 Open Access Article
Open Access ArticleGreen alternative cosolvents to N-methyl-2-pyrrolidone in water polyurethane dispersions†
Lorena Germán *a,
José María Cuevasa,
Rubén Cobosa,
Leyre Pérez-Alvarezb and
José Luis Vilas-Vilelabc
*a,
José María Cuevasa,
Rubén Cobosa,
Leyre Pérez-Alvarezb and
José Luis Vilas-Vilelabc
aGaiker Technology Centre, Basque Research and Technology Alliance (BRTA), Parque Tecnológico de Bizkaia, edificio 202, E-48170 Zamudio, Spain. E-mail: german@gaiker.es
bMacromolecular Chemistry Group (LABQUIMAC), Department of Physical Chemistry, Faculty of Science and Technology, University of the Basque Country UPV/EHU, Barrio Sarriena s/n 48940 Leioa, Spain. E-mail: joseluis.vilas@ehu.eus
cBCMaterials, Basque Center for Materials, Applications and Nanostructures, UPV/EHU Science Park, 48940 Leioa, Spain
First published on 26th May 2021
Abstract
N-Methyl-2-pyrrolidone is a toxic dipolar aprotic solvent widely used in the synthesis of polyurethane dispersions (PUD). Since legislation strongly restricts this substance, green alternatives are essential. Dihydrolevoglucosenone and gamma valerolactone demonstrate comparable performance to that of NMP as cosolvent in the synthesis and the film forming process of PUD.
The use of organic solvents in commercial products like coatings or adhesives is being limited due to their toxicity and environmental hazard.1,2 Aqueous polymeric nanodispersions are suitable alternatives to reduce the use of organic solvents. PUD are extraordinarily interesting due to their equivalent performance to the conventional solvent borne products, but with some additional advantages such as excellent adhesion, tuneable mechanical properties and functionalization, good biocompatibility and potential biodegradability.3–5
These polymeric dispersions are colloidal systems in which polyurethane (PU) particles are dispersed in aqueous media as a continuous phase. Standard polyurethanes are not dispersible in water due to the presence of isocyanates, which are hydrophobic and they also could react with water. Then, polyurethanes dispersible in water need to be modified. Generally, they are obtained by incorporating diol molecules with hydrophilic carboxylic group as internal emulsifier. After synthesising a linear thermoplastic PU prepolymer, the acids groups of the internal emulsifier are neutralized.
This hydrophilic isocyanate-terminated prepolymer is subsequently extended with low molecular weight alcohols or amines to obtain the target polyurethane. The dispersion in water can take place in different stages of the process depending mainly on the chain extender used, i.e. before or after chain extension.
The most common processes for synthesizing PUD are acetone and prepolymer mixing processes. In acetone process, the polymer synthesis requires acetone to obtain a homogeneous and low viscosity reaction system. However, it has the disadvantage of requiring large amounts of acetone and an extra distillation process for removing this solvent from the waterborne dispersion. This makes the industry to commonly discard this strategy.
Nevertheless, in prepolymer mixing process, the medium molecular weight polymer (prepolymer) is prepared through the reaction of di-functional polyols and the internal emulsifier with a molar excess of di-isocyanates. Then, the prepolymer is extended and the dispersion in water is carried out.
In this process, around 12–15 wt% of organic solvent, generally N-methyl-2-pyrrolidone (NMP), is used to reduce the viscosity of the mixture.6 This organic solvent remains in the dispersion in order to promote the coalescence of nanoparticles and the film formation of coatings or adhesives.7 Therefore, any alternative cosolvent to NMP must work as both reaction media and coalesce agent to achieve similar performance and products.
