching vibration peak of the benzene ring C
O ester group respectively. The absorption peaks at 3377 cm−1 and 3615 cm−1 are peaks ascribed to N–H/O–H groups of EA. A strong band at 2274 cm−1 is assigned to the NCO group of MDI43,44 and the stretching vibration peak of the benzene ring C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C at 1522 cm−1. In the spectra of the final PUU product, the absorption peak at 3330 cm−1 that corresponds to N–H group and the characteristic absorption peak at 2274 cm−1 that corresponds to the isocyanate of MDI were disappeared. Furthermore, the newly formed peaks at 1730 and 1525 cm−1 are attributed to the stretching vibration of –NHCOO– and NHCONH– groups, indicating that the MDI, CO and EA are completely reacted and the PUU was successfully synthesized.
C at 1522 cm−1. In the spectra of the final PUU product, the absorption peak at 3330 cm−1 that corresponds to N–H group and the characteristic absorption peak at 2274 cm−1 that corresponds to the isocyanate of MDI were disappeared. Furthermore, the newly formed peaks at 1730 and 1525 cm−1 are attributed to the stretching vibration of –NHCOO– and NHCONH– groups, indicating that the MDI, CO and EA are completely reacted and the PUU was successfully synthesized.
 | ||
| Fig. 1 (A) FTIR spectra of CO, EA, MDI and PUU, and (B) TGA curve of PUU, (C) DSC thermogram of PUU, and (D) XRD diffractogram of PUU. | ||
The existence of PUU thermal stability, is referred to as including the raw materials, i.e. proportions of soft and hard segments, density and type of crosslinking bonds, type of chain extender and the synthesis routes.45 Here, the thermal stability was evaluated by TGA and the results are given in Fig. 1B. Three stages of the thermal decomposition were observed in the TGA-thermogram, where the first stage of weight loss was around 250 °C, is probably due to the vaporization of volatilized products/solvent that might be encapsulated within the PUU chain. The second stage has a maximum degradation rate at 362 °C, involving the complete decomposition of urethane and urea bonds in rigid segments. While the third stage was obtained at 467 °C which is attributed to the thermal decomposition of ester bonds in soft segments. Regarding the glass transition temperature and melting behavior, the DSC analysis showed the glass transition temperature (Tg) at 59 °C and, the melting temperature (Tm) is 456 °C, as depicted in Fig. 1C.
On the other hand, X-ray diffraction analysis (XRD) was carried out to determine the crystalline phase of the prepared PUU. In the diffractograms, two broad diffraction peaks, one large and one small, were observed at 2θ = 21° and 9°, respectively. The two diffraction patterns displayed amorphous nature and did not exhibit crystalline peaks of the PUU Fig. 1D. The amorphous phase of PUU depends upon its structure and the presence of urea groups.
Electrochemical characterization of PUU nanocomposites
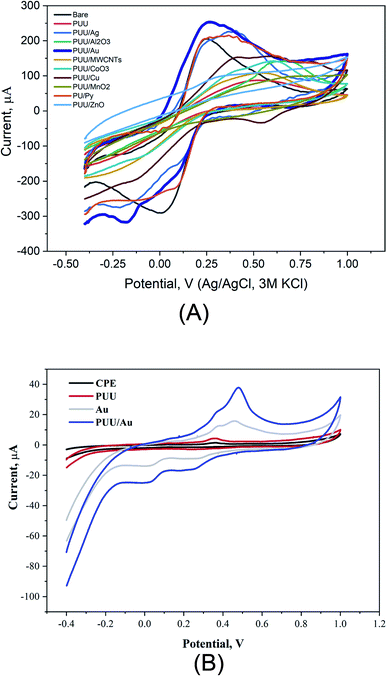
In electrochemical systems, studying electron transfer rate at the electrode/solution interface provides information to understand the role of electrode composition(s) in the studied electrochemical process.46–49 Here, using cyclic voltammetry, redox reactions of ferricyanide, as a standard redox probe was studied at the modified electrodes with the PUU and PUU-nanocomposites (i.e. the PUU doped with each of these nanostructures: (Ag, Au, Pt, Cu, ZnO, CoO3, MnO2, Al2O3, and MWCNTs). As a result, a significant difference between electrochemical signals obtained by the PUU alone and that obtained by the PUU nanocomposites, as shown in Fig. 2A. On the other hand, voltammetric signals of Hg ions at the modified electrodes with AuNPs or PUU/Au were much higher than that obtained by the bare or the PUU based surfaces Fig. 2B. Thus, the use of PUU/Au is promising in the voltammetric recognition of Hg ions. Thus, PUU/Au nanoparticles have been assigned for the voltammetric determination of Hg2+ under further optimization.Morphologic characterization of PUU and PUU-Au
