 Open Access Article
Open Access ArticleTemplate-assisted synthesis of Ag/AgCl hollow microcubes and their composition-dependent photocatalytic activity for the degradation of phenol†
Shiyun Lou ,
Qinglan Chen,
Wan Wang,
Yongqiang Wang
,
Qinglan Chen,
Wan Wang,
Yongqiang Wang and
Shaomin Zhou
and
Shaomin Zhou *
*
Key Laboratory for Special Functional Materials of the Ministry of Education, Henan University, Kaifeng 475004, PR China. E-mail: shaominzhou@yahoo.com; Tel: +86 371 22357375
First published on 2nd August 2021
Abstract
Plasmonic photocatalysts with hollow structures and tunable composition exhibit significant advantages due to their high efficiency in light collection and effective charge transfer across the tight contact heterojunction interface. Herein, hollow Ag/AgCl microcubes were developed by treating nanosheet-assembled hollow Ag microcubes with FeCl3, where a part of Ag at the interface could be in situ transformed and oxidized into AgCl. Equally, by adjusting the concentration of Fe3+ ions, Ag/AgCl hollow microcubes with different compositions could be easily achieved. Electron transfer was favored by a lot of tiny Ag/AgCl heterojunctions induced by the in situ oxidation of the multicrystalline Ag hollow microcube template containing a number of grain boundaries. The designed hollow Ag/AgCl microcubes exhibited strong visible-light adsorption owing to the surface plasmon resonance effect of Ag nanoparticles, in addition to the multiple light-reflections inside the hollow structure. The as-obtained products were then used as visible-light photocatalysts, where the results indicated that 91.6% of phenol was degraded within 150 min under visible light by the as-obtained sample with a Ag to AgCl ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. The superior visible-light photocatalytic activity resulted from the enhancement of the visible light-harvesting and the efficient charge separation at the Ag and AgCl contact interfaces.
3. The superior visible-light photocatalytic activity resulted from the enhancement of the visible light-harvesting and the efficient charge separation at the Ag and AgCl contact interfaces.
1. Introduction
Phenol in wastewater has posed a serious threat to the ecological environment and human health because of its high toxicity to human skin and harm to the central nervous system.1 As a key protocol to remove phenol, photocatalysis has drawn extensive attention of researchers owing to its advantages of rapid decomposition, thorough treatment and no secondary pollution.2,3 However, at present, photocatalysts still face problems such as low utilization efficiency of sunlight and short lifetime of photogenerated carriers, which limit their practical application. Therefore, it is of great significance to design and synthesize photocatalysts for efficient degradation of phenol pollutant in wastewater.In recent years, plasmonic photocatalysts consisting of metallic nanoparticles and semiconducting components exhibit excellent photocatalytic performance owing to the integration of band structures of semiconductor materials, plasmonic properties of noble metals and generation of hetero interfaces.4–6 For example, Ag/TiO2 nanofibers7 and Ag core–TiO2 shell (Ag@TiO2) nanoparticles8 were found to be effective in photocatalytic degradation of phenol. However, interfaces between Ag and TiO2 photocatalysts are not yet well controlled, resulting in uncertainties to improve the efficiency of charge separation at the interface between TiO2 and Ag nanoparticles. Alternatively, a clean and well-defined Ag/AgCl interface can be achieved by in situ synthesis reaction.9,10 It was demonstrated that hot electrons generated by Ag could inject into AgCl in 150 femtoseconds due to the excellent interface, which resulted in a significant improvement in the photocatalytic activity and stability of Ag/AgCl.11–13
It is well known that hollow structures are believed to further improve the catalytic performance of plasmonic photocatalysts thanks to the accessible surface and interior cavity, efficient light collection, shortened distance for carrier transfer and separation, rich surface reaction sites on the shells, and a uniform distribution of metal nanoparticles.14–16 Furthermore, hollow semiconductors are lighter than the corresponding solid structure, and benefit the homogeneous dispersion in the reaction system of photocatalysis. As for hollow plasmonic photocatalysts, the powerful surface plasmonic resonance absorption and intracavity multiple reflections of visible light can improve the total absorption efficiency of photon energies.17
To date, the methods based on templates have been extensively applied in the preparation of hollow nanostructures, and the shape and size of cavities in the obtained hollow structures can be controlled by the designed templates.18 Recently, hollow Ag/AgCl photocatalysts with hollow structures have been prepared and showed superior photocatalytic performance.11,19,20 For example, Ag@AgCl cubic cages were obtained by the sacrificial template method, and exhibited great photocatalytic activity because of their hollow structures.11 Ag@AgCl hollow spheres were synthesized by a chemical reduction approach under light irradiation, where AgNO3 and CCl4 were used as silver and chlorine sources, respectively.19 Hollow cubic AgCl nanostructures were prepared by a one-pot method.20 Thus, recently, most synthesis approaches for Ag/AgCl have been performed through the reduction process. Firstly, AgCl is prepared and then partly transformed to Ag by chemical reduction or photoreduction. Although this method is efficient for the preparation of hollow structure Ag/AgCl, the distribution of hetero interfaces between Ag and AgCl in the shell and the Ag/AgCl ratio are hard to manipulate; meanwhile, the amount and distribution of noble metals in plasmonic photocatalysts have an important influence on the photocatalytic performance.21,22 In fact, an oxidation method was proposed to prepare Ag/AgBr nanowires, which is a simple experimental method, easy to operate, and the molar ratio of Ag to AgBr can be easily adjusted by controlling the concentration of the oxidizing agent composed of halide.23 Furthermore, the in situ oxidation of Ag can form a close connection between Ag and AgBr and it is conducive to effective carrier transfer. In addition, solid Cu2O/Ag/AgCl microcubes were synthesized by a facile oxidation method using Cu2O/Ag microcubes as a template and CuCl2 as an oxidant.24 However, as far as we know, the controllable preparation of hollow Ag/AgCl microcubes by this oxidation method is rarely reported.
