Open Access Article
Open Access ArticleComparison on the immersion corrosion and electrochemical corrosion resistance of WC–Al2O3 composites and WC–Co cemented carbide in NaCl solution
Bin Hana,
Weiwei Donga,
Bowen Fana and
Shigen Zhu *ab
*ab
aCollege of Mechanical Engineering, Donghua University, Shanghai, 201620, PR China. E-mail: sgzhu@dhu.edu.cn
bEngineering Research Center of Advanced Textile Machinery of the Ministry of Education, Shanghai, 201620, PR China
First published on 25th June 2021
Abstract
WC–15 wt% Al2O3 composites were prepared via hot pressing sintering technology. The corrosion behaviors of WC–Al2O3 composites and traditional WC–Co cemented carbide in NaCl solution were studied by immersion corrosion and electrochemical technique. The impedance value of the WC–Al2O3 composite increased more rapidly than WC–Co cemented carbide during the 24 hours, which indicated that WC–Al2O3 composites had a more compact passivation film than WC–Co cemented carbide. The results confirmed that the corrosion resistance of WC–Al2O3 composites was higher than that of WC–Co cemented carbide in NaCl solution. The corrosion mechanisms of WC–Al2O3 composites and WC–Co cemented carbide in NaCl solution were also revealed by SEM, EDS, XPS and Raman. The corrosion products of WC–Al2O3 composites mainly contain WO3, while for WC–Co cemented carbide they are Co(OH)2, Co3O4 and WO3. The different corrosion mechanism of the two materials is attributed to the Al2O3 phase instead of the Co binder, which avoids the galvanic corrosion between the WC phase and the Co binder.
1. Introduction
Conventional WC-based hardmetals consist of a WC phase and a metal binder (Fe, Co, Ni, etc.), which could lead to high hardness and fracture toughness of hardmetals.1–6 Among the conventional WC-based hardmetals, because the metal binder is chemically active and susceptible to corrosion, the development of binderless WC-based hardmetals has become an inevitable demand. Binderless WC-based hardmetals refer to the hard material without or with a small amount of metal binder (<0.5%, mass fraction), which is a new hard material in recent years. Binderless WC-based hardmetals can be used as special sealing materials (such as sealing materials for cooling water pumps in nuclear power stations, sealing materials for petroleum and chemical equipment in acidic and alkaline corrosion environments, etc.), high-pressure jet nozzles, fluid mixers, drilling part and valves.7–9 Our group has previously developed the WC–Al2O3 composite as a new type of binderless WC-based hardmetals and carried out a series of studies. The research content includes: the ratio of WC and Al2O3 composition,10 crystal type and morphology of Al2O3,11 sintering process parameters and two-stage hot pressing sintering process,12,13 rare earth materials,14,15 grain growth inhibitor.16,17 Through these studies, WC–Al2O3 composite with excellent comprehensive mechanical properties have been prepared, which has attracted worldwide attention.The corrosion resistance of materials used in corrosive environment is a very important technical index, which is usually studied by electrochemical method. Electrochemical corrosion resistance of WC-based hardmetals depends on several factors, which includes the binder composition,18,19 WC grain size,20–22 binder content23 and additives.24,25 Human et al.23 confirmed that WC–Co cemented carbide possessed the higher anti-corrosion ability with the decrease of Co content. However, in his other research, it was concluded that the binder phase content had no influence on the corrosion resistance of hardmetals.26 Studies on the corrosion behaviors of binderless hard metals have been rarely reported in domestic and foreign literatures, mainly involving WC–TiC–TaC composite, WC–MgO composite.27,28 Ma Y. et al.27 studied the corrosion resistance of WC–TiC–TaC composite in NaCl solution, and believed that TiC and TaC could form solid solution with WC, which increased the resistivity and resistance of binderless WC-based hard composite and enhanced the corrosion resistance of the composites. Ouyang Chenxin et al.28 studied the corrosion resistance of WC–MgO composite by immersion corrosion and electrochemical methods in alkaline (pH = 13) and acidic (pH = 1) environments, and believed that the corrosion resistance of WC–MgO composites in acidic environment was better than in alkaline environment. Passivation occurred in acidic environment, while no passivation occurred in alkaline environment.
In this work, WC–Al2O3 composites was obtained via hot pressing sintering technology. However, the corrosion resistance of WC–Al2O3 composites in NaCl solution has not been reported. Therefore, the immersion corrosion and electrochemical corrosion resistance of WC–Al2O3 composite in NaCl solution compared to WC–Co hardmetal were researched.