NMP is a dipolar aprotic solvent with excellent coalescence capacity, but its use is restricted,8 i.e., it shall not be placed on the market as a substance on its own or in mixtures in a concentration equal to or greater than 0.3 wt%.9–11 Hence, there is a pressing need to achieve low toxicity alternative solvents with the suitable polarity profile. Structurally homologues substances with different length of carbon side chain like N-ethyl- or N-butyl-2-pyrrolidone (NEP/NBP)12,13 have been promoted as alternatives to NMP. However, this was simply an attempt to use the lack of environmental and health data available at the time to keep ahead of legislation.14
In addition, the studies by M. Schmidt using NBP as alternative cosolvent for PUD, an extra cosolvent is need. The coalescence process is not effective enough and extra cosolvent is incorporated in the final product formulation to complete the coalescence of polyurethane nanoparticles and the film forming.15 This drawback is also the main disadvantage of solvent free PUDs, where the formulation of coatings or adhesive includes additional solvents to achieve proper film forming in the final product.5,16
In this study, two alternative green solvents with improved Health, Environmental and Safety (HES) profile (see detailed information in Table S1 at ESI†) have been selected. Green solvents studied are: Dihydrole-levoglucosenone (CY) and γ-valerolactone (GVL)17,18 (Fig. 1).
Dihydrolevoglucosenone (Cyrene, CY) is a biodegradable compound synthesised in a two-step process from waste cellulose.19 There are evidences that this new substance is an alternative to dipolar aprotic solvents.20 Cyrene is identified as an effective solvent in different chemical applications such as the synthesis of ureas,21 the preparation of membranes,22 metal organic frameworks (MOF) synthesis,23 heteroatom and alkylation reactions, in nucleophilic fluorination reactions20 or in graphene dispersions.24
On the other hand, γ-valerolactone (GVL) is a biodegradable substance obtained from lignocellulosic biomass25 that is widely used in food and perfume industries.26 However, new applications as green solvent in chemical industry have been recently reported: crosscoupling reactions (Hiyama reaction),27 synthesis of formamides,28 synthesis of phosphatidylserine29 or the production of 5-hydroxymethylfurfural30 are some outstanding examples as green solvent.
Physicochemical properties of these green solvents and NMP are collected in Table 1. Polarity profile of alternative solvents is crucial for successful substitution of NMP. Therefore, solvatochromic parameters (Kamlet–Taft and Reichardt scale of polarity ENT)31 and Hansen parameters have been compared, see Fig. 2 and Table 1. Three Kamlet–Taft parameters are: α, which quantifies hydrogen-bond donating ability (acidity), β, hydrogen-bond accepting ability (basicity) and π*, polarity/polarizability. Dipolar aprotic solvents normally possess low Kamlet–Taft (KT) acidity (α ≈ 0), high basicity (β > 0.6), and high polarity (π* > 0.6).32,33 The Reichardt parameter, ENT, is extensively used for measuring empirically the polarity of different systems (organic and ionic liquids, switchable-hydrophilicity solvents, polymers, surfaces, etc.). ENT values measure preferably the solvent's dipolarity/polarizability (given by π*) and hydrogen bond donor acidity (given by α).34,35
| BP (°C) | P (g cm−3) | Viscosity (mPas) | ENT | Kamet–Abboud–Taft solvatochromic parameters | Hansen solubility parameters @ 25 °C | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| π* | α | β | δD (MPa)½ | δH (MPa)½ | δP (MPa)½ | |||||
| NMP | 204 | 1.03 | 1.67 | 0.355 | 0.9 | 0 | 0.75 | 18 | 7.2 | 12.3 |
| CY | 227 | 1.25 | 8.8 | 0.333 | 0.93 | 0 | 0.61 | 18.8 | 6.9 | 10.6 |
| GVL | 207 | 1.05 | 2.2 | 0.301 | 0.83 | 0 | 0.6 | 16.9 | 6.3 | 11.5 |
 | ||
| Fig. 2 Graphic comparison of polarity and volatility parameter of selected green solvents, NMP and other toxic solvents (N,N-dimethylformamide-DMF and acetone) used in the synthesis of polyurethanes. | ||
Selected solvents have similar parameters. The Kamlet–Abboud–Taft polarity scales are useful in correlations with reaction kinetics and equilibria,20 which affect to PU synthesis. Meanwhile, Hansen solubility parameters provide a measure of solvency power,36 which is useful for synthesising PU and coalescence of aqueous PUD. These are represented for three intermolecular forces: dispersions δD; polarity δP and hydrogen bonding δH. The two alternative cosolvents are close to NMP in Hansen space, and solvatochromic parameters, which makes it possible for green alternatives to exhibit the same solubilising properties and reaction rates from similar equilibrium-solvent effects.36
To the best of author's knowledge, there is no previously published work on evaluating these green solvents in PUD as reaction media and coalescence agent cosolvents. This study, thus, opens up new opportunities and understanding of bio-solvents in green chemistry and polyurethane water dispersions.
