Open Access Article
Open Access ArticleHeterogeneous ZnO-containing catalysts for efficient biodiesel production
Anping Wangab,
Wenxuan Quan*a,
Heng Zhangb,
Hu Li *b and
Song Yang
*b and
Song Yang *b
*b
aKey Laboratory for Information System of Mountainous Area and Protection of Ecological Environment of Guizhou Province, Guizhou Normal University, Guiyang, Guizhou 550025, China. Fax: +86 851 8829 2170; Tel: +86 851 8829 2171E-mail: wenxuanq@gznu.edu.cn
bState Key Laboratory Breeding Base of Green Pesticide & Agricultural Bioengineering, Key Laboratory of Green Pesticide & Agricultural Bioengineering, Ministry of Education, State-Local Joint Laboratory for Comprehensive Utilization of Biomass, Center for Research & Development of Fine Chemicals, Guizhou University, Guiyang, Guizhou 550025, China. E-mail: hli13@gzu.edu.cn; jhzx.msm@gmail.com
First published on 8th June 2021
Abstract
Biodiesel is one of the main biofuels used to replace fossil resources, and is mainly produced from esterification and transesterification of fatty acids and oils catalyzed by acids, bases or enzymes. Among the existing catalysts, metal oxides and their derivatives play an important role because of their high catalytic activity and low cost. ZnO is a metal oxide and its related nanomaterials are easy to prepare, which gives ZnO superior reactivity and extensive applications. Suitably modified ZnO nanomaterials typically have high specific surface areas, suitable pore sizes, and enhanced catalytic performance in the production of biodiesel. The present review introduces the application progress of ZnO catalysts in biodiesel preparation. The current shortcomings and future challenges of the basic heterogeneous catalytic systems for biodiesel production are also discussed.
1. Introduction
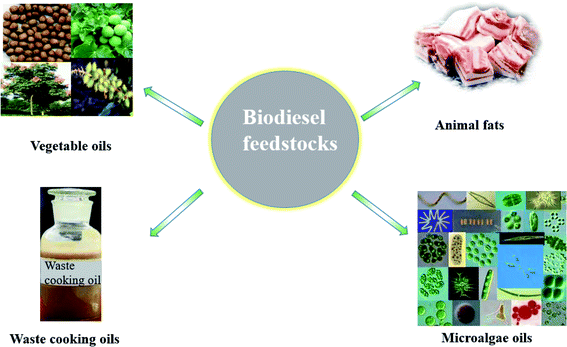
In recent decades, global fossil fuel consumption and greenhouse gas emissions have increased rapidly. Experts predict that by 2050, the existing fossil fuel resources will be exhausted.1–3 Therefore, people are trying to develop renewable green energy in order to deal with the possible energy crisis. This makes biofuels from biomass more and more attractive.Among them, biodiesel is a green, renewable, non-toxic and eco-friendly liquid biofuel.4 At the same time, biodiesel is almost free of sulfur and aromatic substances, biodegradable, and a truly renewable “green energy”.5–10 However, the high cost, especially the cost of raw materials, keeps the price of biodiesel high, which limits its applications. At first, the raw materials of biodiesel were mainly rapeseed oil, soybean oil, and other edible oils, which had a certain impact on national food safety. Later, researchers continued to try to use other non edible oils to produce biodiesel, such as Jatropha curcas oil, Euphorbia oil,11 Xanthium oil,12 parasol oil13 and Koelreuteria integrifoliola oil.14 This expands the source of raw materials for biodiesel production and greatly reduces the production cost. Then, to further reduce the cost, cooking waste oil and animal fat with high acid value are also used to prepare biodiesel. In addition, some researchers tried to use microalgae as raw material for biodiesel production, effectively controlling the cost of raw materials (in Fig. 1). New raw materials are of great significance to alleviate the energy crisis, protect the ecological environment and adjust agricultural structure.15–18
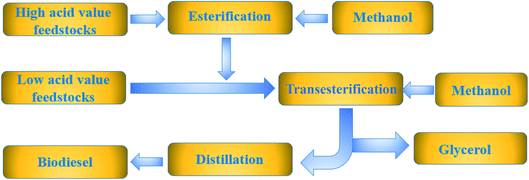
Biodiesel is usually prepared by esterification and transesterification (as can be seen in Fig. 2). The main catalysts are acid,19 alkali20 and enzyme.21 As we all know, heterogeneous catalysts have gradually replaced homogeneous catalysts due to their many advantages, including noncorrosiveness, easy separation and reuse.22–24 At present, various heterogeneous catalysts have been used in the preparation of biodiesel, including metal oxide,25 mixed oxide,26 hydrotalcite,27 ion exchange resin28, carbon based catalyst29 and zeolite.30 However, the traditional solid catalysts have the disadvantages of less active sites, porosity, serious leaching, and high cost. Therefore, in order to prepare high-quality solid catalysts, it has become one of the hot spots to find a green, efficient and stable heterogeneous catalysts for the preparation of biodiesel.31
In the past few years, nanomaterials have become the focus of research because of their unique properties that traditional materials do not have. This is mainly because nanoparticles have the characteristics of high activity, high selectivity, stability, and easy recovery. The particle size of nanoparticles is 1–100 nm. Previous studies have made the preparation of nanomaterials easy. Therefore, nanomaterials as an efficient biodiesel catalyst have been gradually explored. At present, the main nano catalysts used in biodiesel production are calcium oxide, zinc oxide, carbon nanotubes and so on. Among them, zinc oxide is favored for its low density, large specific surface area, acid-base amphoteric and other excellent properties.32 At the same time, some scholars have explored its application in the preparation of biodiesel under the condition of photocatalysis, which has broadened the new method of preparing biodiesel. Generally speaking, the ZnO nanocatalyst directly catalyzes esterification and transesterification, but the yield is low.33,34 Therefore, zinc oxide was modified by different carrier loading, acid–base modification, and other methods to obtain higher biodiesel yield.35,36
In this paper, the application of ZnO catalysts in biodiesel was reviewed, and the related research progress was mentioned. Nano ZnO is also discussed. The preparation method, physical and chemical properties, catalytic effect and reusability of the material. In the past two decades, numerous studies on the application of nano catalysts containing ZnO in biodiesel production were conducted, with satisfactory results. However, as far as we know, the progress of heterogeneous catalysts containing zinc oxide in the preparation of biodiesel has not been reviewed. Therefore, the purpose of this paper is to comprehensively discuss and evaluate the highly efficient functionalized nano ZnO catalyst centered on biodiesel synthesis.
2. ZnO nanocomposites
2.1. Nanocatalysts preparation
ZnO and its composite nanomaterials are usually prepared by coprecipitation, sol–gel method, solvothermal method, template method and impregnation method. Ultimately, the ideal nanocatalysts need to be obtained by calcination at a specific temperature (in Fig. 3 and 4).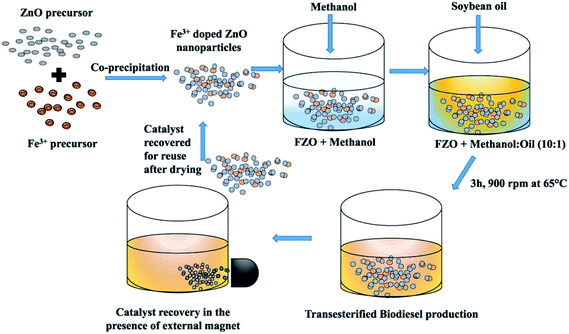 | ||
| Fig. 3 Preparation of bimetallic Fe(III) doped ZnO (FZO) nanoparticles (NPS) catalyst. Reproduced with permission from ref. 37. Copyright 2019 Elsevier. | ||
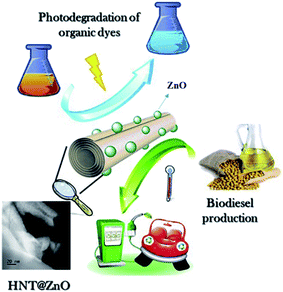 | ||
| Fig. 4 One-pot synthesis of ZnO nanoparticles supported on halloysite nanotubes catalyst. Reproduced with permission from ref. 38. Copyright 2020 Elsevier. | ||
Rakoso et al.39 prepared zinc oxide nanoparticles by coprecipitation method. The milky white solution was formed by using zinc sulfate solution and ammonia water at 80 °C. After centrifugal separation and washing, the precipitation needed to be aged for 24 h. Finally, ZnO catalysts with particle size of 18–23 nm were obtained by calcination at 800 °C for 6 h. Generally, the preparation of ZnO materials by coprecipitation method needs mild conditions, simple steps, uniform particle size and low cost. It is an ideal preparation method.
In addition, sol–gel method is also an attractive method for the preparation of ZnO nanomaterials. Previous reports40–44 have shown that this method has been widely used to prepare ZnO nanomaterials, which is mainly attributed to the high homogeneity of the structure and composition of the metals and metal oxides prepared by this method. Although sol–gel chemistry originates from the hydrolysis and condensation reactions of metal alcohols, it provides many fascinating strategies for preparing materials from the solution precursor. Ultimately, low-temperature chemistry, reproducibility and high surface volume ratio of the resulting products are the advantages of this technology.45
In order to obtain regular mesoporous composites, template method is usually used. Wang et al.46 synthesized zinc oxide nanoparticles by lignin template solid-phase method. Interestingly, the precursor of ZnO was prepared by solid mixing grinding method, and lignin was used as template. By calcination, not only mesoporous ZnO nanoparticles can be rapidly and effectively prepared, but also the microstructure, morphology, size and catalytic activity of the obtained ZnO catalysts can be easily adjusted by changing the calcination temperature.