2. Experimental procedure
2.1 Sample preparation
WC–Al2O3 composite used in this work was fabricated by our group. Table 1 presented the compositions of WC-based composites. Raw materials of WC, Al2O3 (75 μm, 150 μm, respectively) and WC–6Co hardmetal were used in this work. The preparation process of WC–Al2O3 composite have been reported in our previous research by Zhu et al.10 All the samples used for electrochemical corrosion tests were cut into squares from the hot-pressing sintered samples. The exposed area of the electrochemical corrosion samples is 1 cm2.| Composition (wt%) | |||
|---|---|---|---|
| WC | Co | Al2O3 | |
| WC–6Co | 94 | 6 | — |
| WC–15Al2O3 | 85 | — | 15 |
2.2 Immersion corrosion test
According to ASTM standard G31-2004, the specimens were immersed in NaCl solution at 25 °C for obtaining the corrosion rate and macro corrosion morphologies of WC–Co hardmetal and WC–Al2O3 composite, respectively. The mass loss after immersion corrosion each stage (7 days, 14 days, 21 days and 28 days, respectively) was weighed for calculating the corrosion rates. Then the optical images of corroded surface after 7 days were observed by digital camera.2.3 Electrochemical measurements
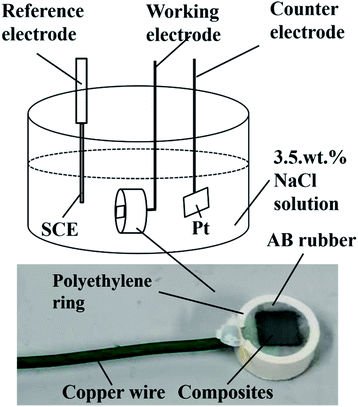
Electrochemical corrosion tests were conducted in NaCl solution at 25 °C and open to air. The electrolyte solution was prepared from sodium chloride crystals and distilled water. Tafel curves, EIS and OCP were measured by electrochemical workstation (CS310H, Wuhan Corrtest Instruments Corp., Ltd). The schematic diagram of the electrochemical three-electrode system is shown in Fig. 1. Saturated calomel electrode was used as the reference electrode. WC matrix composite was used as working electrode. Platinum sheet was used as counter electrode. The area of the electrodes is 1 cm2. The open circuit potential curves were monitored for about 12 h in NaCl solution. Subsequently, the EIS test was conducted on the position of OCP value. The test frequency ranged from 100 kHZ to 1 mHZ. The potentiodynamic polarization was measured in the potential range from −1000 mV vs. SCE to +1500 mV vs. SCE. Refer to the experimental parameters of Konadu et al.38 The scanning rate for all the samples was 2 mV s−1. This scanning rate is typical of those used in literature even though higher scan rates of 5 mV s−1 (ref. 29) has been reported. The scan rate used is not important since the scan rate does not affect the qualitative results.302.4 Microstructural and composition characterization
Prior to microstructural characterization, the specimens were ground with diamond discs from 600 grit to 4000 grit and polished with 1 μm diamond pastes. Then the sample surface was degreased in ethanol by using ultrasonic cleaner. The microstructure and chemical composition of the specimens were observed by SEM (Quanta 250, FEI Co. Ltd., USA) and EDS (AZtec X-Max 20, Oxford Co. Ltd., UK). The phase composition of the specimens was characterized by XRD (D/max-2550PC, Rigaku Co. Ltd., Japan) and Raman spectroscopy (inVia-Reflex, Renishaw Co. Ltd., UK). The composition of the corrosion products of the composites after corrosion test was determined by XPS (Escalab 250Xi, China). The binding energy of C 1s standard carbon was used as the reference peak. XPS spectrum is fitted by XPS peak software.3. Results and discussion
3.1 Microstructure characterization before corrosion tests
The XRD pattern of WC–Co hardmetal and WC–Al2O3 composite are shown in Fig. 2. The local magnified XRD pattern of Fig. 2(a) was faintly observed in Fig. 2(b). In both materials of WC–Co and WC–Al2O3, WC is the main peak. The weak Al2O3 and Co peaks also can be observed, respectively. WC–Al2O3 composite has no phase transition after hot pressing sintering. It can be seen that WC peak is higher intense than Al2O3 and Co peak due to the low amount of Al2O3 and Co.BSE-SEM images of the polished samples are presented in Fig. 3. The uniform microstructure was observed in the sample. As shown in Fig. 3(a), WC–Al2O3 composite is almost completely compact, which indicates that densification has occurred in the sample during the sintering process. WA15 composite had the double phase microstructure. Dark regions are dispersed Al2O3 particles and the surrounding bright regions are WC particles. The microstructure, mechanical performance and frictional wear properties of WC–Al2O3 composite have been reported in our pervious study by Qu et al.11 As presented in Fig. 3(b), Co binder is visible in the WC–Co hardmetal, which uniformly surrounds the WC particles.