In this work, hollow Ag/AgCl microcubes with different compositions were synthesized through an in situ oxidization route. In the designed process, nanosheet-assembled hollow Ag microcubes were prepared as the template, and Ag/AgCl microcubes were obtained by employing FeCl3 as the oxidizer at room temperature. The composition and structure of Ag/AgCl can be adjusted by rationally regulating the experimental conditions. Although AgCl with a hollow nanostructure has been synthesized, this report is the first on hollow Ag/AgCl microcubes synthesized using a nanosheet-assembled multicrystalline Ag microcube template to form a tight contact interface between Ag and AgCl, which is beneficial to the separation of photogenerated carriers. Furthermore, the obtained Ag/AgCl hollow microcubes show outstanding photocatalytic performance for the degradation of phenol.
2. Experimental details
2.1 Materials
Copper acetate (Cu2(CH3COO)2), sodium hydroxide (NaOH), glucose (C6H12O6), nitric acid (HNO3), trisodium citrate (TSC, C6H5Na3O7), silver nitrate (AgNO3), polyvinyl pyrrolidone (PVP), ferric chloride (FeCl3), P25-TiO2, methanol, ethanol and phenol were all purchased from Sinopharm Chemical Reagent Co., Ltd. All chemicals are of analytical reagent grade and need not to be further purified.2.2 Synthesis of Ag/AgCl hollow microcubes
Firstly, uniform Ag hollow microcubes composed of nanosheets were prepared at room temperature using Cu2O microcubes as the template according to the earlier literature of our group.25 The synthesis of uniform Cu2O microcubes was as follows: copper acetate (0.998 g) was dissolved in distilled water (100 mL). After the obtained solution was heated to 70 °C, NaOH solution (5 mL, 0.03 M) and glucose (0.2 g) were added successively under stirring. The mixture was maintained at 70 °C for 60 min. The precipitate was filtered and washed several times with deionized water and ethanol. The prepared Cu2O was used as the template to synthesize Ag hollow microcubes. In a typical synthesis, Cu2O (0.042 g) and trisodium citrate (0.176 g) were dispersed in distilled water (50 mL), and then AgNO3 (0.204 g) was added. After 15 min, dilute HNO3 (10 mL) was quickly injected into the vigorously stirred mixture. The color of the solution changed gradually from brick-red to grey, indicating the formation of silver microcubes. The reaction mixture was stirred for another 30 min after completing the reaction. The precipitate was separated by centrifugation, washed with deionized water and ethanol repeatedly, and dried at 60 °C for 4 h in a vacuum oven. Then, the as-synthesized Ag hollow microcubes and PVP (0.6 g) were dissolved into deionized water (10 mL). Finally, different concentrations of FeCl3 solution (5 mL) were added to the solution containing Ag nanosheets and PVP drop by drop under continuous stirring. The AgCl nanoparticles were in situ formed on the Ag nanosheet surface by the oxidation of Ag and FeCl3, where FeCl3 was selected not only as the Cl− source but also as the oxidizing agent. The solution mixture was magnetically stirred for 30 min to ensure a complete reaction. Using this approach, Ag/AgCl samples with various molar ratios were equally synthesized. The collected Ag/AgCl microcubes were rinsed four times with deionized water and centrifuged at 5000 rpm to get rid of unwanted FeCl3 and PVP. In addition, nitrogen doped TiO2 (N-TiO2) and P25-TiO2/Ag were used as photocatalysts in a controlled test. N-TiO2 was synthesized by calcining P25-TiO2 at 600 °C for 4 h in NH3. P25-TiO2/Ag was prepared according to the literature:26,27 P25-TiO2 powder (100 mg) was taken in different test tubes containing 120 mL water and 30 mL methanol, along with a certain amount of AgNO3 solution (0.01 mM) and Ag–TiO2 were purged with argon for 30 min and irradiated with a 400 W UV lamp under constant magnetic stirring for 2 h. The obtained solutions were centrifuged and washed with deionized water followed by ethanol. The resulting suspensions were separately dried in an oven at 60 °C for 12 h.2.3 Characterization
The crystal phase of the as-obtained products was characterized by X-ray diffraction (XRD) with a powder XRD system (D8-ADVANCE, Bruker, Germany) using Cu Kα radiation (λ = 1.54056 Å). The morphology of Ag/AgCl microcubes was observed using a field-emission scanning electron microscope (FESEM, Nova NanoSEM 450, FEI, USA). The compositions of the products were investigated using an energy-dispersive X-ray spectrometer (EDS) attached to the FESEM instrument. UV-Vis absorption spectra were recorded using a Cary 5000 UV-Vis spectrophotometer. X-ray photoelectronic spectroscopy (XPS) tests were carried out on an X-ray photoelectron spectrometer (XPS, AXIS ULTRA) and the binding energy of the spectrum was corrected by the reference of the C 1s peak (284.6 eV).2.4 Photodegradation experiments