Experimental
Synthesis of the waterborne polyurethane dispersions
NMP and the two green alternatives were used to synthesize anionic aqueous polyurethane dispersions following the same procedure (Fig. 3).Water polyurethane dispersions (PUD) were prepared by prepolymer mixing process using NMP, CY and GVL as cosolvent. In this process small quantity of organic cosolvent is used to reduce the viscosity of the reaction materials. The final polyurethane dispersions content a 12.6 wt% of organic solvent.
In this work, a polycarbonate polyol was selected to carry out the study due to its high hydrolytic and oxidative stability, as well as excellent modulus/strength.37 Polyols used in polyurethane synthesis have a significant effect on final properties of polymer.37 Polycarbonate diol T5651 (1000 g mol−1) produced from 1,6-hexanediol and 1,5-pentanediol by transesterification with ethylene carbonate (Asahi Kasei Corporation) was used as the polyol. Isophorone diisocyanate (IPDI) from Merck was used as the isocyanate and dimethylolpropionic acid (DMPA) (Sigma Aldrich) was the internal emulsifier. Triethylamine (TEA) as neutralizer, 1,4-butanediol as chain extender, and dibutyltin dilaurate (DBTDL) as catalyst, were supplied by Sigma Aldrich.
Polyol, IPDI, DMPA, catalyst and cosolvent reacted in a 250 mL glass-jacketed reactor equipped with a mechanical stirrer at 80 °C for 2.5 h, under nitrogen atmosphere, to synthesize the NCO-terminated prepolymer. TEA was added at lower temperature to perform the neutralization process for 0.5 h. Subsequently, the corresponding chain extender was added, and the synthesis of the polyurethane occurred for 1.5 h. Dispersion was achieved by gradually incorporating water to the polyurethane at 30 °C under vigorous stirring.
Then, a standard formulation was defined in order to analyse the effect of cosolvent alternatives on the synthesis of the polyurethanes and the coalescence of the dispersions. The ratio [NCO]/[OH] in prepolymer was 1.5, which corresponds to 44 wt% of hard segment in the final polyurethane. Internal emulsifier, DMPA, was set to 7 wt% based on the weight of the prepolymer, and the corresponding carboxylic groups were 100% neutralized by the addition of TEA. The solid content in the final waterborne PUD was 30 wt%
Conversion of NCO groups by Fourier-transform infrared spectroscopy (FTIR)
Fourier transform infrared spectra were obtained by using Shimadzu MIRacle 10 spectrometer equipped with ATR (Attenuated total reflection). Spectra were recorded in a range of 4000–400 cm−1 with a nominal resolution of 4 cm−1.Particle size and Z-potential
The particle size distribution was evaluated by Dynamic Light Scattering (DLS) technique from measuring variations in the light scattered by the polyurethane dispersions. The zeta potential was established by phase analysis light scattering from the electrophoretic mobility of the dispersions. Both parameters were measured with a Brookhaven ZetaPALS instrument. PUD samples were diluted with deionized water before measurement at 25 °C.Medium term stability
Medium term stability analysis was carried out with Turbiscan™ LAB Stability Analyser from Formulaction SA. The technology embedded in Turbiscan LAB™ is based on the Multiple Light Scattering (MLS) technique which enables fast and sensitive identification of destabilization mechanisms (such as creaming, sedimentation, flocculation, coalescence…). Turbiscan LAB calculates Turbiscan® Stability Index (TSI), which is a specific parameter developed to compare and characterize the physical stability of samples. TSI value can be associated to the destabilization kinetics analysing its evolution over the time.Stability of PUD was evaluated without dilution at different times to detect destabilization mechanisms and obtain destabilization kinetics.