Xie et al.47 prepared precursors by impregnating LiNO3 solution with nanoparticles of about 60 nm ZnO. Li-Doped ZnO nanomaterials were obtained by calcination at 600 °C for 5 h in air. However, it is regrettable that the stability of the catalyst prepared by impregnation method is poor.
2.2. Characterization of ZnO nanocomposites
The XRD characteristic diffraction lines of ZnO correspond to 31.8° (100), 34.4° (002), 36.3° (101), 47.5° (102), 56.6° (110), 62.8° (103), 66.4° (103), 67.9° (112), 69.1° (201), respectively.48,49 In FT-IR spectra, the strong peak at 457 cm−1 is the characteristic peak of stretching vibration of zinc oxide.50,51 The morphology and particle size of composite ZnO nanomaterials were characterized by SEM and TEM. N2 adsorption and desorption apparatus can provide information on the specific surface area, average pore size and pore volume of catalytic materials. The acid-base content is generally determined by TPD (NH3 or CO2). Through the above characterization process, the physicochemical properties of ZnO nanocomposites can be basically determined, which is very helpful for further application in catalytic chemical reactions.According to Yoo et al.,52 the alkali strength of ZnO (7.2 < H_ < 9.3) is slightly weaker than that of Cao (9.3 < H_ < 15.0). However, the advantage of ZnO is less leaching. The experimental results show that the content of Zn in biodiesel with ZnO as catalyst is only 2.67 mg kg−1, while the content of Ca in biodiesel with CaO as catalyst is astonishing to 1060.5 mg kg−1. This shows that ZnO has good stability and active sites are not easy to be lost, so it is suitable for biodiesel catalysts.
3. Pure ZnO and metal-doped ZnO nanocatalysts
3.1. ZnO nanoparticles
ZnO nanoparticles are usually prepared by a precipitation method, and the specific surface area and pore size of the catalysts are regulated by the activator. Because of the weak acidity and alkalinity of zinc oxide itself, microwave or ultrasound is usually used to assist the production of biodiesel when using zinc oxide as a catalyst to obtain high FAME yield.In recent years, many ZnO nanoparticles with different morphologies have been synthesized and used to catalyze the production of biodiesel. Due to the difference of morphology and structure, the catalytic performance is changed. However, nanostructures and acid–base properties play an important role in the catalytic process of biodiesel. Liu et al.53 have confirmed that exposure of more catalytic sites in ZnO will lead to a significant increase in biodiesel production. For example, the biodiesel yield of granular zinc oxide is only 46.3%, while the yield of 74.5% can be achieved when the catalyst is replaced with nanometer zinc oxide powder under the same other conditions, which is exciting.
Kim et al.54 synthesized biodiesel and ZnO nanoparticles simultaneously by supercritical methanol method. The ZnO nanoparticles formed in situ were used as catalysts for the synthesis of biodiesel. In addition, the formation of ZnO nanoparticles in situ results in the decrease of reaction temperature (from 350 °C to 250 °C) and time (only 10 minutes). However, the particle size of the in situ formed ZnO nanoparticles is smaller than that of the traditional process. This comparison can be seen in Fig. 5, which is more conducive to improving its catalytic performance. It is encouraging that this new developed method has the economic advantages of producing zinc oxide as catalyst and additional by-product, which may provide economic advantages in large-scale production.
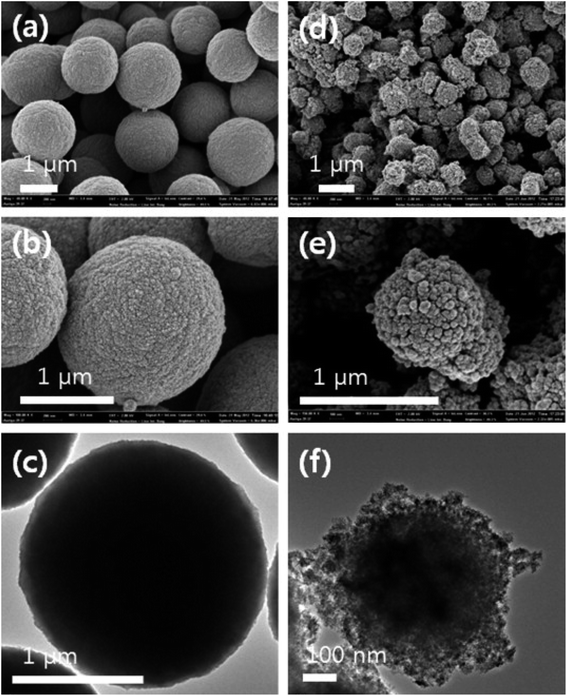 | ||
| Fig. 5 SEM and TEM images of the ZnO nanoparticles synthesized in neat supercritical methanol at 300 °C and 350 bar over a period of 10 min (a–c) and ZnO synthesized during the transesterification of rapeseed oil in supercritical methanol under identical conditions (d–f). Reproduced with permission from ref. 54. Copyright 2013 Elsevier. | ||
The ZnO nanostar material was obtained by microwave-assisted surfactant hydrolysis.55 It can be seen from Fig. 6 that the nanostar with a unique configuration can be used to prepare biodiesel by one pot esterification and transesterification catalysis of high acid value feedstocks. The results showed that zinc oleate was synthesized by the reaction of ZnO nanocatalyst with FFA, finally giving zinc glycerin (Zn-Gly) by reacting with glycerin. It is noted that the Zn-Gly catalyst can be easily recovered and kept active for 5 cycles by re-depositing Zn-Gly on the ZnO nano star catalyst at the end of the reaction. This makes ZnO nanoparticles assume the function of catalyst and catalyst carrier at the same time. In addition, more importantly, the presence of FFA (6 wt%) greatly improves the transesterification rate, making it an effective catalyst for waste edible oil, crude oil and other low-grade raw materials.
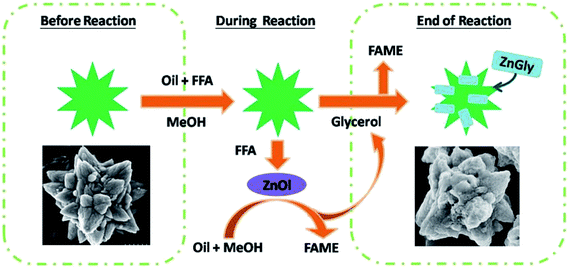 | ||
| Fig. 6 Synthesis of biodiesel catalyzed by ZnO nanostar. Reproduced with permission from ref. 55. Copyright 2016 Elsevier. | ||
Interestingly, biodiesel was prepared by transesterification of olive oil catalyzed by zinc oxide nanorods.56 It is worth mentioning that at 150 °C, the conversion rate of biodiesel is as high as 95%, which is far lower than 250 °C reported by Yoo et al.52 This shows that ZnO nanorods can effectively expose more O2− active sites, which can catalyze transesterification to produce biodiesel under more mild conditions.56
A novel ZnO nanomaterials was prepared by microwave hydrothermal method using PEG-400 as a template, which can be completed in 5 min at 100 °C. X-ray diffraction analysis shows that ZnO nanoparticles form hexagonal wurtzite phase. SEM analysis showed that the morphology of ZnO nanostructures prepared by the NaOH method was quasi-spherical, while NH4OH synthesis was flower-like. It is worth mentioning that the PEG-400 template makes the microcrystalline size increase and the surface area decrease. The surface area (2.03 m2 g−1) of the sample ZONH5P was slightly larger than that of the zinc oxide microdisk (1.86 m2 g−1) obtained by Zhang et al.57 using the traditional hydrothermal method of citric acid. In addition, ZnO nanoparticles were evaluated as the catalyst for biodiesel synthesis with soybean oil at 180 °C. The catalytic efficiency of layered ZONa5P sample for transesterification was 77.82%, while that of flower like ZONH5P was only 52.25%. The results show that the exposed active sites will be different due to the different morphology of ZnO.58
In order to compare the activity of different metal oxide catalytic materials in biodiesel production, Yigezu et al.59 studied the effects of different metal oxides (CO3O4, KOH, MoO3, NiO, V2O5, and ZnO). Although zinc oxide has a conversion of 85.8% in 40 min at 320 °C, it has a good catalytic effect, but too high a temperature is unfavorable for industrialization. This has been proposed to modify the ZnO catalyst to meet the new requirements for catalytic reactions under milder conditions.
The transesterification of WCO catalyzed by ultrasonic-assisted ZnO nanocatalyst and the traditional stirring method was compared. The conversion of FAME was 96% under ultrasound treatment. In the catalytic reaction, the application of microwave can make the reaction conditions milder and obtain better catalytic efficiency. Microwave-assisted catalysis has been tried many times in the preparation of biodiesel, which has proved to be very effective.60
Raghavendra et al.61 used Garcinia gummi-gutta oil to synthesize biodiesel by zinc oxide nanoparticles. The optimum condition of transesterification is at 64 °C for 2 h. The reaction mixture was poured into the separation funnel to separate catalyst, glycerol, and FAME. After the reaction, the yield of biodiesel was 80.1%.