The PUU/Au nanocomposite is being selected as an electrode modifier for the detection of Hg ions, therefore, morphological characterization of the nanocomposite constituents (i.e. the PUU or the PUU/Au) were performed. From the TEM images, a compact and smooth surface of the PUU was observed Fig. 3A. On the other side, a correlation between the TEM and the 3D-SEM images Fig. 3B and C revealed that the PUU structure is modified non-covalently with spherical particles sized with 10 nm that are composed of the AuNPs. | ||
| Fig. 3 (A) TEM image of the PUU morphology, (B) TEM image of the PUU/Au nanocomposite morphology, and (C) SEM image of the PUU/Au nanocomposite-3D structure. | ||
Assay optimization
| Sensing platform | Technique | Detection limit | Linear range | Ref. |
|---|---|---|---|---|
| PPh3/MWCNTs/IL/CPE | SWASV | 9.2 × 10−5 μM | 1 × 10−4–0.15 μM | 51 |
| AuNPs/CFME | DPASV | 0.1 μM | 0.2–50 μM | 52 |
| 12-crown-4-ether/MWCNTs | LSV | 1.25 μM | 25 μM–550 μM | 18 |
| Functionalized gold nanoparticles/reduced graphene oxide | DPV | 7.5 nM | 50 nM–5 μM | 53 |
| Au/DMAET/(SWCNT-PABS) | SWASV | 0.06 μM | 20–250 μM | 54 |
| Mg–Al–TGA LDH/GCE | SWASV | 0.8 nM | 2.0–800 nM | 55 |
| EDTA/CPE | SWV | 16.6 × 10−9 M | 5 × 10−5–35 × 10−5 M | 56 |
| CuO/PVA/GCE | CV | 0.42 nM. | 10–70 μM | 57 |
| 2,2′-(Ethane-1,2-diylbis((2-(azulen-2-ylamino)-2-oxoethyl) azanediyl))diacetic acid | DPV | 3 × 10−10 M | 10−9–5 × 10−7 M | 58 |
| NRGO/GCE | DPASV | 0.58 nM | 1 nM to 800 nM | 59 |
| Thiosemicarbazone ligands | DPV | 7 × 10−10 M | 10−9–10−6 M | 60 |
| ssDNA-cysteine/gold electrode surface. | SWV | 10 (pM) | 100 (nM) −10 (pM) | 61 |
| AuNPs – GC | (SWASV) | 0.42 nM | 0.64–4.00 nM | 62 |
| Poly(aniline-co-o-aminophenol) – PANOA/Au NPs | ASV | 0.23 nM | 0.8–12.0 nM | 63 |
| PUU-Au/CPE | CV | 1.17 nM | 25 nM–775 nM | *This work |
| Sample | Spiked conc. (μg mL−1) | Detected conc. (μg mL−1) | Proposed method-recovery (%) |
|---|---|---|---|
| Tilapia-river Nile | 4 | 3.7 | 93 |
| Tilapia fish farming | 4 | 4 | 100 |
| Catfish-river Nile | 4 | 3.84 | 96 |
| Catfish-fish farming | 4 | 4.4 | 111 |
| Shrimp-Red Sea | 4 | 3.57 | 90 |
| Shrimp-fish farming | 4 | 4.7 | 117 |
| Tuna | 4 | 3.78 | 95 |
| Salmon | 4 | 3.82 | 96 |
Conclusion
Exploiting poly(ester-urethane) urea (PUU) as a new sensing platform for the rapid detection of mercury ions in fish samples was achieved. PUU incorporation with other nano-structures such as gold nanoparticles improved the electrochemical properties and the sensing responses reaching a very low limit of detection. Physicochemical characterizations were completely performed to define the physical and chemical properties, along with the surface morphology and sensing mechanisms. Eventually, assay optimization and real samples analysis using the new electrode were conducted. This new approach is directed to support the use of polymeric hetero-structures for the rapid tracking of hazardous in environmental and biological specimens.Author contributions
Hany Abd El-Raheem: methodology, validation, writing – original draft. Rabeay Y. A. Hassan: conceptualization, supervision, writing and review the manuscript. Rehab Khaled: review the manuscript. Ahmed Farghali: review the manuscript. Ibrahim M. El-Sherbiny: conceptualization, supervision, writing and review the manuscript.Conflicts of interest
The authors confirm that there are no conflicts to declare.References
- F. G. o. C. Environment Canada, Mercury and the environment — Basic facts., 2004 Search PubMed.