Film fabrication
Polyurethane films were prepared by casting 4 g of polyurethane dispersion in a mould and allowing evaporation of the water at room temperature for 7 days. Afterwards, the polyurethane was dried at 50 °C for 24 h. Transparent films obtained were 0.3–0.5 mm thick, see ESI Fig. S1.†Surface quality by atomic force microscopy (AFM)
Study of PUDs surface topography was carried out using an atomic force microscope (Dimension ICON) equipped with Nanoscope control (Buker). Measurements were performed using peak force tapping mode with ScanAsyst (Bruker) cantilever and a nominal resonant frequency of 70 kHz.Dynamic mechanical analysis (DMA)
The dynamic mechanical thermal properties of the film samples were measured in tensile mode at 3 Hz with a DMA 1 METTLER TOLEDO at a heating rate of 3 °C min−1 from −80 to 50 °C and deformation of 20 μm (Fig. S3†). The dimensions of the film samples were 20 mm × 5 mm × 0.4 mm. Glass transition temperature was determined as the maximum value of loss modulus E′′.38Gel permeation chromatography (GPC)
The molecular mass distribution of the PUD films was determined by gel permeation chromatography, using GPC WATERS equipment, with refractive index detector 2414 and two columns TOSOH HR1 and HR3, calibrated with respect to polystyrene standards. Polymer were dissolved in tetrahydrofuran (THF) and 70 μl of the sample was injected with a volume rate of carrier solvent of 1 mL min−1 at 40 °C temperature. The average molecular masses, Mn, Mw and polydispersity index were determined.Results and discussion
Reaction rate was evaluated by monitoring the conversion of NCO groups (∼2260 cm−1) using Fourier-transform infrared spectroscopy (FTIR),39 which is a technique widely used in the synthesis of polyurethane.40–43The polyurethanes synthesized in green solvents achieved similar NCO conversion with time to the polyurethane synthesized in NMP (see ESI†). However, at intermedium reaction times NMP and GVL achieved higher conversion grades than CY. It seems that solvents with the higher boiling point and density could consume more of the supplied heat before distributing among the reactants at the beginning of the reaction process44. Nevertheless, the final conversion rate is not affected, and, in all cases, the conversion of NCO groups was above 0.98 for the same reaction conditions. Therefore, the studied reaction media do not have significant effect on the conversion rate of the process (Fig. 4).
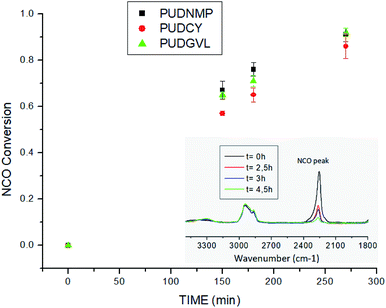 | ||
| Fig. 4 Graphic of NCO conversions in PU synthesis (data in ESI†). | ||
Particle size distribution of PUDs was characterized by Dynamic Light Scattering (DLS). The results reveal that all dispersions have monomodal size distribution with polydispersity (PDI) around 0.1 and d50 < 100 nm, Table 2. Some differences in particle size may result from differences in viscosity of the solvents. Polyurethane particles synthesised in biobased solvents which present higher-viscosity,45 CY with 8.8 mPas and GVL with 2.2 mPas, present a 50% lower particle size than particles synthesised in NMP.
| PUD | Particle size distribution DLS (nm) | Z potential (mV) | Roughness Raa (nm) | DMA Tg (°C) | GPC | ||||
|---|---|---|---|---|---|---|---|---|---|
| PDI | d10b (nm) | d50b (nm) | d90b (nm) | Mw (g mol−1) | PDI | ||||
| a Ra is the arithmetic mean of the absolute values of the height of the surface profile Z(x).52b dn: n% of particles have a diameter below this value. | |||||||||
| PUD_NMP | 0.13 | 60.27 ± 1.72 | 93.57 ± 0.66 | 145.36 ± 3.79 | −45 | 0.340 | −5 | 35.838 | 1.28 |
| PUD_CY | 0.13 | 27.74 ± 0.55 | 43.32 ± 0.32 | 67.68 ± 1.35 | −39 | 0.305 | 0 | 29.323 | 1.29 |
| PUD_GVL | 0.13 | 26.88 ± 0.48 | 42.3 ± 0.36 | 66.59 ± 1.19 | −34 | 0.419 | −5 | 32.221 | 1.19 |
However, the analysis of electrostatic stabilization, analysed by measuring Z-potential, demonstrates excellent stability from values below −30 mV (ref. 46) in all cases, regardless of dispersion particle size. Medium term stability was studied by further analysis using Multiple Light Scattering (MLS) tests using Turbiscan LAB™ optical analyzer47,48 (Fig. 5).