The photocatalytic performance of the as-synthesized Ag/AgCl hollow microcubes was evaluated by the phenol degradation experiments because phenol is a colorless, non-volatile and acute toxic organic pollutant in industrial waste water.28,29 160 mg photocatalysts were suspended in phenol (120 mL, 10 mg L−1) aqueous solution. To achieve the absorption equilibrium, the obtained suspension was stirred for 30 min in the dark before irradiation. The light source was a solar simulator (xenon lamp 500 W) equipped with a cutoff filter (400 nm). During the process of photocatalytic reaction the suspensions (2.0 mL) were taken out at specific irradiation intervals, with removal of the photocatalyst by centrifugation. A Varian high-performance liquid chromatography (HPLC) system was equipped with an ultraviolet detector and it was adjusted at 269 nm to analyze the concentration of phenol. The mobile phase in a C18-reverse phase column was acetonitrile (70%)–water (29.5%)–phosphoric acid (0.5%) and the flow rate was 0.4 mL min−1.3. Results and discussion
3.1 Morphology and structure characterization
Fig. 1a shows that Ag hollow microcubes assembled by nanosheets were successfully prepared. The Ag microcubes were uniform with an average length of about 1.3 μm, and the microcubes presented a relatively rough surface and an internal hollow structure (Fig. 1b and c). Uniform Ag hollow microcubes were selected as the chemical template and reacted with FeCl3. After reaction, the morphologies of the obtained samples were characterized by FESEM. In Fig. 1d, the morphology of the Ag/AgCl product still maintained a uniform microcube structure and had a similar size to the Ag microcube template, which suggested that AgCl nanoparticles were instantly formed on the Ag microcubes. Even though Ag/AgCl microcubes maintained the hollow structure, the surface of Ag/AgCl microcubes changed from nanosheets to nanoparticles with an average size of tens of nanometers because the polycrystalline nanosheets on the surface of original Ag hollow microcubes were composed of Ag nanoparticles.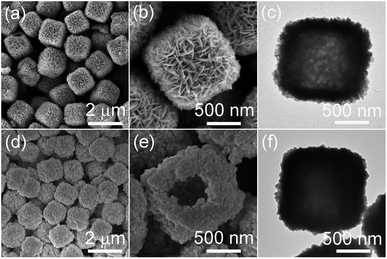 | ||
| Fig. 1 Typical FESEM (a, b, d and e) and TEM (c and f) images of hollow Ag microcubes (a–c) and Ag/AgCl microcubes (d–f). | ||
The XRD profiles of Ag and Ag/AgCl hollow microcubes with various component ratios are shown in Fig. 2. All the peaks of Ag hollow microcubes revealed that Ag was a cubic structure (JCPDS no. 04-0783). From XRD data, it could be seen that as FeCl3 content increased, the XRD peak intensity of Ag slightly decreased until it completely vanished, and that of cubic-structure AgCl (JCPDS no. 31-1238) increased correspondingly. This indicated that Ag/AgCl hollow microcubes with various component proportions can be synthesized by adjusting the quantity of FeCl3.
 | ||
Fig. 2 XRD patterns of Ag and Ag/AgCl microcubes with various compositions (Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AgCl, (a) 3 AgCl, (a) 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (b) 1 1, (b) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (c) 1 1, (c) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and (d) 0 3, and (d) 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). | ||
To investigate the influence of FeCl3 concentration on the morphology of Ag/AgCl hollow microcubes, Ag/AgCl hollow microcubes with different compositions were further studied by FESEM. As shown in Fig. 3, with the increasing ratio of FeCl3 to Ag, the particles on the surface of Ag/AgCl microcubes become denser gradually. The mean size of Ag/AgCl hollow microcubes was about 1.3 μm, indicating that the obtained Ag/AgCl products copied the original hollow Ag microcube template. Though the magnified images show that the final products consisted of nanoparticles, the packing density was significantly different. In Fig. 3e–h, when the ratio of Ag to AgCl was higher than 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the surface of Ag/AgCl microcubes with a large number of pores was composed of loose nanoparticles, but when the ratio of Ag to AgCl was lower than 1
1, the surface of Ag/AgCl microcubes with a large number of pores was composed of loose nanoparticles, but when the ratio of Ag to AgCl was lower than 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, almost no pores could be observed.
3, almost no pores could be observed.