3.2 Corrosion behavior after immersion corrosion test
Fig. 4(a) and (b) illustrates the optical morphologies of WC–Co hardmetal and WC–Al2O3 composite after immersion tests for 7 days in NaCl solution, respectively. As presented in Fig. 4(a), obvious corrosion products appeared on the surface of WC–Co hardmetal. However, as presented in Fig. 4(b), no corrosion layer was visible on the corroded surface of WC–Al2O3 composite. Fig. 4(c) shows the mass loss curve of the samples after immersion corrosion test for 7, 14, 21 and 28 days, respectively. It could be found that the dissolution rate increased obviously with immersion time in the sample of WC–Co hardmetal. However, there were no significant differences in WC–Al2O3 composite at any time point. Hence, it may be speculated that the corrosion resistance of WC–Al2O3 composite in NaCl solution is higher than WC–Co hardmetal. Human et al.23 have reported the corrosion mechanism of WC–Co hardmetal in NaCl solution, while for WC–Al2O3 composite was not yet clear.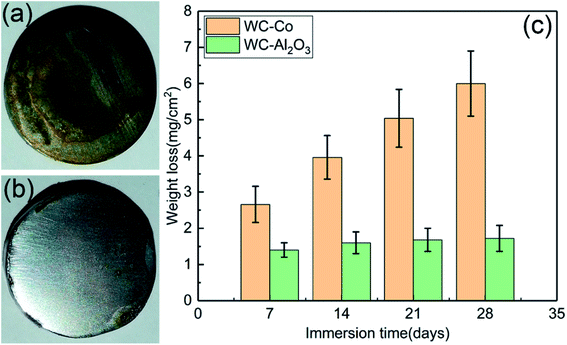 | ||
| Fig. 4 Optical images (a and b), and mass loss (c) of WC–Co and WC–Al2O3 specimens after immersion corrosion test in NaCl solution. | ||
3.3 Electrochemical response
Table 2 presents the corrosion parameters from the OCP and Tafel curves. There is a difference between the value of the open circuit potential and the corrosion potential. The open circuit potential is the electrode potential when no voltage is applied, and the corrosion potential is the potential obtained during the potential scanning process. During the scanning process, the electrode surface is corroded, so there is a difference between the open circuit potential and the corrosion potential. The significant corrosion parameters were obtained according to ASTM standard G5-94. The icorr and Ecorr were determined by Tafel extrapolation. The Ecorr value reflected the thermodynamic characteristic of the composites.34 Martin et al.35 reported that the higher corrosion potential implies better chemical stability and lower corrosion tendency, and the lower icorr reflects lower corrosion rate. It could be observed that the Ecorr of WC–Al2O3 composite was higher than that of WC–Co hardmetal, implying that WC–Co hardmetal has lower corrosion tendency. Because the icorr values can imply the dynamics characteristic of corrosion process, the icorr value reflects the corrosion rate more accurately than Ecorr value. WC–Al2O3 composite shows the lower icorr value, while WC–Co hardmetal shows the higher value. The variation of icorr value could be explained from the following reasons. The main reason was the formation of dense passivation film on the surface of WC–Al2O3 composite, which could decrease the corrosion rate of the composites. In addition, the ipass of WC–Al2O3 composite is lower than WC–Co hardmetal. In short, it can be concluded that WC–Al2O3 composites had more positive Ecorr value, lower icorr and ipass values, indicating that WC–Al2O3 composites possessed better corrosion resistance in NaCl solution from corrosion thermodynamics and corrosion kinetics.
| Samples | EOCP (vs. SCE, mV) | Ecorr (vs. SCE, mV) | icorr (μA cm−2) | ipass (μA cm−2) |
|---|---|---|---|---|
| WC–6Co | −376.2 | −359.6 | 18.25 | 2020.4 |
| WC–15Al2O3 | −238.8 | −312.1 | 32.56 | 8.55 |
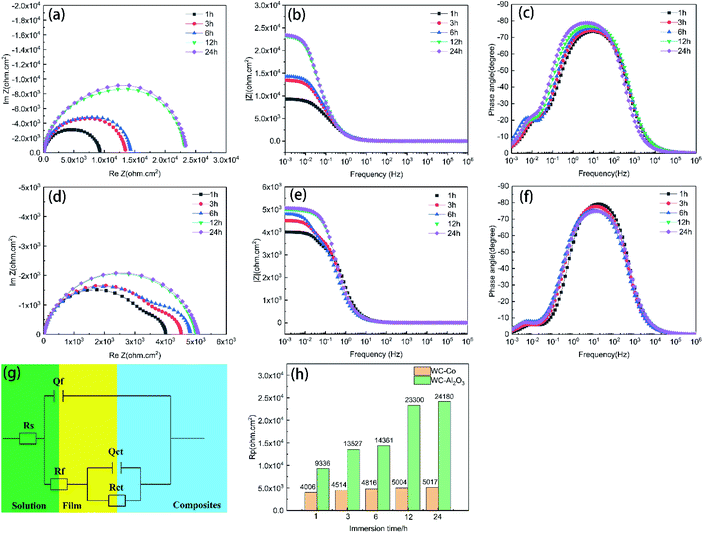
Fig. 7(g) presents the equivalent circuit of WC-based composites. In the circuit, Rct is the resistance of charge transfer; Rs and Rf represent the resistances of corrosion solution and corrosion products, respectively; Qf and Qct are the capacitances of corrosion products and double-charge layer, respectively. The corrosion parameters of WC-based composites during corrosion process were presented in Table 3. The variations in Qf and Rf occur on the composites and reflect the growth of passivation film on the surface of the composites. Compared with WC–Co cemented carbide, the values of decreased Y0 (Qf) of WC–Al2O3 composite in NaCl solution showed more compacted passivation film on the surface of the composites.32 The value of Qct in NaCl solution was higher than the typical of a double layer (1–100 μF cm−2). This phenomenon could be explained by an increase in the number of corrosion products (oxides), which will expand the electrochemical active region, if semiconducting, the double layer could be formed. The most significant corrosion parameter for the composite is the charge transfer resistance (Rct), which is related to Faraday process and it can reflect the corrosion rate of the materials. The higher the Rct, the higher resistance to corrosion. The Rct value of WC–Co cemented carbide in NaCl solution was lower than WC–Al2O3 composites. In addition, the polarization resistance (Rp) is shown in Fig. 7(h). The polarization resistance (Rp) is equal to the sum of the corrosion product films resistance (Rf) and charge transfer resistance (Rct).29,41 The Rp value of WC–Al2O3 composite is about one order of magnitude higher than WC–Co cemented carbide. As the increase of corrosion time, the Rp value of WC–Al2O3 composites increased obviously. However, for WC–Co cemented carbide, Rp value keeps a stable value. Therefore, it could be concluded that compared with WC–Co cemented carbide, WC–Al2O3 composites has higher corrosion resistance. The EIS result is consistent with the polarization curve and the weight-loss result.