3.2. Metal-doped ZnO catalysts
Metal-doped ZnO nanocatalysts are usually prepared by coprecipitation, sol–gel, impregnation and calcination.62 The catalysts obtained by the coprecipitation method are generally nanoparticles, doped more uniformly, as well as have good catalytic activity, and reusability and the preparation method itself needs short time and low cost, so it is an excellent catalyst preparation method.63,64 The catalyst prepared by the sol–gel method takes a longer time, but the active sites of the catalyst are dispersed evenly, which is also a green and environmentally friendly preparation method.65,66 However, the catalytic materials obtained by the impregnation method are relatively uneven, so there are fewer applications now. The preparation of metal-doped ZnO nanomaterials and their application in biodiesel production are introduced (in Table 1).| Entry | Catalyst | Oil | T (°C) | t (h) | M/O | CA (wt%) | Yield (%) | Reuse cycle (%) | Ea (kJ mol−1) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Na-ZnO | Virgin cottonseed oil | 65 | 1.0 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
10 | 99 | — | — | 67 |
| 2 | K-ZnO | WCO | 65 | 0.83 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2.5 | 98 | — | 14.54 | 68 |
| 3 | Cu-ZnO | WCO | 55 | 0.83 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12 | 97.7 | 5(87) | — | 69 |
| 4 | Cu-ZnO | Neem oil | 55 | 1.0 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
10 | 97.1 | 6(73.9) | — | 70 |
| 5 | Ni-ZnO | Castor oil | 55 | 1.0 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
11 | 95.2 | 3(85) | — | 71 |
| 6 | Fe-ZnO | Castor oil | 55 | 0.83 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
14 | 91.0 | 4(90) | 1527 | 72 |
| 7 | Fe-ZnO | Pongamia oil | 55 | 0.83 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12 | 93.0 | — | — | 73 |
| 8 | Mn-ZnO | Mahua oil | 50 | 0.83 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 97 | 5(91) | 181.9 | 74 |
| 9 | Ag-ZnO | Simarouba oil | 64 | 2.0 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.5 | 84.5 | — | — | 75 |
| 10 | Bi-ZnO | Jatropha oil | 65 | 1.0 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
4 | 95 | — | — | 76 |
Ali et al.67 reported different concentration of Na-ZnO catalysts prepared by impregnation. The catalyst prepared with soaking 5% sodium in zinc oxide and calcining at 400 °C. It was used to catalyze cottonseed oil for producing biodiesel. Catalytic activity depends on (i) the amount of sodium leaching, (ii) calcination temperature, (iii) the molar ratio of MeOH to oil, (iv) reaction temperature, and (v) the content of FFA in raw materials. The catalyst is also used for transesterification of various feedstocks (raw cottonseed oil, waste cottonseed oil, mutton fat, Karaza oil and Jatropha oil).
A new heterogeneous zinc oxide catalyst containing potassium was synthesized by precipitation method. The catalyst by K/Zn mole ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 was calcined at 900 °C, providing high catalytic activity. The effects of different reaction parameters on the conversion efficiency of biodiesel were investigated. Under the optimum reaction conditions (catalyst 2.5 wt%, oil–methanol molar ratio 1
2 was calcined at 900 °C, providing high catalytic activity. The effects of different reaction parameters on the conversion efficiency of biodiesel were investigated. Under the optimum reaction conditions (catalyst 2.5 wt%, oil–methanol molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18, 600 rpm at 65 °C for 50 min), the conversion of biodiesel is the highest reached 98%. The kinetics of transesterification at different reaction temperatures (45–65 °C) was studied. The reaction activation energy was 14.54 kJ mol−1, which is a relatively lower value in the transesterification of biodiesel, reflecting the excellent performance of the catalyst.68
18, 600 rpm at 65 °C for 50 min), the conversion of biodiesel is the highest reached 98%. The kinetics of transesterification at different reaction temperatures (45–65 °C) was studied. The reaction activation energy was 14.54 kJ mol−1, which is a relatively lower value in the transesterification of biodiesel, reflecting the excellent performance of the catalyst.68
A novel copper-doped zinc oxide (CZO) nanocomposite has good catalytic performance in the preparation of biodiesel.69,70 The synthesized CZO nanocomposites were characterized by FESEM, and the average particle size was about 80 nm. X-ray diffraction patterns show that there are substitutes for zinc oxide in the hexagonal lattice of nano-copper. Atomic force microscopy (AFM) analysis confirmed that CZO nanoparticles had porous and heterogeneous properties. The maximum yield of biodiesel from waste edible oil catalyzed by CZO nanocomposites is 97.71%. Subsequently, CZO nanomaterials were used to catalyze neem oil to produce biodiesel. The optimum reaction conditions: 10 wt% catalyst, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 oil–methanol ratio at 55 °C for 60 min, and the yield of biodiesel was 97.18%. The yield of biodiesel was 73.95% in the sixth cycle with regenerated CZO nanocatalyst. The reaction kinetics model of biodiesel production was tested, and the activation energy of the reaction is 233.88 kJ mol−1.
10 oil–methanol ratio at 55 °C for 60 min, and the yield of biodiesel was 97.18%. The yield of biodiesel was 73.95% in the sixth cycle with regenerated CZO nanocatalyst. The reaction kinetics model of biodiesel production was tested, and the activation energy of the reaction is 233.88 kJ mol−1.
Baskar group prepared a variety of metal-doped ZnO nanocatalytic materials,68–71 which have important applications in the preparation of FAME from feedstocks with high FFA content. These doped catalysts obviously improved the catalytic performance, and some are functionalized by magnetism, which provides a demonstration for the development of excellent biodiesel catalysts. Nickel-doped zinc oxide nanocatalyst was prepared to catalyze the production of biodiesel by castor oil with high FFA content71 (in Fig. 7). The nanocomposites calcined at 800 °C have good catalytic activity. When the biodiesel yield is high (95.20%), the response surface methodology (RSM) is more accurate.
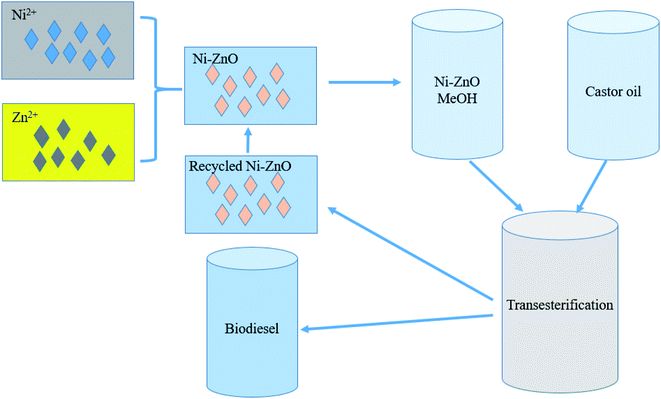 | ||
| Fig. 7 Diagram of Ni-doped ZnO catalyzed transesterification of castor oil for biodiesel production. Reproduced with permission from ref. 71. Copyright 2018 Elsevier. | ||
Baskar team72 used castor oil as raw material and nano-zinc oxide ferromagnetic complex as a heterogeneous catalyst to prepare biodiesel by transesterification. The yield of biodiesel was 91%. Fe(II) doped zinc oxide nanocatalyst is a novel material for heterogeneous catalytic transesterification of biodiesel. Iron(II) doped ZnO nanocatalyst was also used for transesterification of Ponciella oil.73 The transesterification reaction of Pongamia oil produced 93% biodiesel, which was completed at 55 °C for 50 min with 12 wt% catalyst, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) molar ratio of methanol to oil.
1 (v/v) molar ratio of methanol to oil.
Mn-doped ZnO was employed as a catalyst to prepare biodiesel from Mahua oil.74 The catalyst hexagonal structure was confirmed by SEM and XRD. The catalyst particle size was about 24 nm. The results showed that the optimum conditions (7/1 methanol/oil volume ratio and 8% catalyst concentration at 50 °C for 50 min) could afford the maximum yield of biodiesel (97%).
Nagaraju et al.75 prepared biodiesel from Smaruba oil by using zinc oxide and silver-zinc oxide nanoparticles as catalysts. After transesterification, the yields of biodiesel catalyzed by ZnO and Ag-ZnO nanocatalyst were 80.1% and 84.5%, respectively. Compared with zinc oxide nanoparticles, Ag-ZnO nanoparticles have higher catalytic activity and may become potential catalysts for biodiesel production.
Oletoye et al.76 used Bi-ZnO as a solid catalyst to synthesize biodiesel from Jatropha curcas oil. The Bi-ZnO catalyst was prepared by coprecipitation method. The results showed that the catalytic activity of 2.0 wt% Bi supported on zinc oxide was the highest when the molar ratio of methanol to oil was 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the amount of catalyst was 4 wt%. The conversion of Jatropha oil could reach 95% for 1 h. The catalyst was washed with MeOH and at 80 °C dried in the oven, which can be used for further transesterification.