3.2 The composition of hollow Ag/AgCl microcubes
The composition and surface chemical state of Ag/AgCl hollow microcubes can be investigated by EDS and XPS. In Fig. 4, EDS of the Ag/AgCl hollow microcubes exhibited strong peaks of Ag, Cl, and O. The ratios of Ag to AgCl are about 2.24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.8
1, 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.94 and 0 in EDS spectra corresponding to Ag/AgCl microcubes with various compositions (Ag
2.94 and 0 in EDS spectra corresponding to Ag/AgCl microcubes with various compositions (Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AgCl, (a) 3
AgCl, (a) 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (b) 1
1, (b) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (c) 1
1, (c) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and (d) 0
3, and (d) 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). This variation in readings of actual content and observed amount is because the EDS technique takes into account a particular point instead of the whole catalyst surface for analysis, and thus, the metal concentration or percentage may be less than expected.26 In these spectra, when the amount of FeCl3 increases, the ratio of Cl to Ag equally increases, which indicates that more AgCl was generated in the obtained products. This result was consistent with that of XRD mentioned above. XPS spectra of Ag/AgCl hollow microcubes with various compositions are shown in Fig. 5. All binding energies were corrected by the reference value of C 1s (284.6 eV). In Fig. 5, the spectrum of Ag 3d with two peaks (367.0 and 373.0 eV) corresponded separately to the binding energies of Ag 3d5/2 and Ag 3d3/2.30,31 In Fig. 5a, the peaks of Ag 3d deconvolution were respectively 373.5, 374.1, 367.5 and 368.4 eV. The peaks at 374.1 and 368.4 eV were attributed to the peak of metal Ag0, while the bands at 373.5 and 367.5 eV could be attributed to the peak of Ag+.32 In Fig. 5a–c, there were Ag and AgCl in the Ag/AgCl products, but in Fig. 5d, no peak of metal Ag0 was observed, which indicated that Ag has been totally transformed to AgCl. According to the area of fitting peaks, the molar ratios of Ag0 to Ag+ (3
1). This variation in readings of actual content and observed amount is because the EDS technique takes into account a particular point instead of the whole catalyst surface for analysis, and thus, the metal concentration or percentage may be less than expected.26 In these spectra, when the amount of FeCl3 increases, the ratio of Cl to Ag equally increases, which indicates that more AgCl was generated in the obtained products. This result was consistent with that of XRD mentioned above. XPS spectra of Ag/AgCl hollow microcubes with various compositions are shown in Fig. 5. All binding energies were corrected by the reference value of C 1s (284.6 eV). In Fig. 5, the spectrum of Ag 3d with two peaks (367.0 and 373.0 eV) corresponded separately to the binding energies of Ag 3d5/2 and Ag 3d3/2.30,31 In Fig. 5a, the peaks of Ag 3d deconvolution were respectively 373.5, 374.1, 367.5 and 368.4 eV. The peaks at 374.1 and 368.4 eV were attributed to the peak of metal Ag0, while the bands at 373.5 and 367.5 eV could be attributed to the peak of Ag+.32 In Fig. 5a–c, there were Ag and AgCl in the Ag/AgCl products, but in Fig. 5d, no peak of metal Ag0 was observed, which indicated that Ag has been totally transformed to AgCl. According to the area of fitting peaks, the molar ratios of Ag0 to Ag+ (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 0) could be calculated. With the increase of FeCl3, the ratio of Ag0 to AgCl decreased, clearly indicating that Ag0 was oxidized to Ag+. The Cl 2p spectrum in Fig. 5 corresponded to the binding energy of Cl 2p1/2 and Cl 2p3/2, respectively. Furthermore, EDS and XPS results suggested that Ag/AgCl hollow microcubes with controllable components could be obtained by this method.
3 and 0) could be calculated. With the increase of FeCl3, the ratio of Ag0 to AgCl decreased, clearly indicating that Ag0 was oxidized to Ag+. The Cl 2p spectrum in Fig. 5 corresponded to the binding energy of Cl 2p1/2 and Cl 2p3/2, respectively. Furthermore, EDS and XPS results suggested that Ag/AgCl hollow microcubes with controllable components could be obtained by this method.
 | ||
Fig. 4 EDS spectra of Ag/AgCl microcubes with various compositions (Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AgCl, (a) 3 AgCl, (a) 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (b) 1 1, (b) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (c) 1 1, (c) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and (d) 0 3, and (d) 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). | ||
 | ||
Fig. 5 XPS spectra of Ag/AgCl microcubes with various compositions (Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AgCl, (a) 3 AgCl, (a) 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (b) 1 1, (b) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (c) 1 1, (c) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and (d) 0 3, and (d) 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). | ||
3.3 Photocatalytic activity and mechanism
In addition, the UV-Vis absorption spectra of the products with different component ratios are shown in Fig. 6. In the spectra Fig. 6a–c, there was a wide absorption peak in the visible range of 400–700 nm owing to the several reflections inside the interior hollow structure, the increase of the average photon path length, and the surface plasmon resonance effect of Ag.33 In Fig. 6d, the AgCl sample can absorb solar energy with wavelengths shorter than 400 nm and no absorption peak was observed in the visible range.12 | ||
Fig. 6 UV-Vis absorption spectra of Ag/AgCl microcubes with various compositions (Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AgCl, (a) 3 AgCl, (a) 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (b) 1 1, (b) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (c) 1 1, (c) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and (d) 0 3, and (d) 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). | ||
To determine the photocatalytic performance of the synthesized Ag/AgCl hollow microcubes, we carried out photocatalytic phenol degradation experiments under visible light with the obtained products having different component ratios. For comparison, N-TiO2 and P25-TiO2/Ag were used as photocatalysts and their relative information is provided in Fig. S1.† Fig. 7 displays the time-dependent change of the ratio of phenol concentration to initial concentration measured according to the photocatalytic process. It could be seen from Fig. 7 that the photocatalytic activity of Ag/AgCl was the highest with the ratio of Ag to AgCl (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), and more than 91.6% of phenol could be degraded in 150 min. Fig. 7b can be characterized as the first-order rate constant diagram of phenol degradation by various products under visible light, and the illustration showed the degradation rate histogram. It can be seen from the figure that the first-order rate constants are 0.0005, 0.0025, 0.0089, 0.0072, 0.0107, 0.0162 and 0.0037 min−1, corresponding to the blank experiment (without a catalyst), N-TiO2, Ag/P25-TiO2, Ag/AgCl with various compositions (the ratio of Ag to AgCl: 3
3), and more than 91.6% of phenol could be degraded in 150 min. Fig. 7b can be characterized as the first-order rate constant diagram of phenol degradation by various products under visible light, and the illustration showed the degradation rate histogram. It can be seen from the figure that the first-order rate constants are 0.0005, 0.0025, 0.0089, 0.0072, 0.0107, 0.0162 and 0.0037 min−1, corresponding to the blank experiment (without a catalyst), N-TiO2, Ag/P25-TiO2, Ag/AgCl with various compositions (the ratio of Ag to AgCl: 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 1
1, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) and pure AgCl, respectively. The maximum rate constant of AgCl/Ag (the ratio of Ag to AgCl = 1
3) and pure AgCl, respectively. The maximum rate constant of AgCl/Ag (the ratio of Ag to AgCl = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) was about 6.48-fold and 1.82-fold higher than that of N-TiO2 and Ag/P25-TiO2 nanoparticles.