| Samples | Time/h | Rs (Ω cm2) | Qf | Rf (Ω cm2) | Qct | Rct (Ω cm2) | Rp (Ω cm2) | ||
|---|---|---|---|---|---|---|---|---|---|
| Y0 (μF cm−2) | n | Y0 (μF cm−2) | n | ||||||
| WC–15Al2O3 | 1 | 7.38 | 133.4 | 0.86 | 7116 | 1299 | 0.91 | 2220 | 9336 |
| 3 | 7.09 | 133.1 | 0.86 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 270 270 |
1239 | 0.99 | 3257 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 527 527 |
|
| 6 | 6.95 | 129.8 | 0.87 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 460 460 |
1174 | 1 | 3901 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 361 361 |
|
| 12 | 6.77 | 127.9 | 0.90 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250 |
799 | 1 | 7050 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
|
| 24 | 6.81 | 118.6 | 0.90 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 851 851 |
608 | 1 | 7329 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 180 180 |
|
| WC–6Co | 1 | 5.29 | 97.2 | 0.93 | 3389 | 6447 | 1 | 617 | 4006 |
| 3 | 5.37 | 127.9 | 0.91 | 3817 | 9211 | 1 | 697 | 4514 | |
| 6 | 5.45 | 140.4 | 0.88 | 3878 | 8177 | 1 | 937 | 4815 | |
| 12 | 5.30 | 141.6 | 0.88 | 4059 | 66.99 | 1 | 945 | 5004 | |
| 24 | 5.30 | 168 | 0.88 | 4139 | 65.82 | 1 | 932 | 5071 | |
3.4 Characterization of the corroded surface
 | ||
| Fig. 9 (a) EDS mapping scanning of WC–Co cemented carbide; (b) element quantitative analysis of WC–Co hard metal after potentiodynamic polarization tests. | ||
 | ||
| Fig. 10 (a) EDS mapping scanning of WC–Al2O3 composite; (b) element quantitative analysis of WC–Al2O3 composite after potentiodynamic polarization tests. | ||
Turning to WC–Al2O3 composite, as presented in Fig. 8(b) and (d), different corrosion morphologies were observed on the surface of WC–Co cemented carbide and WC–Al2O3 composites. As presented in Fig. 8(d), the compacted passive film was formed on the surface of WC–Al2O3 composite. According to the EDS results of WC–Al2O3 composite (Fig. 11), it could be found that the main elements are W and O, which could correspond in EDS mapping. The O content of the original composite is only 15 wt%, but the O content increases after potentiodynamic polarization tests. Hence, it could be speculated that the corrosion products of WC–Al2O3 composites contains WO3.
Fig. 12 and 13 show the XPS results of WC–Co cemented carbide and WC–Al2O3 composites after potentiodynamic polarization tests, respectively. As illustrated in Fig. 12(a), the peaks of W, Co, O and C could be identified in the XPS spectra of WC–Co cemented carbide. The peaks of Co 2p were determined as Co(OH)2 and Co3O4 (Fig. 12(b)), which is in agreement with the results by Li Zhang et al.18,20 The W 4f peaks corresponded to WC and WO3 (Fig. 12(c)), which implies that WC phase was also suffered certain corrosion after Co binder was dissolved. The C 1s peaks were associated with WC (Fig. 12(d)). The peaks of O 1s corresponded to WO3, Co(OH)2 and Co3O4 (Fig. 12(c)). As illustrated in Fig. 13(a), the peaks of W, Al, O and C elements can be detected in the XPS spectra of WC–Al2O3 composite. The peak of Al 2p correspond to Al2O3 (Fig. 13(b)). Based on W 4f and O 1s, the result indicated that the peak was determined as WO3 (Fig. 13(c) and (d)). It could be inferred that the oxidation reaction of WC phased occurred at the anode. Zhang et al.20 reported that the corrosion product of WC–Co in acidic solution is also WO3. The peak of C 1s was determined as WC (Fig. 13(e)). Hence, it could be concluded that the corrosion product of WC–Al2O3 composites is WO3, which is in agreement with EDS and Raman results.