- P. Krystek and R. Ritsema, Appl. Organomet. Chem., 2004, 18, 640–645 CrossRef CAS.
- Z. Gu, M. Zhao, Y. Sheng, L. A. Bentolila and Y. Tang, Anal. Chem., 2011, 83, 2324–2329 CrossRef CAS PubMed.
- Y. Gong, Y. Liu, Z. Xiong and D. Zhao, Environ. Sci. Technol., 2014, 48, 3986–3994 CrossRef CAS PubMed.
- P. B. Tchounwou, W. K. Ayensu, N. Ninashvili and D. Sutton, Environ. Toxicol., 2003, 18, 149–175 CrossRef CAS PubMed.
- M. H. Mashhadizadeh, S. Ramezani and M. K. Rofouei, Mater. Sci. Eng., C, 2015, 47, 273–280 CrossRef CAS PubMed.
- C. Li, Q. Zhang, S. Kang, Y. Liu, J. Huang, X. Liu, J. Guo, K. Wang and Z. Cong, Environ. Sci. Pollut. Res., 2015, 22, 12490–12500 CrossRef CAS PubMed.
- E. Sumesh, M. Bootharaju and T. Pradeep, J. Hazard. Mater., 2011, 189, 450–457 CrossRef CAS PubMed.
- F. M. Rebelo and E. D. Caldas, Environ. Res., 2016, 151, 671–688 CrossRef CAS PubMed.
- N. Zheng, S. Wang, W. Dong, X. Hua, Y. Li, X. Song, Q. Chu, S. Hou and Y. Li, Bull. Environ. Contam. Toxicol., 2019, 102, 714–720 CrossRef CAS PubMed.
- L. Ou, C. Chen, L. Chen, H. Wang, T. Yang, H. Xie, Y. Tong, D. Hu, W. Zhang and X. Wang, Environ. Sci. Technol., 2015, 49, 6899–6908 CrossRef CAS PubMed.
- Y. Wu, Y.-I. Lee, L. Wu and X. Hou, Microchem. J., 2012, 103, 105–109 CrossRef CAS.
- H. Bagheri and M. Naderi, J. Hazard. Mater., 2009, 165, 353–358 CrossRef CAS PubMed.
- S. S. de Souza, A. D. Campiglia and F. Barbosa Jr, Anal. Chim. Acta, 2013, 761, 11–17 CrossRef CAS PubMed.
- K. Supong and P. Usapein, Water Sci. Technol., 2019, 79, 833–841 CrossRef CAS PubMed.
- T. Hezard, K. Fajerwerg, D. Evrard, V. Collière, P. Behra and P. Gros, J. Electroanal. Chem., 2012, 664, 46–52 CrossRef CAS.
- A. A. Ismaiel, M. K. Aroua and R. Yusoff, Sensors, 2014, 14, 13102–13113 CrossRef PubMed.