As it could be observed, all the dispersions present certain instability in the early days. However, PUD with NMP and CY become stable with time by observing the asymptotical evolution of Turbiscan Stability Index (TSI). As a consequence, the material became stable but probably with slight changes in some characteristics like particle distribution, Z potential or density39 Meanwhile, PUD synthesised with GVL continues the destabilization process for longer time. By these means, extra stabilization effort is probably needed by maybe increasing the concentration of internal emulsifier in PU structure or adding surfactants to dispersion.
PU films were fabricated and characterized by Gel Permeation Chromatography (GPC) and Dynamic Mechanical Analysis (DMA) to determinate polymer molecular weight (Mw) and thermomechanical properties, respectively. Differences in molar mass of PUDs are not representative, and the polydispersity of all the samples was below 1.5, see Table 2. Likewise, similar thermomechanical profiles are observed in polymers synthesized with NMP and green solvents (Fig. S2, at ESI†).
PU are characterised by two incompatible and separated segments, hard and soft domains, where the hard segment formed by isocyanate reacted with internal emulsifier and chain extender, aggregates into domains that act as reinforcing fillers to the soft segment, formed by polyol chains. The degree of phase separation, as well as the concentration of the hard segments, are contributing factors to the properties of PU.49
Therefore, the results obtained in GPC and DMA analysis suggest that the structure of polyurethane, i.e. distribution and separation of soft and hard segment/phases have not been modified by changing the NMP cosolvent by the two green solvents studied.
Coalescence and film formation capacity of new alternative solvents was studied by Atomic Force Microscopy (AFM)50,51 to evaluate surface quality (roughness in Table 2). The alternative cosolvents lead to excellent surface quality with very low roughness (images included in Table S3 at ESI†), which demonstrates that coalescence process and film formation occurs without any additional cosolvent.
Conclusions
Obtained results demonstrate that Dihydrole-levoglucosenone (CY) and γ-valerolactone (GVL) are excellent and more safe and sustainable alternatives to NMP in the synthesis of PUD. Their incorporation in the described prepolymer mixing process as substitute reaction solvents have not significant effect on the structure and the key properties of the achieved polyurethane polymers.Waterborne polyurethane dispersions synthesised with NMP and the green solvents are characterised by similar particle size distributions and short-medium term stability, where increased viscosity slightly reduces the average particle size.
Furthermore, cosolvent action of the sustainable alternatives in film forming of transparent coatings is as effective as NMP (Fig. S1 at ESI†).
Therefore, the two sustainable alternatives are very promising as means of substituting NMP in producing more safe and sustainable waterborne PU dispersions. From these results, there is a favourable opportunity to promote this kind of green solvents from further studies. Other studies with different PUD systems and formulation strategies are in progress to assess these cutting-edge cosolvents, not only in the synthesis process, but also in the performance of final products such as coatings for textile, wood, automotive or packaging sector.
Conflicts of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this communication.Acknowledgements
The authors acknowledge the financial support from Spanish Government in the frame of RETOS program, (RTC-2017-6385–5) and thank CROMOGENIA UNITS for technical and time support on the project. This work has also been supported by the biobased Industries Undertaking (JU) under the European Union's Horizon 2020 research and innovation programme under VIPRISCAR project grant agreement No 790440. The JU receives support from the European Union's Horizon 2020 research and innovation programme and the Bio Based Industries Consortium.Notes and references
- The European Parliament and the Council of the European Union, Off. J. Eur. Union, 2004, 87–96 Search PubMed.
- EU Commission, J. Chem. Inf. Model., 2013, 53, 1689–1699 CrossRef PubMed.
- H. W. Engels, H. G. Pirkl, R. Albers, R. W. Albach, J. Krause, A. Hoffmann, H. Casselmann and J. Dormish, Angew. Chem., Int. Ed., 2013, 52, 9422–9441 CrossRef CAS PubMed.