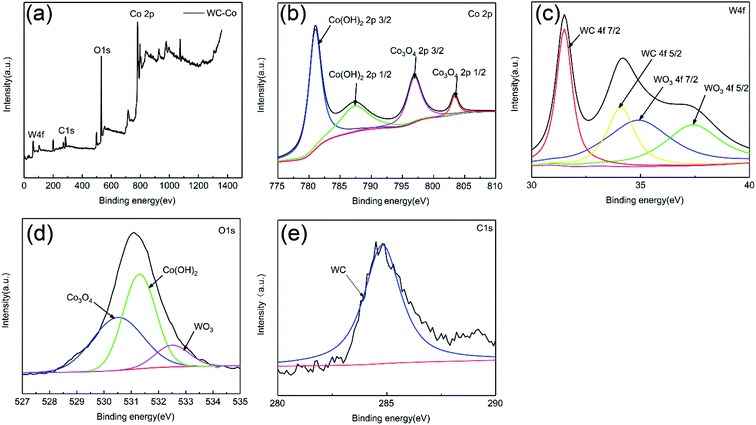 | ||
| Fig. 12 XPS spectra of corroded surface of WC–Co cemented carbide after potentiodynamic polarization tests: (a) wide scan survey spectra, (b) Co 2p, (c) W 4f, (d) O 1s, (e) C 1s. | ||
3.5 Corrosion mechanism
Fig. 14 displays the corrosion current transients of WC–Co cemented carbide and WC–Al2O3 composites in NaCl solution. The applied potentials were −300 and 750 mV vs. SCE in the passivation region for WC–Co and WC–Al2O3 composite, respectively. With the increase of corrosion time, the corrosion current density decreased to a stable value, and the potentiostatic polarization behavior of WC–Al2O3 and WC–Co composite was analyzed. Lekatou et al.41 believed that the current density increased and stabilized to a stable value with immersion time, indicating that the passivation behavior of the material has occurred. As shown in Fig. 14(a) and (b), WC–Co cemented carbide shows the greatest steady current density value with the increasing immersion time, implying that the corrosion behavior of WC–Co cemented carbide is general corrosion corresponding with the dissolution of Co binder. However, as shown in Fig. 14(c) and (d), the current density of WC–Al2O3 composites indicated an obvious fluctuation, which implied that the passivation film of WC–Al2O3 composites was broken in NaCl solution. Hence, it could be inferred that the corrosion behavior of WC–Co cemented carbide in NaCl solution is general corrosion by the dissolution of Co binder, but for WC–Al2O3 composites is general corrosion by the oxidation of WC phase and the dissolution of Al2O3 phase. | ||
| Fig. 14 Current transients of WC–Co (a and c) and WC–Al2O3 (b and d) composites at passivation region in NaCl solution. | ||
Fig. 15 shows the schematic illustrations of the corrosion mechanism for WC–Co cemented carbide and WC–Al2O3 composite, respectively. As presented in Fig. 15(a1), after removing the loose corrosion products, WC skeleton is left, which imply that Co binder has been dissolved. Combined with EDS, XPS and Raman analysis, it can be further confirmed that the corrosion behavior of WC–Co cemented carbide in NaCl solution exhibits general corrosion characteristic. Katiyar et al.31 have reported that Co binder plays cathodic protection role for WC phase, which inhibit the corrosion of WC phase. However, when Co binder was totally dissolved, subsequently WC phase was also oxidized to WO3. Therefore, the corrosion mechanism of WC–Co cemented carbide in NaCl solution is the electrochemical dissolution of Co binder and the chemical oxidation of WC phase.
 | ||
| Fig. 15 Schematic diagram of corrosion mechanism after potentiodynamic polarization tests: (a and a1) WC–Co; (b and b1) WC–Al2O3. | ||
As written in Reaction (1), Co binder would react with water to form CoO.17
| Co + H2O → CoO + 2H+ + 2e− | (1) |
This is oxidized to Co3O4, which is the final product. Therefore, CoO is not detected by Raman and XPS. This process can be expressed as the following reactions:
| 3CoO + H2O → Co3O4 (corrosion products) + 2H+ + 2e− | (2) |
In addition, the most significant corrosion process for WC–Co cemented carbide is, respectively, cobalt dissolution of the anode and oxygenation reaction of the cathode. The reactions are as follows:33
| Co → Co2+ + 2e− | (3) |
| 2H2O + O2 + 4e− → 4OH− | (4) |
Then, the OH− ions react with Co2+ ions to form cobalt hydroxide:
| Co2+ + 2OH− → Co(OH)2 (corrosion products) | (5) |
After Co binder is dissolved, WC phase exposed to NaCl solution begins to corrode. The reaction is as follows:29
| WC + 5H2O → WO3 (corrosion products) + CO2 + 10H+ + 10e− | (6) |
As presented in Fig. 15(b1), after removing the loose corrosion products, the compacted corrosion layer is still on the surface of WC–Al2O3 composites. In addition, the corroded surface of WC–Al2O3 composite showed some cracks, which may be caused by stress due to the polarization process and new phase formation.42 Combined with EDS, XPS and Raman analysis, it can be inferred that the corrosion behavior of WC–Al2O3 composites exhibit the general corrosion characteristic. The corrosion of WC phase can cause the spalling of Al2O3 phase. Sudha et al. and Ouyang et al.28,43 confirmed that ceramic phase (such as Al2O3 and MgO) is a good insulator, and WC phase has excellent conductivity, so WC phase cannot form a galvanic couple with Al2O3 phase. Loto et al.44 reported that chemical corrosion occurred on the surface of Al2O3 phase. Hence, the corrosion process of WC–Al2O3 composites is the electrochemical oxidation of WC phase and the chemical dissolution of Al2O3 phase in NaCl solution. As expressed in Reaction (7), leading to the high hydration of surface film.44