- R. Y. Hassan, M. S. Kamel, H. N. Hassan and E. Khaled, J. Electroanal. Chem., 2015, 759, 101–106 CrossRef CAS.
- G. Zhou, J. Chang, H. Pu, K. Shi, S. Mao, X. Sui, R. Ren, S. Cui and J. Chen, ACS Sens., 2016, 1, 295–302 CrossRef CAS.
- D. Peng, L. Zhang, R.-P. Liang and J.-D. Qiu, ACS Sens., 2018, 3, 1040–1047 CrossRef CAS PubMed.
- R. A. Alfadaly, A. Elsayed, R. Y. A. Hassan, A. Noureldeen, H. Darwish and A. S. Gebreil, Molecules, Basel, Switzerland, 2021, vol. 26 Search PubMed.
- R. Y. A. Hassan and U. Wollenberger, Electroanalysis, 2019, 31, 1112–1117 CrossRef CAS.
- S. Oprea, S. Vlad and A. Stanciu, Eur. Polym. J., 2000, 36, 2409–2416 CrossRef CAS.
- M. Suriati Ghazali, J. O. Akindoyo, M. Beg and A. Nitthiyah Jeyaratnam, 2017.
- R. J. Soto, J. B. Schofield, S. E. Walter, M. J. Malone-Povolny and M. H. Schoenfisch, ACS Sens., 2017, 2, 140–150 CrossRef CAS PubMed.
- W. K. Ward, L. B. Jansen, E. Anderson, G. Reach, J.-C. Klein and G. S. Wilson, Biosens. Bioelectron., 2002, 17, 181–189 CrossRef CAS PubMed.
- H. Abd El-Raheem, R. Y. Hassan, R. Khaled, A. Farghali and I. M. El-Sherbiny, Microchem. J., 2020, 104765 CrossRef.
- P. Cervini, I. A. Mattioli and É. T. G. Cavalheiro, RSC Adv., 2019, 9, 42306–42315 RSC.
- I. A. Mattioli, M. Baccarin, P. Cervini and É. T. G. Cavalheiro, J. Electroanal. Chem., 2019, 835, 212–219 CrossRef CAS.
- J. Pathak, J. Twigg, K. Nugent, D. Ho, E. Lin, P. Mott, C. Robertson, M. Vukmir, T. Epps III and C. Roland, Macromolecules, 2008, 41, 7543–7548 CrossRef CAS.
- C. Roland and R. Casalini, Polymer, 2007, 48, 5747–5752 CrossRef CAS.
- D.-H. Kim, K.-C. Yu, Y. Kim and J.-W. Kim, ACS Appl. Mater. Interfaces, 2015, 7, 15214–15222 CrossRef CAS PubMed.
- S. Brady, D. Diamond and K.-T. Lau, Sens. Actuators, A, 2005, 119, 398–404 CrossRef CAS.
- S. G. Chen, J. W. Hu, M. Q. Zhang and M. Z. Rong, Sens. Actuators, B, 2005, 105, 187–193 CrossRef CAS.
- P. Bosch, A. Fernández, E. F. Salvador, T. Corrales, F. Catalina and C. Peinado, Polymer, 2005, 46, 12200–12209 CrossRef CAS.
- T. Tung, C. Robert, M. Castro, J. Feller, T. Kim and K. S. Suh, Carbon, 2016, 108, 450–460 CrossRef CAS.
- A. Paşahan, S. Köytepe, M. A. Cengiz and T. Seçkin, Polym. Int., 2013, 62, 246–250 CrossRef.
- J. Fang, T. Lin, W. Tian, A. Sharma and X. Wang, J. Appl. Polym. Sci., 2007, 105, 2321–2326 CrossRef CAS.
- P. Cervini, I. A. Mattioli and É. T. Cavalheiro, RSC Adv., 2019, 9, 42306–42315 RSC.
- I. A. Mattioli, M. Baccarin, P. Cervini and É. T. Cavalheiro, J. Electroanal. Chem., 2019, 835, 212–219 CrossRef CAS.