- A. Noreen, K. M. Zia, M. Zuber, S. Tabasum and M. J. Saif, Korean J. Chem. Eng., 2016, 33, 388–400 CrossRef CAS.
- X. Liu, W. Hong and X. Chen, Polymers, 2020, 12, 2875 CrossRef CAS PubMed.
- H. Honarkar, J. Dispersion Sci. Technol., 2018, 39, 507–516 CrossRef CAS.
- J. K. Oh, J. Anderson, B. Erdem and R. Drumright, Prog. Org. Coat., 2011, 72, 253–259 CrossRef CAS.
- European Commission, Commission Regulation (EU) 2018/588, 2018 Search PubMed.
- E. C. Agency, Off. J. Eur. Union, 2018, 61, 63–65 Search PubMed.
- A. Hunt and N. Dale, Int. J. Mol. Sci., 2015, 16, 17101–17159 CrossRef PubMed.
- EPA Document EPA-740-R1-7005 Search PubMed.
- ECHA, ECHA and NEP, https://echa.europa.eu/es/substance-information/-/substanceinfo/100.018.409.
- ECHA and NBP, https://echa.europa.eu/es/registration-dossier/-/registered-dossier/10579/7/2/1.
- L. J. Diorazio, D. R. J. Hose and N. K. Adlington, Org. Process Res. Dev., 2016, 20, 760–773 CrossRef CAS.
- M. Schmidt, PCI-Paint Coatings Ind, 2015, https://www.pcimag.com/articles/100361 Search PubMed.
- P. Berce, S. Skale, T. Razboršek and M. Slemnik, J. Appl. Polym. Sci., 2017, 134, 1–9 CrossRef.
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, Green Chem., 2015, 18, 288–296 RSC.
- F. P. Byrne, S. Jin, G. Paggiola, T. H. M. Petchey, J. H. Clark, T. J. Farmer, A. J. Hunt, C. Robert McElroy and J. Sherwood, Sustainable Chem. Processes, 2016, 4, 1–24 CrossRef.
- G. R. Court, C. H. Lawrence, W. D. Raverty and A. J. Duncan, US Pat., 2012/0111714A1, 2012 Search PubMed.
- J. Sherwood, M. De bruyn, A. Constantinou, L. Moity, C. R. McElroy, T. J. Farmer, T. Duncan, W. Raverty, A. J. Hunt and J. H. Clark, Chem. Commun., 2014, 50, 9650–9652 RSC.
- L. Mistry, K. Mapesa, T. W. Bousfield and J. E. Camp, Green Chem., 2017, 19, 2123–2128 RSC.
- T. Marino, F. Galiano, A. Molino and A. Figoli, J. Membr. Sci., 2019, 580, 224–234 CrossRef CAS.
- J. Zhang, G. B. White, M. D. Ryan, A. J. Hunt and M. J. Katz, ACS Sustainable Chem. Eng., 2016, 4, 7186–7192 CrossRef CAS.
- H. J. Salavagione, J. Sherwood, M. De Bruyn, V. L. Budarin, G. J. Ellis, J. H. Clark and P. S. Shuttleworth, Green Chem., 2017, 19, 2550–2560 RSC.
- W. Fang and H. Sixta, ChemSusChem, 2015, 8, 73–76 CrossRef CAS PubMed.
- D. M. Alonso, S. G. Wettstein and J. A. Dumesic, Green Chem., 2013, 15, 584–595 RSC.
- E. Ismalaj, G. Strappaveccia, E. Ballerini, F. Elisei, O. Piermatti, D. Gelman and L. Vaccaro, ACS Sustainable Chem. Eng., 2014, 2, 2461–2464 CrossRef CAS.
- J. Song, B. Zhou, H. Liu, C. Xie, Q. Meng, Z. Zhang and B. Han, Green Chem., 2016, 18, 3956–3961 RSC.
- Z. Q. Duan and F. Hu, Green Chem., 2012, 14, 1581–1583 RSC.