| Al2O3 + 3H2O → 2Al(OH)3 | (7) |
The adsorption of Cl− onto the WC–Al2O3 composite easily transforms Al(OH)3 to soluble AlCl3. Diffusion of AlCl3 from the surface of samples to electrolyte leads to the corrosion of WC–Al2O3 composite.44
| Al(OH)3 + 2Cl− → AlCl3 + 2OH− | (8) |
The most important anodic and cathodic processes of WC–Al2O3 composites are tungsten carbide oxidation of the anode and oxygenation reaction of the cathode in NaCl solution, respectively. The specific electrochemical reaction is as follows:
| WC + 5H2O → WO3 (corrosion products) + CO2 + 10H+ + 10e− | (9) |
| 2H2O + O2 + 4e− → 4OH− | (10) |
In summary, combined with the results of polarization curve, it could be rigorously inferred that the corrosion current density increased during anodic polarization due to the oxidation of WC phase. WC–Al2O3 composite could form the compacted passivation film after corrosion test in NaCl solution. Hence, it could be confirmed that the corrosion resistance of WC–Al2O3 composite is higher than WC–Co cemented carbide.
4. Conclusion
In this work, the corrosion behavior of WC–Al2O3 composite and WC–Co cemented carbide in NaCl solution were investigated by immersion corrosion test and electrochemical test. Some important conclusions are as follows:(1) WC–Al2O3 composites has higher corrosion resistance than WC–Co cemented carbide in NaCl solution, due to the lower icorr and more positive Ecorr for WC–Al2O3 composites. In addition, the corrosion current density is different from the passivation current density, indicating the passivation trend. The pseudo-passivation of WC–Co cemented carbide was observed in NaCl solution (ipass ≫ 10 μA cm−2). However, WC–Al2O3 composite exhibited the passivation behavior (ipass < 10 μA cm−2).
(2) The EIS results also proved higher corrosion resistance of WC–Al2O3 composite, due to the higher value of polarization resistance (Rp). The impedance value of WC–Al2O3 composite increased more rapidly than WC–Co cemented carbide, indicating that compacted passivation film was formed on the surface of WC–Al2O3 composites, and its stability and compactness was enhanced with the increase of immersion time.
(3) The corrosion behavior of conventional WC–Co cemented carbide is general corrosion with preferential dissolution of Co binder and subsequent oxidation of WC phase. The corrosion products of WC–Co cemented carbide contain mainly Co(OH)2, Co3O4 and a small amount of WO3. In addition, the corrosion mechanism of WC–Al2O3 composite is general corrosion, in which WC phase is oxidized and a small amount of Al2O3 phase is dissolved. The corrosion products of WC–Al2O3 composites contain mainly WO3.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was supported by the Fundamental Research Funds for the Central Universities under grant CUSF-DH-D-2020050.References
- P. K. Katiyar, P. K. Singh, R. Singh and A. L. Kumar, Modes of failure of cemented tungsten carbide tool bits (WC/Co): A study of wear parts, Int. J. Refract. Met. Hard Mater., 2016, 54, 27–38 CrossRef CAS.
- C. L. Cramer, T. G. Aguirre, N. R. Wieber, R. A. Lowden, A. A. Trofimov, H. Wang, J. Yan, M. P. Paranthaman and A. M. Elliott, Binder jet printed WC infiltrated with pre-made melt of WC and Co, Int. J. Refract. Met. Hard Mater., 2020, 87, 105137 CrossRef CAS.
- X. Liu, X. Song, H. Wang, X. Liu, F. Tang and H. Lu, Complexions in WC–Co cemented carbides, Acta Mater., 2018, 149, 164–178 CrossRef CAS.
- Q. Yang, J. Yang, H. Yang and J. Ruan, The effects of fine WC contents and temperature on the microstructure and mechanical properties of inhomogeneous WC-(fine WC–Co) cemented carbides, Ceram. Int., 2016, 42(16), 18100–18107 CrossRef CAS.
- X. Zhang, S. Zhu, T. Shi, H. Ding, Y. Bai and P. Di, Preparation, mechanical and tribological properties of WC–Al2O3 composite doped with graphene platelets, Ceram. Int., 2020, 46(8, Part A), 10457–10468 CrossRef CAS.
- R. Useldinger and U. Schleinkofer, Creep behaviour of cemented carbides—Influence of binder content, binder composition and WC grain size, Int. J. Refract. Met. Hard Mater., 2017, 62, 170–175 CrossRef CAS.