- H. A. El-Raheem, Y. Abdel-Monem, I. El-Sherbiny, K. Abdelal, M. Basuni and H. Youssef, Appl. Math., 2018, 12, 1–11 Search PubMed.
- R. Voegborlo and H. Akagi, Food Chem., 2007, 100, 853–858 CrossRef CAS.
- Q. Zhang, X. Huang, X. Wang, X. Jia and K. Xi, Polymer, 2014, 55, 1282–1291 CrossRef CAS.
- J. Wu, Q. Ge and P. T. Mather, Macromolecules, 2010, 43, 7637–7649 CrossRef CAS.
- P. Król and B. Pilch-Pitera, J. Appl. Polym. Sci., 2007, 104, 1464–1474 CrossRef.
- L. Tang, Y. Wang, Y. Li, H. Feng, J. Lu and J. Li, Adv. Funct. Mater., 2009, 19, 2782–2789 CrossRef CAS.
- R. Y. Hassan, M. A. Sultan, M. M. A. El-Alamin, M. A. Atia and H. Y. Aboul-Enein, Electroanalysis, 2017, 29, 843–849 CrossRef CAS.
- X. Cheng, Y. Yu, Y. Jia and L. Duan, Mater. Des., 2016, 95, 133–140 CrossRef CAS.
- R. H. Mahmoud, F. A. Samhan, M. K. Ibrahim, G. H. Ali and R. Y. A. Hassan, Bioprocess Biosyst. Eng., 2021, 44, 759–768 CrossRef CAS PubMed.
- R. Y. A. Hassan, M. S. Kamel, H. N. A. Hassan and E. Khaled, J. Electroanal. Chem., 2015, 759, 101–106 CrossRef CAS.
- H. Bagheri, A. Afkhami, H. Khoshsafar, M. Rezaei and A. Shirzadmehr, Sens. Actuators, B, 2013, 186, 451–460 CrossRef CAS.
- D. Li, J. Li, X. Jia and E. Wang, Electrochem. Commun., 2014, 42, 30–33 CrossRef CAS.
- N. Wang, M. Lin, H. Dai and H. Ma, Biosens. Bioelectron., 2016, 79, 320–326 CrossRef CAS PubMed.
- G. G. Matlou, D. Nkosi, K. Pillay and O. Arotiba, Sensing and Bio-Sensing Research, 2016, 10, 27–33 CrossRef.
- K. Asadpour-Zeynali and R. Amini, Sens. Actuators, B, 2017, 246, 961–968 CrossRef CAS.
- A. Moutcine and A. Chtaini, Sensing and Bio-Sensing Research, 2018, 17, 30–35 CrossRef.
- A. Karthika, V. R. Raja, P. Karuppasamy, A. Suganthi and M. Rajarajan, Microchem. J., 2019, 145, 737–744 CrossRef CAS.
- G.-O. Buica, A. A. Ivanov, I.-G. Lazar, G.-L. Tatu, C. Omocea, L. Birzan and E.-M. Ungureanu, J. Electroanal. Chem., 2019, 849, 113351 CrossRef CAS.
- L. Li, Y. Qiu, Y. Feng, Y. Li, K. Wu and L. Zhu, J. Electroanal. Chem., 2020, 114121 CrossRef CAS.
- M. D. Raicopol, N. A. Chira, A. M. Pandele, A. Hanganu, A. A. Ivanov, V. Tecuceanu, I. G. Bugean and G.-O. Buica, Sens. Actuators, B, 2020, 128030 CrossRef CAS.
- C. Pal and S. Majumder, Mater. Today: Proc., 2020, 29(4), 1129–1131 CAS.
- T. Hezard, K. Fajerwerg, D. Evrard, V. Collière, P. Behra and P. Gros, J. Electroanal. Chem., 2012, 664, 46–52 CrossRef CAS.
- F. H. Narouei, L. Livernois, D. Andreescu and S. Andreescu, Sens. Actuators, B, 2021, 329, 129267 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra03693a |
| This journal is © The Royal Society of Chemistry 2021 |