3) was about 6.48-fold and 1.82-fold higher than that of N-TiO2 and Ag/P25-TiO2 nanoparticles.
For the good application of a photocatalyst, its stability is as important as its photocatalytic activity. Therefore, the stability of the photocatalyst with the Ag/AgCl ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 was studied by cyclic experiments. As shown in Fig. 7d, even after four cycling runs of phenol photodegradation, the photocatalytic activity has no obvious change, which indicates that the prepared Ag/AgCl hollow microcube photocatalyst has good photocatalytic stability.
3 was studied by cyclic experiments. As shown in Fig. 7d, even after four cycling runs of phenol photodegradation, the photocatalytic activity has no obvious change, which indicates that the prepared Ag/AgCl hollow microcube photocatalyst has good photocatalytic stability.
The photocatalysis mechanism of Ag/AgCl hollow microcubes was disclosed by active species trapping measurements. In this work, isopropyl alcohol (IPA), benzoquinone (BQ) and triethanolamine (TEOA) were applied to scavenge ˙OH, ˙O2− and h+, respectively.34,35 It was found that no obvious decrease of photocatalytic performance was observed when IPA was added, indicating that ˙OH could not change the photocatalytic process. Nevertheless, the degradation efficiencies decreased greatly with the existence of benzoquinone (BQ) and triethanolamine (TEOA), confirming that the main active species were ˙O2− and h+ and played an important role in the process of photocatalysis.
According to the above active species trapping measurement results, a possible photocatalytic mechanism of Ag/AgCl was proposed and is shown in Fig. 8. It is reported that the band gap of AgCl is about 3.25 eV.36,37 Based on UV-Vis reflectance spectrometry in Fig. 6, the band gap of AgCl can be determined by the following equation:38
 | ||
| Fig. 8 The proposed schematic diagram of the Ag/AgCl composite for the photocatalytic degradation of phenol under visible light irradiation. | ||
The mechanism proposed could also be used to explain the dependence of photocatalytic performance on the ratio of Ag to AgCl in our experiment of photocatalytic degradation of phenol. According to the mechanism, photocatalytic performance of Ag/AgCl hollow microcubes was related to the hot electron concentration of Ag and the separation efficiency of photoinduced carriers was dependent on the amount of Ag nanoparticles in Ag/AgCl and on the nanoscale spatial distribution of supported Ag nanoparticles.21,22 Based on the experimental results of photocatalytic degradation of phenol, the as-obtained Ag/AgCl hollow microcubes (the ratio of Ag to AgCl = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) showed the highest photocatalytic capability under visible-light illumination. The reason might be ascribed to two aspects including the content of Ag and the surface porous structure of Ag/AgCl hollow microcubes in our experiments. Ag/AgCl hollow microcubes (the ratio of Ag to AgCl = 3
3) showed the highest photocatalytic capability under visible-light illumination. The reason might be ascribed to two aspects including the content of Ag and the surface porous structure of Ag/AgCl hollow microcubes in our experiments. Ag/AgCl hollow microcubes (the ratio of Ag to AgCl = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) not only have the most visible light absorbance but also more number of pores on the surface which results in higher adsorption capability for phenol molecules than all other Ag/AgCl. However, it still exhibited lower photocatalytic performance than the Ag/AgCl hollow microcubes (the ratio of Ag to AgCl = 1
1) not only have the most visible light absorbance but also more number of pores on the surface which results in higher adsorption capability for phenol molecules than all other Ag/AgCl. However, it still exhibited lower photocatalytic performance than the Ag/AgCl hollow microcubes (the ratio of Ag to AgCl = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), which may be related to the content of Ag in the Ag/AgCl composite microcubes, since photocatalytic performance was also strongly influenced by the content of Ag in previously reported composite catalysts.33,41 For the as-obtained Ag/AgCl composite microcubes, Ag nanoparticles would produce photogenerated hot electrons and holes under visible light illumination, which can be transferred to the conduction band of AgCl.9,12 Consequently, the photocatalytic performance of the AgCl/Ag samples depends intimately on the interaction between AgCl and metallic Ag. It was reported that the interface between the AgCl and Ag would be enlarged with the higher Ag content in the Ag/AgCl composite, thereby increasing hole capture by the negative surface charge on the Ag nanoparticles, which may reduce the efficiency of charge separation.42 In addition, high proportion of Ag in Ag/AgCl hollow microcubes enhances electron capture by neighboring Ag nanoparticles and reduces the photocatalytic activity.21 In a word, the proper content of Ag and surface structure of Ag/AgCl microcubes in our experiments made the Ag/AgCl (the ratio of Ag to AgCl = 1
3), which may be related to the content of Ag in the Ag/AgCl composite microcubes, since photocatalytic performance was also strongly influenced by the content of Ag in previously reported composite catalysts.33,41 For the as-obtained Ag/AgCl composite microcubes, Ag nanoparticles would produce photogenerated hot electrons and holes under visible light illumination, which can be transferred to the conduction band of AgCl.9,12 Consequently, the photocatalytic performance of the AgCl/Ag samples depends intimately on the interaction between AgCl and metallic Ag. It was reported that the interface between the AgCl and Ag would be enlarged with the higher Ag content in the Ag/AgCl composite, thereby increasing hole capture by the negative surface charge on the Ag nanoparticles, which may reduce the efficiency of charge separation.42 In addition, high proportion of Ag in Ag/AgCl hollow microcubes enhances electron capture by neighboring Ag nanoparticles and reduces the photocatalytic activity.21 In a word, the proper content of Ag and surface structure of Ag/AgCl microcubes in our experiments made the Ag/AgCl (the ratio of Ag to AgCl = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) show the highest photocatalytic activities.