- J. Li, J. Cheng, P. Chen, W. Chen and C. Wei, Fabrication of WC–Co cemented carbides with gradient distribution of WC grain size and Co composition by lamination pressing and microwave sintering, Ceram. Int., 2018, 44(10), 11225–11232 CrossRef CAS.
- Q. Yang, S. Yu, C. Zheng, J. Liao, J. Li and L. Chen, et al. Effect of carbon content on microstructure and mechanical properties of WC–10Co cemented carbides with plate-like WC grain, Ceram. Int., 2020, 46(2), 1824–1829 CrossRef CAS.
- Q. Zhang, N. Lin and Y. He, Effects of Mo additions on the corrosion behavior of WC–TiC–Ni hardmetals in acidic solutions, Int. J. Refract. Met. Hard Mater., 2013, 38, 15–25 CrossRef CAS.
- H. Qu, S. Zhu, Q. Li and C. Ouyang, Microstructure and mechanical properties of hot-pressing sintered WC–x vol.%Al2O3 composites, Mater. Sci. Eng., A, 2012, 543, 96–103 CrossRef CAS.
- H. Qu, S. Zhu, Q. Li and C. Ouyang, Influence of sintering temperature and holding time on the densification, phase transformation, microstructure and properties of hot-pressing WC–40 vol.%Al2O3 composites, Ceram. Int., 2012, 38(2), 1371–1380 CrossRef CAS.
- W. Dong, S. Zhu, T. Bai and Y. Luo, Influence of Al2O3 whisker concentration on mechanical properties of WC–Al2O3 whisker composite, Ceram. Int., 2015, 41(10), 13685–13691 CrossRef CAS.
- H. Qu and S. Zhu, Two step hot pressing sintering of dense fine-grained WC–Al2O3 composites, Ceram. Int., 2013, 39(5), 5415–5425 CrossRef CAS.
- H. Qu, S. Zhu, P. Di, C. Ouyang and Q. Li, Microstructure and mechanical properties of WC–40vol%Al2O3 composites hot pressed with MgO and CeO2 additives, Ceram. Int., 2013, 39(2), 1931–1942 CrossRef CAS.
- W. Dong, S. Zhu, H. Qu and X. Liu, Microstructural evolution and mechanical properties of hot-pressed WC–α-Al2O3 with Y2O3 and CeO2, Adv. Appl. Ceram., 2016, 115(6), 316–321 CrossRef CAS.
- W. Dong, S. Zhu, Y. Wang and T. Bai, Influence of VC and Cr3C2 as grain growth inhibitors on WC–Al2O3 composites prepared by hot press sintering, Int. J. Refract. Met. Hard Mater., 2014, 45, 223–229 CrossRef CAS.
- Q. Su, S. Zhu, H. Ding, Y. Bai and P. Di, Effect of the additive VC on tribological properties of WC–Al2O3 composites, Int. J. Refract. Met. Hard Mater., 2018, 75, 111–117 CrossRef CAS.
- H. Scholl, B. Hofman and A. Rauscher, Anodic polarization of cemented carbides of the type [(WC, M): M = Fe, Ni or Co] in sulphuric acid solution, Electrochim. Acta, 1992, 37(3), 447–452 CrossRef CAS.
- A. Ismail and N. Abd Aziz, Corrosion behavior of WC–Co and WC–Ni in 3.5% NaCl at increasing temperature, Appl. Mech. Mater., 2014, 660, 135–139 CAS.
- L. Zhang, Y. Chen, Q. Wan, T. Liu, J. Zhu and W. Tian, Electrochemical corrosion behaviors of straight WC–Co alloys: Exclusive variation in grain sizes and aggressive media, Int. J. Refract. Met. Hard Mater., 2016, 57, 70–77 CrossRef CAS.
- F. J. J. Kellner, H. Hildebrand and S. Virtanen, Effect of WC grain size on the corrosion behavior of WC–Co based hardmetals in alkaline solutions, Int. J. Refract. Met. Hard Mater., 2009, 27(4), 806–812 CrossRef CAS.
- W. J. Tomlinson and N. J. Ayerst, Anodic polarization and corrosion of WC–Co hardmetals containing small amounts of Cr3C2 and/or VC, J. Mater. Sci., 1989, 24, 2348–2352 CrossRef CAS.
- A. M. Human and H. E. Exner, Electrochemical behaviour of tungsten-carbide hardmetals, Mater. Sci. Eng., A, 1996, 209(1), 180–191 CrossRef.
- L. Zhang, Y. Feng, Q. Nan, R. Ke, Q. Wan and Z. Wang, Effects of titanium-based raw materials on electrochemical behavior of Ti(C,N)-based cermets, Int. J. Refract. Met. Hard Mater., 2015, 48, 11–18 CrossRef CAS.
- F. J. J. Kellner, M. S. Killian, G. Yang, E. Spiecker and S. Virtanen, TEM and ToF-SIMS studies on the corrosion behavior of vanadium and chromium containing WC–Co hard metals in alkaline solutions, Int. J. Refract. Met. Hard Mater., 2011, 29(3), 376–383 CrossRef CAS.