1 and the amount of catalyst was 4 wt%. The conversion of Jatropha oil could reach 95% for 1 h. The catalyst was washed with MeOH and at 80 °C dried in the oven, which can be used for further transesterification.
In addition, Co doped ZnO nanoparticles were prepared by a sol–gel method, in which the Co content and magnetism could be precisely controlled.77 The application of gel–sol method will have more uniform and more excellent catalytic activity for metal-doped ZnO nanoparticles.
4. Supported zinc oxide catalysts
4.1. Oxide-supported ZnO catalysts
Metal oxides have important applications in the preparation of biodiesel, such as CaO,78,79 MgO,80 and La2O3.81 However, their reusability is poor and their active sites are easily lost. Based on this situation, more complex metal oxide materials have emerged, among which zinc oxide has more complex metal oxide. This is due to the stable nature of zinc oxide, which can better disperse strong alkaline active sites, improve catalytic activity, and prevent leaching of active sites.82 The application of CaO-ZnO,83 MgO-ZnO,84 and La2O3-ZnO85 catalysts in biodiesel synthesis was introduced (in Table 2).| Entry | Catalyst | Oil | T (°C) | t (h) | M/O | CA (wt%) | Yield (%) | Reuse cycle (%) | Basic density (mmol g−1) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| a The substrate of transesterification is ethanol. | ||||||||||
| 1 | CaO-ZnO | Jatropha oil | 120 | 3.0 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3 | 94 | 4(80) | 1.38 | 88 |
| 2 | CaO-ZnO | Jatropha oil | 120 | 4.0 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3.7 | 97.0 | — | — | 89 |
| 3 | CaO-ZnO | Sunflower oil | 78 | 3.0 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1a 1a |
3.0 | 95.0 | 3(40) | — | 90 |
| 4 | ZnO-CaO | Jatropha oil | 65 | 0.75 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
5.0 | 99 | 5(99) | — | 91 |
| 5 | CaO-ZnO | Sunflower oil | 60 | 4.0 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2 | 95 | — | 0.28 | 92 |
| 6 | K2O/CaO-ZnO | Soybean oil | 60 | 4.0 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6 | 81.1 | — | 93 | |
| 7 | ZnO/Ca (OH)2/KF | Soybean oil | 65 | 1.5 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3 | 97.6 | — | 3.62 | 94 |
Wang et al.86 tried to prepare ZnO-B2O3 catalyst to convert high acid-value raw materials into biodiesel. However, the yield was still less than 20% after reaction at 85 °C for 6 h, which is mainly attributed to the low acid and alkali densities. Therefore, the preparation of strong alkaline catalysts can produce biodiesel under mild conditions.
In order to develop easier transesterification process, Madhuvilakku et al.87 prepared stable and active nano-catalyst of TiO2-ZnO and applied to palm oil transesterification process. The catalyst had a good catalytic activity by testing. Supported by 200 mg catalyst, 98% conversion was obtained under the optimum conditions.
Lee et al.88 studied the activity of CaO-ZnO and ZnO as catalysts for FAME production from Jatropha oil. CaO-ZnO was synthesized by the coprecipitation method. The transesterification activity is closely related to the physicochemical properties of catalysts. The catalytic activities of catalysts were CaO-ZnO (94%) > ZnO (41%). In addition, especially CaO-ZnO catalysts had higher reuse efficiency and catalyst stability for the four transesterification cycles. Under the same reaction conditions, the activity of pure zinc oxide is much lower than that of CaO-ZnO, which is attributed to the alkalinity of zinc oxide that is not strong enough. CaO belongs to strong alkali and has strong catalytic activity. After loading, activity, stability, and reusability of CaO-ZnO are enhanced, indicating that CaO-ZnO is a promising biodiesel catalyst. Similarly, solid base catalyst (CaO-ZnO) was used for transesterification of high acid Jatropha curcas oil. The maximum yield of the CaO-ZnO catalytic reaction was 97.03%.89
The sunflower oil to prepare biodiesel was studied using CaO-ZnO as an efficient catalyst. The yield of fatty acid ethyl ester can reach more than 95% for 3 h with 3 wt% catalyst at 78 °C. However, due to the leaching of calcium, the catalyst has a low resistance to reuse in the reaction, but adding Na2CO3 to the catalyst can obtain concentrated biodiesel after washing with water.90
Kumar and his co-workers91 prepared zinc oxide-calcium oxide catalyst by a simple wet chemical method at 950 °C. The activity of cottonseed oil catalyzed by zinc oxide-calcium oxide in biodiesel production is related to calcination temperature, crystallinity, and alkali strength. The activation energy (Ea) of the reaction is 43 kJ mol−1. The catalyst can be recycled for at least five cycles.
The heterogeneous transesterification kinetics of sunflower oil and WCO from 60 to 96 °C was studied with CaO-ZnO as a catalyst. The mixture of calcium oxide and zinc oxide powders was treated by the mechanochemical method. The heterogeneous CaO-ZnO was obtained by adding water needed to form corresponding hydroxides and calcining in the air at 700 °C. In the process of preparing biodiesel, the reaction rate is greatly affected by mass transfer resistance at a lower temperature. At higher temperature, the effect of mass transfer resistance can be neglected.92
In addition, CaO-ZnO materials prepared by the mechanochemical method93 and calcined CaZn2(OH)6·2H2O94 are also used in FAME production.
Istadi et al.95 reported a novel solid base catalyst (K2O/CaO-ZnO) for the synthesis of FAME with soybean oil. The catalyst was prepared by the coprecipitation method and impregnation method. It was compared with ZnO and CaO-ZnO catalysts to study their activity in transesterification under suitable conditions (catalyst loading 6.0 wt% and the molar ratio of methanol to oil 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 60 °C for 4 h). The catalytic activity of 2.0 wt% K2O/CaO-ZnO was the highest, and the yield of FAME was 81.1%. The alkalinity and surface area of the CaO-ZnO catalyst were improved by adding K2O. Strong basicity can effectively improve the catalytic efficiency, and a large surface area can disperse the active center, which is conducive to mass transfer.
1 at 60 °C for 4 h). The catalytic activity of 2.0 wt% K2O/CaO-ZnO was the highest, and the yield of FAME was 81.1%. The alkalinity and surface area of the CaO-ZnO catalyst were improved by adding K2O. Strong basicity can effectively improve the catalytic efficiency, and a large surface area can disperse the active center, which is conducive to mass transfer.
Fan et al.96 prepared ZnO/Ca(OH)2/KF material for the synthesis of FAME by soybean oil. The effects of KF molar ratio, calcination temperature, catalyst dosage, methanol-oil molar ratio, reaction temperature and reaction time on catalyst activity were investigated. KF interacts with Ca(OH)2 before calcination and forms KCaF3 phase. In addition, the basicity of ZnO/Ca(OH)2/KF is greatly affected by different calcination temperatures, and the activity of the catalyst is closely related to the basicity. Under the conditions of catalyst dosage 3%, methanol/oil ratio 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, reaction time 1.5 h and temperature 65 °C, the yield of biodiesel is 97.6%.
1, reaction time 1.5 h and temperature 65 °C, the yield of biodiesel is 97.6%.
Veiga et al.97 synthesized ZnO-La2O3 catalytic material by coprecipitation method, and used it to prepare biodiesel from non-edible oil. The effects of the precipitator, calcination temperature and the molar ratio of zinc to lanthanum on the catalytic performance of mixed oxides were studied. Increasing zinc oxide content can promote FAME synthesis, and La2(CO3)3, La(HCO3)2 are more effective than La2O3 in the transesterification reaction.
Pasupulety et al.98 synthesized MgO-ZnO catalysts by precipitation method. The catalytic performance of MgO-ZnO catalyze soybean oil to synthesize FAME was studied. The results show that the molar ratio of Mg to Zn is about 3, namely Mg3Zn1. Different types of Mg3Zn1 catalysts were synthesized by coprecipitation, impregnation, and urea hydrolysis, and their preparation methods were studied.
Lee et al.99 used MgO-ZnO as a catalyst and non-edible Jatropha oil as raw material to prepare biodiesel by transesterification reaction. The base of solid MgO-ZnO catalysts with different mole ratios was studied. The physical and chemical properties of MgO-ZnO are better than those of single MgO and ZnO. The catalyst exhibited high catalytic activity (>80%) and had reliable reusability for biodiesel production.
The heterogeneous catalyst ZnO loaded on Al2O3/SiO2 was prepared using a sol–gel approach.100 The yield of biodiesel was 92% after 4 h by sunflower oil as a raw material. Subsequently, the effects of different carriers and calcination temperatures were investigated. The catalyst had good catalytic activity, and the reaction kinetics could be expressed by a first-order reversible reaction model.
Veiga et al.101 used ZnO-Al2O3 with different molar ratios as catalysts for transesterification of soybean oil with methanol. When the reaction temperature was 182.5 °C with the catalyst of 5.0 wt% and 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of alcohol to oil, the optimum yield of biodiesel was 86%, and the catalyst had no obvious leaching.
1 molar ratio of alcohol to oil, the optimum yield of biodiesel was 86%, and the catalyst had no obvious leaching.