3) show the highest photocatalytic activities.
4. Conclusions
In summary, Ag/AgCl hollow microcube photocatalysts with different components and a number of tiny Ag/AgCl heterojunctions were successfully synthesized by the oxidation of a nanosheet-assembled Ag hollow microcube template. By adjusting the quantity of the oxidizing agent FeCl3, the microcubes with various compositions were obtained. The Ag/AgCl hollow microcubes with adjustable compositions could not only result in multiple light reflections in the cavity with an improvement in the visible light absorption ability, but also enhance the separation efficiency of photogenerated charges by regulating the heterojunction interface of Ag/AgCl. Ag/AgCl hollow microcubes with a ratio of Ag to AgCl of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 had the best photocatalytic performance for the degradation of 91.6% of phenol in 150 min, which was mainly ascribed to the efficient light-harvesting for the generation of photoinduced carriers in Ag/AgCl and effective charge separation at the interfaces between Ag and AgCl. This study provides a new possibility for the rational design of other highly efficient composite photocatalysts through structural and component regulation.
3 had the best photocatalytic performance for the degradation of 91.6% of phenol in 150 min, which was mainly ascribed to the efficient light-harvesting for the generation of photoinduced carriers in Ag/AgCl and effective charge separation at the interfaces between Ag and AgCl. This study provides a new possibility for the rational design of other highly efficient composite photocatalysts through structural and component regulation.
Author contributions
Shiyun Lou: conceptualization, investigation, and writing original draft. Qinglan Chen: investigation, formal analysis, and validation. Wan Wang: investigation and data curation. Yongqiang Wang: investigation. Shaomin Zhou: conceptualization, supervision, and project administration.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 51372070 and No. 21371049), Fundamental and Frontier Research Project of Henan Province, China (No. 162300410040), the Key Project of Education Department of Henan Province, China (No. 14B430010), and the Key Scientific and Technological Research Project of Henan Province, China (202102310598).References
- X. Y. Zhang, Y. N. Chen, Q. K. Shang and Y. N. Guo, Copper doping and organic sensitization enhance photocatalytic activity of titanium dioxide: Efficient degradation of phenol and tetrabromobisphenol A, Sci. Total Environ., 2020, 716, 137144 CrossRef CAS PubMed.
- T. Ahmad, J. Iqbal, M. A. Bustam, M. Zulfiqar, N. Muhammad, B. M. Al Hajeri, M. Irfan, H. M. A. Asghar and S. Ullah, Phytosynthesis of cerium oxide nanoparticles and investigation of their photocatalytic potential for degradation of phenol under visible light, J. Mol. Struct., 2020, 1217, 128292 CrossRef CAS.
- M. C. N. Martinez, B. Bajorowicz, T. Klimczuk, A. Zak, J. Luczak, W. Lisowski and A. Zaleska-Medynska, Synergy between AgInS2 quantum dots and ZnO nanopyramids for photocatalytic hydrogen evolution and phenol degradation, J. Hazard. Mater., 2020, 398, 123250 CrossRef PubMed.
- S. W. Li, P. Miao, Y. Y. Zhang, J. Wu, B. Zhang, Y. C. Du, X. J. Han, J. M. Sun and P. Xu, Recent advances in plasmonic nanostructures for enhanced photocatalysis and electrocatalysis, Adv. Mater., 2020, 33(6), 2000086 CrossRef PubMed.
- Z. Z. Lou, S. Kim, M. Fujitsuka, X. G. Yang, B. J. Li and T. Majima, Anisotropic Ag2S-Au triangular nanoprisms with desired configuration for plasmonic photocatalytic hydrogen generation in visible/near-infrared region, Adv. Funct. Mater., 2018, 28(13), 1706969 CrossRef.
- N. Q. Wu, Plasmonic metal-semiconductor photocatalysts and photoelectrochemical cells: a review, Nanoscale, 2018, 10(6), 2679–2696 RSC.
- M. Norouzi, A. Fazeli and O. Tavakoli, Phenol contaminated water treatment by photocatalytic degradation on electrospun Ag/TiO2 nanofibers: Optimization by the response surface method, J. Water Process. Eng., 2020, 37, 101489 CrossRef.
- A. Shet and K. V. Shetty, Solar light mediated photocatalytic degradation of phenol using Ag core-TiO2 shell (Ag@TiO2) nanoparticles in batch and fluidized bed reactor, Sol. Energy, 2016, 127, 67–78 CrossRef CAS.