- J. M. R. Gallo, D. M. Alonso, M. A. Mellmer and J. A. Dumesic, Green Chem., 2013, 15, 85–90 RSC.
- P. G. Jessop, D. A. Jessop, D. Fu and L. Phan, Green Chem., 2012, 14, 1245–1259 RSC.
- A. Duereh, Y. Sato, R. L. Smith and H. Inomata, Org. Process Res. Dev., 2017, 21, 114–124 CrossRef CAS.
- T. Islam, M. Z. Islam Sarker, A. H. Uddin, K. Bin Yunus, R. Prasad, M. A. R. Mia and S. Ferdosh, Anal. Chem. Lett., 2020, 10, 550–561 CrossRef CAS.
- C. Reichardt, Chem. Rev., 1994, 94, 2319–2358 CrossRef CAS.
- J. P. Cerõn-Carrasco, D. Jacquemin, C. Laurence, A. Planchat, C. Reichardt and K. Sraïdi, J. Phys. Org. Chem., 2014, 27, 512–518 CrossRef.
- Hansen solubility parameters : a user's handbook, ed. C. Hansen, 2nd edn, 2013, ISBN 0-8493-7248-8 Search PubMed.
- H. Pandya and P. Mahanwar, Adv. Ind. Eng. Polym. Res., 2020, 3, 102–110 Search PubMed.
- P. J. Achorn and R. G. Ferrillo, J. Appl. Polym. Sci., 1994, 54, 2033–2043 CrossRef CAS.
- M. Ocepek, J. Zabret, J. Kecelj, P. Venturini and J. Golob, Mater. Tehnol., 2015, 49, 495–501 CrossRef.
- L. Poussard, J. Lazko, J. Mariage, J. M. Raquez and P. Dubois, Prog. Org. Coat., 2016, 97, 175–183 CrossRef CAS.
- C. A. Cateto, M. F. Barreiro and A. E. Rodrigues, Ind. Crops Prod., 2008, 27, 168–174 CrossRef CAS.
- J. C. Tilly, A. K. Pervaje, D. L. Inglefield, E. E. Santiso, R. J. Spontak and S. A. Khan, ACS Omega, 2019, 4, 932–939 CrossRef CAS PubMed.
- S. Boufi, M. N. Belgacem, J. Quillerou and A. Gandini, Macromolecules, 1993, 6706–6717 CrossRef CAS.
- S. Hussain and A. Shafeeq, Arabian J. Chem., 2020, 13, 3957–3962 CrossRef.
- X. mei Wang and Q. Li, Prog. Org. Coat., 2019, 131, 285–290 CrossRef.
- G. V. Lowry, R. J. Hill, S. Harper, A. F. Rawle, C. O. Hendren, F. Klaessig, U. Nobbmann, P. Sayre and J. Rumble, Environ. Sci.: Nano, 2016, 3, 953–965 RSC.
- M. G. De Paola, N. Arcuri, V. Calabrò and M. De Simone, Energies, 2017, 10, 354 CrossRef.
- X. Qi, Y. Dong, H. Wang, C. Wang and F. Li, Colloids Surf., A, 2017, 535, 96–104 CrossRef CAS.
- S. M. Cakić, I. S. Ristić and O. Z. Ristić, Polyurethane, IntechOpen, ch. 5, 2012, DOI:10.5772/35800.
- S. M. Cakić, M. Špírková, I. S. Ristić, J. K. B-Simendić, M. M-Cincović and R. Poręba, Mater. Chem. Phys., 2013, 138, 277–285 CrossRef.
- M. Špírková, J. Hodan, J. Kredatusová, R. Poręba, M. Uchman and M. Serkis-Rodzeń, Prog. Org. Coat., 2018, 123, 53–62 CrossRef.
- R. R. L. De, D. A. C. Albuquerque, T. G. S. Cruz, F. M. Yamaji and F. L. Leite, Measurement of the Nanoscale Roughness by Atomic Force Microscopy: Basic Principles and Applications, Atomic Force Microscopy - Imaging, Measuring and Manipulating Surfaces at the Atomic Scale, 2012, InTech, pp. 148–174, 978-953-51-0414-8 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra03157k |
| This journal is © The Royal Society of Chemistry 2021 |