Da Silva et al.102 developed a fixed-bed tubular continuous reactor using ZnO-Al2O3 particles as a catalyst to prepare biodiesel. The catalyst was placed in a 30 cm long tubular reactor. Soybean oil (168 g h−1) and MeOH or EtOH (89 g h−1) were used as raw materials in the reactor, and the temperature was fixed at 100 °C. Under these conditions, soybean oil could be converted into biodiesel with 75% yield in methanol and 35% yield in ethanol. The yield of biodiesel with ethanol was 78% when the temperature rose to 180 °C. Importantly, after reaching a stable state, the transition remained approximately constant over time. It is noteworthy that the fixed bed has remained active for more than 120 h without any leaching or deactivation of the catalyst.
Kesambi (Schleichera oleosa L.) oil was converted into FAME using Al2O3 supported ZnO solid catalyst. ZnO supported Al2O3 catalyst was prepared by precipitation and gel method. The surface area, total pore diameter and average pore size of the catalyst were 71.561 m2 g−1, 0.137 cm3 g−1, and 8.1 nm, respectively. The effects of catalyst dosage, the molar ratio of oil to methanol and reaction time on the yield of biodiesel were studied. The results show that these three variables have significant effects on biodiesel production. The highest yield of biodiesel was 92.29% at 65 °C for 6 h and 4 wt% catalyst, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 molar ratio of oil to methanol.103
12 molar ratio of oil to methanol.103
ZnO-Al2O3 and ZnO-Fe2O3 samples with different loadings (5–20 wt%) were prepared by impregnating the carrier (γ-Al2O3 and α-Fe2O3) with ZnNO3 and calcining at 600 °C. XRD studies of calcined samples show that ZnO has reacted with the carrier to form corresponding spinel, ZnAl2O4, and ZnFe2O4. The results show that the catalyst exhibits good catalytic activity.104
ZnO-Al2O3-La2O3 layered catalyst was synthesized by the coprecipitation method, and calcination at 400 °C. The high specific surface area (180–130 m2 g−1) was obtained in the mixed oxides. With the reaction of oleic acid with MeOH, FAME yield is more than 88% after 1 h reaction at 100 °C.105
4.2. Zeolite-supported ZnO catalysts
Three catalysts, ZnO/zeolite, PbO/zeolite, and MgO were evaluated for the preparation of biodiesel. Under the optimum conditions, biodiesel yield from Jatropha oil by ZnO/zeolite was 93.8%.106Two kinds of catalysts, ZnO/zeolite, and PBO/zeolite, were investigated for the synthesis of biodiesel from Jatropha oil. Zeolite was used as a carrier to minimize the leaching of metal ions in the reaction process. When Jatropha oil containing a large amount of FFA (>10%) was used as a raw material, zinc oxide/zeolite activity was higher.107
4.3. Bentonite-supported ZnO nanocatalysts
Farias and co-workers108 used natural bentonite to prepare biodiesel by microwave-assisted solvothermal acidification, impregnation of CuO, ZnO, and CeO2. The transesterification reaction was carried out in a Parr reactor at 200 °C. The reaction time was 1, 2 and 4 h, respectively. For natural bentonite, the pure material has almost no conversion, but after zinc oxide homogeneous impregnation, the conversion reaches 88%. The acid density of acidified ACD-Zn catalyst is as high as 1.48 mmol g−1. This indicates that zinc oxide impregnated bentonite is an excellent catalyst.4.4. Other carriers ZnO nanocatalysts
Wan et al.109 prepared MnCO3/ZnO alkali catalysts with different molar ratios of manganese to zinc by coprecipitation and used them in the synthesis of biodiesel from subcritical methanol. The 99.25% triglyceride conversion and 94.20% FAME yield were obtained by calcining at 300 °C for 30 min, under reaction conditions with 4 wt% catalyst, 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 methanol/oil molar ratio at 175 °C for 1 h. After 17 cycles of reuse, the yield of FAME remained above 86.26%. After reusing the catalyst, the deactivation is mainly caused by the conversion of small particles of zinc oxide to flake zinc glycerol, which indicates that catalyst morphology change will have a certain impact on the yield.
1 methanol/oil molar ratio at 175 °C for 1 h. After 17 cycles of reuse, the yield of FAME remained above 86.26%. After reusing the catalyst, the deactivation is mainly caused by the conversion of small particles of zinc oxide to flake zinc glycerol, which indicates that catalyst morphology change will have a certain impact on the yield.
When zinc (NO3)2 was impregnated on SiO2 carrier and calcined at 500 °C in air, the number of Lewis acid sites on the SiO2 surface increased. In the process of esterifying FFA into FAME, the activity of ZnO/SiO2 catalyst decreases with the increase of calcination temperature. This is due to the loss of acid sites on the surface of samples during hot sintering. The results show that the surface acid density of Lewis acid on the solid catalyst can be determined by the acidity–basicity electrode with sodium hydroxide as a base. The method is rapid and simple and can provide a cheap method for screening catalyst samples for increasing yield in the FFA esterification process.110
A kind of magnetic ZnO/BiFeO3 was prepared by coprecipitation method. As a cheap and novel magnetic nanocatalyst, which was used to catalyze rapeseed oil to produce biodiesel. The characterization results show that ZnO/BiFeO3 with a saturation magnetization of 13.65 emu g−1 exhibits superparamagnetic behavior at room temperature and can be recycled by external magnets. The optimum process conditions were as follows: methanol/rapeseed oil molar ratio 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, reaction temperature 65 °C, catalyst dosage 4 wt%, and rapeseed oil conversion 95.43%. The catalyst was reused for five times, and the yield was 92.08% after the fifth cycle. The results of CO2-TPD showed that the addition of ZnO to BiFeO3 could improve the basicity and activity.111
1, reaction temperature 65 °C, catalyst dosage 4 wt%, and rapeseed oil conversion 95.43%. The catalyst was reused for five times, and the yield was 92.08% after the fifth cycle. The results of CO2-TPD showed that the addition of ZnO to BiFeO3 could improve the basicity and activity.111
SBA-15 loaded 5-Na/ZnO was prepared by the one-pot method and wet impregnation method to catalyze cottonseed oil for produce biodiesel. The catalyst activity depends on its alkali strength. Under the optimum conditions, 12 wt% catalyst and 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of methanol to oil at 65 °C for 4 h, the yield of FAME was over 98%. Even in the fifth cycle after regeneration, the yield was as high as 74%. Metal leaching was observed in the fourth and fifth cycles. The activation energy of the reaction is 77.3 kJ mol−1.112
1 molar ratio of methanol to oil at 65 °C for 4 h, the yield of FAME was over 98%. Even in the fifth cycle after regeneration, the yield was as high as 74%. Metal leaching was observed in the fourth and fifth cycles. The activation energy of the reaction is 77.3 kJ mol−1.112
5. Modified zinc oxide nanocatalysts
A novel active solid acid catalyst for sulfated zinc oxide (SO42−-ZnO and SO42−/ZnO) was prepared by coprecipitation and impregnation, respectively. The catalytic performance of solid acid catalyst for transesterification of soybean oil with methanol to produce biodiesel was studied (in Table 3). The performance trend of these two catalysts is due to the effect of the combination of sulfonate and ZnO structure to form active acid sites. The catalysts for transesterification of soybean oil with methanol at mild conditions (temperature 65 °C, the molar ratio of methanol to oil 6, catalyst loading 4 wt%) were tested. It was found that the excellent rate of SO42−-ZnO catalyst was 80.19% in 4 h reaction time. Therefore, SO42−-ZnO catalyst has the potential to catalyze soybean oil to produce biodiesel.113Soltani et al.114 synthesized –SO3H functionalized mesoporous ZnO catalyst (SO3H-ZnO) to catalyze the esterification of palm fatty acid distillates (PFAD) containing high FFA (>90%). Mesoporous zinc oxide catalysts were prepared by hydrothermal method, and further functionalized by post-sulfonation treatment. SO3H acid groups were applied on the mesoporous walls (as shown in Fig. 8). The mesoporous SO3H-ZnO catalyst has the unique properties of high specific surface area (305.62 m2 g−1), suitable pore size (3.16 nm) and high acid density (1.72 mmol g−1). The yield of FAME was 95.60% with a methanol-oil molar ratio of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, catalyst 2 wt%, reaction temperature of 120 °C and the reaction time of 90 min. In addition, recyclability experiments show that the used catalyst is most likely to be recycled seven times without further treatment. This indicates that large specific surface area, strong acidic sites, and mesoporous structure have a great influence on the activity of catalytic materials. Modified ZnO nanoparticles have excellent catalytic activity in the production of biodiesel.
1, catalyst 2 wt%, reaction temperature of 120 °C and the reaction time of 90 min. In addition, recyclability experiments show that the used catalyst is most likely to be recycled seven times without further treatment. This indicates that large specific surface area, strong acidic sites, and mesoporous structure have a great influence on the activity of catalytic materials. Modified ZnO nanoparticles have excellent catalytic activity in the production of biodiesel.