- A. M. Human and H. E. Exner, The relationship between electrochemical behaviour and in-service corrosion of WC based cemented carbides, Int. J. Refract. Met. Hard Mater., 1997, 15(1), 65–71 CrossRef CAS.
- Y. Ma, L. Zhang, C. Shan, T. Lei and X. Cheng, Electrochemical corrosion behaviors of binderless carbides, Fenmo Yejin Cailiao Kexue yu Gongcheng/Materials Science and Engineering of Powder Metallurgy, 2011, 16(6), 820–826 Search PubMed.
- C. Ouyang, S. Zhu and D. Y. Li, Corrosion and corrosive wear behavior of WC–MgO composites with and without grain-growth inhibitors, J. Alloys Compd., 2014, 615, 146–155 CrossRef CAS.
- S. Hochstrasser Kurz, Y. Mueller, C. Latkoczy, S. Virtanen and P. Schmutz, Analytical characterization of the corrosion mechanisms of WC–Co by electrochemical methods and inductively coupled plasma mass spectroscopy, Corros. Sci., 2007, 49(4), 2002–2020 CrossRef CAS.
- S. Sutthiruangwong and G. Mori, Corrosion properties of Co-based cemented carbides in acidic solutions, Int. J. Refract. Met. Hard Mater., 2003, 21(3), 135–145 CrossRef CAS.
- P. K. Katiyar and N. S. Randhawa, Corrosion behavior of WC–Co tool bits in simulated (concrete, soil, and mine) solutions with and without chloride additions, Int. J. Refract. Met. Hard Mater., 2019, 85, 105062 CrossRef CAS.
- L. Wei, Y. Liu, Q. Li and Y. F. Cheng, Effect of roughness on general corrosion and pitting of (FeCoCrNi)0.89(WC)0.11 high-entropy alloy composite in 3.5 wt.% NaCl solution, Corros. Sci., 2019, 146, 44–57 CrossRef CAS.
- A. Fazili, M. R. Derakhshandeh, S. Nejadshamsi, L. Nikzad, M. Razavi and E. Ghasali, Improved electrochemical and mechanical performance of WC–Co cemented carbide by replacing a part of Co with Al2O3, J. Alloys Compd., 2020, 823, 153857 CrossRef CAS.
- J. B. Sun, G. A. Zhang, W. Liu and M. X. Lu, The formation mechanism of corrosion scale and electrochemical characteristic of low alloy steel in carbon dioxide-saturated solution, Corros. Sci., 2012, 57, 131–138 CrossRef CAS.
- M. H. Martin and A. Lasia, Influence of experimental factors on the constant phase element behavior of Pt electrodes, Electrochim. Acta, 2011, 56(23), 8058–8068 CrossRef CAS.
- A. M. F. Rocha, A. C. Bastos, J. P. Cardoso, F. Rodrigues, C. M. Fernandes, E. Soares, J. Sacramento, A. M. R. Senos and M. G. S. Ferreira, Corrosion behaviour of WC hardmetals with nickel-based binders, Corros. Sci., 2019, 147, 384–393 CrossRef CAS.
- A. Fazili, M. R. Derakhshandeh, S. Nejadshamsi, L. Nikzad, M. Razavi and E. Ghasali, Improved electrochemical and mechanical performance of WC–Co cemented carbide by replacing a part of Co with Al2O3, J. Alloys Compd., 2020, 823, 153857 CrossRef CAS.
- D. S. Konadu, J. V. D. Merwe, J. H. Potgieter, S. Potgieter-Vermaak and C. N. Machio, The corrosion behaviour of WC–VC–Co hardmetals in acidic media, Corros. Sci., 2010, 52(9), 3118–3125 CrossRef CAS.
- M. Arai, S. Hayashi, K. Yamamoto and S. S. Kim, Raman studies of phase transitions in gas-evaporated WO3 microcrystals, Solid State Commun., 1990, 75(7), 613–616 CrossRef CAS.
- K. Ç. Demir, Corrosion behavior of electrodeposited WO3 thin films, Ceram. Int., 2020, 46(4), 4358–4364 CrossRef CAS.
- A. Lekatou, E. Regoutas and A. E. Karantzalis, Corrosion behaviour of cermet-based coatings with a bond coat in 0.5 M H2SO4, Corros. Sci., 2008, 50(12), 3389–3400 CrossRef CAS.
- S. Guo, R. Bao, J. Yang, H. Chen and J. Yi, Effect of Mo and Y2O3 additions on the microstructure and properties of fine WC–Co cemented carbides fabricated by spark plasma sintering, Int. J. Refract. Met. Hard Mater., 2017, 69, 1–10 CrossRef CAS.
- P. N. Sudha, Fundamental Biomaterials: Ceramics‖Corrosion of Ceramic Materials, 2018 Search PubMed.
- R. T. Loto and A. Adeleke, Corrosion of Aluminum Alloy Metal Matrix Composites in Neutral Chloride Solutions, Journal of Failure Analysis and Prevention, 2016, 16(5), 874–885 CrossRef.
| This journal is © The Royal Society of Chemistry 2021 |