- C. H. An, S. Peng and Y. G. Sun, Facile synthesis of sunlight-driven AgCl:Ag plasmonic nanophotocatalyst, Adv. Mater., 2010, 22(23), 2570–2574 CrossRef CAS PubMed.
- Y. P. Bi and J. H. Ye, In situ oxidation synthesis of Ag/AgCl core-shell nanowires and their photocatalytic properties, Chem. Commun., 2009, 43, 6551–6553 RSC.
- Y. X. Tang, Z. L. Jiang, G. C. Xing, A. R. Li, P. D. Kanhere, Y. Y. Zhang, T. C. Sum, S. Z. Li, X. D. Chen, Z. L. Dong and Z. Chen, Efficient Ag@AgCl cubic cage photocatalysts profit from ultrafast plasmon-induced electron transfer processes, Adv. Funct. Mater., 2013, 23(23), 2932–2940 CrossRef CAS.
- P. Wang, B. B. Huang, X. Y. Qin, X. Y. Zhang, Y. Dai, J. Y. Wei and M. H. Whangbo, Ag@AgCl: A highly efficient and stable photocatalyst active under visible light, Angew. Chem., Int. Ed., 2008, 47(41), 7931–7933 CrossRef CAS PubMed.
- B. Ma, J. Guo, W. Da and K. Fan, Highly stable and efficient Ag/AgCl core–shell sphere: Controllable synthesis, characterization, and photocatalytic application, Appl. Catal., B, 2013, 130, 257–263 CrossRef.
- M. J. Guo, Z. P. Xing, T. Y. Zhao, Y. L. Qiu, B. Tao, Z. Z. Li and W. Zhou, Hollow flower-like polyhedral alpha-Fe2O3/Defective MoS2/Ag Z-scheme heterojunctions with enhanced photocatalytic-fenton performance via surface plasmon resonance and photothermal effects, Appl. Catal., B, 2010, 272, 118978 CrossRef.
- J. Richard-Daniel and D. Boudreau, Enhancing galvanic replacement in plasmonic hollow nanoparticles: Understanding the role of the speciation of metal ion precursors, ChemNanoMat, 2020, 6(6), 907–915 CrossRef CAS.
- A. Genc, J. Patarroyo, J. Sancho-Parramon, N. G. Bastus, V. Puntes and J. Arbiol, Hollow metal nanostructures for enhanced plasmonics: synthesis, local plasmonic properties and applications, Nanophotonics, 2017, 6(1), 193–213 CAS.
- M. Nazemi and M. A. El-Sayed, Plasmon-enhanced photo(electro)chemical nitrogen fixation under ambient conditions using visible light responsive hybrid hollow Au-Ag2O nanocages, Nano Energy, 2019, 63, 103886 CrossRef CAS.
- J. Feng and Y. D. Yin, Self-templating approaches to hollow nanostructures, Adv. Mater., 2019, 31(38), 1802349 CrossRef PubMed.
- P. Wang, B. B. Huang, Z. Z. Lou, X. Y. Zhang, X. Y. Qin, Y. Dai, Z. K. Zheng and X. N. Wang, Synthesis of highly efficient Ag@AgCl plasmonic photocatalysts with various structures, Chem.–Eur. J., 2009, 16(2), 538–544 CrossRef PubMed.
- S. K. Wu, X. P. Shen, Z. Y. Ji, G. X. Zhu, C. J. Chen, K. M. Chen, R. Bu and L. M. Yang, Synthesis of AgCl hollow cubes and their application in photocatalytic degradation of organic pollutants, CrystEngComm, 2015, 17(12), 2517–2522 RSC.
- T. Yoshida, Y. Misu, M. Yamamoto, T. Tanabe, J. Kumagai, S. Ogawa and S. Yagi, Effects of the amount of Au nanoparticles on the visible light response of TiO2 photocatalysts, Catal. Today, 2020, 352, 34–38 CrossRef CAS.
- A. Holm, E. D. Goodman, J. H. Stenlid, A. Aitbekova, R. Zelaya, B. T. Diroll, A. C. Johnston-Peck, K.-C. Kao, C. W. Frank, L. G. M. Pettersson and M. Cargnello, Nanoscale spatial distribution of supported nanoparticles controls activity and stability in powder catalysts for CO oxidation and photocatalytic H2 evolution, J. Am. Chem. Soc., 2020, 142(34), 14481–14494 CrossRef CAS PubMed.
- Y. P. Bi and J. H. Ye, Direct conversion of commercial silver foils into high aspect ratio AgBr nanowires with enhanced photocatalytic properties, Chem.–Eur. J., 2010, 16(34), 10327–10331 CrossRef CAS PubMed.
- S. Y. Lou, W. Wang, L. X. Wang and S. M. Zhou, In-situ oxidation synthesis of Cu2O/Ag/AgCl microcubes with enhanced visible-light photocatalytic activity, J. Alloys Compd., 2019, 781, 508–514 CrossRef CAS.
- Y. Q. Wang, T. Gao, K. Wang, X. P. Wu, X. J. Shi, Y. B. Liu, S. Y. Lou and S. M. Zhou, Template-assisted synthesis of uniform nanosheet-assembled silver hollow microcubes, Nanoscale, 2012, 4(22), 7121–7126 RSC.