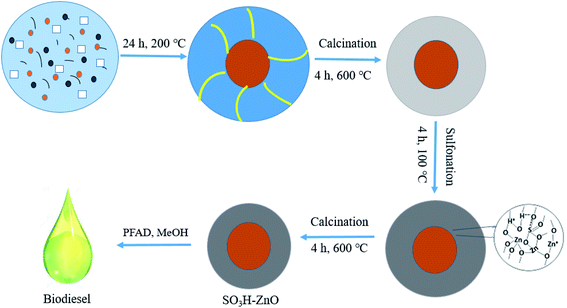 | ||
| Fig. 8 Preparation route of SO3H-ZnO catalyst. Reproduced with permission from ref. 37. Copyright 2017 Elsevier. | ||
Hangaraj et al.115 prepared heteropoly acid supported zinc oxide nanocatalysts by coprecipitation method. The nano-catalyst can catalyze FFA to FAME, and the yield is up to 95% in 5 h. The results showed that the zinc oxide catalyst modified by heteropoly acid had good catalytic activity.
6. Other ZnO catalysts
Corro et al.116 used ZnO/silica as a heterogeneous photocatalyst to esterify high FFA in JCCO with methanol under ultraviolet light. After 10 cycles of esterification, the activity of catalyst remained unchanged, indicating that heterogeneous photocatalysis is a feasible choice for FFA esterification to produce biodiesel. Considering the formation of H+, CH3O·and R-COOH· on the surface of photocatalyst, the possible mechanism of esterification reaction was proposed. The attempt to prepare biodiesel with photocatalyst provides a choice for future industrial production. The application of ZnO/SiO2 photocatalyst shows that ZnO is a kind of catalyst with good application prospects. It can be used not only in conventional catalysts, but also in photocatalysts.Nur et al.117 tried to modify Malaysian dolomite by adding zinc oxide and tin oxide to catalyze palm oil to produce biodiesel. The results show that SnO2 and ZnO modified material is an excellent biodiesel catalyst.
For the first time, Soltani et al.118 synthesized a kind of mixed metal oxides with different molar ratios (Al/(Zn + Al) as mesoporous materials. Then, a solid acid SO3H-ZnAl2O4 catalyst was prepared by post-sulfonation treatment. The optimized mesoporous SO3H-ZnAl2O4 catalyst has unique properties, such as the surface area of 352.39 m2 g−1, the average pore size of 3.10 nm, the total pore volume of 0.13 cm3 g−1, and acid density of 1.95 mmol g−1. The catalytic activity of the catalysts was further investigated through the esterification of PFAD with high FFA (about 90%). The yield of FAME reached 94.65%. In addition, the polymer mesoporous SO3H-ZnAl2O4 catalyst can be reused for eight cycles without obvious loss of activity.
At the same time, Soltani et al.119 prepared nanocrystalline mesoporous CuO-ZnO hollow spheres by hydrothermal method. Mesoporous nanocomposites were prepared by cleverly using glucose as a template. After sulfonation, it was used to catalyze the esterification of PFAD to prepare biodiesel. The physical and chemical properties, structure, structure and thermal properties of the mesoporous copper catalyst was evaluated.
7. Possible reaction mechanism
According to Yan et al.120 ZnO can provide Lewis acid and Lewis base sites, which play catalytic roles in esterification and transesterification, respectively (in Fig. 9 and 10). It is generally believed that O provides the base site, while metal ions provide the acidic site. If the active site is more dispersed, such as doping La2O3, it will have a better catalytic effect.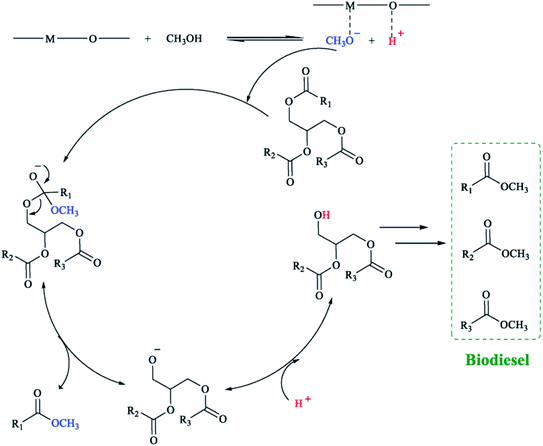 | ||
| Fig. 9 Representation of possible mechanism for transesterification of triglycerides with methanol. M: Lewis base site. Reproduced with permission from ref. 120. Copyright 2009 Elsevier. | ||
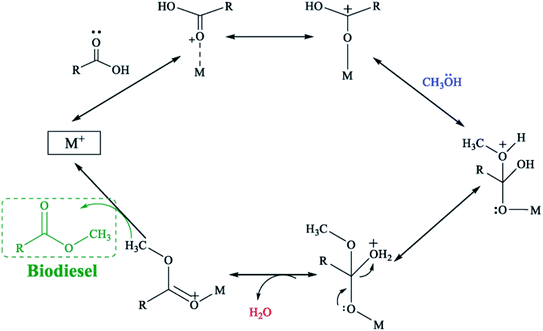 | ||
| Fig. 10 Representation of possible mechanism for esterification of FFAs with methanol. M: Lewis acid site. Reproduced with permission from ref. 121. Copyright 2019 Elsevier. | ||
8. Summary and outlook
In this review, the application of ZnO and its modified nanomaterials in biodiesel preparation was investigated. Because of the simple preparation method, low cost, high catalytic activity, and good stability, this kind of materials attracted people's attention. In order to enhance the activity of zinc oxide, many doping modifications and acid-base modifications were carried out, and relatively satisfactory nanocatalysts of ZnO were obtained. The main factors affecting catalyst activity are the modification method and calcination temperature. Therefore, it is necessary to discuss in detail the influence of calcination temperature on the surface area, pore size and acid-base density during the preparation of catalysts.In general, the good reusability of the catalyst means lower production cost. Obviously, in the doped and oxide supported ZnO composite nanomaterials, due to the leaching of oxide, the reusability of some catalysts containing ZnO is poor. On the other hand, the ZnO composite nano materials with fixed supports are not easy to lose active sites because ZnO is fixed on the carrier, and the reuse performance of the catalyst has been improved obviously, which can meet the production demands. Therefore, it will be the focus of future research to find more stable and efficient composite oxide catalysts for transesterification. At the same time, at this stage, waste cooking oil is more and more used as biodiesel raw material, which generally contains a large amount of FFA and water, requires strong acid and water tolerance of catalysts that will be a focus of future catalysts research and development.
There is no doubt that biodiesel is the best alternative to fossil fuels. However, obtaining heterogeneous catalysts with excellent performance is a way to reduce the cost of biodiesel. Through the above introduction, it is confirmed that zinc oxide and its modified catalyst have good catalytic performance. Pure zinc oxide nanocatalyst has a certain catalytic effect, while supported zinc oxide material has a good catalytic effect. At the same time, the researchers also tried to use photocatalysts to prepare biodiesel, and prepared hollow structure catalysts, indicating that ZnO nanomaterials have important applications in biodiesel preparation.
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This study was financially supported by the Guizhou provincial characteristic key laboratory (QJHKY [2021]002), National Natural Science Foundation of China (21908033), Fok Ying-Tong Education Foundation (161030).References
- Z. E. Tang, S. Lim, Y. L. Pang, H. C. Ong and K. T. Lee, Renewable Sustainable Energy Rev., 2018, 92, 235–253 CrossRef CAS.
- G. Pathak, D. Das, K. Rajkumari and L. Rokhum, Green Chem., 2018, 20, 2365–2373 RSC.
- A. F. Lee, J. A. Bennett, J. C. Manayil and K. Wilson, Chem. Soc. Rev., 2014, 43, 7887–7916 RSC.
- F. Su and Y. Guo, Green Chem., 2014, 16, 2934–2957 RSC.
- M. R. Avhad and J. M. Marchetti, Catal. Rev., 2016, 58, 157–208 CrossRef CAS.
- H. H. Mardhiah, H. C. Ong, H. H. Masjuki, S. Lim and H. V. Lee, Renewable Sustainable Energy Rev., 2017, 67, 1225–1236 CrossRef CAS.
- A. Wang, H. Li, H. Pan, H. Zhang, F. Xu, Z. Yu and S. Yang, Fuel Process. Technol., 2018, 181, 259–267 CrossRef CAS.
- G. Knothe, Prog. Energy Combust. Sci., 2010, 36, 364–373 CrossRef CAS.
- H. Zhang, H. Li, H. Pan, A. Wang, S. Souzanchi, C. C. Xu and S. Yang, Appl. Energy, 2018, 223, 416–429 CrossRef CAS.
- I. M. Lokman, U. Rashid, R. Yunus and Y. H. Taufiq-Yap, Catal. Rev., 2014, 56, 187–219 CrossRef CAS.
- R. Wang, M. A. Hanna, W. W. Zhou, P. S. Bhadury, Q. Chen, B. A. Song and S. Yang, Bioresour. Technol., 2011, 102, 1194–1199 CrossRef CAS PubMed.
- F. Chang, M. A. Hanna, D. J. Zhang, H. Li, Q. Zhou, B. A. Song and S. Yang, Bioresour. Technol., 2013, 140, 435–438 CrossRef CAS PubMed.
- H. Zhang, Q. Zhou, F. Chang, Hu Pan, X. F. Liu, H. Li, D. Y. Hu and S. Yang, Ind. Crop. Prod., 2015, 76, 768–771 CrossRef CAS.
- H. Zhang, H. Li, H. Pan, X. Liu, K. Yang, S. Huang and S. Yang, Energy Convers. Manage., 2017, 138, 45–53 CrossRef CAS.
- Y. Zhou, S. Niu and J. Li, Energy Convers. Manage., 2016, 114, 188–196 CrossRef CAS.
- M. Kirubakaran and V. A. M. Selvan, Renewable Sustainable Energy Rev., 2018, 82, 390–401 CrossRef CAS.
- A. Islam, Y. H. Taufiq-Yap, C. M. Chu, E. S. Chan and P. Ravindra, Process Saf. Environ. Prot., 2013, 91, 131–144 CrossRef CAS.
- B. F. Pinto, M. A. S. Garcia, J. C. S. Costa, C. V. R. de Moura, W. C. de Abreu and E. M. de Moura, Fuel, 2019, 239, 290–296 CrossRef CAS.
- H. Pan, H. Li, H. Zhang, A. Wang and S. Yang, Fuel, 2019, 239, 886–895 CrossRef CAS.
- H. Li, F. Liu, X. Ma, Z. Wu, Y. Li, L. Zhang, S. Zhou and Y. Helian, Energy Convers. Manage., 2019, 180, 401–410 CrossRef CAS.
- S. Rafiei, S. Tangestaninejad, P. Horcajada, M. Moghadam, V. Mirkhani, I. Mohammadpoor-Baltork, R. Kardanpour and F. Zadehahmadi, Chem. Eng. J., 2018, 334, 1233–1241 CrossRef CAS.
- A. Enferadi-Kerenkan, T. O. Do and S. Kaliaguine, Catal. Sci. Technol., 2018, 8, 2257–2284 RSC.
- H. Pan, H. Li, H. Zhang, A. Wang, D. Jin and S. Yang, Energy Convers. Manage., 2018, 166, 534–544 CrossRef CAS.
- Y. Wang, D. Wang, M. Tan, B. Jiang, J. Zheng, N. Tsubaki and M. Wu, ACS Appl. Mater. Interfaces, 2015, 7, 26767–26775 CrossRef CAS PubMed.
- W. Roschat, S. Phewphong, A. Thangthong, P. Moonsin, B. Yoosuk, T. Kaewpuang and V. Promarak, Energy Convers. Manage., 2018, 165, 1–7 CrossRef CAS.
- M. Shi, P. Zhang, M. Fan, P. Jiang and Y. Dong, Fuel, 2017, 197, 343–347 CrossRef CAS.
- A. Navajas, I. Campo, A. Moral, J. Echave, O. Sanz, M. Montes, J. A. Odriozola, G. Arzamenddi and L. M. Gandía, Fuel, 2018, 211, 173–181 CrossRef CAS.
- A. Hykkerud and J. M. Marchetti, Biomass Bioenergy, 2016, 95, 340–343 CrossRef CAS.
- A. B. Fadhil, A. M. Aziz and M. H. Al-Tamer, Energy Convers. Manage., 2016, 108, 255–265 CrossRef CAS.
- H. Li, S. Yang, A. Riisager, A. Pandey, R. S. Sangwan, S. Saravanamurugan and R. Luque, Green Chem., 2016, 18, 5701–5735 RSC.
- R. V. Quah, Y. H. Tan, N. M. Mubarak, M. Khalid and E. C. Abdullah, J. Environ. Chem. Eng., 2019, 7, 103219 CrossRef CAS.
- C. Zhang, L. Yin, L. Zhang, Y. Qi and N. Lun, Mater. Lett., 2012, 67, 303–307 CrossRef CAS.
- B. K. Highina, I. M. Bugaje and B. Umar, J. Pet. Technol. Altern. Fuels, 2011, 2, 146–149 CAS.
- A. V. R. K. Rao, P. Dudhe and V. Chelvam, Catal. Commun., 2021, 149, 10628 CrossRef.
- S. Palanisamy, S. Cheemalapati and S. M. Chen, Int. J. Electrochem. Sci., 2012, 7, 11477–11487 CAS.
- A. C. Alba-Rubio, J. Santamaría-González, J. M. Mérida-Robles, R. Moreno-Tost, D. Martín-Alonso, A. Jiménez-López and P. Maireles-Torres, Catal. Today, 2010, 149, 281–287 CrossRef CAS.
- V. Saxena, S. Sharma and L. M. Pandey, Mater. Lett., 2019, 237, 232–235 CrossRef CAS.
- M. Massaro, M. Casiello, L. D'Accolti, G. Lazzara, A. Nacci, G. Nicotra, R. Noto, A. Pettignano, C. Spinella and S. Riela, Appl. Clay Sci., 2020, 189, 105527 CrossRef CAS.
- S. P. Prakoso and R. Saleh, Mater. Sci. Appl. Chem., 2012, 3, 530–537 Search PubMed.
- M. Vafaee and M. S. Ghamsari, Mater. Lett., 2007, 61, 3265–3268 CrossRef CAS.
- K. Hayat, M. A. Gondal, M. M. Khaled, S. Ahmed and A. M. Shemsi, Appl. Catal., A, 2011, 393, 122–129 CrossRef CAS.
- M. Fu, Y. Li, P. Lu, J. Liu and F. Dong, Appl. Catal., A, 2011, 258, 1587–1591 CAS.
- M. S. Tokumoto, S. H. Pulcinelli, C. V. Santilli and V. Briois, J. Phys. Chem. B, 2003, 107, 568–574 CrossRef CAS.
- A. Erol, S. Okur, B. Comba, Ö. Mermer and M. C. Arıkan, Sens. Actuators, B, 2010, 145, 174–180 CrossRef CAS.
- S. Esposito, Materials, 2019, 124, 668 CrossRef PubMed.
- X. Wang, Y. Zhang, C. Hao, F. Feng, H. Yin and N. Si, Ind. Eng. Chem. Res., 2014, 53, 6585–6592 CrossRef CAS.
- W. Xie, Z. Yang and H. Chun, Ind. Eng. Chem. Res., 2007, 46, 7942–7949 CrossRef CAS.
- W. Raza, M. M. Haque and M. Muneer, Appl. Catal., A, 2014, 322, 215–224 CAS.
- W. Xie and X. Huang, Catal. Lett., 2006, 107, 53–59 CrossRef CAS.
- A. Matei, I. Cernica, O. Cadar, C. Roman and V. Schiopu, Inter. J. Mater. Form., 2008, 1, 767–770 CrossRef.
- J. Wu, X. Shen, L. Jiang, K. Wang and K. Chen, Appl. Catal., A, 2010, 256, 2826–2830 CAS.
- S. J. Yoo, H. S. Lee, B. Veriansyah, J. Kim, J. D. Kim and Y. W. Lee, Bioresour. Technol., 2010, 101, 8686–8689 CrossRef CAS PubMed.
- F. Liu and Y. Zhang, Ceram. Int., 2011, 37, 3193–3202 CrossRef CAS.
- M. Kim, H. S. Lee, S. J. Yoo, Y. S. Youn, Y. H. Shin and Y. W. Lee, Fuel, 2013, 109, 279–284 CrossRef CAS.
- T. L. Kwong and K. F. Yung, Renewable Energy, 2016, 90, 450–457 CrossRef CAS.
- A. Nambo, C. M. Miralda, J. B. Jasinski and M. A. Carreon, React. Kinet., Mech. Catal., 2015, 114, 583–595 CrossRef CAS.
- L. Zhang, J. Zhao, J. Zheng, L. Li and Z. Zhu, Sens. Actuators, B, 2011, 158, 144–150 CrossRef CAS.
- M. R. Quirino, M. J. C. Oliveira, D. Keyson, G. L. Lucena, J. B. L. Oliveira and L. Gama, Mater. Chem. Phys., 2017, 185, 24–30 CrossRef CAS.
- Z. D. Yigezu and K. Muthukumar, Energy Convers. Manage., 2014, 84, 326–333 CrossRef CAS.
- R. Varghese, J. P. Henry and J. Irudayaraj, Environ. Prog. Sustainable Energy, 2018, 37, 1176–1182 CrossRef CAS.
- M. Raghavendra, K. V. Yatish and H. S. Lalithamba, Eur. Phys. J. Plus, 2017, 132, 358 CrossRef.
- S. Yan, S. Mohan, C. DiMaggio, M. Kim, K. S. Ng and S. O. Salley, Fuel, 2010, 89, 2844–2852 CrossRef CAS.
- Y. H. Taufiq-Yap, H. V. Lee, M. Z. Hussein and R. Yunus, Biomass Bioenergy, 2011, 35, 827–834 CrossRef CAS.
- Y. Wang, S. Y. Hu, Y. P. Guan, L. B. Wen and H. Y. Han, Catal. Lett., 2009, 131, 574–578 CrossRef CAS.
- M. M. Zainol, N. A. S. Amin and M. Asmadi, Bioresour. Technol., 2015, 190, 44–50 CrossRef CAS PubMed.
- J. He, H. Li, Y. Liu, W. Zhao, T. Yang, W. Xue and S. Yang, J. Ind. Eng. Chem., 2016, 43, 133–141 CrossRef CAS.