- M. K. Aulakh, R. Sharma, B. Pal and R. Prakash, Photo-induced oxidation and reduction by plasmonic Ag-TiO2 nanocomposites under UV/sunlight, Sol. Energy, 2020, 196, 427–436 CrossRef.
- A. A. Melvin, K. Illath, T. Das, T. Raja, S. Bhattacharyya and C. S. Gopinath, M-Au/TiO2 (M = Ag, Pd, and Pt) nanophotocatalyst for overall solar water splitting: role of interfaces, Nanoscale, 2015, 7(32), 13477–13488 RSC.
- X. L. Yan, T. Ohno, K. Nishijima, R. Abe and B. Ohtani, Is methylene blue an appropriate substrate for a photocatalytic activity test? A study with visible-light responsive titania, Chem. Phys. Lett., 2006, 429(4–6), 606–610 CrossRef CAS.
- F. Hayati, A. A. Isari, M. Fattahi, B. Anvaripour and S. Jorfi, Photocatalytic decontamination of phenol and petrochemical wastewater through ZnO/TiO2 decorated on reduced graphene oxide nanocomposite: influential operating factors, mechanism, and electrical energy consumption, RSC Adv., 2018, 8(70), 40035 RSC.
- X. J. Wen, C. G. Niu, D. W. Huang, L. Zhang, C. Liang and G. M. Zeng, Study of the photocatalytic degradation pathway of norfloxacin and mineralization activity using a novel ternary Ag/AgCl-CeO2 photocatalyst, J. Catal., 2017, 355, 73–86 CrossRef CAS.
- S. Y. Lou, X. B. Jia, Y. Q. Wang and S. M. Zhou, Template-assisted in situ synthesis of porous AgBr/Ag composite microspheres as highly efficient visible-light photocatalyst, Appl. Catal., B, 2015, 176, 586–593 CrossRef.
- N. N. Wang, K. Cheng, Z. F. Xu, P. Li, G. W. Geng, C. C. Chen, D. J. Wang, P. L. Chen and M. H. Liu, High-performance natural-sunlight-driven Ag/AgCl photocatalysts with a cube-like morphology and blunt edges via a bola-type surfactant-assisted synthesis, Phys. Chem. Chem. Phys., 2020, 22(7), 3940–3952 RSC.
- H. Y. Li, T. S. Wu, B. Cai, W. G. Ma, Y. J. Sun, S. Y. Gan, D. X. Han and L. Niu, Efficiently photocatalytic reduction of carcinogenic contaminant Cr(VI) upon robust AgCl:Ag hollow nanocrystals, Appl. Catal., B, 2015, 164, 344–351 CrossRef CAS.
- S. F. Yang, C. G. Niu, D. W. Huang, H. Zhang, C. Lianga and G. M. Zeng, SrTiO3 nanocubes decorated with Ag/AgCl nanoparticles as photocatalysts with enhanced visible-light photocatalytic activity towards the degradation of dyes, phenol and bisphenol A, Environ. Sci.: Nano, 2017, 4(3), 585–595 RSC.
- Y. X. Yang, W. Guo, Y. N. Guo, Y. H. Zhao, X. Yuan and Y. H. Guo, Fabrication of Z-scheme plasmonic photocatalyst Ag@AgBr/g-C3N4 with enhanced visible-light photocatalytic activity, J. Hazard. Mater., 2014, 271, 150–159 CrossRef CAS PubMed.
- Z. K. Xu, L. Han, P. Hu and S. J. Dong, Facile synthesis of small Ag@AgCl nanoparticles via a vapor diffusion strategy and their highly efficient visible-light-driven photocatalytic performance, Catal. Sci. Technol., 2014, 4(10), 3615–3619 RSC.
- J. Tejeda, N. J. Shevchik, W. Braun, A. Goldmann and M. Cardona, Valence bands of AgCl and AgBr: UV photoemission and theory, Phys. Rev. B, 1975, 12, 1557–1566 CrossRef CAS.
- X. Zhang, L. Z. Zhang, T. F. Xie and D. J. Wang, Low-temperature synthesis and high visible-light-induced photocatalytic activity of BiOI/TiO2 heterostructures, J. Phys. Chem. C, 2009, 113(17), 7371–7378 CrossRef CAS.
- P. K. de Boer and R. A. de Groot, Conduction band of the photographic compound AgCl, J. Phys. Chem. A, 1999, 103, 5113–5115 CrossRef CAS.
- J. He, D. W. Shao, L. C. Zheng, L. J. Zheng, D. Q. Feng, J. P. Xu, X. H. Zhang, W. C. Wang, W. H. Wang, F. Lu, H. Dong, Y. H. Cheng, H. Liu and R. K. Zheng, Construction of Z-scheme Cu2O/Cu/AgBr/Ag photocatalyst with enhanced photocatalytic activity and stability under visible light, Appl. Catal., B, 2017, 203, 917–926 CrossRef CAS.
- M. S. Zhu, P. L. Chen and M. H. Liu, Ag/AgBr/graphene oxide nanocomposite synthesized via oil/water and water/oil microemulsions: A comparison of sunlight energized plasmonic photocatalytic activity, Langmuir, 2012, 28(7), 3385–3390 CrossRef CAS PubMed.
- J. Jiang and L. Zhang, Rapid microwave-assisted nonaqueous synthesis and growth mechanism of AgCl/Ag, and its daylight-driven plasmonic photocatalysis, Chem.–Eur. J., 2011, 17(13), 3710–3717 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra03569j |
| This journal is © The Royal Society of Chemistry 2021 |