- A. Ali, P. Khullar and D. Kumar, Energy Sources, Part A, 2014, 36, 1999–2008 CrossRef CAS.
- M. Yadav, V. Singh and Y. C. Sharma, Energy Convers. Manage., 2017, 148, 1438–1452 CrossRef CAS.
- B. Gurunathan and A. Ravi, Bioresour. Technol., 2015, 188, 124–127 CrossRef CAS PubMed.
- B. Gurunathan and A. Ravi, Bioresour. Technol., 2015, 190, 424–428 CrossRef CAS PubMed.
- G. Baskar, I. A. E. Selvakumari and R. Aiswarya, Bioresour. Technol., 2018, 250, 793–798 CrossRef CAS PubMed.
- G. Baskar and S. Soumiya, Renewable Energy, 2016, 98, 101–107 CrossRef CAS.
- G. Baskar, S. Soumiya and R. Aiswarya, Inter. J. Modern Sci. Technol., 2016, 1, 129–137 Search PubMed.
- G. Baskar, A. Gurugulladevi, T. Nishanthini, R. Aiswarya and K. Tamilarasan, Renewable Energy, 2017, 103, 641–646 CrossRef CAS.
- G. Nagaraju, S. A. Prashanth, M. Shastri, K. V. Yathish, C. Anupama and D. Rangappa, Mater. Res. Bull., 2017, 94, 54–63 CrossRef CAS.
- M. A. Olutoye, M. A. B. Suleiman and A. S. Yusuff, Adv. Res., 2016, 1, 1–8 Search PubMed.
- J. El Ghoul, M. Kraini and L. J. El Mir, Mater. Sci., 2015, 26, 2555–2562 CAS.
- X. Liu, H. He, Y. Wang, S. Zhu and X. Piao, Fuel, 2008, 87, 216–221 CrossRef CAS.
- A. Birla, B. Singh, S. N. Upadhyay and Y. C. Sharma, Bioresour. Technol., 2012, 106, 95–100 CrossRef CAS PubMed.
- T. F. Dossin, M. F. Reyniers, R. J. Berger and G. B. Marin, Appl. Catal., B, 2006, 67, 136–148 CrossRef CAS.
- Q. Zhou, H. Zhang, F. Chang, H. Li, H. Pan, W. Xue, D. Hu and S. Yang, J. Ind. Eng. Chem., 2015, 31, 385–392 CrossRef CAS.
- S. G. Kumar and K. K. Rao, Appl. Catal., A, 2017, 391, 124–148 CAS.
- J. Toledo Arana, J. J. Torres, D. F. Acevedo, C. O. Illanes, N. A. Ochoa and C. L. Pagliero, Int. J. Chem. Eng., 2019, 1806017 Search PubMed.
- Y. H. Taufiq-Yap, H. V. Lee, R. Yunus and J. C. Juan, Chem. Eng. J., 2011, 178, 342–347 CrossRef CAS.
- J. Q. He, J. Yin, D. Liu, L. X. Zhang, F. S. Cai and L. J. Bie, Sens. Actuators, B, 2013, 182, 170–175 CrossRef CAS.
- A. Wang, H. Li, H. Zhang, H. Pan and S. Yang, Materials, 2019, 12, 83 CrossRef CAS PubMed.
- R. Madhuvilakku and S. Piraman, Bioresour. Technol., 2013, 150, 55–59 CrossRef CAS PubMed.
- H. Lee, J. Juan, T. Y. Yun Hin and H. Ong, Energies, 2016, 9, 611 CrossRef.
- H. V. Lee and Y. H. Taufiq-Yap, Process Saf. Environ. Prot., 2015, 94, 430–440 CrossRef CAS.
- J. M. Rubio-Caballero, J. Santamaría-González, J. Mérida-Robles, R. Moreno-Tost, M. L. Alonso-Castillo, E. Vereda-Alonso, A. Jiménez-López and P. Maireles-Torres, Fuel, 2013, 105, 518–522 CrossRef CAS.
- D. Kumar and A. Ali, Energy Fuels, 2013, 27, 3758–3768 CrossRef CAS.
- I. Lukić, Ž. Kesić, S. Maksimović, M. Zdujić, H. Liu, J. Krstić and D. Skala, Fuel, 2013, 113, 367–378 CrossRef.
- I. Lukić, Ž. Kesić, S. Maksimović, M. Zdujić, J. Krstić and D. Skala, Chem. Ind. Chem. Eng. Q., 2014, 20, 425–439 CrossRef.
- F. Ouanji, M. Khachani, S. Arsalane, M. Kacimi, M. Halim and A. El Hamidi, Monatshefte für Chemie-Chem. Month., 2016, 147, 1693–1702 CrossRef CAS.
- I. Istadi, S. A. Prasetyo and T. S. Nugroho, Procedia Environ. Sci., 2015, 23, 394–399 CrossRef CAS.
- F. Fan, C. Gao, L. Jia and X. Guo, Res.Chem. Inter., 2014, 40, 157–167 CrossRef CAS.
- P. M. Veiga, C. O. Veloso and C. A. Henriques, Renewable Energy, 2016, 99, 543–552 CrossRef CAS.
- N. Pasupulety, G. L. Rempel and F. T. Ng, Appl. Catal., A, 2015, 489, 77–85 CrossRef CAS.
- H. V. Lee, Y. H. Taufiq-Yap, M. Z. Hussein and R. Yunus, Energy, 2013, 49, 12–18 CrossRef CAS.
- I. Lukić, Ž. Kesić and D. Skala, Chem. Eng. Technol., 2014, 37, 1879–1884 CrossRef.
- P. M. Veiga, A. S. Luna, M. de Figueiredo Portilho, C. de Oliveira Veloso and C. A. Henriques, Energy, 2014, 75, 453–462 CrossRef CAS.
- F. M. da Silva, D. M. Pinho, G. P. Houg, I. B. Reis, M. Kawamura, M. S. Quemel, P. R. Montes and P. A. Suarez, Chem. Eng. Res. Des., 2014, 92, 1463–1469 CrossRef CAS.
- N. P. Asri, S. Soe'eib and B. Poedjojono, Euro-Mediter. J. Environ. Integr., 2018, 3, 3 CrossRef.
- K. Thirunavukkarasu, T. M. Sankaranarayanan, A. Pandurangan, R. V. Shanthi and S. Sivasanker, Catal. Sci. Technol., 2014, 4, 851–860 RSC.
- Y. Carrera, G. Morales-Mendoza, G. Valverde-Aguilar and A. Mantilla, Catal. Today, 2013, 212, 164–168 CrossRef.
- D. Singh, A. Ganesh and S. Mahajani, Clean Technol. Environ. Policy, 2015, 17, 1103–1110 CrossRef CAS.
- D. Singh, R. Bhoi, A. Ganesh and S. Mahajani, Energy Fuels, 2014, 28, 2743–2753 CrossRef CAS.
- A. F. F. Farias, K. F. Moura, J. K. Souza, R. O. Lima, J. D. Nascimento, A. A. Cutrim, E. Longo, A. S. Araujo, J. R. Carvalho-Filho, A. G. Souza and I. M. Santos, Fuel, 2015, 160, 357–365 CrossRef CAS.
- L. Wan, H. Liu and D. Skala, Appl. Catal., B, 2014, 152, 352–359 CrossRef.
- G. Corro, F. Bañuelos, E. Vidal and S. Cebada, Fuel, 2014, 115, 625–628 CrossRef CAS.
- Z. Salimi and S. A. Hosseini, Fuel, 2019, 239, 1204–1212 CrossRef CAS.
- R. Malhotra and A. Ali, Renewable Energy, 2019, 133, 606–619 CrossRef CAS.
- I. Istadi, D. D. Anggoro, L. Buchori, D. A. Rahmawati and D. Intaningrum, Procedia Environ. Sci., 2015, 23, 385–393 CrossRef CAS.
- S. Soltani, U. Rashid, S. I. Al-Resayes and I. A. Nehdi, J. Cleaner Prod., 2017, 144, 482–491 CrossRef CAS.
- B. Thangaraj and S. Piraman, Biofuels, 2016, 7, 13–20 CrossRef.
- G. Corro, U. Pal and N. Tellez, Appl. Catal., B, 2013, 129, 39–47 CrossRef CAS.
- Z. S. Nur, Y. H. Taufiq-Yap, M. R. Nizah, S. H. Teo, O. N. Syazwani and A. Islam, Energy Convers. Manage., 2014, 78, 738–744 CrossRef.
- S. Soltani, U. Rashid, R. Yunus and Y. H. Taufiq-Yap, Fuel, 2016, 178, 253–262 CrossRef CAS.
- S. Soltani, U. Rashid, I. A. Nehdi and S. I. Al-Resayes, Chem. Eng. Technol., 2017, 40, 1931–1939 CrossRef CAS.
- S. Yan, S. O. Salley and K. Y. Simon, Appl. Catal., A, 2009, 353, 203–212 CrossRef CAS.
- H. Zhang, H. Li, Y. L. Hu, K. T. V. Rao, C. B. Xu and S. Yang, Renewable Sustainable Energy Rev., 2019, 114, 109296 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |